Abstract
Kidneys are essential to life but vulnerable to a range of toxicants, including therapeutic drugs and their metabolites. Indeed, nephrotoxicity is often a limiting factor in both drug use and drug development. Most toxicants damage kidneys by one of four mechanisms: damage to the membrane and its junctions, oxidative stress and free radical generation, activation of inflammatory processes, and interference with vascular regulation. Traditionally, animal models were used in preclinical screening for nephrotoxicity, but these can be poorly predictive of human reactions. Animal screens have been joined by simple single-cell–type in vitro assays using primary or immortalized human cells, particularly proximal tubule cells as these are especially vulnerable to toxicants. Recent research, aimed mainly at engineering new kidneys for transplant purposes, has resulted in a method for constructing anatomically realistic mini-kidneys from renogenic stem cells. So far, this has been done only using renogenic stem cells obtained directly from mouse embryos but, in principle, it should be possible to make them from renogenically directed human-induced pluripotent cells. If this can be done, the resulting human-based mini-kidneys would be a promising system for detecting some types of nephrotoxicity and for developing nephroprotective drugs.
Keywords: self-organization, nephrotoxicity, iPS cell, tissue engineering, 3Rs, organogenesis, kidney development, cap mesenchyme, ureteric bud
Introduction
Kidneys are essential to life, receiving and detoxifying about one-fifth of the blood output of each heartbeat. Without working kidneys or an artificial means to replicate their function, humans can live only days. Unfortunately, the kidney is a major target for exogenous toxicant chemicals, ranging from intentionally given drugs to toxicants in foodstuffs, to environmental pollutants.1–4 It is also vulnerable to toxins (ie, toxicants produced by living organisms) that may be eaten in food or inoculated by venomous animals.5,6
The vulnerability of kidneys to get damaged by therapeutic drugs or their metabolites is a serious issue in medicine. In one study, about 1% of all patients admitted to a hospital above the age of 60 developed acute renal failure due to drug-induced nephrotoxicity.7 The relatively high vulnerability of the elderly reflects both age-diminished renal function, reducing margins of safety, and also the number of different drugs that might be given to an older patient at the same time, some of which can act synergistically to cause damage.8–10 As well as providing unexpected emergencies in response to drug treatment, nephrotoxic reactions can be the major limitation to the effectiveness of therapies. In oncology, for example, the dose of drugs such as cisplatin may have to be kept lower than would be optimal for anticancer purposes in order to avoid destroying a patient’s kidneys. Similarly, antibiotics that would otherwise be very useful against infections may have to be avoided, or used at suboptimal doses, in order to protect renal function. For at least some types of nephrotoxicity, the route to damage involves a specific cellular activity, such as an active uptake system that concentrates a toxicant in a cell. This raises the hope of pharmacological intervention so that a protective drug P can block the cellular pathway that makes potentially nephrotoxic toxic drug T so dangerous and allows a higher dose of T to be used.11
Pharmaco-toxicologists need reliable assays for nephrotoxicants for multiple reasons: they need measures of the nephrotoxicity of single new (candidate) compounds, they need measures of the nephrotoxicity of new or existing drugs used in combination, especially those used in common combinations in polypharmacy of the elderly, and they need measures of the reduction in nephrotoxicity achieved by co-administration of drugs designed to be nephroprotective. The choice of assay depends in part on the mechanism of toxicity, which will therefore be considered next in this review.
Mechanisms of Renal Toxicity
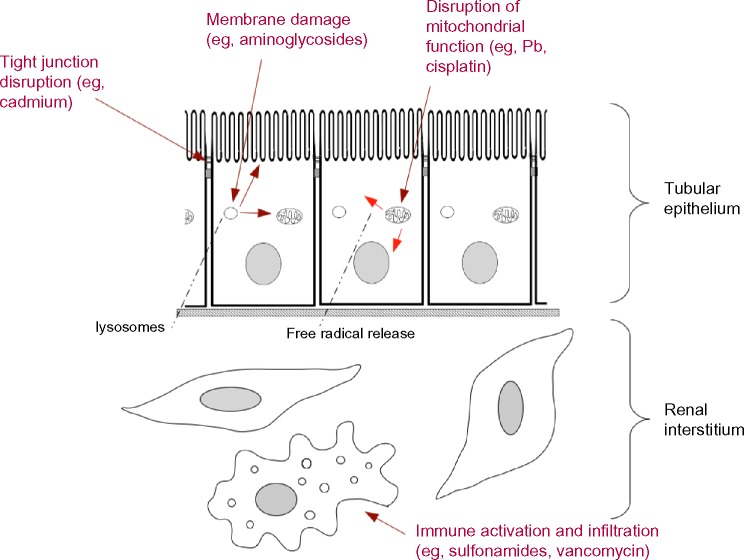
The vulnerability of kidneys to nephrotoxicants is focused mainly (but not exclusively) in three areas. These are (i) the podocytes of the glomerulus, which make the finest element of the glomerular filter through which water and small solutes pass from blood to urine; (ii) the cells of the proximal tubule of the nephron, which recover many “wanted” solutes from the urine and also actively export organic anions and cations; and (iii) the renal interstitium. Fortunately, while the number of different nephrotoxicants is large, most seem to act via a relatively small number of damage pathways (Fig. 1).
Figure 1.
Common mechanisms of damage to cells of the renal tubules and interstitium, together with examples of the toxicants that drive each type of damage. This diagram shows three of the four mechanisms described in the text: not shown, because of the scale of the diagram, is interference with vascular function, as caused by NSAIDS in patients with existing circulatory or renal problems.
Damage to cell membranes and cell–cell junctions
Cell–cell junctions are critical to renal function. Specialized junctions form the filter of the glomerulus, while “standard” tight and adherens junctions of epithelial cells are important in maintaining mechanical integrity of tubule walls and in separating apical and basal zones of epithelial cells. In the proximal tubule, tight junctions are specialized to be somewhat leaky, which is important in paracellular re-uptake of ions such as calcium. Heavy metal toxicants such as cadmium cause a serious disorganization of tight junction proteins.12 Aminoglycoside antibiotics, taken up and targeted initially to lysosomes, disrupt the lysosomal membranes, releasing destructive enzymes into the cytoplasm and also releasing the aminoglycosides to attack membranes of other organelles. Polyaspartic acid is protective against these effects.13 Amphotericin B also disrupts membrane function in the proximal tubule.14 The antiviral compound cidofovir forms a complex with phosphocholine within cells and interferes with normal membrane synthesis, damaging proximal tubule cells.15 One of the probable reasons that proximal tubule cells are so vulnerable to cidofovir, and its relatives adefovir and tenofovir, is that they posses powerful uptake systems for organic anions such as these. This idea is supported by the observation that probenecid, a drug that blocks the organic anion uptake systems, is protective against damage by these toxicants.16
Oxidative stress and free radicals
Many nephrotoxicants cause the production of free radicals. Cadmium ions complexed to metallothionein, for example, are endocytosed by proximal tubular cells using a mechanism requiring megalin.17 Once in the cells, the ions accumulate in mitochondria where they inhibit the respiratory chain and cause production of free radicals.18 This leads, ultimately, to apoptosis of the cells.19 Lead acts in a similar way.20 Cisplatin also acts partially through oxidative stress, although it also affects DNA and RNA synthesis.15
Inflammatory damage
The third mechanism of damage is mediated by the immune/inflammatory system and typically presents as acute interstitial nephritis. The damage develops relatively slowly, typically about 2 weeks after administration of the toxicant, unless a patient has already become sensitive from an earlier exposure.21 There is massive immune infiltration of the interstitium and sometimes the tubules themselves by monocytes, T-cells, and eosinophils, with accompanying tissue edema and often granuloma formation. Tubule lumens are frequently closed off and tubular cells can die or undergo epithelial-to-mesenchymal transition. This type of response is commonly induced by antibiotics (sulfonamides, rifampin, vancomycin, and ciprofloxacin), nonsteroidal anti-inflammatory drugs (NSAIDS), and some diuretics (furosemide and thiazides), although this list is by no means complete.15
Interference with vascular function
Nonsteroidal anti-inflammatory drugs work by inhibiting cyclooxygenase, which is responsible for synthesizing prostaglandins. The problem for the kidney is that prostaglandins play a physiological role in controlling vessel diameter to maintain renal perfusion at the right pressure. In patients with systemic circulatory diseases or chronic kidney disease (which may not yet have been diagnosed), NSAIDS can prevent vasodilation to such an extent that glomeruli are fed with too little blood pressure, causing acute renal failure.15
In addition to these main mechanisms, nephrotoxicants may damage kidneys by forming crystals in urine: antimicrobials and antivirals such as sulpfadiazine, acyclovir, and indinavir are associated with this.15
Existing Assays of Nephrotoxicity and their Limitations
There are three main sources of information on the nephrotoxicity of drugs and other toxicants. The traditional test system is in vivo experimentation on animal models; a more recent innovation is the use of human cell lines and primary cultures; the oldest source is direct clinical observation of patients, but obviously this cannot be used experimentally and it provides most information only when other methods have already failed to identify a danger.
Animal models
The traditional method for preclinical nephrotoxicity testing is the administration of a test substance to mammals, usually rats,22–24 although other animals such as dogs may also be used.25 Assays for nephrotoxicity can be made by measuring the urine volume and composition, plasma composition, postmortem examination of histology, or a combination of these.26–28 Detection of tissue damage from urine alone is considerably helped by the fact that injured kidney tubules secrete the molecule Kim-1.29–31
Animal models have serious problems and limitations. One problem is the obvious ethical one which, even for people who have no ethical qualms about animal use, can still lead to complications of protests and company boycotts organized by people who do. A second problem is the cost of maintaining animal facilities. A third problem – the most serious from the point of view of potential patients – is the poor predictive power of animal studies.32 Meta-analyses of animal tests for adverse drug reactions have shown that human responses are predicted correctly in no more than 50% of experiments.33,34 This creates an urgent and widely recognized need for human cell–based preclinical assays.
Cell lines and primary cultures
A great deal of nephrotoxicity takes place in the proximal tubule.35 This is partly because this cell type is very metabolically active, making it vulnerable to oxidative stressors, and partly because much of that activity is directed to transporting small solutes between the urinary space and the body fluids: this transport can concentrate toxicants in the cells.36,37 The proximal tubule may also be especially vulnerable because the proximal tubules are the first transporting cells to meet the urine, so their uptake of toxicants in the urinary space will reduce the concentration experienced by other cell types located more distally (ie, downstream). Simple, two-dimensional culture of human proximal tubule cells is therefore an obvious potential assay system for nephrotoxicity that escapes the need to guess about the human relevance of animal models.
Much work of this type has been done in one specific human cell line, HK2,38 which was derived from human proximal tubules and immortalized retrovirally.39 The cells have been useful to the study of specific transport systems that are still present and active in the cells but, because other transport systems of the proximal tubule are not active in HK2 cells,40 their use for toxicity studies runs a serious risk of false negatives.
Conditional immortalization of proximal tubule cells has been tried as a way to have a cell line that will replicate in permissive conditions but which can be returned to differentiated “normality” under nonpermissive conditions. An example produced in this manner is the ciPTEC line, which uses a combination of a human telomerse and temperature- sensitive SV40 large T antigen, the temperature sensitivity giving the required conditionality.41 When cultured in nonpermissive conditions, these cells possess a brush border and show active uptake of proteins, phosphate, and organic anions and cations. They show sensitivity to toxicants such as gentamicin,42 suggesting that they may be useful in screening.
Where suitable starting material is available, direct primary culture of human proximal tubule cells can be used. These cells, obtained from human kidneys through biopsy, nephrectomy (including organs intended for transplantation but not in fact used for various reasons), and postmortem, form monolayers that are highly realistic with respect to transport properties.43,44 They are therefore almost ideal for studies of proximal nephrotoxicity, but they live for only 2 weeks or so and they cannot be passaged without quickly losing their physiological properties. Their supply is also somewhat unpredictable.
Toward Anatomically Realistic Models
The culture models described above involve a single-cell type growing in simple two-dimensional culture or in 3- dimensional (3-D) gels.45 They have the advantages of simplicity and accessibility, making them well suited to high-throughput screening, but they lack the interactions between cell types present in the normal kidney, and they also lack anatomical organization. In particular, even where they are affected by nephrotoxicants, they may require much higher concentrations than are needed for the same effect in vivo.46
More realistic are isolated renal proximal tubules, obtained by collagenase digestion of kidneys and density (Percoll)-gated centrifugation. These have been used in studies on nephrotoxicity and on techniques for protecting proximal tubules from known nephrotoxicants.47 Culture of proximal tubule cells on the surfaces of microfluidic channels also offers slightly more realistic conditions, with access to both sides of the epithelium and the possibility of flow.48
Research being done for the ultimate purpose of producing “mini-kidneys” for transplant purposes (with subsequent growth in the host) has resulted in methods to produce much more anatomically realistic organs (Fig. 2A). This has so far been done only with murine systems. The technique makes use of the strong ability of ex fetu renogenic stem cells to organize themselves into a realistic kidney, given appropriate culture conditions. This self-organization is important and removes any need for a scaffold or for precise placement of cells by 3-D printing or a similar technique.49 The stem cells, which are a mixture of epithelial stem cells that will build the collecting duct system and mesenchymal stem cells that will build excretory nephrons and stroma, are first aggregated into a random arrangement by gentle centrifugation. This aggregate is transferred to a filter at the air–medium interface. During the first 24 hours of culture, which has to be supported by ROCK-inhibiting drugs to protect cells from death by anoikis,50 cells form contacts and begin to sort out into epithelial and mesenchymal compartments. Over the next day or so (when the drugs can be removed), small groups of epithelial cells form cysts that elongate into branching tubes, each effectively a mini-collecting duct. Mesenchyme condenses around the tips of the epithelial tubes to make cap mesenchyme, a structure characteristic of branch tips in normal kidney development.51,52 The cap mesenchyme maintains its own population and gives rise to progenitors of excretory nephrons, which go through the normal stages of maturation and connect to the collecting ducts.50
Figure 2.
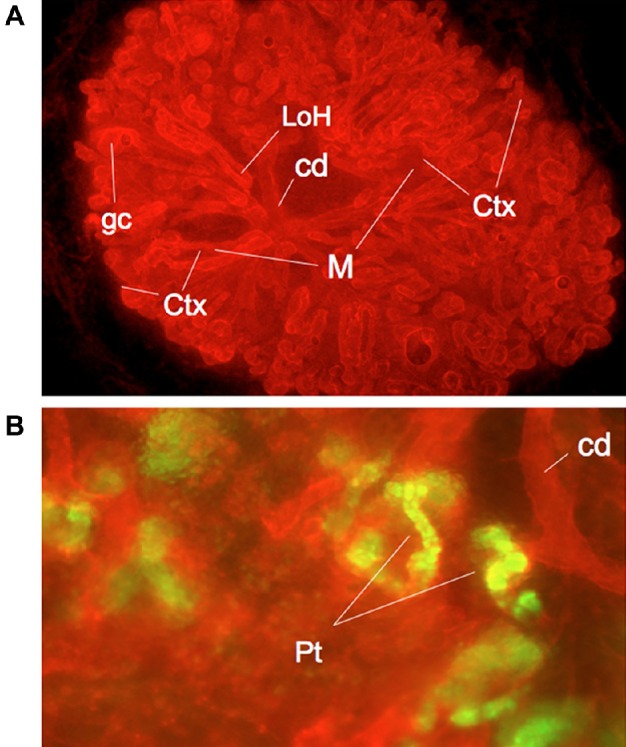
Anatomy and physiology of self-organized, reconstructed mouse kidneys. (A) Shows a low-power view of a kidney that has organized itself from a suspension of separated ex fetu renogenic stem cells. The organ has distinguishable cortex (Ctx) and medulla (M) regions, a branched collecting duct system (cd), glomerular capsules (gc), and nephrons that include loops of Henle (LoH) that descend into the medulla. See Chang and Davies54 for more details. (B) A detailed view of proximal tubules (Pt), which have taken up a fluorescently labeled organic anion, showing that this aspect of proximal tubule physiology is running in culture. These green areas are exactly those vulnerable to toxicant accumulation. See Lawrence and Davies57 for more details. This figure is reproduced from Davies et al.49 under the terms of the Creative Commons 1 CC-BY 4.0: panel (A) was produced by C-Hong Chang and panel (B) by Melanie Lawrence, both in the author’s laboratory.
The system as described makes organotypic microanatomy, but the large-scale structure of the kidney, which is based on a single collecting duct tree, is absent. More realistic anatomy can be produced by an extension of the basic technique: the system is set up as already described, and once the mini-collecting ducts begin to form, one of them is isolated manually and combined with a fresh batch of just the mesenchymal type of stem cell. The mini-collecting duct now develops into a single tree, around which all the cap mesenchymes and the nephrons that they produce are arranged.53 In a suitable culture system,54 the engineered kidney rudiment goes on to produce a distinct cortex and medulla, with loops of Henle descending from the cortex to the medulla as they should.
There are two main missing components to these kidneys. One, a still-unsolved problem, is the ureter: the kidneys as produced above have no exit (in normal development, the ureter enters the kidney from outside as the ureteric bud, which is probably why the ex fetu stem cells that make the kidney itself do not make one). The second is the absence of a circulation. This can be solved to some extent by grafting the rudiment either on to a chick egg chorioallantoic membrane (where it is served by the chick embryo’s circulation55) or under the renal capsule of a host animal, where it connects to the host blood supply and shows glomerular filtration.56
Even without a circulating blood supply, kidney rudiments engineered in this manner and maintained in culture show considerable physiological activity, including the proximal tubule transport of organic anions and cations that is very important to proximal tubule nephrotoxicity57 (Fig. 2B). The presence of this transport activity suggests that these anatomically realistic kidney rudiments might be a good model for the study of nephrotoxicity, and we have preliminary results with responses to cisplatin to suggest that this is indeed so (Ogle, Lawrence, Davies, unpublished data).
Prospects for a Human Engineered Mini-Kidney
All the experiments described above use renogenic stem cells obtained directly from the renogenic region of embryonic day 10.5–11.5 fetal mice. Clearly, obtaining human renogenic stem cells this way would be neither practical, given the precise staging requirements, nor ethically acceptable. The obvious alternative is the use of human-induced pluripotent (hiPS) cells, differentiated to cell states equivalent to the renogenic stem cells currently obtained from mouse fetuses. Considerable progress has been made in developing techniques to make renogenic stem cells58,59 and differentiated renal cells,58 from human pluripotent stem cells, both embryonic and iPS cells. The methods generally work by mimicking the sequence of paracrine signaling molecules that would be experienced by an embryonic cell that follows the branching path of fates from pluripotent epiblast to renogenic stem cell. These include induction of a mesodermal fate (as at gastrulation) by activin, bone morphogenetic proteins (BMPs), and strong Wnt signals, followed by restriction to posterior mesodermal fate by BMP and strong Wnt without activin, followed by specification as intermediate mesoderm by activin, BMP, retinoic acid, and moderate Wnt signaling.60 This is then followed by induction either of mesenchymal renogenic stem cell fate using fibroblast growth factor 958 or induction of the collecting duct stem cell fate using activin and BMP2.61 The production of the collecting duct progenitors is inefficient in this system. Given that the ultimate origin of these cells is the nephric duct, much more anterior in the embryo, it may be better to avoid the posteriorization step outlined above (this question has still to be explored).
So far, nobody has succeeded in making an anatomically realistic kidney rudiment from these cells but realistic microanatomies have been produced, with normal-looking immature nephrons being produced and expressing typical markers.60,62 Combination of the hiPS differentiation techniques described in this section with the techniques for promoting renal self-organization, described in the previous section, might result in realistic all-human mini-kidneys soon.
Given that many nephrotoxins operate through a limited number of pathways, it may also be possible to engineer hiPS cells to self-report damage, for example, through the expression of fluorescent proteins. These could be placed in loci such as Kim-1, expressed in response to a range of kidney injury.29 Alternatively, or perhaps additionally, given that fluorescent proteins of many colors are available, reporters could be placed in loci such as HIF-1 and Nrf2 for oxidative stress.63 It will also be possible to use hiPS cells derived from different patients to explore genetic influences on the responses to toxicants.
Limitations of Culture Models
It is important to note that, great as the potential is for hiPS-derived complex kidney culture models to report some types of nephrotoxic response in a human cell context, other types of toxicity are very unlikely to be detected. Of the list of four main nephrotoxicity mechanisms that were listed earlier in this article, two – damage to membranes and junctions, and oxidative stress – are likely to be replicated faithfully in culture because they are largely autonomous to renal tissue. The other two – inflammatory damage and vascular dysfunction – are not. Inflammation depends for its induction on events in the systemic immune system and is unlikely to be replicated properly in culture. Similarly, it is difficult to see how vascular dysfunction could be detected in a cultured organ that has no circulation: even in systems such as culture on the chick chorioallantoic membrane, which gives the kidney rudiments a circulation, pressures and volumes would be typical of early embryos, not adults.
As with all assay systems, the value of realistic hiPS-derived kidneys will lie in using them for problems for which they are suited. These might include some screening for specific types of nephrotoxicity. They will almost certainly include screening potential protective drugs for their ability to prevent damage by a toxicant (eg, an antibiotic or antiviral compound) known to produce a damage response in the culture system. The current methods of producing and culturing mini- kidneys are labor intensive, so unless this can be automated one day, their use in high-throughput screening would be out of the question and they will be useful only for selected candidate compounds.
No assay, other than the population of patients them-selves, will be perfectly immune from yielding false positive and false negatives. Construction of new human-based assay systems such as mini-kidneys will not change this, but it may provide one more valuable tool, the results from which, if used with proper judgment, will help drug developers to avoid at least a few mistakes that would otherwise be made.
Footnotes
ACADEMIC EDITOR: Karen Pulford, Editor in Chief
FUNDING: The development work described in this review has received funding from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115439, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007–2013) and EFPIA companies’ in kind contribution. The author confirms that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Author discloses no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived the concepts: JAD. Wrote the first draft of the manuscript: JAD. Made critical revisions: JAD. The author reviewed and approved of the final manuscript.
REFERENCES
- 1.Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014;2014:967826. doi: 10.1155/2014/967826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shang P, Chang H, Yue ZJ, et al. Acute kidney injury caused by consumption of melamine-contaminated infant formula in 47 children: a multi-institutional experience in diagnosis, treatment and follow-up. Urol Res. 2012;40:293–8. doi: 10.1007/s00240-011-0422-6. [DOI] [PubMed] [Google Scholar]
- 3.Bandara JM, Wijewardena HV, Bandara YM, Jayasooriya RG, Rajapaksha H. Pollution of River Mahaweli and farmlands under irrigation by cadmium from agricultural inputs leading to a chronic renal failure epidemic among farmers in NCP, Sri Lanka. Environ Geochem Health. 2011;33:439–53. doi: 10.1007/s10653-010-9344-4. [DOI] [PubMed] [Google Scholar]
- 4.Parish GG, Glass R, Kimbrough R. Acute arsine poisoning in two workers cleaning a clogged drain. Arch Environ Health. 1979;34:224–7. doi: 10.1080/00039896.1979.10667403. [DOI] [PubMed] [Google Scholar]
- 5.Kirchmair M, Carrilho P, Pfab R, et al. Amanita poisonings resulting in acute, reversible renal failure: new cases, new toxic Amanita mushrooms. Nephrol Dial Transplant. 2012;27:1380–6. doi: 10.1093/ndt/gfr511. [DOI] [PubMed] [Google Scholar]
- 6.Mello CP, Morais IC, Menezes RR, et al. Bothropoides insularis venom cytotoxicity in renal tubular epithelia cells. Toxicon. 2014;88:107–14. doi: 10.1016/j.toxicon.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Kohli HS, Bhaskaran MC, Muthukumar T, et al. Treatment-related acute renal failure in the elderly: a hospital-based prospective study. Nephrol Dial Transplant. 2000;15:212–7. doi: 10.1093/ndt/15.2.212. [DOI] [PubMed] [Google Scholar]
- 8.Soanker R, Udutha SJ, Subbalaxmi MV, Raju Y. Ganciclovir-tenofovir interaction leading to tenofovir-induced nephrotoxicity. J Pharmacol Pharmacother. 2014;5:265–7. doi: 10.4103/0976-500X.142452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi S, Fleet JL, Bailey DG, et al. Calcium-channel blocker-clarithromycin drug interactions and acute kidney injury. JAMA. 2013;310:2544–53. doi: 10.1001/jama.2013.282426. [DOI] [PubMed] [Google Scholar]
- 10.Perazella MA. Drug use and nephrotoxicity in the intensive care unit. Kidney Int. 2012;81:1172–8. doi: 10.1038/ki.2010.475. [DOI] [PubMed] [Google Scholar]
- 11.Ali BH, Al Za’abi M, Blunden G, Nemmar A. Experimental gentamicin nephrotoxicity and agents that modify it: a mini-review of recent research. Basic Clin Pharmacol Toxicol. 2011;109:225–32. doi: 10.1111/j.1742-7843.2011.00728.x. [DOI] [PubMed] [Google Scholar]
- 12.Jacquillet G, Barbier O, Rubera I, et al. Cadmium causes delayed effects on renal function in the offspring of cadmium-contaminated pregnant female rats. Am J Physiol Renal Physiol. 2007;293:F1450–60. doi: 10.1152/ajprenal.00223.2007. [DOI] [PubMed] [Google Scholar]
- 13.Kaloyanides GJ. Drug-phospholipid interactions: role in aminoglycoside nephrotoxicity. Ren Fail. 1992;14:351–7. doi: 10.3109/08860229209106642. [DOI] [PubMed] [Google Scholar]
- 14.Sawaya BP, Briggs JP, Schnermann J. Amphotericin B nephrotoxicity: the adverse consequences of altered membrane properties. J Am Soc Nephrol. 1995;6:154–64. doi: 10.1681/ASN.V62154. [DOI] [PubMed] [Google Scholar]
- 15.Markowitz GS, Perazella MA. Drug-induced renal failure: a focus on tubulointerstitial disease. Clin Chim Acta. 2005;351:31–47. doi: 10.1016/j.cccn.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Ho ES, Lin DC, Mendel DB, Cihlar T. Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J Am Soc Nephrol. 2000;11:383–93. doi: 10.1681/ASN.V113383. [DOI] [PubMed] [Google Scholar]
- 17.Klassen RB, Crenshaw K, Kozyraki R, et al. Megalin mediates renal uptake of heavy metal metallothionein complexes. Am J Physiol Renal Physiol. 2004;287:F393–403. doi: 10.1152/ajprenal.00233.2003. [DOI] [PubMed] [Google Scholar]
- 18.Reyes JL, Molina-Jijón E, Rodríguez-Muñoz R, Bautista-García P, Debray-García Y, Namorado Mdel C. Tight junction proteins and oxidative stress in heavy metals-induced nephrotoxicity. Biomed Res Int. 2013;2013:730789. doi: 10.1155/2013/730789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stinson LJ, Darmon AJ, Dagnino L, D’Souza SJ. Delayed apoptosis post-cadmium injury in renal proximal tubule epithelial cells. Am J Nephrol. 2003;23:27–37. doi: 10.1159/000066298. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Wang H, Hu M, Cao J, Chen D, Liu Z. Oxidative stress and apoptotic changes in primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol. 2009;83:417–27. doi: 10.1007/s00204-009-0425-z. [DOI] [PubMed] [Google Scholar]
- 21.Murray MD, Brater DC. Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu Rev Pharmacol Toxicol. 1993;1993(33):435–65. doi: 10.1146/annurev.pa.33.040193.002251. [DOI] [PubMed] [Google Scholar]
- 22.Mengs U, Stotzem CD. Renal toxicity of aristolochic acid in rats as an example of nephrotoxicity testing in routine toxicology. Arch Toxicol. 1993;67:307–11. doi: 10.1007/BF01973700. [DOI] [PubMed] [Google Scholar]
- 23.Fleck C, Appenroth D. Renal amino acid transport in immature and adult rats during thallium-induced nephrotoxicity. Toxicology. 1996;106:229–36. doi: 10.1016/0300-483x(95)03194-k. [DOI] [PubMed] [Google Scholar]
- 24.Šebeková K, Dušinská M, Simon Klenovics K, et al. Comprehensive assessment of nephrotoxicity of intravenously administered sodium-oleate-coated ultra-small superparamagnetic iron oxide (USPIO) and titanium dioxide (TiO2) nanoparticles in rats. Nanotoxicology. 2014;8:142–57. doi: 10.3109/17435390.2012.763147. [DOI] [PubMed] [Google Scholar]
- 25.Zhou X, Ma B, Lin Z, et al. Evaluation of the usefulness of novel biomarkers for drug-induced acute kidney injury in beagle dogs. Toxicol Appl Pharmacol. 2014;280(1):30–5. doi: 10.1016/j.taap.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Fleck C, Kretzschel I, Sperschneider T, Appenroth D. Renal amino acid transport in immature and adult rats during chromate and cisplatinum-induced nephrotoxicity. Amino Acids. 2001;20:201–15. doi: 10.1007/s007260170060. [DOI] [PubMed] [Google Scholar]
- 27.Ali BH, Al-Wabel N, Mahmoud O, Mousa HM, Hashad M. Curcumin has a palliative action on gentamicin-induced nephrotoxicity in rats. Fundam Clin Pharmacol. 2005;19:473–7. doi: 10.1111/j.1472-8206.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 28.Sieber M, Hoffmann D, Adler M, et al. Comparative analysis of novel noninvasive renal biomarkers and metabonomic changes in a rat model of gentamicin nephrotoxicity. Toxicol Sci. 2009;109:336–49. doi: 10.1093/toxsci/kfp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–42. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 30.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 31.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–63. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 32.Knight A. Systematic reviews of animal experiments demonstrate poor contributions toward human healthcare. Rev Recent Clin Trials. 2008;3:89–96. doi: 10.2174/157488708784223844. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher AP. Drug safety tests and subsequent clinical experience. J R Soc Med. 1978;71:693–6. doi: 10.1177/014107687807100915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Archibald K, Coleman R, Foster C. Open letter to UK Prime Minister David Cameron and Health Secretary Andrew Lansley on safety of medicines. Lancet. 2011;377:1915. doi: 10.1016/S0140-6736(11)60802-7. [DOI] [PubMed] [Google Scholar]
- 35.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2:1303–53. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagos Y, Wolff NA. Assessment of the role of renal organic anion transporters in drug-induced nephrotoxicity. Toxins (Basel) 2010;2:2055–82. doi: 10.3390/toxins2082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagai J, Takano M. Entry of aminoglycosides into renal tubular epithelial cells via endocytosis-dependent and endocytosis-independent pathways. Biochem Pharmacol. 2014;90:331–7. doi: 10.1016/j.bcp.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y, Connors D, Barber L, Jayachandra S, Hanumegowda UM, Adams SP. Multiplexed assay panel of cytotoxicity in HK-2 cells for detection of renal proximal tubule injury potential of compounds. Toxicol In Vitro. 2009;23:1170–8. doi: 10.1016/j.tiv.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- 40.Jenkinson SE, Chung GW, van Loon E, Bakar NS, Dalzell AM, Brown CD. The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflugers Arch. 2012;464:601–11. doi: 10.1007/s00424-012-1163-2. [DOI] [PubMed] [Google Scholar]
- 41.Wilmer MJ, Saleem MA, Masereeuw R, et al. Novel conditionally immortalized human proximal tubule cell line expressing functional influx and efflux transporters. Cell Tissue Res. 2010;339:449–57. doi: 10.1007/s00441-009-0882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moghadasali R, Mutsaers HA, Azarnia M, et al. Mesenchymal stem cell-conditioned medium accelerates regeneration of human renal proximal tubule epithelial cells after gentamicin toxicity. Exp Toxicol Pathol. 2013;65:595–600. doi: 10.1016/j.etp.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Brown CD, Sayer R, Windass AS, et al. Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicol Appl Pharmacol. 2008;233:428–38. doi: 10.1016/j.taap.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Verhulst A, Sayer R, De Broe ME, D’Haese PC, Brown CD. Human proximal tubular epithelium actively secretes but does not retain rosuvastatin. Mol Pharmacol. 2008;74:1084–91. doi: 10.1124/mol.108.047647. [DOI] [PubMed] [Google Scholar]
- 45.DesRochers TM, Suter L, Roth A, Kaplan DL. Bioengineered 3D human kidney tissue, a platform for the determination of nephrotoxicity. PLoS One. 2013;2013(8):e59219. doi: 10.1371/journal.pone.0059219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El Mouedden M, Laurent G, Mingeot-Leclercq MP, Tulkens PM. Gentamicin-induced apoptosis in renal cell lines and embryonic rat fibroblasts. Toxicol Sci. 2000;56:229–39. doi: 10.1093/toxsci/56.1.229. [DOI] [PubMed] [Google Scholar]
- 47.Lee DW, Faubel S, Edelstein CL. A pan caspase inhibitor decreases caspase-1, IL-1alphaand IL-1beta and protects against necrosis of cisplatin-treated freshly isolated proximal tubules. Ren Fail. 2015;37(1):144–50. doi: 10.3109/0886022X.2014.970194. [DOI] [PubMed] [Google Scholar]
- 48.Jang KJ, Mehr AP, Hamilton GA, et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol (Camb) 2013;5(9):1119–29. doi: 10.1039/c3ib40049b. [DOI] [PubMed] [Google Scholar]
- 49.Davies JA, Chang C-H, Lawrence ML, Mills CG, Mullins JJ. Engineered kidneys: principles, progress and prospects. Adv Regen Biol. 2014;2014(1):24990. [Google Scholar]
- 50.Unbekandt M, Davies JA. Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int. 2010;77:407–16. doi: 10.1038/ki.2009.482. [DOI] [PubMed] [Google Scholar]
- 51.Schreiner F. Ueber die Entwicklung der Amniotenniere. Zeitschr f wiss Zool. 1902;71:1–188. [Google Scholar]
- 52.Reinhoff WF. Development and growth of the metanephros or permanent kidney in chick embryos. Bull Johns Hopkins Hosp. 1922;33:392–406. [Google Scholar]
- 53.Ganeva V, Unbekandt M, Davies JA. An improved kidney dissociation and reaggregation culture system results in nephrons arranged organotypically around a single collecting duct system. Organogenesis. 2011;7:83–7. doi: 10.4161/org.7.2.14881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang CH, Davies JA. An improved method of renal tissue engineering, by combining renal dissociation and reaggregation with a low-volume culture technique, results in development of engineered kidneys complete with loops of Henle. Nephron Exp Nephrol. 2012;121:e79–85. doi: 10.1159/000345514. [DOI] [PubMed] [Google Scholar]
- 55.Davies JA, Chang CH. Engineering kidneys from simple cell suspensions: an exercise in self-organization. Pediatr Nephrol. 2014;29:519–24. doi: 10.1007/s00467-013-2579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xinaris C, Benedetti V, Rizzo P, et al. In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J Am Soc Nephrol. 2012;23:1857–68. doi: 10.1681/ASN.2012050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lawrence M, Davies JA. Transport of organic anions and cations in engineered kidneys and in murine embryonic kidney development. Sci Rep. 2015;5:9092. doi: 10.1038/srep09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lam AQ, Freedman BS, Morizane R, Lerou PH, Valerius MT, Bonventre JV. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol. 2014;25:1211–25. doi: 10.1681/ASN.2013080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takasato M, Er PX, Becroft M, et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 2014;16:118–26. doi: 10.1038/ncb2894. [DOI] [PubMed] [Google Scholar]
- 60.Taguchi A, Nishinakamura R. Nephron reconstitution from pluripotent stem cells. Kidney Int. 2015;87(5):894–900. doi: 10.1038/ki.2014.358. [DOI] [PubMed] [Google Scholar]
- 61.Xia Y, Nivet E, Sancho-Martinez I, et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol. 2013;15:1507–15. doi: 10.1038/ncb2872. [DOI] [PubMed] [Google Scholar]
- 62.Xia Y, Sancho-Martinez I, Nivet E, Rodriguez Esteban C, Campistol JM, Izpisua Belmonte JC. The generation of kidney organoids by differentiation of human pluripotent cells to ureteric bud progenitor-like cells. Nat Protoc. 2014;9:2693–704. doi: 10.1038/nprot.2014.182. [DOI] [PubMed] [Google Scholar]
- 63.Zahlten J, Kim YJ, Doehn JM, et al. Streptococcus pneumoniae-induced oxidative stress in lung epithelial cells depends on pneumococcal autolysis and is reversible by resveratrol. J Infect Dis. 2015;211(11):1822–30. doi: 10.1093/infdis/jiu806. [DOI] [PubMed] [Google Scholar]