Abstract
In mammalian cells, the nuclear lamina is composed of a complex fibrillar network associated with the inner membrane of the nuclear envelope. The lamina provides mechanical support for the nucleus and functions as the major determinant of its size and shape. At its innermost aspect it associates with peripheral components of chromatin and thereby contributes to the organization of interphase chromosomes. The A- and B-type lamins are the major structural components of the lamina, and numerous mutations in the A-type lamin gene have been shown to cause many types of human diseases collectively known as the laminopathies. These mutations have also been shown to cause a disruption in the normal interactions between the A and B lamin networks. The impact of these mutations on nuclear functions is related to the roles of lamins in regulating various essential processes including DNA synthesis and damage repair, transcription and the regulation of genes involved in the response to oxidative stress. The major cause of oxidative stress is the production of reactive oxygen species (ROS), which is critically important for cell proliferation and longevity. Moderate increases in ROS act to initiate signaling pathways involved in cell proliferation and differentiation, whereas excessive increases in ROS cause oxidative stress, which in turn induces cell death and/or senescence. In this review, we cover current findings about the role of lamins in regulating cell proliferation and longevity through oxidative stress responses and ROS signaling pathways. We also speculate on the involvement of lamins in tumor cell proliferation through the control of ROS metabolism.
Keywords: Nuclear lamins, Lamin A, Lamin B, Oxidative stress, Reactive oxygen species (ROS)
Introduction
In mammalian cells, the nuclear lamina is a major determinant of nuclear architecture. The lamina is located at the inner membrane of the nuclear envelope (NE). The major structural components of the lamina in somatic cells are the A-type lamins (LA, LC) and the B-type lamins (LB1, LB2). LA and LC are derived from the single gene LMNA by alternative splicing, and LB1 and LB2 are encoded by two genes LMNB1 and LMNB2, respectively [1]. In embryonic stem cells, the expression of LA and LC is low and they begin to increase at the onset of differentiation and continue to increase to relatively high levels in certain terminally differentiated cell types [2]. In contrast, LB1 and/or LB2 are expressed in all cells throughout development [2]. For example, T-cells and B-cells express only B-type lamins but not A-type lamins [1].
Lamins are type V intermediate filament proteins, which assemble into higher order filamentous structures within the peripheral lamina under the NE [3, 4]. All lamins contain a long central α-helical rod domain, flanked by globular N-terminal (head) and C-terminal (tail) domains. Many lamin subtypes are posttranslationally modified either transiently or permanently (see below). In particular, LA is transiently modified by C-terminal farnesylation and the failure to remove this farnesylation site results in nuclear defects, while B-type lamins tend to be permanently farnesylated. Electron microscopy has revealed that the lamina in Xenopus oocytes appears as a meshwork of ~10–15 nm filaments [5]. Lamin structures organized into meshworks have also been seen in nuclei of mouse cells by super resolution light microscopy [6]. Furthermore, it has been shown that A- and B-type lamin fibrils form separate but interacting meshworks within the lamina [7]. These lamin fibrils play important roles in assembling the lamina and contribute to the size, shape, and mechanical stability of the nucleus. Lamins are also involved in nuclear functions including chromatin organization, DNA replication, DNA repair, and transcription [7–10]. With respect to chromatin organization, the lamins provide anchorage sites for peripheral elements of heterochromatin, which are involved in the local regulation of gene expression [11–13]. Interestingly, silencing LB1 expression in HeLa cells dramatically alters the structure of the LA/C meshworks and induces LA/C-enriched NE blebs [7] that contain transcriptionally inactive gene-rich euchromatin in cancer cells [7].
The functional importance of lamins is further supported by the finding that structural changes in the lamina are among the most dramatic hallmarks of differentiation, cancer and aging and that numerous mutations in the LMNA gene are now known to be responsible for a wide range of genetic disorders called laminopathies. These combined studies suggest that lamins play important roles as key regulators of epigenetic events that may be critical in cellular stress responses. In particular, knowledge is accumulating to show an interdependence between oxidative stress and lamins. For example, oxidative stress modulates the expression and posttranslational modification of lamins. Conversely, mutations of lamin genes and depletion of lamins affect oxidative stress responses. Reactive oxygen species (ROS), major products of oxidative stress, are natural by-products of mitochondrial respiration which are normally eliminated in protective mechanisms such as antioxidant defenses [14–16]. Moderate increases in ROS act as a signaling mechanism to promote cell proliferation and differentiation [14–16]. However, excessive increases in ROS cause damage to DNA, proteins, and lipids, resulting in defects in proliferation and longevity that have been linked to cardiovascular and neurodegenerative diseases, as well as chronic inflammation [17]. Importantly, it is now becoming evident that lamins are involved in modulating ROS to regulate proliferation and longevity.
Here, we discuss current knowledge regarding the involvement of lamins in oxidative stress, cell proliferation, and longevity. Specifically, we focus our attention on the role of lamins in mediating cell proliferation and longevity through oxidative stress responses and ROS signaling pathways. We also consider the possible involvement of this nexus in tumor proliferation.
The Expression and Stability of Lamin Proteins Is Modulated by Oxidative Stress
Several studies have indicated that the expression and stability of lamin proteins is altered in response to oxidative stress, which in turn is tightly coupled to cell proliferation, cellular senescence, apoptosis, and autophagy.
Lamin expression is regulated by the tumor suppressors p53 and retinoblastoma protein (pRb) and by telomere functions; all master regulators of the cell cycle, apoptosis, replicative senescence, and autophagy. For example, LA/C expression is significantly upregulated upon the activation of p53 [18]. The LA mutant progerin, which causes the premature aging disease Hutchinson-Gilford Progeria Syndrome (HGPS) [19] is also expressed during normal aging [20]. Progerin expression is induced by telomere dysfunctions [21]. In contrast, the expression of LB1, but not LB2, is significantly down-regulated during senescence induced by replicative exhaustion, DNA damage, and oncogenic stress [22–24] (Fig. 1). A decrease in LB1 expression has also been observed in HGPS and in atypical progeroid syndromes caused by different mutations in the LMNA gene [25–27]. This decrease in LB1 expression is specifically coupled to senescence, since it does not occur in quiescence induced by serum depletion [22, 23]. The activation of pRb is required for the decrease in LB1 expression in senescence [22], and this is attributable to the fact that the LMNB1 gene is a downstream target of the pRb–E2F pathway [28]. Based on these findings, it would be predicted that LA/C expression increases and LB1 expression decreases with no change in expression of progerin and LB2 when oxidative stress activates p53 and pRb [22] (Fig. 2). However, it has been reported that fibroblasts derived from patients with the recessive autosomal genetic disorder ataxia telangiectasia show an increase in LB1 expression in response to oxidative stress mediated by the activation of p38 mitogen-activated protein (MAP) kinase [29]. It is therefore, possible that the mutation causing ataxia telangiectasia alters the pathways required for regulating LB1 expression levels.
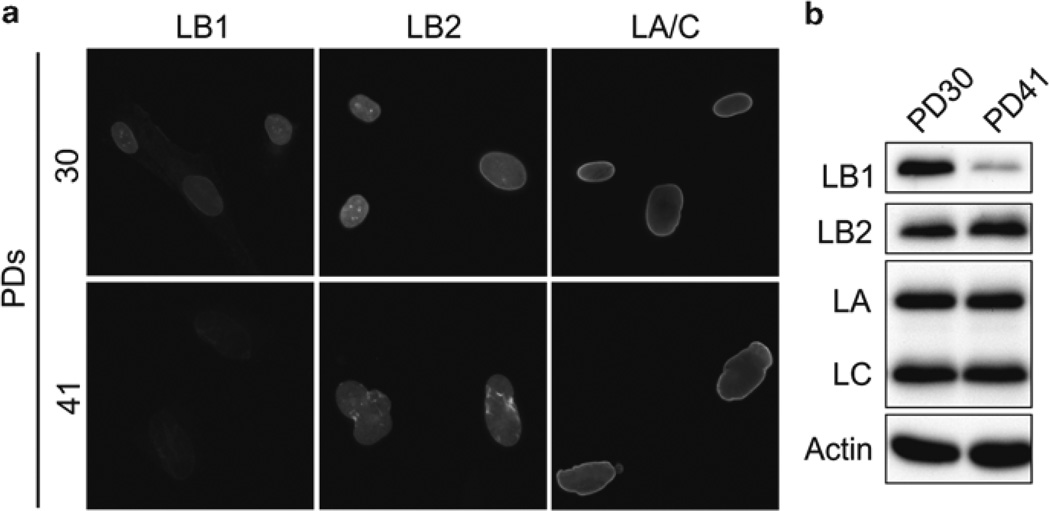
Fig. 1.
The distribution and expression of lamin proteins were determined at population doublings (PD) 30 and 41 in WI-38 human embryonic lung fibroblasts. These cells are proliferating at PD 30 and senescent by PD 41. (a) LA/C, LB1, and LB2 are localized by immunofluorescence. (b) The expression of LA/C, LB1, and LB2 was determined by immunoblotting. The expression of LB1 but not LA/C or LB2 was significantly decreased during replicative senescence (permission to reproduce these data from Cold Spring Harbor Lab Press [22])
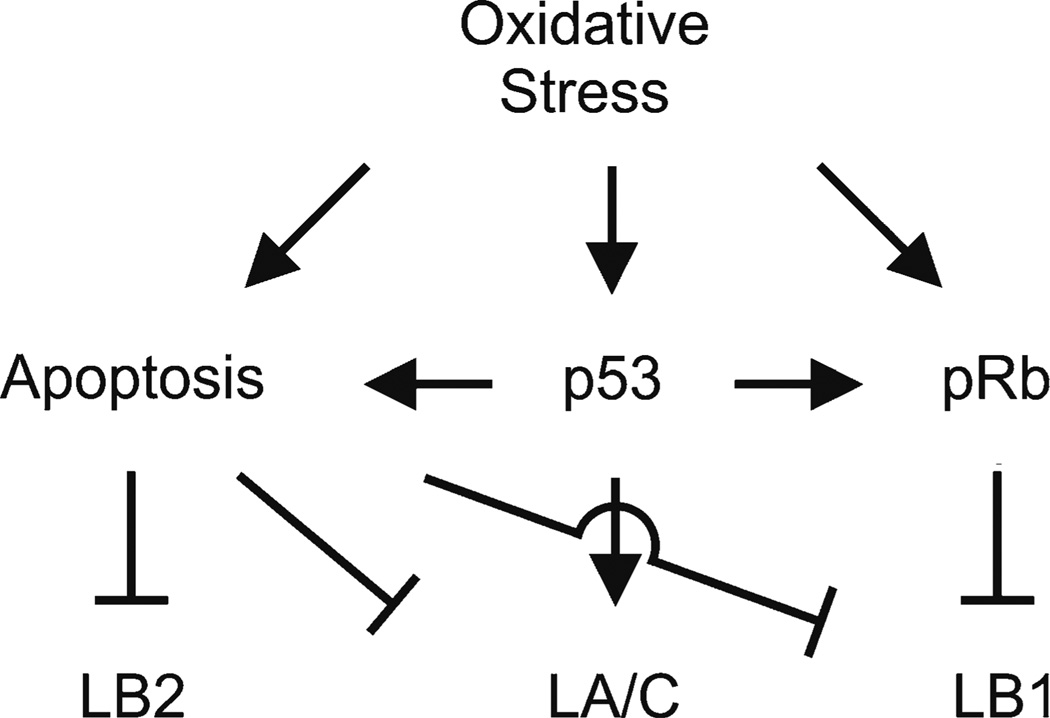
Fig. 2.
A summary of the p53, pRb, and apoptotic pathways known to modulate the levels of lamin expression in response to oxidative stress. Oxidative stress induces an increase in LA/C expression, a decrease in LB1 expression, but no change in the expression of LB2 through the p53 and pRb pathways. Lamin proteins can be cleaved by oxidative-stress induced apoptosis
In addition to the transcriptional regulation of lamin expression described above, other studies have indicated that lamin levels are affected by directed degradation. Rapamycin induces autophagic protein degradation by inactivating the mammalian target of rapamycin (mTOR) pathway [30]. In this fashion, the non-farnesylated form of LA, premature LA (pre-LA), and progerin are degraded by rapamycin treatment [31, 32]. Therefore, it could be that oxygen tension which is upstream of mTOR complex 1 (mTORC1) affects the protein levels of pre-LA and progerin through the mTOR pathway [33]. LB1 is degraded in transformed rat fibroblasts and a human cervical carcinoma cell line after these cells are exposed to a ROS inducer by the ubiquitin–proteasome pathway [34]. The lamina is also known to be broken down by caspases during apoptosis, and lamins are considered to be among the initial nuclear targets cleaved during the apoptotic process [35]. A- and B-type lamins are cleaved at their conserved VEID and VEVD sites by caspase-6 and 3, respectively [36–39]. Furthermore, both serine proteases, granzyme A and B, are known to cleave B-type lamins, whereas only granzyme A but not B appear to cleave A-type lamins [40]. Since oxidative stress can induce apoptosis [41], it is also likely that these cleavages of lamin proteins are induced by oxidative stress (Fig. 2). Together, these studies strongly support the idea that oxidative stress modulates the expression and stability of lamin proteins through transcription and proteolysis.
Posttranslational Modifications of Lamins in Response to Oxidative Stress
Lamins are known to be posttranslationally processed and the resulting modifications are likely to affect their functions, their interactions with each other, and their binding partners [42]. Several studies have indicated that lamins are posttranslationally modified by oxidation and enzymes in response to oxidative stress.
Lamins contain some amino acid residues that could be oxidized. During senescence, increased ROS results in the oxidation of cysteine residues in the LA tail domain, which in turn appears to inhibit the formation of LA inter- and intramolecular disulfide bonds [43]. Additionally, S-thiolation of A-type lamins is induced in isolated rat kidneys subjected to ischemia and reperfusion [44].
Phosphorylation of lamins has been the most extensively studied among many lamin posttranslational modifications. Though it is well known that hyperphosphorylation of lamins drives NE disassembly in mitosis [45–48], little is known regarding interphase phosphorylation of lamins. During interphase A-type lamins are known to be phosphorylated in response to oxidative stress in human neuroblastoma cells [49]. Since extracellular signal-regulated kinase 1/2 (ERK1/2) is activated by oxidative stress [50], it is possible that A-type lamins are phosphorylated by this kinase. In support of this, A-type lamins have been identified as among the most heavily phosphorylated proteins following activation of ERK1/2 [51, 52]. LB1 is also phosphorylated by p38 MAP kinase during senescence induced by oxidative stress, which leads to an increase in LB1 expression [29].
Furthermore, it has been shown that LA posttranslational processing by farnesylation is affected by oxidative stress. This is supported by the accumulation of pre-LA in old vascular smooth muscle cells (VSMCs) but not in young healthy blood vessels [53]. This accumulation of pre-LA correlates with the downregulation of the metallopeptidase Zmpste24/FACE-1, which is required for the processing of pre- LA into mature LA. Since both the mRNA and protein level of Zmpste24 are reduced in response to oxidative stress [53], this affects pre-LA levels. Posttranslational modifications and levels of B-type lamins are also altered by oxidative stress. For example, in transformed and cancer cells, LB1 protein is oxidized by ROS, which mediates the degradation of LB1 protein [34].
It still remains unclear how these posttranslational modifications of lamins induced by oxidative stress affect their structures and functions. However, one study shows that aggregates of LA/C and LB1 are observed in the nucleus during the early response to liver injury induced by oxidative stress [54]. This suggests, but certainly does not prove, that changes in posttranslational modifications of lamins caused by oxidative stress may inhibit lamin assembly into the lamina, which may lead to dysfunctions of the nucleus.
Lamin Functions in Cell Proliferation and Longevity
As mentioned above, the lamina and lamins are known to be involved in various nuclear functions including chromatin organization, DNA replication, DNA repair, and transcription (see “ Introduction ”). Some studies have indicated that the deregulation of these lamin functions by disease causing mutations and/or alterations in the expression levels of the different types of lamins inhibit cell proliferation and induce senescence or cell death.
The functions of A-type lamins in cell proliferation have been most extensively studied in the premature aging diseases, HGPS, and atypical progeroid syndromes. Dermal fibroblasts obtained from progeria patients are commonly found to proliferate slowly and become prematurely senescent [27, 55]. In several transgenic mouse models for HGPS, the mice also show marked reduction in body size and fibroblasts derived from the progeria mice exhibit slow proliferation and premature senescence [56, 57]. Mutations in the gene encoding a pre-LA processing enzyme, Zmpste24 and Zmpste24 knockout mice (Zmpste24−/−) cause a severe progeroid phenotype similar to HGPS [58, 59]. In support of this, the accumulation of pre-LA by silencing Zmpste24 expression or the overexpression of pre-LA accelerates VSMC senescence [53]. Similarly, silencing of LA/C expression slows cell proliferation and induces premature senescence in human diploid fibroblasts (HDFs) [60]. Lmna knockout mice (Lmna−/−) also have proliferation defects within 4 weeks after birth and die by 8 weeks [61].
Alterations in LA/C either caused by silencing or defects in processing by Zmpste24 appear to increase the susceptibility of cells to DNA damage [62–65], most likely resulting in the activation of p53. In support of this, silencing LA/C expression or overexpression of LA and progerin slows proliferation and induces premature senescence in a p53-dependent but not pRb-dependent manner [60, 66] (Fig. 3). In Zmpste24−/− mice, p53 and the p53 target genes are upregulated and induce proliferation defects and premature death [67].
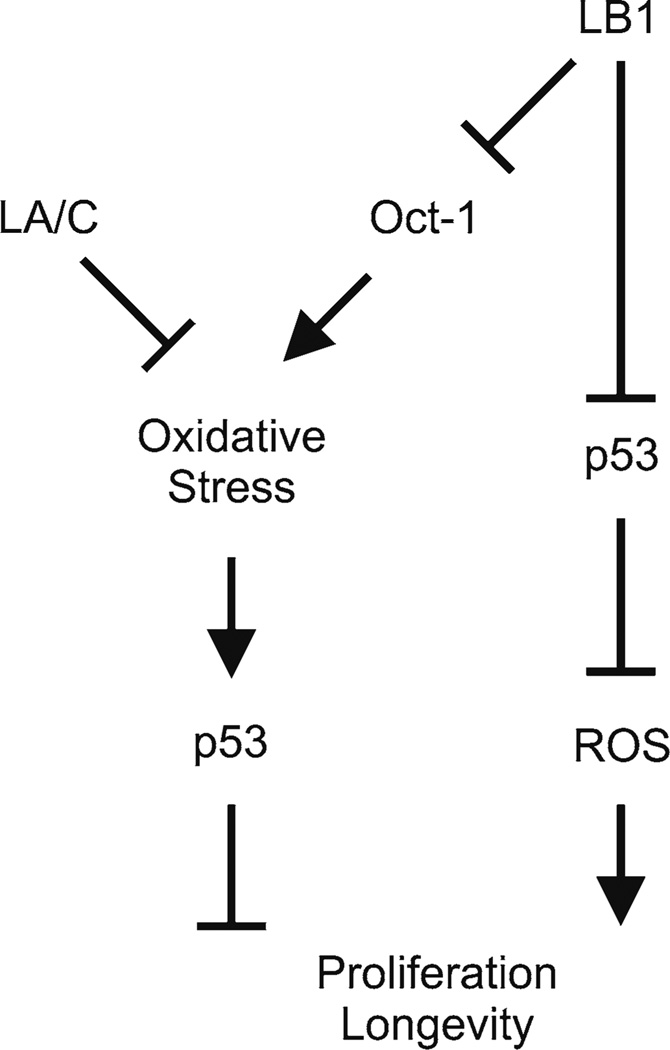
Fig. 3.
A summary of p53- and Oct-1 dependent pathways linking lamin functions to oxidative stress responses, cell proliferation, and longevity. LA/C and LB1 suppress oxidative stress to promote proliferation and longevity through the p53 pathway. The suppression of oxidative stress by LB1 is mediated by Oct-1. LB1 also maintains ROS metabolism through the p53 pathway
Recent studies have shown that B-type lamins are also involved in cell proliferation, differentiation, and longevity. Silencing the expression of LB1 slows cell proliferation in HDFs [22, 24] and induces premature senescence [22]. Lmnb1 mutant mice (Lmnb1Δ/Δ), Lmnb1 knockout mice (Lmnb1−/−), and Lmnb1 / Lmnb2 double knockout mice (Lmnb1−/− Lmnb2−/−) are born, but die immediately after birth due to developmental defects in specific differentiated cell types such as those comprising the lung and brain [68, 69]. Lmnb2−/− mice also have severe brain abnormalities, but these developmental defects are rather minor compared to those in Lmnb1−/− mice [69, 70]. Importantly, mouse embryonic fibroblasts (MEFs) derived from Lmnb1Δ/Δ mice and the mutant mice expressing a polymorphic variant of LB1 responsible for the curly tail phenotype show proliferation defects and prematurely senesce [68, 71]. On the other hand, mice with the conditional Lmnb1−/−Lmnb2−/− restricted to their skin keratinocytes develop normally [72]. These findings support the idea that cell proliferation defects caused by the absence of B-type lamins are most likely to be tissue specific.
As in the case of the A-type lamins, there is evidence that p53 mediates the regulation of proliferation by LB1. Changes in proliferation rates caused by silencing LB1 expression and overexpression of LB1 can be restored by the inactivation of p53 [22, 24] (Fig. 3).
The Involvement of Lamins in Regulating Oxidative Stress
Oxidative stress activates several signaling pathways including the DNA damage response (DDR), the p38 MAP-kinase pathway, and the p53 pathway to induce slow proliferation, senescence, and cell death. Several studies have shown that lamins are involved in responses to oxidative stress.
There is significant evidence that in fibroblasts derived from HGPS patients, ROS levels are higher than those of normal fibroblasts, and these elevated levels of ROS are correlated with slow proliferation rates [73]. In addition, ROS-induced DNA double-strand breaks in HGPS fibroblasts are not repaired normally, and this appears to be related to their slow rate of proliferation [73]. This increase in ROS level in HGPS fibroblasts could be due to cysteine residues missing in progerin, which appear to be hyperoxidized during senescence and may contribute to the suppression of ROS-responsive genes [43]. Furthermore, mesenchymal stem cells (MSCs) overexpressing progerin or LA shows a decrease in expression of an antioxidant SOD2 and a SOD2-dependent increase in mitochondrial ROS, which leads to defects in chondrogenic differentiation potential [74]. Another study also shows that the viability of MSCs and VSMCs which have been differentiated from pluripotent stem cells (iPSCs) derived from HGPS fibroblasts are compromised by oxidative stress [75].
Other mutations in the LMNA gene have been reported to cause the accumulation of pre-LA and/or an increase in oxidative stress. Fibroblasts derived from patients with the LMNA mutations D47Y, L92F, L387V, R399H, L421P, and R482W, causing insulin resistance and/or lipodystrophy, accumulate pre-LA, and this is related to increased oxidative stress and the decreased expression of mitochondrial respiratory chain proteins that trigger premature cellular senescence [76]. Another study shows that cells from lipodystrophy patients with the LA mutations R439C, R482W, and H506D also accumulate pre-LA and show the expression of adipogenic proteins with brown fat-like features, an increased number of mitochondria and the overexpression of thermogenin (uncoupling protein 1, UCP1), which decreases the proton gradient generated in oxidative phosphorylation [77]. Higher levels of ROS are also induced by oxidative stress in fibroblasts from other lipodystrophy patients with a R439C LMNA mutation [78]. In addition, a homozygous LMNA mutation leads to expression of a mutated pre-LA with a deletion of 48 C-terminal amino acids, preventing its farnesylation and maturation. The resulting form of pre-LA is associated with increased oxidative stress and premature senescence [79]. Zmpste24−/− mice also accumulate pre-LA and show an increased mitochondrial response to oxidative stress [80]. Moreover, the mitochondrial proteins related to lipid metabolism, the tricarboxylic acid cycle, and oxidative phosphorylation are all upregulated in these mice. This supports the relationship between defective pre-LA processing and mitochondrial dysfunction, in addition to highlighting the relevance of pre-LA to oxidative damage in lipoatrophy and aging [80]. These results strongly support the idea that dysfunctions of LA/C cause oxidative stress (Fig. 3).
LB1 is also known to be involved in oxidative stress responses. The proliferation defects induced by silencing LB1 expression are accompanied by a p53-dependent reduction of mitochondrial ROS in HDFs, which can be rescued by growth under hypoxic conditions [22] (Fig. 3). RT-PCR analyses show that p53-target genes are altered under the experimental conditions used for silencing LB1 expression. For example, the antioxidant genes SOD1, SOD2, SESN1, SESN2, and GPX1 are all upregulated, resulting in lowering the levels of ROS in LB1-silenced cells [22]. Other than p53, the POU-domain transcription factor Oct-1 also appears to mediate the LB1 regulation of the genes involved in oxidative stress responses [81]. Most importantly, Oct-1 has been shown to associate with the lamina through LB1 [11, 81]. This association is disrupted in the Lmnb1Δ/Δ fibroblasts, causing the elevation of ROS levels [81]. In autosomal dominant leukodystrophy (ADLD) fibroblasts, there is an increased expression of LB1 due to the duplication of the LMNB1 gene, which is also coincident with an increase in the amount of Oct-1 associated with the lamina and a decrease in the nucleoplasmic fraction of Oct-1 by oxidative stress [82]. Since Oct-1 regulates the expression of genes involved in oxidative stress responses by binding to their putative octamer-binding DNA sequences [81], the sequestration of Oct-1 to the lamina by LB1 could be another mechanism by which LB1 modulates ROS levels (Fig. 3).
LB2 appears to regulate mitochondrial functions in neurons. For example, in Lmnb2−/− mice, a neuronal layering abnormality is caused by defective neuronal migration [70]. Inhibition of LMNB2 mRNA translation in Xenopus retinal ganglion cell axons in vivo does not affect guidance but causes axonal degeneration [83]. This is attributable to the finding that a form of axonal LB2 associates with mitochondria and in LB2-deficient axons mitochondria are dysfunctional causing defects in axonal transport [83]. These results suggest that axonally synthesized LB2 plays a crucial role in axon maintenance by promoting mitochondrial functions to protect against oxidative stress.
Possible Pathways Related to Lamin Functions in Response to Oxygen Metabolism and Oxidative Stress Responses
Recent studies have indicated that lamins play important roles in regulating signaling pathways mediated by SIRT1, NF-κB, and mTORC1. These pathways are known to be involved in regulating oxygen metabolism and oxidative stress. Though in many cases, direct biochemical evidence has yet to be provided, these studies imply that lamins may regulate a cellular response to oxidative stress through these pathways.
In this regard, LA has been shown to interact with and activate an NAD-dependent deacetylase, Sirtuin 1 (SIRT1) [84]. SIRT1 deacetylates FOXO3, a member of the FOXO family of Forkhead transcription factors in response to oxidative stress, leading to cell cycle arrest, resistance to oxidative stress, and inhibition of cell death [85]. The presence of progerin or the accumulation of pre-LA in Zmpste24−/− mice induces a decrease in deacetylase activity of SIRT1, leading to rapid depletion of adult stem cells [84]. This scenario might also explain why a mitochondrial response to oxidative stress is increased in Zmpste24−/− mice and fibroblasts derived from HGPS patients [73, 80]. Therefore, it is possible that LA functions in oxidative stress responses are mediated by SIRT1.
It has also been shown that the accumulation of pre-LA activates an ATM- and NEMO-dependent signaling pathway, leading to the activation of NF-κB and secretion of proinflammatory cytokines in Zmpste24−/− and progeria mice (LmnaG609G/ G609G) [86]. The activation of NF-κB suppresses cell death by inducing the expression of genes encoding pro-oxidants [87]. In this fashion, the accumulation of pre-LA might increase the levels of ROS through the activation of NF-κB [80]. It is also possible that NF-κB mediates LA functions in oxidative stress responses.
Cellular oxygen sensing is upstream of the mTOR pathway [88, 89]. Interestingly, Lmna−/− mice show enhanced mTORC1 signaling in cardiac and skeletal muscle cells. Pharmacologic reversal of elevated mTORC1 signaling by rapamycin improves cardiac and skeletal muscle function and the longevity of the mice. In addition, this treatment also alleviates the defective autophagic-mediated degradation in Lmna−/− mice [90]. Based on these findings, it has been suggested that there is molecular cross talk between LA and oxygen sensing mechanisms.
The Prospective for Future Studies of Lamin Functions in Oxidative Stress
We have discussed current evidence for roles of lamins in regulating cell proliferation and longevity through the cellular response to oxidative stress and ROS signaling pathways. Though the lamin-related diseases that we have described are not directly involved in cancer, there are significant implications that lamins could be involved in tumor cell growth and in cancer progression. Many types of cancer cells are known to produce increased reactive ROS compared to normal cells [91]. Cancer cells also develop a unique way to control their proliferation as their increased glucose metabolism is coupled to fast proliferation [22] and their mitochondrial metabolism regulates ROS production which is essential for anchorage-independent growth [14]. Cancer cells are also more susceptible to oxidative stress compared to normal cells [91]. Therefore, progress in understanding how lamins control ROS metabolism in normal and cancer cells will provide new insights for cancer treatment. The modulation of ROS metabolism by changing lamin expression might provide a biochemical basis to design therapeutic strategies, including vectors for gene therapy and small molecule compounds for chemotherapy, to selectively slow cancer development, growth, and progression.
Abbreviations
- ADLD
Autosomal dominant leukodystrophy
- DDR
DNA damage response
- HDFs
Human diploid fibroblasts
- HGPS
Hutchison-Gilford progeria syndrome
- iPSCs
Inducible pluripotent stem cells
- LA
Lamin A
- LB1
Lamin B1
- LB2
Lamin B2
- LC
Lamin C
- MSC
Mesenchymal stem cells
- MEFs
Mouse embryonic fibroblasts
- NE
Nuclear envelope
- ROS
Reactive oxygen species
- VSMCs
Vascular smooth muscle cells
Contributor Information
Takeshi Shimi, Email: takeshi@northwestern.edu.
Robert D. Goldman, Email: r-goldman@northwestern.edu.
References
- 1.Adam SA, Goldman RD. Insights into the differences between the A- and B-type nuclear lamins. Adv Enzyme Regul. 2011 doi: 10.1016/j.advenzreg.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckersley-Maslin MA, Bergmann JH, Lazar Z, Spector DL. Lamin A/C is expressed in pluripotent mouse embryonic stem cells. Nucleus. 2013;4(1):53–60. doi: 10.4161/nucl.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stuurman N, Heins S, Aebi U. Nuclear lamins: their structure, assembly, and interactions. J Struct Biol. 1998;122(1–2):42–66. doi: 10.1006/jsbi.1998.3987. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann H, Foisner R. Intermediate filaments: novel assembly models and exciting new functions for nuclear lamins. Cell Mol Life Sci. 2003;60(8):1607–1612. doi: 10.1007/s00018-003-3004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323(6088):560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- 6.Schermelleh L, Carlton PM, Haase S, Shao L, Winoto L, Kner P, Burke B, Cardoso MC, Agard DA, Gustafsson MG, Leonhardt H, Sedat JW. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science. 2008;320(5881):1332–1336. doi: 10.1126/science.1156947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimi T, Pfleghaar K, Kojima S, Pack C, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T, Goldman RD. The A- and B-type nuclear lamin networks: micro-domains involved in chromatin organization and transcription. Genes Dev. 2008;22(24):3409–321. doi: 10.1101/gad.1735208. doi:22/24/3409 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moir RD, Spann TP, Herrmann H, Goldman RD. Disruption of nuclear lamin organization blocks the elongation phase of DNA replication. J Cell Biol. 2000;149(6):1179–1192. doi: 10.1083/jcb.149.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spann TP, Goldman AE, Wang C, Huang S, Goldman RD. Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription. J Cell Biol. 2002;156(4):603–608. doi: 10.1083/jcb.200112047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shumaker DK, Solimando L, Sengupta K, Shimi T, Adam SA, Grunwald A, Strelkov SV, Aebi U, Cardoso MC, Goldman RD. The highly conserved nuclear lamin Ig-fold binds to PCNA: its role in DNA replication. J Cell Biol. 2008;181(2):269–280. doi: 10.1083/jcb.200708155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, van Steensel B. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453(7197):948–951. doi: 10.1038/nature06947. doi:nature06947 [pii]10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 12.Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452(7184):243–247. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- 13.Zullo JM, Demarco IA, Pique-Regi R, Gaffney DJ, Epstein CB, Spooner CJ, Luperchio TR, Bernstein BE, Pritchard JK, Reddy KL, Singh H. DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell. 2012;149(7):1474–1487. doi: 10.1016/j.cell.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107(19):8788–8793. doi: 10.1073/pnas.1003428107. doi:1003428107 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell EL, Klimova TA, Eisenbart J, Schumacker PT, Chandel NS. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol Cell Biol. 2007;27(16):5737–5745. doi: 10.1128/MCB.02265-06. doi:MCB.02265-06 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011;14(4):537–544. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman-Roblick R, Roblick UJ, Hellman U, Conrotto P, Liu T, Becker S, Hirschberg D, Jornvall H, Auer G, Wiman KG. p53 targets identified by protein expression profiling. Proc Natl Acad Sci U S A. 2007;104(13):5401–5406. doi: 10.1073/pnas.0700794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423(6937):293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312(5776):1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao K, Blair CD, Faddah DA, Kieckhaefer JE, Olive M, Erdos MR, Nabel EG, Collins FS. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J Clin Invest. 2011;121(7):2833–2844. doi: 10.1172/JCI43578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimi T, Butin-Israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA, Shumaker DK, Kosak ST, Chandel NS, Goldman RD. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011;25(24):2579–2593. doi: 10.1101/gad.179515.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell. 2012;23(11):2066–2075. doi: 10.1091/mbc.E11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreesen O, Chojnowski A, Ong PF, Zhao TY, Common JE, Lunny D, Lane EB, Lee SJ, Vardy LA, Stewart CL, Colman A. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J Cell Biol. 2013;200(5):605–617. doi: 10.1083/jcb.201206121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scaffidi P, Misteli T. Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat Med. 2005;11(4):440–445. doi: 10.1038/nm1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kandert S, Luke Y, Kleinhenz T, Neumann S, Lu W, Jaeger VM, Munck M, Wehnert M, Muller CR, Zhou Z, Noegel AA, Dabauvalle MC, Karakesisoglou I. Nesprin-2 giant safeguards nuclear envelope architecture in LMNA S143F progeria cells. Hum Mol Genet. 2007;16(23):2944–2959. doi: 10.1093/hmg/ddm255. doi:ddm255 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Taimen P, Pfleghaar K, Shimi T, Moller D, Ben-Harush K, Erdos MR, Adam SA, Herrmann H, Medalia O, Collins FS, Goldman AE, Goldman RD. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc Natl Acad Sci U S A. 2009;106(49):20788–20793. doi: 10.1073/pnas.0911895106. doi:0911895106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallstrom TC, Mori S, Nevins JR. An E2F1-dependent gene expression program that determines the balance between proliferation and cell death. Cancer Cell. 2008;13(1):11–22. doi: 10.1016/j.ccr.2007.11.031. doi:S1535-6108(07)00370-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barascu A, Le Chalony C, Pennarun G, Genet D, Imam N, Lopez B, Bertrand P. Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J. 2012;31(5):1080–1094. doi: 10.1038/emboj.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao K, Graziotto JJ, Blair CD, Mazzulli JR, Erdos MR, Krainc D, Collins FS. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med. 2011;3(89):89ra58. doi: 10.1126/scitranslmed.3002346. [DOI] [PubMed] [Google Scholar]
- 32.Cenni V, Capanni C, Columbaro M, Ortolani M, D’Apice MR, Novelli G, Fini M, Marmiroli S, Scarano E, Maraldi NM, Squarzoni S, Prencipe S, Lattanzi G. Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria. Eur J Histochem. 2011;55(4):e36. doi: 10.4081/ejh.2011.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiarini A, Whitfield JF, Pacchiana R, Armato U, Dal Pra I. Photoexcited calphostin C selectively destroys nuclear lamin B1 in neoplastic human and rat cells—a novel mechanism of action of a photodynamic tumor therapy agent. Biochim Biophys Acta. 2008;1783(9):1642–1653. doi: 10.1016/j.bbamcr.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Lazebnik YA, Takahashi A, Moir RD, Goldman RD, Poirier GG, Kaufmann SH, Earnshaw WC. Studies of the lamin proteinase reveal multiple parallel biochemical pathways during apoptotic execution. Proc Natl Acad Sci U S A. 1995;92(20):9042–9046. doi: 10.1073/pnas.92.20.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orth K, Chinnaiyan AM, Garg M, Froelich CJ, Dixit VM. The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J Biol Chem. 1996;271(28):16443–16446. [PubMed] [Google Scholar]
- 37.Takahashi A, Alnemri ES, Lazebnik YA, Fernandes-Alnemri T, Litwack G, Moir RD, Goldman RD, Poirier GG, Kaufmann SH, Earnshaw WC. Cleavage of lamin A by Mch2 alpha but not CPP32: multiple interleukin 1 beta-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc Natl Acad Sci U S A. 1996;93(16):8395–8400. doi: 10.1073/pnas.93.16.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slee EA, Adrain C, Martin SJ. Executioner caspase-3, 6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276(10):7320–7326. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- 39.Rao L, Perez D, White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol. 1996;135(6 Pt 1):1441–1455. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D, Beresford PJ, Greenberg AH, Lieberman J. Granzymes A and B directly cleave lamins and disrupt the nuclear lamina during granule-mediated cytolysis. Proc Natl Acad Sci U S A. 2001;98(10):5746–5751. doi: 10.1073/pnas.101329598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29(3–4):323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 42.Simon DN, Wilson KL. Partners and post-translational modifications of nuclear lamins. Chromosoma. 2013;122(1–2):13–31. doi: 10.1007/s00412-013-0399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pekovic V, Gibbs-Seymour I, Markiewicz E, Alzoghaibi F, Benham AM, Edwards R, Wenhert M, von Zglinicki T, Hutchison CJ. Conserved cysteine residues in the mammalian lamin A tail are essential for cellular responses to ROS generation. Aging Cell. 2011;10(6):1067–1079. doi: 10.1111/j.1474-9726.2011.00750.x. [DOI] [PubMed] [Google Scholar]
- 44.Eaton P, Jones ME, McGregor E, Dunn MJ, Leeds N, Byers HL, Leung KY, Ward MA, Pratt JR, Shattock MJ. Reversible cysteine-targeted oxidation of proteins during renal oxidative stress. J Am Soc Nephrol. 2003;14(8) Suppl 3:S290–S296. doi: 10.1097/01.asn.0000078024.50060.c6. [DOI] [PubMed] [Google Scholar]
- 45.Dessev GN, Iovcheva-Dessev C, Goldman RD. Lamin dimers. Presence in the nuclear lamina of surf clam oocytes and release during nuclear envelope breakdown. J Biol Chem. 1990;265(21):12636–12641. [PubMed] [Google Scholar]
- 46.Heald R, McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990;61(4):579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- 47.Mall M, Walter T, Gorjanacz M, Davidson IF, Nga Ly-Hartig TB, Ellenberg J, Mattaj IW. Mitotic lamin disassembly is triggered by lipid-mediated signaling. J Cell Biol. 2012;198(6):981–990. doi: 10.1083/jcb.201205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peter M, Nakagawa J, Doree M, Labbe JC, Nigg EA. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990;61(4):591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura M, Yamada M, Ohsawa T, Morisawa H, Nishine T, Nishimura O, Toda T. Phosphoproteomic profiling of human SH-SY5Y neuroblastoma cells during response to 6-hydroxydopamine-induced oxidative stress. Biochim Biophys Acta. 2006;1763(9):977–989. doi: 10.1016/j.bbamcr.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 51.Lewis TS, Hunt JB, Aveline LD, Jonscher KR, Louie DF, Yeh JM, Nahreini TS, Resing KA, Ahn NG. Identification of novel MAP kinase pathway signaling targets by functional proteomics and mass spectrometry. Mol Cell. 2000;6(6):1343–1354. doi: 10.1016/s1097-2765(00)00132-5. [DOI] [PubMed] [Google Scholar]
- 52.Kosako H, Yamaguchi N, Aranami C, Ushiyama M, Kose S, Imamoto N, Taniguchi H, Nishida E, Hattori S. Phosphoproteomics reveals new ERK MAP kinase targets and links ERK to nucleoporin-mediated nuclear transport. Nat Struct Mol Biol. 2009;16(10):1026–1035. doi: 10.1038/nsmb.1656. [DOI] [PubMed] [Google Scholar]
- 53.Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, Shroff R, Skepper J, Shanahan CM. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121(20):2200–2210. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]
- 54.Singla A, Griggs NW, Kwan R, Snider NT, Maitra D, Ernst SA, Herrmann H, Omary MB. Lamin aggregation is an early sensor of porphyria-induced liver injury. J Cell Sci. 2013 doi: 10.1242/jcs.123026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McClintock D, Gordon LB, Djabali K. Hutchinson-Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody. Proc Natl Acad Sci U S A. 2006;103(7):2154–2159. doi: 10.1073/pnas.0511133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mounkes LC, Kozlov S, Hernandez L, Sullivan T, Stewart CL. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature. 2003;423(6937):298–301. doi: 10.1038/nature01631. [DOI] [PubMed] [Google Scholar]
- 57.Osorio FG, Navarro CL, Cadinanos J, Lopez-Mejia IC, Quiros PM, Bartoli C, Rivera J, Tazi J, Guzman G, Varela I, Depetris D, de Carlos F, Cobo J, Andres V, De Sandre-Giovannoli A, Freije JM, Levy N, Lopez-Otin C. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci Transl Med. 2011;3(106):106ra107. doi: 10.1126/scitranslmed.3002847. [DOI] [PubMed] [Google Scholar]
- 58.Moulson CL, Go G, Gardner JM, van der Wal AC, Smitt JH, van Hagen JM, Miner JH. Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J Invest Dermatol. 2005;125(5):913–919. doi: 10.1111/j.0022-202X.2005.23846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pendas AM, Zhou Z, Cadinanos J, Freije JM, Wang J, Hultenby K, Astudillo A, Wernerson A, Rodriguez F, Tryggvason K, Lopez-Otin C. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet. 2002;31(1):94–99. doi: 10.1038/ng871. [DOI] [PubMed] [Google Scholar]
- 60.Moiseeva O, Bourdeau V, Vernier M, Dabauvalle MC, Ferbeyre G. Retinoblastoma-independent regulation of cell proliferation and senescence by the p53-p21 axis in lamin A/C-depleted cells. Aging Cell. 2011;10(5):789–797. doi: 10.1111/j.1474-9726.2011.00719.x. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147(5):913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzalez-Suarez I, Redwood AB, Perkins SM, Vermolen B, Lichtensztejin D, Grotsky DA, Morgado-Palacin L, Gapud EJ, Sleckman BP, Sullivan T, Sage J, Stewart CL, Mai S, Gonzalo S. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J. 2009;28(16):2414–2427. doi: 10.1038/emboj.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzalez-Suarez I, Redwood AB, Gonzalo S. Loss of A-type lamins and genomic instability. Cell Cycle. 2009;8(23):3860–3865. doi: 10.4161/cc.8.23.10092. [DOI] [PubMed] [Google Scholar]
- 64.Liu B, Wang J, Chan KM, Tjia WM, Deng W, Guan X, Huang JD, Li KM, Chau PY, Chen DJ, Pei D, Pendas AM, Cadinanos J, Lopez-Otin C, Tse HF, Hutchison C, Chen J, Cao Y, Cheah KS, Tryggvason K, Zhou Z. Genomic instability in laminopathy-based premature aging. Nat Med. 2005;11(7):780–785. doi: 10.1038/nm1266. [DOI] [PubMed] [Google Scholar]
- 65.Liu GH, Barkho BZ, Ruiz S, Diep D, Qu J, Yang SL, Panopoulos AD, Suzuki K, Kurian L, Walsh C, Thompson J, Boue S, Fung HL, Sancho-Martinez I, Zhang K, Yates J, 3rd, Izpisua Belmonte JC. Recapitulation of premature ageing with iPSCs from Hutchinson-Gilford progeria syndrome. Nature. 2011;472(7342):221–225. doi: 10.1038/nature09879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kudlow BA, Stanfel MN, Burtner CR, Johnston ED, Kennedy BK. Suppression of proliferative defects associated with processing-defective lamin A mutants by hTERT or inactivation of p53. Mol Biol Cell. 2008;19(12):5238–5248. doi: 10.1091/mbc.E08-05-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varela I, Cadinanos J, Pendas AM, Gutierrez-Fernandez A, Folgueras AR, Sanchez LM, Zhou Z, Rodriguez FJ, Stewart CL, Vega JA, Tryggvason K, Freije JM, Lopez-Otin C. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 2005;437(7058):564–568. doi: 10.1038/nature04019. [DOI] [PubMed] [Google Scholar]
- 68.Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci U S A. 2004;101(28):10428–10433. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, Gaiano N, Ko MS, Zheng Y. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 2011;334(6063):1706–1710. doi: 10.1126/science.1211222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coffinier C, Chang SY, Nobumori C, Tu Y, Farber EA, Toth JI, Fong LG, Young SG. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc Natl Acad Sci U S A. 2010;107(11):5076–5081. doi: 10.1073/pnas.0908790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Castro SC, Malhas A, Leung KY, Gustavsson P, Vaux DJ, Copp AJ, Greene ND. Lamin b1 polymorphism influences morphology of the nuclear envelope, cell cycle progression, and risk of neural tube defects in mice. PLoS Genet. 2012;8(11):e1003059. doi: 10.1371/journal.pgen.1003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang SH, Chang SY, Yin L, Tu Y, Hu Y, Yoshinaga Y, de Jong PJ, Fong LG, Young SG. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum Mol Genet. 2011;20(18):3537–3544. doi: 10.1093/hmg/ddr266. doi:ddr266 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richards SA, Muter J, Ritchie P, Lattanzi G, Hutchison CJ. The accumulation of un-repairable DNA damage in laminopathy progeria fibroblasts is caused by ROS generation and is prevented by treatment with N-acetyl cysteine. Hum Mol Genet. 2011;20(20):3997–4004. doi: 10.1093/hmg/ddr327. [DOI] [PubMed] [Google Scholar]
- 74.Mateos J, De la Fuente A, Lesende-Rodriguez I, Fernandez-Pernas P, Arufe MC, Blanco FJ. Lamin A deregulation in human mesenchymal stem cells promotes an impairment in their chondrogenic potential and imbalance in their response to oxidative stress. Stem Cell Res. 2013;11(3):1137–1148. doi: 10.1016/j.scr.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Zhang J, Lian Q, Zhu G, Zhou F, Sui L, Tan C, Mutalif RA, Navasankari R, Zhang Y, Tse HF, Stewart CL, Colman A. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011;8(1):31–45. doi: 10.1016/j.stem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 76.Caron M, Auclair M, Donadille B, Bereziat V, Guerci B, Laville M, Narbonne H, Bodemer C, Lascols O, Capeau J, Vigouroux C. Human lipodystrophies linked to mutations in A-type lamins and to HIV protease inhibitor therapy are both associated with prelamin A accumulation, oxidative stress and premature cellular senescence. Cell Death Differ. 2007;14(10):1759–1767. doi: 10.1038/sj.cdd.4402197. [DOI] [PubMed] [Google Scholar]
- 77.Bereziat V, Cervera P, Le Dour C, Verpont MC, Dumont S, Vantyghem MC, Capeau J, Vigouroux C, Lipodystrophy Study G. LMNA mutations induce a non-inflammatory fibrosis and a brown fat-like dystrophy of enlarged cervical adipose tissue. Am J Pathol. 2011;179(5):2443–2453. doi: 10.1016/j.ajpath.2011.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verstraeten VL, Caputo S, van Steensel MA, Duband-Goulet I, Zinn-Justin S, Kamps M, Kuijpers HJ, Ostlund C, Worman HJ, Briede JJ, Le Dour C, Marcelis CL, van Geel M, Steijlen PM, van den Wijngaard A, Ramaekers FC, Broers JL. The R439C mutation in LMNA causes lamin oligomerization and susceptibility to oxidative stress. J Cell Mol Med. 2009;13(5):959–971. doi: 10.1111/j.1582-4934.2009.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le Dour C, Schneebeli S, Bakiri F, Darcel F, Jacquemont ML, Maubert MA, Auclair M, Jeziorowska D, Reznik Y, Bereziat V, Capeau J, Lascols O, Vigouroux C. A homozygous mutation of prelamin-A preventing its farnesylation and maturation leads to a severe lipodystrophic phenotype: new insights into the pathogenicity of nonfarnesylated prelamin-A. J Clin Endocrinol Metab. 2011;96(5):E856–E862. doi: 10.1210/jc.2010-2234. [DOI] [PubMed] [Google Scholar]
- 80.Peinado JR, Quiros PM, Pulido MR, Marino G, Martinez-Chantar ML, Vazquez-Martinez R, Freije JM, Lopez-Otin C, Malagon MM. Proteomic profiling of adipose tissue from Zmpste24−/− mice, a model of lipodystrophy and premature aging, reveals major changes in mitochondrial function and vimentin processing. Mol Cell Proteomics. 2011;10(11):M111.008094. doi: 10.1074/mcp.M111.008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malhas AN, Lee CF, Vaux DJ. Lamin B1 controls oxidative stress responses via Oct-1. J Cell Biol. 2009;184(1):45–55. doi: 10.1083/jcb.200804155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Columbaro M, Mattioli E, Maraldi NM, Ortolani M, Gasparini L, D’Apice MR, Postorivo D, Nardone AM, Avnet S, Cortelli P, Liguori R, Lattanzi G. Oct-1 recruitment to the nuclear envelope in adult-onset autosomal dominant leukodystrophy. Biochim Biophys Acta. 2013;1832(3):411–420. doi: 10.1016/j.bbadis.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 83.Yoon BC, Jung H, Dwivedy A, O’Hare CM, Zivraj KH, Holt CE. Local translation of extranuclear lamin B promotes axon maintenance. Cell. 2012;148(4):752–764. doi: 10.1016/j.cell.2011.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu B, Ghosh S, Yang X, Zheng H, Liu X, Wang Z, Jin G, Zheng B, Kennedy BK, Suh Y, Kaeberlein M, Tryggvason K, Zhou Z. Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria. Cell Metab. 2012;16(6):738–750. doi: 10.1016/j.cmet.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 85.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 86.Osorio FG, Barcena C, Soria-Valles C, Ramsay AJ, de Carlos F, Cobo J, Fueyo A, Freije JM, Lopez-Otin C. Nuclear lamina defects cause ATM-dependent NF-kappaB activation and link accelerated aging to a systemic inflammatory response. Genes Dev. 2012;26(20):2311–2324. doi: 10.1101/gad.197954.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reiling JH, Hafen E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev. 2004;18(23):2879–2892. doi: 10.1101/gad.322704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18(23):2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, MacKay VL, An EH, Strong R, Ladiges WC, Rabinovitch PS, Kaeberlein M, Kennedy BK. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4(144):144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]