Abstract
Nearly a century ago, Otto Warburg made the astute observation that the metabolic properties of cancer cells differ markedly from those of normal cells. Several decades passed before the concept of exploiting cancer cell metabolism came into clinical practice with the advent of chemotherapy, the underlying principle of which is to target rapidly dividing cells by interfering with critical processes that are all, on some level, driven by cell metabolism. Although chemotherapy can be quite effective, success rates are highly variable and the adverse effects associated with treatment often outweigh the benefits due to the fact that chemotherapy is indiscriminately cytotoxic against all rapidly dividing cells, cancerous or healthy. During the past several years, a more intricate understanding of cancer cell metabolism has permitted the development of targeted therapies that aim to specifically target cancer cells and spare healthy tissue by exploiting the altered metabolism of cancer cells. The identification of new metabolic targets and the subsequent development of small-molecule inhibitors of metabolic enzymes have demonstrated the utility and promise of targeting cancer cell metabolism as an anticancer strategy. This review summarizes recent advances in the identification and characterization of several metabolic enzymes as emerging anticancer targets.
Keywords: the Warburg effect, cancer metabolism, metabolic enzymes, small-molecule inhibitors, anticancer targets
INTRODUCTION
The recent resurgence of interest in tumor metabolism, a concept pioneered by German physiologist Otto Warburg nearly a century ago, has led to several discoveries concerning specific alterations to cellular metabolism in cancer cells, some of which are requisite for malignant transformation.1,2 In a phenomenon later designated the “Warburg effect,” Warburg observed that cancer cells produce energy primarily by glycolysis in the cytosol rather than by oxidative phosphorylation in mitochondria as in most normal cells. Although normal cells switch to glycolysis for energy production in the absence of oxygen, cancer cells use glycolysis even when oxygen is present (aerobic glycolysis). Leukemia cells, for example, are highly glycolytic despite residing in the bloodstream, in which oxygen is plentiful. Warburg hypothesized that this altered metabolism arose from mitochondrial defects that inhibited their ability to effectively oxidize glucose carbon to carbon dioxide.3
Despite his prescient observations regarding the distinctiveness of tumor metabolism and the suggestion that such alterations could represent targetable vulnerabilities in cancer cells, nearly 80 years passed before Warburg’s hypothesis was revisited. Although recent discoveries have served to reinforce many of his initial postulations, the notion that the metabolic properties of cancer cells are a result of damaged mitochondria has since been refuted. Instead, it has been found that these alterations are in fact a result of oncogene-driven metabolic reprogramming required to support cancer cell proliferation and survival. This helps to illuminate why cancer cells would “choose” glycolysis, a relatively inefficient mode of energy production, over the much more efficient oxidative phosphorylation: glycolysis is quicker, and readily provides energy in the form of adenosine triphosphate (ATP) required by rapidly proliferating cancer cells.4,5
In addition to the expeditious production of energy, aerobic glycolysis also facilitates rapid cell division by providing metabolic intermediates that can be shunted into divaricating pathways, in which they serve as precursors for the anabolic biosynthesis of macromolecules. These include nucleotides, amino acids, and fatty acids, respectively, to produce RNA/ DNA, proteins, and lipids, which are necessary for rapid cell division.3–5 Moreover, glycolytic intermediates can also be diverted into pathways that produce reduced nicotinamide adenine dinucleotide phosphate (NADPH), which not only fuels macromolecular biosynthesis of lipids but also functions as an antioxidant to quench the reactive oxygen species produced during rapid proliferation of cancer cells, which is imperative for the maintenance of cellular redox homeostasis.
Traditional Approaches to Targeting Cancer Cell Metabolism
The concept of exploiting cancer cell metabolism is one that has been in practice for nearly 50 years, since the advent of chemotherapy. The rapid proliferation that characterizes cancer cells is fueled in part by metabolic processes that serve to provide the cell what it requires to grow and divide. The enduring principle of chemotherapy has been to target rapidly dividing cells by interfering with these critical processes that are all, on some level, driven by cell metabolism.
The first use of chemotherapeutic drugs to treat cancer came in the mid-20th century, with the application of nitrogen mustards to treat patients with advanced lymphoma. During World War I, mustard gas was used as a chemical warfare agent by the Imperial German Army. Among the many powerful physiological effects of mustard gas, those afflicted experienced potent hematopoietic suppression, particularly in the leukocyte compartment. It was later reasoned that similar compounds may be useful in treating hematopoietic malignancies that display an overproduction of white blood cells. Indeed, patients with lymphoma who are treated with nitrogen mustards displayed a marked reduction in their white blood cell count and experienced a transitory remission period.6 This opened the door for the development of chemotherapeutic drugs to treat cancer over the next several decades.
The majority of chemotherapeutic drugs can be divided into 5 major classes, all of which function to inhibit cell division: alkylating agents, anthracyclines, plant alkaloids, topoisomerase inhibitors, and antimetabolites. Antimetabolite drugs were among the first effective chemotherapeutic agents to be discovered, and provide the most direct evidence to support the usefulness of disrupting cancer cell metabolism as a treatment strategy. Just as the name suggests, antimetabolites inhibit the use of a metabolite needed for normal cellular metabolic functions. These compounds often masquerade as the metabolite with which they interfere.7
Methotrexate
In 1947, after the discovery that the administration of folic acid conjugates could promote leukemia cell proliferation in patients, Farber and his colleagues found that aminopterin, a chemical analog of folic acid, was effective in treating children with acute lymphoblastic leukemia (ALL). To the best of our knowledge, aminopterin was the first antimetabolite used in cancer treatment, and the first drug shown to induce remission in patients with ALL. Methotrexate soon replaced aminopterin as a chemotherapeutic agent, and is still used in treatment regimens for many cancers.8
Although unknown at the time of their first clinical use, the molecular mechanism of folate analogues was later elucidated. Methotrexate competitively inhibits dihydrofolate reductase, an enzyme essential to tetrahydrofolate synthesis, by catalyzing the conversion of dihydrofolate to active tetrahydrofolate. Folic acid is needed for the de novo synthesis of thymidine, which in turn is required for DNA synthesis.7
5-Fluorouracil
The chemotherapeutic agent 5-fluorouracil (5-FU) has been in clinical use for 40 years, and is used to treat a variety of malignancies including cancers of the colon, rectum, and head and neck. 5-FU primarily functions as an inhibitor of thymidylate synthase, an enzyme that converts deoxyuridine monophosphate (dUMP) into thymidine monophosphate (dTMP). dTMP is subsequently phosphorylated to form thymidine triphosphate for use in DNA replication. The depletion of dTMP by 5-FU thus prevents DNA replication and ultimately results in cell death.7
L-asparaginase
The use of the enzyme L-asparaginase to treat patients with ALL represents a fairly rudimentary example of how the distinctive metabolism of cancer cells has been exploited for therapy. In contrast to normal hematopoietic cells, ALL cells are unable to synthesize the nonessential amino acid asparagine and thus depend on circulating asparagine. L-asparaginase catalyzes the conversion of as-paragine to aspartic acid, thereby depriving the leukemic cell of the circulating asparagine it requires to survive, leading to cell death.9,10 However, its systemic administration may lead to severe side effects, including pancreatitis, hepatic dysfunction, nephrotoxicity, and central nervous system dysfunction.11
Although chemotherapeutic agents can be quite effective, success rates are highly variable. Moreover, the adverse effects associated with chemotherapy often outweigh the benefits. Current regimens can be highly aggressive and are associated with extremely adverse side effects that severely affect quality of life. Because chemotherapeutic agents are indiscriminately cytotoxic, they prove to be equally detrimental to all rapidly proliferating cells, whether healthy or cancerous. Common side effects of chemotherapy result from the damage incurred by rapidly proliferating healthy cells, including alopecia due to effects on hair follicles, myelosuppression due to effects on bone marrow cells, and nausea/vomiting due to effects on the gastric mucosa. Life-threatening side effects include vital organ toxicity and secondary neoplasms.
A New Era of Targeting Cancer Cell Metabolism
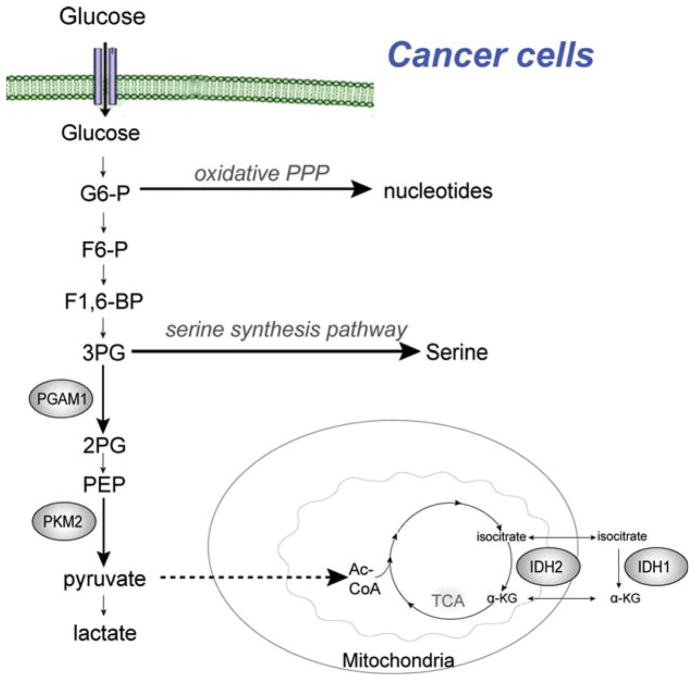
The past decade has seen tremendous advances in the understanding of cancer cell metabolism, as well as a more developed appreciation for its complexity. The molecular characterization of metabolic differences between cancer cells and normal cells has provoked exploration of the therapeutic opportunities these differences might provide. Drug development in this vein has sought to exploit metabolic vulnerabilities in cancer cells, with the aim of developing molecularly targeted therapies against cancer cell-specific metabolic alterations. The past several years has witnessed validation of metabolic enzymes as emerging anticancer targets, such as ATP citrate lyase,12 lactate de-hydrogenase,13 pyruvate dehydrogenase kinase,14–16 and glutaminase.17 Below we will focus on several new targets including pyruvate kinase M2 (PKM2), phosphoglycerate mutase 1 (PGAM1), and isocitrate dehydrogenase (IDH) 1/2 in cancer cell metabolism (Fig. 1).
Figure 1.
New targets in cancer cell metabolism are shown. The glycolytic enzymes pyruvate kinase M2 (PKM2) and phosphoglycerate mutase 1 (PGAM1) as well as the tricarboxylic acid (TCA) cycle enzyme isocitrate dehydrogenase (IDH) 1/2 have been recently identified as new and promising targets for cancer therapy. PKM2 catalyzes the conversion of phosphoenolpyruvate (PEP) into pyruvate while concurrently producing adenosine triphosphate, and has been shown to play a key role in promoting the Warburg effect in tumor cells. PGAM1 catalyzes the conversion of 3-phosphoglycerate (3PG) to 2-phosphoglycerate (2PG), and is uniquely positioned at the branching point between glycolysis and anabolic biosynthesis, making it an attractive anticancer target. PGAM1 activity is increased in many cancers, and its gene expression is believed to be upregulated due to loss of TP53 in cancer cells, because TP53 negatively regulates the PGAM1 level. IDH catalyzes the oxidative decarboxylation of isocitrate, producing α-ketoglutarate (α-KG) in the citric acid cycle. Both IDH1 and IDH2 produce nicotinamide adenine di-nucleotide phosphate (NADPH), and both have been identified as being mutated in human cancers, namely glioma and acute myeloid leukemia. G6-P indicates glucose 6-phosphate; PPP, pentose phosphate pathway; F6-P, fructose 6-phosphate; F1,6-BP, fructose 1,6-bisphosphate; Ac-CoA, acetyl coenzyme A.
PKM2
PK is a glycolytic enzyme that catalyzes the conversion of phosphoenolpyruvate into pyruvate while concurrently producing ATP. The M1 isoform of PK (PKM1) is expressed in most adult tissues, whereas the M2 isoform (PKM2), an alternatively spliced variant of M1, is expressed during embryonic development.18 More recently, it has been shown that cancer cells also express PKM2,18–20 and that PKM2 plays a key role in promoting the Warburg effect in tumor cells.18
PKM2 can adopt 2 possible conformations: an inactive dimer and an active tetramer. Recent studies have shown that oncogenic tyrosine kinase fibroblast growth factor receptor kinase 1 phosphorylates PKM2 at tyrosine 105 to inhibit the formation of active tetrameric PKM2, thereby promoting the formation of the inactive dimer.21 Moreover, PKM2 has been shown to be acetylated on lysine 305 in response to high intracellular glucose levels. Acetylation at K305 decreases PKM2 enzyme activity and promotes its lysosomal-dependent degradation via chaperone-mediated autophagy.22 Together, these results suggest that negative regulation of PKM2 activity is advantageous to cancer cells. When PKM2 is less active, glycolytic flux is decreased. This in turn allows cancer cells to accumulate building blocks and precursors produced in the upper glycolytic process above PKM2, and shunt intermediates into divaricating biosynthetic pathways including the pentose phosphate pathway and the serine biosynthesis pathway, which support cancer proliferation.
This notion prompted the development of several small-molecule PKM2 activators (Table 1). Because PKM2 is expressed in cancer cells and not normal adult tissue, selectively targeting PKM2 should have minimal adverse effects on healthy cells, making it a promising anticancer target. Current PKM2 activators, including TEPP-46, DASA-58, and ML-265, have all been shown to promote constitutive activity of PKM2, mimicking the enzymatic activity of PKM1. Increased PKM2 activity consequently results in decreased cell proliferation under hypoxia and attenuated tumor growth in mice, likely due to decreased anabolic biosynthesis.23,24 To the best of our knowledge, the extent of off-target toxicity induced by PKM2 activators has yet to be fully elucidated, and further studies are warranted to better understand the potential toxicity of PKM2 activators at the whole-organism level.
TABLE 1.
Targeting Metabolic Enzymes for Cancer Therapy
| Target | Agent(s) | Development Stage | Drug Development Platform(s) |
|---|---|---|---|
| PKM2 | TEPP-46 | Preclinical (cell line and animal data) | H1299 lung cancer cells |
| DASA-58 | Preclinical (cell line data only) | H1299 lung cancer cells | |
| ML-265 | Preclinical (cell line and animal data) | H1299 and A549 lung cancer cells | |
| PGAM1 | MJE3 | Preclinical (cell line data only) | MDA-MB-231 breast cancer cells |
| PGMI-004A | Preclinical (cell line and animal data) | Diverse leukemia and solid tumor cells | |
| IDH1 | AGI-5198 | Preclinical (cell line and animal data) | R132H-positive glioma cells |
| IDH2 | AGI-6780 | Preclinical (cell line and animal data) | R140Q-positive AML cells |
Abbreviations: AML, acute myeloid leukemia; IDH1, isocitrate dehydrogenase 1; IDH2, isocitrate dehydrogenase 2, PGAM1, phosphoglycerate mutase 1, PKM2, pyruvate kinase M2.
It is important to note that PKM2 has recently been shown to have nonmetabolic functions implicit in tumor-igenesis as well. In particular, various studies have demonstrated a nuclear role for PKM2 in which it serves to directly regulate transcription of genes encoding tumor-promoting factors including Oct-4,25 hypoxia-inducible factor 1-α (HIF-1α),26 and β-catenin.27 The nonglyco-lytic functions of PKM2 must therefore be accounted for as well during the continued development of small-molecule PKM2 activators and inhibitors.
PGAM1
PGAM1 catalyzes the conversion of 3-phosphoglycerate (3PG) to 2-phosphoglycerate (2PG) during glycolysis. PGAM1 is uniquely positioned at the branching point between glycolysis and anabolic biosynthesis, making it an attractive anticancer target. In many cancers, including hepatocellular carcinoma, colorectal cancer,28,29 and leukemia,30 PGAM1 activity is increased compared with normal tissues. Moreover, PGAM1 gene expression is believed to be upregulated due to loss of TP53 in cancer cells, because TP53 negatively regulates the PGAM1 level.31–33 PGAM1 has been shown to regulate distal metabolic pathways by controlling the metabolite levels of its substrate 3PG and product 2PG, which exert regulatory functions on key metabolic enzymes including 6-phosphogluconate dehydrogenase in the oxidative pentose phosphate pathway and 3-phosphoglycerate dehydrogen-ase in the serine biosynthesis pathway, respectively.30 Thus, the inhibition of PGAM1 not only affects glycolytic flux in cancer cells but also compromises biosynthetic pathways.
There currently exist 2 small-molecule inhibitors of PGAM1, MJE3 and PGMI-004A (Table 1). MJE3 was found to inhibit proliferation of MDA-MB-231 breast cancer cells, and was subsequently shown to target PGAM1 through in situ proteome reactivity profiling. MJE3 inhibits PGAM1 exclusively in intact cells, suggesting that the drug may be modified to its active form in cells.34 This presents a set of limitations with regard to determining inhibitor specificity. The small-molecule PGAM1 inhibitor PGMI-004A was identified through coupled PGAM1 and enolase assays, using a pure in vitro system to overcome the limitations associated with MJE3. PGMI-004A was shown to inhibit proliferation of diverse cancer and leukemia cell lines, as well as primary leukemia cells from patients, without demonstrating any significant toxicity to normal proliferating cells or peripheral blood and bone marrow cells isolated from healthy patients. Moreover, PGMI-004A was shown to be effective in attenuating tumor growth in mice with minimal off-target toxicity at the whole-organism level.30 Together, these results suggest that targeting PGAM1 is a promising anticancer strategy that may produce minimal adverse side effects in humans. However, to the best of our knowledge, the effect of PGAM1 inhibition on normal, metabolically active, postmitotic tissue such as the heart, brain, and skeletal muscle remains to be determined, and represents a potentially sizeable obstacle to be overcome before anti-PGAM1 therapy can be used in humans.
IDH
IDH catalyzes the oxidative decarboxylation of isocitrate, producing α-ketoglutarate in the citric acid cycle. Both IDH1 and IDH2 produce NADPH, in which the former is localized to the cytosol and the latter to the mitochon-dria. Unlike PKM2 and PGAM1, IDH1 and IDH2 have both been identified as mutated in human cancer. Large-scale sequencing studies have revealed that 60% to 90% of patients with secondary gliomas and 12% to 18% of patients with acute myeloid leukemia (AML) have heterozygous mutations in IDH1 or IDH2.35,36 Mutations affecting IDH1 and IDH2 confer neomorphic activity to the enzyme, wherein isocitrate is converted to 2-hydroxyglutarate (2-HG) instead of α-ketoglutarate. It has been reported that 2-HG increased 100-fold in patients with gliomas and AML with IDH mutations, suggesting it could serve as a clinical biomarker.37,38 Subsequent studies have identified 2-HG as an oncome-tabolite, capable of competitively inhibiting α-ketoglutarate–dependent dioxygenases, including his-tone and DNA demethylases, leading to genome-wide hypermethylation and ultimately a block in cellular differentiation.39–42
The identification of IDH mutations and glioma and AML followed by the discovery of 2-HG as an onco-metabolite quickly prompted the development of IDH mutant inhibitors (Table 1). Currently, 2 IDH mutant inhibitors have been developed: AGI-5198, which selectively inhibits IDH1-R132H,43 and AGI-6780, which selectively inhibits IDH2-R140Q.44 Both inhibitors promote cellular differentiation and impair IDH mutant but not IDH wild-type cancer cell proliferation in vitro and in vivo (AGI-5198 in glioma cells and AGI-6780 in leukemia cells).
Future Directions and Remaining Obstacles
In addition to identifying new targets for monotherapy, combination therapies targeting complementary metabolic pathways may result in the enhanced or synergistic inhibition of cancer cell viability. For example, cancer cells rely primarily on glycolysis for ATP production, but inhibiting glycolysis would in theory drive cells toward oxidative phosphorylation as an ATP source. Targeting both glycolysis and oxidative phosphorylation would likely lead to the severe depletion of intracellular ATP levels and, consequently, cell death. This concept has been explored in a prostate cancer model using the glycolytic inhibitor 2-deoxyglucose and the oxidative phosphorylation inhibitor metformin. It was shown that prostate cancer cells displayed significant sensitivity to this combination, whereas normal prostate cells were only moderately affected.45
An alternative strategy involves the combined inhibition of distinct biosynthetic pathways. In cancer cells, glucose and glutamine serve as primary carbon sources for ATP production and biosynthesis.46 Glutamine has recently been shown to be crucial for de novo lipogenesis in cells under hypoxia. Normally, precursors for fatty acid synthesis are generated from glucose-derived pyruvate through the oxygen-dependent tricarboxylic acid cycle. However, proliferating cells undergoing aerobic glycolysis and those grown under hypoxic conditions use reductive carboxylation of glutamine-derived α-ketoglutarate to synthesize lipid precursors, with the latter relying almost exclusively on this pathway for de novo lipogenesis.47 Inhibition of this pathway would thus disrupt de novo lipid biosynthesis in hypoxic tumor cells. Therefore, combined inhibition of the reductive glutamine pathway together with inhibitors of glycolytic flux would block at least 2 different biosynthetic pathways from 2 different carbon sources, which may in turn lead to enhanced or synergistic inhibition of cancer cell viability, particularly under hypoxia.
The major outstanding concern associated with targeting cancer cell metabolism lies in the fact that all cells use the same life-sustaining metabolic networks, and the disruption of any of these metabolic processes has the potential to adversely affect cancer cells and normal cells alike. The majority of metabolic enzymes implicated in the pathogenesis of cancer are not mutated, and are expressed both in transformed cells and normal cells throughout the body. This presents a considerable set of challenges with regard to achieving specificity in targeting cancer cells versus normal cells. However, the altered metabolism in cancer cells does provide a window for therapeutic intervention. Although most metabolic enzymes are not mutated in cancer, there is increasing evidence to suggest that many are aberrantly regulated by oncogenes, which can in turn create addictions to specific metabolic pathways.10 Dissecting how oncogenes drive metabolic enzyme activity will certainly provide insight into potential therapeutic strategies that exploit the altered metabolism in cancer cells. For example, several metabolic enzymes have been shown to be regulated by posttranslational modifications in cancer cells but not normal cells. Oncogenic tyrosine kinase signaling has been well documented to regulate the activity and function of several metabolic enzymes, including PKM2,21 lactate dehydrogenase-A,48 pyruvate dehydrogenase kinase 1,49 and PGAM1.50 This has provided a great deal of insight into how oncogene addiction can in turn regulate cellular metabolism, thereby providing an important distinction between metabolic regulation in cancer cells versus normal cells.
Drug combinations also represent an important avenue to be explored with regard to targeting cancer cells and sparing normal cells. Metabolic reprogamming in cancer cells renders them more reliant on certain metabolic pathways, and thus potentially more sensitive to metabolic inhibitors compared with normal cells. Drug combinations would likely permit reduced drug doses, which may limit the effect metabolic enzyme inhibitors would have on normal, metabolically active cells.
Acknowledgments
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 3.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeVita VT, Jr, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68:8643–8653. doi: 10.1158/0008-5472.CAN-07-6611. [DOI] [PubMed] [Google Scholar]
- 7.Kaye SB. New antimetabolites in cancer chemotherapy and their clinical impact. Br J Cancer. 1998;78(suppl 3):1–7. doi: 10.1038/bjc.1998.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer LM, Miller FR, Rowen MJ, Bock G, Rutzky J. Treatment of acute leukemia with amethopterin (4-amino, 10-methyl pteroyl glutamic acid) Acta Haematol. 1950;4:157–167. doi: 10.1159/000203749. [DOI] [PubMed] [Google Scholar]
- 9.Broome JD. L-Asparaginase: discovery and development as a tumor-inhibitory agent. Cancer Treat Rep. 1981;65(suppl 4):111–114. [PubMed] [Google Scholar]
- 10.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 11.Cairo MS. Adverse reactions of L-asparaginase. Am J Pediatr Hematol Oncol. 1982;4:335–339. [PubMed] [Google Scholar]
- 12.Hatzivassiliou G, Zhao F, Bauer DE, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Le A, Cooper CR, Gouw AM, et al. Inhibition of lactate dehydro-genase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnet S, Archer SL, Allalunis-Turner J, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Cao W, Yacoub S, Shiverick KT, et al. Dichloroacetate (DCA) sensitizes both wild-type and over expressing Bcl-2 prostate cancer cells in vitro to radiation. Prostate. 2008;68:1223–1231. doi: 10.1002/pros.20788. [DOI] [PubMed] [Google Scholar]
- 16.Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99:989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang JB, Erickson JW, Fuji R, et al. Targeting mitochondrial gluta-minase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 19.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Dombrauckas JD, Santarsiero BD, Mesecar AD. Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry. 2005;44:9417–9429. doi: 10.1021/bi0474923. [DOI] [PubMed] [Google Scholar]
- 21.Hitosugi T, Kang S, Vander Heiden MG, et al. Tyrosine phospho-rylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv L, Li D, Zhao D, et al. Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol Cell. 2011;42:719–730. doi: 10.1016/j.molcel.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anastasiou D, Yu Y, Israelsen WJ, et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8:839–847. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh MJ, Brimacombe KR, Anastasiou D, et al. Probe Reports from the NIH Molecular Libraries Program [Internet] Bethesda, MD: National Center for Biotechnology Information; 2010–2012. Mar 16, ML265: A potent PKM2 activator induces tetramerization and reduces tumor formation and size in a mouse xenograft model. updated 2013 May 8. [PubMed] [Google Scholar]
- 25.Lee J, Kim HK, Han YM, Kim J. Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. Int J Biochem Cell Biol. 2008;40:1043–1054. doi: 10.1016/j.biocel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Luo W, Hu H, Chang R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang W, Xia Y, Ji H, et al. Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature. 2011;480:118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren F, Wu H, Lei Y, et al. Quantitative proteomics identification of phosphoglycerate mutase 1 as a novel therapeutic target in hepatocel-lular carcinoma. Mol Cancer. 2010;9:81. doi: 10.1186/1476-4598-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Wang S, Zhang Q, Ding Y. Identification of potential genes/ proteins regulated by Tiam1 in colorectal cancer by microarray analysis and proteome analysis. Cell Biol Int. 2008;32:1215–1222. doi: 10.1016/j.cellbi.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Hitosugi T, Zhou L, Elf S, et al. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell. 2012;22:585–600. doi: 10.1016/j.ccr.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tennant DA, Duran RV, Boulahbel H, Gottlieb E. Metabolic transformation in cancer. Carcinogenesis. 2009;30:1269–1280. doi: 10.1093/carcin/bgp070. [DOI] [PubMed] [Google Scholar]
- 32.Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10:267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 33.Corcoran CA, Huang Y, Sheikh MS. The regulation of energy generating metabolic pathways by p53. Cancer Biol Ther. 2006;5:1610–1613. doi: 10.4161/cbt.5.12.3617. [DOI] [PubMed] [Google Scholar]
- 34.Evans MJ, Saghatelian A, Sorensen EJ, Cravatt BF. Target discovery in small-molecule cell-based screens by in situ proteome reactivity profiling. Nat Biotechnol. 2005;23:1303–1307. doi: 10.1038/nbt1149. [DOI] [PubMed] [Google Scholar]
- 35.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gross S, Cairns RA, Minden MD, et al. Cancer-associated metabo-lite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207:339–344. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin G, Reitman ZJ, Spasojevic I, et al. 2-hydroxyglutarate production, but not dominant negative function, is conferred by glioma-derived NADP-dependent isocitrate dehydrogenase mutations. PLoS One. 2011;6:e16812. doi: 10.1371/journal.pone.0016812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2010;465:966. doi: 10.1038/nature09132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang F, Travins J, DeLaBarre B, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340:622–626. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]
- 45.Ben Sahra I, Laurent K, Giuliano S, et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70:2465–2475. doi: 10.1158/0008-5472.CAN-09-2782. [DOI] [PubMed] [Google Scholar]
- 46.Wellen KE, Lu C, Mancuso A, et al. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 2010;24:2784–2799. doi: 10.1101/gad.1985910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metallo CM, Gameiro PA, Bell EL, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan J, Hitosugi T, Chung TW, et al. Tyrosine phosphorylation of lactate dehydrogenase A is important for NADH/NAD(+) redox homeostasis in cancer cells. Mol Cell Biol. 2011;31:4938–4950. doi: 10.1128/MCB.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hitosugi T, Fan J, Chung TW, et al. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol Cell. 2011;44:864–877. doi: 10.1016/j.molcel.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hitosugi T, Zhou L, Fan J, et al. Tyr26 phosphorylation of PGAM1 provides a metabolic advantage to tumours by stabilizing the active conformation. Nat Commun. 2013;4:1790. doi: 10.1038/ncomms2759. [DOI] [PMC free article] [PubMed] [Google Scholar]