Abstract
Study Objectives:
To evaluate the frequency, severity, and associations of symptoms of attention-deficit/hyperactivity disorder (ADHD) in children with narcolepsy with and without cataplexy.
Design:
Cross-sectional survey.
Setting:
Four French national reference centers for narcolepsy.
Patients:
One hundred eight consecutively referred children aged younger than 18 y with narcolepsy, with (NwC, n = 86) or without cataplexy (NwoC, n = 22), and 67 healthy controls.
Interventions:
The participants, their families, and sleep specialists completed a structured interview and questionnaires about sleep, daytime sleepiness, fatigue, and ADHD symptoms (ADHD-rating scale based upon Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision [DSM-IV-TR] symptoms), and use of psychostimulants for the treatment of narcolepsy (administered in 68.2%). Polysomnographic measures were collected.
Measurements and Results:
Clinically significant levels of ADHD symptoms were found in 4.8% of controls compared with 35.3% in patients with NwoC (P < 0.001) and 19.7% in patients with NwC (P < 0.01). Total ADHD scores were 6.4 (95% confidence interval [CI]: 4.5, 9.0) in controls compared with 14.2 (95% CI: 10.6, 18.9; P < 0.001), in patients with NwoC and 12.2 (95% CI: 9.8, 15.3; P < 0.01) in patients with NwC; subscores of inattention and hyperactivity/impulsivity were also significantly higher in both narcolepsy groups compared with controls. No difference was found between the NwC and NwoC groups for any ADHD measure. ADHD symptom severity was associated with increased levels of sleepiness, fatigue, and insomnia. Compared with the 34 untreated patients, the 73 patients treated with psychostimulants (modafinil in 91%) showed a trend toward lower narcolepsy symptoms but not lower ADHD symptoms.
Conclusions:
Pediatric patients with narcolepsy have high levels of treatment-resistant attention-deficit/hyperactivity disorder (ADHD) symptoms. The optimal treatment for ADHD symptoms in these patients warrants further evaluation in longitudinal intervention studies.
Citation:
Lecendreux M, Lavault S, Lopez R, Inocente CO, Konofal E, Cortese S, Franco P, Arnulf I, Dauvilliers Y. Attention-deficit/hyperactivity disorder (adhd) symptoms in pediatric narcolepsy: a cross-sectional study. SLEEP 2015;38(8):1285–1295.
Keywords: attention-deficit disorder with hyperactivity, methylphenidate, modafinil, narcolepsy, pediatrics
INTRODUCTION
Narcolepsy is characterized by excessive daytime sleepiness (EDS) with or without cataplexy (sudden loss of muscle tone typically triggered by strong emotion), hypnagogic hallucinations and sleep paralysis, and disturbed nocturnal sleep.1 Until recently, and at the time this study was conducted, the disorder was categorized into narcolepsy with cataplexy (NwC) and narcolepsy without cataplexy (NwoC) according to the revised second edition of the International Classification of Sleep Disorders (ICSD).2 ICSD-2-revised has now been replaced by ICSD-3, which classifies the disorder into narcolepsy type 1 (with hypo-cretin deficiency) characterized by EDS and one or both of the following: cataplexy and a mean sleep latency of up to 8 min and two or more sleep onset rapid eye movement periods (SOREMPs) during multiple sleep latency tests (MSLTs) performed according to standard techniques (REM onset within 15 min of sleep onset during preceding nocturnal polysomnography [PSG] may replace one of the SOREMPs during the MSLTs); and a cerebrospinal fluid (CSF) hypocretin-1 level ≤ 110 pg/mL. Narcolepsy type 2 (without hypocretin deficiency) is characterized by EDS and all four of the following: MSLT criteria as per type 1, absence of cataplexy, CSF hypocretin-1 level > 110 pg/ mL (if a lumbar puncture was performed), and EDS not being better explained by other causes.3
Childhood narcolepsy is usually characterized by higher levels of EDS, more frequent spontaneous than emotion-triggered cataplexy, and more frequent secondary forms of the disease. In addition to nocturnal sleep difficulties and EDS, narcolepsy affects metabolic and neuropsychiatric dimensions resulting in obesity,4 depressive symptoms,5 and attention- deficit/hyperactivity disorder (ADHD) symptoms,6 all of which contribute to reduced quality of life7 and lower academic performance in young people.6,8 In this report, we focus on ADHD symptoms in pediatric narcolepsy.
ADHD is characterized by a persistent, age-inappropriate pattern of behavior, which is present in multiple settings (e.g., school and home) and may result in impaired social, educational, or work performance. Symptoms are divided into two categories: inattention (e.g., difficulty sustaining attention, difficulty organizing tasks, excessive distractibility) and/or hyperactivity-impulsivity (e.g., excessive fidgeting, difficulty remaining seated, difficulty waiting turn) and several should have been present before 12 years of age.9 Given that deficits of alertness and sleep disturbances have been hypothesized to contribute to ADHD,10,11 comorbid ADHD symptoms are likely to be found in young people with narcolepsy. ADHD symptoms have been infrequently investigated in narcolepsy, especially in children.12,13 Some retrospective reports in adults with narcolepsy have identified frequent ADHD symptoms in childhood.14,15 Given these findings, there may be common underlying pathophysiological mechanisms linking narcolepsy and ADHD such that treatment for one condition may improve and/or affect the other, especially since treatments for narcolepsy, such as modafinil and methylphenidate,16 are also used in children with ADHD.
We aimed to investigate ADHD symptoms in pediatric narcolepsy, using cross-sectional data from: (1) a large cohort of children and adolescents with narcolepsy (with or without cataplexy) referred to and followed up in four national narcolepsy reference centers in France, and (2) control children. Specifically, we evaluated the frequency and severity of ADHD symptoms, and their associations with EDS, insomnia, fatigue, PSG characteristics, and psychostimulant treatment for narcolepsy. These associations were examined in both univariate and multivariate analyses (e.g., including the effect of age, sex, and body mass index z-score). In patients with clinically significant levels of ADHD symptoms, the burden of depressive symptoms, educational difficulty, and lower quality of life were also assessed. Given the exploratory nature of the study, no a priori hypotheses were formulated.
METHODS
Participants
All children (younger than 12 y) and adolescents (12–18 y) with NwC or NwoC who were consecutively seen in the four French national reference centers for narcolepsy (Hospital Robert-Debré, Paris; Hospital Femme-Mère Enfant, Hospital Pitié-Salpétrière, Paris; Hospital Gui de Chauliac, Montpellier) between August 2008 and March 2011 were invited to take part in a national research program on narcolepsy (NARCOBANK). Patients underwent clinical assessment by the lead physician at each center (ML, PF, IA, YD). Both parents and children signed a written consent form. The study was approved by the local ethics committee (Comité de Protection des Personnes - Ile de France 06). According to a predefined study protocol, clinical and questionnaire data were collected in a computerized database specifically programmed for this study.
To achieve a positive diagnosis of narcolepsy, all patients had a medical interview by the sleep specialist at each center, and underwent PSG followed by MSLTs and class II HLA typing; a subsample underwent measurement of CSF hypocretin-1 levels. Patients were classified as having narcolepsy according to the criteria of ICSD-2-revised,2 including: (1) complaints of EDS for at least 3 mo; (2) symptoms not better explained by other medical or psychiatric disorders; (3) the absence of secondary narcolepsy; (4) the presence of clear-cut cataplexy; and/ or (5) multiple sleep latency during MSLTs lower than 8 min with two or more SOREMPs. NwC and NwoC patients were included, who all met the ICSD-2-revised narcolepsy criteria.
PSG was performed from 20:00–22:00 (children) or from 23:00 to 07:00 (adolescents) and was followed the next day by five (or four at the Lyon study center) standard MSLTs at 09:00, 11:00, 13:00, 15:00, and 17:00, which were terminated after 20 min if no sleep had occurred, and after 15 min asleep if sleep did occur. PSG included at least three (or eight)-lead EEG, two electrooculograms, a chin electromyography, nasal pressure trough cannula, thoracic and abdominal belts, electrocardiography, and transcutaneous oximetry. Sleep stages, arousals, and respiratory events were scored visually in accordance with international criteria.17
HLA class II genotyping was planned to be undertaken in all subjects. A lumbar puncture was performed when clinically necessary or for research purposes with the specific agreement of parent and child. The CSF samples were collected, immediately frozen, and transferred to Gui de Chauliac Hospital (Montpellier) for measurement of hypocretin-1 levels using a standardized radioimmunoassay. Samples were thawed once and CSF hypocretin-1 was determined in all available samples in duplicate using radioimmunoassay kits (Phoenix Peptide, Phoenix Pharmaceuticals, Inc., 330 Beach Road, Burlingame, CA 94010, USA) according to the manufacturer's instructions. The detection limit was 10 pg/mL and intra-assay variability was < 10%. CSF hypocretin-1 levels ≤ 110 pg/mL were considered as low, 110–200 as intermediate, and > 200 as normal.18 All values were cross-referenced to Stanford reference samples (HHMI Stanford University Center for Narcolepsy, Palo Alto, CA).
Healthy young children and adolescents, who were siblings of patients attending Hospital Robert-Debré, Hospital Femme-Mère Enfant, and Hospital Pitié-Salpétrière, were recruited by invitation from study investigators or through advertisements displayed in these and other public hospitals between 2008 and 2011. They were included if they had no current daytime sleepiness validated by a score lower than 9 in a modified (adapted for children and adolescents) sleepiness scale.19 All participants (plus the parents of minors) signed a written consent for the study.
Clinical Examination
A complete physical examination was conducted and found to be normal for both patients with narcolepsy (thus, no patients with secondary narcolepsy were included in this analysis) and for controls. Height and weight measurements were conducted at time of entry in the study. Body mass index (BMI) was calculated (weight/height2) and a z-score computed representing a standardized measure of weight adjusted for height, sex, and age relative to a smoothed reference distribution.20 Using standardized growth curves, overweight, which included obesity, was defined, as per the International Obesity Task Force criteria, as being on or above the centile trajectory corresponding to a BMI of 25 kg/m2 at the age of 18 y. Obesity was defined as being on or above the centile trajectory corresponding to a BMI of 30 kg/m2 at the age of 18 y.21 No clinical assessment or formal diagnosis of ADHD was undertaken.
Interventions
Most patients were offered treatment for narcolepsy following the diagnosis of narcolepsy, which could include psychostimulants, but no specific treatment for elevated ADHD symptoms was initiated. Thus, it is important to note that psychostimulant therapy in this study was optimized solely for the treatment of narcolepsy.
Questionnaire Measures
Daytime sleepiness was evaluated with the Pediatric Daytime Sleepiness Scale (PDSS),22 which has a score range of 0–44. Insomnia symptoms were evaluated using the Insomnia Severity Index (ISI), which has a score range of score 0–28.23 Fatigue was scored with the Chalder fatigue scale24 using the 11-item version (score range of 0–11), which has been validated in children and adolescents.25 Symptoms of ADHD were scored by parents using the ADHD rating scale (ADHD-RS), based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR)26 symptoms with a maximum score of 54.27 In addition to continuous symptom scores, clinically significant levels of total ADHD (i.e., inattention + hyperactive-impulsive symptoms), inattention only, and hyperactive-impulsive only symptoms were calculated using 90th, 93rd, and 90th percentile thresholds, respectively, based on published norms by age and sex.28 Parents were asked if their children needed to repeat a school grade, which was scored as binary yes/no response. The Children's Depression Inventory (CDI), which has a score range of 0–54, was used to assess symptoms of Major Depressive Disorder.29 Health-related quality of life was assessed using a questionnaire developed for adolescents, the VSP-A (“Vécu et Santé Perçue de l'Adolescent” [perceived health-related quality of life in adolescents]),30 which has also been adapted for use in children31; both versions have a score range of 0–100.
Statistical Analysis
Descriptive statistics for continuous variables were expressed as median (min–max) and categorical variables as number (percent). For continuous outcomes, between-group statistical comparisons were conducted within a Generalized Linear Models (GLM) framework using appropriate family and link functions depending upon whether responses and/or residual errors were normally (BMI z-score, quality of life scores) or nonnormally (ADHD-RS, CDI, PDSS, ISI, and fatigue scores) distributed.32 Given that data were obtained from four separate participating study centers, a term for study center was added to all univariate and multivariate models and estimated population marginal means, otherwise known as least-squares (LS) means, and 95% confidence intervals (CIs) were calculated for between-group comparisons to obtain adjusted estimates that took variations attributable to study center into account. For nontransformed variables, LS means were calculated, for log-transformed variables, LS geometric mean ratios (GMRs) were calculated, which were also back-transformed where appropriate for graphical representation. GLM analyses were conducted using the GLM function within the R statistical software platform version 3.0.2 (http://www.R-project.org).
For variables with distributions that could not be satisfactorily transformed to normality (age at database registration, CSF hypocretin-1), nonparametric comparisons were performed using the npar.t.test function for comparisons of two groups and the nparcomp function for comparisons of three or more groups from the nparcomp package in R (http://cran.r-project.org/web/packages/nparcomp/index.html). Differences in proportions and associated 95% CIs were calculated using the diffscoreci function from the PropCIs package in R (http://cran.r-project.org/web/packages/PropCIs/index.html).
Appropriate transformations were performed for PSG variables that were not normally distributed prior to analysis. PSG variables entered into the initial screening model were total sleep time (minutes), sleep efficiency (%), log percent time in sleep stage 1, percent time in sleep stage 2, percent time in sleep stage 3–4, percent time in REM sleep, log latency to sleep onset (minutes), log latency REM sleep onset (minutes), log apnea-hypopnea (AHI) index (log AHI), log mean sleep latency of the MSLT (minutes), and number of episodes of SOREMPs. Missing data were too extensive to include periodic limb movements during sleep.
Exploratory multivariate regression analyses were conducted using GLMs as described previously; however, for initial PSG variable screening, stepwise selection was performed using the stepAIC function from the MASS package in R (http://cran.r-project.org/web/packages/MASS/index.html).
Statistical significance was determined at a level of alpha lower than 0.05, unadjusted for multiple comparisons in this exploratory, cross-sectional study.
RESULTS
Demographic and Physical Characteristics at Database Registration
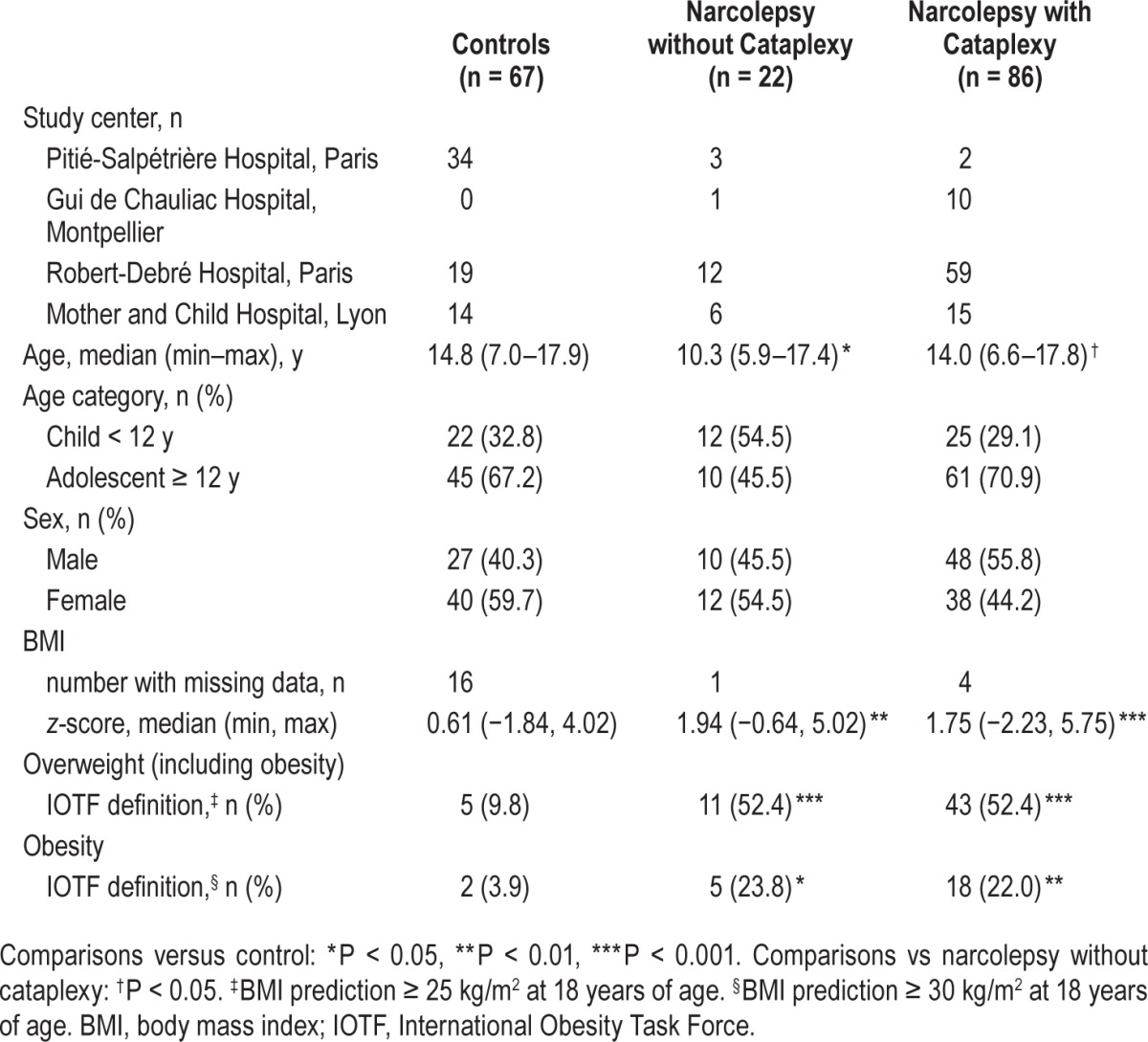
Overall, 108 patients with narcolepsy and 67 control children were recruited. Of these, 78/108 patients with narcolepsy (72.2%) and 63/67 of controls (94.0%) had available ADHDRS scores. Age at database registration was significantly lower in the NwoC group compared with the NwC (P = 0.044) and control groups (P = 0.012). The distribution of male and female children/adolescents was comparable across groups. Compared with the control group, BMI z-score (adjusted for age and sex) was significantly higher in the NwC (least squares [LS]-mean difference 1.17; 95% CI: 0.48, 1.87; P < 0.001) and NwoC group (LS-mean difference 1.22; 95% CI: 0.37, 2.07; P = 0.005). Greater proportions of patients with narcolepsy were overweight or obese compared with controls (Table 1).
Table 1.
Demographic, physical characteristics, and clinical features at database registration.
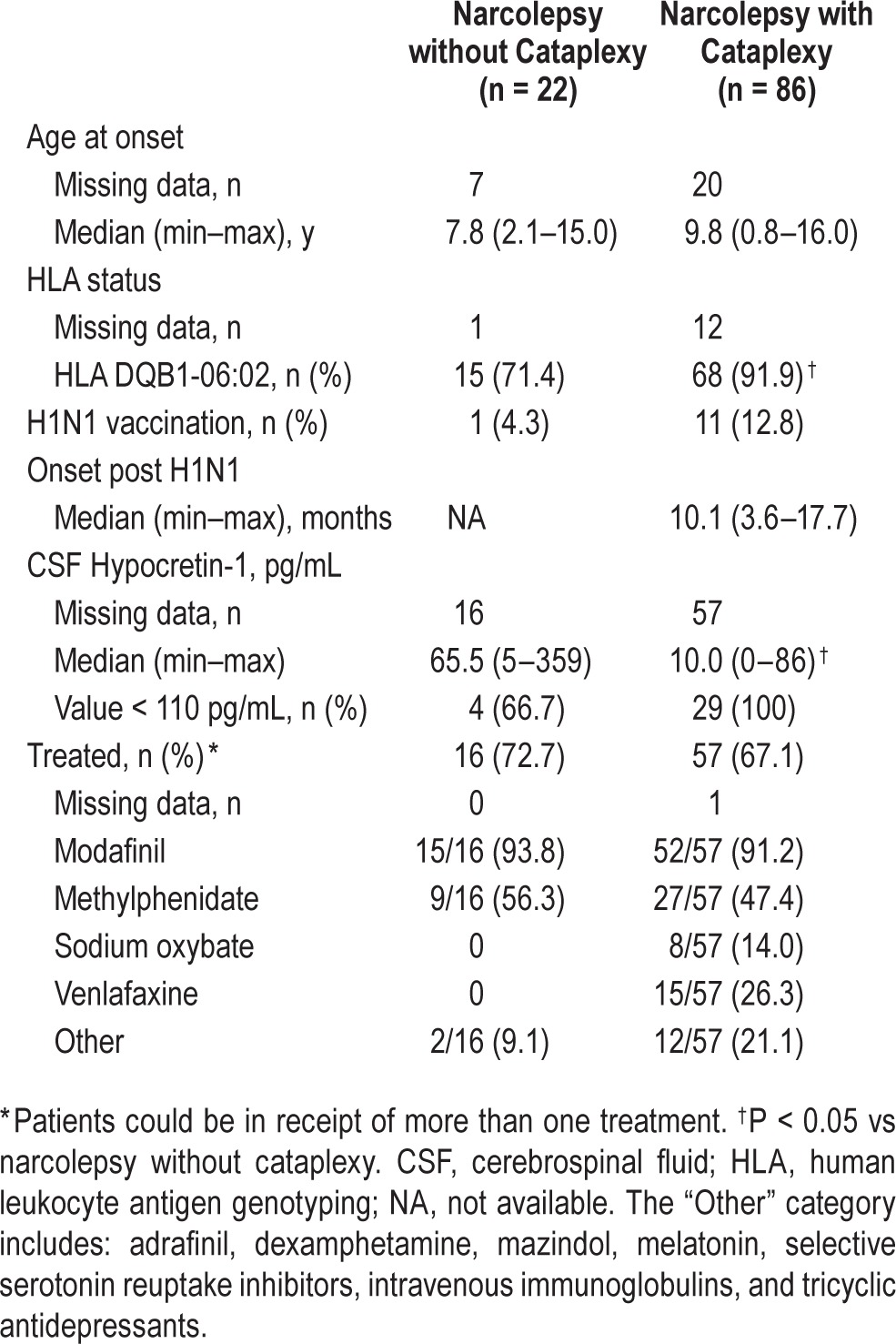
As indicated in Table 2, there were significantly more HLA DQB1*06:02 positive patients in the NwC group than the NwoC group (difference in proportion 20.5 percentage points; 95% CI: 3.6, 42.7; P = 0.040), and CSF hypocretin-1 was significantly lower in the NwC group compared with the NwoC group (P = 0.028). Approximately two-thirds of all patients (73/108; 68.2%) were receiving treatment for narcolepsy. Of those treated, nearly all patients were receiving modafinil; additional treatments included methylphenidate in approximately 50% of patients in both groups, and in the NwC group, sodium oxybate, venlafaxine, and other treatments were used. In treated patients, 68/73 (93.2%) started treatment before ADHD symptom measurement, 2/73 (2.7%) started treatment at the time of ADHD symptom measurement, and 3/73 (4.1%) started treatment shortly after ADHD symptom measurement. In untreated patients, 17/34 (50.0%) had ADHD symptoms measured before the diagnosis of narcolepsy, 12/34 (35.3%) at the time of diagnosis, and 5/34 (14.7%) shortly after diagnosis.
Table 2.
Background clinical features of patients with narcolepsy.
ADHD Symptoms and Categorization in Narcolepsy Versus Controls
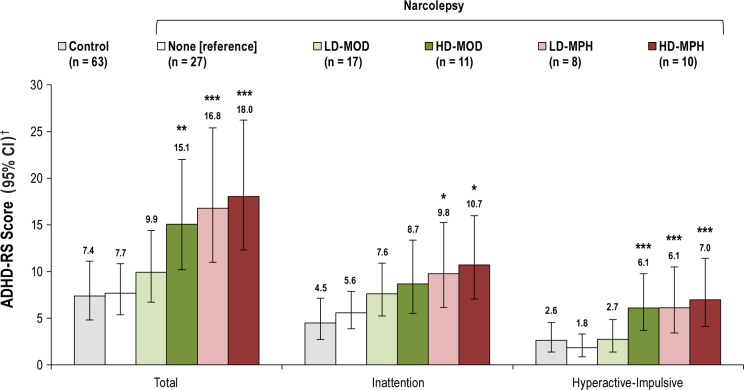
Total ADHD symptoms, inattention symptoms, and hyper-active-impulsive symptoms were significantly elevated in patients with narcolepsy compared with controls, irrespective of treatment received and of the presence of cataplexy (Figure 1A); total ADHD symptoms were twofold higher in NwoC than in controls (least squares geometric mean ratio [LS-GMR] 2.049; 95% CI: 1.409, 2.980; P < 0.001) and 1.8-fold higher in NwC than in controls (LS-GMR 1.788; 95% CI: 1.26, 2.536; P = 0.001). Inattention symptoms were 2.2-fold higher in the NwoC group versus controls (LS-GMR 2.170; 95% CI: 1.498, 3.144; P < 0.001) and 1.9-fold higher in the NwC group versus controls (LS-GMR 1.873; 95% CI: 1.322, 2.654; P < 0.001). Hyperactive-impulsive symptoms were 1.6-fold higher in the NwoC group versus controls (LS-GMR 1.598; 95% CI: 1.048, 2.435; P = 0.029) and 1.5-fold higher in the NwC group versus controls (LS-GMR 1.528; 95% CI: 1.055, 2.211; P = 0.025). There were no significant differences between the two narcolepsy groups for total ADHD, inattention, or hyperactive-impulsive symptoms. In addition, in patients with available CSF hypocretin-1 data (n = 35), no correlation was found between CSF hypocretin-1 level and total ADHD symptom severity, or inattention symptom severity or hyperactive-impulsive symptom severity for either the NwC or NwoC group.
Figure 1.
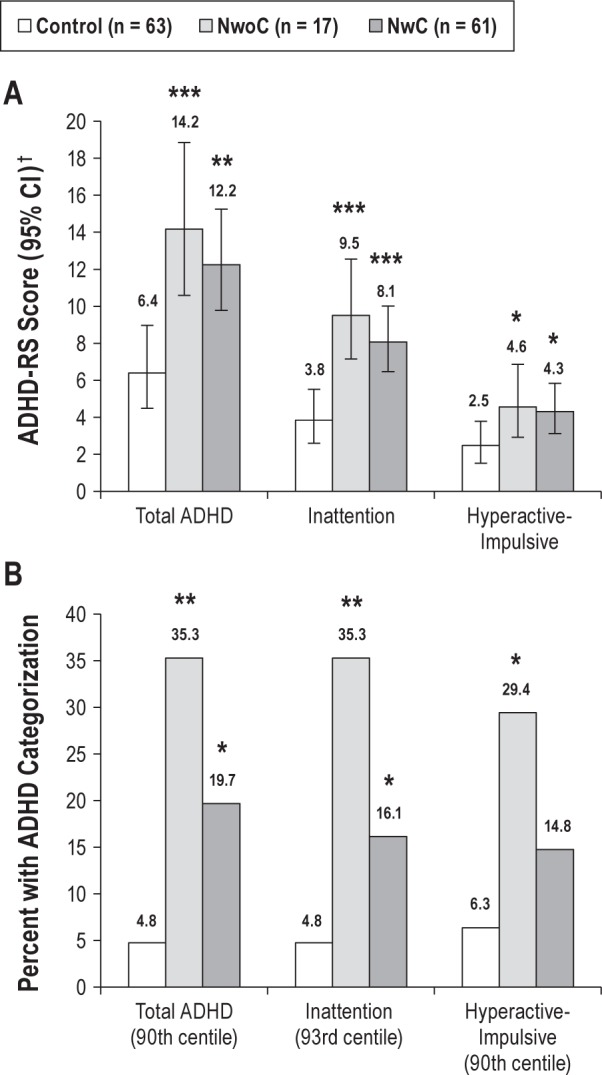
(A) Attention-deficit hyperactivity disorder rating scale (ADHDRS) scores in controls versus patients with narcolepsy. †Data are back-transformed least squares (LS) means and 95% confidence intervals (CIs) adjusted for study center derived from a generalized linear model using Gaussian family and log link. (B) Percent with clinically significant ADHD categorization in controls versus patients with narcolepsy. Comparisons versus controls: *P < 0.05, **P < 0.01, ***P < 0.001. NwC, narcolepsy with cataplexy; NwoC, narcolepsy without cataplexy.
Proportions with clinically significant levels of ADHD symptoms were higher in patients with NwoC and NwC compared with controls (Figure 1B). For the total ADHD category, compared with controls, proportions were significantly higher in patients with NwoC (difference 30.5 percentage points; 95% CI: 11.2, 52.6; P = 0.006) and in patients with NwC (difference 14.9 percentage points; 95% CI: 3.7, 27.3; P = 0.013). For the inattention category, compared with controls, proportions were significantly higher in patients with NwoC (difference 30.5 percentage points; 95% CI: 11.2, 52.6; P = 0.006) and in patients with NwC (difference 11.4 percentage points; 95% CI: 0.7, 23.2; P = 0.048). For the hyperactive-impulsive category, compared with controls, proportions were significantly higher in patients with NwoC (difference 23.1 percentage points; 95% CI: 4.9, 47.6; P = 0.034) and nu -merically higher in patients with NwC (difference 11.4 percentage points; 95% CI: −2.7, 20.4; P = 0.053). There were no significant differences in proportions between the NwoC and NwC groups for the total ADHD, inattention, or hyperactive-impulsive categories.
Associations with Clinically Significant ADHD Symptoms in Narcolepsy
Because no significant differences in levels of ADHD symptoms or categorization were observed between the NwoC and NwC groups, we pooled both groups for subsequent analyses. Patients with clinically significant total ADHD symptoms had greater proportions with school grade repetition compared with patients without clinically significant inattention symptoms, but this difference was not statistically significant (difference 5.0 percentage points; 95% CI: −15.3, 30.4; P = 0.670). Patients with clinically significant total ADHD symptoms had a statistically significant 1.4-fold increase in depressive symptoms (LSGMR 1.395; 95% CI: 1.072, 1.817; P = 0.013) and a statistically significant 10% decrease in quality of life (LS mean difference −10.165; 95% CI: −18.927, −1.403; P = 0.023) compared with patients without clinically significant total ADHD symptoms.
ADHD Symptoms and Narcolepsy Symptoms
Subjectively rated EDS, insomnia, and fatigue were all associated with total ADHD symptoms, regardless of treatment. A 10% increase in EDS was associated with a 5.0% increase in total ADHD symptoms (95% CI: 2.0, 8.1; P = 0.001). A 10% increase in insomnia was associated with a 6.2% increase in total ADHD symptoms (95% CI: 3.8, 8.6; P < 0.001). A 10% increase in fatigue was associated with a 4.7% increase in total ADHD symptoms (95% CI: 2.8, 6.6; P < 0.001).
ADHD Symptoms, Narcolepsy Symptoms, and Psychostimulants
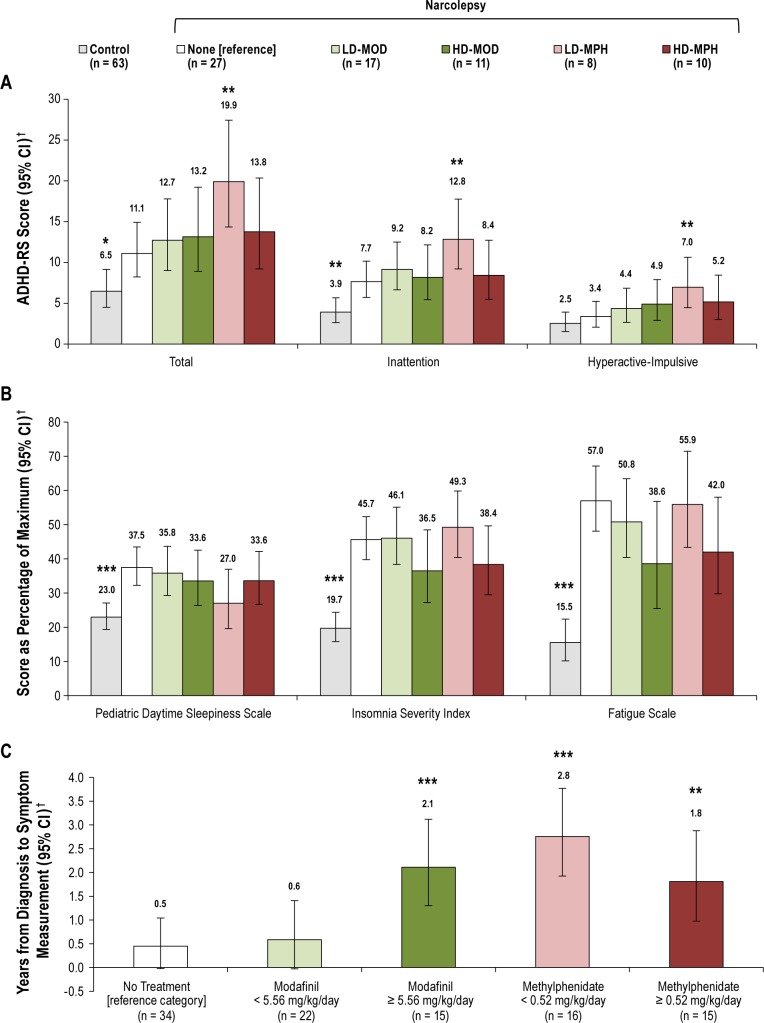
Because a number of psychostimulants used in narcolepsy (e.g., modafinil and methylphenidate) also have activity against inattention and hyperactive-impulsive symptoms,33,34 we sought to examine levels of ADHD symptoms in patients with narcolepsy who were either unexposed or exposed to these treatments. The association between treatment for narcolepsy and ADHD symptoms was analyzed by comparing patients with narcolepsy not receiving treatment (reference category) with patients with narcolepsy receiving modafinil or methylphenidate and with controls (defined as in receipt of no treatment). The modafinil and methylpheni-date groups were split into two groups, those receiving lower versus higher doses, based upon their median values of 5.56 mg/kg/day (range 2.53–15.20) and 0.52 mg/kg/day (range 0.17–3.20), respectively. Of the 22 patients with available ADHD-RS scores receiving methylphenidate, 19 were also receiving modafinil. The numbers of patients in each treatment group were too small to conduct meaningful analyses for the other less commonly used treatments. With untreated narcolepsy patients as the reference category, controls had lower total ADHD symptoms (LS-GMR 0.618; 95% CI: 0.415, 0.922; P = 0.018), indicating that patients with narcolepsy had higher ADHD symptoms regardless of treatment (Figure 2A). In patients receiving modafinil alone at any dose or methylphenidate doses ≥ 0.52 mg/kg/day (+ modafinil in 8/10), total ADHD symptoms were not significantly different from those receiving no treatment. In patients receiving methylphenidate doses < 0.52 mg/kg/day (+ modafinil in 7/8), total ADHD symptoms were higher than in those receiving no treatment (LS-GMR 1.725; 95% CI: 1.217, 2.445; P = 0.002). In patients treated with lower-dose methylphenidate < 0.52 mg/kg/day versus no treatment similar higher levels were observed for inattention (LS-GMR 1.597; 95% CI: 1.136, 2.246; P = 0.007) and hyperactive-impulsive symptoms (LS-GMR 1.819; 95% CI: 1.174, 2.818; P = 0.007).
Figure 2.
(A) Attention-deficit hyperactivity disorder rating scale (ADHD-RS) scores.(B) Other subjective symptoms in controls and patients with narcolepsy by treatment received. (C) Interval in years between diagnosis and questionnaire symptom assessments in patients with narcolepsy by treatment received. †Data are back-transformed least squares (LS) means and 95% confidence intervals (CIs) adjusted for study center derived from a generalized linear model using gaussian family and log link. Comparisons versus patients with narcolepsy not receiving treatment: *P < 0.05, **P < 0.01, ***P < 0.001. HDMOD, higher-dose modafinil (≥ 5.56 mg/kg/day); HD-MPH, higher-dose methylphenidate (≥ 0.52 mg/kg/day) + modafinil in majority; LD-MOD, lower-dose modafinil (< 5.56 mg/kg/day); LD-MPH, lower-dose methylphenidate (< 0.52 mg/kg/day) + modafinil in majority.
We also sought to establish whether a similar or different pattern of treatment was observed for the other narcolepsy symptoms of EDS, insomnia, and fatigue. With untreated narcolepsy patients as the reference category, controls had significantly lower EDS, insomnia, and fatigue, indicating that patients with narcolepsy have higher levels of all three of these symptoms regardless of treatment (Figure 2B). In contrast to the findings with ADHD symptoms, none of the treatment groups were associated with any statistically significant differences in EDS, insomnia, or fatigue compared with patients not receiving treatment (Figure 2B).
Because treatments could have been introduced or added in a stepwise fashion over time during the course of the clinical management of patients with narcolepsy, we analyzed treatments received by the length of time between narcolepsy diagnosis and ADHD symptom measurement. The ‘no treatment’ and low-dose modafinil groups were associated with short time intervals between diagnosis and symptom measurement, whereas the high-dose modafinil and both methylphenidate groups (in conjunction with modafinil in the majority) were significantly associated with longer time intervals between diagnosis and symptoms measurement compared with patients not receiving treatment (Figure 2C).
Multivariate Analyses of the Association between ADHD Symptoms and Other Variables
ADHD symptoms, narcolepsy symptoms, and psychostimulants
In multivariate analyses (including the effect of study centre, EDS, insomnia, fatigue, age, sex, BMI z-score, interval between diagnosis and symptom score measurement, and treatment category), insomnia symptoms, fatigue, and treatment with methylphenidate were associated with elevated inattention symptoms (Table 3). For every 10% increase in insomnia, inattention symptoms increased by 4.5% (95% CI: 0.8, 8.4; P = 0.017); and for every 10% increase in fatigue, inattention symptoms increased by 2.7% (95% CI: 0.1, 5.4; P = 0.041). In addition, both lower and higher dose of methylphenidate were associated with 1.6- and 1.8-fold higher inattention symptoms, respectively (Table 3). For hyperactive-impulsive symptoms, a similar picture was evident. For every 10% increase in insomnia, hyperactive-impulsive symptoms increased by 9.7% (95% CI: 5.3, 11.4; P < 0.001). In addition, higher-dose modafinil, and lower and higher methylphenidate doses were associated with 2.5-, 2.5-, and 2.8-fold higher hyperactive-impulsive symptoms, respectively (Table 3). Multivariate adjusted LS means and 95% CIs for ADHD-RS symptoms by treatment category are shown in Figure 3.
Table 3.
Multivariate Generalized Linear Models analysis of the association of sleep symptoms (daytime sleepiness, insomnia, and fatigue) and stimulants with attention-deficit hyperactivity disorder symptoms in patients with narcolepsy and controls.
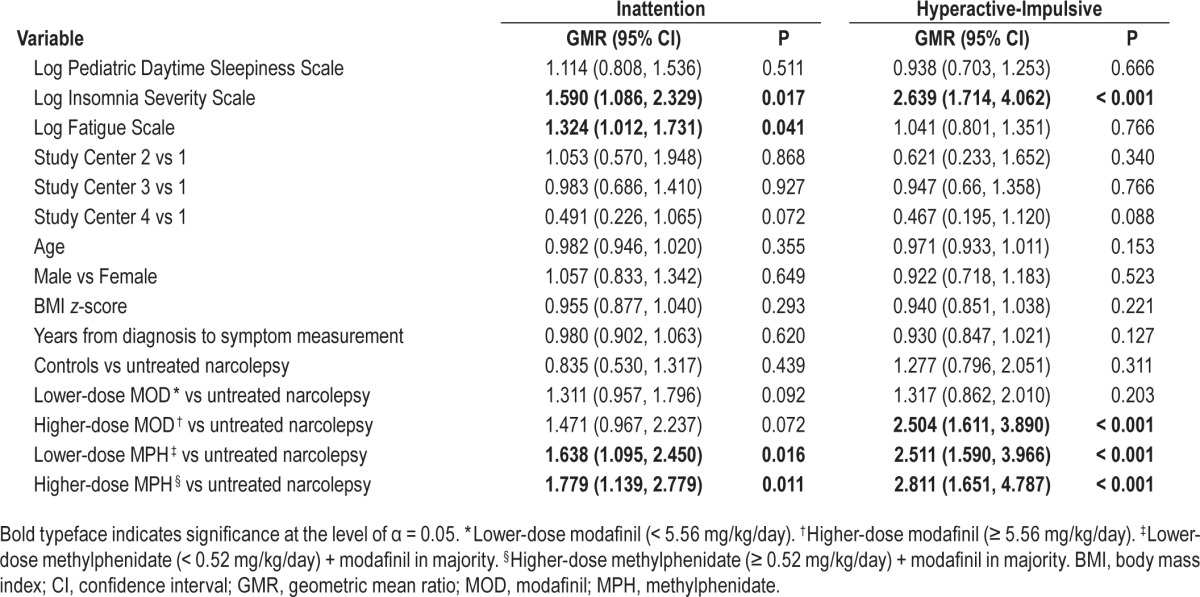
Figure 3.
Multivariate adjusted attention-deficit hyperactivity disorder rating scale (ADHD-RS) scores by treatment received. †Data are back-transformed least squares (LS) means and 95% confidence intervals (CIs) adjusted for study center, daytime sleepiness, insomnia, fatigue, age, sex, BMI z-score, interval between diagnosis and symptom score measurement, and treatment category derived from a generalized linear model using gaussian family and log link. Comparisons versus patients with narcolepsy not receiving treatment: *P < 0.05, **P < 0.01, ***P < 0.001. HD-MOD, higher-dose modafinil (≥ 5.56 mg/kg/day); HD-MPH, higher-dose methylphenidate (≥ 0.52 mg/kg/day) + modafinil in majority; LD-MOD, lower-dose modafinil (< 5.56 mg/kg/day); LD-MPH, lower-dose methylphenidate (< 0.52 mg/kg/day) + modafinil in majority.
ADHD symptoms, narcolepsy symptoms, polysomnographic variables, and psychostimulants
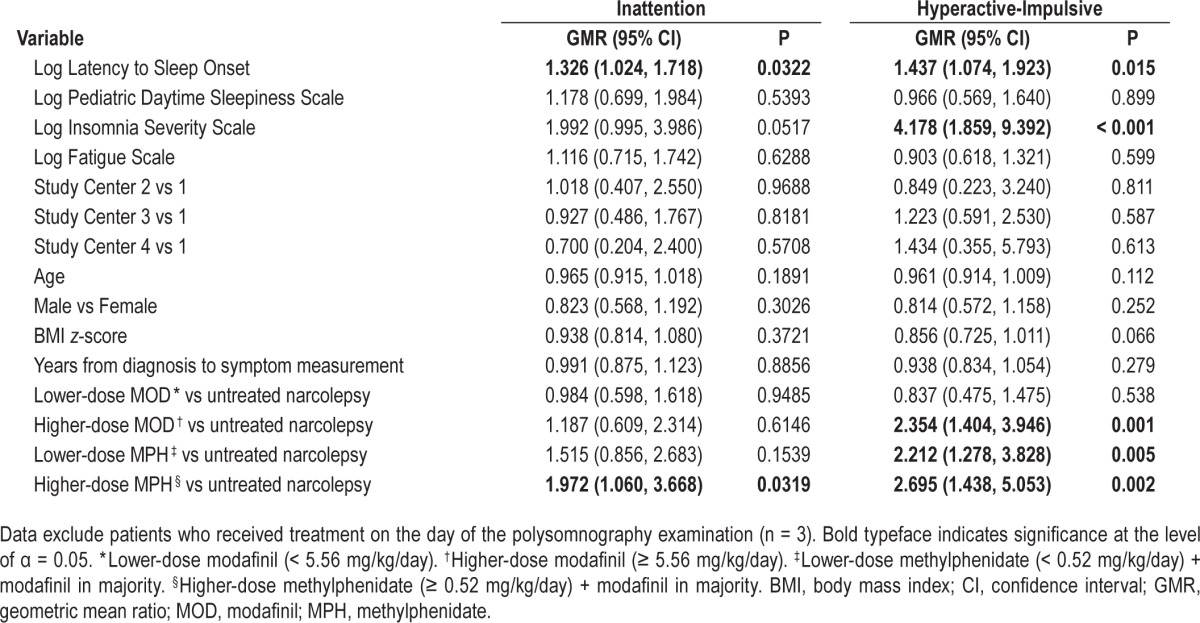
Because PSG measures were not available for controls, these analyses could only be performed in patients with narcolepsy. PSG was performed while not receiving treatment, but took place on average 1.2 y before ADHD-RS questionnaires were administered (49 patients had PSG performed > 6 mo before ADHD symptom measurement, 34 up to 6 mo before, 13 at the time of ADHD symptom measurement, and 8 after ADHD symptom measurement). A stepwise regression analysis was first performed to identify PSG variables potentially associated with total ADHD symptoms while controlling for study center variation. The only variable associated with total ADHD symptoms was log latency to sleep onset; a 10% increase in latency to sleep onset was associated with a 2.1% increase in total ADHD symptoms (95% CI: 0.6, 3.8; P = 0.011). In multivariate analyses (including study center, latency to sleep onset, EDS, insomnia, fatigue, age, sex, BMI z-score, interval between diagnosis and symptom score measurement, and treatment category), latency to sleep onset remained significant; for every 10% increase in latency to sleep onset, inattention symptoms increased by 2.7% (95% CI: 0.2, 5.3; P = 0.032). In addition, higher methylphenidate dose was associated with twofold higher inattention symptoms (Table 4). For hyperactive-impulsive symptoms, a similar picture was evident. For every 10% increase in latency to sleep onset, hyperactive-impulsive symptoms increased by 3.5% (95% CI: 0.7, 6.4; P = 0.015); and for every 10% increase in insomnia, hyperactive-impulsive symptoms increased by 14.6% (95% CI: 6.1, 23.8; P < 0.001). In addition, higher-dose modafinil, lower-dose methylphenidate, and higher-dose methylphenidate were associated with 2.4-, 2.2-, and 2.7-fold higher hyperactive-impulsive symptoms, respectively (Table 4). No correlation between methylphenidate dose and insomnia symptoms (Spearman rho −0.036, P = 0.746) or log latency to sleep onset (rho −0.147, P = 0.157) was demonstrated.
Table 4.
Multivariate Generalized Linear Models analysis of the association of polysomnographic latency to sleep onset, sleep symptoms (daytime sleepiness, insomnia, and fatigue), and stimulants with ADHD symptoms in patients with narcolepsy.
DISCUSSION
In children/adolescents with narcolepsy, ADHD symptoms were approximately twofold higher compared with controls. In terms of clinically significant levels of ADHD symptoms, this threshold was met in approximately 30% of NwoC patients and 15% of NwC patients compared with approximately 5–6% of controls. We showed that ADHD symptoms are associated with a significant burden in young patients with narcolepsy; specifically, patients with clinically significant levels of ADHD symptoms had higher levels of depressive symptoms and lower health-related quality of life.
The overall prevalence of ADHD in controls was similar to that identified both in a meta-analysis of studies examining the prevalence DSM-IV ADHD diagnoses in unselected populations (5.9–7.1%)35 and in a large epidemiological study of ADHD prevalence conducted in France (3.5–5.6%).36 Thus, the elevated rates observed in patients with narcolepsy compared with controls are unlikely to be accounted for by selection bias of controls, especially because these rates are consistent with findings of increased hyperactivity in an international cross-sectional survey of 42 children and adolescents with narcolepsy.6
Although the NwoC group appeared to show a numerically higher level/severity of ADHD symptoms in our study, the difference versus the NwC group was not statistically significant; however, this numeric difference may have resulted from the younger patient age in the NwoC group. In addition, no correlation between CSF hypocretin-1 and ADHD symptoms was demonstrated, suggesting that elevated ADHD symptoms in narcolepsy might be secondary to EDS, fatigue, or nocturnal sleep disturbance rather than a primary phenomenon directly related to hypocretin deficiency. However, very few patients had available CSF measurements, so the lack of any association might represent inadequate statistical power. Despite potential differences between the NwoC and NwC groups, we pooled these groups for subsequent analyses as HLA DQB1*06:02 was present in 71.4% of patients with NwoC, CSF hypocretin-1 was low in both groups, and patient age was lower in the NwoC versus the NwC group, suggesting that patients with NwoC in our cohort were likely to develop cataplexy later.37
Because treatments for narcolepsy, such as modafinil and methylphenidate,16 are also used in children with ADHD,33,34 we investigated ADHD symptoms in patients who were either unexposed or exposed to treatment for narcolepsy. Results from univariate and multivariate analyses differed, tending to support different conclusions as to the effect of psychostimulant therapy for narcolepsy on ADHD symptoms. In univariate analyses, total ADHD, inattention, and hyperactive-impulsive symptoms were significantly higher in patients receiving lower methylphenidate dose compared with patients receiving no treatment. Neither the higher methylphenidate dose nor the modafinil groups were associated with significant differences in ADHD symptoms versus those not receiving treatment. Because of the cross-sectional nature of this study, it was not possible to attribute any causal relationships to these findings; however, a number of possible explanations can be considered. The observation that higher doses of modafinil and methylphenidate were associated with longer time intervals between narcolepsy diagnosis and ADHD symptom measurement compared with those not receiving treatment or those receiving lower-dose modafinil would suggest that during the course of clinical management of patients with narcolepsy, the dose of treatments may be increased and/or new treatments may be added to control narcolepsy symptoms. Interestingly, the lower-dose methylphenidate group was associated with the longest interval and also with the highest ADHD symptom level, which might suggest physician inertia/ reluctance to increase methylphenidate dose. In general, treatments were associated with nonsignificant reductions in narcolepsy symptoms, especially in the higher-dose modafinil and methylphenidate groups, compared with patients receiving no treatment (Figure 2B), but this appeared not to be the case for ADHD symptoms, for which symptoms tended to be higher in all treatment categories and significantly higher in the lower-dose methylphenidate group (Figure 2A). Despite the fact that patients were being treated for narcolepsy and not specifically for ADHD, these findings might suggest that, in contrast to narcolepsy symptoms, ADHD symptoms in patients with narcolepsy might be somewhat resistant to treatment with psycho-stimulants and might require a higher dose of methylphenidate.
In multivariate analyses, however, a different conclusion regarding the effect of psychostimulant therapy for narcolepsy on ADHD symptoms was suggested. On including the effect of study center, EDS, insomnia, fatigue, age, sex, BMI z-score, interval between diagnosis and symptom score measurement, and treatment category; longer latency to sleep onset, subjective insomnia, subjective fatigue, and any use of methylphenidate were modestly associated with higher inattention symptoms. For hyperactive-impulsive symptoms, longer latency to sleep onset, subjective insomnia and use of psycho-stimulants, including higher-dose modafinil and any dose of methylphenidate, were strongly associated with higher hyper-active-impulsive symptoms (Table 3, Table 4, and Figure 3). The multivariate analysis findings with respect to psycho-stimulant use contrasted with the univariate analyses, in which only the lower-dose methylphenidate group was associated with higher ADHD symptoms. Given that low-dose methylphenidate use was associated with the longest interval between diagnosis and ADHD symptom measurement, it is likely that patients receiving lower-dose methylphenidate had longer duration of disease and a correspondingly greater burden of ADHD symptoms. The multivariate analyses included time interval between diagnosis and ADHD symptom measurement, allowing for an assessment of the effect of psychostimulant therapy on ADHD symptoms having adjusted for the potential confounding effect of this and other variables.
The association of ADHD symptoms, and in particular hyperactive-impulsive symptoms, with insomnia and psycho-stimulant therapy in multivariate analyses might suggest that psychostimulant therapy could delay the onset of nocturnal sleep, which in turn could exacerbate ADHD symptoms. This is especially relevant because it is known that ADHD in the absence of narcolepsy is associated with significantly increased objective latency to sleep onset and subjective sleep onset difficulties, night awakenings, difficulties with morning awakenings, and EDS compared with controls.38 However, in the current study, we found no correlation between methylphenidate dose level and either subjectively rated insomnia symptoms or objective PSG latency to sleep onset. Therefore, this hypothesis remains unconfirmed and warrants further investigation in suitably designed longitudinal studies. If confirmed, this would have significant implications with respect to psycho-stimulant therapy in narcolepsy, which, while addressing EDS and fatigue, might exacerbate insomnia. It is important to note that, with the exception of modafinil (and methylphenidate in the United States) in adults, most treatments for narcolepsy are used off-label with no randomized trial evidence to support their use. Therefore, there is an unmet need for well-conducted randomized controlled trials of novel agents in pediatric narcolepsy, such as sodium oxybate, which not only increase daytime alertness, but also consolidate nocturnal sleep.39–42
The strengths of this study are that it employed preplanned data-collection methods and, to our knowledge, represents the largest cohort of pediatric patients with narcolepsy evaluated to date. A number of limitations must be acknowledged. This was a cross-sectional, observational, nonrandomized study, and ADHD symptoms were not measured before the onset of narcolepsy or before and after the initiation of psychostimulants; therefore, causality cannot be attributed to any of the findings, which should be viewed as exploratory and hypothesis-generating in nature. Data on ADHD symptoms were available for only 72.2% of patients with narcolepsy. However, this rate is similar to that found in a large observational study investigating the determinants of mental health outcomes in children, which employed specific interventions to improve response rates.43 Of note, a rate of responding of this magnitude was not found to result in significant differences between non-responders and responders in terms of the associations between individual characteristics and ADHD symptoms.43 Data were obtained from four different study centers; thus, although the effect of study center variation was statistically controlled in both univariate and multivariate analyses, some of the observed differences could have been attributable to this or other unknown sources of variation. No clinical assessment or formal diagnosis of ADHD was undertaken and the age of onset of ADHD symptoms was not known. Regarding PSG measures, it was not possible to assess the association of periodic limb movements of sleep with ADHD symptoms because of the extent of missing data. Although PSG measures excluded patients in receipt of treatment, in a substantial proportion of cases PSG took place a number of years before ADHD-RS questionnaires were measured. The sample size of the NwoC group was too small to reliably assess differences between the NwC and NwoC groups. In addition, sample sizes in the treatment subgroups were also small, limiting the certainty of the findings with respect to the influences of treatment for narcolepsy on ADHD symptoms. CSF hypocretin-1 measurements were available in only a small proportion of patients and serum ferritin levels were not collected. Finally, a small proportion of patients with narcolepsy (12%) had been exposed to H1N1 vaccination, raising the possibility that the inclusion of these patients in the analysis might have altered the pattern of observed associations compared with that in a population of patients with purely idiopathic narcolepsy. To test this possibility, we re-ran the multivariate analysis presented in Table 4 but excluding patients exposed to H1N1 vaccination. We found an identical pattern of significant associations (data not shown), which strongly suggested that the inclusion of patients exposed to H1N1 vaccination had not limited the generalizability of our findings.
In conclusion, pediatric patients with narcolepsy have a high level of ADHD symptoms, approximately twofold higher compared with controls, with a higher burden of depressive symptoms and poorer quality of life. Moreover, in contrast to narcolepsy symptoms, for which some benefit of therapy was observed, ADHD symptoms appeared to be largely unresponsive to psychostimulant therapy. Although univariate analysis suggested that suboptimal doses of methylphenidate might be associated with poor control of ADHD symptoms, multivariate analysis suggested that insomnia, use of higher-dose modafinil, and use of methylphenidate at any dose might be associated with elevated ADHD symptoms, especially hyperactive-impulsive symptoms. It remains unclear, therefore, whether psychostimulant therapy is effective for ADHD symptoms in pediatric narcolepsy and whether hypersomnias and ADHD may or may not share a common underlying pathophysiology. Indeed, the lack of influence of age on ADHD symptoms in young patients with narcolepsy might suggest a different pathophysiological mechanism for ADHD symptoms to that in young patients with ADHD without narcolepsy. Thus, the optimal treatment for ADHD symptoms in pediatric narcolepsy warrants further investigation in longitudinal intervention studies.
DISCLOSURE STATEMENT
This was not an industry supported study. The study is part of a larger study financed by a grant (PHRC AOM07-138, Principal Investigator: Isabelle Arnulf) from the French Health Ministry and supported by Assistance Publique - Hôpitaux de Paris (AP-HP). Dr. Lecendreux has served as a consultant for Bioprojet, Shire, UCB, and Jazz Pharma and has received research support from Shire and UCB and honoraria from UCB and Shire. Dr. Franco has participated in speaking engagements for UCB Pharma. Dr. Arnulf has participated in speaking engagements for and served on the board of UCB Pharma and Jazz. Dr. Dauvilliers has participated in speaking engagements for and served on the board UCB Pharma, Jazz, and Bioprojet. Dr. Konofal is on the speakers' bureau of Shire and Eunethydis Guidelines Europe ADHD. The other authors have indicated no financial conflicts of interest. Institutions where work was performed: Centre Pédiatrique des Pathologies du Sommeil, CHU Robert-Debré, 48 boulevard Serurier, 75019 Paris, France; Service des Pathologies du sommeil, Pavillon Marguerite Bottard, Groupe Hospitalier Pitié-Salpêtrière, 47-83 boulevard de l'Hôpital, 75651 Paris cedex 13, France; Unité de sommeil, Neurologie, Hôpital Gui de Chauliac, 80 avenue Augustin Fliche, 34295 Montpellier Cedex 5, France; and Unité de Sommeil Pédiatrique, Hôpital Femme-Mère Enfant, 59, boulevard Pinel, 69500 Bron, France.
REFERENCES
- 1.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 3.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 4.Inocente CO, Lavault S, Lecendreux M, et al. Impact of obesity in children with narcolepsy. CNS Neurosci Ther. 2013;19:521–8. doi: 10.1111/cns.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inocente CO, Gustin M-P, Lavault S, et al. Depressive feelings in children with narcolepsy. Sleep Med. 2014;15:309–14. doi: 10.1016/j.sleep.2013.08.798. [DOI] [PubMed] [Google Scholar]
- 6.Stores G, Montgomery P, Wiggs L. The psychosocial problems of children with narcolepsy and those with excessive daytime sleepiness of uncertain origin. Pediatrics. 2006;118:e1116–23. doi: 10.1542/peds.2006-0647. [DOI] [PubMed] [Google Scholar]
- 7.Inocente CO, Gustin MP, Lavault S, et al. Quality of life in children with narcolepsy. CNS Neurosci Ther. 2014;20:763–71. doi: 10.1111/cns.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes D., Jr Narcolepsy with cataplexy in early childhood. Clin Pediatr (Phila) 2006;45:361–3. doi: 10.1177/000992280604500409. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 10.Cortese S, Konofal E, Lecendreux M. Alertness and feeding behaviors in ADHD: does the hypocretin/orexin system play a role? Med Hypotheses. 2008;71:770–5. doi: 10.1016/j.mehy.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Lecendreux M, Konofal E, Bouvard M, Falissard B, Mouren-Simeoni MC. Sleep and alertness in children with ADHD. J Child Psychol Psychiatry. 2000;41:803–12. [PubMed] [Google Scholar]
- 12.Oosterloo M, Lammers GJ, Overeem S, de Noord I, Kooij JJ. Possible confusion between primary hypersomnia and adult attention-deficit/ hyperactivity disorder. Psychiatry Res. 2006;143:293–7. doi: 10.1016/j.psychres.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Walters AS, Silvestri R, Zucconi M, Chandrashekariah R, Konofal E. Review of the possible relationship and hypothetical links between attention deficit hyperactivity disorder (ADHD) and the simple sleep related movement disorders, parasomnias, hypersomnias, and circadian rhythm disorders. J Clin Sleep Med. 2008;4:591–600. [PMC free article] [PubMed] [Google Scholar]
- 14.Modestino EJ, Winchester J. A retrospective survey of childhood ADHD symptomatology among adult narcoleptics. J Atten Disord. 2013;17:574–82. doi: 10.1177/1087054713480033. [DOI] [PubMed] [Google Scholar]
- 15.Ohayon MM. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14:488–92. doi: 10.1016/j.sleep.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 16.De la Herran-Arita AK, Garcia-Garcia F. Current and emerging options for the drug treatment of narcolepsy. Drugs. 2013;73:1771–81. doi: 10.1007/s40265-013-0127-y. [DOI] [PubMed] [Google Scholar]
- 17.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 18.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 20.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolland-Cachera MF. Childhood obesity: current definitions and recommendations for their use. Int J Pediatr Obes. 2011;6:325–31. doi: 10.3109/17477166.2011.607458. [DOI] [PubMed] [Google Scholar]
- 22.Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Pietro B. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep. 2003;26:455–8. [PubMed] [Google Scholar]
- 23.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 24.Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37:147–53. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 25.Chalder T, Deary V, Husain K, Walwyn R. Family-focused cognitive behaviour therapy versus psycho-education for chronic fatigue syndrome in 11- to 18-year-olds: a randomized controlled treatment trial. Psychol Med. 2009;40:1269–79. doi: 10.1017/S003329170999153X. [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Text Revision. [Google Scholar]
- 27.Zhang S, Faries DE, Vowles M, Michelson D. ADHD Rating Scale IV: psychometric properties from a multinational study as a clinician-administered instrument. Int J Methods Psychiatr Res. 2005;14:186–201. doi: 10.1002/mpr.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DuPaul GJ, Anastopoulos AD, Power TJ, Reid R, Ikeda MJ, McGoey KE. Parent ratings of attention-deficit/hyperactivity disorder symptoms: factor structure and normative data. J Psychopathol Behav Assessment. 1998;20:83–102. [Google Scholar]
- 29.Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the Children's Depression Inventory. J Abnorm Child Psychol. 1986;14:25–39. doi: 10.1007/BF00917219. [DOI] [PubMed] [Google Scholar]
- 30.Simeoni MC, Auquier P, Antoniotti S, Sapin C, San Marco JL. Validation of a French health-related quality of life instrument for adolescents: the VSP-A. Qual Life Res. 2000;9:393–403. doi: 10.1023/a:1008957104322. [DOI] [PubMed] [Google Scholar]
- 31.Serra-Sutton V, Ferrer M, Rajmil L, Tebe C, Simeoni MC, Ravens-Sieberer U. Population norms and cut-off-points for suboptimal health related quality of life in two generic measures for adolescents: the Spanish VSP-A and KINDL-R. Health Qual Life Outcomes. 2009;7:35. doi: 10.1186/1477-7525-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidian M. ST 732 Applied Longitudinal Data Analysis. Raleigh, NC: North Carolina State University; 2005. Generalized linear models for nonnormal response; pp. 423–64. [Google Scholar]
- 33.Swanson JM, Greenhill LL, Lopez FA, et al. Modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: results of a randomized, double-blind, placebo-controlled, fixed-dose study followed by abrupt discontinuation. J Clin Psychiatry. 2006;67:137–47. doi: 10.4088/jcp.v67n0120. [DOI] [PubMed] [Google Scholar]
- 34.The MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56:1073–86. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 35.Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9:490–9. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lecendreux M, Konofal E, Faraone SV. Prevalence of attention deficit hyperactivity disorder and associated features among children in France. J Atten Disord. 2011;15:516–24. doi: 10.1177/1087054710372491. [DOI] [PubMed] [Google Scholar]
- 37.Andlauer O, Moore Ht, Hong SC, et al. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep. 2012;35:1247–55F. doi: 10.5665/sleep.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J Am Acad Child Adolesc Psychiatry. 2009;48:894–908. doi: 10.1097/CHI.0b013e3181ac09c9. [DOI] [PubMed] [Google Scholar]
- 39.A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep. 2002;25:42–9. [PubMed] [Google Scholar]
- 40.A 12-month, open-label, multicenter extension trial of orally administered sodium oxybate for the treatment of narcolepsy. Sleep. 2003;26:31–5. [PubMed] [Google Scholar]
- 41.Lecendreux M, Poli F, Oudiette D, et al. Tolerance and efficacy of sodium oxybate in childhood narcolepsy with cataplexy: a retrospective study. Sleep. 2012;35:709–11. doi: 10.5665/sleep.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mamelak M, Black J, Montplaisir J, Ristanovic R. A pilot study on the effects of sodium oxybate on sleep architecture and daytime alertness in narcolepsy. Sleep. 2004;27:1327–34. doi: 10.1093/sleep/27.7.1327. [DOI] [PubMed] [Google Scholar]
- 43.de Winter AF, Oldehinkel AJ, Veenstra R, Brunnekreef JA, Verhulst FC, Ormel J. Evaluation of non-response bias in mental health determinants and outcomes in a large sample of pre-adolescents. Eur J Epidemiol. 2005;20:173–81. doi: 10.1007/s10654-004-4948-6. [DOI] [PubMed] [Google Scholar]