Abstract
We previously showed that axoplasmic organelles from the squid giant axon move toward the barbed ends of actin filaments and that KI-washed organelles separated from soluble proteins by sucrose density fractionation retain a 235-kDa putative myosin. Here, we examine the myosin-like activities of KI-washed organelles after sucrose density fractionation to address the question whether the myosin on these organelles is functional. By electron microscopy KI-washed organelles bound to actin filaments in the absence of ATP but not in its presence. Analysis of organelle-dependent ATPase activity over time and with varying amounts of organelles revealed a basal activity of 350 (range: 315–384) nmoles Pi/mg/min and an actin-activated activity of 774 (range: 560–988) nmoles/mg/min, a higher specific activity than for the other fractions. By video microscopy washed organelles moved in only one direction on actin filaments with a net velocity of 1.11 ± .03 μm/s and an instantaneous velocity of 1.63 ± 0.29 μm/s. By immunogold electronmicroscopy, 7% of KI-washed organelles were decorated with an anti-myosin antibody as compared to 0.5% with non-immune serum. Thus, some axoplasmic organelles have a tightly associated myosin-like activity.
Keywords: motility, organelles, myosin
INTRODUCTION
Because little protein synthesis occurs in the axon or in its synaptic terminals, proteins used in the axon must be synthesized in the cell body and transported down the axon [Grafstein, 1995]. The giant axon of the squid has provided an ideal system to study the molecular mechanisms of axonal transport of membrane proteins because its enormous diameter allows pure axoplasm to be extruded from the axon in large enough quantities, 1–1.5 μ/cm of axon, for both biochemical analyses and assay of movements by video microscopy [Vale et a1., 1985b; Allen et a1., 1982; Brady et al., 1982]. The mechanisms of transport in axoplasm and the protein motors that mediate them turn out to be common to many cell types. Studies of organelle transport in the giant axon of the squid have revealed universal mechanisms mediated by homologous classes of proteins [Vale et a1., 1986].
Microtubule motors are thought to mediate the fast transport of membrane-bound vesicles to and from the cell body of both axons and dendrites of neurons [Elluru et al., 1995; Vallee and Bloom, 1991]. However, evidence is accumulating indicating that microtubule based motors are not the whole story for organelle transport. Injection of gelsolin, a calcium-activated actin filament–severing protein, disrupts axoplasmic transport [Brady et al., 1984]. More recently, organelles in axoplasm have been shown to move on invisible tracks presumed to be axoplasmic actin [Kuznetsov et al., 1992] and to interact directly with and move along exogenous actin filaments [Bearer et al., 1993; Langford et al., 1994]. Squid ganglia are known to contain at least one myosin [See and Metuzals, 1976] which, however, has been proposed to form thick filaments and to have no native association with organelles. There are likely to be a number of other myosins present in axoplasm [Bement et al., 1994] any of which could associate non-specifically with organelles and mediate motility in vitro. Thus, it remains to be seen whether the actin-based motility in extruded axoplasm is due to an organelle-associated actin-based motor, or to non-physiologic adherence of axoplasmic myosins to organelles occurring during extrusion.
To test whether organelles have a tightly associated actin-based motor, it is necessary to separate them from other axoplasmic myosins and show that they retain the ability to move on actin filaments. Organelles can be separated from soluble axoplasmic proteins by sedimentation through a sucrose density gradient [Schroer et al., 1988]. Solubilization of axoplasm in 0.6 M KI prior to fractionation dissociates filamentous components so that they are not heavy enough to enter the gradient. Furthermore, passage through 0.6 M potassium iodide (KI) renders non-specific association of soluble myosins with organelles unlikely. Such a KI step is routinely used in the solubilization of myosins, permitting separation from actin and other cytoskeletal proteins [Pollard, 1982]. Organelles prepared this way retain kinesin and the ability to move toward the plus ends of microtubules, but lose retrograde motility [Schnapp et al., 1992]. We have previously shown that KI-washed organelles co-sediment with a 235-kDa protein recognized by an anti-myosin antibody, whereas five other putative myosins remain in the supernatant [Bearer et al., 1993]. The next step is to determine whether these isolated organelles have myosin-like enzymatic activity such as ATP-sensitive actin binding, actin-activated ATPase activity, and motility on actin filaments. If they do, then a likely candidate for the motor would be the 235-kDa protein, since no other myosin has yet been detected on isolated organelles. Here we show that some KI-washed fractionated organelles display myosin-like activities and are decorated by the anti-myosin antibody.
MATERIALS AND METHODS
Squid (Loligo pealeii) and horseshoe crabs (Limulus polyphemus) were obtained through the Marine Resources Center at the Marine Biological Laboratory, Woods Hole, MA. Squid giant axons were dissected in Ca-free seawater and stored in liquid nitrogen until use.
Isolation of Organelles
Axoplasm was extruded from thawed axons and triturated in 50–100 ml of 1/2 × motility buffer [Vale et al., 1985a,b] containing 1 mM DTT and the protease inhibitors benzamidine, leupeptin, pepstatin, and aprotinin, all at 10 μg/ml [Bearer, 1991, 1995]. To release proteins and organelles from the dense axoplasmic matrix, extruded axoplasm was incubated in 0.6 M KI in 1/2 × buffer for 10 min followed by 1:1 dilution in 1/2 × containing protease inhibitors [Schroer et al., 1988; Schnapp et al., 1992]. KI-treated axoplasm layered on top of a three-step sucrose gradient (45%, 15%, 12%) was centrifuged at 180,000g for 1.5 h at 4°C in a 50.1 SW Beckman rotor. To isolate KI-washed organelles for in vitro motility assays, 100 μl of axoplasm from 50–70 cm of axons were used. Motility assays were performed within hours of extrusion. Fractions were removed by side puncture according to markings made on the tubes at the sucrose interfaces before centrifugation. Concentration of protein in each fraction was determined by Bradford assay (BioRad, Hercules, CA).
Acrosomal Processes
Acrosomal processes were isolated from horseshoe crab sperm [Tilney et al., 1981] followed by an additional 1O-min incubation in 1 M KCl on ice to eliminate flagellae, centrifugation for 7 min at 15,000g and resuspension in assay buffer (AB: 25 mM Imidazole, 25 mM KCl, 4 mM MgCl2, 1 mM EGTA, pH 7.4). Electron microscopy (EM) of thin sections and negatively stained preparations confirmed that flagellae were absent.
Binding of Organelles to Actin Filaments
Actin was nucleated off fresh acrosomal processes on EM grids [Pollard, 1986]. After polymerization, grids were washed and blocked by flotation for 5 min on droplets of polymerization buffer (PB: 25 mM Imidazole, 50 mM KCl, 1 mM EGTA and 2 mM MgCl2 [Pollard, 1986]) containing 0.04% Carnation Instant Milk. Grids were then floated on a drop of 15% sucrose fraction diluted 1:1 in 1/2 × buffer or AB with or without 2 mM ATP. After 10 min, grids were passed through two 1O-μl droplets of 1 mg/ml bacitracin (Sigma, St. Louis, MO) and then stained in 2% uranyl acetate. In controls, no acrosomal processes or polymerized actin (AP-actin) was used, and grids were simply blocked and exposed to organelles. Additionally, organelles were treated with and without ATP and examined by negative stain. This latter control experiment demonstrated that the ATP had no effect on organelle stability or morphology. To determine the numbers of organelles bound to actin, 100 plumes were selected at random at a magnification too low to visualize organelles, the magnification was increased, and the numbers of organelles adherent to the plume were counted. Results are from three separate experiments.
ATPase Assay
ATPase activity was measured at low actin concentration according to Bearer et al. [1993]. Soluble phosphate was measured colorimetrically [Taussky and Shorr, 1953]. In a second method, ATPase activity of 10-μl aliquots of fractions was measured using 50 μl of a 5 mg/ml actin stock and ATPase buffer in a volume of 1 ml at RT [Cheney et al., 1993a]. Amounts of released phosphate were measured after 30 min. Activity was normalized for the total amount of protein present as determined by the Bradford assay (BioRad, Hercules, CA). Specific activity of the organelle fraction was more precisely defined by measuring phosphate release over 10 min with 350 μg of actin for increasing amounts of organelles: 10, 20, 50, and 100 μl of the 15% fraction containing from 0.4 to 0.5 mg/ml of protein. Ten minutes was chosen as the optimal time point for these measurements according to a time course of phosphate release for 20 μl (10 μg) of organelle fraction at 2, 5, 10, 15, and 30 min which showed that phosphate release increased linearly up to 10 min and then leveled off.
Motility Assays
Rabbit muscle actin purified to 99.9% purity by sodium dodecyl sulfate (SDS) gels [Pardee and Spudich, 1982] was polymerized off the ends of acrosomal processes in solution [Bearer et al., 1993]. To acquire longer filaments, the actin concentration was increased to 20 μM, the incubation time was increased to 18 h, and phalloidin was added at least 4 h prior to use at a final concentration of 0.3 μM.
For video microscopy, 5 μl of AP-actin was perfused into a narrow chamber and allowed to adhere for 5 min. After two washes with 5 μl of PB containing 0.04% Carnation Instant Milk, 10 μl of the 15% fraction from the sucrose gradient to which 1 μl of 0.1 M ATP had been added was introduced into the chamber. Chambers were observed immediately by either a Nikon inverted microscope equipped with a 100× lens with a 1.4 N.A. and Nomarski optics or a Zeiss inverted scope similarly equipped [Bearer et al., 1993]. Images were captured onto Beta Max video tape after enhancement by a Hamamatsu Argus 50. For combined fluorescence-DIC, AP-actin was incubated with rhodamine-labeled phalloidin and imaged through a Xybion image intensifier. All processes visible by DIC were also detected by phalloidin fluorescence. For velocity measurements sequences were transferred to an optical memory disc recorder (Panasonic) and measured frame by frame on a monitor.
Immunogold Cytochemistry
KI-washed organelles were obtained from the 15% sucrose fraction as described above. Glow-discharged carbon-Formvar-coated copper grids were incubated on a 2-μl droplet of the organelle sample for 1 min followed by blocking with 0.1% bovine serum albumin (BSA) in 1/2× motility buffer. Blocked grids were incubated on drops of 1:50, 1:100, or 1:200 dilution of anti-myosin antibody for 4 h, washed 3 times for 5 min each, and then incubated for 1 h on drops of 1:10 dilution of protein A gold (Auroprobe EM Protein A G15, Amersham Corp., Arlington Heights, IL). These washes and dilutions were all in BSA blocking buffer. Non-immune antibody and protein A gold without primary antibody were used in parallel to process grids for each experiment. Samples were washed in Tris-buffered saline (20 mM Tris-HCl, pH 7.0, and 0.85% saline) 5 times for 5 min each, stained in 1% aqueous uranyl acetate, viewed, and photographed with a JEOL 200-CX.
For each data point ten fields were selected in the electron microscope at magnifications too low to visualize the gold particles, photographed at 27,000×, and printed at 75,000×. Fifty to 100 organelles (membrane-bound particles) were selected at random in the field on the photomicrograph, and gold particles on them were counted. The result for each data point represents the percent of organelles in ten micrographs marked by one or more gold particles.
RESULTS
ATP-Sensitive Actin Binding of KI-Washed Organelles
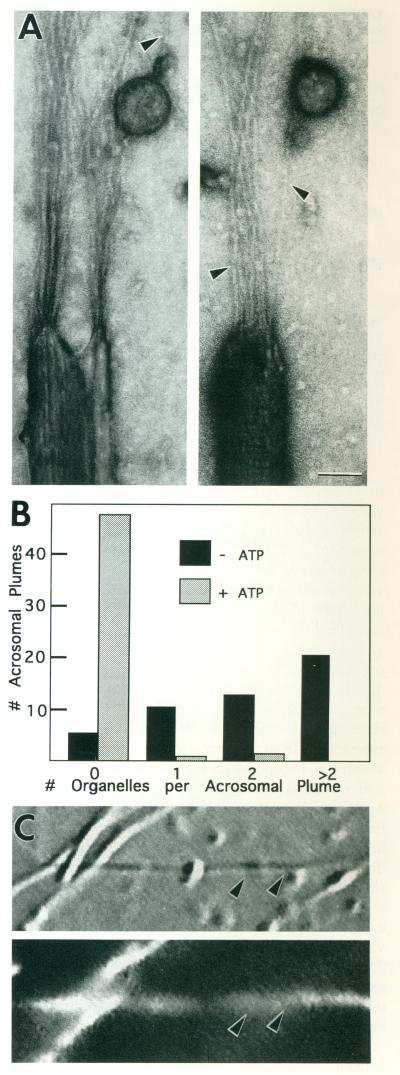
Organelles were separated from other soluble components of axoplasm by a three-step sucrose density gradient after suspension of the extruded axoplasm in 0.6 M KI. Organelles are selectively enriched in the 15% fraction of such a gradient and have been shown to retain kinesin and to move in the plus ends of microtubules [Schroer et al., 1988; Schnapp et al., 1992]. KI-washed organelles from this 15% fraction, hereafter referred to as “washed organelles,” bound to actin filaments polymerized off Limulus sperm acrosomal processes adherent to an electron microscope grid [Tilney et al., 1981; Bonder et al., 1983; Pollard, 1986]. These filaments served as substrate for organelle binding studies. After blocking, grids carrying polymerized actin were floated onto droplets of organelles with or without ATP for 15 min, washed, and negatively stained. In the absence of ATP, organelles decorated the newly polymerized actin filaments but not the surface of the grid nor the acrosomal process (Fig. 1A). In the presence of ATP, virtually no organelles were found on the plumes. Counts of organelles on actin plumes showed that a marked increase in the association of organelles with actin filaments occurred in the absence of ATP (Fig. 1B).
Fig. 1.
ATP-sensitive binding of KI-washed organelles to acrosomal plumes. A: Actin filaments nucleated off the actin bundle of acrosomal processes of Limulus sperm on EM grids were incubated with mM Mg-ATP. In the absence of ATP, membrane-bound vesicles were frequently associated with the newly polymerized actin filaments (arrowheads) extending in a plume from the end of individual acrosomal processes. Scale bar = 50 nm. B: The histogram compares the number of organelles attached to plumes selected at random from preparations with and without ATP. Assays were performed in 1/2× motility buffer [Vale et al., 1985a]. C: DIC (top) and corresponding fluorescence (bottom) image of a field of rhodamine-phalloidin–stained acrosomal processes with actin filaments polymerized off their ends. Example shows a typical preparation containing a type of long actin-containing plume (arrowheads) developed for the motility assay (Fig. 2).
Actin-Activated Mg-ATPase Activity of the Organelle Fraction
Myosin hydrolyzes ATP, harvesting the chemical energy to produce movement along actin filaments. Myosin is the only known enzyme to increase its rate of ATP hydrolysis in the presence of actin filaments. Thus, enhanced hydrolysis of ATP by the addition of actin and magnesium ions in low salt demonstrates the presence of active myosins in mixtures of proteins [Pollard, 1982]. We measured actin-activated ATPase activities across the four fractions of the sucrose gradient: the cytosol, containing the solublized proteins that fail to enter the gradient; the 12% fraction; the 15% fraction enriched in organelles; and the 45% fraction which includes the pellet. Total protein was also measured in order to calculate specific-activities of each fraction. As expected after KI solubilization, the majority of the protein was found in the cytosol (200 μl of 2.5 mg/ml, or 62% of total protein), whereas the 12% sucrose fraction had 26% (200 μl of 1.1 mg/ml). In contrast, the organelle-containing 15% fraction had only 100 μl of 0.1 mg/ml, 1% of the total, whereas the 45% sucrose cushion had 60 μl of 1.4 mg/ml, 10% of the total. In three separate fractionation experiments, the total protein in the organelle fraction varied from 0.1 to 0.5 μg/ml.
All fractions had a significant baseline ATPase activity in the absence of actin. Addition of small amounts (50 μg) of actin moderately enhanced activity in all fractions (five experiments). The cytosol had the highest proportional increase with addition of actin (1.7-fold increase), whereas the phosphate released by each of the three sucrose fractions increased 1.2–1.3-fold. With addition of fivefold more actin (250 μg), specific activity (nmol Pi released/mg protein/min) increased by 1.1-, 2.5-, 6.3-, and 8-fold over baseline for cytosol and the 12%, 15%, and 45% fractions, respectively (Table I).
TABLE I.
Specific Activities of Actin-Dependent Mg-ATPase in Sucrose Density Fractions of Axoplasm*
| Protein source | nmol Pi released/ 30 min |
μg prteina |
Specific activity (nmo1Pi/mg/min) |
|---|---|---|---|
| Cytosol | |||
| −actin | 21 | 25 | 28 |
| + actin | 23 | 25 | 31 |
| 12% sucrose | |||
| −actin | 11.6 | 11 | 35 |
| + actin | 28.4 | 11 | 86 |
| 15% sucroseb | |||
| −actin | 4 | 1 | 132 |
| + actin | 25 | 1 | 834 |
| 45% sucrose | |||
| −actin | 2.1 | 1.4 | 50 |
| + actin | 16.8 | 1.4 | 400 |
Samples with (+) or without (−) 250 μg of actin were incubated for 30 min in 30 mM HEPES (pH 7.6), 20 mM NaCl, 2 mM MgCl2, 4 mM EGTA, and 10 mM Mg-ATP. Phosphate release in control tubes containing buffer plus ATP (for −actin) or buffer plus ATP and actin (for +actin) was subtracted from experimental values.
Protein concentrations determined by Bradford assay.
Organelle fraction.
Since the amount of protein in the organelle fraction was very low compared to the other fractions of the gradient, baseline measurements of phosphate release shown in Table I are at the limit of detection for the organelle fraction. We therefore performed two series of experiments to determine more accurately the specific activities of the organelle fraction. First, dependence of phosphate release upon the amount of organelle protein was measured by comparing phosphate release with and without 350 μg of actin with 3.4- and 6.8-fold more organelle protein than that used in Table I. Buffer conditions, temperature, and total volume of the reaction were as described in Table I. Phosphate enzymatically released by the organelle fractions was determined by subtracting from all experimental measurements the amount of phosphate present in the organelle fractions without added ATP. Furthermore, phosphate contributed by the added ATP was subtracted from measurements of baseline phosphate, and that contributed by ATP plus actin was subtracted from actin-activated measurements. This analysis yielded a mean baseline activity of 350 (range: 315–384) nmoles Pi/mg/min and actin-activated activity of 774 (range: 560–988) nmoles/mg/min (two experiments), an increase of ~2.2-fold. This degree of variability is not surprising since these experiments used 250 cm of squid axons representing approximately 40 axons from 20 individuals collected at different times over the summer when variability in axon diameter and organelle motility is known to occur.
Second, a 30-min time course of phosphate release with and without 350 μg of actin in 20 μl of the organelle fraction containing 5 μg of protein was obtained (two experiments). Data points were collected at 2,5, 10, 15, and 30 min, and absorbance measurements were again corrected for the contributions of organelle fraction alone, ATP, and ATP plus actin. Baseline phosphate release increased linearly for 10 min from 25 (range: 20–30) to 44 (40–47) nmoles and then continued to increase, although at a slower rate, to 55 (50–59) nmoles at 30 min. Addition of actin substantially increased the phosphate released at early time points (2.6-fold at 2 min), but had less of an effect at later times (1.7-fold at 30 min).
Washed Organelles Move on Actin Filaments
Native organelles in diluted axoplasm move toward the barbed ends of plumes of actin filaments nucleated off the acrosomal process of Limulus sperm [Bearer et al., 1993; Langford et al., 1994]. Measurements of organelle velocity on actin plumes is complicated by their short length (~1 μm) and by the tangled actin filaments in the plume which prohibit excursions over long distances. Although acrosomal process preparations are reported to be free of flagellae, there were some in our preparations. These flagellar microtubule contaminants were abolished by washing acrosomal processes in 1 M KCl before resuspension in assay buffer, as determined by electron microscopy of KCl-treated preparations by both negative stain and by thin section. Although this treatment removed microtubules, it did not dissociate the acrosomal actin bundles, but did fray them slightly [Bullitt et al., 1988]. To increase their lengths, actin plumes were nucleated off the ends of aerosomal processes in 20 μM actin for 18 h, followed by stabilization in phalloidin for 4 h. The plumes stained strongly with rhodamine phalloidin showing that they contained actin (Fig. 1C). All filaments seen by DIC were also fluorescent, showing that none of the visible filaments were microtubules. Electron microscopy of negatively stained preparations showed that the extended plumes were composed of parallel arrays of long actin filaments (not shown). At this high concentration of actin, filaments arise from both ends of the aerosomal processes. Thus, these plumes are not useful for determining the direction of movements.
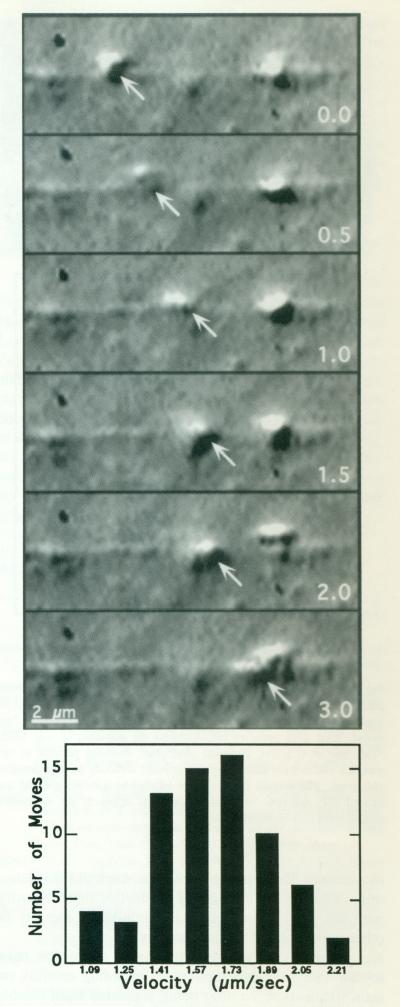
KI-washed organelles, like all myosins studied so far [Cheney et al., 1993b], moved in only one direction on any particular actin plume (Fig. 2), presumably toward the barbed end [Bearer et al., 1993; Langford et al., 1994]. Organelles moved discontinuously, with numerous short stops and starts—the overall velocity of five different organelles moving on the same process over a distance of 24 μm was 1.11 μm/s (±0.03 μm). When only moves continuous throughout a sequence of ten video frames were measured, the velocity was 1.63 μm/s (±0.29 μm) as computed from 69 individual video sequences (Fig. 2, lower panel). We attribute the discontinuous aspect of the movement to interactions of the organelle with other actin filaments in the substrate; these interactions appear to account for the even slower overall movements previously reported [Bearer et al., 1993].
Fig. 2.
KI-washed organelles move on actin filaments. Upper: High-resolution DIC video sequence of a KI-washed organelle moving along an actin plume. This organelle moves, slows down at 1.5 s, and then speeds up again. Overall, it moves approximately 3 μm in 3 s. The actin plume was nucleated off the end of a KCl-washed acrosomal process, KI-washed, isolated organelles in 1/2× buffer were combined with pre-formed actin filaments in polymerization buffer containing ATP. The numbers in the right lower corner of each image represent the time in seconds. Lower: Movements of seven individual organelles moving on two different extended actin plumes were analyzed by measuring the distanced traveled during a ten-frame sequence in order to determine an instantaneous as opposed to an overall velocity. The ten frames were selected for inclusion if the organelle moved continuously from the first through the tenth frame; 69 such sequences were measured. Velocities were divided into categories according to the smallest incremental distance that could be measured on the video screen.
Immunocytochemistry of Organelles With Anti-Myosin Antibody
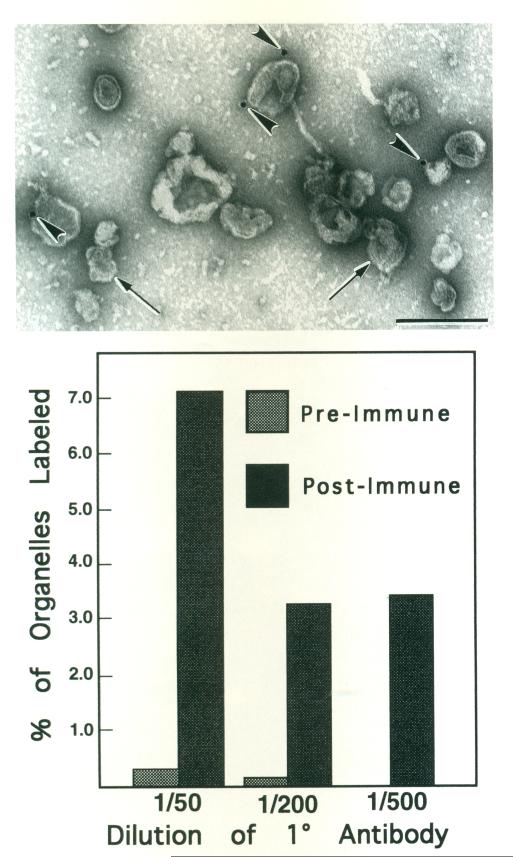
We previously reported that organelles are labeled by a polyclonal antibody to scallop muscle myosin II heavy chain [Bearer et al., 1993; Walliman and Szent-Györgyi, 1981], but the proportion of organelles labeled was not analyzed. Here KI-washed organelles attached to surfaces of electron microscope grids were treated with the anti-myosin antibody followed by protein A-gold (Fig. 3, top panel). A small number of organelles, recognized by their limiting membrane, had one to two gold particles closely apposed to their surfaces. Only 7% of the organelles were marked by one or more gold particles, even at the highest concentration of primary antibody (1:50). In contrast, negligible labeling occurred with non-immune antiserum.
Fig. 3.
Colloidal gold labeling of KI-washed isolated organelles with anti-myosin antibody. Top: Micrograph of a representative field of negatively stained organelles defined by their limiting membrane (arrows). Four gold particles (arrowheads) are apparent in this field. Scale bar = 0.5 μm. Bottom: Histogram showing percent of organelles which were labeled by anti-myosin antibody or non-immune antiserum. The percent of organelles labeled by anti-myosin did not exceed 7%. Whereas very little label was apparent on organelles treated with non-immune antiserum.
DISCUSSION
Organelles in the squid giant axon appear to have a myosin on their surfaces because they move along actin filaments in axoplasmic spreads [Kuznetsov et al., 1992; Bearer et al., 1993]. The myosin II heavy chain antibody used in our studies recognizes several bands in squid ganglia and five bands in axoplasm, one of which copurifies with the KI-washed organelles described here [Bearer et al., 1993]. This ~235-kDa band, which might share features of the myosin head domain with myosin II, could well represent the motor for these organelle movements. However, the previous work did not determine whether the KI-washed organelles that co-purify with this putative myosin retain actin-based motility or other indications of myosin activity.
Here we show that fractionated organelles have myosin-like enzymatic activities, including motility on actin filaments, even after being separated from soluble myosins and most other axoplasmic proteins by differential centrifugation in the presence of potassium iodide (KI). Loading solublized axoplasm onto the gradient in the presence of KI decreases the likelihood that the sticky surfaces of KI-washed organelles would attract non-specific interactions with solubilized myosins. The sucrose gradient then acts as a physical barrier between the organelles that sediment into it and the soluble proteins, including myosins, that do not. Thus, in contrast to previous studies of organelle motility where extraneous proteins, including myosins not normally associated with organelles, could associate with them as they diffuse through unfractionated proteins out to the periphery of an axoplasmic spread [Langford et al., 1994], or where the axoplasm was simply diluted and triturated [Bearer et al., 1993], the present experiments show that myosin-like activity associated with the organelle fraction is likely to be tightly associated with organelles.
Three types of experiments demonstrate that the KI-washed organelles have myosin-like activity. First, the KI-washed organelles bind actin filaments in an ATP-sensitive manner. Yeast mitochondria also display this behavior, which appears to be functionally significant, because mitochondria are abnormally distributed in yeast mutants lacking actin filaments [Lazzarino et al., 1994]. Second, the organelle fraction shows a significant basal ATPase activity independent of actin. This activity is further enhanced (approximately twofold) by addition of actin. The high basal level (350 nmoles/mg protein/min) can be attributed to the presence of mitochondria and vesicles carrying ion transporters as well as to the presence of some endogenous actin, which would activate ATPase activity independent of additional exogenous actin. For this reason, the twofold increase in ATpase activity with addition of actin is likely to represent only a fraction of the true actin-dependent activity in this fraction. Furthermore, the buffer conditions we used, although optimal for chick myosin V [Cheney et al., 1993a], may not be optimal for the squid organelle myosin. Finally, any loss of light chains during KI solubilization could effect ATPase activity of myosins [Collins et al., 1990; Tan et al., 1992].
Third, the motility of the KI-washed organelles shows that they have an active actin-base,d motor on their surfaces, further supporting the 235-kDa protein as the organelle motor, since it is currently the only identified myosin on these organelles [Bearer et al., 1993]. The anti-myosin antibody used to detect this 235-kDa band was raised against the heavy chain of scallop striated muscle myosin II and recognizes a wide range of invertebrate myosin IIs, presumably cross-reacting with conserved epitopes in the head domain [Walliman and Szent-Györgyi, 1981]. However, this antibody is unlikely to recognize all unconventional myosins [Cheney et al., 1993b; Goodson and Spudich, 1993; Bement et al., 1994], although the fact that it detects five bands ranging from 150 to 240 kDa in squid axoplasm [Bearer et al., 1993] argues that it does recognize at least some unconventional myosins in addition to myosin II. Therefore, is possible that other myosins not recognized by this antibody are also present on these organelles.
The instantaneous velocity of KI-washed organelles on actin filaments (1.63 ± 0.3 μ/s) is the fastest yet reported for an unconventional myosin [Collins et al., 1990; Cheney et al., 1993a], but is within the range of myosin II [Kron and Spudich, 1986]. Since velocities of different myosins vary, the relatively uniform net velocity (1.11 ± .03 μm/s) of KI-washed organelles along actin filaments argues against their having more than one active myosin. A similar velocity was reported for organelle movements in axoplasmic spreads on invisible tracks that apparently represent endogenous actin filaments [Kuznetsov et al., 1992]. However, a velocity of 1.1 μm/s is half that of KI-washed organelles moving toward the plus ends of microtubules in vitro [Schnapp et al., 1992].
By immunogold cytochemistry, only 7% of organelles were labeled With the anti-myosin antibody, suggesting that not every organelle has a recognizable myosin on their surface. However, 7% is likely to be an underestimate of the actual number of organelles with myosins on their surfaces for several reasons. First, the antibody used, as mentioned above, is unlikely to recognize all unconventional myosins. Additionally, washing the organelles in KI would be expected to strip some of the myosin from their surfaces. Finally, during adherence to the grid, only half of the organelle surface is exposed for immunogold labeling. If surface proteins are mobile in the plane of the organelle membrane, they might slide around to the highly charged ionic environment on the attached side becoming inaccessible to the antibody.
Our results show that axoplasmic organelles have a tightly associated actin-based motor, probably a myosin, on their surfaces. Are there tracks for such a motor in the axon? Indeed, axoplasm contains a large amount of actin that is thought to be in the form of short (0.55- to 0.79-μm) filaments [Fath and Lasek, 1988]. Recently, we have found that long bundles of actin filaments run parallel to the microtubule bundles and interweave among them along the length of the squid giant axon (Bearer and Reese, unpublished). These could serve as tracks for myosin-powered organelle movements from the deep axoplasm out to the cortex of the axon, where in other cells oriented actin filaments are known to provide tracks to the cell surface [Fath and Burgess, 1993], or could be a substrate for the movements of organelles such as mitochondria along the length of the axon (Hollenbeck, in press). However, the net rate of actin-based movements of organelles in vitro reported here is 1.1 ± 0.03 mm/s, only half that of smaller organelles moving in the intact axon [Allen et al., 1982; Vale et al., 1985a]. It should not be assumed, however, that KI-washed organelles correspond to the small organelles seen in the intact axon. They could represent vesiculated endoplasmic reticulum, which is known to move on actin filaments in other systems [Kachar and Reese, 1988]. A necessary next step will be to determine the orientation and precise distribution of actin filaments in the axon as well as to identify which filaments serve as substrates for organelle motility.
ACKNOWLEDGMENTS
We thank John Chludzinski for help with the photography and Ben Greenfield, Bonnie Reese, and Kasia Hammar for help in the laboratory. Peter Bent and Jeff-Larson from Nikon Instruments and Philip Presley from Zeiss Instruments also provided help with microscopy, and Jorge Morreira helped with immunolabeling. We are particularly grateful to Andrew Szent-Györgyi, who has been unstintingly generous with his polyclonal antibody to scallop myosin II as well as with his advice. Finally, we would like to thank Paul Gallant and Ayse Dosemenci for their reviews. This work was supported in part by NIH GM47368 and Council for Tobacco Research grant 3192.
REFERENCES
- Allen RD, Metuzals J, Tasaki I, Brady ST, Gilbert SP. Fast axonal transport in squid giant axon. Science. 1982;218:1127–1128. doi: 10.1126/science.6183744. [DOI] [PubMed] [Google Scholar]
- Bearer EL. Direct observation of actin filament severing by gelsolin and binding by gCap39 and CapZ. J. Cell Biol. 1991;115:1629–1638. doi: 10.1083/jcb.115.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer EL. Cytoskeletal domains in the activated platelet. Cell Motil. Cytoskeleton. 1995;30:50–66. doi: 10.1002/cm.970300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer EL, DeGiorgis JA, Bodner RA, Kao AW, Reese TS. Evidence for myosin motors on organelles in squid axoplasm. Proc. Natl. Acad. Sci. U.S.A. 1993;90:11252–11256. doi: 10.1073/pnas.90.23.11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement WM, Hasson T, Wirth JA, Cheney RE, Mooseker MS. Identification and overlapping expression of multiple unconventional myosin genes in vertebrate cell types. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6549–6553. doi: 10.1073/pnas.91.14.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonder EM, Fishkind DJ, Mooseker MS. Direct measurement of critical concentrations and assembly rate constants at the two ends of an actin filament. Cell. 1983;34:491–501. doi: 10.1016/0092-8674(83)90382-3. [DOI] [PubMed] [Google Scholar]
- Brady ST, Lasek RI, Allen RD. Fast axonal transport in extruded axoplasm from squid giant axon. Science. 1982;218:1129–1131. doi: 10.1126/science.6183745. [DOI] [PubMed] [Google Scholar]
- Brady ST, Lasek RJ, Allen RD, Yin HL, Stossel TP. Gelsolin inhibition of fast axonal transport indicates a requirement for actin microfilaments. Nature. 1984;310:56–58. doi: 10.1038/310056a0. [DOI] [PubMed] [Google Scholar]
- Bullitt ESA, DeRosier DJ, Coluccio LM, Tilney LG. Three-dimensional reconstruction of an actin bundle. J. Cell Biol. 1988;107:597–611. doi: 10.1083/jcb.107.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney RE, O’Shea MK, Heuser JE, Coelho MV, Wolenski JS, Espreafico EM, Forscher P, Larson RE, Mooseker MS. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 1993a;75:13–23. doi: 10.1016/S0092-8674(05)80080-7. [DOI] [PubMed] [Google Scholar]
- Cheney RE, Riley MA, Mooseker MS. Phylogenetic analysis of the myosin superfamily. Cell Motil. Cytoskeleton. 1993b;24:215–223. doi: 10.1002/cm.970240402. [DOI] [PubMed] [Google Scholar]
- Collins K, Sellers JR, Matsudaira P. Calmodulin dissociation regulates brush border myosin I (110-kD calmodulin) mechanochemical activity in vitro. J. Cell Biol. 1990;110:1137–1147. doi: 10.1083/jcb.110.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellmu RG, Bloom GS, Brady ST. Fast axonal transport of kinesin in the rat visual system. Mol. Cell. Biol. 1995;6:21–40. doi: 10.1091/mbc.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath KR, Burgess DR. Golgi-derived vesicles from developing epithelial cells bind actin filaments and possess myosin-I as a cytoplasmically oriented peripheral membrane protein. J. Cell Biol. 1993;120:117–127. doi: 10.1083/jcb.120.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath KR, Lasek RJ. Two classes of actin microfilaments are associated with the inner cytoskeleton of axons. J. Cell Biol. 1988;107:613–621. doi: 10.1083/jcb.107.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson HV, Spudich JA. Molecular evolution of the myosin family: relationships derived from comparisons of amino acid sequences. Proc. Natl. Acad. Sci. U.S.A. 1993;90:659–663. doi: 10.1073/pnas.90.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstein B. Axonal transport: Function and mechanisms. In: Waxman SG, Kocsis JD, Stys PK, editors. The Axon. Oxford U. Press; New York: 1995. pp. 185–199. [Google Scholar]
- Kachar B, Reese TS. The mechanism of cytoplasmic streaming in Charcean algal cells: sliding of endoplasmic reticulum along actin filaments. J. Cell Biol. 1988;106:1545–1552. doi: 10.1083/jcb.106.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron SJ, Spudich JA. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc. Natl. Acad. Sci. U.S.A. 1986;83:6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov SA, Langford GM, Weiss DO. Actin-dependent organelle movement in squid axoplasm. Nature. 1992;356:722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Langford GM, Kuznetsov SA, Johnson D, Cohen DL, Weiss DG. Movement of axoplasmic organelles on actin filaments assembled on acrosomal processes: evidence for a barbed-end-directed organelle motor. J. Cell Sci. 1994;107:2291–2298. doi: 10.1242/jcs.107.8.2291. [DOI] [PubMed] [Google Scholar]
- Lazzarino DA, Boldogh I, Smith MG, Rosand J, Pon LA. Yeast mitochondria contain ATP-sensitive, reversible actin-binding activity. Mol. Cell. Biol. 1994;5:807–818. doi: 10.1091/mbc.5.7.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J. Cell Biol. doi: 10.1083/jcb.131.5.1315. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee JD, Spudich JA. Purification of muscle actin. Methods Enzymol. 1982;85:164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Pollard TD. Purification of Nonmuscle Myosins. Methods Enzymol. 1982;85:331–356. doi: 10.1016/0076-6879(82)85033-7. [DOI] [PubMed] [Google Scholar]
- Pollard TD. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J. Cell Biol. 1986;103:2747–2754. doi: 10.1083/jcb.103.6.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp BJ, Reese TS, Bechtold R. Kinesin is bound with high affinity to squid axon organelles that move to the plus-end of microtubules. J. Cell Biol. 1992;119:389–399. doi: 10.1083/jcb.119.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer TA, Schnapp BJ, Reese TS, Sheetz MP. The role of kinesin and other soluble factors in organelle movement along microtubules. J. Cell Biol. 1988;107:1785–1792. doi: 10.1083/jcb.107.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See YS, Metuzals J. Purification and characterization of squid brain myosin. J. Biol. Chem. 1976;251:7682–7689. [PubMed] [Google Scholar]
- Tan JL, Ravid S, Spudich JA. Control of nonmuscle myosins by phosphorylation. Annu. Rev. Biochem. 1992;61:721–759. doi: 10.1146/annurev.bi.61.070192.003445. [DOI] [PubMed] [Google Scholar]
- Taussky HH, Shorr E. A microcolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem. 1953;202:675–685. [PubMed] [Google Scholar]
- Tilney LG, Bonder EM, DeRosier DJ. Actin filaments elongate from their membrane-associated ends. J Cell Biol. 1981;90:485–494. doi: 10.1083/jcb.90.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985a;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD, Schnapp BJ, Reese TS, Sheetz MP. Movement of organelles along filaments dissociated from axoplasm of the squid giant axon. Cell. 1985b;40:449–454. doi: 10.1016/0092-8674(85)90159-x. [DOI] [PubMed] [Google Scholar]
- Vale RD, Scholey JM, Sheetz MP. Kinesin: possible biological roles for a new microtubule motor. Trends Biochem. Sci. 1986;11:464–468. [Google Scholar]
- Vallee RB, Bloom GS. Mechanisms of fast axonal transport. Annu. Rev. Neurosci. 1991;14:59–92. doi: 10.1146/annurev.ne.14.030191.000423. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Szent-Györgyi AG. An immunological approach to myosin light-chain function in thick filament linked regulation. 1. Characterization, specificity, and cross-reactivity of anti-scallop myosin heavy- and light-chain antibodies by competitive, solid-phase radioimmunoassay. Biochemistry. 1981;20:1176–1187. doi: 10.1021/bi00508a020. [DOI] [PubMed] [Google Scholar]