Highlight
Metabolite profiling of heat-acclimated seedlings revealed accumulation of raffinose and polyunsaturated triacylglycerols. Dynamic heat-induced triacylglycerol accumulation was independent of heat shock factors and appears to be involved in lipid remodelling.
Abstract
Heat acclimation enables plants to tolerate and survive short-term heat stress on hot days. In Arabidopsis thaliana, a genetically programmed heat shock response can be rapidly triggered in the temperature range of 32–38°C through activation of heat shock transcription factors (HSF). The heat shock response leads to heat acclimation and confers short-term protection against temperatures above 40°C. However, little is known about metabolic adjustments during heat acclimation.
Untargeted metabolite analyses of A. thaliana seedlings revealed that levels of polyunsaturated triacylglycerols (TG) rapidly and dramatically increase during heat acclimation. TG accumulation was found to be temperature dependent in a temperature range of 32–50°C (optimum at 42°C) and reversible after a return from 37°C to normal growth temperatures. Heat-induced TGs accumulated in extra-chloroplastic compartments and increased in both roots and shoots to a similar extent. Analysis of mutants deficient in all four HSFA1 master regulator genes or the HSFA2 gene revealed that TG accumulation was not dependent on HSFs. Moreover, the TG response was not limited to heat stress because drought and salt stress also triggered an accumulation of TGs, but not short-term osmotic, cold, and high light stress. Lipid analysis revealed that heat-induced accumulation of TGs was not due to massive de novo fatty acid synthesis. It is hypothesized that TGs serve as transient stores for fatty acids that may be required for membrane remodelling during heat acclimation.
Introduction
Plants are exposed to daily and seasonal fluctuations of ambient temperatures. Growth at suboptimal temperatures requires short- and long-term adaptations of physiology and metabolism. During the day, temperatures are typically lowest in the morning and reach a maximum in the afternoon. Plants and other organisms have the inherent capacity to temporarily survive temperatures above the optimal growth temperature without prior acclimation (basal thermotolerance) and an ability to acquire tolerance to otherwise lethal heat temperatures (acquired thermotolerance) (Larkindale et al., 2005). Basal thermotolerance may strongly vary depending on the plant species. For instance, the optimal growth temperature of Arabidopsis thaliana is 22°C but seedlings have been reported to tolerate higher temperatures for different time periods: 30°C up to 5 days (Chen et al., 2006), 38°C for 16h (Clarke et al., 2009) and 45°C for up to 45min (Yeh et al., 2012). Short-term acquired thermotolerance (SAT) requires acclimation of Arabidopsis seedlings at moderately elevated temperatures of 32–38°C for 1h (Liu and Charng, 2012). During heat acclimation, a genetically programmed heat shock response (HSR) is triggered. This is characterized by rapid activation of heat shock transcription factors (HSF), which trigger a massive accumulation of a battery of heat shock proteins (HSP), chiefly involved in protein folding and protection. In addition, enzymes involved in redox regulation such as ascorbate peroxidase 2 (APX2) and metabolic enzymes such as galactinol synthase 1 (GOLS1) are strongly induced (Liu et al., 2011). After acclimation at 38°C for 1.5h, seedlings survived exposure to 45°C for 2h, a condition that is lethal for non-acclimated plants (Hong and Vierling, 2000; Larkindale and Vierling, 2008). In nature, temperatures rise gradually during the day and, typically, there is sufficient time to acclimate in the temperature range 31–38°C before potentially lethal temperatures above 40°C are reached in the afternoon. Usually, high temperature stress lasts only a few hours before temperatures decrease during the night. SAT is a perfectly evolved mechanism that helps plants to cope with this situation. HSR genes are rapidly and transiently induced at moderately elevated temperatures and, hence, SAT is fully reversible and lasts for up to 72h (Charng et al., 2006).
Plant HSFs comprise three evolutionary conserved classes: A, B, and C. The HSF class A possesses short peptide motifs enriched with aromatic and large hydrophobic amino acid residues embedded in acidic surrounding, which are essential for their activation (Kotak et al., 2004). In A. thaliana, four HSFA genes belong to the HSFA1 subclass (HSFA1a, HSFA1b, HSFA1d, and HSFA1e). HSFA1 are constitutively present and can be activated rapidly at moderately elevated temperatures. They have been termed the master regulators of the HSR because hsfa1abd triple and hsfa1abde quadruple mutants are unable to induce HSR or, therefore, acquire thermotolerance (Liu et al., 2011; Yoshida et al., 2011). Heat activation of HSFA1 triggers the transcription of inducible HSFs. Among them, HSFA2 is required for the extension of short-term heat acclimation (Charng et al., 2007) and mediates amplification of a subset of heat-responsive genes such as HSPs (including HSP101, HSP26.5, HSP22-ER, HSP18.1, HSP70b), APX2, and GOLS1 as well as some drought- and cold-regulated genes (Schramm et al., 2006).
Although regulation of metabolic enzymes after activation of HSR has been reported in several studies, limited information on heat-responsive metabolites could be found in the literature. After up-regulation of GOLS1 at 37°C, raffinose and galactinol were found to accumulate rapidly in Arabidopsis leaves (Panikulangara et al., 2004). As far as could be determined, only one large metabolite profiling analysis has been performed to study heat-induced changes of metabolic pathways in Arabidopsis. Studying basal thermotolerance at 40°C for up to 4h, Kaplan et al. (2004) identified 80 metabolite features that increased significantly (P < 0.05) after heat treatment for 2h. Only four known metabolites (galactinol, raffinose, pipecolic acid, and digalactosylglycerol) and four non-identified compounds showed a strong accumulation (fold change >4). However, short-term exposure to 40°C for only 1h can be lethal for A. thaliana seedlings (Larkindale and Knight, 2002) and, hence, the observed metabolic changes may reflect severe disturbance of cellular homoeostasis.
For identification of metabolites specifically regulated upon short-term moderate heat, the metabolome of 2-week-old A. thaliana seedlings grown at the optimal growth temperature (22°C) was compared with seedlings that were shifted to the optimal acclimation temperature (37°C) for 2h. To investigate the heat response on the metabolome level, untargeted metabolite analyses were performed using ultra-performance liquid chromatography (UPLC) coupled with a quadrupole time-of-flight mass spectrometer (qTOF-MS).
Material and methods
Plant material and growth conditions
A. thaliana wild-type ecotypes ‘Columbia-0’ and ‘Wassilewskija’ were grown in a growth chamber under an 8h/16h short-day cycle at 22°C (160 µE) for 2 weeks. Plants were grown on agar plates with Murashige & Skoog (MS) medium basal mixture including MES buffer (pH 5.7; Duchefa Biochemie BV, Haarlem, Netherlands), containing 3% sugar and 1.2% agarose. The mutant line gols1 was kindly provided by Schoeffel (Panikulangara et al., 2004); the hsfa1abde quadruple mutant was kindly provided by Ohama and Yamaguchi-Shinozaki (Yoshida et al., 2011). Additionally, hsfa2 (SALK_008978) was used.
Heat treatment and abiotic stress treatments
For heat treatment, plants grown on agar plates (100 seeds per plate) were transferred 2h after the onset of light to a growth chamber set at 37°C for the time indicated. All seedlings grown on a plate were harvested, immediately shock frozen with liquid nitrogen, and stored at −80°C until extraction.
To determine the temperature dependency of the sugar and triacylglycerol (TG) markers, seedlings were transferred to liquid Murashige & Skoog media 1 day before the treatments. Eight seedlings were pooled and heated at different temperatures (22–50°C) in a heating block for 2h. For cold treatment, plates were transferred to a refrigerator set to 4°C for 2h. High light treatment was carried out by irradiating the seedlings with high quantum flux density (650 µE m−2 s−1) for 2h. For dehydration treatments, plants were gently removed from the plates and allowed to dry on paper for 2h. For osmotic and salt stress, seedlings were transferred to liquid Murashige & Skoog media 1 day before the experiment. Seedlings (eight seedlings were pooled) were treated with water, 125mM NaCl, or 250mM mannitol for 2h prior to harvesting.
Chloroplast isolation
Chloroplasts in 3-week-old A. thaliana seedlings, grown on Murashige & Skoog agar containing 1% sucrose, were isolated according to Aronsson and Jarvis (2002). All manipulations were done at 4°C or on ice. Plants were transferred into 15mL of isolation buffer [20mM Hepes KOH, pH 8.0, 5mM ethylenediaminetetraacetic acid (EDTA), 5mM MgCl2 × H2O, 10mM NaHCO3, and 0.3M sorbitol], ground, and filtered three times in succession. The lysate was centrifuged at 950g for 4min and the pellet was gently resuspended in 2mL of isolation buffer. The sample was then layered on top of a discontinuous Percoll gradient (75% Percoll in gradient mix, 28% Percoll in gradient mix with 14% water; gradient mix: 25mM Hepes KOH, 10mM EDTA). After centrifugation at 1300g for 6min the green band that appeared at the interface of the Percoll layers was collected. The chloroplast-rich fraction was diluted with 10mL wash buffer (50mM HEPES KOH, pH 8.0, 3mM MgSO4, and 0.3M sorbitol) and centrifuged at 950g for 4min to remove the Percoll. Chloroplasts were resuspended in 200 µL wash buffer and a modified Bligh and Dyer extraction was carried out.
Metabolite analysis
All solvents were at least HPLC grade and were purchased from Biosolve (Valkenswaard, Netherlands). Seedlings (100mg, pooled from one plate) were shock-frozen in liquid nitrogen and extracted two times with 600 µl of chloroform/methanol/water (3:2:1, v/v) using a ball mill at 21 Hz for 10min (Retsch, Haan, Germany). As internal standards (IS), 0.2 µg of α, α-[1,1’-D2] trehalose (Sigma Aldrich, Taufkirchen, Germany) and 0.24 µg of tridecanoyl-triacylglycerol (TG30:0; Larodan, Solna, Sweden) were added into a sample. The aqueous phase was used for the analysis of hydrophilic metabolites and the organic phase was used for lipid analysis. The organic phase was evaporated in a vacuum concentrator at 40°C. The residue was resuspended in 100 µL isopropanol for analysis.
For untargeted metabolite analysis, the organic and aqueous phases were analysed with an ACQUITY UPLC system coupled to a Synapt G2 HDMS qTOF-MS (all Waters, Eschborn, Germany). Chromatographic separation of the organic phase was carried out on a BEH C18 column (2.1×100mm, 1.7 μm; Waters) with a linear binary solvent gradient of 30−100% eluent B over 10min at a flow rate of 0.3mL min−1. Eluent A consisted of 10mM ammonium acetate in water/acetonitrile (60:40, v/v) and eluent B consisted of 10mM ammonium acetate in isopropanol/acetonitrile (90:10, v/v). Chromatographic separation of the aqueous phase was performed on a BEH amide column (1.7 µm, 2.1×100mm; Waters) according to the Waters application note WA60126 with modifications. Briefly, elution was performed using a linear solvent-strength gradient (0.2mL min−1 at 35°C) from 75% to 45% acetonitrile containing 1% ammonium hydroxide in 10min.
After chromatographic separation, hydrophilic and lipophilic metabolites were detected by MS coupled with an electrospray ionisation (ESI) source operated in positive and negative modes. The ESI capillary voltage was set to 0.8kV and nitrogen (at 350°C, flow rate of 800L h−1) was used as desolvation gas. The quadrupole was operated in a wide-band RF mode, and data was acquired over the mass range of 50−1200Da. Two discrete and independent interleaved acquisition functions were automatically created. The first function collected the low energy data where molecule ions were acquired while the second function collected the fragments of the molecule ion (high energy data) by using a collision energy ramp from 15 to 35eV (MSE). MassLynx, MarkerLynx, and QuanLynx (version 4.1; all Waters) were used to acquire and process chromatograms.
Response factors for TG54:9, TG54:6, TG54:3, TG54:0, TG51:0, and TG48:0 (Larodan) were determined using TG30:0 as IS. Because the response factors were in the range of 0.8–1.1, a response factor of 1 was used for semi-quantitative analysis of TGs. The inter-day repeatability of peak areas were <6% at a concentration of 100ng/sample (n = 3) and the determined linear range spanned two orders of magnitude (regression coefficient >0.995 between 10 and 1000ng/sample) using standard solutions of TG54:9, TG54:6, TG54:3, TG54:0, TG51:0, and TG48:0.
For the targeted analysis of the defined lipid species (Supplementary Table S1), glycerophosphoethanolamine (PE) 34:0, glycerophosphocholin (PC) 34:0, monogalactosyldiacylglycerol (MGDG) 36:0, digalactosyldiacylglycerol (DGDG) 36:0, and diacylglycerol (DG) 34:0 (2.4 µg/sample) were used as IS for each lipid class. For semi-quantitative analysis, peak areas of the analytes and ISs were determined in the extracted total ion chromatogram and lipid concentrations were calculated by using a response factor of 1 for each analyte/IS pair.
For the determination of raffinose and galactinol in the aqueous phases, multiple reaction monitoring (MRM) was applied using a Waters Micromass Quattro Premier triple quadrupole MS coupled to UPLC (Waters). The operational parameter of chromatographic separation was identical to the ones used for the untargeted metabolite analysis. The sugars were ionized in an ESI source operated in the negative mode. The collision-induced dissociation of each compound for MRM was performed using argon as the collision gas with a flow rate of 0.3mL min−1 and a pressure of 3.0×10−3 mbar. The following MRM transitions were monitored: m/z 503→179 at a retention time (RT) of 5.33min for raffinose, m/z 341→179 at RT of 5.79min for galactinol, and m/z 343→180 at RT of 4.51min for D2-trehalose (IS).
Chlorophyll content was measured according to Aronsson and Jarvis (2002). Briefly, the lipophilic extract was used and diluted 1 to 10 with isopropanol and measured at absorption wavelengths of 647nm and 664nm with NanoDrop1000 (Thermo Fisher Scientific, Dreieich, Germany).
For total fatty acid analysis, shock-frozen seedlings (100mg) were extracted with 500 µL of isopropanol containing 10% potassium hydroxide and 5 µg heptadecanoic acid (IS) in a ball mill (21 Hz, 10min). The supernatant was incubated at 60°C for 1h. After centrifugation, the pH of the supernatant was adjusted to 6 and analysed by UPLC–qTOF-MS (Waters) in the negative ESI mode. Analytes were eluted on a BEH C18 column (1.7 µm, 2.1×100mm; Waters) at 40°C using an eluent from 70% to 100% acetonitrile (acidified with 0.1% formic acid) within 10min at a flow rate of 0.3mL min−1. The fatty acid RTs, mass-to-charge ratios (m/z), and response factors are included in Supplementary Table S1.
Results
Untargeted metabolite analysis revealed accumulation of raffinose, galactinol, and polyunsaturated triacylglycerols in heat-acclimated seedlings
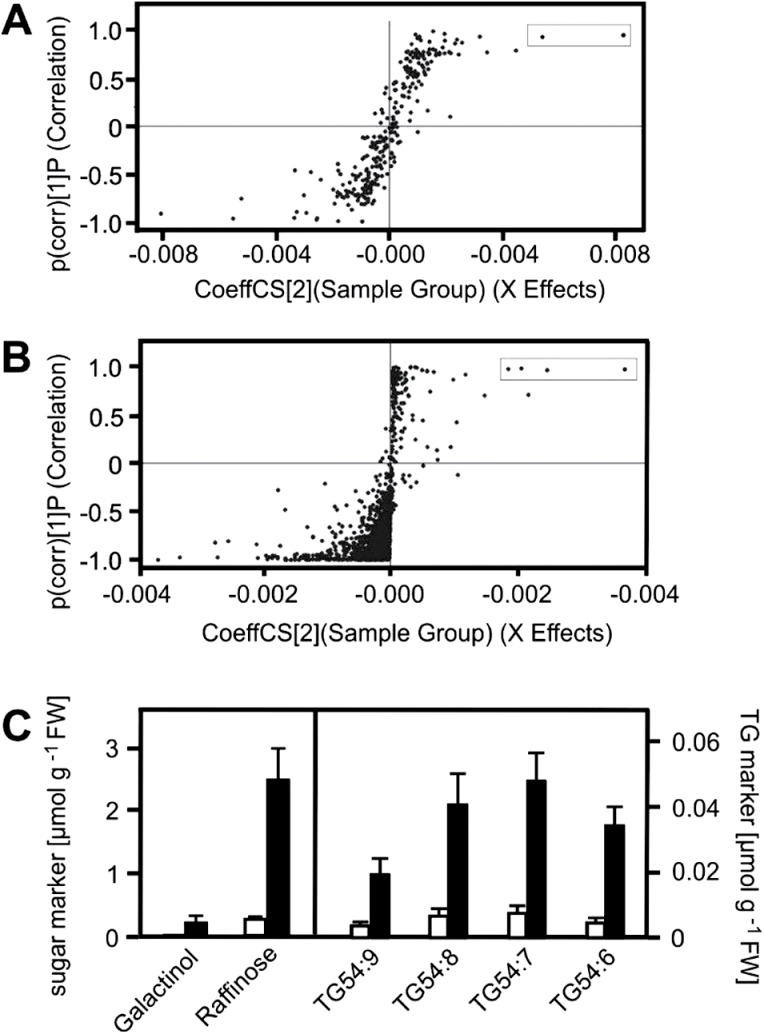
In order to identify metabolic pathways responding to elevated temperature, global metabolite profiles of heat-acclimated and control seedlings were compared. Two-week-old A. thaliana seedlings grown on agar plates were shifted to 37oC for 2h (Supplementary Fig. S1). Metabolites of heat-treated and untreated seedlings were isolated with liquid-liquid extraction to recover hydrophilic and lipophilic metabolites simultaneously. Hydrophilic metabolites recovered in the aqueous phase were analysed by hydrophilic interaction liquid chromatography coupled to MS and lipophilic metabolites in the organic phase were separated by reversed phase liquid chromatography coupled to MS. In total, 248 and 987 metabolite features were detected in the aqueous and organic phases, respectively. Discrimination in the metabolome of treated and control seedlings was observed using principal component analysis (Supplementary Fig. S2). Orthogonal partial least square discriminant analysis revealed significant accumulation of two hydrophilic and four lipophilic metabolites (termed as markers) in the acclimated seedlings (Fig. 1).
Fig. 1.
Identification of heat-responsive hydrophilic and lipophilic metabolites using orthogonal partial least square discriminant analysis (OPLS-DA). Two-week-old control (22°C) and heat-acclimated (37°C, 2h) seedlings grown on agar plates were extracted with liquid-liquid extraction. OPLS-DA was performed on the detected hydrophilic (A) and lipophilic (B) metabolite features. Significantly heat-induced metabolites are presented in boxes and were identified as raffinose, galactinol, and polyunsaturated TG species (C). Levels of the sugar and TG markers in control (white columns) and heat-acclimated (black columns) seedlings are shown (C). Data represent means ± SD, n = 9.
The structures of the six identified markers were identified by comparing their accurate masses and fragmentation patterns with metabolome databases (Supplementary Table S2). The Metabolite and Tandem MS Database (METLIN) listed 45 and 34 hits for the two hydrophilic metabolites displaying m/z of 503.161 and 341.108, respectively. After determining the elemental compositions of the two heat-responsive hydrophilic metabolites by using isotope abundance distribution, all hits were identified as oligosaccharide derivatives. Literature on plant heat-stress responses suggested raffinose and galactinol as possible metabolites, which was confirmed by comparing the RTs and fragmentation patterns of the markers to authentic reference materials.
The four lipophilic markers with m/z of 890.7238, 892.7394, 894.7551, and 896.7707 were identified as ammonium adducts of TG using METLIN and the Database of Lipid Metabolites and Pathways Strategy (LIPID MAPS). In addition, collision-induced fragmentation of the lipophilic markers revealed a loss of fatty acyls, resulting in abundant formation of diacylglycerol (DG) ions and protonated fatty acid fragments (Supplementary Table S2) that are characteristic for TGs (Murphy et al., 2007). The four heat-responsive TGs were identified (Supplementary Table S2) as trioctadecatrienoyl-glycerol (TG54:9), dioctadecatrienoyl-octadecadienoyl-glycerol (TG54:8), dioctadecadienoyl-octadecatrienoyl-glycerol (TG54:7), and octadecenoyl-octadecadienoyl-octadecatrienoyl-glycerol (TG54:6). The structure elucidation was confirmed using commercially available TG54:9 and TG54:6 as reference compounds.
Accumulation of predominantly polyunsaturated TG species after heat acclimation
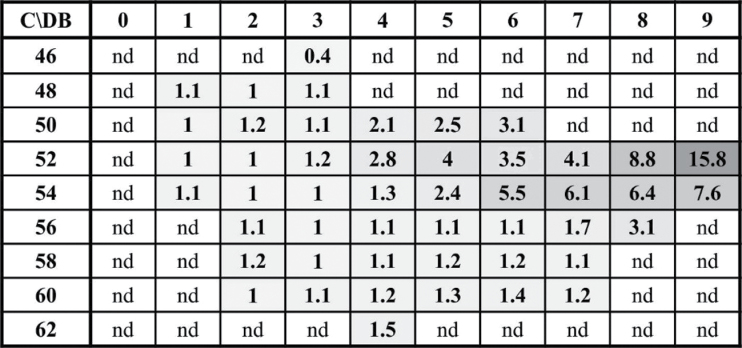
To investigate if TG species other than the TG markers accumulated during heat acclimation (37°C, 2h), endogenous TGs were identified and quantified using an in-house developed database (unpublished). RT-aligned molecule ion and fragment spectra led to the identification of 48 TGs specified by the number of carbons and double bonds of the acyl chains. Determination of (Supplementary Table S3) all RT-aligned fragments resulting from neutral losses of fatty acyls in the pre-processed data were obligatory. After short validation of the analysis method, levels of the 48 TGs were determined in heat-acclimated and control seedlings (Supplementary Table S3). Out of the 48 TG species, 17 increased by 1.5- to 16-fold at 37°C (Fig. 2). Out of the most abundant TG species (levels higher than 2.5 mol%), the four most strongly induced species were the TG markers (TG54:9, TG54:8, TG54:7, and TG54:6). By contrast, no significant accumulation was observed for TG species comprising saturated and/or very long chain fatty acyls. Thus, to characterize the TG response to heat acclimation, we determined total levels of TG markers in the following experiments.
Fig. 2.
Fold increase of identified TGs after heat acclimation. TGs were plotted according to the number of carbons (rows) and double bonds (columns) of the fatty acids esterified to glycerol. Fold changes after heat acclimation (37°C, 2h) are shown. C = number of carbon atoms in the fatty acyls, DB = number of double bonds in the fatty acyls, nd = non-detected. Data represent means ± SD, n=9.
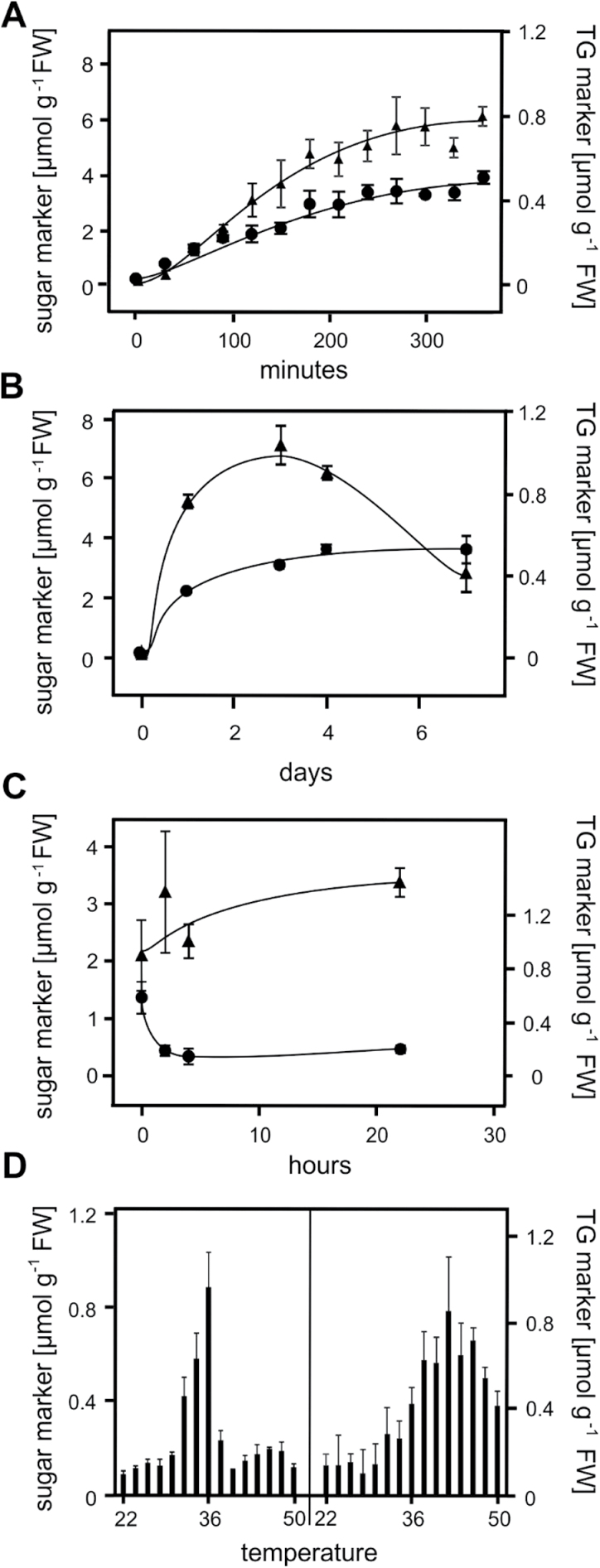
Time- and temperature-dependent accumulation of sugar and TG markers in response to elevated temperature
To study the time dependency of the marker responses, sugar and TG markers were determined in 2-week-old Arabidopsis seedlings after treatment at 37°C for different exposure times (Fig. 3A). Already after 30min, a 6-fold increase in the levels of the sugar and TG markers was determined. Levels of the markers steadily increased up to 6h. Upon long-term exposure to 37°C (Fig. 3B), levels of TG markers remained high for at least 7 days while levels of the sugar markers declined after 4 days. When heat-acclimated seedlings (37°C, 2h) were returned to 22°C (Fig. 3C), levels of sugar markers remained constant for at least 24h while levels of TG markers dropped to nearly basal levels after 2h.
Fig. 3.
Time- and temperature-dependent responses of sugar (▲) and TG (●) markers in Arabidopsis seedlings. Seedlings exposed to 37°C for the times indicated were analysed (A and B). Heat-acclimated seedlings (37°C, 2h) were transferred back to the growth condition (22°C) for the times indicated (C). Sugar and TG markers were determined in seedlings treated at different temperatures (22°C to 50°C) for 2h (D). Data represent means ± SD, n = 4.
To investigate if the responses of the markers were dependent on the temperature, seedlings in liquid media were treated at different temperatures ranging from 22°C to 50°C for 2h (Fig. 3D). All markers accumulated at a temperature above 30°C. The highest accumulation was determined at 36°C for the sugar markers (0.8 µmol g−1 fresh weight) and 42°C for the TG markers (0.85 µmol g−1 fresh weight). Interestingly, the sugar markers did not accumulate at temperatures above 38°C. By contrast, an increase of the TG markers was observed even at 50°C.
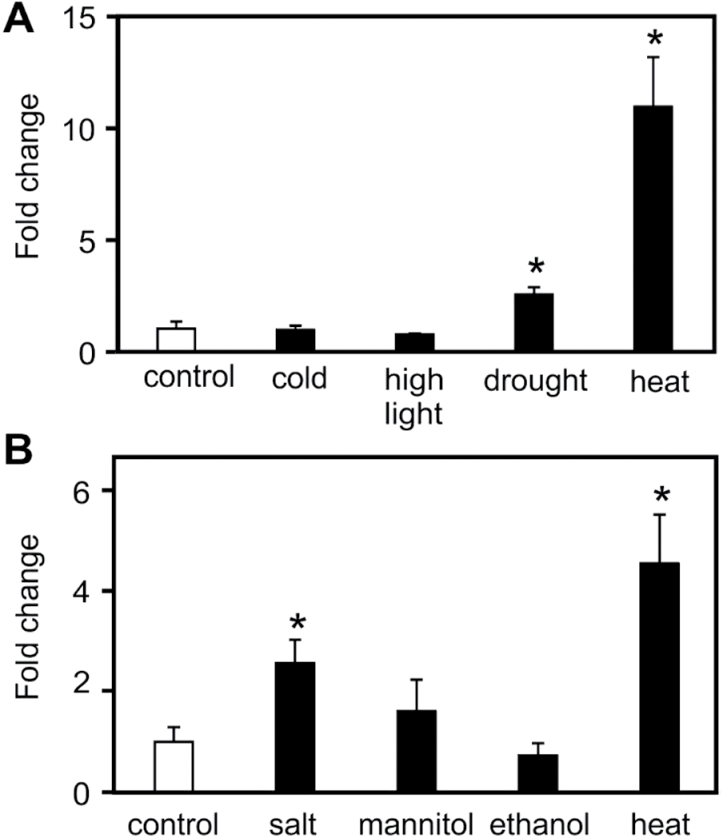
Significant accumulation of TGs after short-term heat, drought, and salt stress but not after short-term cold, osmotic, or high light stress
Raffinose has been reported to increase after several abiotic stresses such as heat, cold, drought, salt, and oxidative stress (Taji et al., 2002; Nishizawa et al., 2008). Therefore, accumulation of TG markers was examined to determine if it is a general stress response or specifically up-regulated by heat. Two-week-old seedlings grown on agar plates were subjected to low temperature (4°C) as well as elevated light intensity (900 µE m−2 s−1) and drought (seedlings were placed on filter paper) for 2h (Fig. 4A). In another series of experiments, seedlings were transferred to liquid Murashige & Skoog media and exposed to salt stress (125mM NaCl), osmotic stress (250mM mannitol), and 1% ethanol for 2h (Fig. 4B). As shown in Fig. 4, heat appeared to be the strongest inducer of TG markers under these test conditions. However, levels of TG markers were also significantly increased after salt and drought stress (2.6- and 2.5-fold, respectively). By contrast, no significant induction of TG markers was determined after osmotic, cold, high light, or ethanol treatment.
Fig. 4.
Accumulation of TG markers after different abiotic stresses. Two-week-old seedlings grown on agar plates were exposed to cold (4°C, 2h), high light (900 µE m−2 s−1, 2h), drought (seedlings placed on filter paper), or heat (37°C, 2h) stress (A). Seedlings grown in liquid Murashige & Skoog medium were treated with salt (125mM NaCl, 2h), mannitol (250mM, 2h), ethanol (1%, 2h), or heat (37°C, 2h) stress (B). Data represent means ± SD, n = 4.
Heat-induced TG accumulation is independent of HSFs and raffinose biosynthesis
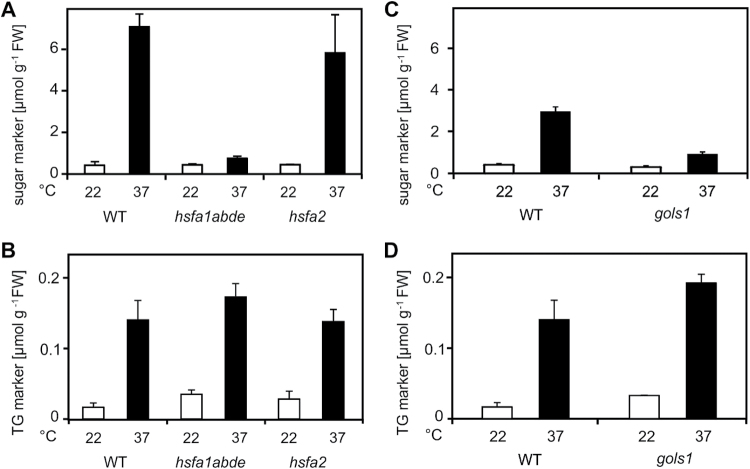
In order to determine whether the accumulation of sugar and TG markers after heat acclimation is induced in an HSF-dependent fashion, mutant Arabidopsis plants lacking HSFA1 or HSFA2 were examined. No significant accumulation of raffinose was determined in the hsfa1abde quadruple mutant, while raffinose levels increased to wild-type levels in the hsfa2 mutant when seedlings were treated at 37°C for 2h (Fig. 5A). By contrast, accumulation of TG markers was not dependent on HSFA1 and HSFA2 transcription factors (Fig. 5B). To investigate if the accumulation of TG markers is dependent on raffinose biosynthesis, the gols1 mutant deficient in heat-induced raffinose synthesis was characterized (Fig. 5C). Levels of the TG markers in heat-acclimated gols1 were similar to levels in the heat-acclimated wild type (Fig. 5D). These results indicate that TG markers accumulate independently of HSFs and of raffinose biosynthesis.
Fig. 5.
Accumulation of TG markers in hsfa1abde, hsfa2, and gols1 mutant Arabidopsis seedlings after heat acclimation. Levels of sugar and TG markers were determined in 2-week-old hsfa1abde, hsfa2, and gols1 seedlings kept at 22°C (white bar) and 37°C for 2h (black bar). Data represent means ± SD, n = 4.
TG markers accumulate predominantly in extra-chloroplastic compartments
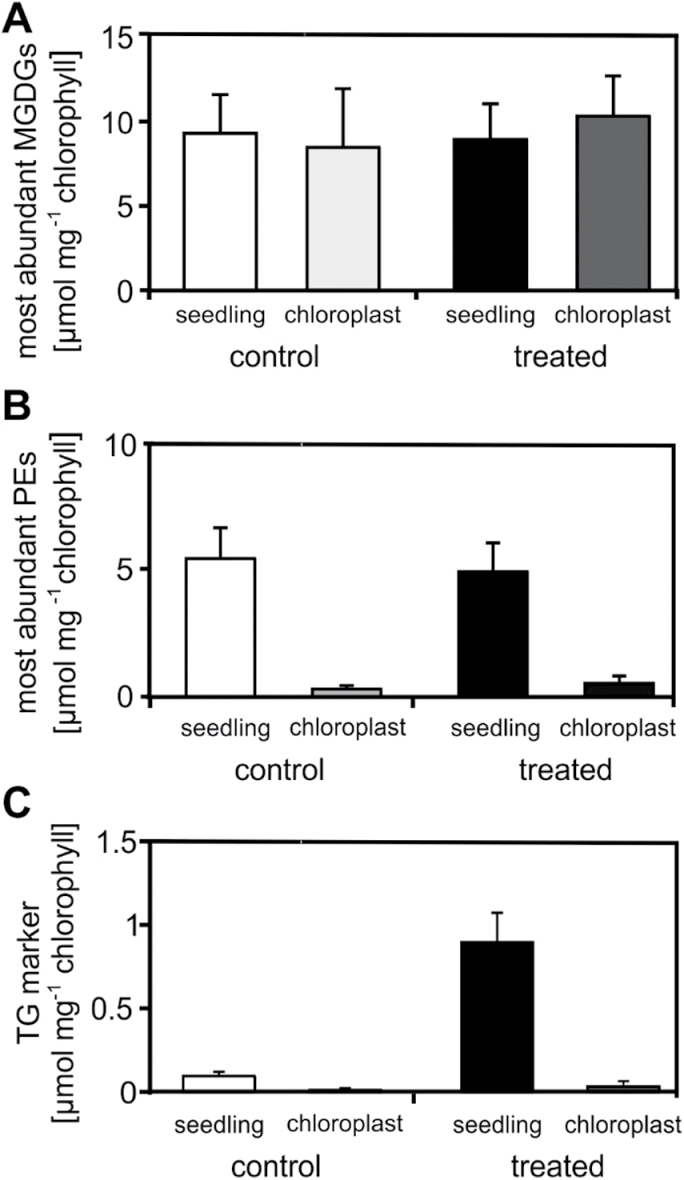
In Arabidopsis leaves, TGs may either accumulate in the cytosol or chloroplasts. To localize the subcellular accumulation of TGs after heat acclimation, TG markers were determined in isolated chloroplasts and whole seedlings. Levels of TG markers as well as compartment-specific lipids were determined and normalized to chlorophyll content to compare the two different preparations. The reliability of the chloroplast isolation was confirmed by analysing the most abundant plastid-specific monogalactosyldiacylglycerols (MGDGs) with the acyl combinations 18:3–18:3 and 18:3–16:3. As expected, no significant differences in the levels of the two most abundant MGDG species between seedlings and chloroplast fractions were determined (Fig. 6A). To further assess the purity of the preparations, the most abundant PEs (PE36:5, PE36:4, PE34:3, PE34:2), which do not occur in plastids, were analysed. The contamination of PEs in the chloroplast fractions was <5% (Fig. 6B). TG markers accumulated 3.5-fold in the chloroplasts and 9-fold in the extra-chloroplastic compartments (Fig. 6C). TG markers were determined at a concentration of 32 nmol mg−1 chlorophyll in chloroplasts and 900 nmol mg−1 chlorophyll in seedlings after heat treatment (Supplementry Table S4). Considering the contamination of the chloroplast fraction with extra-plastidic components (5%), results suggest that the vast majority of TG markers accumulated in the cytosol. In addition, TG markers were analysed separately in shoots and roots. Interestingly, there was a similar increase in levels (8- to 10-fold) and similar absolute levels in both tissues after heat acclimation (Fig. 7).
Fig. 6.
TG markers accumulated predominantly in extra-chloroplastic compartments after heat acclimation. Chloroplasts of control (white columns) and heat-acclimated (37°C, 2h; black columns) seedlings were isolated and most abundant MGDGs (A), PEs (B), and TG markers (C) were determined relative to chlorophyll levels. The determined MGDGs and PEs were MGDG36:6, MGDG34:4, PE36:5, PE36:4, PE34:3, and PE34:2. Data represent means ± SD, n = 3.
Fig. 7.
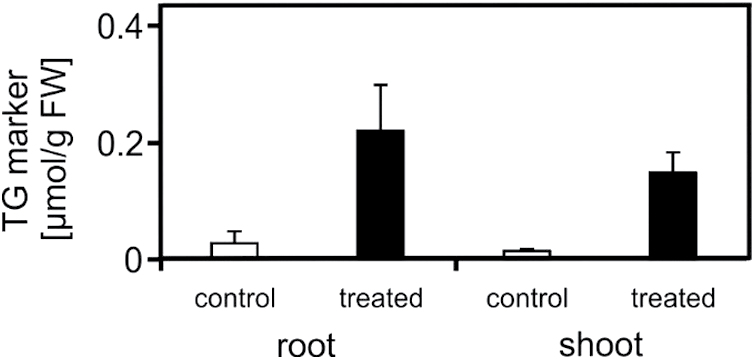
Levels of TG markers in roots and shoots. Roots and shoots of control (22°C, white columns) and heat-acclimated (37°C, 2h, black columns) seedlings were harvested; shoots were separated from roots and TG markers were analysed. Data represent means ± SD, n = 4.
Total membrane lipid composition remained unaltered after heat acclimation
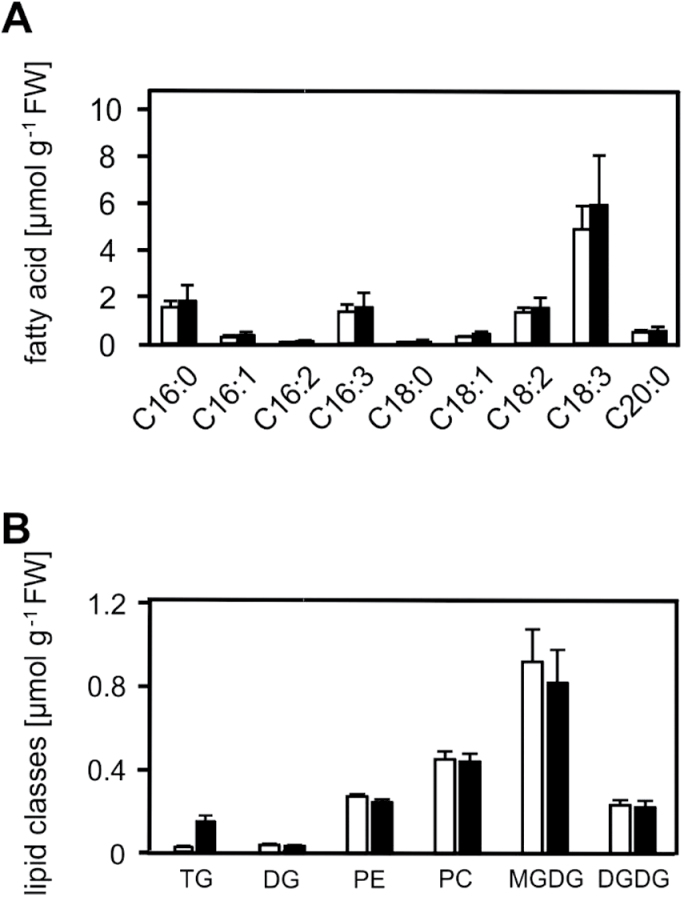
Rapid heat-induced TG accumulation could be either due to increased TG biosynthesis or reduced TG breakdown. With respect to TG biosynthesis, TGs can be synthesized by de novo synthesis of fatty acids and incorporation into TGs through the Kennedy pathway, or by channelling fatty acids from structural lipids to TGs (membrane remodelling). To this end, total fatty acids in heat-acclimated (37°C, 2h) and control seedlings (22°C) were determined. Levels of total fatty acids (esterified and free fatty acids) in heat-acclimated and control seedlings were comparable (Fig. 8A). To test whether fatty acids required for TG synthesis are derived from membrane lipid breakdown, levels of membrane lipids including MGDGs, DGDG, PEs, and PC as well as DG and TGs were determined (Fig. 8B and Supplementary Table S5). Except for TG markers, however, no significant change in the lipid composition was observed after heat acclimation.
Fig. 8.
Levels of total fatty acids, neutral lipids, and membrane lipids after heat acclimation. Total fatty acids (A), TG markers and complex lipids (B) from control (22°C; white columns) and heat-acclimated (37°C, 2h; black columns) seedlings. Data represent means ± SD, n = 4 for the determination of total fatty acids, and n = 9 for the determination of complex lipids.
Discussion
Rapid TG accumulation during heat acclimation
Untargeted and targeted metabolite analyses were performed to identify metabolic pathways that were regulated during heat acclimation. There was a dramatic accumulation of the sugars raffinose and galactinol as well as of the most abundant TG species within 30min after a temperature shift from 22°C to 37°C. Accumulation of raffinose and galactinol after moderate heat has already been described (Kaplan et al., 2004; Panikulangara et al., 2004). In addition, Kaplan et al. identified pipecolic acid and digalactosylglycerol as heat-induced metabolites in 5-week-old Arabidopsis plants at 40°C (Kaplan et al., 2004). These metabolites, however, were not increased in this study using 2-week-old A. thaliana seedlings after 2h at 37°C. As far as can be determined, the massive accumulation of TGs after heat acclimation in A. thaliana has not been characterized before. Levels of TG markers increase extremely rapidly and were sensitive to temperature increases (Fig. 3D). Moreover, levels of TG markers rapidly dropped after the return to basal temperatures (Fig. 3C). Notably, the heating method (growth chamber or heating block) and growth conditions (seedlings grown on agar plates or transferred in liquid medium 1 day before treatment) as well as the time to harvest the seedling outside the growth chamber had a strong impact on TG levels. Therefore, some variations of mean TG levels in different experiments may reflect differences in experimental conditions.
The raffinose pathway is known to be up-regulated by a variety of stresses in the absence of heat. For instance, drought, salt, and cold stress are induced by expression of GOLS1 or GOLS3, leading to the accumulation of raffinose and galactinol (Taji et al., 2002). While TG accumulation was triggered by drought and salt stress, other short-term stresses such as cold, high light, and osmotic stress did not induce a significant accumulation of TGs in Arabidopsis seedlings (Fig. 4). It has been shown that TGs increase upon long-term cold treatment (Moellering et al., 2010), desiccation (Gasulla et al., 2013), senescence (Watanabe et al., 2013), and Pseudomonas infection (Zoeller et al., 2012) in Arabidopsis. Collectively, data suggest that accumulation of TGs, similar to the raffinose response, is a stress response that is not limited to elevated temperatures.
Heat-induced TG accumulation is not part of the genetically programmed HSR
The four master HSFA1s are essential for up-regulation of a series of heat-inducible HSFs and HSPs as well as anti-oxidative enzymes such as APX2 and metabolic enzymes such as GOLS1 (Liu et al., 2011). Out of the four HSFA1s, only HSFA1a and b are essential for rapid heat-induced transcription of GOLS1 and raffinose accumulation, because GOLS1 expression levels are strongly reduced in hsf1ab double mutants (Busch et al., 2005). In agreement with these findings, a lack of raffinose accumulation in heat-treated hsfa1abde was observed (Fig. 5A). In addition, HSFA2, a heat- and stress-induced transcription factor, also contributes to GOLS1 expression because it is reduced in heat-treated hsfa2 mutant plants (Busch et al., 2005; Nishizawa et al., 2006). Transgenic plants overexpressing HSFA2 overexpress GOLS1 and over-accumulate raffinose and galactinol (Nishizawa et al., 2008). However, under the experimental conditions in this study, hsfa2 mutant seedlings still accumulated wild-type levels of raffinose during heat acclimation (Fig. 5A). Hsfa1 and hsfa2 mutant plants displayed wild-type-like accumulation of TG markers in response to heat (Fig. 5B), suggesting that TG accumulation is not part of the genetically programmed HSR. Additionally, gols1 mutant plants that are unable to accumulate raffinose and galactinol did not over-accumulate TGs in response to heat (Fig. 5D), suggesting that carbons are not re-directed from the sugar pathway to the TG pathway.
TG accumulation occurs in the cytosol of Arabidopsis seedlings
TG biosynthesis and accumulation has been best studied in Arabidopsis seeds but also occurs in mature leaves. In leaves, TGs have been identified in thylakoid-associated plastoglobuli in chloroplasts as well as in oil bodies in the cytosol (Martin and Wilson, 1984; Lippold et al., 2012). Plastoglobuli are lipid bodies, containing mainly TGs, carotenoids, and prenyl quinones (Steinmuller and Tevini, 1985). Their swelling and formation is often associated with stress responses such as drought (Eymery and Rey, 1999), nitrogen starvation (Gaude et al., 2007), and, notably, moderate heat stress (Zhang et al., 2010). In plastids, phytyl ester synthases 1 and 2 can acylate DGs and might be involved in TG synthesis in chloroplasts (Lippold et al., 2012). Given that plastoglobuli have been shown to accumulate upon abiotic stress responses, in particular after heat stress, heat-responsive TGs may accumulate in chloroplasts. However, lipid analyses of isolated chloroplasts and seedlings revealed that TG markers did not accumulate in chloroplasts, suggesting cytosolic assembly and storage of TGs after heat stress (Fig. 6). In addition, TGs accumulate to a similar extent in roots and shoots after heat acclimation (Fig. 7), indicating extra-chloroplastic storage of TGs. Similar to TGs, raffinose is also synthesized in the cytosol (Schneider and Keller, 2009) and is also heat inducible in roots (Panikulangara et al., 2004).
Hence, TGs are likely synthesized through cytosolic pathways. Enzymes of the endoplasmic reticulum–localized Kennedy pathway involved in TG biosynthesis, like acyl-CoA:G3P acyltransferase, lysophosphatidic acid acyltransferase, and DG acyltransferase catalyse the sequential acylation of glycerol in developing seeds. An alternative TG biosynthesis pathway in the endoplasmic reticulum involves acyl-CoA independent transacylase phospholipid: DG acyltransferase that converts PCs and DGs to TGs (Chapman and Ohlrogge, 2012).
In order to clarify if accumulation of TG markers originates from de novo synthesis, levels of free and esterified fatty acids were analysed. Levels of total fatty acids (free and esterified) did not significantly change during heat acclimation (Fig. 8A), suggesting that TG accumulation is not driven by massive de novo fatty acid synthesis. Alternatively, accumulation of TG markers may reflect lipid remodelling, i.e. channelling of fatty acids from constitutive membrane lipids to TGs. However, significant changes were not observed in most abundant membrane lipid species, including PCs, MGDGs, DGDGs, PEs, and DGs after heat acclimation (Fig. 8B). These analyses indicate that a dramatic disturbance of membrane lipid homeostasis did not occur. However, the fatty acid pool in TGs was 1–8% compared to the total fatty acid pool in structural membrane lipids and, hence, fine-tuning of lipid pools might have escaped detection. Because TG markers predominantly contain polyunsaturated fatty acids, it is likely that polyunsaturated fatty acids are released from structural lipids and used for transient assembly of TGs during membrane remodelling.
After short-term heat treatment, slightly altered levels of MGDGs have been reported that did not result in discrimination when using multivariate statistical analysis (Burgos et al., 2011). Therefore, small but potentially relevant changes in absolute lipid levels might have escaped detection. Another possibility is that heat-induced accumulation of TGs simply reflects decreased degradation and turnover of the cellular TG pool due to reduced fatty acid consumption during heat stress. It remains to be clarified how the TG pool is regulated through transcriptional or post-transcriptional mechanisms. Notably, transcripts of key proteins involved in cytosolic TG biosynthesis, such as phospholipid: DG acyltransferase 1 and DG acyltransferase, are not up-regulated during heat acclimation (Hruz et al., 2008), indicating that rapid modulation of the TG pool may be regulated by post-transcriptional events.
Functional significance of heat-induced raffinose and TG accumulation
Raffinose has been proposed to act as an osmoprotective solute (Panikulangara et al., 2004) and an antioxidant (Nishizawa et al., 2008) in abiotic stresses. Arabidopsis transgenic plants that constitutively overexpress GOLS1 or GOLS2 and accumulate raffinose and galactinol are more resistant to cold, salt, oxidative, and drought stress (Taji et al., 2002; Nishizawa et al., 2008). However, gols1-mutant plants that are unable to accumulate raffinose and galactinol after heat stress were fully competent to acclimate to heat stress (Panikulangara et al., 2004). Hence, up-regulation of the raffinose pathway appears to protect plants from a variety of abiotic stresses but not from heat stress.
The biological significance of TG accumulation during heat stress is not known. Previous studies have demonstrated activation of TG synthesis and a concurrent decrease in galactolipids and phospholipids in ozone-fumigated spinach leaves (Sakaki et al., 1990a, b ; Sakaki et al., 1990c), drought-stressed cotton leaves (El-Hafid et al., 1989), and cold-stressed (Moellering et al., 2010) as well as senescent Arabidopsis plants (Kaup et al., 2002; Watanabe et al., 2013). Severe stresses may trigger degradation of structural membrane lipids with small head groups like MGDGs and PCs through induction of galactolipases and phospholipases (Kaup et al., 2002; Welti et al., 2002; Moellering et al., 2010). The degradation products like free fatty acids and DGs may then be converted to TGs. An accumulation of DGs, which form inverted micellar structures, may introduce small areas with unstable negative curvatures into bilayers that can lead to the fusion of apposed membrane bilayers (Goni and Alonso, 1999; Gasulla et al., 2013). In addition, high levels of free fatty acids that display detergent-like amphiphilic properties are not well tolerated by plant cells. Thus, removal of DGs and fatty acids by formation of TG-rich oil bodies might contribute to membrane stabilization (Moellering et al., 2010; Gasulla et al., 2013). It has also been proposed that fatty acids derived from thylakoid galactolipid breakdown are transiently stored in TGs prior to conversion into phloem-mobile sucrose (Kaup et al., 2002; Watanabe et al., 2013). To clarify a possible function of TG accumulation for heat acclimation and resistance, stress resistance of transgenic plants that over-accumulate TGs or are deficient in stress-induced TG accumulation remains to be investigated.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Thermotolerance assay. Schemes of heat stress regimes for the thermotolerance assay is shown in panel A. Arabidopsis seedlings (100 seeds/plate) were grown at 22°C (a) and heat acclimated at 37°C for 2h (b). Acclimated (c) and non-acclimated (d) seedlings were then shifted to 45°C for 2h. The survival rate was measured 5 days after the heat treatment (B). Data represent means ± SD, n = 4.
Fig. S2. Principal components analysis (PCA) revealed a discrimination in the metabolome of heat-acclimated and control seedlings. Metabolite features of the hydrophilic (A) and hydrophobic (B) compounds were subjected to PCA. The first component in the PCA plots differentiated the metabolite features of heat-acclimated from control seedlings (n = 4).
Table S1. Identification parameters (m/z and RT) of identified lipids.
Table S2. Parameters used for the identification of heat-responsive markers.
Table S3. Amount of the identified TGs in control and heat acclimated (37°C, 2h) seedlings. Data represent means ± SD, n = 9.
Table S4. Lipid analysis of control and heat acclimated (37°C, 2h) samples in chloroplast fractions and in seedlings. Levels of the most abundant MGDGs (A), PEs (B), and TG markers (C) were determined in total seedling extracts and isolated chloroplasts and normalized to chlorophyll content. Data represent means ± SD, n = 3.
Table S5. Levels of complex lipids in heat-acclimated (37°C, 2h) and control seedlings (22°C). Data represent means ± SD, n = 9.
Acknowledgement
SPM was supported by a grant of the German Excellence Initiative to the Graduate School of Life Sciences, University Wuerzburg. SPM and MJM were also supported by the Deutsche Forschungsgemeinschaft (GK 1342). We would like to thank Maria Lesch and Matthias Freund for technical help, and Frank Waller for proof reading.
References
- Aronsson H, Jarvis P. 2002. A simple method for isolating import-competent Arabidopsis chloroplasts. FEBS Letters 529, 215–220. [DOI] [PubMed] [Google Scholar]
- Burgos A, Szymanski J, Seiwert B, Degenkolbe T, Hannah MA. 2011. Analysis of short-term changes in the Arabidopsis thaliana glycerolipidome in response to temperature and light. The Plant Journal 66, 656–668. [DOI] [PubMed] [Google Scholar]
- Busch W, Wunderlich M, Schoffl F. 2005. Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana . The Plant Journal 41, 1–14. [DOI] [PubMed] [Google Scholar]
- Chapman KD, Ohlrogge JB. 2012. Compartmentation of triacylglycerol accumulation in plants. The Journal of Biological Chemistry 287, 2288–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT. 2007. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis . Plant Physiology 143, 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charng YY, Liu HC, Liu NY, Hsu FC, Ko SS. 2006. Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. Plant Physiology 140, 1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Burke JJ, Velten J, Xin Z. 2006. FtsH11 protease plays a critical role in Arabidopsis thermotolerance. The Plant Journal 48, 73–84. [DOI] [PubMed] [Google Scholar]
- Clarke SM, Cristescu SM, Miersch O, Harren FJ, Wasternack C, Mur LA. 2009. Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana . New Phytologist 182, 175–187. [DOI] [PubMed] [Google Scholar]
- El-Hafid L, Pham AT, Zuily-Fodil Y, da Silva JV. 1989. Enzymatic breakdown of polar lipids in cotton leaves under water stress: I. Degradation of monogalactosyl-diacylglycerol. Plant Physiology and Biochemistry 27. [Google Scholar]
- Eymery F, Rey P. 1999. Immunocytolocalization of CDSP 32 and CDSP 34, two chloroplastic drought-induced stress proteins in Solanum tuberosum plants. Plant Physiology and Biochemistry 37, 305–312. [Google Scholar]
- Gasulla F, Vom Dorp K, Dombrink I, Zahringer U, Gisch N, Dormann P, Bartels D. 2013. The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: a comparative approach. The Plant Journal 75, 726–741. [DOI] [PubMed] [Google Scholar]
- Gaude N, Brehelin C, Tischendorf G, Kessler F, Dormann P. 2007. Nitrogen deficiency in Arabidopsis affects galactolipid composition and gene expression and results in accumulation of fatty acid phytyl esters. The Plant Journal 49, 729–739. [DOI] [PubMed] [Google Scholar]
- Goni FM, Alonso A. 1999. Structure and functional properties of diacylglycerols in membranes. Progress in Lipid Research 38, 1–48. [DOI] [PubMed] [Google Scholar]
- Hong SW, Vierling E. 2000. Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proceedings of the National Academy of Sciences of the United States of America 97, 4392–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P. 2008. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Advances in Bioinformatics 2008, 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL. 2004. Exploring the temperature-stress metabolome of Arabidopsis . Plant Physiology 136, 4159–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup MT, Froese CD, Thompson JE. 2002. A role for diacylglycerol acyltransferase during leaf senescence. Plant Physiology 129, 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak S, Port M, Ganguli A, Bicker F, von Koskull-Doring P. 2004. Characterization of C-terminal domains of Arabidopsis heat stress transcription factors (Hsfs) and identification of a new signature combination of plant class A Hsfs with AHA and NES motifs essential for activator function and intracellular localization. The Plant Journal 39, 98–112. [DOI] [PubMed] [Google Scholar]
- Larkindale J, Hall JD, Knight MR, Vierling E. 2005. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiology 138, 882–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Knight MR. 2002. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene and salicylic acid. Plant Physiology 128, 682–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Vierling E. 2008. Core genome responses involved in acclimation to high temperature. Plant Physiology 146, 748–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippold F, vom Dorp K, Abraham M, Holzl G, Wewer V, Yilmaz JL, Lager I, Montandon C, Besagni C, Kessler F, Stymne S, Dormann P. 2012. Fatty acid phytyl ester synthesis in chloroplasts of Arabidopsis . The Plant Cell 24, 2001–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HC, Charng YY. 2012. Acquired thermotolerance independent of heat shock factor A1 (HsfA1), the master regulator of the heat stress response. Plant Signaling and Behavior 7, 547–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HC, Liao HT, Charng YY. 2011. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis . Plant Cell and Environment 34, 738–751. [DOI] [PubMed] [Google Scholar]
- Martin BA, Wilson RF. 1984. Subcellular localization of TAG synthesis in spinach l. Lipids 19, 117–121. [DOI] [PubMed] [Google Scholar]
- Moellering ER, Muthan B, Benning C. 2010. Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 330, 226–228. [DOI] [PubMed] [Google Scholar]
- Murphy RC, James PF, McAnoy AM, Krank J, Duchoslav E, Barkley RM. 2007. Detection of the abundance of diacylglycerol and triacylglycerol molecular species in cells using neutral loss mass spectrometry. Analytical Biochemistry 366, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa A, Yabuta Y, Yoshida E, Maruta T, Yoshimura K, Shigeoka S. 2006. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. The Plant Journal 48, 535–547. [DOI] [PubMed] [Google Scholar]
- Nishizawa A, Yabuta Y, Shigeoka S. 2008. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiology 147, 1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikulangara TJ, Eggers-Schumacher G, Wunderlich M, Stransky H, Schoffl F. 2004. Galactinol synthase1. A novel heat shock factor target gene responsible for heat-induced synthesis of raffinose family oligosaccharides in Arabidopsis . Plant Physiology 136, 3148–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki T, Kondo N, Yamada M. 1990. a Free fatty acids regulate two galactosyltransferases in chloroplast envelope membranes isolated from spinach leaves. Plant Physiology 94, 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki T, Kondo N, Yamada M. 1990. b Pathway for the synthesis of triacylglycerols from monogalactosyldiacylglycerols in ozone-fumigated spinach leaves. Plant Physiology 94, 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki T, Saito K, Kawaguchi A, Kondo N, Yamada M. 1990c. Conversion of monogalactosyldiacylglycerols to triacylglycerols in ozone-fumigated spinach leaves. Plant Physiology 94, 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Keller F. 2009. Raffinose in chloroplasts is synthesized in the cytosol and transported across the chloroplast envelope. Plant and Cell Physiology 50, 2174–2182. [DOI] [PubMed] [Google Scholar]
- Schramm F, Ganguli A, Kiehlmann E, Englich G, Walch D, von Koskull-Doring P. 2006. The heat stress transcription factor HsfA2 serves as a regulatory amplifier of a subset of genes in the heat stress response in Arabidopsis . Plant Molecular Biology 60, 759–772. [DOI] [PubMed] [Google Scholar]
- Steinmuller D, Tevini M. 1985. Composition and function of plastoglobuli: I. Isolation and purification from chloroplasts and chromoplasts. Planta 163, 201–207. [DOI] [PubMed] [Google Scholar]
- Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K. 2002. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana . The Plant Journal 29, 417–426. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Balazadeh S, Tohge T, Erban A, Giavalisco P, Kopka J, Mueller-Roeber B, Fernie AR, Hoefgen R. 2013. Comprehensive dissection of spatiotemporal metabolic shifts in primary, secondary, and lipid metabolism during developmental senescence in Arabidopsis . Plant Physiology 162, 1290–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang X. 2002. Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis . The Journal of Biological Chemistry 277, 31994–32002. [DOI] [PubMed] [Google Scholar]
- Yeh CH, Kaplinsky NJ, Hu C, Charng YY. 2012. Some like it hot, some like it warm: phenotyping to explore thermotolerance diversity. Plant Science 195, 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Ohama N, Nakajima J, Kidokoro S, Mizoi J, Nakashima K, Maruyama K, Kim JM, Seki M, Todaka D, Osakabe Y, Sakuma Y, Schoffl F, Shinozaki K, Yamaguchi-Shinozaki K. 2011. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Molecular Genetics and Genomics 286, 321–332. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wise RR, Struck KR, Sharkey TD. 2010. Moderate heat stress of Arabidopsis thaliana leaves causes chloroplast swelling and plastoglobule formation. Photosynthesis Research 105, 123–134. [DOI] [PubMed] [Google Scholar]
- Zoeller M, Stingl N, Krischke M, Fekete A, Waller F, Berger S, Mueller MJ. 2012. Lipid profiling of the Arabidopsis hypersensitive response reveals specific lipid peroxidation and fragmentation processes: biogenesis of pimelic and azelaic acid. Plant Physiology 160, 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.