Abstract
Melanoma has traditionally been viewed as an ultra-violet (UV) radiation induced malignancy. While UV is a common inducing factor, other endogenous stresses such as metal ion accumulation or the melanin pigment itself, may provide alternative pathways to melanoma progression. Eumelanosomes within melanoma often exhibit disrupted membranes and fragmented pigment which may be due to alterations in their amyloid-based striatial matrix. The melanosomal amyloid can itself be toxic, especially in combination with reactive oxygen species (ROS) and reactive nitrogen species (RNS) generated by endogenous NADPH oxidase (NOX) and nitric oxide synthase (NOS) enzymes; a toxic mix that may initiate melanomagenesis. Further understanding of the loss of the melanosomal organization, the behavior of the exposed melanin, and the induction of ROS/RNS in melanomas may provide critical insights into this deadly disease.
The color of our skin and hair is largely determined by variations in the two main melanin types, black/brown eumelanin and blond/red pheomelanin [1]. The ratio of these two melanin types is also a major predictor of melanoma susceptibility, with the darker pigmented population significantly less susceptible to skin cancers of all types [2, 3]. But the connection between melanoma and pigmentation is unusual, for instance squamous cell carcinomas and other non-melanoma skin cancers are relatively common in both black and white albinos and yet the development of cutaneous melanoma is rare [4, 5]. These individuals still have melanocytes, but they cannot make melanin; perhaps the carcinogenic progression to melanoma depends on the presence of the pigment itself. Even so-called amelanotic melanomas generate melanin; in cultured human melanoma cells and melanocytes pigmentation is observed only when the darker eumelanin is detectable, even when substantial amounts of the lighter pheomelanin are present [6].
Reactive Oxygen Species (ROS) and melanin
The link of melanin generation with melanoma seems at first counter-intuitive, as melanin pigment is in general protective [2, 7, 8]. But the synthesis of melanin has long been recognized as involving cytotoxic molecules and is tightly compartmentalized within pigment-producing cells [9–11]. Both melanocytes and melanoma cells exhibit higher basal levels of ROS as compared to keratinocytes and fibroblasts [12–15]; the source of these ROS, at least in part, results from the melanosome and its contained melanin [16, 17]. Oxidative stress has also been linked to pigmentation disorders, such as vitiligo [18, 19]. Conversely, inhibiting melanin synthesis by N phenylthiourea reduces intracellular ROS in melanocytes [15].
Of the two types of pigment, the black/brown eumelanin plays the major role in protecting skin cells from UV radiation [20]. In contrast, the yellow/red pheomelanin is much less protective; as the pheomelanin to eumelanin ratio increases in isolated melanosomes, the UV absorption capacity decreases [21]. Melanocytes with high pheomelanin content can become pro-oxidant, particularly in the presence of UV radiation [22, 23] and/or metal ions [22, 24]. The fair skin color and and red hair phenotype are associated with non-functional melanocortin 1 receptor gene (Mc1R) [25]; melanocytes from these individuals showed increased ROS generation upon UV radiation [26, 27]. These characteristics of pheomelanin pose a major attributable risk for melanoma for the fair skinned. But still skin cancer, and especially melanoma, are the exception rather than the rule. However, most such individuals, including those with repeated sun-burns and possibly other environmental exposures, never developeg melanoma. Clearly other factors in addition to UV radiation and the pigment itself contribute to melanomagenesis.
Melanosomes and pigment regulation
In melanocytes the pigments are generated within suborganelles called melanosomes through a complex series of tightly regulated processes, controlled by over 120 genes [28, 29]. Ultrastructural investigations have shown distinct differences between eumelanin- and pheomelanin-containing melanosomes. Eumelanosomes are ellipsoidal in shape and display a proteinaceous striatial matrix upon which eumelanin is deposited and ordered in the early stages of development. Pheomelanosomes are typically spherical and the pigment has a coarser granular appearance. Pheomelanosomes also contain significantly more protein than eumelanosomes [30], and the amorphous protein matrix is decidedly more mobile than the fibrillar matrix in eumelanosomes [31]. Melanosomes of both types appear similar before melanization, and contain small vesiculo-globular bodies that appear intimately involved in the melanization process [32, 33]. In early stage eumelanosomes, well-formed fibrils or striations are observed, upon which the black melanin is deposited. This is illustrated in a transmission electron microscopy (TEM) image of a heavily pigmented normal human melanocyte cell line (Figure 1). In melanosomes of mixed phenotypic individuals, pheomelanosomes are observed with striatial fibrils but exhibited spotty and incomplete melanization [34].
Figure 1. In situ transmission electron microscopy images of melanocyte and melanoma cells in culture.
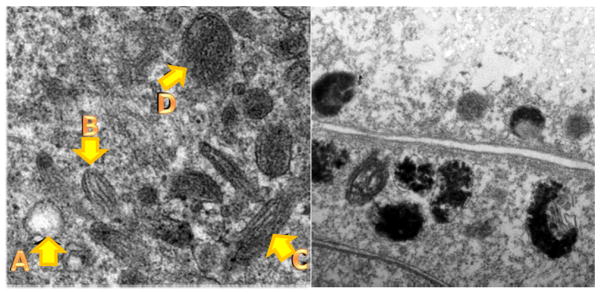
Left panel, Stages of normal melanosomes in heavily pigmented melanocyte A: Stage 1, B: Stage 2, C: Stage 3, D: Stage 4. Right panel, abnormal melanosomes in MNT1 melanoma cells: note disruption of structure and difficulty in identifying stages. Adapted from our work Gidanian et al., 2008 [56].
Also shown in Figure 1 are malformed melanosomes isolated from the heavily pigmented melanoma cell line MNT1, with dramatically altered melanosome structures. It has been recognized for some time that melanosomal genesis is altered early in melanoma progression, including abnormal disposition of melanin [35] and a loss of membrane integrity [11, 36–38]. The presence of the melanin precursor cysteinyldopa in the blood has long been recognized as a clinical marker of melanoma progression [39]. There are also rare reports of generalized melanosis as a complication of melanoma, in which melanin precursors are secreted into the tissue of patients, resulting in hyperpigmentation [40]. Melanoma cells of several types demonstrate pro-oxidant behaviors not seen in normal melanocytes or other cancer cell lines [16, 17]. This suggests that an essential change had occurred within transformed melanoma cells that render its melanin more reactive and susceptible to oxidative stress, which we propose is tied to the loss of melanosomal organization (Figure 2). Detailed relationships among the different melanin subtypes and new information on melanosomal proteins is discussed below.
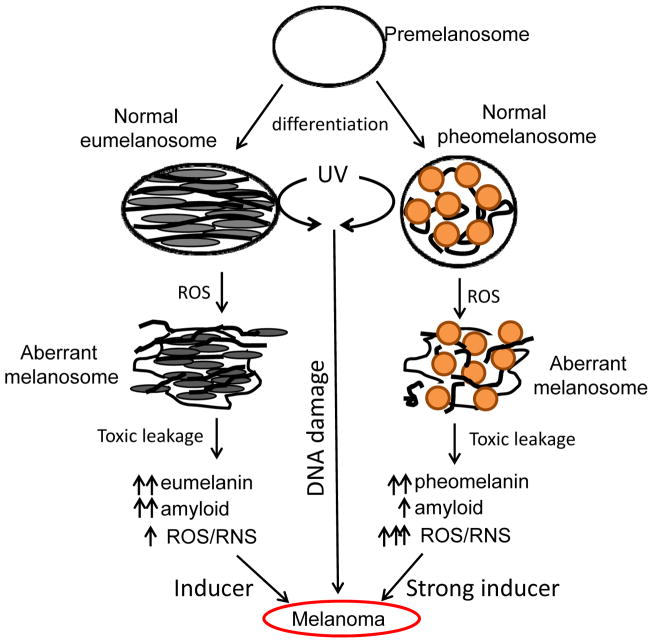
Figure 2. UV/ROS induces aberrant pheomelanosome and eumelanosome structural changes and leads to melanoma transformation/progression.
Pre-melanosomes mature and differentiate into eumelanin-contained eumelanosomes and pheomelanin-contained pheomelanosomes, both of which can undergo aberrant structural changes in the presence of UV or ROS, leading to leaking of melanosomal contents (melanins, melanin synthesis intermediates, amyloids, etc) which can further react with ROS and become more toxic. Eumelanosomes may generate more amyloids and less ROS as compared to pheomelanosomes.
The amyloid-melanin connection
Recent work has shown that the filaments that form the striatial matrix in early stage eumelanosomes are in fact amyloid fibrils [41, 42]. Amyloid is a broad term applied to aggregates of proteins that form extended “cross β” fibrils associated with a number of age-related degenerative diseases such as Parkinson’s and Alzheimer’s diseases [43]. Although the amyloid proteins associated with the various neuro-degenerative diseases are different, the accumulated fibrils identified in each disease share a common parallel β-strand structural motif [44, 45].
The initial evidence for amyloids in melanocytes came from the discovery that the melanosomal-associated protein Pmel is cleaved in normal melanosomes into two fragments, called Mα and Mβ, and that its overexpression in non-pigment cells resulted in the formation of striations within multivesicular bodies[46]. Subsequently the Pmel-derived amyloid fibrils were characterized in normal melanocytes [47]. Mutations of the same proprotein convertase Furin was found in a rare familial amyloidal disease [48], and. Maturation of eumelanosome depends on the cleavage of Pmel [49], and it is fragment Mα which self-assembles to form the striatial fibrils within the early stage eumelanosomes [41]. An important characteristic of the nonpathogenic Pmel-amyloid is that the fibrils form at mildly acidic pH (~5.0) and dissolve at neutral pH, which allows for a reversible aggregation-disaggregation process [50, 51]. Thus loss of melanosomal membrane integrity may affect the stability of the striatial amyloid matrix, as illustrated in Figure 2.
The loss of melanosomal integrity observed in melanoma is particularly intriguing, as similar membranal disruptions are also observed in amyloid diseases, attributed to the action of protofibril precursors which form pores that span the membrane [52–54]. Although pheomelanosomes lack the striatial matrix of the early stage eumelanosomes, both evolve from common precursor premelanosomes and are often found within the same cells [32, 55]. We hypothesize that melanosomal abnormalities seen in melanoma may be due to a loss of or change in amyloid fibril formation. Using antibody stains our initial observations document the presence of amyloid precursors in several melanoma cell lines; these include both heavily pigmented black MNT1 cells [56], and amelanotic SK-Mel28 cells [57]. If substantiated, then amyloid dysfunction may be a characteristic early pathology in melanoma, potentially related to both carcinogenesis and to the high rate of mutation and chemo-resistance of this deadly cancer. These ideas suggest several diverse lines of investigation.
Are mutations of proteins regulating the striatial matrix in eumelanosomes involved in melanoma? Pmel itself was first identified from inbred mice (silver) which have accelerated graying of their hair [58]. The expression of a variety of melanosomal proteins has been shown to become altered in malignant melanoma [59, 60]. Mutations in Pmel result in loss of melanin production and damage to the cell membrane, possibly due to changes in the formation of pathological amyloid aggregates [61]. Several mutations of melanin-synthesis related genes are also melanoma-associated, such as in genes for the melanocortin receptor 1 (Mc1R), MiTF, agouti (ASIP) and tyrosinase [62–65]. Mc1R serves as a switch between eumelanin and pheomelanin synthesis, and therefore it plays an essential role in skin color [62, 66–69], although its exact role in melanomagenesis is still under debate [26, 70, 71].
Might the aggregation of melanin by deposition on amyloid fibrils inhibit its pro-oxidant and cytotoxic behavior? If so, then this would explain the varied behaviors of isolated eumelanin and pheomelanins [13, 21, 22, 24, 72, 73]. Melanosomes purified from melanoma generate ROS under ambient conditions, while melanosomes from highly pigmented human melanocytes do not [27]. Disturbed melanin synthesis and chronic oxidative stress are present in dysplastic nevi, a possible first transformative step towards melanoma [72].
-
Is there a connection between ROS/RNS and the loss of melanosomal integrity? Ultraviolet radiation induces an inflammatory response in skin [74], and melanoma tumors themselves often show macrophage and neutrophil infiltration. Melanocytic cells express NADPH Oxidase (NOX), a key player in the generation of oxidative stress, mainly as NOX1 and NOX4 enzymes and their subunits [75–77]. NOX1 is expressed in all melanoma cell lines examined at a higher level than normal human melanocytes [76], but NOX4 was only detected in a subset of metastatic melanoma samples [76, 77]. NOX1 protein levels increase after UVR in a primary melanoma cell line (Liu-Smith and Meyskens, unpublished data), and may be a major source of UV-induced ROS in dysplastic nevi [78].
The neuronal form of Nitric Oxide Synthase, nNOS, is found in melanogenic cells and its expression is much higher in melanoma cell lines than in normal melanocytes [79]. Inhibiting nNOS by specific inhibitors led to decreased xenografted melanoma tumor growth in vivo [79, 80]. Similarly, NADPH Oxidase (NOX) activity is induced by UV radiation, and NOX1 protein levels are higher in melanoma cells than in normal melanocytes [76, 78]. It is well known that the toxicity of NO is dramatically enhanced in conjuction with mitochondria-generated ROS [81]. Hence the ROS/RNS pool from various sources may form a detelerious feedback circuit for melanomagenesis, resulting from the leaking of melanosome contents (Figure 2).
Likewise, there is a strong connection between oxidative stress and the formation of amyloid deposits in neurodegenerative diseases [82–85], where the direct binding of Cu(II) ions to the β-amyloid is often implicated [86]. Early studies indicated that β-amyloid deposition caused activation of NOX and release of ROS in a variety of cell lines [87, 88]. Cu ions are abundant in melanosomes because they are required for tyrosinase activity; the combination of amyloid, Cu, and ROS/RNS may form a vicious cycle that serves as a carcinogenic threat to melanocytes [89]. Nevertheless, endogenous ROS can act as preventive agents for melanomagenesis as they kill damaged cells and prevent transformation [90]; in other venue endogenous ROS can be signals for cell proliferation and promote transformation [78, 91]; therefore the function of ROS in melanomagenesis is indeed complicated.
Is melanoma an amyloid disease and vice versa? Melanocytes are derived from the neural crest during differentiation, and thus may be susceptible to causative factors related to neurodegenerative amyloid-based diseases. For instance, it is known that Parkinson’s disease patients have a higher risk for melanoma, and vice versa [92, 93]. Therefore we speculate that the neuromelanin in neuronal cells may also exhibit similar redox capacity as eumelanin in the melanocytes, because melanocytes are also of neural crest origin. Emerging evidence implies that neuromelanin is cytoprotective when contained within neuronal cells but causes cytotoxic effect when released by damaged neuron cells [94].
Taken together, the melanomsomal amyloid toxicity may be summarized into several aspects: 1) amyloid changes the structure of the melanosomes and leads to leaking of the melanomsomal contents including amyloid itself, melanin and melanin intermediates; 2) amyloid interaction with ROS and RNS augments the detelerious effect of these species; 3) amyloid may disrupt the cellular membrane leading to altered signal transduction, as has been proposed in other cell types [95, 96].
Conclusions
This viewpoint offers alternative etiological explanations for melanomagenesis, i.e., that amyloidal dysfunction in combination with ROS/RNS generated in situ (via NOX and NOS) may lead to melanomagenesis. Figure 3 charts the relationships among melanin phenotypes and carcinogenic risk, as well as potential links to neurodegenerative diseases. While UV is a common inducing factor via alterations in DNA repair, other stresses such as metal ion or pesticide exposure, may provide alternative stresses that involve other pathways to melanoma progression. The low risk population for melanoma includes dark-skinned individuals who predominantly produce eumelanin, and fair-skinned individuals with predominantly pheomelanin pigment who have low exposure to UV (Figure 3). Fair skinned pheomelanonic individuals are more susceptible to UV-induced stress, especially high dose blistering childhood sun-burns or intermittent adult life sun-burns that produce moles or freckles. For all populations, unmanaged oxidative stress increases risk for melanoma. The correlation of melanoma and Parkinson’s disease may also imply a common causative factor of ROS-amyloid dysfunction in neuronal cells [92]. Further understanding of consequences from the loss of melanosomal organization and the effects of the exposed melanin on melanocytes and neuronal cells may provide critical new insights into both diseases of the melanocytes and neurons.
Figure 3. Summary of melanoma risk with melanin types and its potential link with neurodegenerative diseases.
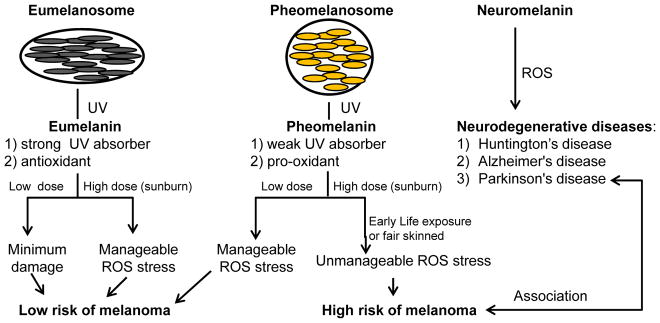
Eumelanin strongly absorbs UV radiation and serves as an antioxidant, hence in dark skinned individuals the UV/ROS induced stress is well managed and melanomas are rare. In pheomelanin-predominant light skinned individuals, pheomelanin does not efficiently absorb UV radiation, and upon UV radiation pheomelanin becomes a pro-oxidant. Hence, with high UV doses the stress is difficult to manage and melanoma risk increases greatly in these individuals. A potential link of UV/ROS-induced amyloid toxicity is suggested by the shared risk of some individuals for cutaneous melanoma and Parkinson’s disease.
Acknowledgments
This work is supported by Chao Family Cancer Center Seed Grant (CCSG, P30 CA 62230, to FLM and FLS), NCI K07 (CA160756 to FLS) and the Waltmar Foundation (to FLM and FLS). PJF acknowledges support from Baylor University.
References
- 1.Thong HY, Jee SH, Sun CC, Boissy RE. The patterns of melanosome distribution in keratinocytes of human skin as one determining factor of skin colour. Br J Dermatol. 2003;149(3):498–505. doi: 10.1046/j.1365-2133.2003.05473.x. [DOI] [PubMed] [Google Scholar]
- 2.Alaluf S, Atkins D, Barrett K, et al. The impact of epidermal melanin on objective measurements of human skin colour. Pigment Cell Res. 2002;15(2):119–26. doi: 10.1034/j.1600-0749.2002.1o072.x. [DOI] [PubMed] [Google Scholar]
- 3.Wood SR, Berwick M, Ley RD, et al. UV causation of melanoma in Xiphophorus is dominated by melanin photosensitized oxidant production. Proc Natl Acad Sci U S A. 2006;103(11):4111–5. doi: 10.1073/pnas.0511248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yakubu A, Mabogunje OA. Skin cancer in African albinos. Acta Oncol. 1993;32(6):621–2. doi: 10.3109/02841869309092440. [DOI] [PubMed] [Google Scholar]
- 5.Luande J, Henschke CI, Mohammed N. The Tanzanian human albino skin. Natural history. Cancer. 1985;55(8):1823–8. doi: 10.1002/1097-0142(19850415)55:8<1823::aid-cncr2820550830>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.del Marmol V, Ito S, Jackson I, et al. TRP-1 expression correlates with eumelanogenesis in human pigment cells in culture. FEBS Lett. 1993;327(3):307–10. doi: 10.1016/0014-5793(93)81010-w. [DOI] [PubMed] [Google Scholar]
- 7.Gloster HM, Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55(5):741–60. doi: 10.1016/j.jaad.2005.08.063. quiz 761–4. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Simon JD. Isolation and biophysical studies of natural eumelanins: applications of imaging technologies and ultrafast spectroscopy. Pigment Cell Res. 2003;16(6):606–18. doi: 10.1046/j.1600-0749.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 9.Nelson M, Foxwell AR, Tyrer P, Dean RT. Protein-bound 3,4-dihydroxy-phenylanine (DOPA), a redox-active product of protein oxidation, as a trigger for antioxidant defences. Int J Biochem Cell Biol. 2007;39(5):879–89. doi: 10.1016/j.biocel.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Land EJ, Ramsden CA, Riley PA. Quinone chemistry and melanogenesis. Methods Enzymol. 2004;378:88–109. doi: 10.1016/S0076-6879(04)78005-2. [DOI] [PubMed] [Google Scholar]
- 11.Borovansky J, Mirejovsky P, Riley PA. Possible relationship between abnormal melanosome structure and cytotoxic phenomena in malignant melanoma. Neoplasma. 1991;38(4):393–400. [PubMed] [Google Scholar]
- 12.Meyskens FL, Jr, McNulty SE, Buckmeier JA, et al. Aberrant redox regulation in human metastatic melanoma cells compared to normal melanocytes. Free Radic Biol Med. 2001;31(6):799–808. doi: 10.1016/s0891-5849(01)00650-5. [DOI] [PubMed] [Google Scholar]
- 13.Meyskens FL, Jr, Farmer P, Fruehauf JP. Redox regulation in human melanocytes and melanoma. Pigment Cell Res. 2001;14(3):148–54. doi: 10.1034/j.1600-0749.2001.140303.x. [DOI] [PubMed] [Google Scholar]
- 14.Meyskens FL, Jr, Chau HV, Tohidian N, Buckmeier J. Luminol-enhanced chemiluminescent response of human melanocytes and melanoma cells to hydrogen peroxide stress. Pigment Cell Res. 1997;10(3):184–9. doi: 10.1111/j.1600-0749.1997.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins NC, Grossman D. Role of melanin in melanocyte dysregulation of reactive oxygen species. Biomed Res Int. 2013:908797. doi: 10.1155/2013/908797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farmer PJ, Gidanian S, Shahandeh B, et al. Melanin as a target for melanoma chemotherapy: pro-oxidant effect of oxygen and metals on melanoma viability. Pigment Cell Res. 2003;16(3):273–9. doi: 10.1034/j.1600-0749.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 17.Meyskens FL, Jr, Farmer PJ, Yang S, Anton-Culver H. New perspectives on melanoma pathogenesis and chemoprevention. Recent Results Cancer Res. 2007;174:191–5. doi: 10.1007/978-3-540-37696-5_16. [DOI] [PubMed] [Google Scholar]
- 18.Laddha NC, Dwivedi M, Mansuri MS, et al. Role of oxidative stress and autoimmunity in onset and progression of vitiligo. Exp Dermatol. 2014;23(5):352–3. doi: 10.1111/exd.12372. [DOI] [PubMed] [Google Scholar]
- 19.Denat L, Kadekaro AL, Marrot L, et al. Melanocytes as instigators and victims of oxidative stress. J Invest Dermatol. 2014;134(6):1512–8. doi: 10.1038/jid.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65(5):671–5. doi: 10.1016/j.jhevol.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Peles DN, Simon JD. The ultraviolet absorption coefficient of melanosomes decreases with increasing pheomelanin content. J Phys Chem B. 2010;114(29):9677–83. doi: 10.1021/jp102603b. [DOI] [PubMed] [Google Scholar]
- 22.Panzella L, Leone L, Greco G, et al. Red human hair pheomelanin is a potent pro-oxidant mediating UV-independent contributory mechanisms of melanomagenesis. Pigment Cell Melanoma Res. 2014;27(2):244–52. doi: 10.1111/pcmr.12199. [DOI] [PubMed] [Google Scholar]
- 23.Mitra D, Luo X, Morgan A, et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491(7424):449–53. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panzella L, Szewczyk G, d’Ischia M, et al. Zinc-induced structural effects enhance oxygen consumption and superoxide generation in synthetic pheomelanins on UVA/visible light irradiation. Photochem Photobiol. 2010;86(4):757–64. doi: 10.1111/j.1751-1097.2010.00726.x. [DOI] [PubMed] [Google Scholar]
- 25.Beaumont KA, Shekar SN, Cook AL, et al. Red hair is the null phenotype of MC1R. Hum Mutat. 2008;29(8):E88–94. doi: 10.1002/humu.20788. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Malek ZA, Knittel J, Kadekaro AL, et al. The melanocortin 1 receptor and the UV response of human melanocytes--a shift in paradigm. Photochem Photobiol. 2008;84(2):501–8. doi: 10.1111/j.1751-1097.2008.00294.x. [DOI] [PubMed] [Google Scholar]
- 27.Swope V, Alexander C, Starner R, et al. Significance of the melanocortin 1 receptor in the DNA damage response of human melanocytes to ultraviolet radiation. Pigment Cell Melanoma Res. 2014;27(4):601–10. doi: 10.1111/pcmr.12252. [DOI] [PubMed] [Google Scholar]
- 28.Crippa R, Horak V, Prota G. Chemistry of Melanins. In: Editor BA, editor. Alkaloids. New York: Academic Press; 1989. [Google Scholar]
- 29.Ganesan AK, Ho H, Bodemann B, et al. Genome-wide siRNA-based functional genomics of pigmentation identifies novel genes and pathways that impact melanogenesis in human cells. PLoS Genet. 2008;4(12):e1000298. doi: 10.1371/journal.pgen.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Hong L, Wakamatsu K, et al. Comparison of structural and chemical properties of black and red human hair melanosomes. Photochem Photobiol. 2005;81(1):135–44. doi: 10.1562/2004-08-03-RA-259.1. [DOI] [PubMed] [Google Scholar]
- 31.Thureau P, Ziarelli F, Thevand A, et al. Probing the motional behavior of eumelanin and pheomelanin with solid-state NMR spectroscopy: new insights into the pigment properties. Chemistry. 2012;18(34):10689–700. doi: 10.1002/chem.201200277. [DOI] [PubMed] [Google Scholar]
- 32.Jimbow K, Oikawa O, Sugiyama S, Takeuchi T. Comparison of eumelanogenesis and pheomelanogenesis in retinal and follicular melanocytes; role of vesiculo-globular bodies in melanosome differentiation. J Invest Dermatol. 1979;73(4):278–84. doi: 10.1111/1523-1747.ep12531650. [DOI] [PubMed] [Google Scholar]
- 33.Wolnicka-Glubisz A, Pecio A, Podkowa D, et al. Pheomelanin in the skin of Hymenochirus boettgeri (Amphibia: Anura: Pipidae) Exp Dermatol. 2012;21(7):537–40. doi: 10.1111/j.1600-0625.2012.01511.x. [DOI] [PubMed] [Google Scholar]
- 34.Jimbow K, Ishida O, Ito S, et al. Combined chemical and electron microscopic studies of pheomelanosomes in human red hair. J Invest Dermatol. 1983;81(6):506–11. doi: 10.1111/1523-1747.ep12522838. [DOI] [PubMed] [Google Scholar]
- 35.Curran RC, McCann BG. The ultrastructure of benign pigmented naevi and melanocarcinomas in man. J Pathol. 1976;119(3):135–46. doi: 10.1002/path.1711190303. [DOI] [PubMed] [Google Scholar]
- 36.Rhodes AR, Seki Y, Fitzpatrick TB, Stern RS. Melanosomal alterations in dysplastic melanocytic nevi. A quantitative, ultrastructural investigation. Cancer. 1988;61(2):358–69. doi: 10.1002/1097-0142(19880115)61:2<358::aid-cncr2820610227>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Amicarelli F, Bonfigli A, Zarivi O, et al. Efflux of cytotoxic species within lipo-melanosome membrane. Ann N Y Acad Sci. 1988;551:141–3. doi: 10.1111/j.1749-6632.1988.tb22331.x. [DOI] [PubMed] [Google Scholar]
- 38.Choi H, Kim M, Ahn SI, et al. Regulation of pigmentation by substrate elasticity in normal human melanocytes and melanotic MNT1 human melanoma cells. Exp Dermatol. 2014;23(3):172–7. doi: 10.1111/exd.12343. [DOI] [PubMed] [Google Scholar]
- 39.Wakamatsu K, Kageshita T, Furue M, et al. Evaluation of 5-S-cysteinyldopa as a marker of melanoma progression: 10 years’ experience. Melanoma Res. 2002;12(3):245–53. doi: 10.1097/00008390-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Tsukamoto K, Furue M, Sato Y, et al. Generalized melanosis in metastatic malignant melanoma: the possible role of DOPAquinone metabolites. Dermatology. 1998;197(4):338–42. doi: 10.1159/000018028. [DOI] [PubMed] [Google Scholar]
- 41.Watt B, van Niel G, Raposo G, Marks MS. PMEL: a pigment cell-specific model for functional amyloid formation. Pigment Cell Melanoma Res. 2013;26(3):300–15. doi: 10.1111/pcmr.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rochin L, Hurbain I, Serneels L, et al. BACE2 processes PMEL to form the melanosome amyloid matrix in pigment cells. Proc Natl Acad Sci U S A. 2013;110(26):10658–63. doi: 10.1073/pnas.1220748110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol. 2004;6(11):1054–61. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 44.Benzinger TL, Gregory DM, Burkoth TS, et al. Two-dimensional structure of beta-amyloid(10–35) fibrils. Biochemistry. 2000;39(12):3491–9. doi: 10.1021/bi991527v. [DOI] [PubMed] [Google Scholar]
- 45.Nelson R, Sawaya MR, Balbirnie M, et al. Structure of the cross-beta spine of amyloid-like fibrils. Nature. 2005;435(7043):773–8. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Theos AC, Watt B, Harper DC, et al. The PKD domain distinguishes the trafficking and amyloidogenic properties of the pigment cell protein PMEL and its homologue GPNMB. Pigment Cell Melanoma Res. 2013;26(4):470–86. doi: 10.1111/pcmr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fowler DM, Koulov AV, Alory-Jost C, et al. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4(1):e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huff ME, Balch WE, Kelly JW. Pathological and functional amyloid formation orchestrated by the secretory pathway. Curr Opin Struct Biol. 2003;13(6):674–82. doi: 10.1016/j.sbi.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Berson JF, Theos AC, Harper DC, et al. Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J Cell Biol. 2003;161(3):521–33. doi: 10.1083/jcb.200302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfefferkorn CM, McGlinchey RP, Lee JC. Effects of pH on aggregation kinetics of the repeat domain of a functional amyloid, Pmel17. Proc Natl Acad Sci U S A. 2010;107(50):21447–52. doi: 10.1073/pnas.1006424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGlinchey RP, Shewmaker F, Hu KN, et al. Repeat domains of melanosome matrix protein Pmel17 orthologs form amyloid fibrils at the acidic melanosomal pH. J Biol Chem. 2011;286(10):8385–93. doi: 10.1074/jbc.M110.197152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 53.Harper JD, Lansbury PT., Jr Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- 54.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192(1):106–13. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inazu M, Mishima Y. Detection of eumelanogenic and pheomelanogenic melanosomes in the same normal human melanocyte. J Invest Dermatol. 1993;100(2 Suppl):172S–175S. [PubMed] [Google Scholar]
- 56.Gidanian S, Mentelle M, Meyskens FL, Jr, Farmer PJ. Melanosomal damage in normal human melanocytes induced by UVB and metal uptake--a basis for the pro-oxidant state of melanoma. Photochem Photobiol. 2008;84(3):556–64. doi: 10.1111/j.1751-1097.2008.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watabe H, Valencia JC, Yasumoto K, et al. Regulation of tyrosinase processing and trafficking by organellar pH and by proteasome activity. J Biol Chem. 2004;279(9):7971–81. doi: 10.1074/jbc.M309714200. [DOI] [PubMed] [Google Scholar]
- 58.Kwon BS, Halaban R, Kim GS, et al. A melanocyte-specific complementary DNA clone whose expression is inducible by melanotropin and isobutylmethyl xanthine. Mol Biol Med. 1987;4(6):339–55. [PubMed] [Google Scholar]
- 59.Jimbow M, Kanoh H, Jimbow K. Characterization of biochemical properties of melanosomes for structural and functional differentiation: analysis of the compositions of lipids and proteins in melanosomes and their subfractions. J Invest Dermatol. 1982;79(2):97–102. doi: 10.1111/1523-1747.ep12500034. [DOI] [PubMed] [Google Scholar]
- 60.Akutsu Y, Jimbow K. Development and characterization of a mouse monoclonal antibody, MoAb HMSA-1, against a melanosomal fraction of human malignant melanoma. Cancer Res. 1986;46(6):2904–11. [PubMed] [Google Scholar]
- 61.Watt B, Tenza D, Lemmon MA, et al. Mutations in or near the transmembrane domain alter PMEL amyloid formation from functional to pathogenic. PLoS Genet. 2011;7(9):e1002286. doi: 10.1371/journal.pgen.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdel-Malek Z, Suzuki I, Tada A, et al. The melanocortin-1 receptor and human pigmentation. Ann N Y Acad Sci. 1999;885:117–33. doi: 10.1111/j.1749-6632.1999.tb08669.x. [DOI] [PubMed] [Google Scholar]
- 63.Sturm RA. Skin colour and skin cancer - MC1R, the genetic link. Melanoma Res. 2002;12(5):405–16. doi: 10.1097/00008390-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 64.Maccioni L, Rachakonda PS, Scherer D, et al. Variants at chromosome 20 (ASIP locus) and melanoma risk. Int J Cancer. 2013;132(1):42–54. doi: 10.1002/ijc.27648. [DOI] [PubMed] [Google Scholar]
- 65.Gudbjartsson DF, Sulem P, Stacey SN, et al. ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat Genet. 2008;40(7):886–91. doi: 10.1038/ng.161. [DOI] [PubMed] [Google Scholar]
- 66.Swope VB, Jameson JA, McFarland KL, et al. Defining MC1R regulation in human melanocytes by its agonist alpha-melanocortin and antagonists agouti signaling protein and beta-defensin 3. J Invest Dermatol. 2012;132(9):2255–62. doi: 10.1038/jid.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sturm RA, Box NF, Ramsay M. Human pigmentation genetics: the difference is only skin deep. Bioessays. 1998;20(9):712–21. doi: 10.1002/(SICI)1521-1878(199809)20:9<712::AID-BIES4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 68.Sturm RA, Teasdale RD, Box NF. Human pigmentation genes: identification, structure and consequences of polymorphic variation. Gene. 2001;277(1–2):49–62. doi: 10.1016/s0378-1119(01)00694-1. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Borron JC, Olivares C. Melanocortin 1 receptor and skin pathophysiology: beyond colour, much more than meets the eye. Exp Dermatol. 2014;23(6):387–8. doi: 10.1111/exd.12310. [DOI] [PubMed] [Google Scholar]
- 70.Song X, Mosby N, Yang J, et al. alpha-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes. Pigment Cell Melanoma Res. 2009;22(6):809–18. doi: 10.1111/j.1755-148X.2009.00615.x. [DOI] [PubMed] [Google Scholar]
- 71.Henri P, Beaumel S, Guezennec A, et al. MC1R expression in HaCaT keratinocytes inhibits UVA-induced ROS production via NADPH oxidase- and cAMP-dependent mechanisms. J Cell Physiol. 2012;227(6):2578–85. doi: 10.1002/jcp.22996. [DOI] [PubMed] [Google Scholar]
- 72.Pavel S, van Nieuwpoort F, van der Meulen H, et al. Disturbed melanin synthesis and chronic oxidative stress in dysplastic naevi. Eur J Cancer. 2004;40(9):1423–30. doi: 10.1016/j.ejca.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 73.Morgan AM, Lo J, Fisher DE. How does pheomelanin synthesis contribute to melanomagenesis?: Two distinct mechanisms could explain the carcinogenicity of pheomelanin synthesis. Bioessays. 2013;35(8):672–6. doi: 10.1002/bies.201300020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rangwala S, Tsai KY. Roles of the immune system in skin cancer. Br J Dermatol. 2011;165(5):953–65. doi: 10.1111/j.1365-2133.2011.10507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Govindarajan B, Sligh JE, Vincent BJ, et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117(3):719–29. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu F, Gomez Garcia AM, Meyskens FL., Jr NADPH oxidase 1 overexpression enhances invasion via matrix metalloproteinase-2 and epithelial-mesenchymal transition in melanoma cells. J Invest Dermatol. 2012;132(8):2033–41. doi: 10.1038/jid.2012.119. [DOI] [PubMed] [Google Scholar]
- 77.Yamaura M, Mitsushita J, Furuta S, et al. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 2009;69(6):2647–54. doi: 10.1158/0008-5472.CAN-08-3745. [DOI] [PubMed] [Google Scholar]
- 78.Liu-Smith F, Dellinger R, Meyskens FL., Jr Updates of reactive oxygen species in melanoma etiology and progression. Arch Biochem Biophys. 2014 doi: 10.1016/j.abb.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Z, Misner B, Ji H, et al. Targeting nitric oxide signaling with nNOS inhibitors as a novel strategy for the therapy and prevention of human melanoma. Antioxid Redox Signal. 2013;19(5):433–47. doi: 10.1089/ars.2012.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang H, Li H, Yang S, et al. Potent and Selective Double-Headed Thiophene-2-carboximidamide Inhibitors of Neuronal Nitric Oxide Synthase for the Treatment of Melanoma. J Med Chem. 2014;57(3):686–700. doi: 10.1021/jm401252e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shiva S, Moellering D, Ramachandran A, et al. Redox signalling: from nitric oxide to oxidized lipids. Biochem Soc Symp. 2004;71:107–20. doi: 10.1042/bss0710107. [DOI] [PubMed] [Google Scholar]
- 82.Pappolla MA, Chyan YJ, Omar RA, et al. Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer’s disease: a chronic oxidative paradigm for testing antioxidant therapies in vivo. Am J Pathol. 1998;152(4):871–7. [PMC free article] [PubMed] [Google Scholar]
- 83.Smith MA, Hirai K, Hsiao K, et al. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J Neurochem. 1998;70(5):2212–5. doi: 10.1046/j.1471-4159.1998.70052212.x. [DOI] [PubMed] [Google Scholar]
- 84.Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol Aging. 2002;23(5):655–64. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 85.Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med. 1997;23(1):134–47. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 86.Mayes J, Tinker-Mill C, Kolosov O, et al. beta-Amyloid Fibrils in Alzheimer’s Disease are not Inert When Bound to Copper Ions but can Degrade Hydrogen Peroxide and Generate Reactive Oxygen Species. J Biol Chem. 2014 doi: 10.1074/jbc.M113.525212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bianca VD, Dusi S, Bianchini E, et al. beta-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease. J Biol Chem. 1999;274(22):15493–9. doi: 10.1074/jbc.274.22.15493. [DOI] [PubMed] [Google Scholar]
- 88.Andersen JM, Myhre O, Aarnes H, et al. Identification of the hydroxyl radical and other reactive oxygen species in human neutrophil granulocytes exposed to a fragment of the amyloid beta peptide. Free Radic Res. 2003;37(3):269–79. doi: 10.1080/1071576021000046631. [DOI] [PubMed] [Google Scholar]
- 89.Leuner K, Schutt T, Kurz C, et al. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid Redox Signal. 2012;16(12):1421–33. doi: 10.1089/ars.2011.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Franke JC, Plotz M, Prokop A, et al. New caspase-independent but ROS-dependent apoptosis pathways are targeted in melanoma cells by an iron-containing cytosine analogue. Biochem Pharmacol. 2010;79(4):575–86. doi: 10.1016/j.bcp.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 91.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–90. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bertoni JM, Arlette JP, Fernandez HH, et al. Increased melanoma risk in Parkinson disease: a prospective clinicopathological study. Arch Neurol. 2010;67(3):347–52. doi: 10.1001/archneurol.2010.1. [DOI] [PubMed] [Google Scholar]
- 93.Olsen JH, Friis S, Frederiksen K. Malignant melanoma and other types of cancer preceding Parkinson disease. Epidemiology. 2006;17(5):582–7. doi: 10.1097/01.ede.0000229445.90471.5e. [DOI] [PubMed] [Google Scholar]
- 94.Zucca FA, Basso E, Cupaioli FA, et al. Neuromelanin of the human substantia nigra: an update. Neurotox Res. 2014;25(1):13–23. doi: 10.1007/s12640-013-9435-y. [DOI] [PubMed] [Google Scholar]
- 95.Sirangelo I, Irace G, Balestrieri ML. Amyloid toxicity and platelet-activating factor signaling. J Cell Physiol. 2013;228(6):1143–8. doi: 10.1002/jcp.24284. [DOI] [PubMed] [Google Scholar]
- 96.Cecchi C, Stefani M. The amyloid-cell membrane system. The interplay between the biophysical features of oligomers/fibrils and cell membrane defines amyloid toxicity. Biophys Chem. 2013;182:30–43. doi: 10.1016/j.bpc.2013.06.003. [DOI] [PubMed] [Google Scholar]