Abstract
Introduction
Few data are available in regards to the prevalence of pulmonary hypertension (PH) in the broad spectrum of COPD. This study was aimed at assessing the prevalence of PH in a cohort of COPD patients across the severity of airflow limitation, and reporting the hemodynamic characteristics at rest and during exercise.
Methods
We performed a retrospective analysis on COPD patients who underwent right-heart catheterization in our center with measurements obtained at rest (n=139) and during exercise (n=85). PH was defined as mean pulmonary artery pressure (mPAP) ≥25 mmHg and pulmonary capillary wedge pressure <15 mmHg. Exercise-induced PH (EIPH) was defined by a ratio of ΔmPAP/Δcardiac output >3.
Results
PH was present in 25 patients (18%). According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification, PH prevalence in GOLD 2 was 7% (3 patients); 25% (14 patients) in GOLD 3; and 22% (8 patients) in GOLD 4. Severe PH (mPAP ≥35 mmHg) was identified in four patients (2.8%). Arterial partial oxygen pressure was the outcome most strongly associated with PH (r=−0.29, P<0.001). EIPH was observed in 60 patients (71%) and had a similar prevalence in both GOLD 2 and 3, and was present in all GOLD 4 patients. Patients with PH had lower cardiac index during exercise than patients without PH (5.0±1.2 versus 6.7±1.4 L/min/m2, respectively; P=0.001).
Conclusion
PH has a similar prevalence in COPD patients with severe and very-severe airflow limitation, being associated with the presence of arterial hypoxemia. In contrast, EIPH is highly prevalent, even in moderate COPD, and might contribute to limiting exercise tolerance.
Keywords: pulmonary hypertension, right heart catheterization, cardiac index, GOLD
Introduction
Pulmonary hypertension (PH) is a relevant complication in the natural history of patients with chronic obstructive pulmonary disease (COPD), since its presence is associated with shorter survival, increased risk of exacerbations, and greater use of health care resources.1 The actual prevalence of PH in COPD remains unsettled and varies widely according to the targeted population, the definition applied, and the diagnostic approach used to identify PH.2 Right-heart catheterization is the gold standard to diagnose PH.3 In COPD, most hemodynamic studies have been performed in patients with advanced disease in whom PH is expected to occur more frequently, with a prevalence of PH ranging from 23%–91%.4–12 Information on the prevalence of PH in patients with milder disease is scarce. Based on the former Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric classification,13 Hilde et al14 reported a prevalence of 5%, 27%, and 53% in patients with GOLD stages 2, 3, and 4, respectively.
Patients with COPD may develop PH during exercise, a condition associated with an increased risk for subsequently developing PH at rest.12 Current understanding of the hemodynamic response of the pulmonary circulation to exercise in patients with COPD and its impact over exercise intolerance is limited. Studies have shown a cardiovascular limitation to exercise in patients with severe PH which seems to be distinct from the ventilatory exhaustion usually observed in COPD.15,16
In order to expand our understanding of the hemodynamic profile of PH across the spirometric degrees of COPD, we analyzed data from 139 COPD patients assessed by right-heart catheterization at rest. A subset of these patients (n=85) had hemodynamic measurements during exercise. The main objective of the present study was to assess the prevalence of PH in this cohort of patients according to the GOLD spirometric grades. Secondary aims were to describe the hemodynamic characteristics at rest and during exercise, and to analyze the potential relationships between pulmonary hemodynamics and lung function tests.
Methods
This was a retrospective study, which included data from seven previously reported studies.17–23 All studies were approved by our institutional ethics committee and the procedures were performed after obtaining written informed consent. All involved patients were clinically stable (ie, at least 3 months from the last COPD exacerbation), without cardiac failure or other coexisting chronic respiratory disease, and received regular treatment for COPD, including long-term oxygen if it was deemed necessary. No patient was treated with PH targeted therapy, namely prostanoids, endothelin-receptor antagonists, or phosphodiesterase-5 inhibitors. All were current (n=53) or former (n=86) smokers (≥10 pack-year). Patients were classified according to the GOLD spirometric grade system.24
PH was defined by a mean pulmonary artery pressure (mPAP) ≥25 mmHg and a pulmonary capillary wedge pressure (PCWP) <15 mmHg.2 Severe PH was considered when mPAP was ≥35 mmHg or ≥25 mmHg along with a cardiac index (CI) <2.0 L/min/m2, according to the most updated clinical classification of PH in chronic lung diseases.3 An abnormal increase of mPAP during exercise was considered when the change in mPAP ([ΔmPAP] in mmHg) was >3- times greater than the change in cardiac output ([CO] in L/min) (ΔCO).14,25 As age may influence exercise mPAP, we also analyzed the mPAP values during exercise according to those reported by Kovacs et al26 who established the upper limit of normal as 30 mmHg for subjects <50 years and as 46 mmHg for subjects ≥50 years.
Measurements
Lung function
Forced spirometry, static lung volumes, and single-breath carbon monoxide diffusing capacity (DLco) were measured according to the American Thoracic Society/European Respiratory Society recommendations,27 using our own predicted equations.28–29 Arterial blood gas samples were drawn for measuring partial pressure of arterial oxygen (PaO2), partial pressure of arterial carbon dioxide (PaCO2), and pH at rest and during exercise.
Right-heart catheterization
A triple-lumen Swan–Ganz catheter (Edwards Laboratories, Santa Ana, CA, USA) was placed into the pulmonary artery under pressure wave monitoring (M1166A; Hewlett-Packard, Boeblingen, Germany). Transducers were zeroed at the level of right atrium. Right atrial pressure (RAP), pulmonary artery pressure (PAP), and PCWP were measured at the end of expiration. CO was measured by thermodilution and expressed as the mean of three measurements. CI, pulmonary vascular resistance (PVR), and total pulmonary resistance were calculated using standard formulae.30
Exercise measurements
Prior to catheterization, the highest workload that patients could tolerate was determined by an incremental exercise test on a cycle ergometer in 85 subjects. The workload was increased using a ramp of 5 Watts every minute until the maximal load limited by symptoms was achieved. Hemodynamic and gas exchange measurements were performed in the semirecumbent position at a workload equivalent to 60% of the maximal workload tolerated in the previous incremental test.
Statistical analysis
Data are expressed as mean ± standard deviation unless otherwise stated. One-way analysis of variance with Bonferroni correction was used to compare more than two groups. Relationships between variables were assessed using Pearson’s or Spearman’s correlation coefficients depending on data distribution. Changes in hemodynamic data from rest to exercise were compared using paired t-tests. The Mann–Whitney U-test was used to assess CI during exercise in patients with and without PH. Estimations of risk for PH were made using odds ratios and their 95% confidence intervals from univariate logistic regression models for PaO2, DLco, PaCO2, inspiratory capacity, or forced expiratory volume in 1 second (FEV1). In order to assess a multivariate model, we used a forward stepwise procedure with a criterion of 0.05 to select variables for the final model. All data were analyzed with SPSS version 18 and P-values <0.05 were considerate as statistically significant.
Results
The means (± standard deviation) of demographic and lung function measurements for all participants are given in Table 1 as a function of the current GOLD spirometric classification.24 One hundred and thirty-nine patients (134 men, 5 women) were included. Mean age was 63±8 years. The mean of post-bronchodilator FEV1 was 41%±16% of predicted; PaO2 was 69±12 mmHg; PaCO2 was 40±6; and alveolar–arterial oxygen partial pressure difference (AaPO2) was 32±10 mmHg. Thirty-seven patients were under long-term oxygen therapy.
Table 1.
Anthropometric and functional characteristics of 139 patients grouped according to GOLD spirometric grades
| All | GOLD grade
|
ANOVA P-value | |||
|---|---|---|---|---|---|
| 2 | 3 | 4 | |||
| N | 139 | 46 | 56 | 37 | |
| Sex, M/F | 134/5 | 46/0 | 53/3 | 35/2 | |
| Age (yr) | 63±8 | 63±8 | 63±7 | 60±7 | NS |
| BMI (m/kg2) | 25±4 | 24±4 | 26±3 | 24±4 | NS |
| FVC (% pred) | 72±18 | 82±14 | 71±17* | 60±17*,≠ | <0.001 |
| FEV1 (L) | 1.3±0.6 | 1.9±0.5 | 1.2±0.2* | 0.7±0.2*,≠ | <0.001 |
| FEV1 (% pred) | 41±16 | 59±7 | 39±6* | 20±4*,≠ | <0.001 |
| FEV1/FVC (%) | 40±15 | 52±8 | 41±14* | 25±8*,≠ | <0.001 |
| TLC (% pred) | 116±21 | 113±17 | 112±22 | 124±22≠ | 0.004 |
| RV (% pred) | 200±75 | 156±44 | 191±69* | 266±68*,≠ | <0.001 |
| IC (% pred) | 62±18 | 76±16 | 63±16* | 47±12*,≠ | <0.001 |
| RV/TLC (%) | 61±11 | 52±8 | 59±9* | 71±8*,≠ | <0.001 |
| IC/TLC ratio | 0.26±0.07 | 0.32±0.06 | 0.27±0.07* | 0.18±0.05*,≠ | <0.001 |
| DLco (% pred) | 57±25 | 72±23 | 59±21* | 36±15*,≠ | <0.001 |
| PaO2 (mmHg) | 69±12 | 77±10 | 68±11* | 62±11*,≠ | <0.001 |
| PaCO2 (mmHg) | 40±6 | 37±4 | 41±6* | 45±7*,≠ | <0.001 |
| AaPO2 (mmHg) | 32±10 | 30±11 | 32±9 | 34±10 | NS |
Notes: Data are presented as means ± standard deviation. Significant differences within each variable are indicated as:
different from GOLD grade 2;
different from GOLD grade 3.
Abbreviations: AaPO2, alveolar–arterial oxygen partial pressure difference; ANOVA, analysis of variance; BMI, body mass index; DLco, diffusing capacity for carbon dioxide; FEV1, post-bronchodilator forced expiratory volume in 1 second; FEV1/FVC, post-bronchodilator forced expiratory volume in 1 second/post-bronchodilator forced vital capacity; FVC, post-bronchodilator forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; IC, inspiratory capacity; IC/TLC, inspiratory capacity/total lung capacity ratio; M/F, male/female; NS, not significant; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; pred, predicted; RV, residual volume; RV/TLC, residual volume/total lung capacity ratio; TLC, total lung capacity.
Hemodynamic characteristics at rest
Table 2 shows the hemodynamic measurements at rest. The mean mPAP of the whole cohort was 20±8 mmHg. PH was present in 25 patients (18% of cases of the whole cohort). Most of them had severe or very-severe airflow limitation, although PH prevalence was similar in GOLD 3 and 4.
Table 2.
Pulmonary hemodynamics at rest of 139 patients grouped according to the GOLD spirometric grades
| All | GOLD grade
|
ANOVA P-value |
|||
|---|---|---|---|---|---|
| 2 | 3 | 4 | |||
| N | 139 | 46 | 56 | 37 | |
| mPAP (mmHg) | 20±8 | 18±6 | 21±9 | 21±6 | NS |
| PCWP (mmHg) | 6±4 | 7±4 | 6±4 | 5±3 | NS |
| RAP (mmHg) | 2.9±3.2 | 3.4±3.5 | 2.1±3.2 | 2.7±2.7 | NS |
| CO (L/min/m2) | 5.6±1.4 | 6.3±1.5 | 5.5±1.3* | 4.7±0.8*,≠ | <0.001 |
| CI (L/min/m2) | 3.2±0.8 | 3.6±0.8 | 3.1±0.7* | 2.8±0.6*,≠ | <0.001 |
| TPR (dyn⋅s⋅cm−5) | 306±158 | 244±105 | 322±197* | 355±191*,≠ | 0.004 |
| PVR (dyn⋅s⋅cm−5) | 218±143 | 160±88 | 233±185* | 264±97*,≠ | 0.003 |
| TPG | 14±7 | 12±5 | 15±8 | 16±5*,≠ | 0.02 |
| Patients with PH, n (%) | 25 (18) | 3 (7) | 14 (25) | 8 (22) | |
Notes: Data are presented as mean ± standard deviation. Significant differences within each variable are indicated as:
different from GOLD grade 2;
different from GOLD grade 3.
Abbreviations: ANOVA, analysis of variance; CI, cardiac index; CO, cardiac output; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; mPAP, mean pulmonary artery pressure; NS, not significant; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RAP, right atrial pressure; TPG, transpulmonary pressure gradient; TPR, total pulmonary resistance.
Mean PVR was normal in GOLD 2 and 3, although with a wide dispersion, and elevated in GOLD 4. RAP, PCWP, and CI were on average within normal range in all GOLD grades. The CI correlated with FEV1 (r=0.38, P<0.001). Severe PH was noticed in four patients (2.8% of the whole cohort). All of them were male. Their lung function and hemodynamic characteristics are listed in Table 3.
Table 3.
Subject characteristics in four COPD patients with severe pulmonary hypertension
| Age (years) | FEV1 (% pred) | DLco (% pred) | PaO2 (mmHg) | PaCO2 (mmHg) | mPAP (mmHg) | PCWP (mmHg) | PVR (dyn⋅s⋅cm−5) | CI (L/min/m2) |
|---|---|---|---|---|---|---|---|---|
| 58 | 35 | 34 | 44 | 46 | 35 | 4 | 594 | 2.75 |
| 65 | 51 | 24 | 50 | 35 | 43 | 6 | 566 | 2.69 |
| 58 | 46 | 22 | 50 | 38 | 38 | 2 | 1,080 | 1.79 |
| 61 | 39 | 21 | 46 | 44 | 61 | 11 | 941 | 2.38 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CI, cardiac index; DLco, diffusing capacity for carbon dioxide; FEV1, post-bronchodilator forced expiratory volume in 1 second; mPAP, mean pulmonary artery pressure; NS, not significant; PaO2, partial pressure of arterial oxygen; PaCO2, partial pressure of arterial carbon dioxide; PCWP, pulmonary capillary wedge pressure; pred, predicted; PVR, pulmonary vascular resistance.
Hemodynamic characteristics during exercise and changes in blood gases
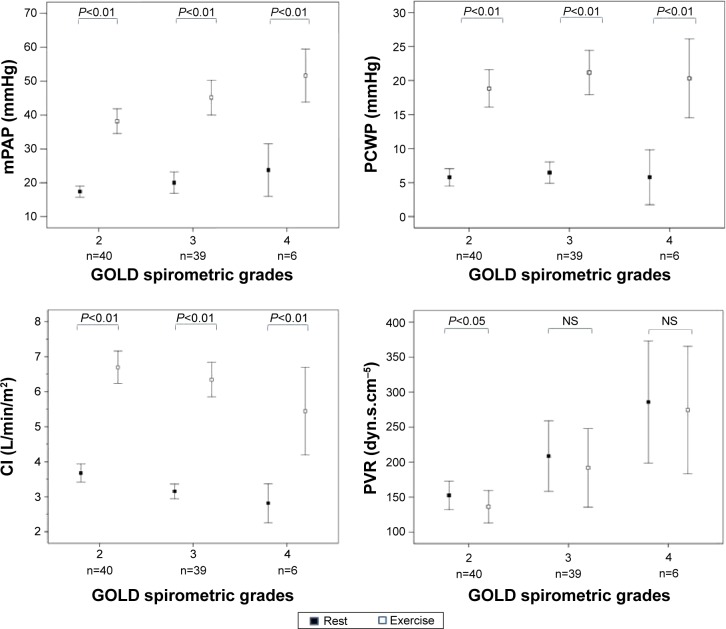
Both mPAP and PCWP increased significantly during exercise in all GOLD grades (Figure 1; Table 4). An abnormal increase of mPAP during exercise, defined by a ΔmPAP/ΔCO ratio >3, was observed in 60 patients (71%): 28 patients (70%) were in GOLD 2, 26 (67%) in GOLD 3, and the remaining six patients in GOLD 4. During exercise, patients with PH, compared with those without PH, had lower CI (5.0±1.2 and 6.7±1.4 L/min/m2, respectively; P=0.001) (Figure 2). Patients with associated PH had, as compared with patients without PH, lower PaO2 and higher PaCO2 both at rest and during exercise (Table 5).
Figure 1.
Changes from rest to peak exercise in the hemodynamic variables in 85 patients who underwent exercise testing, grouped according GOLD stage.
Note: P-value differences from rest to exercise (paired t-tests).
Abbreviations: CI, cardiac index; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; NS, not significant.
Table 4.
Pulmonary hemodynamics during submaximal exercise in patients grouped to the GOLD spirometric grades (n=85)
| All | GOLD grade
|
ANOVA P-value | |||
|---|---|---|---|---|---|
| 2 | 3 | 4 | |||
| Hemodynamics during exercise, N | 85 | 40 | 39 | 6 | |
| mPAP (mmHg) | 42±14 | 38±11 | 45±15 | 52±7 | 0.019 |
| PCWP (mmHg) | 20±9 | 19±9 | 21±10 | 20±6 | NS |
| CO (L/min/m2) | 11.8±2.8 | 12.2±2.8 | 11.7±2.8 | 9.5±2.2 | NS |
| CI (L/min/m2) | 6.5±1.5 | 6.7±1.5 | 6.3±1.5 | 5.4±1.1 | NS |
| TPR (dyn⋅s⋅cm−5) | 315±174 | 265±105 | 345±215 | 454±146* | 0.014 |
| PVR (dyn⋅s⋅cm−5) | 171±134 | 136±72 | 191±173 | 274±86 | 0.02 |
| TPG (mmHg) | 22±11 | 19±8 | 24±13 | 31±7* | 0.02 |
| ΔmPAP/ΔCO (mmHg/L/min) | 4.8±3.7 | 4.1±2.2 | 5.4±4.8 | 6.4±2.7 | |
| Abnormal mPAPa, n (%) | 29 (34) | 8 (20) | 17 (46) | 4 (67) | |
| ΔmPAP/ΔCO >3, n (%) | 60 (71) | 28 (70) | 26 (67) | 6 (100) | |
Notes: Data are presented as mean ± standard deviation.
mPAP: <50 years, >30 mmHg; ≥50 years, >46 mmHg. According to Kovacs et al.26 Significant differences within each variable are indicated as:
different from GOLD grade 2.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; Δ, change; ANOVA, analysis of variance; CI, cardiac index; CO, cardiac output; mPAP, mean pulmonary artery pressure; NS, not significant; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; TPG, transpulmonary pressure gradient; TPR, total pulmonary resistance.
Figure 2.
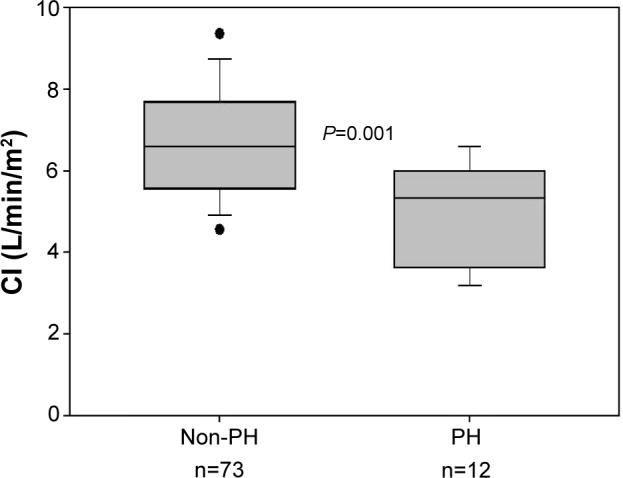
Cardiac index during exercise in patients with and without pulmonary hypertension (n=85).
Note: P-value indicates comparison between PH and non-PH (Mann–Whitney U-test).
Abbreviations: CI, cardiac index; PH, pulmonary hypertension.
Table 5.
Arterial blood gas profile at rest and during exercise in 85 patients with or without pulmonary hypertension
| No PH (n=73) | PH (n=12) | P-value | |
|---|---|---|---|
| PaO2 (mmHg) | 76±8 | 65±14 | 0.020 |
| PaO2 exercise (mmHg) | 74±12 | 54±11 | 0.001 |
| PaCO2 (mmHg) | 38±4 | 44±6 | 0.006 |
| PaCO2 exercise (mmHg) | 41±8 | 47±6 | 0.004 |
Note: Data are presented as mean ± standard deviation.
Abbreviations: PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; PH, pulmonary hypertension.
Variables associated with PH
In the univariate analysis, the following outcomes were associated with the presence of PH at rest: PaO2; DLco (% predicted); PaCO2; inspiratory capacity (% predicted); and FEV1 (% predicted). In the stepwise multiple regression model, PaO2 was the only covariate associated with the presence of PH (Table 6).
Table 6.
Univariate and multivariate logistic regression analysis of variables associated with pulmonary hypertension
| Independent variable | Univariate analysis
|
Multivariate analysis*
|
||
|---|---|---|---|---|
| OR (CI 95%) | P-value | OR (95% confidence interval) | P-value | |
| PaO2 (mmHg) | 0.92 (0.89–0.96) | <0.001 | 0.92 (0.88–0.97) | 0.001 |
| DLco (mmHg) | 0.86 (0.78–0.94) | 0.002 | ||
| PaCO2 (mmHg) | 1.07 (1.01–1.15) | 0.025 | ||
| IC (% predicted) | 0.97 (0.94–0.99) | 0.028 | ||
| FEV1 (% predicted) | 0.96 (0.93–0.99) | 0.026 | ||
Note:
From forward stepwise procedure.
Abbreviations: DLco, diffusing capacity for carbon dioxide; FEV1, post-bronchodilator forced expiratory volume in 1 second; IC, inspiratory capacity; OR, odds ratio; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen.
Discussion
The current study, which includes pulmonary hemodynamic measurements at rest and during exercise in a stable population of COPD across the whole spirometric spectrum of severity, identified several findings. First, the overall prevalence of PH in our cohort was relatively low (18%). Second, patients in GOLD 3 and 4 had similar a prevalence of PH. Third, a significant increase of both mPAP and PCWP during exercise was noticed in all patients with a high proportion of an “exercise hypertensive response” in the entire cohort. And fourth, CI during exercise was affected by the presence of PH.
Data about the prevalence of PH in COPD, particularly in patients with moderate-to-severe COPD, is limited. The prevalence found in our study was lower than in a recent study by Hilde et al14 who reported a prevalence of PH of 27% in stable patients in GOLD 2–4. Interestingly, the prevalence of PH observed in the present study in patients with GOLD 2 and 3 was similar to that reported by Hilde et al.14 In contrast, the prevalence of PH in patients with GOLD 4 was lower in our study (22%) than in Hilde’s study (53%). This discrepancy may be due to the fact that the current GOLD spirometric classification, which we used, does not consider arterial blood gas measurements for the definition of GOLD 4.24 Accordingly, in patients with severe-to-very-severe COPD, the degree of airflow limitation is not related to the presence of PH. What makes a difference regarding PH in those patients is the presence of concomitant abnormal pulmonary gas exchange. In fact, if we classify patients in our series according to the previous GOLD classification, the prevalence of PH in GOLD 4 stage is 33%, in line with that reported by Hilde et al.14 That said, we cannot overlook that the current A-B-C-D GOLD classification, based on a multidimensional assessment of airflow limitation, symptoms, and risk of exacerbations,24 might more accurately identify differences in PH prevalence between categories. Nevertheless, the retrospective nature of our study precludes such a combined assessment.
As previously reported, the degree of PH in this cohort was of mild-to-moderate magnitude with a well preserved CO, and mPAP correlated with airflow obstruction severity.1,31 In the whole cohort, both resting PCWP and RAP were within normal limits. The prevalence of patients with severe PH (2.8%) in our study is comparable with other reported series.6,7,9 Likewise, patients with severe PH showed less severe airflow limitation, more severe arterial hypoxemia, and lower DLco than patients with moderate PH. It is important to identify these patients with predominant vascular involvement since they represent a clinically distinct phenotype within the COPD population that carries poor prognosis.6,9,32
The exercise-induced increase in mPAP was interpreted according to the increase in CO as proposed by recent reports.14,25 The latter proposal is based on the concept that during exercise, the pulmonary microcirculation is progressively recruited under physiological conditions, resulting in the maintenance of relatively low arterial pressure despite increasing flow.33 The abnormal increase in mPAP during exercise in COPD patients has been documented in different studies.12,14,16,34 In spite of the fact that there is no current definition of exercise-induced PH, we observed that 71% of the patients developed a “hypertensive response”, defined on the basis of pressure/flow relationships. Such an abnormal vascular response was noticed even in moderate grades of COPD severity, whereas it was present in all GOLD 4 patients. The clinical significance of the increased PAP during exercise remains to be established. Conceivably, it could limit exercise capacity. In fact, an increased mPAP has been associated with poor results in the 6 minutes walking test.5,10 Furthermore, a PAP threshold >30 mmHg during exercise is a strong predictor for the development of PH in the long-term.12 We also found that PVR does not decrease during exercise in GOLD grades 3 and 4, in contrast with what occurs in healthy subjects.35 This fall in PVR during exercise is explained by the distensibility of resistive pulmonary vessels. Reduced pulmonary artery compliance observed in COPD might explain this response.14 Furthermore, CI during exercise was lower in patients with associated PH. Although ventilatory impairment is the main factor limiting exercise tolerance in COPD, it cannot be disregarded that hemodynamic alterations may also contribute to exercise intolerance. Indeed, Boerrigter et al15 reported a distinctive exercise pattern in COPD patients with severe PH (mPAP >40 mmHg). Although in our study the limited number of patients with severe PH precluded an analysis in this subset of patients, the finding of lower CI values during exercise in patients with PH concurs with the findings by Boerrigter et al.15
Pulmonary gas exchange impairment was associated with the presence of PH. However, the fact that patients without arterial hypoxemia also had PH in our cohort underlines the concept that the genesis of PH is complex and multifactorial.36 The weak correlation in COPD between PH and lung function measurements has been largely known and fits with our results.7,11,34 This makes it difficult in daily clinical practice to use predictive models to identify patients with pretest probability of presenting PH. Skjørten et al37 suggested that PaO2 values at rest and peak exercise below 71 mmHg and 64 mmHg, respectively, indicate the need for further evaluation of coexisting PH. In our study, we failed to find an adequate model with functional variables that could help to predict the presence of PH.
Our investigation has some limitations. First, there is the possibility that selection bias might have influenced the significance of our findings due to its retrospective, single-center, and observational design. However, because the purpose of the study was to describe PH and hemodynamic characteristics in COPD patients with different grades of airflow limitation, we believe that the population addresses the needs of the research question. Secondly, since there were no patients with mild airflow limitation (GOLD grade 1) in the study, our findings may not be extensively applicable to this population. Thirdly, the number of women included was small, such that our observations may not be generalized to all individuals with COPD. This sex bias is due to the lower prevalence of COPD in women than in men in our country and the underdiagnoses of COPD in women.38,39 Finally, we do not exclude that previous oxygen therapy or vasodilator treatment in these patients could influence the hemodynamic measurements.
Conclusion
In summary, the present study that analyzed a representative cohort of COPD patients with varying degrees of airflow limitation severity by means of right-heart catheterization shows that PH at rest is uncommon in patients with moderate airflow limitation but has a similar prevalence to patients with severe and very-severe airflow obstruction from other studies, highlighting that airflow limitation is a poor predictor of PH occurrence. In advanced COPD, the coexistence of pulmonary gas exchange impairment is of great influence on the development of PH. In contrast, an abnormal vascular response to exercise was observed in the majority of patients, even in those with mild airflow limitation, highlighting the notion that pulmonary vascular derangement is an early event in the natural history of COPD. Progression of these abnormalities may lead to the development of PH that restrains the increase of CO during exercise, which might contribute to limiting exercise tolerance.
Acknowledgments
The authors would like to thank to Dr Ignasi Garcia-Olivé and Dr Diego Rodriguez for their assistance in the statistical analysis. This paper was supported by grants from the Societat Catalana de Pneumologia, Sociedad Española de Neumología y Cirugía Torácica and Instituto de Salud Carlos III (EC07/90049).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Barberà JA, Peinado VI, Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J. 2003;21(5):892–905. doi: 10.1183/09031936.03.00115402. [DOI] [PubMed] [Google Scholar]
- 2.Hoeper MM, Barberà JA, Channick RN, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S85–S96. doi: 10.1016/j.jacc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D109–D116. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 4.Oswald-Mammosser M, Apprill M, Bachez P, Ehrhart M, Weitzenblum E. Pulmonary hemodynamics in chronic obstructive pulmonary disease of the emphysematous type. Respiration. 1991;58(5–6):304–310. doi: 10.1159/000195950. [DOI] [PubMed] [Google Scholar]
- 5.Sims MW, Margolis DJ, Localio AR, Panettieri RA, Kawut SM, Christie JD. Impact of pulmonary artery pressure on exercise function in severe COPD. Chest. 2009;136(2):412–419. doi: 10.1378/chest.08-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531–1536. doi: 10.1378/chest.127.5.1531. [DOI] [PubMed] [Google Scholar]
- 7.Scharf SM, Iqbal M, Keller C, et al. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med. 2002;166(3):314–322. doi: 10.1164/rccm.2107027. [DOI] [PubMed] [Google Scholar]
- 8.Doi M, Nakano K, Hiramoto T, Kohno N. Significance of pulmonary artery pressure in emphysema patients with mild-to-moderate hypoxemia. Respir Med. 2003;97(8):915–920. doi: 10.1016/s0954-6111(03)00115-x. [DOI] [PubMed] [Google Scholar]
- 9.Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189–194. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- 10.Cuttica MJ, Kalhan R, Shlobin OA, et al. Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir Med. 2010;104(12):1877–1882. doi: 10.1016/j.rmed.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Andersen KH, Iversen M, Kjaergaard J, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant. 2012;31(4):373–380. doi: 10.1016/j.healun.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(2):219–224. doi: 10.1164/ajrccm.164.2.2006129. [DOI] [PubMed] [Google Scholar]
- 13.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 14.Hilde JM, Skjørten I, Hansteen V, et al. Haemodynamic responses to exercise in patients with COPD. Eur Respir J. 2013;41(5):1031–1041. doi: 10.1183/09031936.00085612. [DOI] [PubMed] [Google Scholar]
- 15.Boerrigter BG, Bogaard HJ, Trip P, et al. Ventilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertension. Chest. 2012;142(5):1166–1174. doi: 10.1378/chest.11-2798. [DOI] [PubMed] [Google Scholar]
- 16.Pynnaert C, Lamotte M, Naeije R. Aerobic exercise capacity in COPD patients with and without pulmonary hypertension. Respir Med. 2010;104(1):121–126. doi: 10.1016/j.rmed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Barberà JA, Roger N, Roca J, Rovira I, Higenbottam TW, Rodriguez-Roisin R. Worsening of pulmonary gas exchange with nitric oxide inhalation in chronic obstructive pulmonary disease. Lancet. 1996;347(8999):436–440. doi: 10.1016/s0140-6736(96)90011-2. [DOI] [PubMed] [Google Scholar]
- 18.Roger N, Barberà JA, Roca J, Rovira I, Gómez FP, Rodriguez-Roisin R. Nitric oxide inhalation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156(3 Pt 1):800–806. doi: 10.1164/ajrccm.156.3.9611051. [DOI] [PubMed] [Google Scholar]
- 19.Agustí AG, Barberá JA, Roca J, Wagner PD, Guitart R, Rodriguez-Roisín R. Hypoxic pulmonary vasoconstriction and gas exchange during exercise in chronic obstructive pulmonary disease. Chest. 1990;97(2):268–275. doi: 10.1378/chest.97.2.268. [DOI] [PubMed] [Google Scholar]
- 20.Ribas J, Díaz O, Barberà JA, et al. Invasive exercise testing in the evaluation of patients at high-risk for lung resection. Eur Respir J. 1998;12(6):1429–1435. doi: 10.1183/09031936.98.12061429. [DOI] [PubMed] [Google Scholar]
- 21.Ribas J, Jiménez MJ, Barberà JA, et al. Gas exchange and pulmonary hemodynamics during lung resection in patients at increased risk: relationship with preoperative exercise testing. Chest. 2001;120(3):852–859. doi: 10.1378/chest.120.3.852. [DOI] [PubMed] [Google Scholar]
- 22.Peinado VI, Gómez FP, Barberà JA, et al. Pulmonary vascular abnormalities in chronic obstructive pulmonary disease undergoing lung transplant. J Heart Lung Transplant. 2013;32(12):1262–1269. doi: 10.1016/j.healun.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Blanco I, Gimeno E, Munoz PA, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med. 2010;181(3):270–278. doi: 10.1164/rccm.200907-0988OC. [DOI] [PubMed] [Google Scholar]
- 24.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 25.Saggar R, Lewis GD, Systrom DM, Champion HC, Naeije R, Saggar R. Pulmonary vascular response to exercise: a haemodynamic observation. Eur Respir J. 2012;39(2):231–234. doi: 10.1183/09031936.00166211. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34(4):888–894. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 27.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 28.Roca J, Sanchis J, Agusti-Vidal A, et al. Spirometric reference values for a Mediterranean population. Bull Eur Physiopathol Respir. 1986;22(3):217–224. [PubMed] [Google Scholar]
- 29.Roca J, Rodriguez-Roisin R, Cobo E, Burgos F, Perez J, Clausen JL. Single-breath carbon monoxide diffusing capacity prediction equations from a Mediterranean population. Am Rev Respir Dis. 1990;141(4 Pt 1):1026–1032. doi: 10.1164/ajrccm/141.4_Pt_1.1026. [DOI] [PubMed] [Google Scholar]
- 30.Nelson LD, Rutherford EJ. Principles of hemodynamic monitoring. In: Pinsky MR, Dhainaut JF, editors. Pathophysiologic Foundations of Critical Care. Baltimore, MD: Williams and Wilkins; 1993. pp. 3–22. [Google Scholar]
- 31.Barberà JA. The pulmonary vasculature of COPD. In: Rennard SI, Rodríguez-Roisin R, Huchon G, Roche N, editors. Clinical management of chronic obstructive lung disease. 2nd ed. New York: Informa Healthcare; 2008. pp. 189–205. [Google Scholar]
- 32.Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J. 2013;41(6):1292–1301. doi: 10.1183/09031936.00079512. [DOI] [PubMed] [Google Scholar]
- 33.Lau EM, Manes A, Celermajer DS, Galiè N. Early detection of pulmonary vascular disease in pulmonary arterial hypertension: time to move forward. Eur Heart J. 2011;32(20):2489–2898. doi: 10.1093/eurheartj/ehr160. [DOI] [PubMed] [Google Scholar]
- 34.Christensen CC, Ryg MS, Edvardsen A, Skjønsberg OH. Relationship between exercise desaturation and pulmonary haemodynamics in COPD patients. Eur Respir J. 2004;24(4):580–586. doi: 10.1183/09031936.04.00118303. [DOI] [PubMed] [Google Scholar]
- 35.Kovacs G, Olschewski A, Berghold A, Olschewski H. Pulmonary vascular resistances during exercise in normal subjects: a systematic review. Eur Respir J. 2012;39(2):319–328. doi: 10.1183/09031936.00008611. [DOI] [PubMed] [Google Scholar]
- 36.Peinado VI, Pizarro S, Barberà JA. Pulmonary vascular involvement in COPD. Chest. 2008;134(4):808–814. doi: 10.1378/chest.08-0820. [DOI] [PubMed] [Google Scholar]
- 37.Skjørten I, Hilde JM, Melsom MN, Hansteen V, Steine K, Humerfelt S. Pulmonary artery pressure and PaO2 in chronic obstructive pulmonary disease. Respir Med. 2013;107(8):1271–1279. doi: 10.1016/j.rmed.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 38.Miravitlles M, Soriano JB, Garcia-Rio R, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64(10):863–868. doi: 10.1136/thx.2009.115725. [DOI] [PubMed] [Google Scholar]
- 39.Ancochea J, Miravitlles M, García-Río F, et al. Underdiagnoses of chronic obstructive pulmonary disease in women: quantification of the problem, determinants and proposals for action. Arch Bronconeumol. 2013;49(6):223–229. doi: 10.1016/j.arbres.2012.11.010. English, Spanish. [DOI] [PubMed] [Google Scholar]