Abstract
Study Objectives:
To determine the associations of 25-hydroxyvitamin D (25(OH)D) concentration with sleep continuity, quality, and symptoms, and to explore race/ethnic variation.
Design:
Cross-sectional study.
Setting:
Multi-Ethnic Study of Atherosclerosis (MESA).
Participants:
There were 1,721 adults.
Measurements and Results:
Sleep outcomes were measured by polysomnography, actigraphy, and questionnaires. Serum 25(OH)D concentration was expressed by clinical thresholds (< 20, 20–29, ≥ 30 ng/mL) and continuously. Using linear regression, we determined the associations between 25(OH)D concentration and sleep duration, efficiency, and symptoms, and assessed race/ethnic variation. Mean age was 68.2 ± 9.1 y, and 37.2% were white, 27.7% African American, 11.9% Chinese Americans, and 23.2% Hispanic. Mean 25(OH)D concentration was 25.4 ± 10.5 ng/mL. 25(OH)D deficient participants had the shortest sleep duration, lowest sleep efficiency, and highest sleepiness scores. After adjusting for demographics, obesity, and health habits, deficient individuals slept an average of 13.0 min (95% confidence interval, −22.8, −3.2) shorter than sufficient individuals. Race/ethnic-stratified analyses indicated that the strongest associations were in African Americans, in whom adjusted sleep duration was 25.6 ± 11.7 min shorter in deficient versus sufficient individuals (P = 0.04), and in Chinese Americans, adjusted apnea-hypopnea index (AHI) was 7.5 ± 3.3 events/h higher in deficient versus sufficient individuals.
Conclusion:
Overall, there were modest associations between 25-hydroxyvitamin D (25(OH)D) concentration and sleep traits. However, race-stratified analyses suggested the association between 25(OH)D concentration and sleep traits varied by race/ethnicity. Vitamin D deficiency was most strongly associated with short sleep duration in African Americans and with elevated apnea-hypopnea index in Chinese Americans, suggesting that race/ethnicity may modify these associations.
Citation:
Bertisch SM, Sillau S, de Boer IH, Szklo M, Redline S. 25-hydroxyvitamin D concentration and sleep duration and continuity: multi-ethnic study of atherosclerosis. SLEEP 2015;38(8):1305–1311.
Keywords: health disparities, MESA, sleep disorders, vitamin D
INTRODUCTION
Growing awareness of the high prevalence of low serum 25-hydroxyvitamin D (25(OH)D),1 its associations with chronic medical conditions2,3 (e.g., cardiovascular disease; asthma), and its role in immune function4 have sparked increasing interest in vitamin D's role in extraskeletal health. The vitamin D receptor is widely distributed throughout many tissues, including brain regions involved in sleep regulation and central nervous system inflammatory signaling.5–7 Furthermore, low 25(OH)D levels may affect medical conditions associated with pain symptoms (e.g., osteomalacia) that deleteriously impact sleep.8,9 To this extent, several case reports have suggested associations between low 25(OH)D levels and excessive daytime sleepiness, clinical improvement in hypersomnia and sleep patterns with vitamin D supplementation.5,6 Nascent research has explored associations between 25(OH)D and subjective sleep indices,10 symptoms,11 and obstructive sleep apnea (OSA).12,13
Although there is biologic plausibility to these clinical observations, there are limited data regarding the relationship between 25(OH)D and objective sleep duration, continuity, and symptoms. Furthermore, because vitamin D is obtained primarily from sunlight exposure, fortified foods, and supplements, and then activated by hydroxylation, certain populations with high rates of sleep disturbance, including older adults, people with limited sunlight exposure, as well as those with dark skin pigmentation and obesity, which limit vitamin D synthesis and circulating levels, respectively, are also at risk for low 25(OH)D concentration. Elucidating variation in the associations between 25(OH)D concentration and sleep across race/ethnic groups is especially important given known race/ethnic differences in sleep traits,14–18 25(OH)D concentrations, and the associations of 25(OH)D with clinical outcomes.19–21 In this study of 1,721 Multi-Ethnic Study of Atherosclerosis (MESA) participants, we examined associations of 25(OH)D with objectively measured sleep duration, continuity, architecture, and apnea-hypopnea index (AHI), and in patient-reported symptom severity in this racially/ethnically diverse cohort of men and women.
METHODS
Data Source/Study Population
MESA is a multicenter, community-based cohort study of clinical and subclinical cardiovascular disease.22 From 2000–2002, MESA enrolled 6,814 adults aged 45–84 y from six US field centers (New York and Bronx counties, NY; Baltimore and Baltimore County, MD; Forsyth County, NC; Chicago, IL; St Paul, MN; and Los Angeles, CA). By design, MESA recruited a racially/ethnically diverse sample (38% white, 28% African American, 22% Hispanic, and 12% Asian, predominantly Chinese American), without cardiovascular disease. At baseline (Exam 1), MESA collected information on sociodemographics, medical history, health habits, and medications using self-administered questionnaires and standardized interviews, as well as performed anthropometry and phlebotomy. Serial examinations have been performed over time. At MESA Exam 5 (2010–2013), MESA participants (other than those reporting regular use of oral devices, nocturnal oxygen, or nightly positive airway pressure devices), were invited to participate in the MESA Sleep Ancillary Study, which consisted of polysomnography (PSG), 7-day wrist actigraphy, and sleep questionnaire data collected during an in-home examination. Of 4,077 participants approached, 147 (3.6%) were ineligible and 141 (3.6%) lived too far away to participate. Of the remaining 3,789 participants, 2,261 (59.7%) participated in the sleep examination. In total, 2,060 participants had successful PSG data, 2,156 had actigraphy data, and 2,240 participants completed sleep questionnaires. Compared to participants in MESA Exam 5 who did not undergo the sleep examination, participants in the sleep examination were slightly younger (68.4 versus 71.0 y), less likely to be white (36.1% versus 44.5%), less likely to be smokers (7.1% versus 8.4%), and less likely to have chronic obstructive pulmonary disease (1.6% versus 2.5%). However, they were comparable in regard to sex, body mass index (BMI), physician-diagnosed sleep apnea, asthma, diabetes, and prior myocardial infarction. In this analysis, we excluded participants with < 5 nights of actigraphy data (n = 127), missing data on sleep stages obtained via PSG (n = 202), or missing baseline serum 25(OH)D levels (n = 89). Our final sample (n = 1,721) included all MESA-Sleep participants who successfully completed PSG and ≥ 5 days of actigraphy, with 25(OH)D levels, and complete data on covariates of interest. No covariate had ≥ 5% of missingness.
The institutional review boards at all participating centers approved the study, and all participants gave written informed consent.
25-Hydroxyvitamin D
Fasting serum was collected from MESA participants at Exam 1 in 2000–2002, and stored at −80°C. Total 25(OH)D [sum of 25(OH)D2 and 25 (OH)D3] was measured using high-liquid chromatography-tandem mass spectrometry with internal standards at the University of Washington as described previously.21 Calibration was confirmed using National Institute of Standards and Technology's standard reference material 972.23 25-hydroxyvitamin D is known to be stable for long periods at −80°C.24
Given substantial seasonal variation in 25(OH)D concentration, we defined our primary exposure of interest as mean annualized total serum 25(OH)D concentration. This value was estimated from a single baseline value using a cosinor model previously developed and validated in MESA.21 To account for the sinusoidal pattern of 25(OH)D throughout the year, the cosinor model fits 25(OH)D as a sine wave over time, a method that leads to less variance compared with standard seasonal adjustment and predicts 25(OH) over time more precisely compared to baseline values carried forward.21 We categorized 25(OH) D as deficient (< 20 ng/mL), insufficient (20–29 ng/ mL), or sufficient (≥ 30 ng/mL) based on established definitions.25 We additionally considered 25(OH)D as a continuous linear variable.
Sleep Outcomes
Actigraphy
Subjects were asked to wear an actigraphy device (Acti-watch Spectrum; Philips Respironics, PA) on the non-dominant wrist for 7 consecutive days while completing a sleep diary over the same period. A minimum of 4 weekdays and 1 weekend day were required for analysis. Data were scored at a central reading center (Brigham and Women's Hospital [BWH]). The sleep period was manually identified based on information from a self-activated event marker, sleep diary, and light sensor. The actigraphy data were processed in 30-sec epochs to classify sleep or wake periods using the Actiware-Sleep v. 5.59 analysis software (Mini Mitter Co., Inc., Bend, OR) and a validated algorithm that weights the activity counts in relationship to the level of activity in the surrounding 2-min time periods to yield weighted values of activity counts.26 Sleep onset was determined based on 5 min of immobile time and sleep offset as the last epoch within the rest interval that met the activity criterion for sleep by the weighted activity algorithm. Intrascorer reliability for average sleep duration and sleep efficiency were 0.91 and 0.97, respectively.
Polysomnography
Participants underwent a single night, unattended 15-channel PSG at home using a portable monitor (Somté PSG, Computmedics Ltd., Abbotsford, Victoria, Australia), utilizing methods adapted from the Sleep Heart Health Study.27 Recorded channels included electroencephalograms (Fz, Cz, Oz, C4, M1), bilateral electrooculograms, electrocardiogram, chin electromyogram, thoracic and abdominal respiratory inductance plethysmography, airflow (via oral/nasal thermistor and nasal pressure transducer), oxyhemoglobin saturation (finger pulse oximetry), leg movements, and body position. Studies were scored at a centralized sleep reading center (BWH), for review and scoring by registered polysomnologists, using American Academy of Sleep Medicine guidelines.28 Interscorer and intrascorer reliability for the reported summary measures was excellent, with intraclass correlation coefficients generally exceeding 0.90.
The primary sleep outcomes of interest were actigraphydefined average sleep duration and average sleep maintenance efficiency during the sleep period and PSG-determined proportion of total sleep time in stages N3 and rapid eye movement (REM), each analyzed as continuous outcomes. Secondary sleep outcomes included Epworth Sleepiness Scale (ESS) score,29 Women's Health Initiative Insomnia Rating Scale (WHIIRS) score (range 0 to 20; higher score = more symp -toms),30 and AHI as continuous outcomes. The AHI was calculated as the number of all obstructive apneas plus hypopneas associated with at least 3% oxygen desaturation per hour of sleep. The “4% AHI” was calculated according to established criteria.28
Additional Covariates of Interest
Sociodemographic characteristics and health habits included baseline sex, race/ethnicity (white, Hispanic, African American, Chinese American), educational attainment (high school graduate or less, some post-high school education, bachelors or graduate degree), as well as family income (< $25,000, $25,000–$39,999, $40,000–$74,999, and ≥ $75,000/y), physical activity, any self-reported current alcohol use (yes/no), and smoking status (current, former, never) from Exam 5. Physical activity was estimated as total reported intentional exercise performed in a usual week in metabolic equivalent-minutes, collected using the MESA Typical Week Physical Activity Survey, adapted from the Cross-Cultural Activity Participant Study.31 As a measure of adiposity, we used waist circumference and calculated BMI (kg/m2) from anthropometry conducted at MESA Exam 5. Hypnotics (i.e., benzodiazepine receptor agonists, trazodone) and antidepressant use (i.e., selective serotonin uptake inhibitor, serotonin-norepinephrine reuptake inhibitor, tricyclic antidepressant, monoamine oxidase inhibitor) were categorized (yes/no) based on medication reconciliation with prescription bottles at the Exam 5 visit. Glomerular filtration rate was estimated from serum creatinine and demographic data collected at Exam 5, using the Chronic Kidney Disease Epidemiology Collaboration equation.32 Information on history of osteoarthritis (yes/no) and asthma (yes/ no) was based on self-report at Exam 1; Depression score was based on the Center for Epidemiologic Studies Depression Scale33 at Exam 5.
Statistical Analyses
Given limited missing data, we employed complete-case analyses. We used the chi-square test to compare baseline characteristics across 25(OH)D categories. To quantify the association between 25(OH)D and sleep outcomes, we fit separate multiple linear regression models for each sleep outcome. Variance estimates in the models of average sleep duration and average sleep maintenance efficiency were weighted by the number of nights of successful actigraphic recordings. Our primary multivariable models included age, race/ethnicity, sex, waist circumference, and examination site (Model 1). Secondary models additionally included covariates that were possible confounders specified a priori, including educational attainment, family income, self-reported physical activity level (log transformed continuous variable), smoking status, and alcohol intake (Model 2). Because some chronic medical conditions may confound or mediate the association between 25(OH)D and sleep, we examined the impact of further adjusting for antidepressant use, depression score, history of osteoarthritis, history of asthma, and glomerular filtration rate (Model 3). We additionally fit each model with 25(OH) D parameterized as a linear continuous effect. As bioactive forms of 25(OH)D vary by race/ethnicity,19 we included a race/ ethnicity interaction term (race/ethnicity*vitamin D category) in our models and additionally stratified analyses by race/ ethnicity. To account for multiple hypothesis testing, overall mean differences among 25(OH)D categories were tested with an F-test. Pairwise differences among the 25(OH)D categories, specifically deficient versus sufficient and insufficient versus sufficient, were examined with t tests. All analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Sample Characteristics
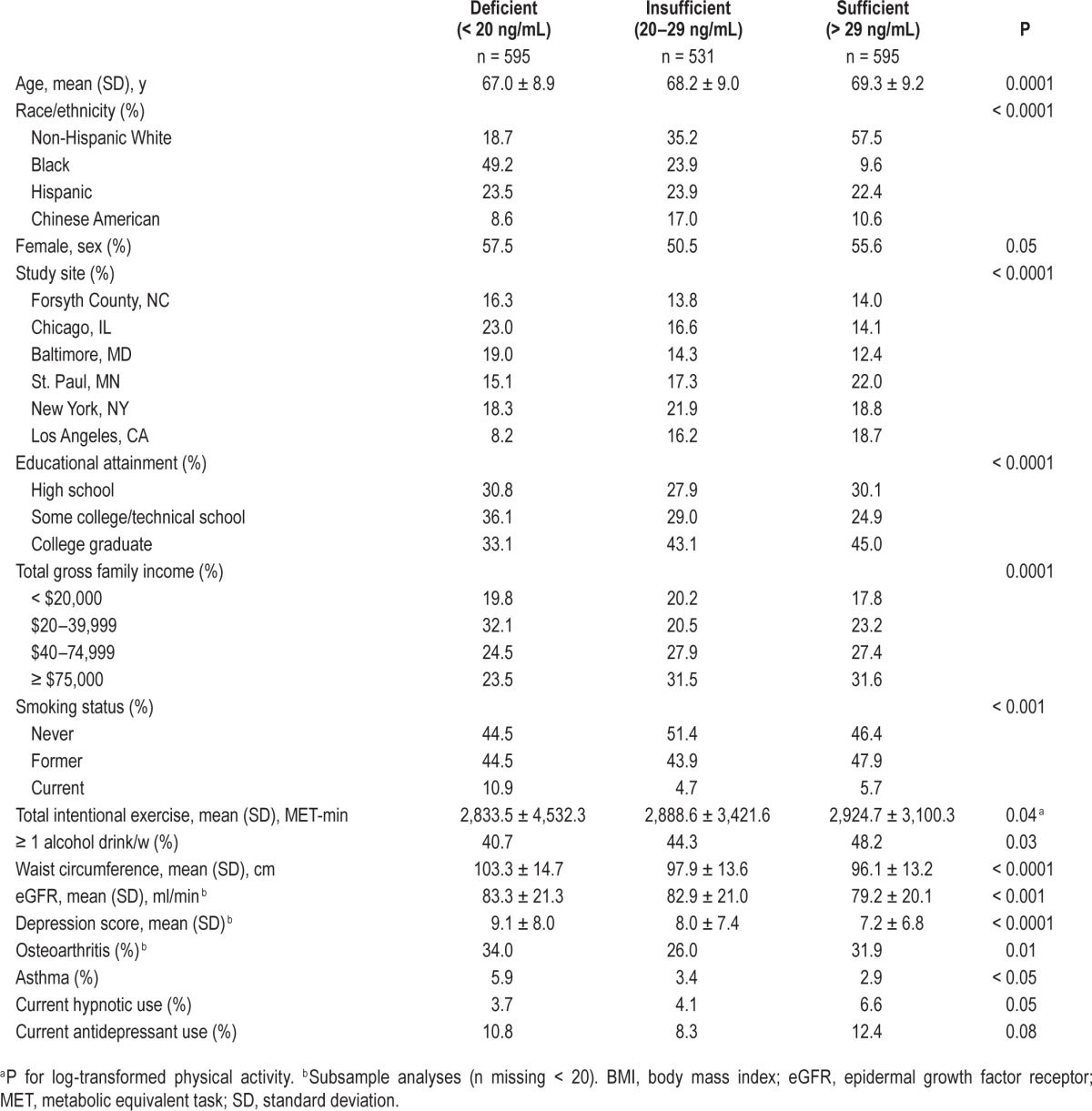
Of the 1,721 MESA participants in our sample, 34.6% were 25(OH)D deficient (< 20 ng/mL) and 30.9% 25(OH)D insufficient (20–29 ng/mL). Mean age was 68.2 ± 9.1 y, and 37.2% were white, 27.7% African American, 11.9% Chinese American, and 23.2% Hispanic. Compared with 25(OH)D sufficient participants, those with lower 25(OH)D were younger, to be of African American race/ethnicity, and had lower educational level, family income, physical activity levels, and alcohol use (Table 1). Participants with lower 25(OH)D also had higher mean waist circumference and depression scores, and higher rates of current smoking and asthma.
Table 1.
Sample characteristics by serum 25-hydroxyvitamin D concentration (n = 1,721).
25(OH) and Sleep Outcomes
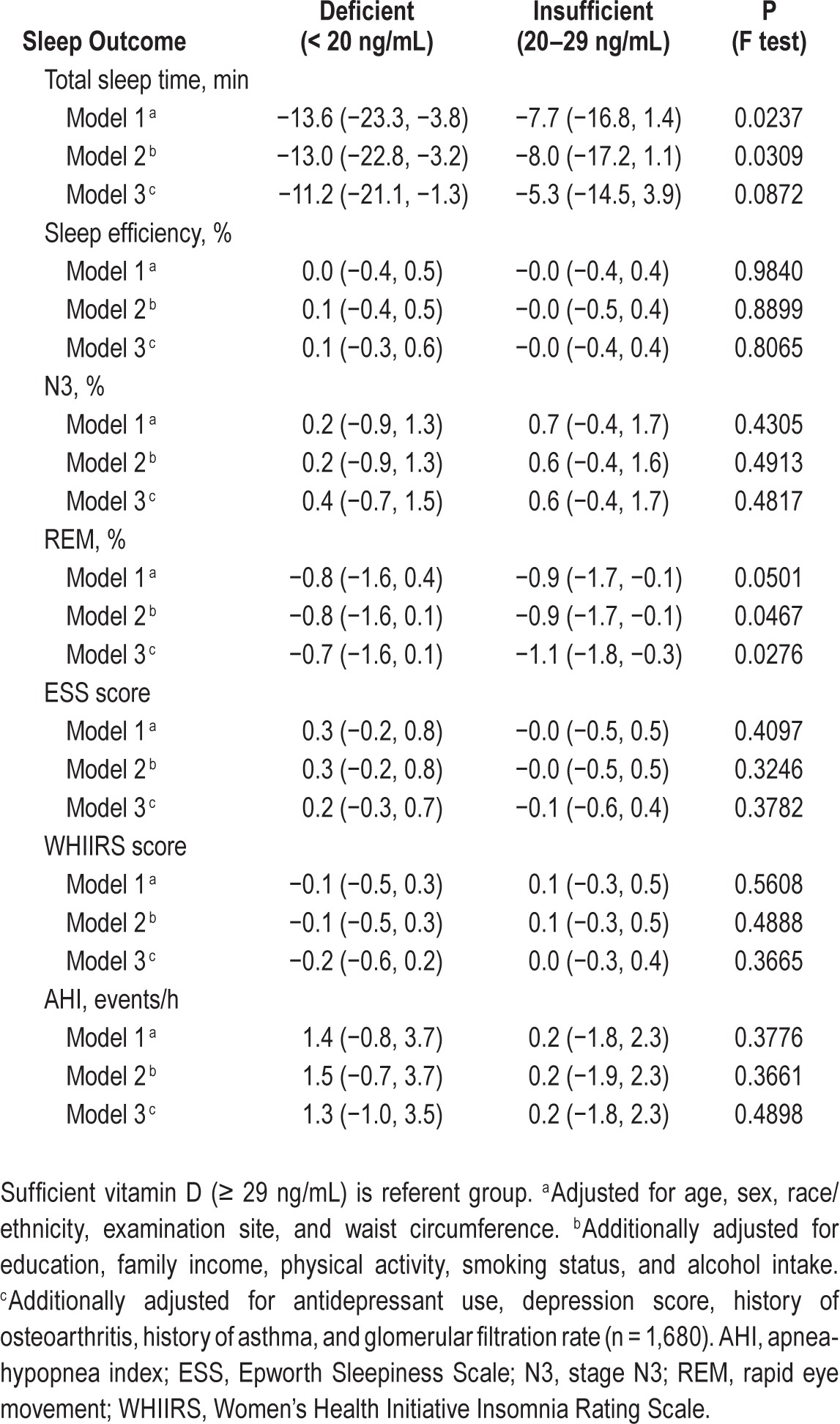
Mean number of usable nights of actigraphy was 7.0 (range 5–12), and did not vary by 25(OH)D category. In unadjusted analyses, participants with 25(OH)D deficiency had shorter sleep duration, lower proportion of time in REM sleep, higher ESS scores, and higher AHI (Table 2). In models adjusted for age, sex, race/ethnicity, waist circumference, and examination site (Model 1), in models additionally adjusted for education, family income, physical activity, smoking status, and alcohol intake (Model 2), 25(OH)D deficiency was significantly associated with shorter sleep duration (Table 3). With further adjustment for additional covariates, some of which may be in intermediate pathways, this association was attenuated by approximately 15% and was no longer significant. Insufficient 25(OH)D was modestly associated with a lower proportion in REM. In adjusted models, we did not find evidence of an association between 25(OH)D concentration and sleep efficiency, proportion in N3, ESS, WHIIS, AHI, or 4% AHI. Using 25(OH) D as a continuous linear variable did not substantively change our results.
Table 2.
Sleep outcomes by serum 25-hydroxyvitamin D concentration, unadjusted associations.
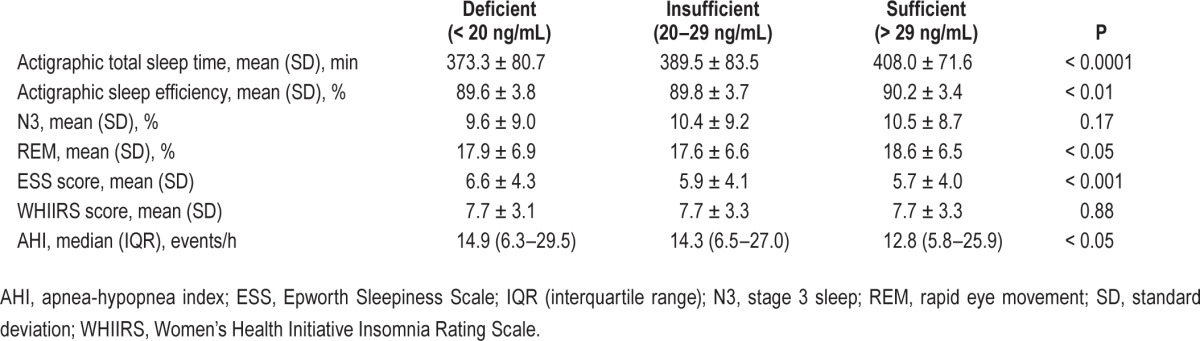
Table 3.
Adjusted mean differences and 95% confidence interval in sleep outcomes by 25(OH)D concentration in the Multi-Ethnic Study of Atherosclerosis.
We further explored variation between 25(OH)D and sleep outcomes by race/ethnicity. In African Americans, 25(OH) D was significantly associated with shorter average sleep duration, with 25(OH)D deficiency associated with 25.6 ± 11.7 fewer minutes (95% confidence interval, −48.5, −2.7; P = 0.03), after adjustment for age, sex, examination site, and waist circumference (Model 1), with similar point estimates with further adjustment of potential confounders and meditators in Models 2 (P < 0.05) and 3 (P = 0.06). In contrast, in race/ ethnicity stratified analyses, there was no association between 25(OH)D and sleep duration within other race/ethnicities, (test for heterogeneity [race/ethnicity*vitamin D category] for total sleep time P = 0.07 for a linear trend model). Though we also found a modest association between 25(OH)D and proportion REM in non-Hispanic whites, the average difference in REM was < 2%. Among Chinese Americans, 25(OH) deficiency was associated with an AHI that was 7.06 (95% confidence interval, 0.66–13.46) events/hour higher AHI compared with 25(OH) D sufficient participants. Further adjustment in models 2 and 3 generally had no appreciable influence on effect estimates. Testing of heterogeneity for AHI was not significant. We did not find evidence of an association between 25(OH)D concentration and other sleep outcomes in race/ethnic-stratified multivariable models. Using 25(OH)D as a continuous linear variable did not substantively change our results in our race/ ethnic-stratified models.
DISCUSSION
In this large, multirace/ethnic, community-based cohort, we evaluated associations between 25(OH)D concentrations and several clinically important sleep traits, observing a modest association between 25(OH)D deficiency with shorter sleep duration measured objectively using actigraphy. Notably, exploratory race/ethnicity specific analysis suggested that this association may be restricted to African Americans, in whom 25(OH)D deficiency was associated with an mean reduction of 25 min in average nightly sleep duration. Although variations in sleep efficiency, sleep stages, sleepiness, and AHI by 25(OH)D levels were also observed in the entire cohort, these associations were largely explained by confounding influences. Last, Chinese American participants with 25(OH)D deficiency had an AHI about 7 events/h higher than 25(OH) D sufficient individuals of Chinese American race/ ethnicity.
Particular interest has been given to variation in 25(OH)D levels across individuals of different racial/ ethnic groups as a potential explanatory factor for health disparities.34,35 In our exploratory race/ethnic stratified analyses, we found that low 25(OH)D concentration may be associated with shorter sleep duration among African American participants and in higher AHI among Chinese American MESA participants. Potential explanations for our findings include race/ethnic variation in vitamin D metabolism, such as variations in vitamin D receptor activity, vitamin D binding proteins, or other factors influencing vitamin D activation,36 as well as other potential unmeasured mediators. In support, previous work from MESA demonstrated an association between lower 25(OH) D concentration and greater risk of incident coronary artery disease among white and Chinese participants, but not among black or Hispanic participants.20 Of interest, in age, sex, and BMI-adjusted analyses in MESA, sleep duration was shortest among African Americans, whereas AHI was highest among Chinese Americans.37 Although there are several potential explanations for these race/ethnic differences in sleep traits, the current analysis suggests a novel mechanism or at least a potential biomarker to explain racial/ethnic differences in sleep outcomes.
The observed decrease by 20–30 min in average objective sleep duration in 25(OH) D deficient African Americans compared to 25(OH) D sufficient individuals is comparable in duration to the effect of benzodiazepines on objective sleep duration in clinical trials of older adults with insomnia.38 Additionally, a recent clinical trial demonstrated that behaviorally extending sleep by about 30 minutes reduced systolic blood pressure 7 mmHg more than a control intervention (sleep hygiene).39 With emerging epidemiologic evidence supporting short sleep duration as a risk factor for cardiometabolic morbidity and mortality,40–43 our finding of an association between vitamin D deficiency and reduced sleep duration within African Americans, a population with both higher cardiovascular risk and shorter sleep duration relative to other race/ethnic groups in the United States,44–47 may have significant public health implications.
Few studies have examined the association between serum 25(OH)D and sleep outcomes, and none has examined variation across race/ethnic groups. A prior study of national survey data observed no relationship between serum 25(OH)D and sleep duration relied on self-reported sleep duration,10 which may result in misclassification. One large community-based cohort study did not find an association between 25(OH)D48 and AHI after adjusting for traditional sleep apnea risk factors, but that study was restricted to elderly men, most of whom were non-Hispanic whites. Our observed association between AHI and 25(OH)D to AHI in Chinese Americans is consistent with to two previous studies reporting an association of serum 25(OH)D to AHI and to sleepiness in small clinic-based samples.11,13 Emerging data indicate that individuals of Asian ancestry may be at increased risk for sleep apnea at lower levels of BMI than other groups.49,50 It is possible that the relatively stronger association among Chinese Americans appear due to fewer “competing risks” associated with obesity.
Study Strengths and Limitations
The study had a number of major strengths, including the multirace/ethnic sample drawn from sites across the United States and use of standardized and rigorously measured sleep outcomes, including those obtained from both PSG and actigraphy. We also rigorously controlled for multiple potential confounders. The study also had several limitations. Although 25(OH)D measurements were made using precise and accurate methods, measurements preceded the collection of sleep outcomes by an average of 10.3 y (range 8.5–12.5 y). However, serum 25(OH)D concentrations have been reported to remain relatively stable over long periods51 and thus, may be considered a relatively stable exposure. Nonetheless, misclassification of long-term vitamin D exposure would likely bias toward the null. Additionally, we also measured total, rather than free or bioavailable vitamin D, and the associations between free or other bioactive forms of vitamin D and sleep outcomes may differ from the associations we found between total 25(OH) D concentration with sleep outcomes. We chose to use mean annualized 25(OH)D to account for seasonal variation in vitamin D levels. This may have attenuated our ability to detect associations between 25(OH)D and sleep outcomes that may vary seasonally. However, more than one-third of our sample had a mean annualized 25(OH)D < 20 ng/mL. In addition to the potential limitations of our staggered cross-sectional design, given the association 25(OH)D with several outcomes, many individuals in the original cohort with low 25(OH)D concentrations may have died before Exam 5, thereby leading to selection bias. Similarly, the MESA Sleep Ancillary Study excluded MESA participants on treatment for OSA (n = 103), and hence our models using AHI as an outcome were subject to selection bias; however, given the low proportion of participants excluded (2%), risk of bias is low. Furthermore, although the participation rate in the MESA Sleep Ancillary study was relatively high (60%), the possibility of selective participation may exist. However, demographic, health, and sleep characteristics were generally similar among MESA participants who did and did not participate in the MESA Sleep Ancillary study. Our results also need to be interpreted in the context of testing of multiple hypotheses. Given that our outcomes were likely to be highly correlated with one another, we did not account for multiple hypotheses testing between our models. This potentially may have led to type I error, and hence our results should be interpreted with caution and results should be replicated in independent samples. To our knowledge, this was the first study to explore race/ethnic variations among 25(OH)D concentrations and several sleep outcomes, and thereby our secondary analyses of race/ethnic variations, particularly among race/ethnic groups with smaller sample sizes (e.g., Chinese American) was largely exploratory.
CONCLUSIONS
With recent interest in vitamin D's role in extraskeletal health and rapid increase in vitamin D testing, sleep physicians frequently encounter patients with symptoms often attributed to low vitamin D. Over four racial/ethnic groups, we found that vitamin D deficiency was modestly associated with shorter duration of sleep, but not other subjective or objective sleep traits. However, our study is the first to suggest that low 25(OH)D status may be most strongly associated with shorter objectively measured sleep duration in African Americans and higher AHI in Chinese Americans. Although our findings should be considered preliminary and require replication in other cohorts, our findings suggest that race/ethnicity may modify vitamin D's associations with sleep duration and AHI. Further research is needed to elucidate whether changes in 25(OH)D may influence sleep duration in groups such as African Americans, who are at increased risk of low 25(OH)D levels, shorter sleep duration, and related adverse health outcomes.
DISCLOSURE STATEMENT
This study was supported by the National Center for Complementary and Integrative Health (NCCIH) of the National Institutes of Health under Award Number K23AT005104 and by the National Heart Lung and Blood Disorders: R01HL098433 and R01HL096875. This research was also supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. de Boer has received research support from Abbvie. Dr. Redline has received research support from ResMed Foundation and equipment use from Philips Respironics and ResMed. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Litonjua AA. Vitamin D deficiency as a risk factor for childhood allergic disease and asthma. Curr Opin Allergy Clin Immunol. 2012;12:179–85. doi: 10.1097/ACI.0b013e3283507927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Song Y, Manson JE, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–29. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen CJ, Adams JS, Bikle DD, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012;33:456–92. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gominak SC, Stumpf WE. The world epidemic of sleep disorders is linked to vitamin D deficiency. Med Hypotheses. 2012;79:132–5. doi: 10.1016/j.mehy.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 6.McCarty DE. Resolution of hypersomnia following identification and treatment of vitamin d deficiency. J Clin Sleep Med. 2010;6:605–8. [PMC free article] [PubMed] [Google Scholar]
- 7.McCarty DE, Chesson AL, Jr, Jain SK, Marino AA. The link between vitamin D metabolism and sleep medicine. Sleep Med Rev. 2014;18:311–9. doi: 10.1016/j.smrv.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Endocrinol Metab Clin North Am. 2010;39:321–31. doi: 10.1016/j.ecl.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y, Winzenberg T, Nguo K, Lin J, Jones G, Ding C. Association between serum levels of 25-hydroxyvitamin D and osteoarthritis: a systematic review. Rheumatology (Oxford) 2013;52:1323–34. doi: 10.1093/rheumatology/ket132. [DOI] [PubMed] [Google Scholar]
- 10.Shiue I. Low vitamin D levels in adults with longer time to fall asleep: US NHANES, 2005-2006. Int J Cardiol. 2013;168:5074–5. doi: 10.1016/j.ijcard.2013.07.195. [DOI] [PubMed] [Google Scholar]
- 11.McCarty DE, Reddy A, Keigley Q, Kim PY, Marino AA. Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med. 2012;8:693–7. doi: 10.5664/jcsm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kheirandish-Gozal L, Peris E, Gozal D. Vitamin D levels and obstructive sleep apnoea in children. Sleep Med. 2014;15:459–63. doi: 10.1016/j.sleep.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mete T, Yalcin Y, Berker D, et al. Obstructive sleep apnea syndrome and its association with vitamin D deficiency. J Endocrinol Invest. 2013;36:681–5. doi: 10.3275/8923. [DOI] [PubMed] [Google Scholar]
- 14.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor GT, Lind BK, Lee ET, et al. Variation in symptoms of sleep-disordered breathing with race and ethnicity: the Sleep Heart Health Study. Sleep. 2003;26:74–9. [PubMed] [Google Scholar]
- 16.Song Y, Ancoli-Israel S, Lewis CE, Redline S, Harrison SL, Stone KL. The association of race/ethnicity with objectively measured sleep characteristics in older men. Behav Sleep Med. 2011;10:54–69. doi: 10.1080/15402002.2012.636276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandner MA, Petrov ME, Rattanaumpawan P, Jackson N, Platt A, Patel NP. Sleep symptoms, race/ethnicity, and socioeconomic position. J Clin Sleep Med. 2013;9:897–905. doi: 10.5664/jcsm.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powe CE, Evans MK, Wenger J, et al. Vitamin D–Binding Protein and Vitamin D Status of Black Americans and White Americans. N Engl J Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310:179–88. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachs MC, Shoben A, Levin GP, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;97:1243–51. doi: 10.3945/ajcn.112.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 23.Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr. 2008;88:511S–12S. doi: 10.1093/ajcn/88.2.511S. [DOI] [PubMed] [Google Scholar]
- 24.Agborsangaya C, Toriola AT, Grankvist K, et al. The effects of storage time and sampling season on the stability of serum 25-hydroxy vitamin D and androstenedione. Nutr Cancer. 2010;62:51–7. doi: 10.1080/01635580903191460. [DOI] [PubMed] [Google Scholar]
- 25.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 26.Oakley NR. Validation with polysomnography of the Sleepwatch sleep/ wake scoring algorithm used by the Actiwatch activity monitoring system. Technical Report to Mini Mitter Co. Inc. 1997 [Google Scholar]
- 27.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 28.Iber C, Ancoli-Israel S, Chesson A, Jr., Quan SF for the American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 29.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 30.Levine DW, Dailey ME, Rockhill B, Tipping D, Naughton MJ, Shumaker SA. Validation of the Women's Health Initiative Insomnia Rating Scale in a multicenter controlled clinical trial. Psychosom Med. 2005;67:98–104. doi: 10.1097/01.psy.0000151743.58067.f0. [DOI] [PubMed] [Google Scholar]
- 31.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8:805–13. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 34.Grant WB, Peiris AN. Possible role of serum 25-hydroxyvitamin D in black-white health disparities in the United States. J Am Med Dir Assoc. 2010;11:617–28. doi: 10.1016/j.jamda.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Kritchevsky SB, Tooze JA, Neiberg RH, et al. 25-Hydroxyvitamin D, parathyroid hormone, and mortality in black and white older adults: the health ABC study. J Clin Endocrinol Metab. 2012;97:4156–65. doi: 10.1210/jc.2012-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norris KC, Williams SF. RAce/ethnicity, serum 25-hydroxyvitamin D, and heart disease. JAMA. 2013;310:153–5. doi: 10.1001/jama.2013.7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Wang R, Zee PC, Lutsey PL, Javaheri S, Alcantara C, Williams MA, Redline S. Racial/ethnic differences in sleep disordered breathing in normal weight, overweight, and obese adults: the Multi-Ethnic Study of Atherosclerosis. Sleep. 2014;37:A142. (Abstract Suppl) [Google Scholar]
- 38.Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335–50. doi: 10.1007/s11606-007-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haack M, Serrador J, Cohen D, Simpson N, Meier-Ewert H, Mullington JM. Increasing sleep duration to lower beat-to-beat blood pressure: a pilot study. J Sleep Res. 2013;22:295–304. doi: 10.1111/jsr.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 41.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 42.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 43.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension analyses of the first national health and nutrition examination survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 44.Mezick EJ, Hall M, Matthews KA. Sleep duration and ambulatory blood pressure in black and white adolescents. Hypertension. 2012;59:747–52. doi: 10.1161/HYPERTENSIONAHA.111.184770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zizi F, Pandey A, Murrray-Bachmann R, et al. Race/ethnicity, sleep duration, and diabetes mellitus: analysis of the National Health Interview Survey. Am J Med. 2012;125:162–7. doi: 10.1016/j.amjmed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grandner MA, Buxton OM, Jackson N, Sands-Lincoln M, Pandey A, Jean-Louis G. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep. 2013;36:769–79E. doi: 10.5665/sleep.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014;15:42–50. doi: 10.1016/j.sleep.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goswami U, Ensrud K, Paudel ML, Redline S, et al. Vitamin D concentrations and obstructive sleep apnea in a multicenter cohort of older male [abstract] American Journal of Respiratory and Critical Care Medicine. 2014;Vol. 189 Meeting Abstracts. [Google Scholar]
- 49.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119:62–9. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 50.Li KK, Kushida C, Powell NB, Riley RW, Guilleminault C. Obstructive sleep apnea syndrome: a comparison between Far-East Asian and white men. Laryngoscope. 2000;110:1689–93. doi: 10.1097/00005537-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 51.Sonderman JS, Munro HM, Blot WJ, Signorello LB. Reproducibility of serum 25-hydroxyvitamin D and vitamin D-binding protein levels over time in a prospective cohort study of black and white adults. Am J Epidemiol. 2012;176:615–21. doi: 10.1093/aje/kws141. [DOI] [PMC free article] [PubMed] [Google Scholar]


