Abstract
The adverse effects of maternal alcohol use during pregnancy represent a spectrum of growth restriction, facial dysmorphology, and neurocognitive challenges in the offspring. The continuum of diagnoses is referred to as fetal alcohol spectrum disorders (FASD). Short palpebral fissures, a smooth philtrum, and a thin vermilion border of the upper lip comprise the three cardinal facial features of FASD. Early attempts to define a smooth philtrum and thin vermilion border of the upper lip were subjective. Astley and colleagues introduced a 5-point Likert-scaled lip/philtrum guide based on Caucasian North American subjects as an objective tool for the evaluation of the facial dysmorphology in FASD. This Caucasian guide has been incorporated into all current diagnostic schemes for FASD. However, broad international clinical experience with FASD indicates racial and ethnic differences with respect to the facial morphology. Because of the substantial number of children with FASD in South Africa among the Cape Coloured (mixed race) population in the Western Cape Province, we developed a specific lip/philtrum guide for that population. The guide incorporates a 45-degree view of the philtrum that enables an enhanced 3-dimensional evaluation of philtral height not possible with a frontal view alone. The guide has proven to be a more specific and sensitive tool for evaluation of the facial dysmorphology of FASD in the Cape Coloured population than the use of the previous North American Caucasian guide and points to the utility of racial and ethnic-specific dysmorphology tools in the evaluation of children with suspected FASD.
Keywords: fetal alcohol syndrome, fetal alcohol spectrum disorders, lip/philtrum guide, alcohol abuse, prenatal alcohol abuse, diagnostic tool
INTRODUCTION
Since the identification of fetal alcohol syndrome (FAS) 40 years ago [Jones and Smith, 1973], significant efforts have been made to clarify the diagnostic criteria. While the operationalization of the criteria have been debated, the basic features comprising a diagnosis of FAS have remained unchanged: growth restriction, a specific pattern of minor anomalies of the face (short palpebral fissures, smooth philtrum, and thin vermilion border of the upper lip), and neurocognitive deficits [Hoyme et al., 2005; Sratton et al., 2000]. Yet clinicians soon recognized that many children adversely affected by prenatal alcohol exposure did not display all of the cardinal features of FAS. It is now recognized that the effects of alcohol on the developing fetus represent a continuum of disability best termed fetal alcohol spectrum disorders (FASD).
One of the difficulties clinicians encountered in making diagnoses of FASD was the subjectivity of describing two of the cardinal facial features of FAS, the smooth philtrum, and thin vermilion border of the upper lip. Originally designed for research purposes, Astley and Clarren first introduced an innovative “lip/philtrum guide” as an objective tool for assessing the morphology of the philtrum and vermilion border of the upper lip [Astley and Clarren, 1995]. The lip/philtrum guide began as a three-point Likert scale [Astley and Clarren, 1995] that evolved into a 5-point scale a year later [Astley and Clarren, 1996]. On the 5-digit Likert scale, a score of 5 represents the flattest philtrum and thinnest vermilion border of the upper lip. A score of 1 represents the most prominent philtral columns and most defined upper lip. All photographs for the original 5-point Likert scale were selected from Caucasian patients evaluated through University of Washington diagnostic clinics; however, the authors did not specify how these particular photos were selected for inclusion on the lip/philtrum guide [Astley and Clarren, 1996].
In 2004, Astley published a revised Caucasian lip/philtrum guide with higher-resolution photographs and also included a new African –American guide [Astley, 2004]. The author states “the five ranks in the African–American Guide were set to be comparable to the percentile cutoffs used in the Caucasian lip/philtrum guide” [Astley, 2004]. However, the method of selecting photos for inclusion was not specified. The addition of a population-specific lip/philtrum guide acknowledged the differences in facial morphology across racial groups and possible improvements that might be needed in current approaches for assessing facial dysmorphology.
FASD in South Africa
South Africa has the highest documented prevalence of FASD in the world [May et al., 2000, 2007, 2013; Viljoen et al., 2005; Urban et al., 2008]. The latest estimates of school-based studies in the Western Cape Province documented a 135.1–207.5 per 1,000 rate of FASD [May et al., 2013]. This same methodology has been applied in other countries, producing much lower rates. A recent report from a Midwestern city in the United States found a rate of 5.9–8.4 per 1,000 children for FAS and 24–48 per 1,000 children for FASD [May et al., 2014]. Comparable rates to this US rate were found in Italy [May et al., 2006, 2011].
Approximately four million people reside in the Western Cape Province, including 57% Cape Coloured (mixed race), 18% black, 25% white, and 1% other individuals [May et al., 2013]. A recent genome-wide analysis study demonstrated that the Cape Coloured population is genetically unique, with Khoesan (Bushmen) genes predominating ([Khoesan (32–43%], Bantu-speaking African [20–36%], European [21–28%], and Asian [9–11%]) [de Wit et al., 2010]. This differs from the African–American population, which is genetically 80% sub-Saharan African, 19% European, and 1% Native American [Genographic Project, 2015]. Prevalence studies of FASD in the Western and Northern Cape provinces have primarily investigated the Cape Coloured population and have used previously published Caucasian North American lip/philtrum guides [May et al., 2000, 2007, 2013; Viljoen et al., 2005; Urban et al., 2008]. The Caucasian guides have been used rather than Astley's African–American guide, because the Caucasian guides have had significantly more widespread field-testing in domestic and international FASD prevalence studies, and since neither the Caucasian nor the African–American guide heretofore has been an exact “fit” for the Cape Coloured population.
Because of the many studies and the high prevalence of FASD in the Cape Coloured population in the Western Cape Province, there is both a need and an opportunity for the development of a more sensitive lip/philtrum guide that is specific to that population. The purpose of this project was to use an expert dysmorphology team, experienced in FASD diagnosis, and the several thousand clinical photographic records from studies of FASD in the Cape Coloured population to formulate a racially specific lip/philtrum guide, and then to test it by comparing results with that of the original Astley/Clarren guide produced from Caucasian North American subjects.
METHODS
Participants
To produce the guide, an expert dysmorphology team analyzed 400 photographs of South African children of Cape Coloured racial background from the Western Cape Province obtained during diagnostic evaluations of first grade learners in NIH-funded epidemiology studies for a potential FASD.
Procedures
Creation of the South African cape coloured lip/philtrum guide
Two photographic views of each child were evaluated for the new guide: a frontal view and a 45-degree view (to better illustrate the height of the philtral columns). The photographs were arranged in order from those of children with the smoothest philtrum and thinnest vermilion border of the upper lip (a score of 5) in a linear fashion to those of children with the most prominent philtral columns and fullest upper lip with the most prominent cupid’s bow (a score of 1). Intermediate morphology scores of 2, 3, and 4 marked the quartiles of the upper lip morphology observed in the photographs (Fig 1).
FIG. 1.
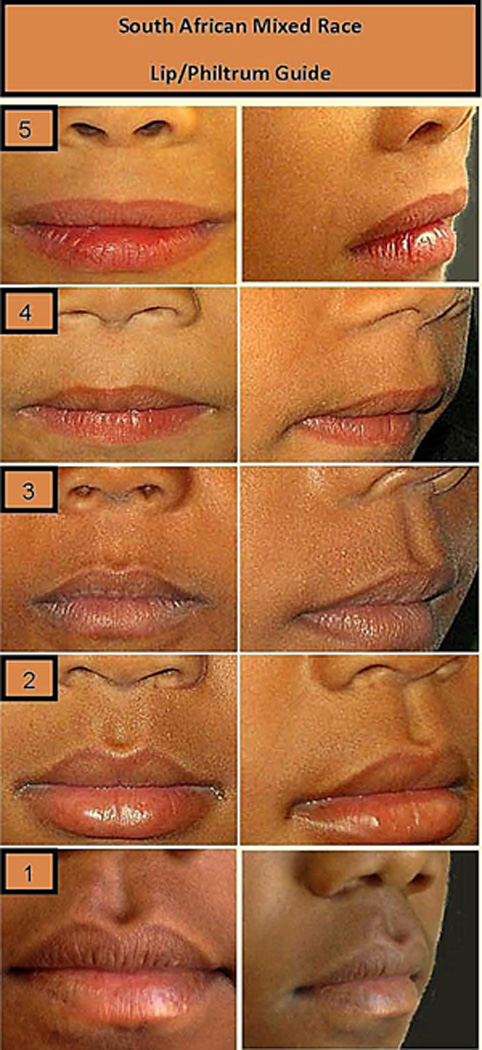
South African Cape Coloured (mixed race) Lip/philtrum guide.
Validation of the lip/philtrum guide
Once the new Cape Coloured lip/philtrum guide was produced, it was used simultaneously with the Astley/Clarren North American Caucasian lip/philtrum guide to score philtrum and vermilion border morphology in the evaluation of 1,057 school children in the Western Cape Province for a potential FASD. In these studies, a team of five experienced dysmorphologists independently assigned two sets of scores for each child: scores for the vermilion border of the upper lip and philtrum using the Caucasian Astley/Clarren guide and similar scores using the new South African Cape Coloured Lip/Philtrum Guide.
Statistical analysis of the paired data was performed via the IBM Statistical Package for the Social Sciences (SPSS), version 20 [IBM, 2011].
RESULTS
For the overall sample, the two guides produced an excelled Cronbach's α reliability of 0.966 and a highly significant Pearson correlation coefficient of 0.793 for the philtrum and 0.954 and 0.781 for the vermilion (Table I). The mean philtrum score was 3.36 (±0.023) for the Astley/Clarren guide and 3.43 (±0.027) for the South African Cape Coloured (SA) guide (t=−4.042, P < 0.0001, df = 1056), a significant difference in means, with the SA guide producing a higher mean. For the vermilion, the mean for the Astley/Clarren guide was 3.41 (±0.025) and the SA guide 3.50 (t = −5.321, P < 0.0001, df = 1056), again a significantly higher or more sensitive score. In rating both features, the result produced by the SA guide was significantly higher, therefore producing indicators of slightly greater hypoplasia.
TABLE I.
Comparison, With Cronbach Alpha Values, of Scores Provided by Dysmorphologists Utilizing the Astley/Clarren Lip/Philtrum Guide and the South African Lip/Philtrum Guide
| Sample | a | r | Astley Mean | FASER-SA Mean | t | p | df |
|---|---|---|---|---|---|---|---|
| Total Sample | |||||||
| Philtrum | 0.966 | 0.793 | 3.36 | 3.43 | −4.042 | <0.001 | 1056 |
| Vermilion | 0.954 | 0.781 | 3.41 | 3.50 | −5.321 | <0.001 | 1056 |
| Coloureds | |||||||
| Philtrum | 0.969 | 0.806 | 3.39 | 3.44 | −3.381 | 0.001 | 930 |
| Vermilion | 0.953 | 0.791 | 3.45 | 3.54 | −5.068 | <0.001 | 930 |
| Blacks | |||||||
| Philtrum | 0.953 | 0.778 | 3.23 | 3.33 | −2.073 | 0.041 | 109 |
| Vermilion | 0.977 | 0.763 | 3.08 | 3.15 | −1.268 | 0.207 | 109 |
| Boys | |||||||
| Philtrum | 0.967 | 0.862 | 3.41 | 3.47 | −2.935 | 0.003 | 521 |
| Vermilion | 0.960 | 0.838 | 3.39 | 3.47 | −3.652 | <0.001 | 521 |
| Girls | |||||||
| Philtrum | 0.964 | 0.721 | 3.31 | 3.39 | −2.869 | 0.004 | 534 |
| Vermilion | 0.947 | 0.723 | 3.42 | 3.53 | −3.903 | <0.001 | 534 |
When similar statistical analyses were performed by ethnicity (Coloured or Black) both the philtrum and vermilion scales were found to be highly reliable, and correlate most highly for the philtrum (α = 0.969, r = 0.806) and for the vermilion border (α = 0.953, r = 0.791) assessments of the Coloured children. The mean scores for the Coloured children were higher for the SA guide and the differences were again significant (t = −3.381, P < 0.001, df = 930; t = −5.068, P < 0.0001, df = 930) indicating more sensitivity of the SA guide than the Astley/Clarren guide to detecting these cardinal features of FASD in the Coloured population. They also were consistent in the ability to produce higher means and measures of reliability and correlation for Blacks, boys, and girls.
DISCUSSION
In virtually every comparison, the South African Cape Coloured guide produced a significantly higher mean score than the original guide, and the correlation with the Astley/Clarren rating was highest for boys and lowest for girls. The SA guide is therefore more sensitive to detecting these clinical features of FASD in the Coloured population of the Western Cape. The SA guide utilizes two innovations: (i) actual South African-specific, Cape Coloured physical features (photos) and, (ii) in addition to the frontal view, a view taken at a 45-degree angle for better gauging the height of the philtral columns has been added. The SA guide appears to be a more sensitive tool for assessing all children in the Western Cape population, but most specifically among the Coloured children who comprise 88% of this sample of school children. But because the new guide produces a higher mean measure of hypoplasia of the philtrum and upper lip with all group comparisons, its increased sensitivity is likely due to introduction of the 45-degree angle picture rather than to the racial specificity.
CONCLUSION
Based on these data, development of new lip/philtrum guides utilizing 45-degree angle images and racially specific philtrum/ vermilion rating scales should be considered for each unique population studied.
Acknowledgments
Grant sponsor: National Institutes of Health (NIH) National Institute on Alcohol Abuse, and Alcoholism (NIAAA); Grant numbers: R01 AA11685, RO1/UO1 AA01115134.
Footnotes
These data were presented in part in abstract form at: the Western Society for Pediatric Research (Hoyme DB, Hoyme HE, Jones KL, Robinson LK, Manning MA, Bezuidenhout H, Marais AS, Kalberg W, Gossage J, and May PA: A South African Mixed Race Lip/Philtrum Guide for Diagnosis of Fetal Alcohol Spectrum Disorders. J Investigative Med, 2010) and the 5th International Conference on FASD, Vancouver, BC, 2013.
REFERENCES
- Astley SJ, Clarren SK. A fetal alcohol syndrome screening tool. Alcohol Clin Exp Res. 1995;19:1565–1571. doi: 10.1111/j.1530-0277.1995.tb01025.x. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. Acase definition and photographic screening tool for the facial phenotype of fetal alcohol syndrome. J Pediatr. 1996;129:33–41. doi: 10.1016/s0022-3476(96)70187-7. [DOI] [PubMed] [Google Scholar]
- Astley SK. FASD 4-Digit Diagnostic Code TM. [Accessed on January 11, 2015];FAS Diagnostic and Prevention Network. https://depts.washington.edu/fasdpn/htmls/4-digit-code.htm. 2004 [Google Scholar]
- de Wit E, Delport W, Rugamika CE, Meintjes A, Möoller M, van Helden PD, Seoighe C, Hoal EG. Genome-wide analysis of the South African Coloured population in the Western Cape. Hum Genet. 2010;128:145–153. doi: 10.1007/s00439-010-0836-1. [DOI] [PubMed] [Google Scholar]
- Genographic Project. [Accessed on January 11, 2015];The National Geographic Society. https://genographic.nationalgeographic.com.
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckely DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBMSPSS Statistics for Windows [computer program] Version 20.0. Armonk, NY: IBM Corp.; 2011. [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Public Health. 2000;90:1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Gossage JP, Kalberg WO, Hoyme HE, Robinson LK, Coriale G, Jones KL, del Campo M, Tarani L, Romeo M, Kodituwakku PW, Deiana L, Buckley D, Ceccanti M. Epidemiology of FASD in a province in Italy: Prevalence and characteristics of children in a random sample of schools. Alcohol Clin Exp Res. 2006;30:1562–1575. doi: 10.1111/j.1530-0277.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NC, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Coriale G, Kalberg WO, Hoyme HE, Aragón AS, Buckley D, Stellavato C, Gossage JP, Robinson LK, Jones KL, Manning M, Ceccanti M. Prevalence of children with severe fetal alcohol spectrum disorders in communities near Rome, Italy: Rates are substantially higher than previous estimates. Int J Environ Res Public Health. 2011;8:2331–2351. doi: 10.3390/ijerph8062331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013;37:818–830. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul-Rahman A, Adam MP, Robinson LK, Manning M, Hoyme HE. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134:855–866. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sratton K, Howe C, Battaglia F. Fetal alcohol syndrome: Diagnosis, epidemiology, prevention and treatment. Washington, DC: National Academy Press; 2000. [Google Scholar]
- Urban M, Chersich MF, Fourie LA, Chetty C, Olivier L, Viljoen D. Fetal alcohol syndrome among grade 1 schoolchildren in Northern Cape Province: Prevalence and risk factors. S Afr Med J. 2008;98:877–882. [PubMed] [Google Scholar]
- Viljoen DL, Gossage JP, Brooke L, Adnams CM, Jones KL, Robinson LK, Hoyme HE, Snell C, Khaole NC, Kodituwakku P, Asante KO, Findlay R, Quinton B, Marais AS, Kalberg WO, May PA. Fetal alcohol syndrome epidemiology in a South African community: A second study of a very high prevalence area. J Stud Alcohol. 2005;66:593–604. doi: 10.15288/jsa.2005.66.593. [DOI] [PMC free article] [PubMed] [Google Scholar]


