Abstract
Background
Understanding how lay people perceive the causes of mortality and their associated risk factors is important for public health. In resource-limited settings, where verbal autopsy (VA) is used as the most expedient method of determining cause of death, it is important to understand how pre-existing concepts of cause of death among VA-informants may influence their VA-responses and the consequential impact on cause of death assessment. This study describes the agreement between VA-derived causes of death and informant-perceived causes and associated influential factors, which also reflects lay health literacy in this setting.
Method
Using 20 years of VA data (n=11,228) from the Agincourt Health and Demographic Surveillance System (HDSS) site in rural South Africa, we explored the agreement between the causes of death perceived by the VA-informants and those assigned by the automated Inter-VA tool. Kappa statistics and concordance correlation coefficients were applied to measure agreement at individual and population levels, respectively. Multivariable regression models were used to explore factors associated with recognised lay perceptions of causes of mortality.
Results
Agreement between informant-perceived and VA-derived causes of death at the individual level was limited, but varied substantially by cause of death. However, agreement at the population level, comparing cause-specific mortality fractions was higher, with the notable exception of bewitchment as a cause. More recent deaths, those in adults aged 15–49 years, deaths outside the home, and those associated with external causes showed higher concordance with InterVA.
Conclusion
Overall, informant perception of causes of death was limited, but depended on informant characteristics and causes of death, and to some extent involved non-biomedical constructs. Understanding discordance between perceived and recognised causes of death is important for public health planning; low community understanding of causes of death may be detrimental to public health. These findings also illustrate the importance of using rigorous and standardised VA methods rather than relying on informants’ reported causes of death.
Keywords: community perception, causes of death, verbal autopsy
Lay opinions about health and disease reflect conceptual models applied by individuals or communities to derive reasons for ill-health and causes of death. Examining divergence between lay perceptions of health and disease and established medical convention can illuminate modern health challenges and inequalities (1). An holistic approach to understanding health and disease also supports strategies set by national and international health decision makers for enhancing self-management of health and empowers people to seek out and handle information responsibly (2, 3).
In resource-poor settings, assessing how people make appropriate health decisions at home or at work, in the community, in the health system, and the political arena (4) – an overall concept of ‘health literacy’ – requires good understanding of how lay people perceive health, illness, and causes of death. In South Africa, as in other nations, emerging health problems, epidemics, and chronic illnesses mean that understanding people's perceptions and their health-related behaviour is particularly essential for effective medical practice and public health interventions (2, 5, 6). In this African context, different cultural and ethnic groups may disagree about symptomatology of particular diseases (1). These disagreements can advance into multifarious conceptual models about health and related causes of mortality across different groups. Consequently, community perceptions of disease processes may diverge from those of health professionals (1, 7). Swami et al. suggested that some communities tend to describe their poor health inconsistently with medical evidence, but such views persist since they form part of the conceptual knowledge of that community (1). Employing traditional methods to assess community understanding of related causes of mortality in Africa has resulted in a broad spectrum of outcomes (8–12). Nonetheless, no studies have yet sought standardised tools such as the verbal autopsy (VA) to explore people's perception of causes of death in African settings, which is the intention of this research.
VA is a widely used approach for ascertaining causes of death in settings that lack complete physician certification (13, 14). Trained fieldworkers use standardised interviews with informants (usually caretakers or close relatives of the deceased) to gather information on the signs, symptoms, and circumstances of death, and this information is then used to identify a probable medical cause of death. The automated InterVA-4 tool, which has been validated in several settings, has shown substantial benefits over physician reviews, particularly its consistency over time and place (15, 16).
The VA method relies to a large extent on lay informants to elicit a specific cause of death. Informant-given responses to VA questions can be influenced by the informant's own perception of causes of death which could ultimately impact on the InterVA assessment of cause of death (14). Evidence of relying on lay reports as a basis for national estimates of cause-specific mortality is not yet proven, with both lay reports and physicians failing to distinguish between common causes of deaths such as TB and HIV/AIDS in some instances (17).
Using 20 years of VA data from the Agincourt Health and Socio-Demographic Surveillance System (HDSS) site in South Africa, this study aims to describe the agreement between objective VA-derived causes of death with the informant-perceived causes gathered during the VA interview. The study also explores informant-related background and socio-economic factors contributing to the derived level of agreement whereby public health implications of misperception of causes of mortality in the rural South African context are also addressed.
Methods
Study population and context
In this empirical study, data were generated from longitudinal observations covering the period from March 1992 to December 2011 in the Agincourt HDSS, in Mpumalanga Province, South Africa. The Agincourt HDSS, described in detail elsewhere (18), has been operating as a monitoring system for around 90,000 inhabitants living in approximately 16,000 households across 26 villages, since 1992. The HDSS procedures for capturing vital events [births, deaths, in-and out-migrations, and other individual characteristics such as marital status, nationality, education, and socio-economic status (SES)] also include VA interviews to establish likely causes of death.
Verbal autopsy
During this 20-year period, 12,206 deaths in the Agincourt HDSS were routinely followed-up by the trained fieldworkers to conduct VA with next of kin or caregiver, and VAs were successfully completed in 11,228 cases (92.0%). Standardised interviews in the local language (Shangaan) were used to elicit signs, symptoms, and circumstances of the terminal illness. During the VA interviews, interviewers also sought respondents’ opinions about the causes of death (5). We refer to this here as the ‘respondent-reported cause of death’ (RRCoD).
Until fairly recently, VA material was usually interpreted into causes of death by presenting individual interview data to local physicians (physician-certified verbal autopsy, PCVA) (19). However, to address time, cost, and reliability issues associated with PCVA (9), computerised approaches that are faster, cheaper, and more consistent have been developed and are now in widespread use (20). The most widely used is InterVA, a Bayesian-based model using a probability matrix to generically estimate the most likely cause(s) of death from VA data (21). Developed from a combination of existing data and input from an expert panel, InterVA-4 is freely available resource (www.interva.net) which is fully compliant with the WHO 2012 VA standard (22). Equivalence between InterVA-4 cause of death assignments and the PCVA approach has also been demonstrated in a large-scale international study (23). Within the Agincourt HDSS, the VA archives from 1992 have been retrospectively processed using InterVA-4 (24). We refer to this source here as the ‘verbal autopsy cause of death’ (VACoD).
Data management
HDSS data on the deceased's background and characteristics were taken directly from the Agincourt database. The recorded deaths were categorised into four age groups (0–14, 15–49, 50–64, and 65+years), two origins (in-migrants from Mozambique and their direct descendants, or South African), and three levels of education measured by the numbers of completed years of study (none, 1–7, and 8–15 years). Relationship between the VA respondent and the deceased was classified in six groups (parent, grandparent, spouse, child, sibling, and other non-relatives). Other HDSS information pertaining to the main dwelling, sanitation, power, and livestock (all with given ordinal scores) was handled to create a SES factor representing household wealth in quintiles. Individual level data from the VA interviews comprising details on any previous illness that had occurred, duration of the final illness (acute:<2 weeks and chronic:≥2 weeks), cumulative number of deaths in the household (one death, two deaths, and three or more deaths at one household), time (1992–1996, 1997–2001, 2002–2006, and 2007–2011), and place of death (home, hospital/health facility, or other) were recorded. Any therapeutic approach prior to death (western or traditional, or both) and the RRCoD was also retrieved from the VA interview data (5).
Exploring agreement at individual level
Each individual VA was processed using InterVA-4 model which assigns up to three likely causes of death per WHO VA cause categories (22). RRCoDs were given as a single cause in most cases. For comparing RRCoD and VACoD, a total of 17 causes of death groups were generated including the indeterminate group, as listed in Table 1. Both the RRCoD and VACoD data were re-categorised into these 17 groups; individual agreement was achieved for any case where there was an RRCoD and VACoD category in common. Cohen's kappa statistic (κ) was used to compare the proportions of agreement between two readings which are made by two different approaches, that is, InterVA and RRCoD, taking into account the proportion of agreement that would be expected due to chance.
Table 1.
Proportions of deaths as assigned to VACoD, for 6,721 deaths in the Agincourt HDSS, South Africa, with RRCoD and 4,507 without RRCoD
| RRCoD given | RRCoD not given | |||
|---|---|---|---|---|
|
|
|
|||
| Cause of death category | n | % | n | % |
| Accidental causes | 394 | 5.9 | 48 | 1.1 |
| Homicide or suicide | 356 | 5.3 | 102 | 2.3 |
| Nervous system disease | 30 | 0.4 | 6 | 0.1 |
| Diarrhoeal disease | 175 | 2.6 | 69 | 1.5 |
| Maternal causes | 23 | 0.3 | 15 | 0.3 |
| Indeterminate | 368 | 5.5 | 282 | 6.3 |
| HIV/TB | 2,579 | 38.4 | 2,078 | 46.1 |
| Stroke | 224 | 3.3 | 63 | 1.4 |
| Cardiovascular disease | 256 | 3.8 | 156 | 3.5 |
| Neoplasm | 416 | 6.2 | 457 | 10.1 |
| Endocrine or malnutrition | 186 | 2.8 | 133 | 3.0 |
| Neonatal or congenital | 166 | 2.5 | 124 | 2.8 |
| Chronic liver disease | 29 | 0.4 | 36 | 0.8 |
| Chronic respiratory disease | 273 | 4.1 | 182 | 4.0 |
| Acute infectious disease | 221 | 3.3 | 124 | 2.8 |
| Acute respiratory disease | 870 | 12.9 | 521 | 11.6 |
| Other | 159 | 2.4 | 111 | 2.5 |
VACoD=verbal autopsy causes of death; RRCoD=respondent-reported cause of death.
The binomial agreement (agreement or not) between VACoD and RRCoD was analysed against the informant's background, characteristics, and socio-economic factors for each cause of death category using multivariable logistic regression with a p-value cut-off point of 0.05. The multivariable regression model building followed a ‘forward’ selection approach carried on the selected variables of interest. The likelihood-ratio test was performed to compare the log likelihood of the two models every time a new variable was added and best model-fit was therefore retained. Missing values in both ‘illness duration’ and ‘therapeutic approach’ factors were coded ‘unknown’ since they considerably refer to accidental, homicide, or suicides.
Estimating agreement at population level
Population-level cause-specific mortality fractions (CSMF) were derived from the InterVA-4 output to estimate the proportion that each cause of death category contributed to the total number of deaths (5). Differences between proportions and 95% confidence intervals of those differences were calculated for each cause of death group between VACoD and RRCoD. A concordance correlation coefficient (CCC) for the CSMFs from VACoD and RRCoD was also computed using Lin's CCC with 95% CI (25). Based on Pearson's correlation, the CCC quantifies the agreement between the two measures (23).
Ethical clearance
The study data were obtained from the Agincourt HDSS where on-going ethical clearance has been granted by the University of Witwatersrand's Committee for Research on Human Subjects (No. M960720 & M110138). The principle of informed consent was fully respected with the right for refusal or withdrawal from interviews at both individual and household levels. No specific additional ethical clearance was required for this study.
Results
Of 11,228 deaths for which a VA was successfully completed, InterVA-4 assigned a single cause of death to 9,434 individuals (84.0%), two likely causes to 1,108 individuals (9.9%), and three likely causes to 36 cases (0.3%). There were a further 650 cases (5.8%) specifically designated ‘indeterminate’ by InterVA-4, generally reflecting a lack of specific VA data or contradictory evidence. Of the 11,228 cases with VA findings, 6,721 (59.9%) recorded specific RRCoDs (including ‘do not know’ reports, but excluding reiteration of symptoms that were not actually causes of death) during VA interviews. Table 1 shows the VACoD distribution for both VA interviews where respondents did or did not report a cause of death.
For the VA interviews where RRCoD was reported, 6,645 (98.8%) gave a single cause (of which 2,292 were ‘do not know’), 73 (1.1%) reported two causes, and three (0.1%) reported 3 or 4 causes. Overall, there was agreement on at least one cause between RRCoD and VACoD in 2,080 cases (30.9%). Table 2 shows VACoD and RRCoD by cause category, together with the agreement between RRCoD and VACoD, and the associated kappa statistic. Kappa (κ)>0.4 is considered moderate-to-good agreement (26). Bewitchment as RRCoD was reported for 865 cases, which had no possibility of being designated as a VACoD cause. Over 40% of the bewitchment cases were associated with HIV/TB as a VACoD.
Table 2.
Proportions of deaths with individual agreement between VACoD and RRCoD, for 6,721 deaths in the Agincourt HDSS, South Africa
| Cause of death category | VACoD n |
RRCoD n |
Agreement% (95% CI) |
Kappa statistic (κ) (95% CI) |
|---|---|---|---|---|
| Accidental causes | 407 | 557 | 79.1 (75.2–83.1) | 0.64 (0.61–0.68) |
| Homicide or suicide | 367 | 358 | 69.8 (65.0–74.4) | 0.69 (0.65–0.73) |
| Nervous system disease | 32 | 127 | 53.1 (35.6–70.7) | 0.21 (0.12–0.29) |
| Diarrhoeal disease | 186 | 464 | 45.2 (38.0–52.3) | 0.23 (0.18–0.27) |
| Maternal causes | 23 | 18 | 34.8 (14.9–54.7) | 0.39 (0.20–0.58) |
| Indeterminate | 374 | 2,292 | 34.5 (29.6–39.4) | 0.001 (−0.013–−0.016)a |
| HIV/TB | 2,857 | 903 | 29.6 (27.9–31.4) | 0.30 (0.28–0.32) |
| Stroke | 252 | 211 | 27.4 (21.9–32.9) | 0.27 (0.22–0.33) |
| Cardiovascular disease | 301 | 264 | 22.1 (17.4–26.8) | 0.20 (0.15–0.25) |
| Neoplasm | 476 | 254 | 19.2 (15.7–22.9) | 0.21 (0.16–0.25) |
| Endocrine or malnutrition | 215 | 197 | 13.3 (8.7–17.9) | 0.11 (0.06–0.16) |
| Neonatal or congenital | 171 | 39 | 11.2 (6.5–16.0) | 0.18 (0.11–0.25) |
| Chronic liver disease | 36 | 46 | 8.6 (−0.8–18.0)a | 0.07 (−0.01–−0.15)a |
| Chronic respiratory disease | 282 | 67 | 5.8 (3.1–8.6) | 0.08 (0.04–0.12) |
| Acute infectious disease | 247 | 83 | 4.5 (1.9–7.1) | 0.05 (0.01–0.09) |
| Acute respiratory disease | 953 | 27 | 1.1 (0.4–1.8) | 0.01 (0.002–0.02) |
| Other | 189 | 28 | 1.1 (−0.4–2.5)a | 0.01 (−0.01–0.04)a |
| Bewitchment | – | 865 | – | – |
| Total | 4,514 | 4,510 | – | – |
Individual deaths can contribute to more than one category, where so described by InterVA-4 or the respondent's report.
Not significantly different from zero agreement.
VACoD=verbal autopsy causes of death; RRCoD=respondent-reported cause of death.
Results from a multivariable logistic regression model of background factors in relation to agreement between RRCoD and VACoD are presented in Table 3. Increased accurate perception of causes of mortality was associated with deaths of people aged 15–49 years, deaths in more recent time periods, deaths with longer illness durations (or those with unknown illness duration, which on closer inspection were largely accidents, homicides, and suicides), and deaths which did not occur at home.
Table 3.
Background characteristics for 6,721 deaths in the Agincourt HDSS, South Africa, showing multivariable OR for agreement between RRCoD and VACoD, including ‘unknowns’
| Background characteristics | Number of deaths (%) | OR (95% CI) | |
|---|---|---|---|
| Age group | 0–14 (child) | 1,322 (19.7) | Ref |
| 15–49 (reproductive age) | 2,560 (38.1) | 1.46 (1.15–1.86)a | |
| 50–64 (adult) | 1,429 (21.3) | 1.06 (0.81–1.38) | |
| >65 (elder) | 1,405 (20.9) | 0.98 (0.74–1.29) | |
| Sex | Male | 3,573 (53.2) | Ref |
| Female | 3,148 (46.8) | 1.04 (0.93–1.17) | |
| Origin | Mozambican origin | 2,061 (30.7) | Ref |
| South African origin | 4,660 (69.3) | 1.09 (0.96–1.24) | |
| Respondent | Child | 867 (12.9) | Ref |
| Parent | 2,418 (36.0) | 0.89 (0.72–1.10) | |
| Spouse/sibling | 2,132 (31.7) | 1.12 (0.92–1.36) | |
| Other | 1,304 (19.4) | 1.16 (0.95–1.42) | |
| Years of education | None | 2,707 (32.8) | Ref |
| 1–7 | 1,465 (21.8) | 1.14 (0.96–1.34) | |
| 8–15 | 1,591 (23.7) | 1.11 (0.92–1.34) | |
| Other/too young | 1,458 (21.7) | 0.86 (0.68–1.09) | |
| Period of death | 1992–1996 | 766 (11.4) | Ref |
| 1997–2001 | 1,161 (17.3) | 1.03 (0.83–1.29) | |
| 2002–2006 | 1,802 (26.8) | 1.26 (1.02–1.55)a | |
| 2007–2011 | 2,992 (44.5) | 1.21 (0.99–1.48) | |
| Cumulative household deaths | 1 Person | 2,744 (40.8) | Ref |
| 2 People | 2,137 (31.8) | 1.01 (0.89–1.15) | |
| 3 People or more | 1,840 (27.4) | 1.07 (0.93–1.22) | |
| Illness duration | <2 weeks (acute) | 1,530 (22.8) | Ref |
| >2 weeks (chronic) | 4,026 (59.9) | 1.16 (1.01–1.34)a | |
| Unknown | 1,165 (17.3) | 3.59 (2.96–4.34)a | |
| Previous illness | No | 4,522 (67.3) | Ref |
| Yes | 2,199 (32.7) | 1.12 (0.98–1.28) | |
| Therapeutic approach | None | 775 (11.5) | Ref |
| Western only | 2,576 (38.3) | 1.14 (0.93–1.40) | |
| Traditional only | 164 (2.4) | 0.67 (0.43–1.06) | |
| Western and traditional | 2,457 (36.6) | 0.98 (0.79–1.21) | |
| Unknown | 749 (11.1) | 1.23 (0.97–1.55) | |
| Place of death | Home | 2,795 (41.6) | Ref |
| Hospital | 3,071 (45.7) | 1.18 (1.04–1.34)a | |
| Other locations | 855 (12.7) | 1.56 (1.30–1.88)a |
Odds ratio significantly different from unity.
OR=odds ratio; VACoD=verbal autopsy causes of death; RRCoD=respondent-reported cause of death.
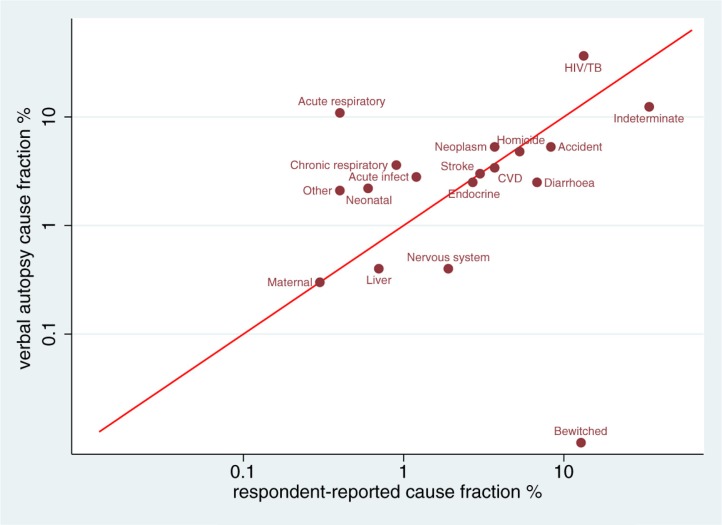
To assess agreement at the population level, CSMFs derived from the VA using the InterVA-4 models were compared with those obtained from RRCoD as presented in Table 4, together with the CSMF difference and 95% confidence interval. Concordance between the VACoD and RRCoD fractions is shown in Fig. 1, with a CCC of 0.43 (95% CI: 0.04–0.83; p=0.03).
Table 4.
CSMF from VACoD and RRCoD for 6,721 deaths in Agincourt HDSS, South Africa
| Cause-specific mortality fraction | |||
|---|---|---|---|
|
|
|||
| Cause of death category | VACoD% | RRCoD% | Difference% (95% CI) |
| HIV/TB | 36.5 | 13.3 | −23.2 (−24.6–−21.8)a |
| Indeterminate | 12.4 | 34.1 | 21.7 (20.3−23.1)a |
| Acute respiratory disease | 10.9 | 0.4 | −10.5 (−11.3−9.7)a |
| Neoplasm | 5.3 | 3.7 | −1.5 (−2.2−0.8)a |
| Accidental causes | 5.3 | 8.3 | 3.0 (2.2−3.8)a |
| Homicide or suicide | 4.8 | 5.3 | 0.5 (−0.2−1.2) |
| Chronic respiratory disease | 3.6 | 0.9 | −2.7 (−3.2−2.2)a |
| Cardiovascular disease | 3.4 | 3.7 | 0.3 (−0.3−0.9) |
| Stroke | 3.0 | 3.0 | 0.1 (−0.5−0.6) |
| Acute infectious disease | 2.8 | 1.2 | −1.6 (−2.1−1.1)a |
| Endocrine or malnutrition | 2.5 | 2.7 | 0.2 (−0.3−0.7) |
| Diarrhoeal disease | 2.5 | 6.8 | 4.3 (3.6−5.0)a |
| Neonatal or congenital | 2.2 | 0.6 | −1.6 (−2.0−1.2)a |
| Others | 2.1 | 0.4 | −1.7 (−2.1−1.3)a |
| Chronic liver disease | 0.4 | 0.7 | 0.3 (0.1−0.6)a |
| Nervous system disease | 0.4 | 1.9 | 1.5 (1.1−1.9)a |
| Maternal | 0.3 | 0.3 | 0.0 (−0.2−0.2) |
| Bewitched | 0.0 | 12.8 | 12.8 (12.0−13.6)a |
Difference significantly different from zero.
CSMF=cause-specific mortality fractions; VACoD=verbal autopsy causes of death; RRCoD=respondent-reported cause of death.
Fig. 1.
Concordance (log-log scale) between CSMF determined by VA and respondent reports, in relation to the line of equivalence, for 6,721 deaths in the Agincourt HDSS, South Africa. CSMF=cause-specific mortality fractions; VA= verbal autopsy.
Discussion
In this African setting, where most deaths pass undocumented or unattended by professionals, reliable information on cause of death is scarce (27). The relatively robust nature of the InterVA-4 tool, and particularly its consistency over time and place, made it the choice for the reference group here (15, 16, 21, 28, 29). During the early part of the period covered by this dataset, a massive epidemic of HIV/AIDS occurred in Agincourt, which subsided latterly as HIV treatment options took effect (16). This may well have influenced local perceptions of causes of death over time (30). Interestingly, Table 1 shows that the RRCoDs were less likely to be given for deaths due to HIV/TB, neoplasm, or acute respiratory diseases, perhaps reflecting a degree of stigmatisation or the entrenched cultural beliefs with respect to some causes.
Exploring pre-existing concepts about causes of deaths among VA respondents is crucial for documenting mortality and understanding patterns of death for public health planning (14). Moreover, it reflects on how local beliefs may influence care-seeking, public health prevention, and impact of health care service interventions in this setting. Not least, a VA respondent's perceptions of cause of death may sway responses to specific questions in a VA interview, and thereby affect conclusions on cause of death. The limited perception of causes of mortality revealed here, with only a fairly small proportion of respondents providing a medically plausible cause of death, is not surprising and may also be similar in other national or regional African settings (31, 32). African countries have some of the lowest literacy rates in the world, particularly among females, though South Africa has some of the highest literacy rates in the continent, at around 80% (33). How literacy affects the ability to read, understand, and act on healthcare information – holistic concept of health literacy (34) – is a more complex issue. The amount of medical information available to VA respondents is also likely to vary with the circumstances of death. Causes for deaths which occurred at home were reported less reliably than those occurring in hospital, as shown in Table 3.
The comparability of the derived causes of death categories at the individual level between both approaches reflected a broad range of agreements. Not surprisingly, causes such as accidents, homicides, and suicides, associated with minimal needs for understanding underlying biomedical processes, were more reliably reported by VA respondents. In a large proportion of cases, VA respondents simply stated that they did not know any cause of death, in much greater numbers than were assigned by VA to be indeterminate. In many of the cases excluded from the overall dataset, for which no eligible cause of death (including ‘do not know’) was given by the respondent, a major symptom of the final illness was reported instead. This appears to reflect a lack of understanding about the concept of cause of death, as distinct from observed symptoms, reinforcing the importance of standardised VA methods for determining cause of death. Perceptions about connections between symptoms and disease processes that may lead to death can cause inconsistency in recognising causes of death (35). The substantial proportion of deaths attributed to witchcraft in this population also emphasises that non-biomedical models of thinking may be applied in many cases (5).
Perhaps surprisingly, there were relatively lower levels of agreement on respiratory and infectious causes. Studies elsewhere have suggested that pneumonia, particularly in childhood, can achieve good lay recognition, and it is unclear why that might not be the case here (31, 32). The pluralistic behaviour in seeking healthcare in the Agincourt area reflects the need for a systematic measure in assessing individuals’ acts throughout the course of illness, which may ultimately explore peoples’ misunderstandings of disease processes (36, 37).
The HIV/TB cause category was the leading cause of death during the study period, and the epidemic dynamics, together with substantial stigmatisation, certainly contributed to understandings and expressions of cause of death, both for adults and children (38). This was partly reflected in the tendency to attribute HIV/AIDS mortality to witchcraft. Other research in the Agincourt reported death attributed to witchcraft was 70% higher in children than adults (5). HIV/AIDS, and by association tuberculosis and pneumonia, may be considered socially sensitive conditions in which people may favour secrecy. In a random survey of 2,500 residents, in the town of Carletonville, South Africa, participants were offered a free and anonymous HIV test, but not one accepted (39). Another study showed that 92% of the 726 HIV-positive patients had not told anyone about their status (40). Anti-retroviral therapies were not as widely available through the public health services, albeit stigmatisation and the concept that people with AIDS are ‘dead before dying’ contributed to more than 80% of South Africans needing anti-retroviral drugs to still be without treatment by 2006 (41).
South Africa remains in the midst of a profound health transition shaped by changing burdens of communicable, non-communicable, perinatal, maternal, and external causes of death. During the past 20 years of political transition – which coincides with the time span of this dataset – South Africa has seen the rise of non-communicable diseases driven by increases in related risk factors (42). The behavioural and social determinants of non-communicable diseases and their associated risk factors are important (43), and may be a key area for health education campaigns.
VA techniques are generally applied to ascertain cause of death patterns in communities where there is insufficient health infrastructure for all deaths to be routinely and consistently assigned a cause by physicians. Although VA has its limitations, it has been shown on a large scale that standardised VA interviews with automatic processing of causes of death achieve substantially equivalent findings to VAs assessed by physicians (44). While this study into VA respondents’ own perceptions of causes of death is interesting, it is also clear that this source provided a much less complete and consistent picture of cause-specific mortality. For national decision makers and epidemiologists, this study emphasises the importance of applying rigorous and consistent VA methods rather than relying on individual opinions (21, 22). Translating cause-specific mortality data into effective policy making remains an on-going challenge (45).
Acknowledgements
Analyses are based on data collected by the MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt). Support for the Agincourt site comes from the School of Public Health and Faculty of Health Sciences, University of the Witwatersrand, and the Medical Research Council, South Africa, with core funding from the Wellcome Trust, UK (Grants 058893/Z/99/A; 069683/Z/02/Z; 085477/Z/08/Z) and contributions from the National Institute on Aging (NIA) of the NIH, William and Flora Hewlett Foundation, and Andrew W Mellon Foundation, USA. The authors thank the MRC/Wits-Agincourt management team for their support of this project.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Authors' contributions
Conceived and designed the study: LHA, EF, MP, KK, PB. Processed and analyzed the data, and wrote the first draft of the manuscript: LHA. All authors contributed to the writing of the manuscript and approved its final form for submission.
References
- 1.Swami V, Arteche A, Chamorro-Premuzic T, Maakip I, Stanistreet D, Furnham A. Lay perceptions of current and future health, the causes of illness, and the nature of recovery: explaining health and illness in Malaysia. Br J Health Psychol. 2009;14:519–40. doi: 10.1348/135910708X370781. [DOI] [PubMed] [Google Scholar]
- 2.Keikelame MJ, Swartz L. Lost opportunities to improve health literacy: observations in a chronic illness clinic providing care for patients with epilepsy in Cape Town South Africa. Epilepsy Behav. 2013;26:36–41. doi: 10.1016/j.yebeh.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Barney LJ, Griffiths KM, Banfield MA. Explicit and implicit information needs of people with depression: a qualitative investigation of problems reported on an online depression support forum. BMC Psychiatry. 2011;11:88. doi: 10.1186/1471-244X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw A, Ibrahim S, Reid F, Ussher M, Rowlands G. Patients’ perspectives of the doctor–patient relationship and information giving across a range of literacy levels. Patient Educ Couns. 2009;75:114–20. doi: 10.1016/j.pec.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Fottrell E, Tollman S, Byass P, Golooba-Mutebi F, Kahn K. The epidemiology of ‘bewitchment’ as a lay-reported cause of death in rural South Africa. J Epidemiol Community Health. 2011;66:704–9. doi: 10.1136/jech.2010.124305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Ambruoso L, Byass P, Qomariyah SN, Ouédraogo M. A lost cause? Extending verbal autopsy to investigate biomedical and socio-cultural causes of maternal death in Burkina Faso and Indonesia. Soc Sci Med. 2010;71:1728–38. doi: 10.1016/j.socscimed.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Posel D, Kahn K, Walker L. Living with death in a time of AIDS: a rural South African case study. Scand J Public Health. 2007;35(69 Suppl):138–46. doi: 10.1080/14034950701356443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mugure G, Karama M, Kyobutungi C, Karanja S. Correlates for cardiovascular diseases among diabetic/hypertensive patients attending outreach clinics in two Nairobi slums, Kenya. Pan Afr Med J. 2014;19:261. doi: 10.11604/pamj.2014.19.261.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nuwaha F. People's perception of malaria in Mbarara, Uganda. Trop Med Int Health. 2002;7:462–70. doi: 10.1046/j.1365-3156.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- 10.O'Neill S, Gryseels C, Dierickx S, Mwesigwa J, Okebe J, d'Alessandro U, et al. Foul wind, spirits and witchcraft: illness conceptions and health-seeking behaviour for malaria in the Gambia. Malar J. 2015;14:167. doi: 10.1186/s12936-015-0687-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chizimba R, Christofides N, Chirwa T, Singini I, Ozumba C, Sikwese S, et al. The Association between multiple sources of information and risk perceptions of tuberculosis, Ntcheu District, Malawi. PLoS One. 2015;10:e0122998. doi: 10.1371/journal.pone.0122998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sambo M, Lembo T, Cleaveland S, Ferguson HM, Sikana L, Simon C, et al. Knowledge, attitudes and practices (KAP) about rabies prevention and control: a community survey in Tanzania. PLoS Negl Trop Dis. 2014;8:e3310. doi: 10.1371/journal.pntd.0003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baiden F, Bawah A, Biai S, Binka F, Boerma T, Byass P, et al. Setting international standards for verbal autopsy. Bull World Health Organ. 2007;85:570–1. doi: 10.2471/BLT.07.043745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fottrell E, Byass P. Verbal autopsy: methods in transition. Epidemiol Rev. 2010;32:38–55. doi: 10.1093/epirev/mxq003. [DOI] [PubMed] [Google Scholar]
- 15.Bauni E, Ndila C, Mochamah G, Nyutu G, Matata L, Ondieki C, et al. Validating physician-certified verbal autopsy and probabilistic modeling (InterVA) approaches to verbal autopsy interpretation using hospital causes of adult deaths. Popul Health Metr. 2011;9:49. doi: 10.1186/1478-7954-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leitao J, Desai N, Aleksandrowicz L, Byass P, Miasnikof P, Tollman S, et al. Comparison of physician-certified verbal autopsy with computer-coded verbal autopsy for cause of death assignment in hospitalized patients in low-and middle-income countries: systematic review. BMC Med. 2014;12:22. doi: 10.1186/1741-7015-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reniers G, Araya T, Davey G, Nagelkerke N, Berhane Y, Coutinho R, et al. Steep declines in population-level AIDS mortality following the introduction of antiretroviral therapy in Addis Ababa, Ethiopia. AIDS. 2009;23:511. doi: 10.1097/QAD.0b013e32832403d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn K, Collinson MA, Gómez-Olivé FX, Mokoena O, Twine R, Mee P, et al. Profile: Agincourt health and socio-demographic surveillance system. Int J Epidemiol. 2012;41:988–1001. doi: 10.1093/ije/dys115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozano R, Freeman MK, James SL, Campbell B, Lopez AD, Flaxman AD, et al. Performance of InterVA for assigning causes of death to verbal autopsies: multisite validation study using clinical diagnostic gold standards. Popul Health Metr. 2011;9:50. doi: 10.1186/1478-7954-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai N, Aleksandrowicz L, Miasnikof P, Lu Y, Leitao J, Byass P, et al. Performance of four computer-coded verbal autopsy methods for cause of death assignment compared with physician coding on 24,000 deaths in low-and middle-income countries. BMC Med. 2014;12:20. doi: 10.1186/1741-7015-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byass P, Chandramohan D, Clark SJ, D'Ambruoso L, Fottrell E, Graham WJ, et al. Strengthening standardised interpretation of verbal autopsy data: the new InterVA-4 tool. Glob Health Action. 2012;5 doi: 10.3402/gha.v5i0.19281. 19281, doi: http://dx.doi.org/10.3402/gha.v8.19281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leitao J, Chandramohan D, Byass P, Jakob R, Bundhamcharoen K, Choprapawon C, et al. Revising the WHO verbal autopsy instrument to facilitate routine cause-of-death monitoring. Glob Health Action. 2013;6 doi: 10.3402/gha.v6i0.21518. 21518, doi: http://dx.doi.org/10.3402/gha.v8.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byass P, Herbst K, Fottrell E, Ali MM, Odhiambo F, Amek N, et al. Comparing verbal autopsy cause of death findings as determined by physician coding and probabilistic modelling: a public health analysis of 54 000 deaths in Africa and Asia. J Glob Health. 2015;5:010402. doi: 10.7189/jogh.05.010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabudula CW, Tollman S, Mee P, Ngobeni S, Silaule B, Gómez-Olivé FX, et al. Two decades of mortality change in rural northeast South Africa. Glob Health Action. 2014;7 doi: 10.3402/gha.v7.25596. 25596, doi: http://dx.doi.org/10.3402/gha.v8.25596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence I, Lin K. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–68. [PubMed] [Google Scholar]
- 26.Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005;85:257–68. [PubMed] [Google Scholar]
- 27.Byass P. Usefulness of the Population Health Metrics Research Consortium gold standard verbal autopsy data for general verbal autopsy methods. BMC Med. 2014;12:23. doi: 10.1186/1741-7015-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tadesse S, Tadesse T. Evaluating the performance of interpreting verbal autopsy 3.2 model for establishing pulmonary tuberculosis as a cause of death in Ethiopia: a population-based cross-sectional study. BMC Public Health. 2012;12:1039. doi: 10.1186/1471-2458-12-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ndila C, Bauni E, Nyirongo V, Mochamah G, Makazi A, Kosgei P, et al. Verbal autopsy as a tool for identifying children dying of sickle cell disease: a validation study conducted in Kilifi district, Kenya. BMC Med. 2014;12:65. doi: 10.1186/1741-7015-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cramm JM, Finkenflügel HJM, Møller V, Nieboer AP. TB treatment initiation and adherence in a South African community influenced more by perceptions than by knowledge of tuberculosis. BMC Public Health. 2010;10:72. doi: 10.1186/1471-2458-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangesho P, Shayo E, Makunde W, Keto G, Mandara C, Kamugisha M, et al. Community knowledge, attitudes and practices towards tuberculosis and its treatment in Mpwapwa District, central Tanzania. Tanzan Health Res Bull. 2007;9:38–43. [PubMed] [Google Scholar]
- 32.Montgomery MR. Perceiving mortality decline. Popul Dev Rev. 2000;26:795–819. [Google Scholar]
- 33.Kickbusch IS. Health literacy: addressing the health and education divide. Health Promot Int. 2001;16:289–97. doi: 10.1093/heapro/16.3.289. [DOI] [PubMed] [Google Scholar]
- 34.Center for Health Care Strategies. What is health literacy? Fact Sheet #1. Hamilton, NJ: CHCS; 2013. [Google Scholar]
- 35.Komaroff AL. Symptoms: in the head or in the brain? Ann Intern Med. 2001;134(9 Pt1):783–5. doi: 10.7326/0003-4819-134-9_part_1-200105010-00016. [DOI] [PubMed] [Google Scholar]
- 36.Pronyk P, Makhubele M, Hargreaves J, Tollman S, Hausler H. Assessing health seeking behaviour among tuberculosis patients in rural South Africa. Int J Tuberc Lung Dis. 2001;5:619–27. [PubMed] [Google Scholar]
- 37.Peltzer K, Henda N. Traditional birth attendants HIV/AIDS and safe delivery in the Eastern Cape, South Africa–evaluation of a treating programme. S Afr J Obstet Gynaecol. 2006;12:140–5. [Google Scholar]
- 38.Gray L, Newell M, Thorne C, Peckham C, Levy J. Fluctuations in symptoms in human immunodeficiency virus-infected children: the first 10 years of life. Pediatrics. 2001;108:116–22. doi: 10.1542/peds.108.1.116. [DOI] [PubMed] [Google Scholar]
- 39.Ashforth A. An epidemic of witchcraft? The implications of AIDS for the post-apartheid state. Afr Stud. 2002;61:121–43. [Google Scholar]
- 40.Pawinski R, Lalloo U, Jinabhai C, Bobat R. Community-based approach to HIV treatment. Lancet. 2002;359:624. doi: 10.1016/S0140-6736(02)07723-1. [DOI] [PubMed] [Google Scholar]
- 41.Niehaus I. Death before dying: understanding AIDS stigma in the South African Lowveld. J South Afr Stud. 2007;33:845–60. [Google Scholar]
- 42.Mayosi BM, Flisher AJ, Lalloo UG, Sitas F, Tollman SM, Bradshaw D. The burden of non-communicable diseases in South Africa. Lancet. 2009;374:934–47. doi: 10.1016/S0140-6736(09)61087-4. [DOI] [PubMed] [Google Scholar]
- 43.Dalal S, Beunza JJ, Volmink J, Adebamowo C, Bajunirwe F, Njelekela M, et al. Non-communicable diseases in sub-Saharan Africa: what we know now. Int J Epidemiol. 2011;40:885–901. doi: 10.1093/ije/dyr050. [DOI] [PubMed] [Google Scholar]
- 44.Oti SO, Kyobutungi C. Verbal autopsy interpretation: a comparative analysis of the InterVA model versus physician review in determining causes of death in the Nairobi DSS. Popul Health Metr. 2010;8:21. doi: 10.1186/1478-7954-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byass P, Kahn K, Fottrell E, Collinson MA, Tollman SM. Moving from data on deaths to public health policy in Agincourt, South Africa: approaches to analysing and understanding verbal autopsy findings. PLoS Med. 2010;7:e1000325. doi: 10.1371/journal.pmed.1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]