Abstract
BACKGROUND
Bowel obstruction is a common complication of late-stage abdominal cancer, especially colon cancer, which has been investigated predominantly in small, single-institution studies.
OBJECTIVE
We used a large, population-based data set to explore the surgical treatment of bowel obstruction and its outcomes after hospitalization for obstruction among patients with stage IV colon cancer.
DESIGN
This was a retrospective cohort study.
SETTING AND PATIENTS
We identified 1004 patients aged 65 years or older in the Surveillance, Epidemiology and End Results-Medicare database diagnosed with stage IV colon cancer January 1, 1991 to December 31, 2005, who were later hospitalized for bowel obstruction.
MAIN OUTCOME MEASURES
We describe outcomes after hospitalization and analyzed the associations between surgical treatment of obstruction and outcomes.
RESULTS
Hospitalization for bowel obstruction occurred a median of 7.4 months after colon cancer diagnosis, and median survival after obstruction was approximately 2.5 months. Median hospitalization for obstruction was about 1 week and in-hospital mortality was 12.7%. Between discharge and death, 25% of patients were readmitted to the hospital at least once for obstruction, and, on average, patients lived 5 days out of the hospital for every day in the hospital between obstruction diagnosis and death. Survival was 3 times longer in those whose obstruction claims suggested an adhesive obstruction origin. In multivariable models, surgical compared with nonsurgical management was not associated with prolonged survival (p = 0.134).
LIMITATIONS
Use of an administrative database did not allow determination of quality of life or relief of obstruction as an outcome, nor could nonsurgical interventions, eg, endoscopic stenting or octreotide, be assessed.
CONCLUSIONS
In this population-based study of patients with stage IV colon cancer who had bowel obstruction, overall survival following obstruction was poor irrespective of treatment. Universally poor outcomes suggest that a diagnosis of obstruction in the setting of advanced colon cancer should be considered a preterminal event.
Keywords: Bowel obstruction, Colon cancer, Surgery, Palliative care, Terminal care, Prognosis, Survival
Bowel obstruction (BO) is a complication of advanced abdominal cancer that causes bloating, pain, nausea, and vomiting, and it significantly reduces quality of life.1,2 Care of these terminally ill patients is challenging and must incorporate an understanding of the quality and quantity of life remaining, overall patient health, success and morbidity of treatment options, and goals of patient care. To that end, there has been little been little systematic research on the incidence and risk factors of BO in the advanced cancer patient; there has been no systemic study of the effectiveness of various treatment strategies to palliate BO, which include surgical3 and nonsurgical approaches,4–11 or of outcomes after hospital discharge. What little evidence exists to guide clinical decisions comes from small, single-institution studies, most single-armed, highly selective, and retrospective in nature. In part, because of this lack of clinical data, recommendations for treatment of patients with BO stem from expert opinion1; there is no consensus as to indications for 1 treatment over another, and no formal guidelines for the management of patients with BO.2,12
We recently performed a population-based study of incidence and risk factors associated with hospitalization for BO13 in a cohort of patients with stage IV colon cancer with the use of the Surveillance, Epidemiology, and End Results (SEER)-Medicare database.14 Among 12,553 patients with stage IV colon cancer, we identified 1004 (8.0%) who were hospitalized with BO after cancer diagnosis. In this follow-up study, we examine the treatment patterns for BO in these 1004 individuals. We report outcomes after hospitalization for BO and examine survival experience after BO in elderly patients with stage IV colon cancer.
METHODS
Data Source
We analyzed data from the SEER-Medicare database.14 The SEER database tracks patients with cancer in approximately 14% of the US population up to 1999, and 26% from 2000 forward. Surveillance, Epidemiology, and End Results database contains demographic information and records of tumor histology, location, stage, and survival. Medicare provider review (MedPAR) files summarize all services rendered to an inpatient hospital beneficiary from admission through discharge. Physician and outpatient billing files contain procedure and diagnosis codes for services rendered.
Cohort Selection
We used International Classification of Disease, 9th Revision (ICD-9) codes15 to identify individuals >65 years with a pathologically confirmed first diagnosis of stage IV colon adenocarcinoma between January 1, 1991, and December 31, 2005, considering International Classification of Diseases for Oncology, 3rd Edition codes 814, 821, 822, 825, 826, 848, and 857 to indicate adenocarcinoma. Study subjects with unknown month of diagnosis and those eligible for Medicare for reasons other than age were excluded. We also excluded individuals enrolled in a health maintenance organization at any time from colon cancer diagnosis to death, because billing claims for these patients may not have been submitted to Medicare for reimbursement.
Patient Characteristics
We extracted patient characteristics, including age, sex, race, marital status, month of cancer diagnosis, and tumor characteristics from SEER. Age and comorbidity were calculated at the time of first hospitalization for obstruction; all other patient and tumor characteristics reflect conditions at cancer diagnosis. We categorized age in 5-year increments, and race as white, black, and other or unknown. We considered divorced, separated, single, and widowed subjects to be unmarried. Cancer diagnosis date was designated as the 15th day of the month of diagnosis. We classified tumors into proximal/right-sided (cecum, ascending colon, hepatic flexure, and transverse colon) and distal/left-sided (splenic flexure, descending, and sigmoid colon). Tumors were classified by histology as mucinous (International Classification of Diseases for Oncology, 3rd Edition 848) or nonmucinous, and tumor grade as well/ moderately differentiated, poorly differentiated or undifferentiated, and unknown. Cases were grouped by nodal involvement into N0 (no lymph nodes involved by tumor), N1 (1–3 nodes involved), N2 (>3 nodes involved), and unknown according to American Joint Committee on Cancer staging criteria.16
We categorized patients as having had primary tumor resection (PTR) if we found a Medicare claim for colon resection within 6 months of diagnosis. If PTR was coincident with a claim for obstruction or perforation, we considered those conditions present at cancer diagnosis. We excluded patients with a history of BO before cancer diagnosis. From the remaining cohort, we identified patients hospitalized at an acute-care hospital after cancer diagnosis in which a diagnosis code for BO was used in a MedPAR inpatient hospital claim (560.81, 560.89, 560.9). We further identified the subset of patients in whom the diagnosis code specific to adhesive BO (560.81) was ever used in a MedPAR claim (“ever-adhesive”). Finally, we assessed patients’ chemotherapy treatment from MedPAR, physician, and outpatient claims files.17
Assessment of Comorbid Disease
To assess the prevalence of comorbid disease at the time of obstruction, we used the Klabunde adaptation of the Charlson comorbidity index.18 We weighted 19 health conditions identified by ICD-9 diagnosis codes in Medi-care inpatient and outpatient claims and calculated a composite score for each patient.
Characteristics of Surgical Treatment for BO
We extracted information on treatment of BO by searching physician and hospital claims files for Level II Healthcare Common Procedure Coding System (HCPCS): Current Procedural Terminology and ICD-9 procedure codes. We considered a claim for gastroenterostomy or entero-enterostomy (ICD-9: 44.3, 44.31, 44.38, 44.39; HCPCS: 43820, 44130), bowel resection (ICD-9: 45.6, 45.61, 45.62, 45.63,45.7, 45.71, 45.72, 45.73, 45.74, 45.75, 45.76, 45.78, 45.79, 45.8, 48.4, 48.41, 48.49, 48.5, 48.6, 48.61, 48.62, 48.63, 48.64, 48.65, 48.69; HCPCS: 44120, 44125, 44140, 44145, 44147, 44157, 44158, 44160, 44202, 44204, 44205, 44207, 44211, 44625, 44626), enterostomy (ICD-9: 46.0, 46.01, 46.02, 46.03, 46.1, 46.10, 46.11, 46.13, 46.14, 46.2, 46.20, 46.21, 46.22, 46.23, 46.24; HCPCS: 44021, 44141, 44143, 44144, 44146, 44150, 44210, 44151, 44155, 44156, 44187, 44310, 44188, 44320, 44206, 44208, 44210, 44212, 44300, 44310, 44316, 44320), and lysis of peritoneal adhesions (ICD-9: 54.5, 54.51; HCPCS: 44005, 44180) to represent surgical therapies. We did not consider a claim for laparotomy or laparoscopy to constitute surgical therapy in the absence of secondary procedure codes.
Outcomes
We used MedPAR hospital claims to calculate length of stay, considering transfers to another acute care hospital as part of the same hospitalization. We considered any hospital claim that postdated the initial claim for obstruction to constitute readmission. We used Medicare date of death to calculate days of life remaining after obstruction and the ratio of days in to days out of the hospital.
Statistical Analysis
Associations between baseline demographic and clinical variables and surgical vs nonsurgical treatment of BO were compared by the use of χ2 tests. We compared differences in time to obstruction by using the Mann-Whitney U test. We used Kaplan-Meier curves to compare hospital length of stay and overall and postobstruction survival times. When presenting postobstruction survival curves, we adjusted for covariates by using the method described by Cole and Hernán.19 When we analyzed length of stay, patients dying in the hospital were censored; when we analyzed survival, patients alive at last follow-up were censored. Among those deceased at last follow-up, we compared total days in and out of a hospital by using the Mann-Whitney U test. We modeled survival after obstruction by using Cox proportional hazards models, and we used reverse stepwise regression to build a final model. We retained predictors significantly associated with survival and those whose removal changed parameter estimates by ≥10%. All statistical tests were 2-sided, and we considered a p value of <0.05 statistically significant. Analyses were performed by using SAS 9.2 statistical software (SAS Institute, Cary, NC).
RESULTS
We identified1004 patients hospitalized for BO after diagnosis of stage IV colon adenocarcinoma between January 1, 1991 and December 31, 2005. Of these patients, 281 (28.0%) received surgical treatment while hospitalized; the characteristics of the cohort with BO, stratified by treatment type, are shown in Table 1. Those who received surgical vs nonsurgical therapy were similar for most characteristics, including age and comorbidity; tumor pathologic and treatment characteristics appeared similar as well. There was a reduction in the use of surgical therapy over time: 32.4% of BOs between 1991 and 1996 received surgical therapy in comparison with 25.2% from 1997 to 2001 and 26.6% from 2002 to 2006 (p for trend 0.088).
TABLE 1.
Characteristics of patients with stage IV colon cancer in SEER-Medicare, 1991–2005, with postdiagnosis bowel obstruction, stratified by management strategy
| Overall n = 1004
|
Nonsurgical management n = 723
|
Surgical management n = 281
|
|||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | χ2p | |
| Age at obstruction | 0.542 | ||||||
| 65–69 | 164 | (16.3) | 119 | (16.5) | 45 | (16.0) | |
| 70–74 | 305 | (30.4) | 228 | (31.5) | 77 | (27.4) | |
| 75–79 | 260 | (25.9) | 185 | (25.6) | 75 | (26.7) | |
| ≥80 | 275 | (27.4) | 191 | (26.4) | 84 | (29.9) | |
| Sex | 0.052 | ||||||
| Male | 453 | (45.1) | 340 | (47.0) | 113 | (40.2) | |
| Female | 551 | (54.9) | 383 | (53.0) | 168 | (59.8) | |
| Race | 0.437 | ||||||
| White | 843 | (84.0) | 603 | (83.4) | 240 | (85.4) | |
| Other/unknown | 161 | (16.0) | 120 | (16.6) | 41 | (14.6) | |
| Marital status | 0.716 | ||||||
| Married | 563 | (56.1) | 408 | (56.4) | 155 | (55.2) | |
| Unmarrieda | 441 | (43.9) | 315 | (43.6) | 126 | (44.8) | |
| Comorbidity at obstruction | 0.891 | ||||||
| None | 490 | (48.8) | 353 | (48.8) | 137 | (48.8) | |
| Charlson index = 1 | 284 | (28.3) | 202 | (27.9) | 82 | (29.2) | |
| Charlson index ≥2 | 230 | (22.9) | 168 | (23.2) | 62 | (22.1) | |
| Year of obstruction | 0.097 | ||||||
| 1991–1996 | 311 | (31.0) | 210 | (29.0) | 101 | (35.9) | |
| 1997–2001 | 302 | (30.1) | 226 | (31.3) | 76 | (27.0) | |
| 2002–2006 | 391 | (38.9) | 287 | (39.7) | 104 | (37.0) | |
| Histology | 0.707 | ||||||
| Nonmucinous | 822 | (81.9) | 594 | (82.2) | 228 | (81.1) | |
| Mucinous | 182 | (18.1) | 129 | (17.8) | 53 | (18.9) | |
| Grade | 0.551 | ||||||
| Well and moderately differentiated | 579 | (57.7) | 411 | (56.8) | 168 | (59.8) | |
| Poorly differentiated | 282 | (28.1) | 204 | (28.2) | 78 | (27.8) | |
| Unknown | 143 | (14.2) | 108 | (14.9) | 35 | (12.5) | |
| Cancer site | 0.056 | ||||||
| Left | 374 | (37.3) | 253 | (35.0) | 121 | (43.1) | |
| Right | 574 | (57.2) | 427 | (59.1) | 147 | (52.3) | |
| NOS | 56 | (5.6) | 43 | (5.9) | 13 | (4.6) | |
| Obstruction at cancer diagnosis | 0.246 | ||||||
| No | 843 | (84.0) | 601 | (83.1) | 242 | (86.1) | |
| Yes | 161 | (16.0) | 122 | (16.9) | 39 | (13.9) | |
| Primary tumor resection | 0.446 | ||||||
| No | 192 | (19.1) | 134 | (18.5) | 58 | (20.6) | |
| Yes | 812 | (80.9) | 589 | (81.5) | 223 | (79.4) | |
| Chemotherapy before obstruction | 0.106 | ||||||
| No | 354 | (35.3) | 265 | (36.7) | 89 | (31.7) | |
| Yes | 650 | (64.7) | 458 | (63.3) | 192 | (68.3) | |
| Node stage | 0.619 | ||||||
| N0 | 169 | (16.8) | 115 | (15.9) | 54 | (19.2) | |
| N1 | 239 | (23.8) | 172 | (23.8) | 67 | (23.8) | |
| N2 | 352 | (35.1) | 259 | (35.8) | 93 | (33.1) | |
| Missing | 244 | (24.3) | 177 | (24.5) | 67 | (23.8) | |
| Cancer diagnosis to obstruction, days, median (IQR) | 228 | (99–477) | 213 | (91–448) | 271 | (128–568) | <0.001b |
Data shown are number and percentage, except where indicated.
NOS = not otherwise specified; IQR = interquartile range; SEER = Surveillance, Epidemiology, and End Results.
Includes 18 with unknown marital status (2.3%).
All subjects and all events, Mann-Whitney U test.
Outcomes after hospitalization for BO are shown in Table 2. Patients were hospitalized approximately 1 week, and 12.7% died in the hospital. Median survival after hospitalization for BO was only 73 days among all patients; 6-month and 1-year survivals were 30% and 16%. Of the 817 who survived the initial hospitalization and died during the follow-up period, 201 (24.6%) were readmitted to an inpatient facility with a diagnosis code for obstruction at least once before death. Hospital length of stay was longer among patients treated surgically compared with those treated nonsurgically (11 vs 7 days, p < 0.001), although 30-day mortality was higher among those not receiving surgery during hospitalization for BO (30.8% vs 18.5%, p = 0.003). The absolute difference in median survival between those treated surgically vs nonsurgically was 56 days; however, those receiving surgery spent more days in the hospital during the final period of life. Ultimately, the ratio of time in to time out of the hospital was approximately 1:5 in both populations.
TABLE 2.
Outcomes after hospitalization for bowel obstruction in 1004 patients with stage IV colon cancer in SEER-Medicare, 1991–2005, stratified by management strategy
| All patients n = 1004
|
Nonsurgical management n = 723
|
Surgical management n = 281
|
|||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p | |
| Hospitalization length of staya | 8 | (4–14) | 7 | (4–12) | 11 | (6–18) | <0.001 |
| One or more days in ICU | 164 | 16.3 | 59 | 8.6 | 105 | 37.3 | <0.001 |
| Hospital readmissionsb | 0.076 | ||||||
| None | 374 | 45.8 | 281 | 47.1 | 93 | 42.3 | |
| 1 | 227 | 27.8 | 153 | 25.6 | 74 | 33.6 | |
| 2 or more | 216 | 26.4 | 163 | 27.3 | 53 | 24.1 | |
| Survival from claim, days, median (IQR)c | 73 | (24–239) | 61 | (21–206) | 118 | (37–277) | <0.001 |
| Days in hospital, median (IQR)c | 15 | (7–27) | 13 | (6–24) | 21 | (13–32) | <0.001 |
| Days out of hospital, median (IQR)d | 64 | (19–200) | 53 | (17–176) | 108 | (35–242) | <0.001 |
| Ratio of days out to days in hospitald | 4.6 | (1.5–12.9) | 4.5 | (1.4–13.5) | 4.7 | (2.0–12.2) | 0.695 |
Actual numbers are not reported because some cells contain counts less than 11. Data shown are number and percentage, except where indicated as median and interquartile range.
IQR = interquartile range; ICU = intensive care unit; SEER = Surveillance, Epidemiology, and End Results.
Lifetest, treating death as censored.
Among those who survived the first hospitalization and died between hospitalization for first obstruction and the end of follow-up (597 nonsurgical and 220 surgical)
Lifetest, censored if alive.
Among those who survived the first hospitalization and died before the end of follow-up (597 nonsurgical and 220 surgical), Mann-Whitney U test.
Patients who ever had a code for adhesive obstruction (“ever adhesive”) comprised 16.3% (n = 164) of the obstructed cohort and accounted for 40.5% of surgically treated events. Median survival was nearly 3 times longer than in the never adhesive group (179 vs 63 days, p < 0.001).
Predictors of Postobstruction Survival
A univariable Cox proportional hazards model of survival after hospitalization for obstruction is shown in Table 3. In univariable analysis, surgical treatment of BO was associated with longer postobstruction survival (HR 0.81, p = 0.003). The distinction of an “ever adhesive” obstruction was even more strongly related to favorable survival (HR 0.63 vs “never adhesive,” p < 0.001). High tumor grade and high nodal stage were associated with worse postobstruction survival, whereas tumor histology was unrelated to survival. Receipt of chemotherapy both before (HR 0.78, p < 0.001) and after obstruction (HR 0.76, p < 0.001) increased postobstruction survival; nearly 98% of those receiving chemotherapy before BO also received it after hospitalization. Sex, race, marital status, comorbidity, and age at obstruction were unrelated to postobstruction survival.
TABLE 3.
Univariable Cox proportional hazards analysis of the association between characteristics of bowel obstruction, tumor features, and cancer treatment and postobstruction survival in 877 patients with stage IV colon cancer discharged after hospitalization for bowel obstruction
| Unadjusted HR | 95% CI | |
|---|---|---|
| Characteristics related to presentation and treatment of BO | ||
| Time from cancer diagnosis to obstruction | ||
| Each 30 days | 0.99 | 0.99–0.99 |
| Management of BO | ||
| Nonsurgical | Ref. | |
| Surgical | 0.76 | 0.65–0.89 |
| Ever adhesion | ||
| No | Ref. | |
| Yes | 0.61 | 0.50–0.74 |
| Cancer presentation and treatment | ||
| Obstruction at diagnosis | ||
| No | Ref. | |
| Yes | 1.06 | 0.88–1.28 |
| Primary tumor resection | ||
| No | Ref. | |
| Yes | 0.76 | 0.64–0.90 |
| Chemotherapya | ||
| No | Ref. | |
| Yes | 0.83 | 0.72–0.95 |
| Tumor features | ||
| Histology | ||
| Nonmucinous | Ref. | |
| Mucinous | 1.01 | 0.84–1.20 |
| Grade | ||
| Well/moderately differentiated | Ref. | |
| Poorly differentiated | 1.25 | 1.07–1.46 |
| Unknown | 1.28 | 1.05–1.57 |
| Cancer site | ||
| Left | Ref. | |
| Right | 1.20 | 1.04–1.39 |
| NOS | 1.70 | 1.23–2.36 |
| Node stage | ||
| N0 | Ref. | |
| N1 | 1.64 | 1.31–2.05 |
| N2 | 2.00 | 1.62–2.46 |
| Unknown | 2.18 | 1.74–2.73 |
| Patient characteristics | ||
| Ageb | ||
| 65–69 | Ref. | |
| 70–74 | 1.07 | 0.87–1.32 |
| 75–79 | 0.86 | 0.70–1.07 |
| ≥80 | 1.06 | 0.86–1.31 |
| Sex | ||
| Male | Ref. | |
| Female | 1.07 | 0.93–1.23 |
| Race | ||
| White | Ref. | |
| Other/unknown | 1.04 | 0.87–1.26 |
| Marital status | ||
| Married | Ref. | |
| Unmarriedc | 1.05 | 0.92–1.21 |
| Comorbidity at obstruction | ||
| None | Ref. | |
| Charlson index = 1 | 1.19 | 1.01–1.40 |
| Charlson index ≥2 | 1.17 | 0.98–1.40 |
| Year of obstruction | ||
| 1991–1996 | Ref. | |
| 1997–2001 | 0.87 | 0.73–1.03 |
| 2002–2006 | 0.83 | 0.70–0.98 |
BO = bowel obstruction; NOS = not otherwise specified.
Before BO.
Age and comorbid score and year of obstruction represent conditions at the time of diagnosis of obstruction; other patient, tumor, and treatment characteristics reflect conditions at the time of cancer diagnosis.
Includes 18 with unknown marital status (2.3%).
Two multivariable Cox proportional hazards models are shown in Table 4, differing based on the inclusion of an “ever adhesive” variable. In both models of postobstruction survival, receipt of chemotherapy, tumor grade and site, and node status remained important predictors of postobstruction survival, as did time elapsed between cancer diagnosis and hospitalization for obstruction (HR 0.99 for each month elapsed, p = 0.003). In the first model, which did not distinguish obstructions based on the appearance of billing codes indicating adhesive disease, surgical treatment of obstruction was associated with improved post-BO survival (HR 0.80, p = 0.003). In the second model, which did distinguish obstructions by the use of adhesive obstruction codes, “ever adhesive” status was significantly associated with improved survival after hospitalization for obstruction (HR 0.67, 95% CI: 0.56–0.81). In this model, surgical treatment of BO was no longer a significant predictor of postobstruction survival (HR 0.91, 95% CI: 0.77–1.06), whereas the relationships between survival and time elapsed between cancer diagnosis and obstruction, chemotherapy, and tumor grade, site, and node stage were essentially unchanged. Adjusted survival curves comparing the surgical with nonsurgical and “ever adhesive” with “never adhesive” groups are shown in Figures 1 and 2. In multivariable models PTR at diagnosis (p = 0.90) and year of hospitalization for obstruction (p = 0.217) were no longer associated with survival and were removed from the models.
TABLE 4.
Two multivariable Cox proportional hazards analyses of the association between the characteristics of bowel obstruction, tumor features, and cancer treatment and postobstruction survival, differing by distinction of ever use of adhesive obstructive codes
| Adjusted HR | 95% CI | Adjusted HR | 95% CI | |
|---|---|---|---|---|
| Characteristics related to presentation and treatment of BO | ||||
| Time from cancer diagnosis to obstruction | ||||
| Each 30 days | 0.99 | 0.99–1.00 | 0.99 | 0.99–1.00 |
| Management of BO | ||||
| Nonsurgical | Ref. | |||
| Surgical | 0.80 | 0.69–0.92 | ||
| Ever adhesion | ||||
| No | Ref. | |||
| Yes | 0.67 | 0.56–0.80 | ||
| Cancer presentation and treatment | ||||
| Chemotherapya | ||||
| No | Ref. | Ref. | ||
| Yes | 0.82 | 0.72–0.94 | 0.83 | 0.73–0.95 |
| Tumor features | ||||
| Histology | ||||
| Nonmucinous | ||||
| Mucinous | ||||
| Grade | ||||
| Well/moderately differentiated | Ref. | Ref. | ||
| Poorly differentiated | 1.20 | 1.04–1.40 | 1.20 | 1.03–1.40 |
| Unknown | 1.00 | 0.81–1.23 | 0.99 | 0.80–1.23 |
| Cancer site | ||||
| Left | Ref. | Ref. | ||
| Right | 1.09 | 0.95–1.25 | 1.09 | 0.95–1.25 |
| NOS | 1.68 | 1.24–2.28 | 1.63 | 1.21–2.21 |
| Node stage | ||||
| N0 | Ref. | Ref. | ||
| N1 | 1.55 | 1.26–1.92 | 1.51 | 1.22–1.87 |
| N2 | 1.87 | 1.53–2.29 | 1.84 | 1.50–2.25 |
| Unknown | 2.11 | 1.69–2.64 | 1.99 | 1.59–2.49 |
BO = bowel obstruction; NOS = not otherwise specified.
Before BO.
FIGURE 1.
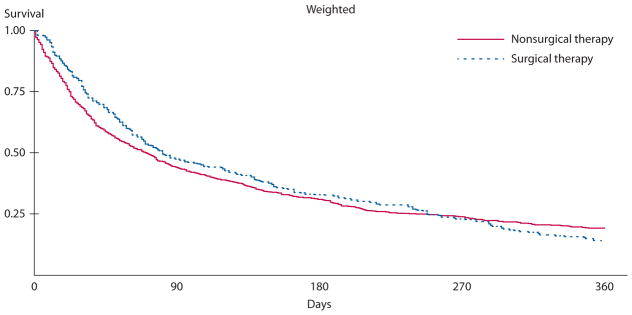
Postobstruction survival among 1004 patients with stage IV colon cancer in SEER-Medicare, 1991 to 2005, who were later hospitalized for bowel obstruction, stratified by treatment, and adjusted for time to obstruction, adhesion status, chemotherapy, tumor grade, and nodal stage. SEER = Surveillance, Epidemiology, and End Results.
FIGURE 2.
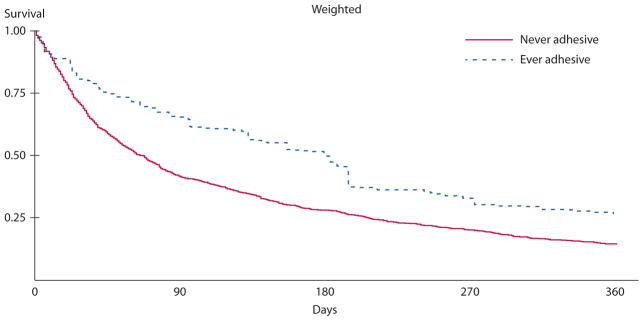
Postobstruction survival among 1004 patients with stage IV colon cancer in SEER-Medicare, 1991 to 2005, who were later hospitalized for bowel obstruction, stratified by any claim for adhesive bowel obstruction, and adjusted for time to obstruction, surgical vs nonsurgical therapy, chemotherapy, tumor grade, and nodal stage. SEER = Surveillance, Epidemiology, and End Results.
DISCUSSION
We examined treatment and outcomes after hospitalization for BO in 1004 patients in SEER-Medicare diagnosed with stage IV colon cancer between 1991 and 2005.Twenty-eight percent of patients were treated surgically for BO, a trend that decreased over time. Hospitalization for obstruction occurred a median of 7 months after cancer diagnosis, and median survival after obstruction was less than 3 months. Treatment required about a week in the hospital, and 12.7% of patients admitted with obstruction died in the hospital. Once discharged from the hospital, 60% of patients returned home; however, 25% were readmitted for obstruction at least once during the remaining months of life. During this terminal period, the overall ratio of days in the hospital to days out of the hospital was about 1:5 and did not differ by surgical vs nonsurgical treatment of BO (p = 0.87). Finally, when controlling for potential confounders, “ever adhesive” disease (HR 0.67, 95% CI: 0.56–0.80) and receipt of chemotherapy at any time (HR 0.66, 95% CI: 0.58–0.76) were favorably associated with post-BO survival; however, surgical treatment of obstruction was not.
The median postobstruction survival of 73 days in this group is within the 2- to 7-month median range reported in previous hospital-based studies of BO.20–24 Only 16% of our cohort was alive 1 year after hospitalization for BO, which is also consistent with many retrospective series.2,20,25 Retrospective series have also reported inhospital mortality rates of between 5% and 38% in patients treated operatively and nonoperatively for BO,26,27 similar to our 12.7% in-hospital mortality and indicative of the nutrition depletion and debilitated functional status common in this population.8
Among those ultimately discharged from the hospital, life after hospitalization for BO was care intensive: nearly one-quarter were readmitted with diagnostic codes for BO between initial obstruction and death. This finding is consistent with reports on frequency of recurrence of obstructive symptoms after initial hospitalization, which ranged from 21% to 50%.16,22,26–29
Although there are no formalized guidelines for treatment of BO in the patient who has advanced cancer,2,12 expert opinion suggests that surgical treatment should be undertaken only when a patient’s estimated life expectancy is greater than 2 months.25,30,31 Additional contraindications to surgical therapy relate to the location, multifocality, and severity of obstruction, the presence of ascites or cachexia, and poor performance status1—factors that we are unable to capture in SEER-Medicare. Therefore, although we present outcomes stratified by treatment, these comparisons are unavoidably biased. Consistent with expected patient selection for invasive treatment, survival after obstruction was longer in the group treated surgically than in the group treated nonsurgically, although in-hospital mortality was higher. Finally, the frequency of hospital readmission for BO was lower in the group treated surgically, consistent with retrospective series arguing that surgery for BO is more durable than medical management alone.32 Despite slightly more favorable outcomes in this group, those treated surgically spent more time in-hospital for all causes during the postobstruction period; as a result, the ratio of time spent in the hospital during the terminal stage of disease was equivalent between treatment strategies, consistent with Chan and Woodruff.23 Finally, in a multivariable model including prognostic tumor characteristics, surgical treatment of BO was not associated with prolonged survival.
We defined BO with the use of both specific (560.81) and nonspecific ICD-9 diagnosis codes (560.89,560.9). The justification for including both coding schemes was, in part, based on evidence that the cause of BO in patients with advanced cancer is not always apparent: 4 reports between 1969 and 1997 noted that 24% to 37% of obstructions in patients with previous cancer were attributable to new primary cancer or benign causes.21,26–28,33 In these series, cause was predominantly determined by laparotomy, and only 28% of our study cohort underwent surgery. One of the aforementioned publications indicated that CT scan was part of the workup of at least some patients,27 and 1 radiology series has demonstrated that the cause of obstruction was accurately determined in 12 of 13 patients with malignant obstruction34; however, other studies evaluating the predictive value of CT in determining the cause of obstruction have included few patients with malignancy.35,36
Despite this difficulty in determining the cause of BO nonoperatively, distinguishing these entities in patients with advanced cancer can be meaningful. A population-based study of 32,583 patients admitted for BO suggested that 75% of benign obstructions will resolve with medical therapy alone,37 whereas retrospective studies indicate that nonoperative intervention is much less successful among patients with a history of malignancy (24%–32%).26,28,29,38 In series distinguishing benign from malignant causes of obstruction in patients with cancer, the prognosis after surgical treatment for benign obstruction is superior to survival after treatment of malignant obstruction.21,26 Consistent with these publications, we found that median postobstruction survival among patients with adhesive disease after cancer diagnosis (“ever adhesive”) was 3 times longer than among “never adhesive” patients (179 vs 63 days, p < 0.001). Although it is likely that some adhesive obstructions were misclassified as nonspecific and vice versa, we had no reason to believe that this misclassification would be differential by survival. Thus, the survival benefit we observed may understate the true benefit associated with adhesive obstruction.
Although there were differences in prognosis based on the cause of obstruction, absolute survival after BO was universally poor, consistent with the natural history of advanced colon cancer and similar to findings of an institutional series whose patient population closely resembled our own: among 62 surgically treated patients with colon cancer, predominantly with stages III and IV disease, Spears and colleagues21 noted a median survival of only 6 months following surgery for benign obstructions vs 2 months when obstruction was secondary to malignancy, results that parallel our “ever adhesive” and “never adhesive” groups.
Our conclusions are strengthened by the population-based and relatively large sample size provided by SEER-Medicare. We were able to track 94% of patients from obstruction to death, enabling a deeper look into the postobstruction experience than previous hospital-based studies. We were also able to evaluate a nonselected cohort; previous studies have often reported only on patients treated surgically and have failed to include those not treated surgically or those whose operative interventions were unsuccessful.2
There are several limitations to our study. Owing to a lack of specific procedure codes, we were unable to assess the effectiveness of several nonsurgical therapeutic strategies. For instance, endoscopic stenting has emerged as an option to palliate BO,4–7 but we could not assess stenting because no specific International Classification of Diseases, Ninth Revision, Clinical Modification code for insertion of a colonic stent existed until 2009.39 For similar reasons, we were unable to assess the use of octreotide.8–11 Further, we could not determine the indication for the use of gastrostomy tubes (ie, decompression vs feeding), nor could we assess the effectiveness of nasogastric suctioning. Most importantly, we were unable to assess the indications for surgery and, therefore, could not adjust treatment analysis for patient selection. Finally, the use of SEER-Medicare does not allow us to assess the success of various treatments in the palliation of obstructive symptoms, arguably the most important outcome in treating this disease, yet one that is rarely addressed in the literature.2,12
CONCLUSION
Our results suggest that BO is a preterminal event and that patients should be informed that median survival under any treatment strategy is poor. The length and intensity of hospital care for BO, and the possible need for readmission for obstruction, should be incorporated into medical decision making. In this setting, it appears that palliative approaches to care seeking to maximize the quality of remaining life are well justified.
Acknowledgments
Funding/Support: This study was supported in part by a fellowship from NCI (R25-CA094061) to M.W. and a fellowship from NCI (T32-CA09529) to S.J.M.
Footnotes
Financial Disclosure: None reported.
Poster presentation at the meeting of the Society of Epidemiologic Research, Minneapolis, MN, June 27 to 30, 2012.
References
- 1.Ripamonti C, Twycross R, Baines M, et al. Working Group of the European Association for Palliative Care. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer. 2001;9:223–233. doi: 10.1007/s005200000198. [DOI] [PubMed] [Google Scholar]
- 2.Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Systematic review of surgery in malignant bowel obstruction in advanced gynecological and gastrointestinal cancer. The Systematic Review Steering Committee. Gynecol Oncol. 1999;75:313–322. doi: 10.1006/gyno.1999.5594. [DOI] [PubMed] [Google Scholar]
- 3.Pothuri B, Vaidya A, Aghajanian C, Venkatraman E, Barakat RR, Chi DS. Palliative surgery for bowel obstruction in recurrent ovarian cancer: an updated series. Gynecol Oncol. 2003;89:306–313. doi: 10.1016/s0090-8258(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 4.Lee HJ, Hong SP, Cheon JH, et al. Long-term outcome of palliative therapy for malignant colorectal obstruction in patients with unresectable metastatic colorectal cancers: endoscopic stenting versus surgery. Gastrointest Endosc. 2011;73:535–542. doi: 10.1016/j.gie.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 5.Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051–2057. doi: 10.1111/j.1572-0241.2004.40017.x. [DOI] [PubMed] [Google Scholar]
- 6.Caceres A, Zhou Q, Iasonos A, Gerdes H, Chi DS, Barakat RR. Colorectal stents for palliation of large-bowel obstructions in recurrent gynecologic cancer: an updated series. Gynecol Oncol. 2008;108:482–485. doi: 10.1016/j.ygyno.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Lee KM, Choi SJ, Shin SJ, et al. Palliative treatment of malignant gastroduodenal obstruction with metallic stent: prospective comparison of covered and uncovered stents. Scand J Gastroenterol. 2009;44:846–852. doi: 10.1080/00365520902929849. [DOI] [PubMed] [Google Scholar]
- 8.DeBernardo R. Surgical management of malignant bowel obstruction: strategies toward palliation of patients with advanced cancer. Curr Oncol Rep. 2009;11:287–292. doi: 10.1007/s11912-009-0040-4. [DOI] [PubMed] [Google Scholar]
- 9.Mangili G, Aletti G, Frigerio L, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer. 2005;15:830–835. doi: 10.1111/j.1525-1438.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- 10.Mangili G, Franchi M, Mariani A, et al. Octreotide in the management of bowel obstruction in terminal ovarian cancer. Gynecol Oncol. 1996;61:345–348. doi: 10.1006/gyno.1996.0154. [DOI] [PubMed] [Google Scholar]
- 11.Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage. 2004;28:412–416. doi: 10.1016/j.jpainsymman.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Kucukmetin A, Naik R, Galaal K, Bryant A, Dickinson HO. Palliative surgery versus medical management for bowel obstruction in ovarian cancer. Cochrane Database Syst Rev. 2010:CD007792. doi: 10.1002/14651858.CD007792.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winner M, Mooney SJ, Hershman DL, et al. Incidence and predictors of bowel obstruction in stage 4 colon cancer patients: a population-based study. JAMA Surg. doi: 10.1001/jamasurg.2013.1. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 15.American Medical Association. International Classification of Diseases, 9th Revision. Chicago: AMA Press; 2008. [Google Scholar]
- 16.Annest LS, Jolly PC. The results of surgical treatment of bowel obstruction caused by peritoneal carcinomatosis. Am Surg. 1979;45:718–721. [PubMed] [Google Scholar]
- 17.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 suppl):IV–55–IV–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 18.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 19.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Helyer LK, Law CH, Butler M, Last LD, Smith AJ, Wright FC. Surgery as a bridge to palliative chemotherapy in patients with malignant bowel obstruction from colorectal cancer. Ann Surg Oncol. 2007;14:1264–1271. doi: 10.1245/s10434-006-9303-6. [DOI] [PubMed] [Google Scholar]
- 21.Spears H, Petrelli NJ, Herrera L, Mittelman A. Treatment of bowel obstruction after operation for colorectal carcinoma. Am J Surg. 1988;155:383–386. doi: 10.1016/s0002-9610(88)80095-3. [DOI] [PubMed] [Google Scholar]
- 22.Wright FC, Chakraborty A, Helyer L, Moravan V, Selby D. Predictors of survival in patients with non-curative stage IV cancer and malignant bowel obstruction. J Surg Oncol. 2010;101:425–429. doi: 10.1002/jso.21492. [DOI] [PubMed] [Google Scholar]
- 23.Chan A, Woodruff RK. Intestinal obstruction in patients with widespread intraabdominal malignancy. J Pain Symptom Manage. 1992;7:339–342. doi: 10.1016/0885-3924(92)90086-w. [DOI] [PubMed] [Google Scholar]
- 24.Walsh HP, Schofield PF. Is laparotomy for small bowel obstruction justified in patients with previously treated malignancy? Br J Surg. 1984;71:933–935. doi: 10.1002/bjs.1800711206. [DOI] [PubMed] [Google Scholar]
- 25.Krebs HB, Goplerud DR. Surgical management of bowel obstruction in advanced ovarian carcinoma. Obstet Gynecol. 1983;61:327–330. [PubMed] [Google Scholar]
- 26.Butler JA, Cameron BL, Morrow M, Kahng K, Tom J. Small bowel obstruction in patients with a prior history of cancer. Am J Surg. 1991;162:624–628. doi: 10.1016/0002-9610(91)90123-u. [DOI] [PubMed] [Google Scholar]
- 27.Woolfson RG, Jennings K, Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Arch Surg. 1997;132:1093–1097. doi: 10.1001/archsurg.1997.01430340047006. [DOI] [PubMed] [Google Scholar]
- 28.Ketcham AS, Hoye RC, Pilch YH, Morton DL. Delayed intestinal obstruction following treatment for cancer. Cancer. 1970;25:406–410. doi: 10.1002/1097-0142(197002)25:2<406::aid-cncr2820250219>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Osteen RT, Guyton S, Steele G, Jr, Wilson RE. Malignant intestinal obstruction. Surgery. 1980;87:611–615. [PubMed] [Google Scholar]
- 30.Castaldo TW, Petrilli ES, Ballon SC, Lagasse LD. Intestinal operations in patients with ovarian carcinoma. Am J Obstet Gynecol. 1981;139:80–84. doi: 10.1016/0002-9378(81)90416-6. [DOI] [PubMed] [Google Scholar]
- 31.Clarke-Pearson DL, Chin NO, DeLong ER, Rice R, Creasman WT. Surgical management of intestinal obstruction in ovarian cancer. I. Clinical features, postoperative complications, and survival. Gynecol Oncol. 1987;26:11–18. doi: 10.1016/0090-8258(87)90066-7. [DOI] [PubMed] [Google Scholar]
- 32.Bryan DN, Radbod R, Berek JS. An analysis of surgical versus chemotherapeutic intervention for the management of intestinal obstruction in advanced ovarian cancer. Int J Gynecol Cancer. 2006;16:125–134. doi: 10.1111/j.1525-1438.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- 33.Weiss SM, Skibber JM, Rosato FE. Bowel obstruction in cancer patients: performance status as a predictor of survival. J Surg Oncol. 1984;25:15–17. doi: 10.1002/jso.2930250105. [DOI] [PubMed] [Google Scholar]
- 34.Megibow AJ, Balthazar EJ, Cho KC, Medwid SW, Birnbaum BA, Noz ME. Bowel obstruction: evaluation with CT. Radiology. 1991;180:313–318. doi: 10.1148/radiology.180.2.2068291. [DOI] [PubMed] [Google Scholar]
- 35.Frager D, Medwid SW, Baer JW, Mollinelli B, Friedman M. CT of small-bowel obstruction: value in establishing the diagnosis and determining the degree and cause. AJR Am J Roentgenol. 1994;162:37–41. doi: 10.2214/ajr.162.1.8273686. [DOI] [PubMed] [Google Scholar]
- 36.Taourel PG, Fabre JM, Pradel JA, Seneterre EJ, Megibow AJ, Bruel JM. Value of CT in the diagnosis and management of patients with suspected acute small-bowel obstruction. AJR Am J Roentgenol. 1995;165:1187–1192. doi: 10.2214/ajr.165.5.7572500. [DOI] [PubMed] [Google Scholar]
- 37.Foster NM, McGory ML, Zingmond DS, Ko CY. Small bowel obstruction: a population-based appraisal. J Am Coll Surg. 2006;203:170–176. doi: 10.1016/j.jamcollsurg.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Helmkamp BF, Kimmel J. Conservative management of small bowel obstruction. Am J Obstet Gynecol. 1985;152(6 pt 1):677–679. doi: 10.1016/s0002-9378(85)80045-4. [DOI] [PubMed] [Google Scholar]
- 39.Varadarajulu S, Roy A, Lopes T, Drelichman ER, Kim M. Endoscopic stenting versus surgical colostomy for the management of malignant colonic obstruction: comparison of hospital costs and clinical outcomes. Surg Endosc. 2011;25:2203–2209. doi: 10.1007/s00464-010-1523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


