Abstract
Study Objectives:
To investigate the association between sleep disordered breathing (SDB) and severe chronic periodontitis.
Design:
Cross-sectional data analysis from the Hispanic Community Health Study/Study of Latinos.
Setting:
Community-based setting with probability sampling from four urban US communities.
Participants:
12,469 adults aged 18–74 y.
Interventions:
None.
Measurements and Results:
Severe chronic periodontitis was defined using the Centers for Disease Control and Prevention/American Academy of Periodontology case classification based on full-mouth periodontal assessments performed by calibrated dentists. SDB was evaluated in standardized home sleep tests, and defined as the number of apnea plus hypopnea events associated with ≥ 3% desaturation, per hour of estimated sleep. SDB was quantified using categories of the apnea-hypopnea index (AHI): 0.0 events (nonapneic); 0.1–4.9 (subclinical); 5.0–14.9 (mild); and ≥ 15 (moderate/severe). Covariates were demographic characteristics and established periodontitis risk factors. C-reactive protein was a potential explanatory variable. Using survey estimation, multivariable binary logistic regression estimated odds ratios (OR) and 95% confidence limits (CL). Following adjustment for confounding, the SDB and periodontitis relationship remained statistically significant, but was attenuated in strength and no longer dose-response. Compared with the nonapneic referent, adjusted odds of severe periodontitis were 40% higher with subclinical SDB (OR = 1.4, 95% CL: 1.0, 1.9), 60% higher with mild SDB (OR = 1.6, 95% CL: 1.1, 2.2) and 50% higher with moderate/severe SDB (OR = 1.5, 95% CL: 1.0, 2.3) demonstrating an independent association between SDB and severe periodontitis.
Conclusions:
This study identifies a novel association between mild sleep disordered breathing and periodontitis that was most pronounced in young adults.
Citation:
Sanders AE, Essick GK, Beck JD, Cai J, Beaver S, Finlayson TL, Zee PC, Loredo JS, Ramos AR, Singer RH, Jimenez MC, Barhart JM, Redline S. Periodontitis and sleep disordered breathing in the Hispanic Community Health Study/Study of Latinos. SLEEP 2015;38(8):1195–1203.
Keywords: apnea-hypopnea index, periodontal disease, Hispanic, survey, epidemiology
INTRODUCTION
Chronic periodontitis is an inflammatory disease initiated by the host immune response to dysbiotic biofilm in tissues surrounding the teeth. Progression is characterized by ulceration of gingival epithelium, loss of periodontal ligament attachment to the tooth surface, and destruction of the alveolar bone. Bleeding of ulcerated epithelium gives rise to bacterial invasion of connective tissue and recurrent transient bacteremia with higher circulating levels of proinflammatory and prothrombotic markers.1,2 The disease is associated with systemic chronic inflammatory disorders including type II diabetes, metabolic syndrome, atherosclerosis, and cardiovascular disease.3–8 Chronic periodontitis has public health significance as the major cause of tooth loss.9 In 2009–2010 prevalence of severe chronic periodontitis in the US population aged 30 y or older was 8.5%,10 based on the case classification jointly developed by the Centers for Disease Control and Prevention and the American Academy of Periodontology (CDC-AAP).11
Sleep disordered breathing (SDB) encompasses a continuum of upper airway obstructive abnormalities ranging from primary snoring to more severe forms such as obstructive sleep apnea (OSA). Moderate to severe OSA is causally implicated in the development of hypertension and, like periodontitis, is strongly associated with cardiovascular disease.
Several studies point to the comorbidity of these two diseases, but their evidence is limited by weaknesses in study design, measurement imprecision and small sample size.12–16 Nonetheless, the relationship has biological plausibility through at least three potential mechanisms. One mechanism is dry mouth due to oral breathing (extrinsic cause) or prescription medications (intrinsic cause). It is common for adults with SDB to breathe through their mouth and many patients report morning xerostomia, i.e., the subjective perception of dry mouth. During oral breathing, gravity pulls the tongue backward, restricting oropharyngeal region volume, increasing upper airway resistance17 and markedly increasing SDB severity. Dry mouth may reduce the effectiveness of bacterial clearance of the oral tissues, leading to greater colonization of periodontal microbiota. One of the few studies to examine the periodontal consequences of extrinsic xerostomia studied 2,077 healthy young adults, finding greater accumulation of biofilm and significantly more bleeding of gingival tissue on probing—two clinical signs of periodontal disease—among the 8.8% with xerostomia.18
A second potential mechanism linking SDB and periodontitis is oxidative stress. Even minor respiratory events are sufficient to disturb oxidative homeostasis and induce release of excess reactive oxygen species. Oxidative stress is a recognized feature of OSA19 and heightened oxidative stress in already inflamed periodontal tissues could augment the breakdown of periodontal attachment. Moreover, individuals with periodontitis have a decreased antioxidant capacity both locally and systemically20,21 to counter the harmful effects of oxidative stress. Periodontitis itself induces oxidative stress as a consequence of the release of excess proteolytic enzymes in an aberrant host inflammatory response to the bacterial challenge.22
A third potential mechanism is systemic inflammation. Both periodontitis and SDB involve local inflammation in the oral cavity and upper airway region. In addition, both involve systemic inflammatory processes measurable as elevated levels of proteins such as C-reactive protein.23–25 Because high-sensitivity CRP (hs-CRP) is a biomarker of systemic inflammation and is associated with SDB25 and periodontitis,24,26 it make play a role in the SDB and periodontitis relationship.
This study sought to build on previous investigations of this relationship in a large, well-designed population-based cohort of Hispanic/Latino adults using objective measures of SDB and examiner-verified periodontitis. Periodontitis is highly prevalent in this population relative to other racial/ethnic groups. Nationally representative data from NHANES 2009– 2010 estimate severe periodontitis prevalence is 6.3% among non-Hispanic whites, 13.2% among non-Hispanic Blacks, and 17.3% among Mexican Americans.10 The primary aim of this cross-sectional analysis was to estimate the strength of association between SDB and chronic periodontitis across a range of SDB severities. The secondary aim was to investigate C-reactive protein in this relationship. We hypothesized that a positive relationship between SDB and chronic periodontitis might be explained by elevated C-reactive protein levels.
METHODS
Study Population and Recruitment
The Hispanic/Latino Community Health Study/Study of Latinos (HCHS/SOL) is a multisite, population-based prospective cohort study designed to evaluate disease burden and health risk factors in the diverse, rapidly growing Hispanic/Latino population. Details of the study design27 and sampling methods28 have been described. In summary, sampling used a two-stage area household probability design. Screened individuals who self-identified as Hispanic or Latino were recruited from a random sample of households in neighboring census tracts of four cities with large Hispanic/Latino populations (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA). The major heritage groups were Mexican (n = 6,471), Puerto Rican (n = 2,728), Cuban (n = 2,348), Central American (n = 1,730), Dominican (n = 1,460), and South American (n = 1,068).
Sample weights were adjusted for nonresponse and were calibrated to the 2010 US Census for age, sex, and Hispanic background, so that estimates were generalizable to census tracts sampled in these four urban communities. Between 2008 and 2011 approximately 4,000 people aged 18–74 y at the time of screening were recruited from each study site to yield a final sample size of 16,415 individuals. This study was approved by the Institutional Review Boards at each field center where all subjects gave written consent.
Interviews, Clinical Examination, and Laboratory Procedures
Questionnaires obtained information about demographic characteristics and cigarette smoking. Height was measured to the nearest centimeter and body weight, measured with the Tanita analyzer (Tanita Corp. of America, Skokie, IL), to the nearest 0.1 kg. Blood samples, including plasma glucose (fasting and after a 2-h oral glucose load) were collected according to standardized protocols. Following a ≥ 8 h fast, all participants had a 2-h oral glucose tolerance test (OGTT) except those with fasting plasma glucose (FPG) > 150 mg/dL and in whom diabetes had been previously diagnosed. Diabetes was defined as either FPG ≥ 126 mg/dL (7 mmol/L), 2-h OGTT ≥ 200 mg/dL (11.2 mmol/L), glycosylated hemoglobin (A1C) level ≥ 6.5% (48 mmol/mol), or use of hypoglycemic agents according to scanned/transcribed antidiabetic medications. Participants with glucose levels elevated above normal, but not meeting criteria for diabetes, were classified as pre-diabetic based on impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) using the following criteria. IFG was defined as FPG of 100–125 mg/dL (5.6–5.9 mmol/L). IGT was defined as 2-h plasma glucose (2-h PG) on the 75-g OGTT of 140–199 mg/dL (7.8–11.0 mmol/L). High-sensitivity C-reactive protein was measured in serum on a Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation, Indianapolis, IN) using an immunoturbidimetric method (Roche Diagnostics Corporation).
Periodontitis Assessment and Classification
Prior to the periodontal assessment, dentists assisted by trained recorders performed a count of remaining teeth.
Edentulous participants, i.e., those with no remaining teeth, and individuals requiring prophylactic antibiotic coverage for periodontal probing were ineligible for periodontal assessment. Examiners assessed probing depth (PD) and gingival recession at six sites per tooth (distal-facial, midfacial, mesial-facial, mesial-lingual, midlingual, and distal-lingual) for each fully erupted tooth present, except third molars. These parameters were used to calculate the clinical attachment level (CAL), which is the measure of loss of attachment from the tooth root surface and a marker of the extent of connective tissue destruction. Examiners at each site were calibrated for periodontal assessments so that measurements were comparable and were recalibrated together each year (2008–2010) against a gold standard referent who had participated in NHANES examinations. The mean interclass correlation coefficient (ICC), percent agreement, and Kappa statistic for periodontal pocket depth (PD) measures within 1 mm across all examiners were 0.95, 95.8, and 0.94, respectively and ranged from 0.90–0.96, 92.1–96.7, and 0.88–0.96, respectively between each examiner and the reference. The mean ICC, percent agreement, and kappa for clinical attachment level (CAL) within 1mm across all examiners were 0.86, 92.8, and 0.84, respectively and ranged from 0.56–0.93, 84.3–98.2 and 0.88–0.96, respectively between each examiner and the referent.
Severe periodontitis was defined according to the classification developed by CDC-AAP.10 It defines severe periodontitis as two or more interproximal sites with clinical attachment loss ≥ 6 mm (not on same tooth) and ≥ 1 interproximal site with pocket depth ≥ 5 mm. We also examined other definitions of periodontitis including mild and moderate levels of disease, extent scores, attachment loss, and probing pocket depth with and without bleeding. In all analyses the strength and direction of the relationships were very similar, irrespective of the choice of outcome and irrespective of whether it was measured on a continuous, ordinal, or binary scale. This consistency supports the validity of our findings.
Sleep Disordered Breathing
The protocol for evaluating SDB in the HCHS/SOL is described elsewhere.29 Briefly, participants were instructed in the use of the home sleep testing monitor—Apnea Risk Evaluation System (ARES Unicorder 5.2; B-Alert, Carlsbad, CA)—which they wore unsupervised for a single in-home overnight sleep test. This device is a limited-channel portable sleep monitoring device for evaluation of SDB. It measures blood oxygen saturation (SpO2) and pulse rate (reflectance pulse oximetry), airflow (by nasal cannula connected to a pressure transducer), snoring levels (calibrated acoustic microphone), head movement, and head position (accelerometers). Certified polysomnologists at a central reading center manually edited artifacts, identified periods of sleep, and annotated each respiratory event with its associated oxyhemoglobin desaturation. Respiratory events were identified as a ≥ 50% reduction in airflow lasting ≥ 10 sec; events were further annotated based on level of desaturation linked to each event. The apnea-hypopnea index (AHI) is the number of apneas plus hypopneas per estimated sleep hour. Interscorer and intrascorer reliability was excellent (AHI ICC = 0.99). Good agreement between the AHI measured by this monitor and by polysomnography (PSG) has been demonstrated in a prior validation study (rho = 0.70, 95% confidence interval (CI): 0.47–0.92).30
We examined multiple SDB parameters to establish the consistency of its relationship with severe periodontitis: the AHI with associated desaturations of greater or equal to 3% and 4%; total time spent in apnea/hypopnea events; percent of time that blood oxygen saturation level was < 90% of baseline level; snoring at varying thresholds (> 30, > 40, > 50, > 60 decibels); and total time spent in loud snoring. In this analysis we report results using the AHI derived as the average number of apnea and hypopnea events with oxygen desaturations ≥ 3% per estimated hour of sleep. AHI severity thresholds of 0 events (nonapneic); 0.1–4.9 (subclinical); 5.0–14.9 (mild); and ≥ 15 (moderate/severe) were applied, following a precedent set by the Wisconsin Sleep Cohort Study.31,32 Because only 4.1% of participants had ≥ 30 events per hour, severe SDB was not separately examined. We also assessed snoring sounds, classified as the percentage of time during which snoring levels exceeded 30 decibels (dB), divided into quartiles.
Statistical Analysis
Statistical analysis of these baseline HCHS/SOL data was conducted using Stata 13 SE 13.1 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). Survey estimation took account of the complex sampling design. Standard descriptive analysis characterized unadjusted associations between demographic and health characteristics with SDB categories and severe chronic periodontitis. Age-stratified analysis investigated potential confounding and effect modification. Because snoring was not significantly associated with severe periodontitis in models adjusted for age, snoring was not included in multivariable models. Using binary logistic regression, odds ratios (ORs) for severe periodontitis were modeled with their corresponding 95% confidence limits (CL) across SDB severity categories reported, using AHI = 0.0 as the referent. Model 1 fitted categories of AHI, adjusted for age. Model 2 additionally adjusted for sex and Hispanic/Latino background to which Model 3 added established periodontitis risk factors of smoking, diabetes, body mass index, and tooth loss along with hs-C-reactive protein as an explanatory variable. A final model added the product term of age*SDB categories and effect modification was determined by the Wald test. Predicted probabilities for SDB categories at specified ages, adjusted for covariates, were plotted to aid interpretation.
RESULTS
Of the 16,415 study participants, 1,946 either did not complete a sleep study or had insufficient sleep data for analysis. An additional 1,886 participants did not complete a periodontal assessment or were excluded from periodontal probing on medical grounds. An additional 114 were excluded for other missing data. In this analytic sample of 12,469 participants, weighted prevalence of severe periodontitis was 8.4%
Table 1 describes sociodemographic, behavioral, and health characteristics of the study target population. It is a young adult population, dominated by Mexican background. Lifetime non-smokers comprised nearly two-thirds of the sample. Less than a quarter had a body mass index within the healthy range and about half the sample was prediabetic or diabetic. Table 1 also describes the prevalence of SDB severity categories by participant characteristics. Approximately a quarter (24.3%) met minimal clinical criteria for OSA, defined as an AHI ≥ 5. In addition, 56.8% demonstrated subclinical disease (AHI 0.1–4.9). Illustrating the strong age-gradient in SDB, prevalence of moderate/severe OSA (AHI ≥ 15) increased tenfold from 2.5% among those aged 18–34 y to 23.0% among adults aged 55 y or older. Prevalence of moderate/severe OSA was twice as high in men (13.0%) as in women (5.8%). Prevalence of SDB at any severity level was at least twofold greater for adults with snoring, diabetes, obesity, or high hs-CRP than their healthy counterparts.
Table 1.
Relationship between sleep disordered breathing categories and demographic and health-related characteristics in the Hispanic Community Health Study/Study of Latinos, United States 2008–2011 (n = 12,469).
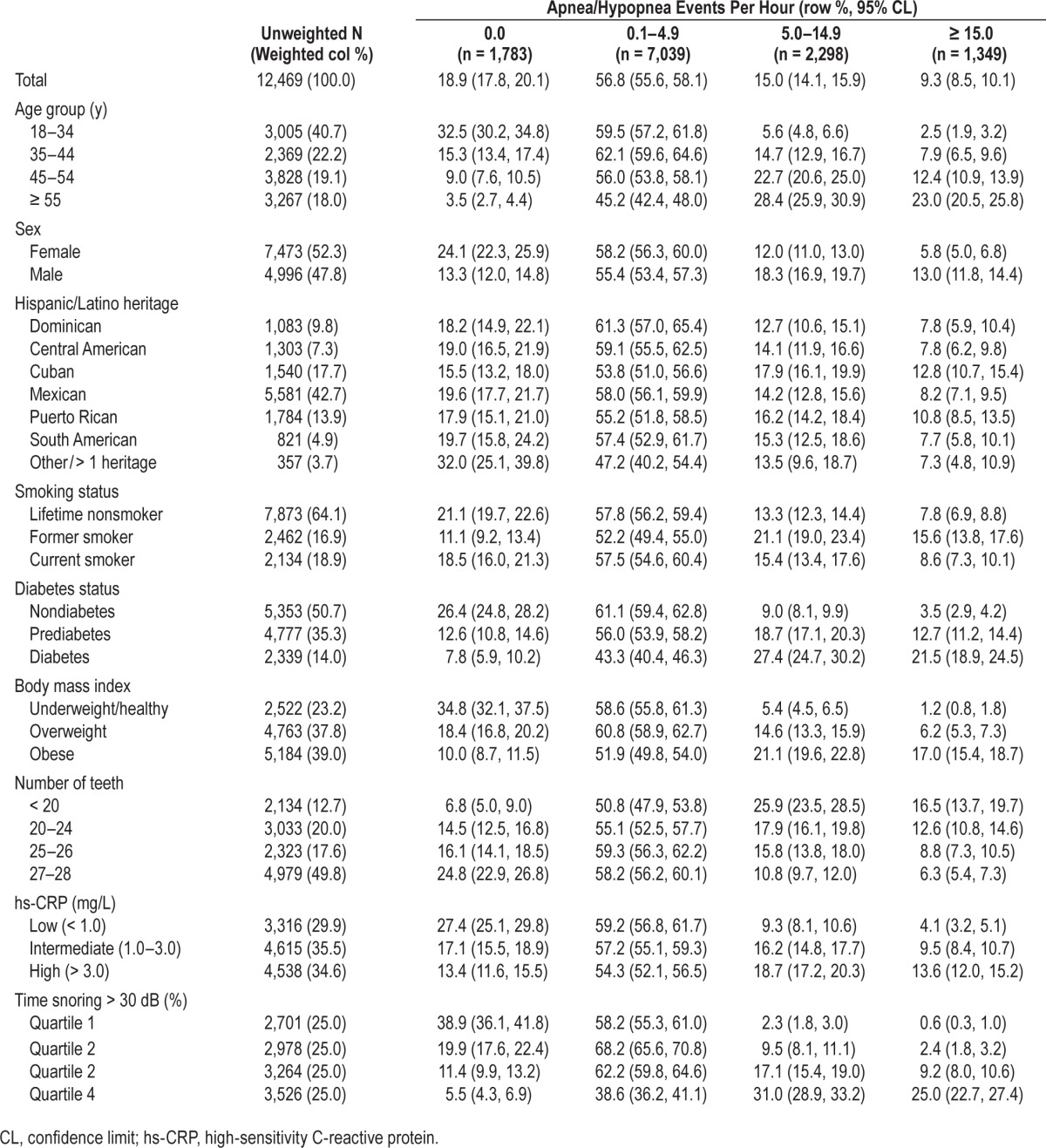
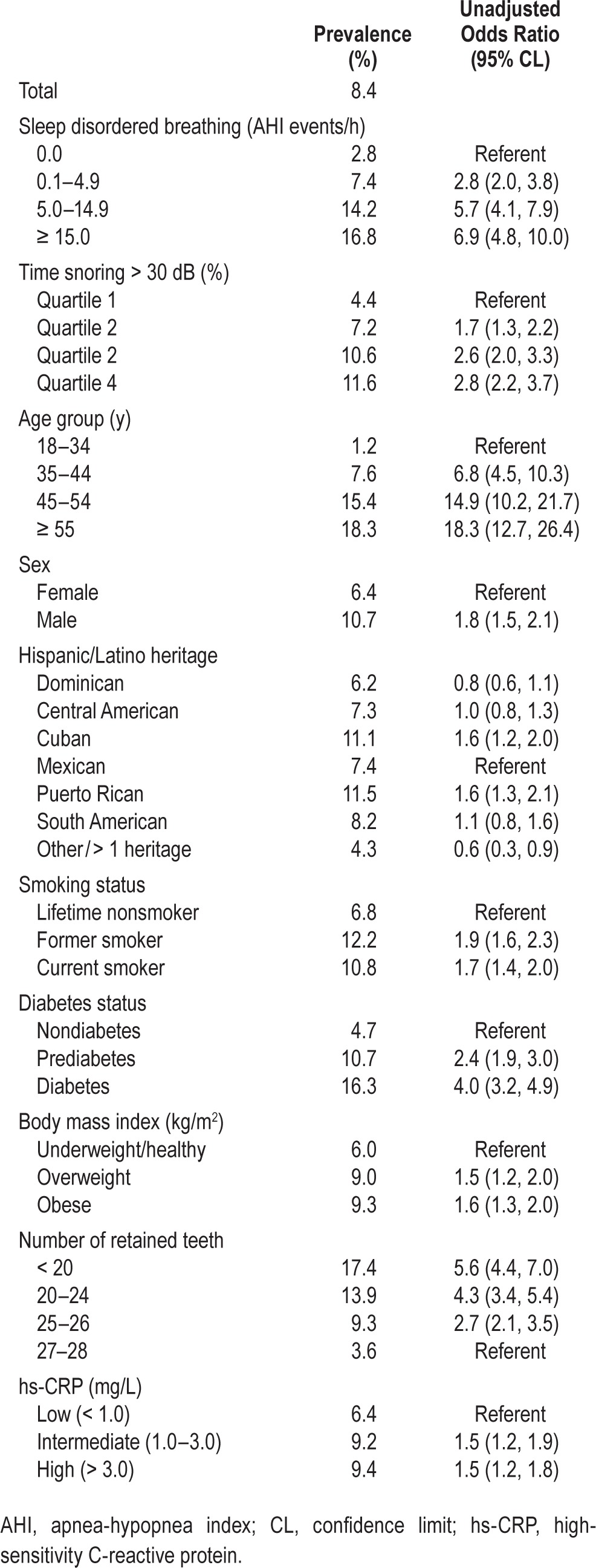
Table 2 depicts the relationship between participant characteristics and severe periodontitis prevalence. There was a positive relationship of a higher prevalence of severe periodontitis prevalence with increasing SDB severity (P < 0.001 in adjusted Wald test for trend). Crude odds of severe periodontitis were approximately sevenfold higher at an AHI ≥ 15 compared with AHI 0.0 (OR = 6.9, 95% CL: 4.8, 10.0). Snoring frequency was less strongly associated with severe periodontitis than AHI severity. Consistent with a well-established literature, severe periodontitis prevalence was associated with male sex, older age, diabetes and obesity, less tooth retention, and elevated C-reactive protein.
Table 2.
Unadjusted associations of demographic and health-related characteristics with prevalence (%) of severe periodontitis and odds ratios (95% confidence limits) for severe periodontitis, Hispanic Community Health Study/Study of Latinos, United States 2008–2011 (n = 12,469).
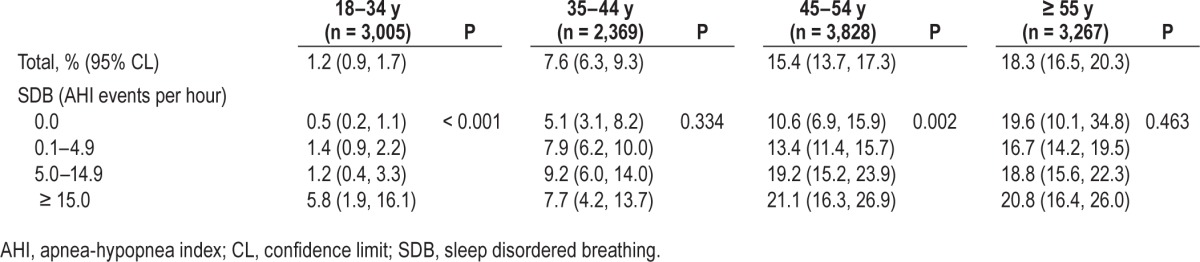
We investigated the presence of effect modification by sex and age. There was a significant effect modification by age (P < 0.001 for effect modification) (Table 3). The SDB and periodontitis relationship was strongest in the youngest stratum, aged 18–34 y, among whom severe periodontitis prevalence increased 11-fold from AHI 0.0 (0.5%) to AHI ≥ 15 (5.8%). There was no apparent relationship between SDB and severe periodontitis prevalence above the age of 55 y.
Table 3.
Prevalence (%) of severe chronic periodontitis (95% confidence limits) according to sleep disordered breathing, stratified by age group in the Hispanic Community Health Study/Study of Latinos, United States 2008–2011 (n = 12,469).
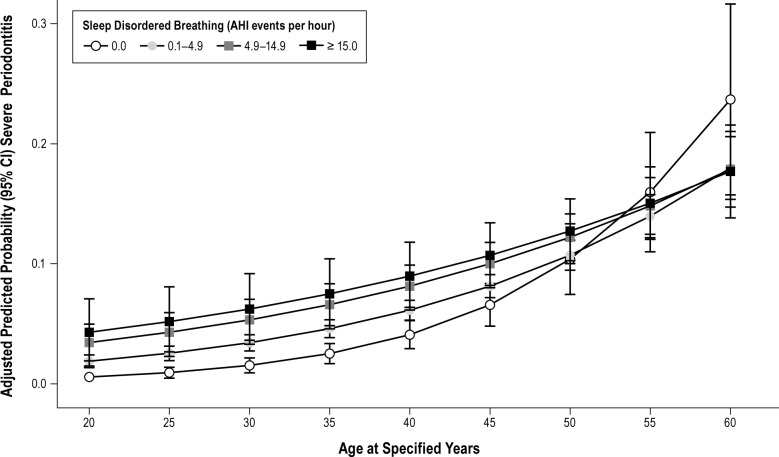
In multivariable analysis for all 12,469 subjects (Table 4), Model 1 estimated odds ratios of severe periodontitis for SDB severity categories, using AHI 0.0 as the referent, with adjustment for age. In the fully adjusted model (Model 3) periodontitis prevalence reached a plateau at mild SDB. hs-C reactive protein did not significantly attenuate the strength of association with SDB. Compared with the nonapneic referent, adjusted odds of severe periodontitis were 40% higher with subclinical SDB (OR = 1.4, 95% CL: 1.0, 1.9), 60% higher with mild SDB (OR = 1.6, 95% CL: 1.1, 2.2), and 50% higher with moderate/ severe SDB (OR = 1.5, 95% CL: 1.0, 2.3) demonstrating an independent association between SDB and severe periodontitis. We added an interaction term of age in years and SDB categories to the fully adjusted multivariable model. The predicted mean probabilities of severe periodontitis depicted at specified ages are plotted in Figure 1. Up to the age of 50 y probability of severe periodontitis increased with increasing SDB severity, but was not apparent above the age of 55 y (P for effect modification < 0.001).
Table 4.
Odds of severe periodontitis (95% confidence limits) according to sleep disordered breathing severity, Hispanic Community Health Study/Study of Latinos, United States 2008–2011 (n = 12,469).
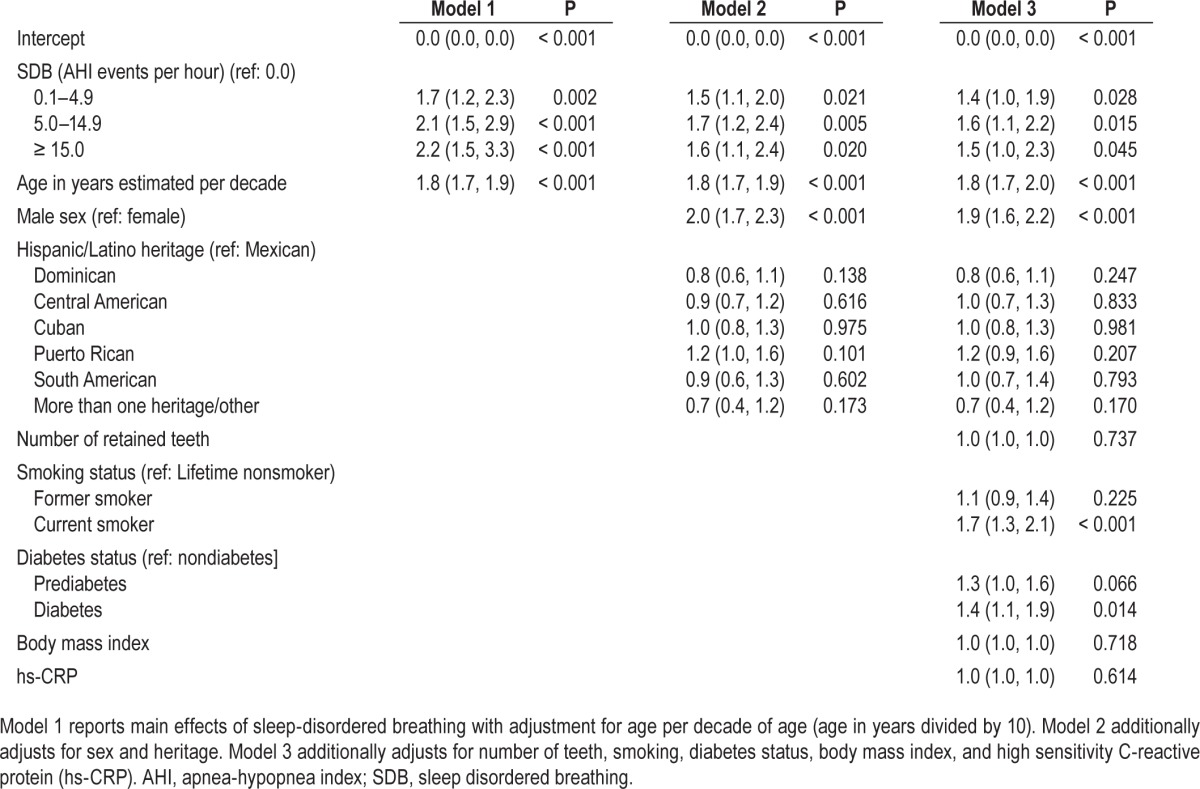
Figure 1.
Relationship of sleep disordered breathing (SDB) severity, assessed by the apnea-hypopnea index and chronic severe periodontitis (n = 12,469). Estimates are expressed at specified ages of 20, 25, 30, 35, 40, 45, 50, 55, and 60 y, stratified at severity thresholds. Predicted estimates were obtained from a binary logistic regression model that adjusted for age, sex, and heritage, number of retained teeth, smoking, diabetes, body mass index, and high-sensitivity C-reactive protein. Findings show a monotonic relationship of greater probability of severe periodontitis at greater SDB severity. There is an effect modification of age (P < 0.001) such that SDB has a stronger relationship with severe periodontitis in younger adults and that this relationship weakens at older ages. AHI, apnea-hypopnea index; CI, confidence interval.
DISCUSSION
Key Findings
In this large population-based cohort of Hispanic/Latino adults, disordered breathing during sleep was positively and significantly associated with severe periodontitis. The relationship was most pronounced at mild levels of SDB severity and was strongest in younger adults. Findings were consistent for men and women and across the major Hispanic/Latino background subgroups. The relationship was independent of tooth loss and established risk factors for periodontitis including age, male sex, smoking, diabetes, and obesity. Contrary to expectation, hs-CRP did not explain this relationship. Following adjustment for confounding, the SDB and periodontitis relationship remained statistically significant, but was attenuated in strength slightly. Any elevation in AHI above zero was associated with an approximate 50% increase in odds of severe periodontitis, irrespective of SDB severity.
Comparison with Published Findings
The co-occurrence of these two diseases was first reported in treatment-naïve OSA patients diagnosed by PSG. Among those patients, prevalence of examiner-verified periodontitis was foldfour higher than expected based on estimates obtained in a recent national survey.12 Even allowing for selection bias, small sample size and use of historical controls, an effect size of this magnitude is highly suggestive of a true association. A subsequent much larger study (n = 29,284) confirmed this relationship, albeit with a smaller effect size. However that study drew subjects from an administrative database without standardized measurement protocols and with no adjustment for confounding.14 Misclassification bias was a limitation of that study as it was in a smaller case control study that relied on the four-item STOP (snoring, daytime tiredness, observed apnea, and high blood pressure) questionnaire to define OSA.14 The STOP was developed to screen surgical patients. Its high rate of false positives is acceptable in the surgical context, but for epidemiologic purposes, misclassification bias from measurement error jeopardizes the validity of findings. To our knowledge, there has been only one community-based study examining the association between periodontitis and OSA. That Korean study (n = 687) showed that in adults in whom OSA was diagnosed by PSG (AHI ≥ 5) had greater than twice the odds of chronic periodontitis than healthy controls, but the association only found in adults aged 55 y or older (adjusted OR = 2.51; 95% CI: 1.37, 4.62) and was not found in younger adults (adjusted OR = 1.46; 95% CI: 0.80, 2.68).33 However, because the youngest adults in that study were 47 y of age, it was not possible to compare effects in young adults.
Consideration of Possible Mechanisms
Several pathophysiological mechanisms may contribute to the relationship between SDB and severe periodontitis. Because both diseases are associated with systemic inflammation, it is plausible that periodontitis mediates the activation of inflammatory pathways in SDB or vice versa. Although this study found that hs-CRP was elevated in SDB and periodontitis, the fact that CRP did not attenuate the strength of association between SDB and periodontitis suggests that CRP is an unlikely mediator of this relationship. Other inflammatory proteins common to both conditions should be explored including interleukin-6 and soluble intercellular adhesion molecule 1.34–36
Another potential mechanism is the side effects of prescription medications. Medications for hypertension such as diuretics and alpha and beta blockers are common causes of decreased salivary flow. Drugs such as calcium channel blockers, phenytoins, and cyclosporine induce an overgrowth of gingival tissue. However, this relationship requires careful consideration. Periodontal diseases encompass a broad spectrum of diseases ranging in severity from gingivitis to severe periodontitis. Although hyposalivation is an established risk factor for dental caries and a recognized cause of gingival inflammation, neither hyposalivation nor gingival inflammation are risk factors for periodontitis. Moreover, gingival overgrowth does not initiate periodontal loss of attachment or promote periodontal destruction. Two reviews have examined the literature on the relationship between medication and periodontal diseases. These reviews find that although some drugs increase gingival inflammation, medication has minimal effect on the much more serious condition of periodontitis. In fact, immunosuppressants, nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2 inhibitors tend to dampen down inflammatory reactions and protect against destructive loss of alveolar bone.37,38 Although an investigation of the effect of medication on this relationship is beyond the scope of this analysis, the topic warrants further exploration in future studies.
A third mechanism could be related to intermittent hypoxia. A hallmark of periodontitis is irreversible and accumulative destruction of alveolar bone. An interesting finding in one study was that chronic intermittent hypoxia may stimulate bone remodeling, preserving bone density in elderly adults39 and in laboratory rats under experimental conditions.40 Although speculative, it is possible that a protective effect of hypoxia against bone loss is one reason for the muted relationship of SDB and periodontitis among older adults. A less speculative explanation involves patterns of age-specific prevalence and severity of OSA; prevalence tends to increase to around age 55 y and then plateau, whereas OSA severity peaks at around age 55 y and then decreases.41
Strengths of the Study
The HCHS/SOL is the first large-scale community-based study to examine the relationship between SDB and periodontitis. It is also the first to examine this relationship in a Hispanic/ Latino population where associations may differ from those in other populations such as the Asian population studied in the Korean Genome and Epidemiology Study. The 2010 National Health Interview Survey noted marked differences in health and risk factor profiles between Hispanic and non-Hispanic white populations. Age-adjusted estimates show higher prevalence of obesity and greater probability of lifetime nonsmoking in Mexican Americans than non-Hispanic whites. In addition, Hispanic had higher rates of diabetes and lower rates of asthma and other respiratory disorders. Cancer, rather than heart disease, was the main cause of death in Hispanics. Because obesity is associated with both SDB and periodontitis and because smoking and diabetes are causally implicated in periodontitis, findings in this Hispanic/Latino population may differ from similar studies conducted in the majority white population.42
The use of rigorous established protocols permitted objective assessments of the relationship between SDB and severe periodontitis. Being population-based, findings are free of clinical selection biases. Moreover, because of probability sampling, findings are generalizable beyond the selected sample to the urban-dwelling Hispanic/Latino population in these geographical regions. Unlike prior research, as in the Korean Genome and Epidemiology Study,33 the HCHS/SOL recruited Hispanic/Latino adults over a wide age distribution, shedding light on health issues in a growing segment of the US population. This analysis of baseline data was by necessity cross-sectional, as such, the study design limits the ability to establish temporal sequence or causal inference. We expect that SDB increases risk for periodontitis, but the relationship could be bidirectional or it could be that periodontitis influences sleep breathing.
Limitations
Prescribed drugs are important in the management of SDB. If medications for SDB are also associated with periodontitis, then potential for confounding exists. A limitation of this study is that it did not take account of prescribed medication. Likewise, findings must be interpreted in light of the limitation that analysis did not account for potential confounding of alcohol consumption. Evidence of an independent effect of alcohol on periodontitis is weak and longitudinal studies are lacking at this time.43 Yet, if alcohol consumption is greater in some groups than others, its omission from analytic models could either overestimate or underestimate the true association between SDB and periodontitis. The failure to adjust for unknown and unmeasured confounders limits the ability of these regression models to fully account for their separate and joint effects and will result in residual confounding.
Irrespective of whether SDB influences periodontitis or is a consequence of it, even if a small percentage of one disorder is attributable to the other, the public health significance is considerable, given the high population prevalence of both disorders. Only 1.3% of adults with OSA in this cohort reported a clinical diagnosis of OSA.29 Future follow-up of the cohort will allow investigators to observe the natural history of SDB and build on to these findings.
Screening for undiagnosed SDB in dental settings may be a valuable public health strategy. Dental personnel already conduct limited screening for chronic conditions such as hypertension and diabetes – medical disorders that have been associated with untreated SDB. Like these conditions, SDB is common and screening is easy to administer through questionnaires and clinical examination for skeletal and soft-tissue characteristics that have been linked to SDB.
It is possible that treatment of SDB would improve the health of the periodontium in patients with periodontitis or the response of the patients to periodontal therapy. Because both periodontitis and the use of oral appliances for the treatment of SDB are under the purview of dentists, future studies might determine whether oral appliance therapy has a therapeutic benefit on periodontal parameters.
CONCLUSION
This study identified a novel association between mild SDB and periodontitis in young adults. Long-term trials with continuous positive airway pressure could consider including change in periodontitis as a potential secondary outcome.
DISCLOSURE STATEMENT
This was not an industry sponsored study. Dr. Loredo has received research support as consultant from Advance Sleep Medicine Inc. and other support from AmeriSleep Diagnostic Services Inc for interpretation of sleep studies. Dr. Essick has received research equipment on loan from Airway Management Inc./VirtuOx and research materials on loan from SleepI-mage. Dr. Zee is a consultant for Jazz and Merck. Dr. Redline has received research equipment and/or research support from Philips Respironics and ResMed Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Center on Minority Health and Health Disparities, the National Institute of Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements.
Footnotes
A commentary on this article appears in this issue on page 1153.
REFERENCES
- 1.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35:277–90. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 2.Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Periodontol. 2013;84:S51–69. doi: 10.1902/jop.2013.134006. [DOI] [PubMed] [Google Scholar]
- 3.Beck JD, Slade G, Offenbacher S. Oral disease, cardiovascular disease and systemic inflammation. Periodontol 2000. 2000;23:110–20. doi: 10.1034/j.1600-0757.2000.2230111.x. [DOI] [PubMed] [Google Scholar]
- 4.Dietrich T, Sharma P, Walter C, Weston P, Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Periodontol. 2013;84:S70–84. doi: 10.1902/jop.2013.134008. [DOI] [PubMed] [Google Scholar]
- 5.Southerland JH, Moss K, Taylor GW, et al. Periodontitis and diabetes associations with measures of atherosclerosis and CHD. Atherosclerosis. 2012;222:196–201. doi: 10.1016/j.atherosclerosis.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Periodontol. 2013;84:S113–34. doi: 10.1902/jop.2013.134005. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med. 2008;23:2079–86. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Aiuto F, Sabbah W, Netuveli G, et al. Association of the metabolic syndrome with severe periodontitis in a large U.S. population-based survey. J Clin Endocrinol Metab. 2008;93:3989–94. doi: 10.1210/jc.2007-2522. [DOI] [PubMed] [Google Scholar]
- 9.Phipps KR, Stevens VJ. Relative contribution of caries and periodontal disease in adult tooth loss for an HMO dental population. J Public Health Dent. 1995;55:250–2. doi: 10.1111/j.1752-7325.1995.tb02377.x. [DOI] [PubMed] [Google Scholar]
- 10.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–20. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 11.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78:1387–99. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 12.Gunaratnam K, Taylor B, Curtis B, Cistulli P. Obstructive sleep apnoea and periodontitis: a novel association? Sleep Breath. 2009;13:233–9. doi: 10.1007/s11325-008-0244-0. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad NE, Sanders AE, Sheats R, Brame JL, Essick GK. Obstructive sleep apnea in association with periodontitis: a case-control study. J Dent Hyg. 2013;87:188–99. [PubMed] [Google Scholar]
- 14.Keller JJ, Wu CS, Chen YH, Lin HC. Association between obstructive sleep apnoea and chronic periodontitis: a population-based study. J Clin Periodontol. 2013;40:111–7. doi: 10.1111/jcpe.12036. [DOI] [PubMed] [Google Scholar]
- 15.Loke W, Girvan T, Ingmundson P, Verrett R, Schoolfield J, Mealey BL. Investigating the association between obstructive sleep apnea (OSA) and periodontitis. J Periodontol. 2015;86:232–43. doi: 10.1902/jop.2014.140229. [DOI] [PubMed] [Google Scholar]
- 16.Nizam N, Basoglu OK, Tasbakan MS, Nalbantsoy A, Buduneli N. Salivary cytokines and the association between obstructive sleep apnea syndrome and periodontal disease. J Periodontol. 2014;85:e251–8. doi: 10.1902/jop.2014.130579. [DOI] [PubMed] [Google Scholar]
- 17.Meurice JC, Marc I, Carrier G, Series F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med. 1996;153:255–9. doi: 10.1164/ajrccm.153.1.8542125. [DOI] [PubMed] [Google Scholar]
- 18.Mizutani S, Ekuni D, Tomofuji T, et al. Relationship between xerostomia and gingival condition in young adults. J Periodontal Res. 2015;50:74–9. doi: 10.1111/jre.12183. [DOI] [PubMed] [Google Scholar]
- 19.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467–84. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- 20.Chapple IL, Milward MR, Dietrich T. The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J Nutr. 2007;137:657–64. doi: 10.1093/jn/137.3.657. [DOI] [PubMed] [Google Scholar]
- 21.Konopka T, Krol K, Kopec W, Gerber H. Total antioxidant status and 8-hydroxy-2'-deoxyguanosine levels in gingival and peripheral blood of periodontitis patients. Arch Immunol Ther Exp (Warsz) 2007;55:417–22. doi: 10.1007/s00005-007-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nibali L, Donos N. Periodontitis and redox status: a review. Curr Pharm Des. 2013;19:2687–97. doi: 10.2174/1381612811319150003. [DOI] [PubMed] [Google Scholar]
- 23.Kumari M, Pradeep A, Priyanka N, Kalra N, Naik SB. Crevicular and serum levels of monocyte chemoattractant protein-4 and high-sensitivity C-reactive protein in periodontal health and disease. Arch Oral Biol. 2014;59:645–53. doi: 10.1016/j.archoralbio.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Slade GD, Offenbacher S, Beck JD, Heiss G, Pankow JS. Acute-phase inflammatory response to periodontal disease in the US population. J Dent Res. 2000;79:49–57. doi: 10.1177/00220345000790010701. [DOI] [PubMed] [Google Scholar]
- 25.Grandner MA, Buxton OM, Jackson N, Sands-Lincoln M, Pandey A, Jean-Louis G. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep. 2013;36:769–79E. doi: 10.5665/sleep.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzsimmons TR, Sanders AE, Bartold PM, Slade GD. Local and systemic biomarkers in gingival crevicular fluid increase odds of periodontitis. J Clin Periodontol. 2010;37:30–6. doi: 10.1111/j.1600-051X.2009.01506.x. [DOI] [PubMed] [Google Scholar]
- 27.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–41. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–9. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;189:335–44. doi: 10.1164/rccm.201309-1735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louis J, Auckley D, Miladinovic B, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120:1085–92. doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–52. [PubMed] [Google Scholar]
- 32.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 33.Seo WH, Cho ER, Thomas RJ, et al. The association between periodontitis and obstructive sleep apnea: a preliminary study. J Periodontal Res. 2013;48:500–6. doi: 10.1111/jre.12032. [DOI] [PubMed] [Google Scholar]
- 34.Chin K, Nakamura T, Shimizu K, Mishima M, Miyasaka M, Ohi M. Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. Am J Med. 2000;109:562–7. doi: 10.1016/s0002-9343(00)00580-5. [DOI] [PubMed] [Google Scholar]
- 35.Maeder MT, Strobel W, Christ M, et al. Comprehensive biomarker profiling in patients with obstructive sleep apnea. Clin Biochem. 2015;48:340–6. doi: 10.1016/j.clinbiochem.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Barros SP, Suruki R, Loewy ZG, Beck JD, Offenbacher S. A cohort study of the impact of tooth loss and periodontal disease on respiratory events among COPD subjects: modulatory role of systemic biomarkers of inflammation. PLoS One. 2013;8:e68592. doi: 10.1371/journal.pone.0068592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seymour RA. Effects of medications on the periodontal tissues in health and disease. Periodontol 2000. 2006;40:120–9. doi: 10.1111/j.1600-0757.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 38.Alani A, Seymour R. Systemic medication and the inflammatory cascade. Periodontol 2000. 2014;64:198–210. doi: 10.1111/j.1600-0757.2012.00454.x. [DOI] [PubMed] [Google Scholar]
- 39.Sforza E, Thomas T, Barthelemy JC, Collet P, Roche F. Obstructive sleep apnea is associated with preserved bone mineral density in healthy elderly subjects. Sleep. 2013;36:1509–15. doi: 10.5665/sleep.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guner I, Uzun DD, Yaman MO, et al. The effect of chronic long-term intermittent hypobaric hypoxia on bone mineral density in rats: role of nitric oxide. Biol Trace Elem Res. 2013;154:262–7. doi: 10.1007/s12011-013-9722-8. [DOI] [PubMed] [Google Scholar]
- 41.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 42.Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat. 2012;10:1–207. [PubMed] [Google Scholar]
- 43.Shepherd S. Alcohol consumption a risk factor for periodontal disease. Evid Based Dent. 2011;12:76. doi: 10.1038/sj.ebd.6400808. [DOI] [PubMed] [Google Scholar]