Abstract
Study Objectives:
To determine the impact of averaging window-length on the “desaturation” indexes (DIs) obtained via overnight pulse oximetry (SpO2) at high altitude.
Design:
Overnight SpO2 data were collected during a 10-day sojourn at high altitude. SpO2 was obtained using a commercial wrist-worn finger oximeter whose firmware was modified to store unaveraged beat-to-beat data. Simple moving averages of window lengths spanning 2 to 20 cardiac beats were retrospectively applied to beat-to-beat SpO2 datasets. After SpO2 artifacts were removed, the following DIs were then calculated for each of the averaged datasets: oxygen desaturation index (ODI); total sleep time with SpO2 < 80% (TST < 80), and the lowest SpO2 observed during sleep (SpO2 low).
Setting:
South Base Camp, Mt. Everest (5,364 m elevation).
Participants:
Five healthy, adult males (35 ± 5 y; 180 ± 1 cm; 85 ± 4 kg).
Interventions:
N/A.
Measurements and Results:
49 datasets were obtained from the 5 participants, totalling 239 hours of data. For all window lengths ≥ 2 beats, ODI and TST < 80 were lower, and SpO2 low was higher than those values obtained from the beat-to-beat SpO2 time series data (P < 0.05).
Conclusions:
Our findings indicate that increasing oximeter averaging window length progressively underestimates the frequency and magnitude of sleep disordered breathing events at high altitude, as indirectly assessed via the desaturation indexes.
Citation:
Cross TJ, Keller-Ross M, Issa A, Wentz R, Taylor B, Johnson B. The impact of averaging window length on the “desaturation” indexes obtained via overnight pulse oximetry at high altitude. SLEEP 2015;38(8):1331–1334.
Keywords: pulse oximetry, sleep, high altitude, moving averages
INTRODUCTION
Sojourning at high altitude (> 2,400 m) is often accompanied by repeating episodes of apneas/hypopneas during sleep; i.e., sleep disordered breathing.1 The prevalence of sleep disordered breathing may be indirectly assessed via overnight pulse oximetry (SpO2).2–5 Overnight SpO2 provides insight into the manifestation of disordered breathing patterns during sleep by the calculation of the “desaturation” indexes (DIs). Some commonly used DIs are the oxygen desaturation index (ODI; events per hour) and the total sleep time (TST) below a certain threshold of SpO2. For overnight SpO2 studies performed at sea level, it is well known that DIs are underestimated during episodes of sleep disordered breathing when a pulse oximeter's averaging window is set too wide.6–14 While these observations may have clear implications for the interpretation of overnight SpO2 at sea level, the impact of averaging window length on the DIs obtained at high altitude is unknown.
This short report was designed to explore the influence of averaging window length on DIs obtained from overnight SpO2 data collected at high altitude. Data were obtained from five young, healthy adults during a 10-day sojourn at South Base camp, Mt. Everest (5,364 m elevation). Simple moving averages of window lengths spanning 2 to 20 cardiac beats were retrospectively applied to beat-to-beat SpO2 collected during sleep. DIs obtained from each averaged dataset were: the oxygen desaturation index (ODI); total sleep time spent below SpO2 of 80% (TST < 80); and the lowest SpO2 value observed during sleep (SpO2 low). Based on the findings of studies at sea level,6–14 we expected that ODI and TST < 80 would fall and SpO2 low would rise as averaging window length was increased from 2 to 20 cardiac beats.
METHODS
Participants and Ethical Approval
Five healthy adult males (35 ± 5 yr; 180 ± 1 cm; 85 ± 4 kg) participated in the present study. Prior to the study period, participants underwent health screening to ensure they were nonobese, physically active, nonsmokers, with no history of cardiac, metabolic and/or pulmonary disease. None of the participants had a previous history of central or obstructive sleep apnea. All participants provided written informed consent to participate in the study, which had been approved by the Institutional Review Board of the Mayo Clinic.
Data Collection and Analyses
Participants were studied during a recent expedition to South Base Camp on Mt. Everest (5,364 m elevation). Overnight SpO2 was recorded using a wrist-worn finger pulse oximeter (WristOx2, Model 3150, Nonin Medical Inc., Plymouth, MN, USA). The firmware of the oximeter was modified to store unaveraged beat-to-beat values of SpO2. Only contiguous datasets ≥ 5 h were included in the present study. Simple moving averages were retrospectively applied to the raw, preprocessed beat-to-beat SpO2 time series. The window length of these moving averages varied from 2 to 20 cardiac beats in single-beat increments. Cardiac beats were used as the time basis for the moving averages rather than seconds. This approach ensured that any effect of averaging window length on the DIs was due solely to the value of n, and not to variations in heart rate within and between participants. Once the “averaged” SpO2 time series had been created, each dataset was analyzed for artifacts and desaturation events.
Artifacts and DIs
Artifacts were identified as single-beat values that were either ≤ 30% or had changed by more than ± 4%/s from the previous beat.15 An artifact index was determined by dividing the observed number of artifacts by the total number of heart beats during the corresponding overnight recording. Once the artifact index had been determined, artifacts were removed from the dataset before calculation of the DIs. The minimum value of SpO2 observed within the “artifact-free” time series was reported as the lowest SpO2 (SpO2 low). The total sleep time spent below SpO2 of 80% was expressed as a percentage of total sleep duration, yielding the score TST < 80. Desatu-ration events were identified as a fall in SpO2 > 3% below a proceeding 20-s baseline, lasting for a duration ≥ 10 s.15 Importantly, the “minimum duration” criterion served to minimize spurious event detection due to breath-by-breath variations in SpO2 during sleep. The oxygen desaturation index (ODI) was calculated as the number of events (dips) per hour.
Statistical Analyses
Repeated measures analyses of variance were used to examine the influence of averaging window-length on artifact index, SpO2 low, TST < 80, and ODI. Pair-wise comparisons were assessed using the Bonferroni post hoc adjustment. Results are presented as means ± 95% confidence interval (CI95%). Statistical analyses were considered significant if P < 0.05.
RESULTS
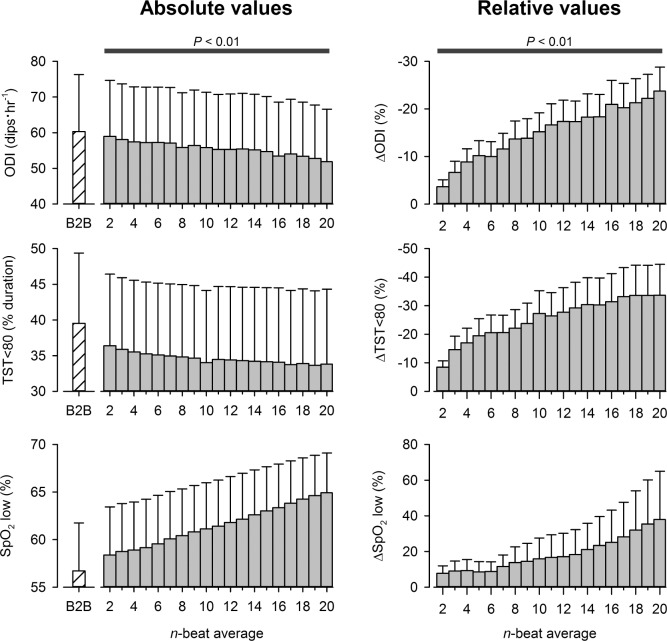
A total of 49 datasets were obtained from the 5 participants, totalling 239 h of data. The average basal SpO2 and heart rate for all datasets were 79% ± 6% and 72 ± 2 beats/min, respectively. The artifact index decreased with progressively wider moving average windows (Figure 1, P < 0.05). The influence of averaging-window length on the DIs at high altitude is displayed in Figure 2. For all averaging window lengths, the calculated ODI, and TST < 80 were lower than those obtained from the “artifact-free” beat-to-beat SpO2 time series (P < 0.05). In contrast, SpO2 low was higher for all window lengths than those values derived from the “artifact-free” beat-to-beat SpO2 data (P < 0.05).
Figure 1.
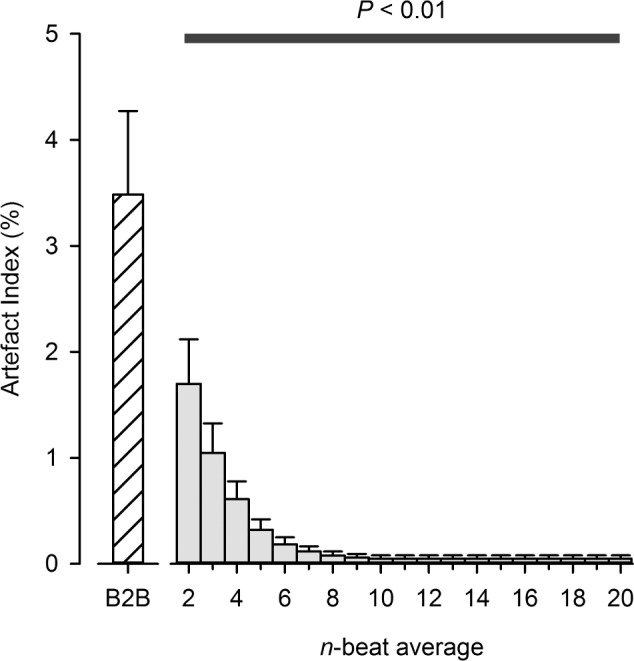
The impact of averaging window-length on the prevalence of SpO2 artifacts during sleep at high-altitude. The black-bar denotes that all averaging windows ≥ 2 beats wide produced artifact indexes that were significantly lower than those determined from the raw, beat-to-beat SpO2 dataset, P < 0.01. B2B, beat-to-beat data.
Figure 2.
The impact of averaging window-length on the “desaturation” indexes obtained during sleep at high-altitude. The black-bar denotes that all averaging windows ≥ 2 beats wide produced desaturation indexes that were significantly different from those obtained with the “artifact-free,” beat-to-beat SpO2 dataset, P < 0.01. ODI, oxygen desaturation index; TST < 80, total time spent below an arterial O2 saturation value of 80%; SpO2 low, the lowest SpO2 observed during sleep; B2B, beat-to-beat data; Δ, absolute change in value expressed relative (%) to B2B data.
DISCUSSION
The novel findings of this report demonstrate that varying averaging window length does indeed produce different DIs calculated from overnight SpO2 at high altitude. These observations are in agreement with those made at lowland, whereby increasing the averaging window length of a pulse oximeter progressively reduces the frequency and magnitude of arterial O2 desaturation events recorded during sleep.6–14 Our results highlight the need for investigators to carefully consider their choice of oximeter averaging window length when reporting DIs obtained during sleep at high altitude.
Artifacts and DIs
New-generation pulse oximeters do not typically allow the user to record and/or display SpO2 data on a beat-to-beat basis. A major reason for this constraint is that raw, beat-to-beat SpO2 data is often confounded by motion artifact. The presence of such “noise” adversely affects calculation of DIs. A simple approach to reducing the prevalence of SpO2 artifacts is to apply a moving average to beat-to-beat data. Certainly, we show in Figure 1 that a 2-beat wide moving average effectively reduces the artifact index by 50% and, moreover, that artifact index approximates zero when n ≥ 10 beats. This “robustness” to SpO2 artifact may be ascribed to the low-pass filtering effect of moving averages.16 On this point, however, moving averages are regarded as exceptionally poor low-pass filters. The effective cutoff frequency and stop-band attenuation of a moving average filter are dependent on its window length (i.e., n). An example of this relationship is illustrated in Figure 3. In general, cutoff frequency decreases and stop-band attenuation improves as window length is increased. For this reason, it is difficult to choose a value for n which appropriately separates noise (i.e., SpO2 artifact) from meaningful signal content (i.e., desaturation events). This problem is highlighted in the present study. Our findings demonstrate that while a 10-beat moving average yielded a desirable level of artifact rejection (artifact index < 0.1%), the corresponding DIs were markedly different from those obtained with the “artifact-free” beat-to-beat SpO2 datasets (see Figure 2). What, then, is an appropriate value for n?
Figure 3.
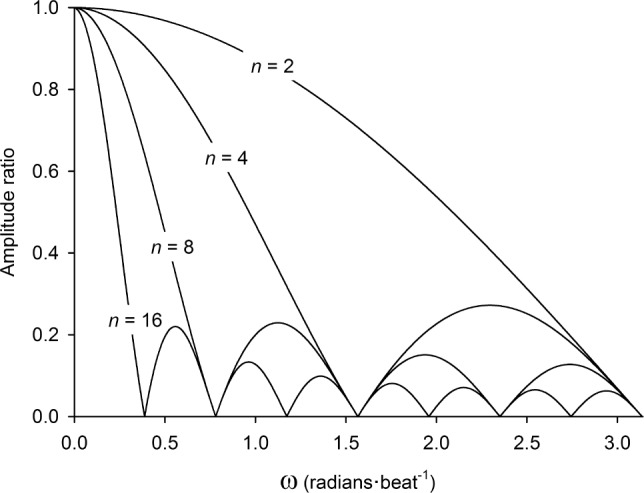
Frequency responses of simple moving averages with varying window lengths. An amplitude ratio of 1.00 indicates that 100% of the amplitude-content in the original signal (i.e., raw beat-to-beat SpO2) is preserved in the filtered signal (i.e., “averaged” SpO2) at the corresponding angular frequency (ω). Conversely, an amplitude ratio of 0.00 indicates that the amplitude-content of the original signal has been completely attenuated by the moving average filter, at the given ω.
The AASM Manual for Scoring of Sleep and Associated Events suggests that overnight SpO2 data be recorded using a window length ≤ 3 s at a heart rate of 80 beats/min, or roughly 4 beats.17 However, Figure 2 illustrates that a window-length as small as 4 beats appreciably underestimates ODI and TST < 80 by 10% to 15%. Further to this point, our data reveals that any value of n ≥ 2 produces DIs that are significantly different from those obtained with the “artifact-free” beat-to-beat SpO2 time series. Thus, it may be tempting to state that the optimal value for n is 1: that is, beat-to-beat SpO2 data with artifacts removed. However, because no clinical outcomes were assessed in this study, the assertion that n = 1 is optimal must be considered as conjecture at this point. Further studies are required to substantiate this idea.
Implications of Our Findings
Although sleep disordered breathing may explain the daytime somnolence and mental fatigue suffered by native lowlanders sojourning at high altitude,18–20 its role in the etiology of altitude sickness is much less certain. For example, it appears that the manifestation of altitude sickness is strongly related to the degree of nocturnal hypoxic exposure (approximated here by TST < 80).21,22 However, there is evidence to suggest that a high frequency of desaturation/resaturation events during sleep (i.e., high ODI) may lessen nocturnal hypoxic exposure by raising mean SpO2 throughout the night, potentially facilitating altitude tolerance.1,23 With the above in mind, we caution future investigators to be cognisant of the effect that averaging window length bears on the calculation of ODI, TST < 80, and SpO2 low at high altitude.
Methodological Considerations
We chose to examine the impact of averaging window-length on the DIs by averaging the participants' beat-to-beat SpO2 data post hoc. The effect of averaging window length on the DIs has been typically examined by recording SpO2 data from simultaneous pulse oximeters on one subject, with each oximeter device set to a different averaging-time.6–8,11–14 Our approach was different in that averaging window length was varied post hoc using beat-to-beat SpO2 data collected during sleep. The advantages of our approach were two-fold: (1) the effect of averaging window-length on the DIs could be examined using data recorded from the same device; and (2) a greater range of window lengths could be explored because n was not limited to the firmware settings of the device. It is also worth mentioning that only equally weighted, “simple” moving averages were used in our analysis. The weighting-scheme of moving averages used by a pulse oximeter varies between device manufacturers (exponential weighting, etc.). It is emphasized, however, that no matter which particular “type” of moving average is used, a very similar effect of window length on the DIs will be observed: namely that ODI and TST < 80 will decrease, and SpO2 low increase, as n is lengthened.
Summary
The present study demonstrates that increasing the window length of an oximeter's moving average leads to a progressive underestimation of the DIs obtained during sleep while sojourning at high altitude. Although moving averages may improve a pulse oximeter's tolerance to unwanted noise, this robustness to artifact comes at the price of underestimating the frequency of desaturation/resaturation events and magnitude of nocturnal hypoxic exposure during sleep at high altitude. This point is particularly important for future investigators seeking to examine potential relationships between DIs and markers of altitude sickness.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was part of a larger Everest Expedition funded by The North Face Company and The National Geographic Society, and the Mayo Clinic through a grant from the Leslie and Lou Gonda families. We appreciate the technical help from Alex Kasak, the clinical expertise of Douglas Summerfield, and all the accompanying members of our laboratory who worked hard to help prepare for the project. This expedition was the 2012 Legacy Climb led by climber Conrad Anchor to celebrate the 50th anniversary of the 1963 American Mt. Everest Expedition.
REFERENCES
- 1.Ainslie PN, Lucas SJ, Burgess KR. Breathing and sleep at high altitude. Respir Physiol Neurobiol. 2013;188:233–56. doi: 10.1016/j.resp.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Bennett JA, Kinnear WJ. Sleep on the cheap: the role of overnight oximetry in the diagnosis of sleep apnoea hypopnoea syndrome. Thorax. 1999;54:958–9. doi: 10.1136/thx.54.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson LG, Ambrogetti A, Gyulay SG. Prediction of sleep-disordered breathing by unattended overnight oximetry. J Sleep Res. 1999;8:51–5. doi: 10.1046/j.1365-2869.1999.00134.x. [DOI] [PubMed] [Google Scholar]
- 4.Netzer N, Eliasson AH, Netzer C, Kristo DA. Overnight pulse oximetry for sleep-disordered breathing in adults: a review. Chest. 2001;120:625–33. doi: 10.1378/chest.120.2.625. [DOI] [PubMed] [Google Scholar]
- 5.Ryan PJ, Hilton MF, Boldy DA, et al. Validation of British Thoracic Society guidelines for the diagnosis of the sleep apnoea/hypopnoea syndrome: can polysomnography be avoided? Thorax. 1995;50:972–5. doi: 10.1136/thx.50.9.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davila DG, Richards KC, Marshall BL, et al. Oximeter's acquisition parameter influences the profile of respiratory disturbances. Sleep. 2003;26:91–5. [PubMed] [Google Scholar]
- 7.Farre R, Montserrat JM, Ballester E, Hernandez L, Rotger M, Navajas D. Importance of the pulse oximeter averaging time when measuring oxygen desaturation in sleep apnea. Sleep. 1998;21:386–90. doi: 10.1093/sleep/21.4.386. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed SJM, Rich W, Finer NN. The effect of averaging time on oximetry values in the premature infant. Pediatrics. 2010;125:e115–21. doi: 10.1542/peds.2008-1749. [DOI] [PubMed] [Google Scholar]
- 9.Vagedes J, Bialkowski A, Wiechers C, Poets CF, Dietz K. A conversion formula for comparing pulse oximeter desaturation rates obtained with different averaging times. PLoS One. 2014;9:e87280. doi: 10.1371/journal.pone.0087280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vagedes J, Poets CF, Dietz K. Averaging time, desaturation level, duration and extent. Arch Dis Child Fetal Neonatal Ed. 2013;98:F265–6. doi: 10.1136/archdischild-2012-302543. [DOI] [PubMed] [Google Scholar]
- 11.Rheineck-Leyssius AT, Kalkman CJ. Advanced pulse oximeter signal processing technology compared to simple averaging. II. Effect on frequency of alarms in the postanesthesia care unit. J Clin Anesth. 1999;11:196–200. doi: 10.1016/s0952-8180(98)00133-0. [DOI] [PubMed] [Google Scholar]
- 12.Rheineck-Leyssius AT, Kalkman CJ. Advanced pulse oximeter signal processing technology compared to simple averaging. I. Effect on frequency of alarms in the operating room. J Clin Anesth. 1999;11:192–5. doi: 10.1016/s0952-8180(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 13.Rheineck-Leyssius AT, Kalkman CJ. Influence of pulse oximeter settings on the frequency of alarms and detection of hypoxemia: theoretical effects of artifact rejection, alarm delay, averaging, median filtering or a lower setting of the alarm limit. J Clin Monit Comput. 1998;14:151–6. doi: 10.1023/a:1007431305610. [DOI] [PubMed] [Google Scholar]
- 14.Davila DG, Richards KC, Marshall BL, et al. Oximeter performance: the influence of acquisition parameters. Chest. 2002;122:1654–60. doi: 10.1378/chest.122.5.1654. [DOI] [PubMed] [Google Scholar]
- 15.Taha BH, Dempsey JA, Weber SM, et al. Automated detection and classification of sleep-disordered breathing from conventional polysomnography data. Sleep. 1997;20:991–1001. doi: 10.1093/sleep/20.11.991. [DOI] [PubMed] [Google Scholar]
- 16.Smith SW. The scientist and engineer's guide to digital signal processing. 2nd ed. San Diego, CA: California Technical Publishing; 1999. Moving average filters; p. 277. [Google Scholar]
- 17.Berry RB, Brooks R, Gamaldo CE, et al. Darien, IL: American Academy of Sleep Medicine; 2013. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Version 2.0. www.aasmnet.org. [Google Scholar]
- 18.Beaumont M, Batejat D, Pierard C, et al. Zaleplon and zolpidem objectively alleviate sleep disturbances in mountaineers at a 3,613 meter altitude. Sleep. 2007;30:1527–33. doi: 10.1093/sleep/30.11.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weil JV. Sleep at high altitude. High Alt Med Biol. 2004;5:180–9. doi: 10.1089/1527029041352162. [DOI] [PubMed] [Google Scholar]
- 20.de Aquino Lemos V, Antunes HK, dos Santos RV, Lira FS, Tufik S, de Mello MT. High altitude exposure impairs sleep patterns, mood, and cognitive functions. Psychophysiology. 2012;49:1298–306. doi: 10.1111/j.1469-8986.2012.01411.x. [DOI] [PubMed] [Google Scholar]
- 21.Erba P, Anastasi S, Senn O, Maggiorirni M, Bloch KE. Acute mountain sickness is related to nocturnal hypoxemia but not to hypoventilation. Eur Respir J. 2004;24:303–8. doi: 10.1183/09031936.04.00006504. [DOI] [PubMed] [Google Scholar]
- 22.Burgess KR, Johnson P, Edwards N, Cooper J. Acute mountain sickness is associated with sleep desaturation at high altitude. Respirology. 2004;9:485–92. doi: 10.1111/j.1440-1843.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 23.Nespoulet H, Wuyam B, Tamisier R, et al. Altitude illness is related to low hypoxic chemoresponse and low oxygenation during sleep. Eur Respir J. 2012;40:673–80. doi: 10.1183/09031936.00073111. [DOI] [PubMed] [Google Scholar]