Abstract
Background:
Previous research has demonstrated a relation between insufficient sleep and overall obesity. Waist circumference (WC), a measure of central adiposity, has been demonstrated to improve prediction of health risk. However, recent research on the relation of insufficient sleep duration to WC in adults has yielded inconsistent findings.
Objectives:
To assess the magnitude and the consistency of the relation of insufficient sleep and WC
Methods:
A systematic search of Internet and research databases using Google Scholar, Medline, PubMed, and PsycINFO through July 2013 was conducted. All articles in English with adult human subjects that included measurements of WC and sleep duration were reviewed. A random effects meta-analysis and regression analyses were performed. Heterogeneity and publication bias were checked. Results are expressed as Pearson correlations (r; 95% confidence interval).
Results:
Of 1,376 articles, 30 met inclusion criteria and 21 studies (22 samples for a total of 56,259 participants) provided sufficient data for meta-analysis. Results showed a significant negative relation between sleep duration and WC (r = −0.10, P < 0.0001) with significant heterogeneity related to sleep comparison method. Potential moderators of the relation between sleep duration and WC were not significant. Funnel plots showed no indication of publication bias. In addition, a fail-safe N calculation indicated that 418 studies with null effects would be necessary to bring the overall mean effect size to a trivial value of r = −0.005.
Conclusions:
Internationally, cross-sectional studies demonstrate a significant negative relation between sleep duration and waist circumference, indicating shorter sleep durations covary with central adiposity. Future research should include prospective studies.
Citation:
Sperry SD, Scully ID, Gramzow RH, Jorgensen RS. Sleep duration and waist circumference in adults: a meta-analysis. SLEEP 2015;38(8):1269–1276.
Keywords: central adiposity, meta-analysis, obesity, sleep duration, waist circumference
INTRODUCTION
Aspects of modern society, including longer work hours, increased shift work, and 24/7 access to activities that compete with sleep, have led to a trend toward shortened sleep duration.1 In the United States in 2001, 38% of adults reported getting 8 h of sleep per night, and, by 2009, that percentage had dropped to only 28%.2 Several studies have demonstrated that insufficient sleep is associated with increased risk for poor physical health (including hypertension,3–5 metabolic syndrome,6 type 2 diabetes,6,7 and hypercholesterolemia8) and mental health (including depressed mood, anxiety, and cognitive deficits7). In parallel with the decrease in sleep, the prevalence of obesity has increased. In 2009–2010, 78 million US adults ages 20 y and older were obese.9 The World Health Organization has identified obesity as a worldwide epidemic.10 Like insufficient sleep, obesity has also been linked to increased health risk for many diseases, including heart disease, stroke, metabolic syndrome, type 2 diabetes, and certain types of cancer.10,11
Previous studies on the relation of obesity to short sleep duration focused on measures of overall obesity, such as body mass index (BMI).12 However, recent research suggests that measures of central adiposity (i.e., excessive adipose tissue gained around the waist or abdomen above healthy guidelines), such as waist circumference (WC), may provide better prediction of health risk.13–16 For example, a logistic regression analysis of both BMI and WC as continuous variables found WC to be a significant predictor of almost all of the examined comorbidities (hypertension, hypercholesterolemia, high low-density lipoprotein, high triacylglycerol, and metabolic syndrome) in both men and women, whereas BMI was no longer a predictor of comorbidity, despite its significant association when analyzed alone.14 Current research on the relation between sleep duration and WC has yielded mixed findings that are difficult to interpret, due to variability in target populations and measurement methodology. This meta-analysis was designed to examine the consistency and magnitude of the association of insufficient sleep duration with WC.
METHODS
Computerized literature searches of research through July 2013 were conducted using online searches of the Internet and research databases using Google Scholar, Medline, PubMed, and PsycINFO. Search terms used to capture articles were “adipos*” (to capture terms “adipose” and “adiposity”) and “waist” and “sleep”, in all fields. All articles dealing with human participants were selected. All research articles in English were reviewed. Reference lists of articles obtained were also reviewed for additional relevant articles. A total of 1,376 research articles were reviewed at the level of abstract or full article by two independent reviewers (SDS and IDS) to determine if inclusion criteria were met.
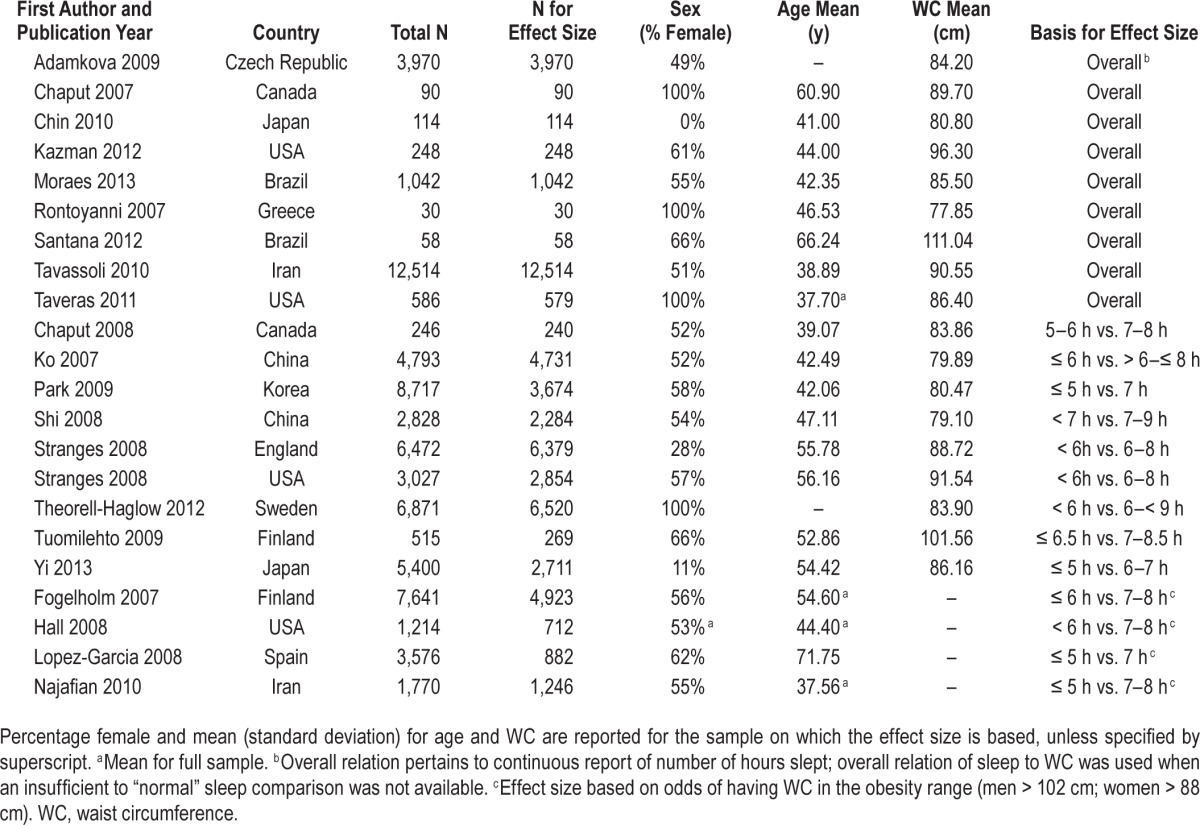
Articles were selected for inclusion in this meta-analysis if the article: (1) was a research study of adults (age 18 y and older); (2) indicated collection of measurements of WC and sleep duration, even if this relation was not a focus of analyses; and (3) reported the necessary information for computation of an effect size. Of the 1,376 articles reviewed, 49 studies were selected. After full and thorough review, eight studies were excluded because they were based on the same sample as other studies, and an additional 11 were excluded because they did not collect relevant data on WC or sleep duration. Of the remaining 30 articles, 17 were identified that referenced measurements on WC and sleep duration, but lacked the necessary information for computation of an effect size. Authors of these 17 studies were contacted via email, and eight responded to provide the necessary data. This process resulted in a total of 21 articles reporting on 22 samples using data collected from 56,259 participants. Table 1 presents a list of included studies, their date of publication, country of study, sample size, sex, mean age, mean WC, and the source for the effect size.
Table 1.
Studies included in meta-analysis.
Methodological Issues in Sleep and WC Measurement
Researchers examining the association of central adiposity and sleep have used a number of different approaches to measuring sleep and WC. Analytic approaches also vary, with some researchers examining the overall relation of sleep duration to WC and other researchers examining the effect of insufficient sleep on WC through a “short” versus “normal” sleep comparison. Methodological issues in the measurement of central adiposity and sleep, as well as how these issues were coded in our meta-analytic review, are detailed in the following paragraphs.
Objective means of assessing sleep, such as actigraphic recordings during sleep and polysomnographic studies, provide reliable measurement of sleep duration; however, these studies place burden on participants and can be time-consuming and costly. Sleep duration is very commonly and less optimally measured through a variety of self-report methods. In this meta-analytic review, all but two of the studies relied solely on self-report of sleep duration. As indicated in the previous paragraph, approaches to the analysis of the relation of sleep duration to WC also vary. Some studies separate data into several sleep duration groups in order to compare “short” and “normal” sleepers on health factors such as WC.6,17–27 Other researchers explore the overall relation of sleep to WC by conducting analyses that treat both sleep and WC as continuous variables.28–36 In this meta-analytic review, studies that used an insufficient versus “normal” sleep comparison were coded in terms of how these groups were created to permit examination of whether differences in sleep duration categories contributed to significant heterogeneity among effect sizes.
The majority of research studies examining WC as a measure of central adiposity use tape measure assessments because of their ease and proven clinical utility.37 Multiple approaches exist regarding the site on the body at which WC is measured, with measurements taken at the narrowest portion of the waist (the “minimal” waist17,19,29,31), the umbilicus,6,27,30 and, most commonly, at the midpoint between the iliac crest and the lowest rib margin,18,20–23,25,26,28,34,35 as recommended by the World Health Organization.15 Measurement site was coded to investigate whether the effect sizes varied in terms of WC measurement site.
Potential Moderators of the Relation of Sleep and Central Adiposity
Age and sex affect adiposity and may affect health risk prediction based on WC.38 To date, research findings as to how sex influences the relation of sleep duration and central adiposity are mixed.18,19,35 To allow for exploration of the moderating effect of sex on the relation of sleep duration and WC, all studies in this meta-analytic review were coded as to sex composition (percentage of female participants) to permit inclusion of this variable in meta-regression analyses. In an attempt to shed light on the age-related inconsistencies18,22,25 reported, studies also were coded for the mean age of participants to examine if this variable moderated the association of sleep duration and WC.
Articles selected for inclusion in the meta-analysis were coded separately by two independent reviewers (SDS and IDS) using a standard coding sheet, based on a detailed coding manual. Initial agreement on effect size between the two coders was high (intraclass coefficient = 0.998). Discrepan -cies between coded datasets were resolved through consensus meetings in which the article and data were reexamined and final coding was resolved. Three separate operational approaches to effect sizes were used in these studies: overall relation between sleep duration and WC; insufficient to “normal” sleep comparison predicting continuous WC; and insufficient to “normal” sleep comparison predicting obese/not obese (see Table 1 for the basis of each individual study). Because this meta-analysis was focused on the relation of insufficient sleep to WC, effect sizes for the comparison of “short” and “normal” sleep were used whenever sufficient data were reported. A subset of studies comparing insufficient to normal sleep based effect sizes on the odds of having WC in the obesity range. When data for an insufficient versus “normal” sleep comparison were not available, effect sizes based on the overall relation of sleep duration to WC were used. These three approaches were assigned numerical codes to permit for analyses to examine whether sleep comparison method contributed to heterogeneity of effect sizes.
Selection of a Random-Effects Model Method
A random-effects model was selected as the best fit to the studies under consideration. A random-effects model assumes that the studies included in the meta-analysis are estimating multiple population effects, and that variability between effect sizes is due to sampling error plus variability in the population of effects.39 It is plausible that the underlying mechanisms linked to the association of insufficient sleep and WC differ across populations due to variation in cultural factors in diet, physical activity, and work hours, as well as to ethnic differences in body fat distribution.40–42 Because this review includes studies conducted in thirteen different countries with different cultural and ethnic factors, it seems likely that effect sizes from these studies are estimating multiple population effects. Unlike fixed-effects models, random-effects models do not assume that all effect sizes in the meta-analysis estimate the same population value.43
Studies Reporting Multiple Effect Sizes
To ensure statistical independence, each study was permitted to contribute only one effect size.39 Because this meta-analysis was focused on the relation of WC to insufficient sleep, whenever possible, the effect size selected for inclusion compared an insufficient sleep group to a normal sleep group. When studies did not report data that permitted a comparison of insufficient to normal sleep, the effect size on the overall relation of WC to sleep duration was used. Five studies reported statistics separately for men and women; these effect sizes were averaged.18,21,27,34,35 One study32 reported statistics from self-report and objective assessment of sleep duration; effect sizes calculated from these statistics were averaged to yield a single effect size. One article24 reported statistics from two separate samples (one in England and one in the United States); a single effect size was calculated and included from each sample. This approach yielded a total 22 effect sizes that were then used to calculate mean effect size.
Computation and Statistical Analysis of Effect Sizes
Statistical data (correlations, odds ratios, standardized betas, and means and standard deviations) on the relation of sleep duration to WC were extracted from each article and converted to Pearson correlation coefficients (r).39 Pearson correlation coefficients were then transformed to ZFISHER for all statistical calculations, as the distribution of r becomes skewed at extreme values.39,44,45 To compute mean effect size across the 22 samples included in this meta-analysis, each ZFISHER effect size was weighted by the inverse of its variance, in order to account for sample size. The 95% confidence interval for each effect size was calculated. The homogeneity test based on the Q statistic was calculated using a random-effects model to examine whether the effect sizes used for the combined mean effect size were estimates from the same distribution. In the random-effects model, the variance associated with each effect size has two components, one that is associated with subject-level sampling error and a second component that is due to random-effects variance.39 The random-effects variance component for these analyses was estimated through an iterative method based on maximum likelihood.46 If significant heterogeneity was revealed by significant Q statistics, analyses using the meta-analog to analysis of variance (ANOVA) were planned to determine whether heterogeneity resulted from differences in the way different studies operationalized the assessment of the relation between sleep duration and WC.
Moderator Analyses
If significant heterogeneity was revealed by significant Q statistics, the meta-analytic analog to ANOVA39 was planned to explore the effect of differences in how the relation of sleep duration to WC was operationalized. This statistical approach enables the partitioning of the total variance to distinct between- and within-category sources. The Qw statistic was used to estimate heterogeneity in effect sizes within categories. Similarly, the QB statistic was used to determine if there was a significant difference in mean-weighted effect sizes between categories. Several studies (k = 13) provided data allowing the comparison of an insufficient sleep group to a normal sleep group.6,17–27 Within these studies, four studies based their effect size on the odds of having WC in the obesity range (defined as men > 102 cm, or women > 88 cm).6,18,20,21 For studies lacking needed information to yield an effect size based on an insufficient sleep to normal sleep comparison (k = 9), an effect size based on the overall relation of sleep duration to WC was calculated.28–36 Potentially moderating variables were tested using multiple meta-regression models.39 These meta-regression models are capable of examining multiple moderating variables simultaneously, even if they are correlated. The variables examined included the following factors: mean age, sex, and WC measurement site.
Publication Bias
Meta-analytic results may be affected by exclusively analyzing results from published studies, which often are biased in favor of significant results that can inflate effect size estimates.47 Fail-safe numbers47 were calculated to estimate the effect of sampling bias and estimate tolerance for null results. Additionally, funnel plots48 were created to evaluate potential bias due to underrepresentation of studies with small samples.39
RESULTS
In this meta-analysis, 22 effect sizes were computed from 21 studies based on a total of 56,259 participants. All studies were published between 2007 and 2013.
Meta-Analytic Results
This random-effects meta-analysis across k = 22 effect sizes from studies that tested the association between sleep duration and WC produced a weighted mean effect size of r = −0.10 (95% confidence interval = −0.14, −0.05; Z = −4.51, P < 0.0001). See Figure 1 for a forest plot of the effect sizes from each study and the overall effect size. The Q statistic, which follows the χ2 distribution, was examined as a measure of homogeneity. Significant heterogeneity was found for the association between sleep duration and WC, Q (22) = 424.55, P < 0.0001, indicating between-study variability. Therefore, moderator analyses were conducted in an effort to systematically account for the heterogeneity among effect sizes.
Figure 1.
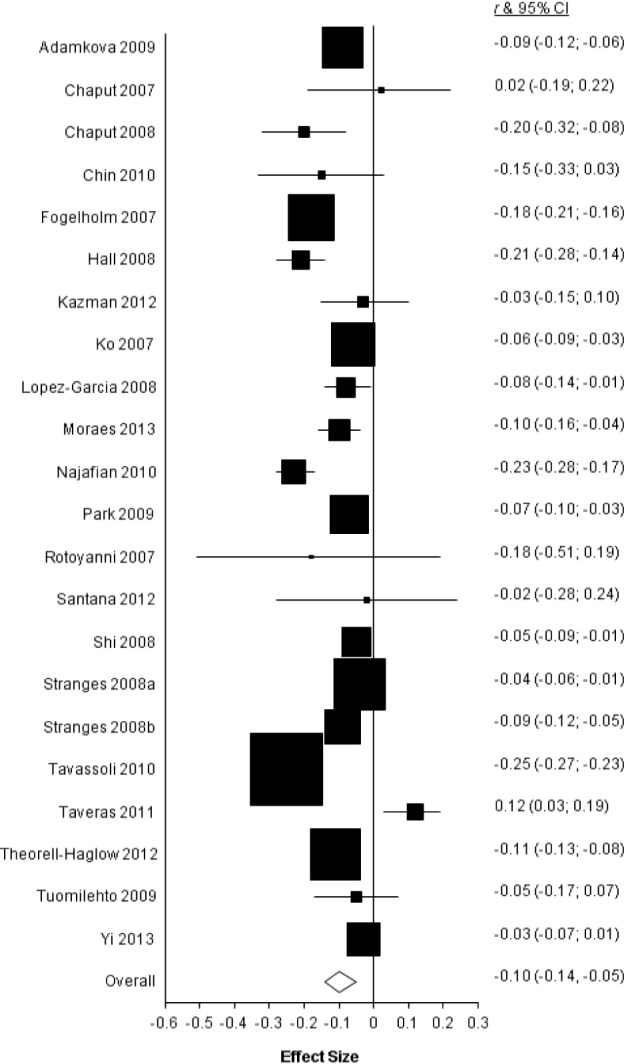
Forest plot of the associations between short duration of sleep and waist circumference. r and 95% confidence interval (CI) indicate Pearson correlation and 95% CIs. Size of the markers is proportional to the weight given each individual study.
Moderator Analyses
The meta-analytic analog to ANOVA (MetaF)39 was used to determine whether the association between sleep duration and WC differed based on how sleep comparison method (that is, the relation of sleep duration to WC) was operationalized. The analysis showed a non-significant Qw statistic, Qw (19) = 20.74, P > 0.05, indicating homogeneity in the residual variability within each method of exploring the sleep duration to WC relation. However, the QB statistic, which is the meta-analog to the F statistic in ANOVA, was significant, QB (2) = 6.26, P < 0.05, indicating a significant between-groups effect. This suggests that the relation between sleep duration and WC was significantly different across the three approaches to categorizing the relation of sleep duration to WC.
An examination of the mean effect sizes based on each categorization approach revealed that the largest weighted mean effect size was found in studies that utilized an insufficient to normal sleep comparison when predicting obesity (r = −0.18; 95% confidence interval = −0.25, −0.11; Z = −4.91, P < 0.0001). Studies examining the overall relation of sleep duration to WC yielded a weighted mean effect size of r = −0.09 (95% confidence interval = −0.14, −0.03; Z = −2.86, P < 0.01). Studies that used an insufficient to “normal” sleep comparison when predicting continuous WC yielded a weighted mean effect size of r = −0.07 (95% confidence interval = −0.12, −0.02; Z = −2.98, P < 0.01). The Qw statistics within each of the three approaches were all nonsignificant, indicating that there was not significant heterogeneity within the studies using these three different approaches to measuring the relation of sleep duration to WC.
The meta-analytic analog to weighted least squares regression was used to determine if other variables (mean age, sex, and WC measurement site) were significant independent predictors of the effect size. Regression analyses focused on mean age, sex, and WC measurement site were based on a reduced number of studies because data specific to the sample on which the effect size was based were not always available. When the predictors were entered independently, none of the relations between the effect size and examined variables were significant. The relation between mean age and effect size showed a trend towards significance (P = 0.05).
Publication Bias
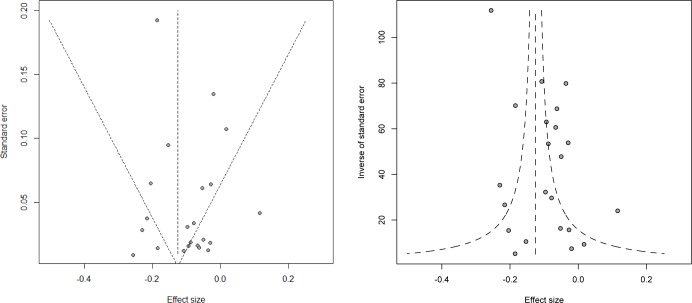
To examine the possibility that publication bias affected results of this meta-analysis, the fail-safe N 47 was calculated and funnel plots were created and examined. The fail-safe N was 418, which represents the number of additional studies with an effect size of zero needed to bring the overall effect size to the trivial value of −0.005.39 However, it should be noted that calculations for fail-safe N, unlike the calculations within meta-analysis, are not weighted according to sample size or variance and assume that the effect size is the same across study populations, unlike random-effects models. To further explore the possibility that publication bias affected results of this meta-analysis, funnel plots were generated. Figure 2 presents funnel plots, which represent a scatterplot of treatment effect plotted against standard error of the treatment effect and inverse variance of the treatment effect (weight).49 Examination of the funnel plots confirms that publication bias does not appear to be a concern with this set of studies. The effect sizes are distributed symmetrically around the weighted mean value. There is one effect size from a large-sample study that is noticeably large, but the aforementioned analyses are essentially unchanged when this study is omitted.
Figure 2.
Funnel plots for the effect of sleep duration on waist circumference.
DISCUSSION
This meta-analysis is the first to quantify the magnitude and consistency of the relation of insufficient sleep to WC, a measure of central adiposity with demonstrated sensitivity in the prediction of health risk. Across 22 studies utilizing data collected from 56,259 participants, a random-effects model approach yielded a small but significant negative relation (r = −0.10; P < 0.0001) between sleep duration and WC, in which shorter sleep durations are associated with an increase in central adiposity. Despite significant heterogeneity related to sleep comparison method across the 22 studies, there was a consistent pattern of findings of a significant negative relation between sleep duration and WC, providing empirical support that shorter sleep durations are linked to an increase in central adiposity. The emergence of this pattern across all studies, despite methodological and analytic variances, is notable. Studies that explore the overall relation of sleep to WC may underestimate this association, as some research20,23 has demonstrated significant curvilinear relations between WC and sleep duration, with both insufficient and longer duration sleep resulting in increased WC. Studies that dichotomize sleep duration into groups rather than analyze sleep duration as a continuous variable may also reduce their statistical power to detect effects.50
In this review, studies that used an insufficient to normal sleep comparison in the prediction of WC in the obesity range yielded the largest mean effect size (r = −0.18), reflecting that the significant association between insufficient sleep and WC is demonstrated most clearly in individuals who are obese. The insufficient to “normal” sleep comparison predicting obese/ not obese likely provides the most insight into biologically meaningful associations between sleep duration and WC. Established guidelines for obesity (defined by the World Health Organization as WC > 102 cm in men, or WC > 88 cm in women) reflect thresholds at which there is increased risk for disease (e.g., cardiovascular disease, hypertension, type 2 diabetes, and hypercholesterolemia) and mortality. A recent meta-analysis examining the relation of insufficient sleep to obesity measured by BMI found a comparable association (pooled odds ratio of 1.55, which equates to an r of 0.17).12 Further research is needed to clarify which obesity measures provide the best tools to assess specific health risk. Recent meta-analyses have shown WC to outperform BMI in the prediction of cardiovascular risk51 and waist-to-height measures to outperform both WC and BMI in the prediction of cardiometabolic health risk.52 The similar magnitude relation found in this review suggests that WC and BMI may perform similarly in sleep research.
Other potential moderators of the relation of sleep duration to WC (including the following: mean age, sex, and WC measurement site) were examined but were not found to be significant. However, meta-regression analyses were limited by insufficient data across all examined studies. Therefore these findings are preliminary and should be an area of focus for future research.
Limitations in Research
Despite areas of strength, it should be noted that this meta-analysis has certain weaknesses and limitations. Meta-analytic findings are limited by the quality of the studies included. Some of the studies that were reviewed did not supply sufficient data to permit their inclusion. Most of the studies (95.5%) included in this meta-analysis were cross-sectional in nature, and these studies do not provide information on temporal sequence or causality. Nonetheless, these results are important in that they permit assessment of the current body of research and suggest directions for future study.
The discrepancies in effect sizes of the relation between sleep duration and WC across the studies may result from a number of study limitations. These studies varied in their definitions of insufficient sleep and normal sleep, as well as their approach to how the relation of sleep duration to WC was characterized (i.e., whether effect sizes were based on the overall relation of sleep duration to WC, an insufficient to normal sleep comparison, or an insufficient to normal sleep comparison predicting obesity), a factor that was found to underlie significant variability among study findings. Only two studies30,32 actually used objective means such as polysomnography or wrist actigraphy to measure sleep duration, with most studies instead relying on self-report. Self-reported sleep duration has been noted to be quite inaccurate among insufficient sleep sufferers.53 Self-report may be a biased proxy for actual sleep duration and may underestimate the strength of the relation between sleep duration and central adiposity. Also, the studies reviewed did not assess sleep quality as an issue separate from sleep duration, making it impossible to assess whether poor sleep quality has an effect on WC that is distinct from the effect of shortened sleep duration. Critically, none of the studies reviewed attempt to determine the length of time the participant has suffered from disordered sleep, which may directly affect the degree to which the disordered sleep has resulted in metabolic and physiological changes, such as central fat mass gain.
Although several potential moderators of the relation of insufficient sleep and WC have been identified in previous research, exploration of these moderators was hampered by inconsistent reporting of these variables across the studies included in this meta-analytic review. For example, only 14 of the 22 studies included in this meta-analysis included data on the mean age of the participants for the sample on which the effect size was based. Only 19 of the studies reported sex composition for the sample on which the effect size was based. More commonly, studies reported these demographic variables only for the full sample. Sample characteristics as to racial composition were seldom reported in these studies, making analyses for potential moderation based on this factor impossible. Underreporting on these sample characteristics limits our ability to assess whether these factors are significant moderators in the relation of sleep duration and WC.
Future Directions
Research on the relation of insufficient sleep and central adiposity has yielded interesting findings to date. Because of the finding of a significant association between insufficient sleep and WC, this meta-analytic review suggests several potential directions for future research.
Standardized Assessment
Currently, measurement and definitional issues limit interpretation of findings from research on the relation between insufficient sleep and central adiposity. Standardized operational definitions of insufficient and normal sleep across studies would provide a better means for cross-study comparison. Use of objective medical assessments, such as actigraphy or polysomnographic studies, would permit researchers to make more credible claims about the relation of actual (rather than self-reported) sleep duration to central adiposity. For studies using self-report, well-validated sleep questionnaires, such as the Pittsburgh Sleep Quality Index54 would permit researchers to quantify sleep duration and quality, as well as obtain rates of specific reported sleep problems in the past month.
Increased Exploration of Moderating Factors
Information about known potential moderators of the relation of insufficient sleep and WC, including information on age, sex, ethnicity, and race, was not reported for many of the studies included in this review. Future research would benefit from better reporting of these characteristics for all statistical comparisons made. Also, the research studies examined in this meta-analysis seldom reported whether participants had diagnosed medical conditions that have established relations with sleep problems, central adiposity, or both conditions. In particular, future research should assess the presence of obstructive sleep apnea (OSA) and snoring, which have been shown to have associations with obesity and insufficient sleep. Also, most of the studies examined did not include any assessment of psychological factors that may influence the association of sleep duration and WC. Inclusion of assessments of stress, depression, and anxiety may elucidate important psychological variables that may play an important role in this relationship. For example, rumination at bedtime may delay sleep onset, leading to a decrease in sleep quality and overall sleep duration. High levels of stress or depressed mood may decrease physical activity or increase food consumption, both of which could contribute to central adiposity. Given that this meta-analysis supports a significant relation between sleep duration and WC, it will be important to expand research to areas that will allow us to better understand factors that moderate this relation and may provide avenues for targeted intervention.
Focus on the Future
Because of the emergence of both insufficient sleep and central weight gain in childhood, this research area could benefit from an increased number of studies focused on younger populations. Prospective longitudinal studies examining the relation of central adiposity and insufficient sleep over time could help to establish the directionality of the association of insufficient sleep and central weight gain. These studies could assist in clarifying how the relation of sleep duration and WC is influenced by age and sex. Finally, prospective studies could help illuminate how insufficient sleep and WC lead to negative health outcomes.
Opportunities for Intervention
The findings from this meta-analytic review, which document a significant relation between insufficient sleep and WC, suggest directions for health promotion interventions. Given the demonstrated relation between WC and health risk, for individuals with central adiposity, interventions aimed at improving sleep may prove beneficial in reducing WC and improving overall health. Research into evidence-based interventions55 to treat conditions such as insomnia that result in insufficient sleep has led to established practice parameters for behavioral interventions with demonstrated efficacy, including stimulus control therapy, relaxation training, sleep restriction, cognitive behavioral therapy, paradoxical intention, and biofeedback. Inclusion of behavioral health team members in primary care settings may prove to be an effective means of providing medical patients with treatments that have been shown to be efficacious in improving sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
Footnotes
A commentary on this article appears in this issue on page 1159.
REFERENCES
Studies included in the meta-analysis: 6, 17–36.
- 1.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Sleep Foundation. Washington, DC: National Sleep Foundation; 2009. Sleep in America poll. [Google Scholar]
- 3.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II study. Hypertension. 2007;50:694–701. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 5.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colten HR, Altevogt BM, editors. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: The National Academies Press; 2006. [PubMed] [Google Scholar]
- 8.Gangwisch JE, Malaspina D, Babiss LA, et al. Short sleep duration as a risk factor for hypercholesterolemia: analyses of the National Longitudinal Study of Adolescent Health. Sleep. 2010;33:956. doi: 10.1093/sleep/33.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogden CL, Carroll MD, Kit BK, Flegal KM. Hyattsville, MD: National Center for Health Statistics; 2012. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief no 82. [PubMed] [Google Scholar]
- 10.World Health Organization. Geneva: World Health Organization; 1998. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation on Obesity. [PubMed] [Google Scholar]
- 11.Overweight and obesity: adult obesity facts. Centers for Disease Control website. [Accessed December 16, 2014]. http://www.cdc.gov/obesity/data/adult.html. Published April 27, 2012. Updated September 9, 2014.
- 12.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ardern CI, Katzmarzyk PT, Janssen I, Ross R. Discrimination of health risk by combined body mass index and waist circumference. Obes Res. 2003;11:135–42. doi: 10.1038/oby.2003.22. [DOI] [PubMed] [Google Scholar]
- 14.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379–84. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Geneva: 2008. Dec 8–11, Waist Circumference and Waist–hip Ratio: Report of a WHO Expert Consultation. [Google Scholar]
- 16.Zhu S, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am J Clin Nutr. 2002;76:743–9. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 17.Chaput JP, Després JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a six-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–23. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fogelholm M, Kronholm E, Kukkonen-Harjula K, Partonen T, Partinen M, Härmä M. Sleep-related disturbances and physical inactivity are independently associated with obesity in adults. Int J Obes (Lond) 2007;31:1713–21. doi: 10.1038/sj.ijo.0803663. [DOI] [PubMed] [Google Scholar]
- 19.Ko GTC, Chan JCN, Chan AWY, et al. Association between sleeping hours, working hours and obesity in Hong Kong Chinese: the ‘better health for better Hong Kong’ health promotion campaign. Int J Obes (Lond) 2007;31:254–60. doi: 10.1038/sj.ijo.0803389. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-García E, Faubel R, León-Muñoz L, Zuluaga MC, Banegas JR, Rodríguez-Artalejo F. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr. 2008;87:310–6. doi: 10.1093/ajcn/87.2.310. [DOI] [PubMed] [Google Scholar]
- 21.Najafian J, Mohammadifard N, Dana Siadat Z, Sadri G, Ramazani M, Nouri F. Association between sleep duration and body mass index and waist circumference. Iran J Med Sci. 2010;35:140–4. [Google Scholar]
- 22.Park SE, Kim HM, Kim DH, Kim J, Cha BS, Kim DJ. The association between sleep duration and general and abdominal obesity in Koreans: data from the Korean National Health and Nutrition Examination Survey, 2001 and 2005. Obesity. 2009;17:767–71. doi: 10.1038/oby.2008.586. [DOI] [PubMed] [Google Scholar]
- 23.Shi Z, McEvoy M, Luu J, Attia J. Dietary fat and sleep duration in Chinese men and women. Int J Obes (Lond) 2008;32:1835–40. doi: 10.1038/ijo.2008.191. [DOI] [PubMed] [Google Scholar]
- 24.Stranges S, Dorn JM, Shipley MJ, et al. Correlates of short and long sleep duration: a cross-cultural comparison between the United Kingdom and the United States the Whitehall II study and the Western New York health study. Am J Epidemiol. 2008;168:1353–64. doi: 10.1093/aje/kwn337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theorell-Haglöw J, Berglund L, Janson C, Lindberg E. Sleep duration and central obesity in women–Differences between short sleepers and long sleepers. Sleep Med. 2012;13:1079–85. doi: 10.1016/j.sleep.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Tuomilehto H, Peltonen M, Partinen M, et al. Sleep duration, lifestyle intervention, and incidence of type 2 diabetes in impaired glucose tolerance: the Finnish diabetes prevention study. Diabetes Care. 2009;32:1965–71. doi: 10.2337/dc08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi S, Nakagawa T, Yamamoto S, et al. Short sleep duration in association with CT-scanned abdominal fat areas: the Hitachi Health Study. Int J Obes (Lond) 2013;37:129–34. doi: 10.1038/ijo.2012.17. [DOI] [PubMed] [Google Scholar]
- 28.Adamkova V, Hubacek JA, Lanska V, et al. Association between duration of the sleep and body weight. Physiol Res. 2009;58:S27–31. doi: 10.33549/physiolres.931853. [DOI] [PubMed] [Google Scholar]
- 29.Chaput JP, Després JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Québec Family Study. Obesity. 2007;15:253–61. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 30.Chin K, Oga T, Takahashi KI, et al. Associations between obstructive sleep apnea, metabolic syndrome, and sleep duration, as measured with an actigraph, in an urban male working population in Japan. Sleep. 2010;33:89. doi: 10.1093/sleep/33.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazman JB, Abraham PA, Zeno SA, Poth M, Deuster PA. Self-reported sleep impairment and the metabolic syndrome among African Americans. Ethn Dis. 2012;22:410–5. [PubMed] [Google Scholar]
- 32.Moraes W, Poyares D, Zalcman I, et al. Association between body mass index and sleep duration assessed by objective methods in a representative sample of the adult population. Sleep Med. 2013;14:31–8. doi: 10.1016/j.sleep.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Rontoyanni VG, Baic S, Cooper AR. Association between nocturnal sleep duration, body fatness, and dietary intake in Greek women. Nutrition. 2007;23:773–7. doi: 10.1016/j.nut.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Santana AA, Pimentel GD, Romualdo M, et al. Sleep duration in elderly obese patients correlated negatively with intake fatty. Lipids Health Dis. 2012;11:1–6. doi: 10.1186/1476-511X-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tavassoli AA, Gharipour M, Khosravi A, et al. Gender differences in obesogenic behaviour, socioeconomic and metabolic factors in a population-based sample of Iranians: the IHHP study. J Health Popul Nutr. 2010;28:602–9. doi: 10.3329/jhpn.v28i6.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taveras EM, Rifas-Shiman SL, Rich-Edwards JW, Gunderson EP, Stuebe AM, Mantzoros CS. Association of maternal short sleep duration with adiposity and cardiometabolic status at 3 years postpartum. Obesity. 2010;19:171–8. doi: 10.1038/oby.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.North American Association for the Study of Obesity and the National Heart, Lung, and Blood Institute. Bethesda, MD: National Institutes of Health; 2000. The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. [Google Scholar]
- 38.Stevens J, Katz EG, Huxley RR. Associations between gender, age and waist circumference. Eur J Clin Nutr. 2010;64:6–15. doi: 10.1038/ejcn.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipsey MW, Wilson DB. Thousand Oaks, CA: Sage Publications; 2001. Practical meta-analysis. [Google Scholar]
- 40.Carroll JF, Chiapa AL, Rodriquez M, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity. 2008;16:600–7. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 41.Lear SA, Toma M, Birmingham CL, Frohlich JJ. Modification of the relationship between simple anthropometric indices and risk factors by ethnic background. Metabolism. 2003;52:295–301. doi: 10.1016/s0026-0495(03)00196-3. [DOI] [PubMed] [Google Scholar]
- 42.Zhu S, Heymsfield SB, Toyoshima H, Wang Z, Pietrobelli A, Heshka S. Race-ethnicity-specific waist circumference cutoffs for identifying cardiovascular disease risk factors. Am J Clin Nutr. 2005;81:409–15. doi: 10.1093/ajcn.81.2.409. [DOI] [PubMed] [Google Scholar]
- 43.Field AP, Gillett R. How to do a meta-analysis. Br J Math Stat Psychol. 2010;63:665–94. doi: 10.1348/000711010X502733. [DOI] [PubMed] [Google Scholar]
- 44.Hedges LV, Olkin I. Orlando, FL: Academic Press; 1985. Statistical methods for meta-analysis. [Google Scholar]
- 45.Kim S, Jorgensen RS, Thibodeau R. Shame, guilt, and depressive symptoms: a meta-analytic review. Psychol Bull. 2011;137:68–96. doi: 10.1037/a0021466. [DOI] [PubMed] [Google Scholar]
- 46.Overton RC. A comparison of fixed-effects and mixed (random-effects) models for meta-analysis tests of moderator variable effects. Psychol Methods. 1998;3:354–79. [Google Scholar]
- 47.Rosenthal R. The “file drawer problem” and tolerance for null results. Psychol Bull. 1979;86:638–43. [Google Scholar]
- 48.Light RJ, Pillemer DB. Cambridge, MA: Harvard University Press; 1984. Summing up: the science of reviewing research. [Google Scholar]
- 49.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 50.Cohen J. The cost of dichotomization. App Psych Meas. 1983;7:249–53. [Google Scholar]
- 51.Lee CMY, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61:646–53. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13:275–86. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 53.Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138:77–101. doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 55.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. The diagnosis and management of insomnia in clinical practice: a practical evidence-based approach. Can Med Assoc J. 2000;162:210–6. [PMC free article] [PubMed] [Google Scholar]