Abstract
The Sleep Apnea cardioVascular Endpoints (SAVE) study is an ongoing investigator-initiated and conducted, international, multicenter, open, blinded endpoint, randomized controlled trial that was designed to determine whether treatment of obstructive sleep apnea (OSA) with continuous positive airways pressure (CPAP) can reduce the risk of serious cardiovascular (CV) events in patients with established CV disease (clinical trial registration NCT00738179). The results of this study will have important implications for the provision of health care to patients with sleep apnea around the world. The SAVE study has brought together respiratory, sleep, CV and stroke clinicians-scientists in an interdisciplinary collaboration with industry and government sponsorship to conduct an ambitious clinical trial. Following its launch in Australia and China in late 2008, the recruitment network expanded across 89 sites that included New Zealand, India, Spain, USA, and Brazil for a total of 2,717 patients randomized by December 2013. These patients are being followed until December 2015 so that the average length of follow-up of the cohort will be over 4 y. This article describes the rationale for the SAVE study, considerations given to the design including how various cultural and ethical challenges were addressed, and progress in establishing and maintaining the recruitment network, patient follow-up, and adherence to CPAP and procedures. The assumptions underlying the original trial sample size calculation and why this was revised downward in 2012 are also discussed.
Clinical Trials Registration Number:
Australia New Zealand Clinical Trials Registry Number:
ACTRN12608000409370.
Citation:
Antic NA, Heeley E, Anderson CS, Luo Y, Wang J, Neal B, Grunstein R, Barbe F, Lorenzi-Filho G, Huang S, Redline S, Zhong N, McEvoy RD. The sleep apnea cardiovascular endpoints (SAVE) trial: rationale, ethics, design, and progress. SLEEP 2015;38(8):1247–1257.
Keywords: OSA, cardiovascular, outcomes, clinical trial
BACKGROUND AND RATIONALE FOR THE SAVE STUDY
Obstructive sleep apnea (OSA), which is characterized by repeated episodes of complete or partial upper airway obstruction during sleep leading to transient hypoxemia, arousal from sleep, tachycardia, and a surge in systemic and pulmonary arterial blood pressure (BP), was first widely recognized as a clinical disorder in the 1970s. OSA, defined as more than 15 apneas and hypopneas per hour of sleep, was shown in early studies to affect approximately 7% of adults in the general population1 and 30–60% of patients with known cardiovascular (CV) disease.2–4 However, as rates of obesity rise worldwide, recent studies suggest that approximately 10–15% of adults may suffer from moderate-severe OSA.5,6
In a landmark Australian study by Sullivan and colleagues in 1981, nasal continuous positive airway pressure (CPAP) was shown to be a highly effective treatment for patients with OSA7 by improving levels of daytime alertness and well-being through alleviating upper airway obstruction and returning sleep quality and blood oxygen levels to normal. The major goal of OSA treatment has been to relieve patients of debilitating daytime sleepiness and socially disruptive snoring, and for patients who initially accept CPAP therapy, long-term adherence is 70–80%.8 However, there has been increasing evidence of a causal relationship between OSA and CV disease through several potential pathways of physiological disturbance during sleep.9 Animals exposed to intermittent hypoxia similar to those experienced by patients with OSA have shown sustained elevations in BP, central nervous system damage, and abnormalities of glucose and lipid metabolism.10–13 Clinical and community-based studies have shown OSA to be independently associated with hypertension, glucose dysregulation, and ischemic and cerebrovascular disease.14–18 Short-term CPAP treatment of OSA has been shown to result in small reductions in systemic19,20 and pulmonary artery blood pressure,21,22 and improvements in some other biomarkers of CV risk,23 but not all such intervention studies had been positive.24 A large longitudinal but nonrandomized study completed prior to the launch of SAVE suggested that CPAP therapy might substantially reduce the risk of CV events.16
Since the launch of the study in 2008, several large longitudinal studies have reported independent associations between OSA and incident CV disease25–31 and mortality,32–35 further strengthening the rationale for the trial. Larger randomized controlled trials with longer follow-up have also confirmed small BP reductions with CPAP.19,36,37
There thus exists substantial evidence that OSA may increase the risk of premature CV disease including myocardial infarction and stroke, and that CPAP treatment may reduce these risks. However, there are many examples in medicine where non-randomized studies pointed strongly to a likely benefit of a particular therapeutic intervention (e.g., vitamin E and CV disease, hormone replacement therapy and CV disease, and most recently of renal denervation and hypertension) only for large-scale clinical trials to subsequently show no benefit, or even harm, from such treatment.38–41 A large-scale, hard endpoint, randomized controlled trial is therefore required to determine whether the pathophysiological disorder of OSA has a modifiable effect on premature CV disease.
The need for adequately powered clinical trials of OSA treatment focused on CV outcomes was well recognized in the late 2000s.9,42 The 2008 joint American Heart Association and American College of Cardiologists Foundation scientific statement on sleep apnea9 concluded that although the association between OSA and CV disease appears strong, the observational nature of much of the evidence and the possibility of residual confounding by visceral obesity weakened the overall case in favor of a causal link. The statement concluded that rigorous intervention studies in support of a benefit from OSA treatment were missing, which hampered progress in this field, a view that has been strongly endorsed by a more recent international consensus statement.43 The SAVE trial was therefore designed to bridge this gap.
Clinical Equipoise
The Steering Committee consulted widely with respiratory and CV physicians in several countries in their design of the SAVE study to gauge the level of uncertainty over whether to prescribe OSA treatment specifically for CV risk reduction in adults with established CV disease. Not surprisingly, a wide range of opinions were obtained but consensus at the time was that there was insufficient evidence to recommend OSA treatment routinely for CV risk reduction. Substantial clinical equipoise existed on this question during 2005–2008, and it has not changed sufficiently to alter the opinion of the SAVE investigators or the broader international sleep medicine community.
Background Planning
Planning for the SAVE study began in 2006 assisted by a research grant from the Respironics Foundation. An academic partnership was formed between Australian sleep and respiratory clinicians-scientists at the Adelaide Institute for Sleep Health (AISH) of Flinders University and investigators at The George Institute of Global Health of the University of Sydney, to prepare the ground work for the trial. With considerable experience in large-scale international CV clinical trials (e.g., ADVANCE and INTERACT 1 and 244,45), The George Institute was able to provide the necessary expertise and research infrastructure to mount a study of this size. The AISH had considerable experience in clinical sleep research and, with the assistance of the Australasian Sleep Trials Network, provided the necessary expertise in sleep apnea diagnosis and CPAP treatment. With equipment grants from the sleep diagnostic and device companies Compumedics and ResMed, a necessary preliminary study to test the validity of a simple screening device for diagnosing OSA in potentially eligible patients for the trial was conducted in Shanghai.46 After further engagement with experts in sleep, respiratory, and CV disease around the world, the study design and research plan were finalized. Further funding from the Respironics Foundation then allowed the study to proceed.
Trial Design
The SAVE study was designed as an international, multi-center, open, blinded endpoint assessment, randomized controlled trial to determine whether treatment of OSA with CPAP on top of best medical care compared to best medical care alone can reduce the risk of serious CV events in patients with established CV disease. In order for the results to be able to influence clinical practice, the study was powered to detect a treatment effect on hard CV endpoints that included myocardial infarction and stroke rather than surrogate markers of CV risk such as blood pressure (BP), lipids and glucose metabolism. Moreover, given multiple overlapping potential pathogenic pathways whereby OSA may lead to CV events, we considered this approach assessing the effects of CPAP treatment on a composite of downstream CV events was preferable to assuming a dominant mechanism(s) for increased CV risk.
We considered it impractical to continue patients randomized to the control group on sham CPAP over several years. The control group is thus usual care and the study an open label one. To allow for other factors that may affect cardiovascular outcomes (e.g., weight change, use of antihypertensive medication) and that could potentially differ between the two groups during the study, we are tracking medication changes and lifestyle modifications such as patient weight, level of exercise, and smoking history throughout the trial.
Because of the relatively high rate of CV events in patients with established CV disease, the number of patients required to assess the treatment effects in a secondary prevention trial is less. If we assume a 1% annual event rate in a primary prevention trial we would need two to three times as many participants. Although a secondary prevention trial in OSA might target some pathogenic mechanisms that are less relevant to primary CV disease prevention (e.g., sudden nocturnal death due to ventricular tachycardia/fibrillation in patients with existing ischemic heart disease), it nevertheless provides highly relevant information to inform clinical practice with plausible translation of results into primary prevention. Furthermore, the smaller sample size and shorter duration of exposure to assess effects on outcomes provides reassurance about feasibility and efficiency in completing such a trial.
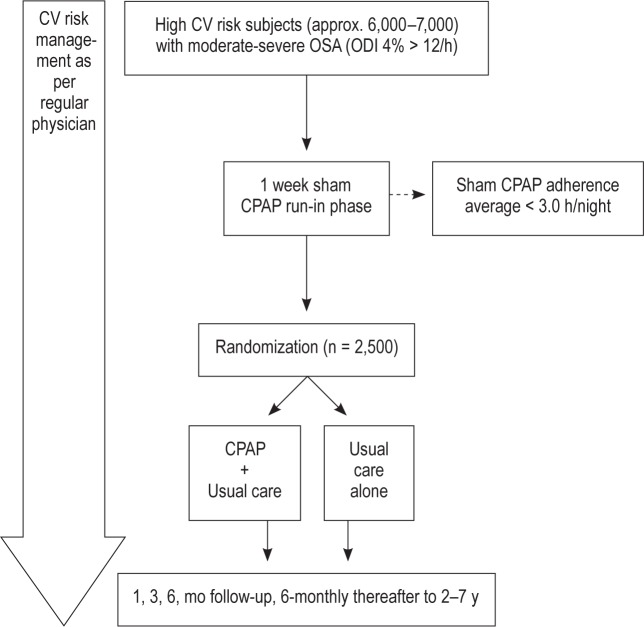
Table 1 summarizes the primary and secondary endpoints in the SAVE study, and Figure 1 outlines the overall design schema. The primary endpoint is a composite of CV events that includes CV death, nonfatal myocardial infarction, non-fatal stroke, and any hospitalization (or presentation at hospital to allow detailed diagnostic assessment) for unstable angina, heart failure, or transient ischemic attack (TIA). Based on prior studies in similar high risk populations, the event rate for this composite endpoint was estimated at 6% per annum.47
Table 1.
Primary and secondary endpoints in the SAVE study.

Figure 1.
SAVE design scheme. CPAP, continuous positive airway pressure; CV, cardiovascular; ODI, oxygen desaturation index.
Patient Inclusion and Exclusion Criteria
Table 2 summarizes the eligibility criteria for the SAVE study. Patients were included with established CV disease and co-occurring moderate-severe OSA that was diagnosed by a simple overnight screening device (Apnea Link, Resmed, Bella Vista Sydney, Australia) which also allowed other forms of sleep disordered breathing (e.g., obesity hypoventilation syndrome, overlapping hypoxemic lung disease or Cheyne-Stokes Respiration (CSR) to be excluded on the basis of the pattern of nasal airflow disturbance, awake resting and overnight oximetry measurement, and other clinical features. Patients were also excluded if they were judged to be very sleepy or if they reported a sleepiness-related accident in the previous 6 months (see ethical issues in the following paragraphs).
Table 2.
Subject eligibility and recruitment procedures.

Patient Recruitment
We recognized that recruitment would be a challenging component of SAVE where patients require screening for a diagnosis of OSA. Despite the high frequency (30–60%) of OSA in groups with high CV risk,9,48–51 we estimated that two to three patients with existing CV disease would need to be screened to identify one eligible patient (i.e., 10,000–15,000 patients) to achieve the required sample size. We realized this would not be easily achieved by relying solely on referrals to sleep medicine services, where patients with OSA are generally symptomatic, expect to be offered treatment, and have a low frequency of comorbid CV disease. Although recruiting patients directly from CV clinics was more attractive, this would require CV/ stroke clinicians to develop new skills in OSA diagnosis and CPAP treatment and/or form strong collaborative working relationships with local respiratory/sleep services. We therefore decided to extend an invitation to a wide range of these clinician groups to participate in the study, provide training and support in sleep diagnostics and CPAP therapy where needed, and encourage collaboration at national and local levels. Such interdisciplinary collaboration will be needed if the results of the SAVE study prove to be positive.
DIAGNOSIS OF OSA BY AMBULATORY SLEEP APNEA MONITORING
In-laboratory polysomnography (PSG) was not feasible for screening and diagnosing OSA in such a large number of participants required for the SAVE study. Moreover, many potential recruitment sites in China do not have access to PSG and most sleep medicine services elsewhere are heavily booked with clinical work. Even if PSG facilities could be made available at sites, we considered the cost and effort to train staff and standardize recording and scoring techniques between laboratories (or centralise scoring) was prohibitive for the type of study envisioned. There was increasing evidence that simplified home screening devices could be used to identify moderate-severe OSA with a high degree of certainty, at least in populations with high pretest probability of disease, with many sleep experts advocating such an approach in clinical practice and research.52 Because the frequency of OSA in the proposed study population was likely to be high, it was decided to validate a simple, automated two-channel (oximetry and nasal pressure) screening device (the ApneaLink, ResMed) in a high CV risk community setting in China with the view to using this device in the main study. This China-Australian collaborative validation study showed that the automatically calculated oxygen desaturation index (oximetry) and apnea-hypopnea index (AHI) (nasal pressure) had equally high diagnostic accuracy for moderate-severe OSA when compared with full PSG simultaneously performed in the homes of 143 patients at high risk of CV in Shanghai. Because the ApneaLink Oximetry had a lower technical failure rate (e.g., loss of signal because of sensor displacement) than nasal pressure recordings, it was the preferred primary diagnostic method for identifying patients with OSA for SAVE.46 The nasal pressure trace can be used to exclude patients whose predominant pattern of sleep disordered breathing pattern is symmetrical waxing and waning of flow indicating possible CSR. An algorithm was developed by the study core laboratory at the Adelaide Institute for Sleep Health (AISH) to identify patients with predominantly CSR using nasal pressure signals from the ApneaLink device (Table 3). Subsequent enhancement of ApneaLink software has also proved useful in this regard.53
Table 3.
Procedure for checking Cheyne-Stokes respiration pattern.

ETHICAL AND SAFETY CONCERNS
Excessive Daytime Sleepiness and Accident Risk
The main ethical question was whether withholding CPAP treatment in patients who screen positive for OSA and have the potential to benefit from improved daytime vigilance and well-being would place them at significant risk of future accident. To minimize such risk, it was decided to exclude patients who held a commercial driver's licence, reported an accident (or near-miss accident) because of sleepiness in the previous 6 mo, or who had marked daytime sleepiness as evident by high scores (> 15) on the Epworth Sleepiness Scale (ESS) (Table 2). Additionally, systems were developed to closely monitor patient safety during the course of follow-up, where all patients are asked about any recent accidents or uptake of employment as a commercial driver, and the ESS questionnaire was completed at each study visit. The core laboratory monitors the following key data: if an ESS is found to exceed 15 in either usual care or CPAP-treated patients, a simple pro forma questionnaire is used to collect information on possible contributors to daytime sleepiness covering sleep patterns and estimated total sleep time, sedative medications or excessive alcohol use, emergent depression, or in the case of those on CPAP, the adequacy of therapy in terms of adherence and control of OSA. The core laboratory director then communicates either directly with the principal investigator of the relevant site to advise on the management of the excessive sleepiness, or in China indirectly via a sleep expert independent of the SAVE study to assist and adjudicate on cases where there remains clinical uncertainty as to the cause of daytime sleepiness. The intent is to ensure that severely sleepy patients are not prevented from receiving a potentially effective therapy, yet at the same time avoiding unnecessary crossover from the usual care arm to CPAP by carefully considering and managing causes of sleepiness deemed not to be primarily related to OSA. At enrollment, all patients were fully informed about the nature of the study and the possible symptomatic benefits of CPAP and the option to seek treatment outside of the trial if they wished. Both the patient and his or her responsible physician were required to be comfortable about random allocation to CPAP treatment or usual care.
Patients with Very Severe OSA
Another concern was whether, despite equipoise regarding the advisability of OSA treatment for CV risk reduction, it was reasonable to deny CPAP treatment to patients with very severe OSA. Following discussions with the lead investigators, it was decided to exclude patients whose overnight oximetry study showed > 10% of the time with oxygen saturations < 80%. These patients were recommended for further investigation and/or treatment by their physician independent of the study.
Conduct of Clinical Trials in Low Resource Settings
There has been debate about the ethics of investigators or pharmaceutical companies from advanced economies conducting clinical trials of relatively complex or expensive treatments in socioeconomically disadvantaged populations where treatments are either not relevant to their health care system or are so expensive as to put them beyond the reach of most of the population. The 2002 statement by the Nuffield Council of Bio-ethics report provided us with useful guidance on these ethical issues in designing the study. The statement recommends that:54
studies respect the cultural values of host countries and the rights of the participants to be fully informed and to give their consent willingly without coercion or undue inducement;
the research, wherever possible, should be relevant to the host country and is not detrimental to the health or welfare of the participants;
the development of local expertise in health care is integral to the conduct of the research and that any changes are sustainable in the local context after the research is completed; and
the sponsoring researchers do not take advantage of the vulnerabilities created by poverty, or lack of infrastructure or resources.
A guiding principle is that there should be a thorough process of consultation with clinicians and researchers in the host countries and, if necessary, the government or health authorities in those countries. Also, ethics committees in both the sponsoring and host countries must approve the protocols.
We consider the SAVE study meets these recommendations after we undertook detailed discussions with key opinion leaders in sleep, respiratory, and CV medicine in each of the participating countries to ensure the study protocol adheres to ethical and regulatory standards, was sensitive to cultural norms, and that the research question being addressed was relevant to local health care needs. CV disease is the major cause of mortality in developing as well as developed countries and will increase in line with the demographic transitions of aging, urbanization, and adoption of Western lifestyles. Thus, the findings from the SAVE study will be highly relevant to future health care in the host countries, with the simple home diagnostic technique for detecting OSA ideally suited for routine care in large populations with limited health care resources. Agreement was reached with Philips Respironics to ensure CPAP treatment would be available to the control group, should the results prove positive at the end of the study. The study has been approved by the Ethics Committees of all the participating sites in each of the host countries.
Use of a Sham Run-in Phase
Another issue we considered was that adherence to CPAP therapy might be a significant problem in a study lasting over several years, particularly considering that most subjects were likely to report little or no daytime sleepiness and therefore would be unlikely to experience significant symptomatic benefit. In addition, clinical studies indicate that as many as 30% of patients with OSA refuse CPAP treatment outright or in the first few weeks of therapy.55 To exclude those patients who would be unwilling or unlikely to adhere to CPAP therapy, we decided to use a 1-w sham CPAP run-in phase. Sham CPAP is designed to deliver only a very low, nontherapeutic pressure to the airway,56 and previous clinical studies have shown similar short-term adherence levels with sham and active CPAP. We assumed that if patients were sufficiently motivated and able to wear a CPAP mask for a minimum average period of 3 h per night, they would be more likely to comply with active treatment in the long term. The inclusion of a sham device runin phase prior to randomization is consistent with other long-term clinical trials that aim to maximize efficiency in detecting a treatment effect by excluding people who are unable to tolerate the treatment or adhere to procedures,57 and in the case of OSA it simulates current clinical practice in which patients are frequently offered an initial trial of CPAP to ascertain their acceptance or otherwise of the treatment.
RANDOMIZATION
Randomization was Web based via a password-protected, fully secure study website. A minimization program was used to stratify treatment allocation by site, type of CV disease (cardiac or cerebrovascular) and by a measure of daytime sleepiness severity (ESS score < 11 or ≥ 11). After eligibility in the study was confirmed, the patient was registered and assigned to a particular randomised group.
CPAP Commencement and Follow-up
Patients randomized to receive CPAP were given further written information about the treatment and were asked to watch a short DVD designed to educate them about the importance of the trial and adherence to CPAP. Next, they were provided with an AutoPAP machine (M or PR series Remstar, Phillips Respironics, Murrayville, PA) from which data were downloaded via smart cards after 1 w to check that it was technically satisfactory, and that the average usage time was > 3 h per night, with average leak < 60 L/min. If patients failed to meet these criteria, any technical or mask-fit problems were resolved and the patient was asked to repeat the study. After this a fixed CPAP pressure was set at the 90th centile pressure, patients received a telephone call after 1 w to troubleshoot any difficulties and at later times if any problems arose. All participants were reviewed at 1, 3, and 6 mo, and contacted 6 months thereafter until the close of the study. The core laboratory monitors trends in CPAP adherence stratified by country, site, and patient levels. An automatic email is sent to the site investigator if any of their patients show any of the following conditions: CPAP usage drop to < 3 h per night, high mask leak (> 60 L/min), or high (> 15/h) residual AHI as measured by the CPAP device. The core laboratory also provides a helpline for specific advice to investigators.
Sample Size Calculations
We originally estimated that 5,000 patients would need to be recruited to detect a 20% relative risk (RR) reduction in the primary endpoint in the CPAP group compared with the usual care group with 80% power (α 0.05) assuming: (1) a 2-y recruitment period with an average follow-up of 4 y; (2) 6% annual event rate for the primary endpoint, and (3) loss to follow-up of 10% in each arm. A meta-regression of the association between AHI and the risk of CV disease based on data extracted from five prior epidemiological studies available in 20084,16,17,27,58 suggested that the risk of CV events increased by 16% for every 10 events per hour increase in AHI. We estimated that a 32% RR reduction in major CV events would occur among participants in SAVE who are likely to have a mean AHI of 45 events per hour (American Academy of Sleep Medicine “Chicago” scoring criteria) or an oxygen desaturation index (> 3%, 30 events per hour) that would be decreased to about 25 events per hour with CPAP treatment, assuming an average usage of 3–4 h per night. However, the trial was powered more conservatively to detect a 20% RR reduction (α 0.05, β 0.80) to allow for potential for 20% non adherence to treatment and 10% loss to follow-up in each group.
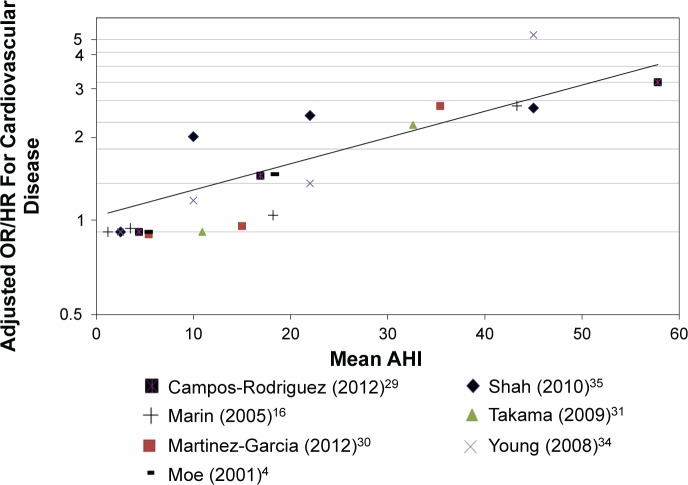
In 2012, we reassessed the assumptions inherent in the power calculation based upon: (1) a review of the accumulated blinded study data, specifically the severity of OSA in participants, adherence to CPAP, the overall primary CV endpoint rate and loss to follow-up; and (2) an updated meta-regression of longitudinal studies of CV events according to OSA severity (AHI) which shows that CV risk increases by 25–32% for every 10 events per hour increase in AHI from 19 to 29 (Figure 3). This relationship is considerably stronger than our original estimate, and agrees closely with another regression analysis by Loke et al.59 that reported that a 10-unit incremental increase in AHI was associated with an odds ratio (OR) of 1.36 (95% confidence interval [CI] 1.26 to 1.43) for the outcomes of stroke and CV death (Figure 2).
Figure 3.
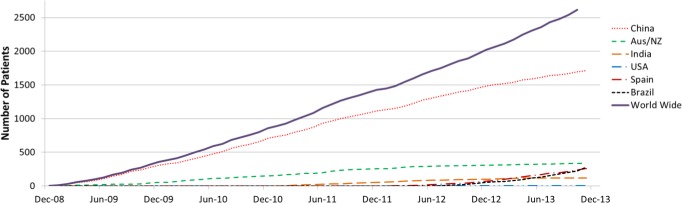
Cumulative recruitment of participants randomized into the SAVE trial.
Figure 2.
Predicted odds ratio for cardiovascular events based on metaregression coefficients (n = 7 studies). Two researchers independently conducted literature searches for papers on the association of sleep apnea and cardiovascular disease/events. Studies needed to report adjusted odds or hazard ratios (OR, HR) stratified by groups with reports of mean apnea-hypopnea index (AHI) or range of AHI in the stratification. Twenty-four studies were identified, 17 were excluded (2 were cross sectional studies, 12 did not report on a composite cardiovascular outcome, e.g., only stroke, one was a subsequent publication of the same population included, one had no stratification or reports of AHI, one reported RDI only), and seven studies were included in the meta-regression. One researcher extracted data and conducted the meta-regression on the log OR/HR. Normal probability plot of shrunken residuals was conducted to confirm that there were no notable outliers as such a normal random effects model was sufficient. Sensitivity analyses were conducted removing the cross-sectional study. All analyses were conducted using STATA version 12. From the meta-regression coefficients it is possible to estimate the log OR of cardiovascular event (CVE) at a given AHI using the formula log OR = 0.1321137 + 0.0223362AHI. Based on the coefficients from the meta-regression we calculated that for a drop in AHI from 29 to 19 the resulting CVE risk reduction is 25%.
Adherence to CPAP treatment was also greater than we had initially expected, varying from an average of 3–3.5 h per night (including the patients who had ceased using CPAP) in July 2012. At this time, the average oxygen desaturation index (ODI) at baseline was 28/h (which approximates to an AHI of ∼35–40/h) but was expected to decrease by approximately 15/ hour with CPAP adherence at an average of 3–3.5 h/night by the end of the study. The overall primary un adjudicated CV event rate was 6.08%, which would equate to an event rate of 6.86% in the usual care group if our underlying assumptions over the potential treatment effect were correct.
The updated power calculation indicated a sample size of 2,500 would be needed to detect a 25% RR reduction (α 0.05, β 0.80) in the primary composite CV endpoint. Assumptions inherent in this calculation were: (1) updated recruitment period of 5 y; (2) an average follow-up of 4.5 y and a minimum follow-up of 2 y for the last patient recruited at the end of 2013; (3) an annual event rate in the usual care group of 6.86%; and (4) an average CPAP adherence of 3 h per night. Based on these considerations the Executive Committee recommended that the study be repowered to detect a 25% reduction in the RR of the primary composite CV event endpoint.
PROGRESS OF THE SAVE STUDY
Funding for the trial has included a grant of US$5million from the Respironics Foundation in 2008 and grants of AUS$4.2million and US$1million from the Australian National Health and Medical Research Council (NHMRC) and Philips Respironics, respectively, during 2010–2013. Patient enrollment closed on December 1, 2013 with a total of 2,717 randomized patients recruited from 89 hospitals worldwide (48 in China, 13 in Australia, 5 in New Zealand, 9 in India, 5 in Spain, 8 in Brazil, and 1 in the US). Follow-up of these patients will continue until December 2015. Figure 3 shows the pattern of enrollment over the 5-y recruitment phase, with 62% of patients included from China. We initially envisioned recruiting 0.8–1.0 patients per site per month but this proved difficult to achieve and despite minor protocol amendments in 2009, 2011, and 2013 designed to lessen the burden of data collection and data entry on investigators, and expansion of the international network to include sites in India (2010) Spain (2011), USA (2011), and Brazil (2012), the average rate of recruitment remained at 0.7 per active site per month equating to a total of 45 per month.
Impediments to recruitment varied over time, and between sites and countries. The major difficulty reported by investigators related to the complexity and time consuming nature of the screening process. Despite changes made in early 2009 to reduce the amount of data collection and entry, the study still required a necessary and unavoidable high level of technical competency covering the diagnosis of OSA, use of the sham CPAP run-in, and implementation of therapeutic (and adherent) CPAP. Access to a suitable high CV risk patient pool was also a problem at some sites. Generally, recruitment was highest at sites with both ready access to the required type of patients and a highly motivated and organized study coordinator with sufficient time to devote to research. We tried to maximize investigator engagement through regular communications by newsletter, meetings, and telephone calls to highlight the importance of the study, report on progress, and suggest strategies to overcome barriers. The addition of the Spanish and Brazilian sites to the study has bolstered recruitment and offset investigator fatigue in other countries.
We recently reported trial data showing intention-to-treat average daily CPAP adherence of 3.3 h at 12 mo60 which exceeds our original estimates and is greater than has been reported in other CV populations.61–64 Investigators remained blind to all CV outcome data. The challenge is to maintain or, if possible, increase CPAP adherence for the remainder of the trial. The current “better-than-expected” adherence is likely caused by a combination of factors: use of a sham run-in phase to exclude subjects unable to tolerate mask treatment; an OSA study population which by virtue of the serious nature of their CV problems is motivated to comply with the study procedures despite OSA symptoms; and the intensive training and support in CPAP therapy provided to site investigators.
TRIAL MANAGEMENT
The trial is managed by an Executive Committee (Appendix) with overall responsibility for the design and conduct of the study and an Operations Committee is responsible for day-to-day operational matters. A Principal Investigator (PI) for each participating country or region advises these Committees on relevant national regulatory issues, clinical practice standards, and patient recruitment strategies. The CV trials expertise and management skills of The George Institute have been crucial to the successful conduct of the study. Project Managers in each country provide valuable links with investigators and are able to advise and monitor issues that are often country specific and which may not be readily identifiable to either the Principal Investigator or Operations Committee staff based in Australia. For example, CPAP usage was limited at times in some cities in India due to frequent planned nocturnal power outages used to manage stretched power grids. As staff in some countries had limited experience and training in OSA and CPAP therapy, we needed to provide comprehensive start-up training in sleep medicine, OSA and CPAP, and extensive and ongoing support and education during the course of the trial.
An independent Data Safety Monitoring Board (DSMB) also monitors rates of self-reported accidents in the CPAP-treated and usual care groups. As part of their regular oversight of the study, the DSMB is responsible for safeguarding the interests of trial participants, assessing the safety and efficacy of the interventions during the trial, and for monitoring the overall conduct of the trial. The DSMB has met annually to review study progress, has undertaken one planned interim analysis, and concurred with the Executive Committee's decision to reduce the sample size. It has not identified any safety concerns to date and has recommended continuation of the trial.
SUMMARY AND CONCLUSIONS
As the largest clinical trial in the field of sleep apnea research to date, SAVE would not have been made possible without the early start-up and ongoing support from industry and peer-reviewed competitive research grant funding from the NHMRC of Australia. However, our study has been conceived and designed independent of the sponsors and the SAVE Investigators have autonomy with respect to the conduct of the study and in analysing and reporting of the data. We believe the SAVE trial is of considerable interest to the fields of sleep, respiratory, and CV medicine. Our projections are that the last patient visit will occur in late 2015 and the main results will be announced in late 2016. If the results prove that the treatment of OSA reduces the risk of CV events in a high-risk patient group, it will pave the way for a paradigm shift in CV disease management with global reach.
DISCLOSURE STATEMENT
The major sponsor of the project was Philips Respironics untied grant (direct funding plus CPAP equipment) with additional sponsorship from ResMed (diagnostic equipment), Fisher and Paykel (direct funding) and the Australian National Health and Medical Research Council (Project grant 1006501 and 1060078) and the Australasian Sleep Trials Network. Dr. Antic has received research funding from the National Health and Medical Research Council of Australia, Philips Respironics, and Fisher and Paykel; equipment donations from ResMed, Philips Respironics, and SomnoMed; and lecture fees and payment for development of educational presentations from ResMed, Astra Zeneca, and GSK. Dr. McEvoy has received research funding from the National Health and Medical Research Council of Australia, Philips Respironics, and Fisher and Paykel; equipment donations from ResMed, Philips Respironics, AirLiquide, and SomnoMed; and lecture fees from Philips Respironics. Dr. Heeley has received funding from the National Health and Medical Research Council of Australia. Dr. Anderson has received speaker fee and travel expenses to attend a Global Stroke Symposium from General Electric. He has received research support from the National Health and Medical Research Council of Australia, Compumedics, Philips Respironics, and Resmed. Dr. Neal has received research support from National Health and Medical Research Council of Australia, Compumedics, Phillips Respironics, Resmed, Roche, Jannsen, Merck Shering Plough, Servier, Abbvie, Dr. Reddy's Laboratories, Abbot, and Novartis. Dr. Barbe has received research support from the Spanish Government and Resmed. Dr. Grunstein has received research support from National Health and Medical Research Council of Australia. Dr. Lorenzi-Filho has received research support from Resmed, Philips Respironics, and the Brazilian Government. Dr. Redline has received research support from Dymedix Inc, use of equipment from Philips Respironics, and is the incumbent of an endowed professorship donated to Harvard Medical School by Dr. Peter Farrell, the founder and Board Chairman of ResMed Inc. The other authors have indicated no financial conflicts of interest. There has been no off-label use of devices.
ACKNOWLEDGMENTS
Philips-Respironics Foundation is the major benefactor of the SAVE study. Project grant funding (numbers 1006501 and 1060078) has been obtained from the NHMRC of Australia in 2010 and 2014. Philips-Respironics has donated all the CPAP equipment for the study. ResMed has donated home sleep apnea diagnostic screening devices. Fisher and Paykel and the Australasian Sleep Trials Network have provided additional monetary support. SAVE is supported by the Adelaide Institute for Sleep Health and The George Institute for Global Health. The SAVE PI (McEvoy) and Co-PI (Anderson) are supported by Practitioner and Senior Principal Research Fellowships, respectively, from the NHMRC. Bruce Neal is supported by an Australian Research Council Future Fellowship (DP100100295) and a National Health and Medical Research Council of Australia Senior Research Fellowship (APP100311). With Co-PI Anderson he holds an NHMRC Program Grant (APP1052555). The authors gratefully acknowledge the contribution of the recruitment networks and PIs, DSMB and Executive Committees (Appendix). The authors also acknowledge Dr. D.P. White who provided invaluable support and advice to the SAVE investigators since the study's inception.
APPENDIX
Principal Investigators and Coordinators (According to Country and Center)
Australia
Sir Charles Gairdner Hospital: N. McArdle; Repatriation General Hospital: R.D. McEvoy; Royal Prince Alfred Hospital: C.S. Anderson; The Prince Charles Hospital: J. Douglas; Lyell McEwin Hospital: M. Arstall; Eastern Clinical Research Unit: A. Young; Flinders Medical Centre: D. Chew; Caulfield Clinical Trials Centre: M. Naughton; Royal Melbourne Hospital: J. Goldin; Cardiovascular Centre Adelaide: P. Sanders; Monash Medical Centre: G. Hamilton; Concord Repatriation General Hospital: A. Corbett; Austin Hospital: M. Barnes.
Brazil
Instituto do Coracao (Incor): G. Lorenzi Filho; Pronto Socorro Cardiologico de Pernambuco: R.P. Pedrosa; Instituto Dante Pazzanese de Cardiologia: C. Amodeo; Instituto do Sono – AFIP: L.R.A. Bittencourt; Clinica de Pneumologia d Medicina do Sono: A. Petruco; Hospital Universitario - Sao Paulo: L. Drager; Hospital Beneficencia Portuguesa: P. Genta; Labsono - Diagnostico e Solucoes em Sono: S. Fagondes.
China
The First Affiliated Hospital of Guangzhou Medical College (Respiration Disease Research Centre): Y. Luo; Guangdong Provincial People's Hospital: Q. Ou; The First Affiliated Hospital of Nanjing Medical University: X. Zhang; The Second Affiliated Hospital of Soochow University: R. Chen; Fuwai Hospital: Z. Liu; Xuzhou Central Hospital: G. Chen; Hejian Municipal People's Hospital: B. Du; Jiangsu Provincial Hospital of State Organ – Cardiology Department: Z. Pan; Peking University Shougang Hospital: W.Gao; The Second Affiliated Hospital of Hebei Medical University: L. Tai; Jiangsu Provincial Hospital of State Organ: G. Lu; Inner Mongolia Baotou City Central Hospital: Y. Li; The Third Hospital of Hebei Medical University: H. Wang; Peking Union Medical College Hospital – Respiratory Department: Y. Xiao; The Second Affiliated Hospital of Guangzhou Medical College: E. Xu; The People's Hospital of Guangxi Zhuang Autonomous Region: J. Liu; Zhongshan Hospital of Fudan University: S. Li; General Hospital of Tianjin Medical University: B. Chen; Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine: X. Guo; First Affiliated Hospital of Chinese Medical University: W. Wang; The First People's Hospital of Foshan: G. Zhen; Beijing Shijitan Hospital – Department of Neurology: M. He; The Second Hospital of Shanxi Medical University: B. Wang; The First Affiliated Hospital of Baotou Medical College: L. Wu; Fengtian Hospital: S. Wang; Beijing Haidian Hospital: F. Yu; Shanghai Huadong Hospital: H. Zhu; Shanghai Shidong Hospital: X. Liu; Shanghai East Hospital: Y. Liang; Beijing Tongren Hospital - Department of Neurology: X. Zhang; The Fifth Affiliated Hospital, Sun Yat –Sen University: Z. Li; Beijing Shijitan Hospital – Sleep Center: L. Pan; Zhejiang Hospital: G. Qin; No. 260 PLA Hospital of China: S. Tian; The First Affiliated Hospital of Shanxi Medical University: S. Ren; Beijing Friendship Hospital, Capital Medical University: K. Chen; Beijing Police Hospital: G. Xiao; Ji Shui Tan Hospital: X. Zhao; Sino-Japan Friendship Hospital: J. Lin; Shanghai Ruijin Hospital: M.Li; Zengcheng People's Hospital: R. Zou; The First People's Hospital of Changzhou: C. Li; 301 Hospital - Department of Cardiology: L. Gai; Nanjing Jiangning Hospital: X. Zhang; The First People's Hospital of Wujiang: Q. Wu; Peking University First Hospital: Y. Huang; Beijing Tongren Hospital, Cardiology Department: L. Li; Tai Yuan City Centre Hospital: P. Wang;
India
All India Institute of Medical Sciences: M. Tripathi; B Y L Nair Hospital: J.M. Joshi; Christian Medical College and Hospital: J.D. Pandian; Global Hospital: M. Samiuddin; Vijaya Health Center: S. Kumar; P S G Hospital: R. Palaniyappan; King Edverd Memorial Hospital: P. Kerkar; Medicit Hospital: A. Bordoloi; Chest Clinic, Sri Rama Krishna Medical Centre: M.K. Thekkinkattil.
New Zealand
Christchurch Hospital: M. Hlavac; Dunedin Hospital: B. Brockway; Hutt Hospital: K. Ferrier; Waikato Hospital: C. Chang; Tauranga Hospital: N. Graham.
Spain
Hospital Universitario de Guadalajara: O. Mediano; Hospital Parc Tauli: M.J. Masdeu; Hospital Doce De Octubre, Madrid: M.J.D de Atauri; Hospital Santa Maria: F. Barbé; Hospital Txagorritxu: J. Durán.
United States of America
Mayo Clinic: S. Caples.
Executive Committee: R.D. McEvoy (PI), C.S. Anderson (co-PI), R.R. Grunstein, B. Neal, S.G. Huang, N.S. Zhong, J.G. Wang, J. Hedner, G. Lorenzi-Filho, S. Redline, E. Heeley.
National Leaders: China - Steering Committee: N. Zhong, S. Huang, X. Zhang, Q. He, Y. Xiao, B. Chen, J. Wang, Y. Huang.
Data Safety and Monitoring Committee: G. Jennings (Chair), Q. Li, G. Marks and K.S. Wong.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Bassetti C, Aldrich MS. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep. 1999;22:217–23. doi: 10.1093/sleep/22.2.217. [DOI] [PubMed] [Google Scholar]
- 3.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. Journal of hypertension. 2001;19:2271–7. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Mooe T, Franklin KA, Holmstrom K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. 2001;164:1910–3. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 5.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11:441–6. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 8.Collard P, Pieters T, Aubert G, Delguste P, Rodenstein DO. Compliance with nasal CPAP in obstructive sleep apnea patients. Sleep Med Rev. 1997;1:33–44. doi: 10.1016/s1087-0792(97)90004-6. [DOI] [PubMed] [Google Scholar]
- 9.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 10.Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Thorne LN, Punjabi NM, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97:698–706. doi: 10.1161/01.RES.0000183879.60089.a9. [DOI] [PubMed] [Google Scholar]
- 12.Iiyori N, Alonso LC, Li J, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175:851–7. doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher EC, Lesske J, Qian W, Miller CC, 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19:555–61. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- 14.He QY, Feng J, Zhang XL, et al. Relationship of daytime blood pressure and severity of obstructive sleep apnea among Chinese: a multi-center investigation in China. Chinese Med J. 2010;123:18–22. [PubMed] [Google Scholar]
- 15.McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med. 2007;175:190–5. doi: 10.1164/rccm.200602-270OC. [DOI] [PubMed] [Google Scholar]
- 16.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 17.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 18.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–17. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 19.Barbe F, Duran-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–26. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 20.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–23. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 21.Arias MA, Garcia-Rio F, Alonso-Fernandez A, Martinez I, Villamor J. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled crossover study. Eur Heart J. 2006;27:1106–13. doi: 10.1093/eurheartj/ehi807. [DOI] [PubMed] [Google Scholar]
- 22.Sajkov D, Wang T, Saunders NA, Bune AJ, McEvoy RD. Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:152–8. doi: 10.1164/ajrccm.165.2.2010092. [DOI] [PubMed] [Google Scholar]
- 23.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–12. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 24.Kohler M, Ayers L, Pepperell JC, et al. Effects of continuous positive airway pressure on systemic inflammation in patients with moderate to severe obstructive sleep apnoea: a randomised controlled trial. Thorax. 2009;64:67–73. doi: 10.1136/thx.2008.097931. [DOI] [PubMed] [Google Scholar]
- 25.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 27.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 28.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–52. [PubMed] [Google Scholar]
- 29.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156:115–22. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Garcia MA, Campos-Rodriguez F, Soler-Cataluna JJ, Catalan-Serra P, Roman-Sanchez P, Montserrat JM. Increased incidence of nonfatal cardiovascular events in stroke patients with sleep apnoea: effect of CPAP treatment. Eur Respir J. 2012;39:906–12. doi: 10.1183/09031936.00011311. [DOI] [PubMed] [Google Scholar]
- 31.Takama N, Kurabayashi M. Influence of untreated sleep-disordered breathing on the long-term prognosis of patients with cardiovascular disease. Am J Cardiol. 2009;103:730–4. doi: 10.1016/j.amjcard.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 32.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 34.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin Sleep Cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath. 2010;14:131–6. doi: 10.1007/s11325-009-0298-7. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–15. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 37.Duran-Cantolla J, Aizpuru F, Montserrat JM, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. 2010;341:c5991. doi: 10.1136/bmj.c5991. [DOI] [PubMed] [Google Scholar]
- 38.Bakris GL, Townsend RR, Liu M, et al. Impact of renal denervation on 24-hour ambulatory blood pressure: results from SYMPLICITY HTN-3. J Am Coll Cardiol. 2014;64:1071–8. doi: 10.1016/j.jacc.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 40.Cook NR, Albert CM, Gaziano JM, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167:1610–8. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159:824–34. doi: 10.7326/0003-4819-159-12-201312170-00729. [DOI] [PubMed] [Google Scholar]
- 42.Somers V. Sleep - a new cardiovascular frontier. N Engl J Med. 2005;353:2070–3. doi: 10.1056/NEJMe058229. [DOI] [PubMed] [Google Scholar]
- 43.Gottlieb DJ, Craig SE, Lorenzi-Filho G, et al. Sleep apnea cardiovascular clinical trials-current status and steps forward: the International Collaboration of Sleep Apnea Cardiovascular Trialists. Sleep. 2013;36:975–80. doi: 10.5665/sleep.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Group AC, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 45.Anderson C, Heeley E, Heritier S, et al. Statistical analysis plan for the second INTEnsive blood pressure Reduction in Acute Cerebral hemorrhage Trial (INTERACT2): a large-scale investigation to solve longstanding controversy over the most appropriate management of elevated blood pressure in the hyperacute phase of intracerebral hemorrhage. Int J Stroke. 2013;8:327–8. doi: 10.1111/ijs.12004. [DOI] [PubMed] [Google Scholar]
- 46.Gantner D, Ge JY, Li LH, et al. Diagnostic accuracy of a questionnaire and simple home monitoring device in detecting obstructive sleep apnoea in a Chinese population at high cardiovascular risk. Respirology. 2010;15:952–60. doi: 10.1111/j.1440-1843.2010.01797.x. [DOI] [PubMed] [Google Scholar]
- 47.Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–40. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 48.Peker Y, Kraiczi H, Hedner J, Loth S, Johansson A, Bende M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J. 1999;14:179–84. doi: 10.1034/j.1399-3003.1999.14a30.x. [DOI] [PubMed] [Google Scholar]
- 49.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in women: occurrence and association with coronary artery disease. Am J Med. 1996;101:251–6. doi: 10.1016/S0002-9343(96)00122-2. [DOI] [PubMed] [Google Scholar]
- 50.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest. 1996;109:659–63. doi: 10.1378/chest.109.3.659. [DOI] [PubMed] [Google Scholar]
- 51.Lee CH, Khoo SM, Tai BC, et al. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest. 2009;135:1488–95. doi: 10.1378/chest.08-2336. [DOI] [PubMed] [Google Scholar]
- 52.Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovas Dis. 2009;51:434–51. doi: 10.1016/j.pcad.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Weinreich G, Armitstead J, Topfer V, Wang YM, Wang Y, Teschler H. Validation of ApneaLink as screening device for Cheyne-Stokes respiration. Sleep. 2009;32:553–7. doi: 10.1093/sleep/32.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The ethics of research related to healthcare in developing countries. Avaiable from http://www.nuffieldbioethics.org/go/ourwork/developingcountries/publication_309.html.2002.
- 55.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farre R, Hernandez L, Montserrat JM, Rotger M, Ballester E, Navajas D. Sham continuous positive airway pressure for placebo-controlled studies in sleep apnoea. Lancet. 1999;353:1154. doi: 10.1016/S0140-6736(99)01056-9. [DOI] [PubMed] [Google Scholar]
- 57.Pablos-Mendez A, Barr RG, Shea S. Run-in periods in randomized trials: implications for the application of results in clinical practice. JAMA. 1998;279:222–5. doi: 10.1001/jama.279.3.222. [DOI] [PubMed] [Google Scholar]
- 58.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5:720–8. doi: 10.1161/CIRCOUTCOMES.111.964783. [DOI] [PubMed] [Google Scholar]
- 60.Chai-Coetzer CL, Luo YM, Antic NA, et al. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep. 2013;36:1929–37. doi: 10.5665/sleep.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180:36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 62.Hsu CY, Vennelle M, Li HY, Engleman HM, Dennis MS, Douglas NJ. Sleep-disordered breathing after stroke: a randomised controlled trial of continuous positive airway pressure. J Neurol Neurosurg Psychiatry. 2006;77:1143–9. doi: 10.1136/jnnp.2005.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. 2011;42:1062–7. doi: 10.1161/STROKEAHA.110.597468. [DOI] [PubMed] [Google Scholar]
- 64.Bravata DM, Concato J, Fried T, et al. Continuous positive airway pressure: evaluation of a novel therapy for patients with acute ischemic stroke. Sleep. 2011;34:1271–7. doi: 10.5665/SLEEP.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]