Highlight
OsHrd3 plays crucial roles in protein quality control of seed storage proteins through polyubiquitination of a cyteine-rich prolamin, RM1, during rice seed maturation.
Key words: Prolamins, protein body, protein quality control, seed storage protein, unfolded protein response.
Abstract
Large amounts of seed storage proteins (SSPs) are produced in the maturing endosperm of rice seeds. Rice SSPs are synthesized as secretory proteins on the rough endoplasmic reticulum (ER), and are transported and deposited into protein complexes called protein bodies (PB-I and PB-II). Due to the high production of SSPs, unfolded SSPs may be generated during this process. However, it was previously unclear how such unfolded proteins are selected among synthesized products and removed from the ER to maintain protein quality in the endosperm. Since Hrd3/SEL1L recognizes unfolded proteins in yeast and mammalian protein quality control systems, the role of OsHrd3 in protein quality control in rice endosperm was investigated. Co-immunoprecipitation experiments demonstrated that OsHrd3 interacts with components of the Hrd1 ubiquitin ligase complex such as OsOS-9 and OsHrd1 in rice protoplasts. Endosperm-specific suppression of OsHrd3 in transgenic rice reduced the levels of polyubiquitinated proteins and resulted in unfolded protein responses (UPRs) in the endosperm, suggesting that OsHrd3-mediated polyubiquitination plays an important role in ER quality control. It was found that a cysteine-rich 13kDa prolamin, RM1, was polyubiquitinated in wild-type (WT) seeds but not in OsHrd3 knockdown (KD) seeds. RM1 formed aberrant aggregates that were deposited abnormally in OsHrd3 KD seeds, resulting in deformed PB-I. Therefore, the quality of protein bodies is maintained by polyubiquitination of unfolded SSPs through the Hrd1 ubiquitin ligase system in rice endosperm.
Introduction
Seed storage proteins (SSPs) are a source of the nitrogen, sulphur, and carbon required for the germination and growth of seedlings prior to photosynthesis. Rice SSPs are synthesized on the rough endoplasmic reticulum (rER) and translocated into the ER lumen, followed by deposition in two different types of protein bodies, PB-I and PB-II (Tanaka et al., 1980; Krishnan et al., 1986). PB-I is a 1–2 μm, spherical proteinaceous granule derived from the ER. PB-I is composed of cysteine-rich (10, 13, and 16kDa) and cysteine-poor (13kDa) prolamins (Ogawa et al., 1987). PB-II is an irregularly shaped, 2–4 μm protein storage vacuole that has high electron density. PB-II is formed via the Golgi apparatus or by precursor-accumulating (PAC) vesicles from the ER, into which glutelins and globulin are deposited (Takahashi et al., 2005). Glutelin is synthesized as proglutelin in the ER lumen and subsequently processed into mature acidic and basic subunits in PB-II. Large amounts of SSPs are produced in the ER of rice endosperm and, thus, the ER in rice endosperm is densely packed with polypeptides. In such a crowded cellular environment, unfolded proteins are often produced through stochastic errors during protein synthesis or by perturbation due to adverse environmental changes (Gershenson and Gierasch, 2011; Hartl et al., 2011). The accumulated unfolded proteins may form aggregations, resulting in perturbed cellular homeostasis when these unfolded proteins bind to other proteins. However, there may be unknown molecular mechanisms that serve to clean up the unfolded proteins and to maintain functional, healthy proteostasis in the ER of rice endosperm.
Protein quality control systems are mechanisms used to maintain healthy proteostasis in the ER (Brodsky, 2012). ER chaperones, folding enzymes, and chaperone-like proteins play central roles in protein quality control, and these proteins mediate the repair of improperly/incompletely folded polypeptides. In contrast, severely damaged unfolded polypeptides are recognized and sequestered for degradation via a process known as ER-associated degradation (ERAD). The unfolded polypeptides are then retrotranslocated from the ER to the cytoplasm and marked with ubiquitins by a catalytic cascade of E1 (ubiquitin-activating enzyme), E2 (ubiquitin-conjugating enzyme), and E3 (ubiquitin ligase) activities (Hochstrasser, 1996; Hershko and Ciechanover, 1998). Since proteosome delivery and degradation require the ubiquitination of the substrate, unfolded polypeptides, the ubiquitination enzymes are crucial for ERAD. Finally, polyubiquitinated, unfolded polypeptides are degraded by proteosomes.
Increasing numbers of components of the ERAD machinery have been identified in plants. The AAA ATPase Cdc48/p97 is a direct contributor to the retrograde translocation of ERAD substrates at an intermediate step preceding proteosomal protein degradation in mammalian cells and yeast (Braun et al., 2002; Jarosch et al., 2002). Plant Cdc48 is involved in the retrograde transport of plant ERAD substrates such as the mutant forms of mildew resistance o (MLO) protein and mutated ricin (Müller et al., 2005; Marshall et al., 2008). The isolation of suppressor mutants for a brassinosteroid-insensitive mutant revealed that a number of ERAD-related genes similar to those in yeast and mammals are involved in the degradation of the mutated brassinosteroid receptor. These include genes encoding UDP-glucose:glycoprotein glucotransferase (UGGT), a plant-specific calreticulin, and a homologue of yeast Hrd3/mammalian SEL1L (Jin et al., 2007, 2008; Su et al., 2011). Hrd3/SEL1L is a component of the Hrd1 E3 ligase complex and is involved in substrate recruitment (Carvalho et al., 2006; Denic et al., 2006). Arabidopsis Hrd3 is necessary for the degradation of mutated brassinosteroid receptor BRI, which is implicated in the HRD pathway in plants (Su et al., 2011). Furthermore, ER-resident chaperones such as calnexin and BiP interact with the mutated brassinosteroid receptors Bri1-5 and Bri1-9 (Jin et al., 2007; Hong et al., 2008). Arabidopsis ubiquitin conjugase UBC32 is an ERAD component that is involved in brassinosteroid-mediated salt stress tolerance (Cui et al., 2012). These studies clearly demonstrate that plants have ERAD machinery that can degrade aberrant proteins caused by genetic mutations. However, it is still unclear whether the plant ERAD machinery is involved in protein quality control and the degradation of unfolded proteins derived from wild-type (WT) proteins, which is caused by stochastic errors during protein synthesis or perturbation by adverse environmental changes under normal conditions.
In this work, the roles of OsHrd3 in protein quality control in rice endosperm were investigated. Transgenic rice with suppressed expression of OsHrd3 under the control of an endosperm-specific promoter were generated. The results reveal that OsHrd3 is required for the polyubiquitination of unfolded proteins, including the cysteine-rich 13kDa prolamin RM1, in rice endosperm. Thus, significant amounts of unfolded RM1 are produced under normal conditions, and the removal of unfolded RM1 is achieved through the involvement of OsHrd3, which is required for proper formation of PB-I in rice endosperm.
Materials and methods
Construction of pasmids
To make the following plasmid constructs, the coding region of OsHrd3 was amplified from a rice full-length cDNA clone (AK067004) by PCR using the primers shown in Supplementary Table S1 available at JXB online. The Ubip-3× FLAG-GluBter vector was constructed by inserting the 3× FLAG tag fragment of the 2×35S-3× FLAG-Nos vector (Ohta et al., 2013) into the KpnI and SacI sites of the Ubip-GFP-GluBter vector. To produce OsHrd3-FLAG, the coding region of OsHrd3 was excised from OsHrd3–green fluorescent protein (GFP) by digestion with XmaI and inserted into the XmaI site of the Ubip-3× FLAG-GluBter vector. The 2×HA tag sequence fragment was amplified using the primers shown in Supplementary Table S1, and the DNA fragment was inserted into the KpnI and SacI sites of the Ubip-GluBter and the 2×35S-Nos vectors to produce the Ubip-2×HA-GluBter and the 2×35S-2×HA-Nos vectors, respectively. The coding regions of OsHrd1 and OsOS9 were amplified by PCR using the primers listed in Supplementary Table S1. The DNA fragments were inserted into the KpnI site of the Ubip-2×HA-GluBter vector to produce OsHrd1-HA. OsOS9-HA was constructed by inserting the PCR fragment into the NcoI site of the 2×35S-2×HA-Nos vector.
Endosperm-specific knockdown lines for OsHrd3 (OsHrd3 KD) were generated by RNA interference. The gene fragment containing coding sequences (base pairs 1843–2087) and 357bp of 3′ untranslated region (UTR) in OsHrd3 was amplified by PCR using the primers listed Supplementary Table S1 at JXB online, and connected with the intron sequence from the rice aspartic protease gene (Kuroda et al., 2010) to express intron-containing hairpin RNA. The construct was linked downstream of the 16kDa prolamin promoter and inserted into a modified pHm43GW binary vector (Wakasa et al., 2011).
Rice transformation
Transgenic rice plants (Oryza sativa L. cv. Kita-ake) were generated by Agrobacterium-mediated transformation (Goto et al., 1999), and lines exhibiting suppressed expression of OsHrd3 were screened by real-time PCR (RT-PCR) analysis of OsHrd3 transcript levels in developing transgenic seeds [14 days after flowering (DAF)]. The T3 generation of homozygous plants of a representative line (line 3) was analysed.
Immunoprecipitation experiments
The transient expression assay was carried out as described previously (Kawakatsu et al., 2009). Protoplasts were prepared from cultured rice cells (Oc cells). To analyse the interaction between OsHrd3 and other proteins, co-immunoprecipitation (Co-IP) experiments were carried out essentially as described (Ohta et al., 2013), except that 1% digitonin was used in the extraction buffer instead of 0.5% Triton X-100.
Protein extraction and immunoblot analysis
Extraction of total proteins from mature WT and transgenic rice seeds and immunoblot analysis were performed as described previously (Ohta et al., 2013). To analyse the aggregates, total proteins were extracted from mature WT and OsHrd3 KD seeds using SDS–urea buffer without 2-mercaptoethanol. The extracts were centrifuged at 20 400 g for 10min at room temperature, and the supernatants were collected (fraction S). The pellets were again extracted with SDS–urea buffer containing 5% 2-mercaptoethanol, and the soluble fractions were collected by centrifugation as described above (fraction P). Before SDS–PAGE analysis, the proteins in the S fraction were reduced in SDS–urea buffer containing 5% 2-mercaptoethanol. Rabbit polyclonal antibodies (anti-OsBiP1, anti-OsBiP4&5, anti- OsPDIL2-3, anti-GluA, anti-GluB, anti-GluC, anti-Glb, anti-10k, anti-16k, anti-RM, anti-RM2, anti-RM4, and anti-RM9) were prepared previously (Yasuda et al., 2006; Oono et al., 2010; Wakasa et al., 2012).
RNA extraction and RT-PCR analysis
Total RNA was extracted from seeds as previously described (Takaiwa et al., 1987). RT-PCR analysis was carried out as described (Wakasa et al., 2012) using Go-Taq polymerase (Promega, WI, USA) with gene-specific primers for OsbZIP39, OsbZIP50, OsbZIP60, OsBiP4, and 17S rRNA (Hayashi et al., 2012), and for OsHrd3 (listed in Supplementary Table S1 at JXB online).
Confocal immunohistochemical analysis
Maturing WT and OsHrd3 KD seeds were harvested at 18 DAF and used for immunocytochemical analysis as described (Ohta et al., 2013).
Detection and immunoprecipitation of polyubiquitinated proteins
Developing seeds (14 DAF) from WT and OsHrd3 KD plants were surface-sterilized with 50% hypochlorous acid for 30min, and the hulls were aseptically removed from the sterilized seeds. The dehulled seeds (eight grains) were incubated in half-strength Murashige and Skoog (MS) liquid medium containing 100 μM MG132 overnight and then incubated in MS liquid medium containing 20 μM PR-619 b (Seiberlich et al., 2012) for 1h at 28 °C with gentle shaking. Total proteins were extracted from the treated seeds with SDS–urea buffer containing 5% 2-mercaptoethanol. The extracts were subjected to immunoblot analysis using polyclonal rabbit antibodies against ubiquitin–protein conjugates (Enzo Life Science, NY, USA).
For immunoprecipitation of polyubiquitinated proteins, dehulled seeds treated with MG132 and PR-619 were homogenized in 800 μl of extraction buffer containing 50mM TRIS-HCl, pH 7.5, 150mM NaCl, 0.5% Triton X-100, 5mM EDTA, 20mM N-ethylmaleimide, and 1× Complete mini EDTA-free Protease Inhibitor Cocktail (Roche, Switzerland). The homogenates were centrifuged at 20 400 g for 10min at 4 °C and the supernatants were collected. The supernatants were mixed with immobilized anti-multiubiquitin mAb-magnetic beads (MBL, Japan) for 3h at 4 °C to immunoprecipitate the polyubiquitinated proteins. The beads were washed three times with NET buffer containing 50mM TRIS-HCl, pH 7.5, 150mM NaCl, and 0.1% NP-40. The immunoprecipitated samples were eluted with SDS loading buffer containing 2% SDS, 62.5mM TRIS-HCl pH 6.8, and 5% 2-mercaptoethanol. The samples were denatured at 65 °C for 10min and subjected to immunoblot analysis.
Results
Interaction between OsHrd3 and both OsHrd1 and OsOS-9
A database survey revealed that the rice genome encodes a homologue of Hrd3/SEL1L (Os03g0259300) containing a signal peptide, nine Sel1-like repeats, and a C-terminal transmembrane anchor. Thus, Os03g0259300 was designated as OsHrd3. It was confirmed that OsHrd3 is an ER-resident type I membrane protein (Supplementary Fig. S1 at JXB online).
Hrd3/SEL1L is a component of the Hrd1 ubiquitin ligase complex. In yeast, the Hrd1 complex consists of Hrd3p and Der1p, including the ER lectin Yos9p bound to Hrd3p (Carvalho et al., 2006; Denic et al., 2006). Rice genes encoding homologues of the yeast Hrd1 and Yos9p were found in the database (RAP-DB, http://rapdb.dna.affrc.go.jp/). To examine the possibility that OsHrd3 also forms a complex with these proteins, the interaction between OsHrd3 and both OsHrd1 (Os06g0301000) and OsOS-9 (Os06g0644800), which are putative homologues of yeast Hrd1 and Yos-9p, respectively, was investigated. OsHrd1 and OsOS-9 were fused with 2× HA tag sequence at their C-termini. Plasmid DNA harboring OsHrd3-FLAG, together with OsHrd1-HA or OsOS-9-HA, was then transfected into rice protoplasts and Co-IP analysis was performed with an antibody against FLAG tag (Ohta et al., 2013). As shown in Fig. 1, OsHrd1-HA and OsOS-9-HA co-precipitated with OsHrd3-FLAG, demonstrating that OsHrd1 and OsOS-9 can interact with OsHrd3. These data suggest that OsHrd3 could form a complex with OsHrd1 and OsOS-9.
Fig. 1.
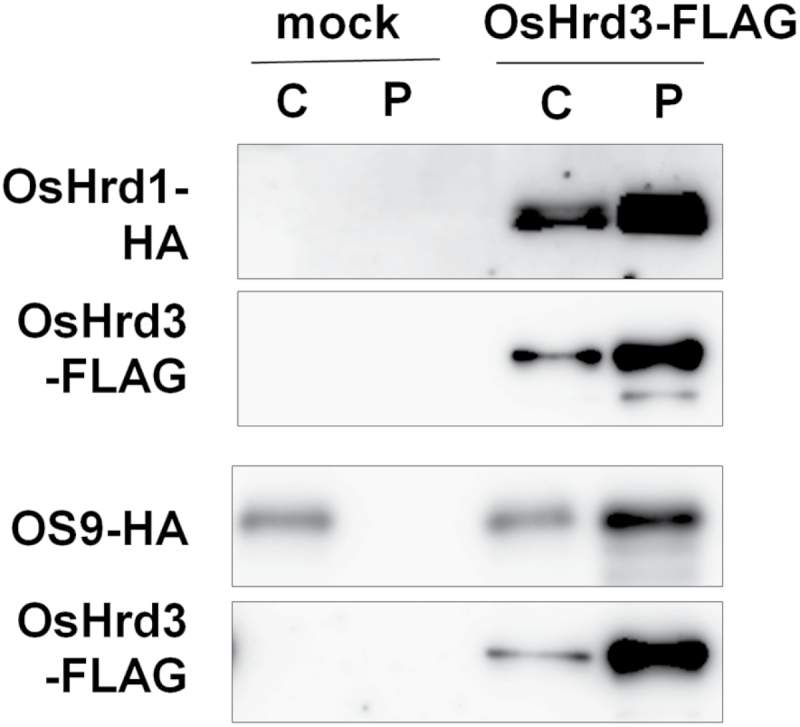
Interaction between OsHrd3 and components of the Hrd1 ubiquitin ligase complex. Immunoprecipitation of OsHrd3-FLAGs. Protein extracts from rice protoplasts expressing OsHrd1-HA, OsOS-9-HA, and OsHrd3-FLAG were subjected to immunoprecipitation using anti-FLAG M2 magnetic beads. The immunoprecipitates were analysed by immunoblot analysis using anti-FLAG–horseradish peroxidase (HRP) and anti-HA–HRP conjugated antibodies. C represents 2% (v/v) of the starting crude lysate used for immunoprecipitation. P represents immunoprecipitated proteins.
OsHrd3 is involved in polyubiquitination in rice endosperm
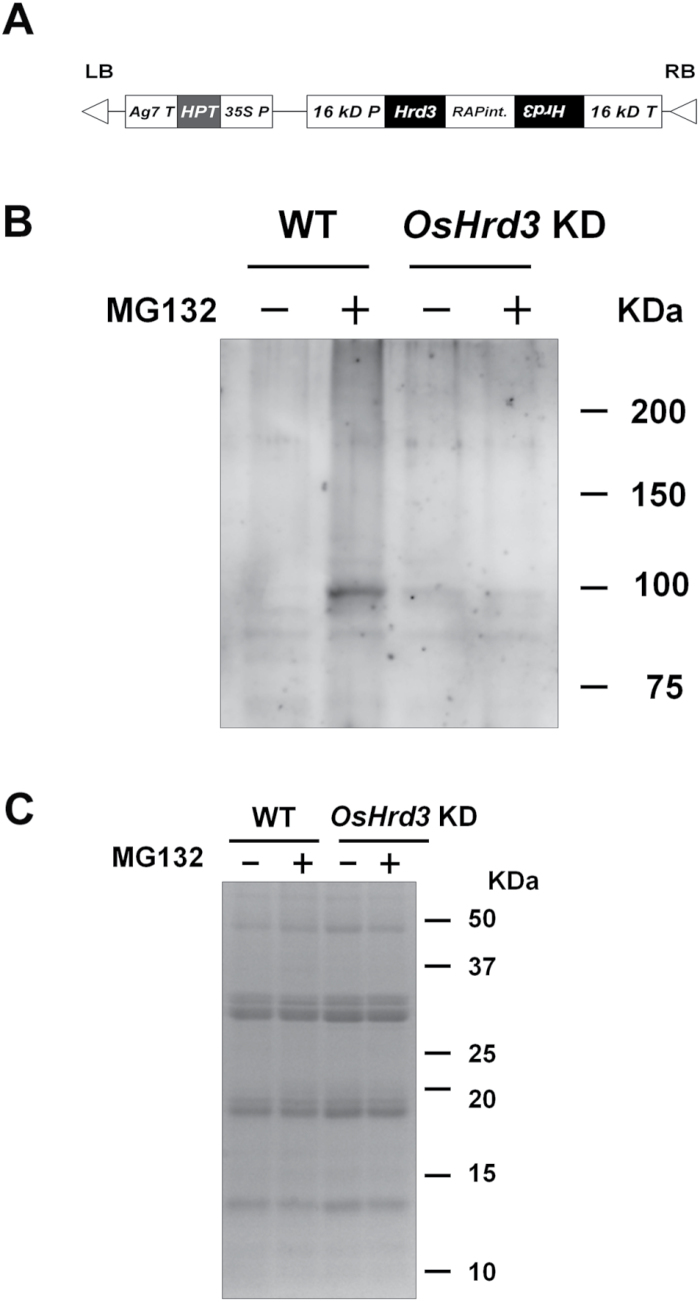
Maturing rice seeds produce a large amount of SSPs. Thus, a significant amount of unfolded protein is likely to be produced by stochastic errors during protein synthesis, perturbation by adverse environmental changes, and an imbalance in stoichiometry among components of the protein bodies. However, it is unclear how the ER in developing seeds discriminates and removes such unfolded proteins. To elucidate the role of a quality control system in rice seeds, transgenic rice plants were generated with suppressed expression of OsHrd3 in the endosperm under the control of the 16kDa prolamin (Os03g0766200) promoter (Fig. 2A). Since mRNAs for OsHrd3 and 16kDa prolamin were detected at 7DAF (Fig. 3B; Supplementary Fig. S2D at JXB online), the 16kDa prolamin promoter is suitable for suppression of OsHrd3 expression. RT-PCR analysis showed that the level of OsHrd3 transcript was lower in OsHrd3 KD seeds than in WT seeds (Fig. 3B).
Fig. 2.
OsHrd3 is required for polyubiquitination of unfolded proteins in rice endosperm. (A) The construct used for OsHrd3 knockdown (OsHrd3 KD); 35S P, Cauliflower mosaic virus 35S promoter (AF485783); HPT, hygromycin phosphotransferase coding region (K01193); Ag7 T, gene 7 terminator (AF85783); 16 kD P, promoter region of the gene encoding 16kDa prolamin (AY427574); RAPint, an intron from the rice aspartic protease gene (D32165); 16 kD T, 16kDa prolamin terminator. (B and C) Levels of polyubiquitinated proteins are reduced in OsHrd3 KD seeds. Seeds (14 DAF) from wild-type (WT) and OsHrd3 KD plants were dehulled and treated with either 0.1% dimethylsulphoxide (–) or 100 μM MG132 (+) for 24h and then treated with 20 μM PR-619 for 1h. Then, total proteins were extracted from the seeds with SDS–urea buffer containing 2-mercaptoethanol. The total proteins were separated by SDS–PAGE, followed by immunoblot analyses using an antibody against ubiquitin–protein conjugates (B) or Coomassie Brilliant Blue staining (C).
Fig. 3.
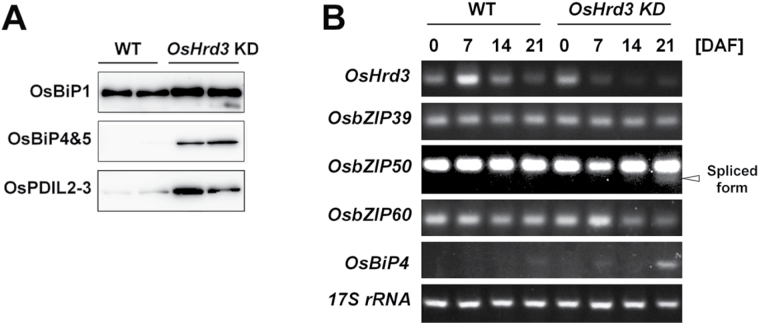
Unfolded protein responses in OsHrd3 KD seeds. (A) Immunoblot analysis of total proteins extracted from mature WT and OsHrd3 KD seeds. Total proteins were extracted from mature seeds with SDS–urea buffer containing 2-mercaptoethanol. The total proteins were separated by SDS–PAGE, followed by immunoblot analyses using antibodies against ER-resident chaperones. The two tracks in the WT and OsHrd3 KD represent different samples from different seeds. (B) Induction of ER stress-related genes during seed development in WT and OsHrd3 KD seeds. Total RNAs were isolated from seed tissues at 0, 7, 14, and 21 days after flowering (DAF). Transcript levels were estimated by semi-quantitative RT-PCR. 17S rRNA was analysed as a loading control. The arrowhead indicates mature transcript produced by unconventional splicing of precursor OsbZIP50 transcript.
To examine the possibility that OsHrd3 is involved in the ubiquitination of unfolded proteins in rice endosperm, the levels of polyubiquitinated proteins in rice endosperm were analysed. When maturing seeds were harvested at 14 DAF from WT and OsHrd3 KD plants and treated with the proteosome inhibitor MG132 and the deubiquitinase inhibitor PR-619, the levels of polyubiquitinated proteins increased in the treated WT only compared with the non-treated control WT and OsHrd3 KD seeds (Fig. 2B, C). These results demonstrate that OsHrd3 is involved in the polyubiquitination of unfolded proteins in rice endosperm, and this protein is a component of the Hrd1 ubiquitin ligase complex.
Induction of unfolded protein response in OsHrd3 KD seeds
Since the polyubiquitination of unfolded proteins is impaired in OsHrd3 KD seeds, the unfolded proteins in these seeds are likely to reside in the ER as a result of the suppression of retrograde transport of these proteins to the cytoplasm. Thus, the unfolded protein response (UPR) was evaluated in OsHrd3 KD seeds. Mature seeds from OsHrd3 KD plants displayed an abnormal phenotype, with a slightly floury and shrunken appearance (Supplementary Fig. S2A at JXB online), and the grain weight of the OsHrd3 KD seeds was significantly lower than that of WT seeds (P=0.002 by t-test, Supplementary Fig. S2B), suggesting that the UPR occurred in these seeds.
To ascertain that the UPR was induced in the OsHrd3 KD seeds, the levels of ER-resident chaperones in mature seeds were investigated. Immunoblot analyses demonstrated that the levels of UPR-responsive OsBiP4 and OsBiP5 (Wakasa et al., 2012) were higher in the OsHrd3 KD seeds than in the WT (Fig. 3A). Furthermore, the levels of OsBiP1 and OsPDIL2-3 were also higher in the OsHrd3 KD seeds than in the WT (Fig. 3A). See Supplementary Fig. S2C at JXB online for a loading control of this experiment. RT-PCR analysis revealed unconventional splicing of OsbZIP50 mRNA (Hayashi et al., 2012) in OsHrd3 KD seeds at 21 DAF concomitant with the induction of OsBiP4 (Fig. 3B). These results indicate that the OsIRE1/OsbZIP50 signalling pathway for the UPR was activated in OsHrd3 KD seeds and that the OsIRE1/OsbZIP50 signalling pathway partly accounts for the ER stress responses in OsHrd3 KD seeds. In contrast, the transcript levels of OsbZIP39 and OsbZIP60 were not affected in OsHrd3 KD seeds (Fig. 3B). Thus, the UPR is induced in OsHrd3 KD seeds, implying that the unfolded proteins reside in the ER of OsHrd3 KD seeds and that OsHrd3 is necessary for removing unfolded proteins from the ER in rice endosperm.
Accumulation of aberrant aggregations in OsHrd3 KD seeds
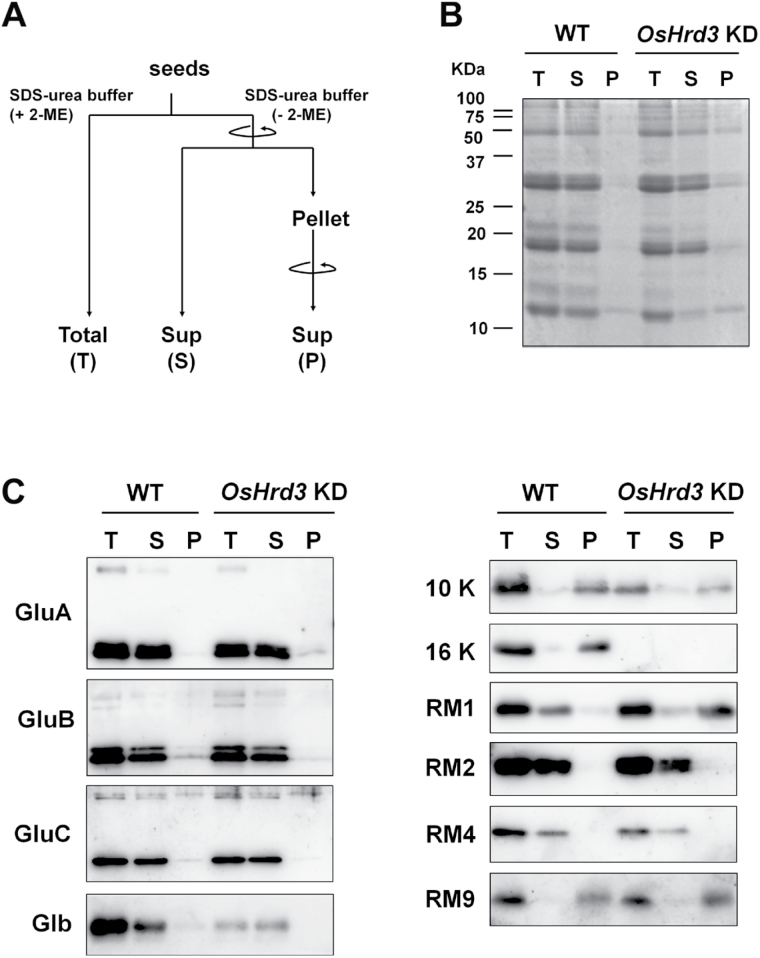
To compare the levels of SSPs in OsHrd3 KD versus WT mature seeds, proteins were extracted from these seeds using SDS–urea buffer containing 2-mercaptoethanol (fraction T in Fig. 4A). Immunoblot analysis showed that the accumulation of 26kDa globulin (Glb) and 16kDa prolamin (16 k) was dramatically reduced in the OsHrd3 KD seeds (Fig. 4C). The mRNA levels for Glb and 16 k were also down-regulated in maturing seeds (14–21 DAF) from OsHrd3 KD (Supplementary Fig. S2D at JXB online), implying that the reduced accumulation of Glb and 16 k was related to a decrease in transcript levels. Although levels of 10kDa prolamin (10 k) and 13kDa prolamin, RM4, were also decreased in the OsHrd3 KD seeds, the levels of other SSPs examined here were not affected in the OsHrd3 KD seeds (Fig. 4C).
Fig. 4.
Aberrant aggregation of RM1 in OsHrd3 KD seeds. (A) Schematic representation of the experiment. Total proteins were extracted from mature wild-type (WT) and OsHrd3 KD seeds with SDS–urea buffer without 2-mercaptoethanol and fractionated into the supernatant (S) and pellet (P) by centrifugation. The resulting pellets were again extracted with SDS–urea buffer containing 2-mercaptoethanol to collect the solubilized proteins, and proteins in the S fraction were denatured in the presence of 2-mercaptoethanol. For a control, total proteins (T) were extracted from mature WT and OsHrd3 KD seeds with SDS–urea buffer supplemented with 2-mercaptoethanol. (B) SDS–PAGE analysis of the T, S, and P fractions derived from WT and OsHrd3 KD seeds. Total proteins of the T, S, and P fractions were subjected to immunoblot analyses using antibodies against rice seed storage proteins. (C) Immunoblot analysis of the total (T), soluble (S), and pellet (P) fractions derived from WT and OsHrd3 KD seeds. Total proteins of the T, S, and P fractions were subjected to immunoblot analyses using antibodies against rice seed storage proteins.
Unfolded proteins in mammalian cells tend to form aggregations through cross-linking of interchain disulphides (Machamer and Rose, 1988). To assess this possibility in plants, proteins were extracted from mature seeds using SDS–urea buffer without 2-mercaptoethanol to maintain the aggregation of unfolded proteins caused by aberrant disulphide bond formation (Fig. 4). Most of the SSPs from both WT and OsHrd3 KD seeds were detected in the soluble (S) fraction (Fig. 4). In contrast, cysteine-rich proteins such as 10kDa prolamin (10 k), 13kDa prolamin (RM9), and 16kDa prolamins (16 k) were detected in the pellet (P) fractions from both WT and OsHrd3 KD seeds (Fig. 4C). Although another cysteine-rich 13kDa prolamin, RM1, was detected in the S fraction from WT seeds, most of the RM1 in OsHrd3 KD seeds was detected in the P fraction (Fig. 4C). This result suggests that the cysteine residues of RM1 form aberrant disulphide bonds in OsHrd3 KD seeds. These results imply that OsHrd3 is required for the removal of unfolded SSPs, such as RM1.
Deformed PB-I in OsHrd3 KD seeds
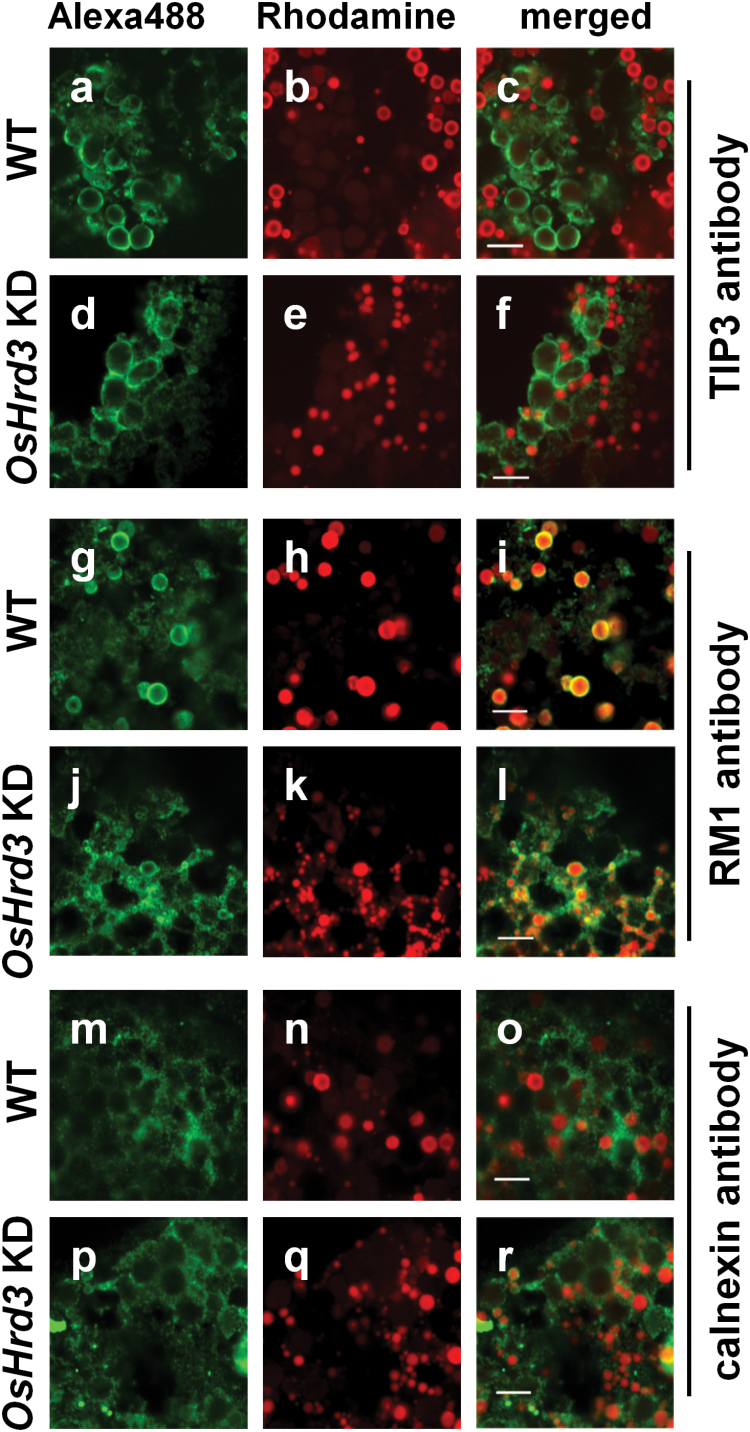
To investigate whether the intracellular structure and protein body formation were altered in OsHrd3 KD seeds, indirect immunohistochemical analysis was carried out using confocal microscopy (Fig. 5). The staining pattern of the PB-II marker TIP3 antibody (Takahashi et al., 2004) in OsHrd3 KD seeds was almost the same as that in WT seeds (Fig. 5a, c, d, f). In contrast, rhodamine staining revealed that PB-I was smaller in OsHrd3 KD seeds than in WT seeds (Fig. 5b, c, e, f). These results demonstrate that PB-I, but not PB-II, is severely affected in OsHrd3 KD seeds.
Fig. 5.
Aberrant protein body-I (PB-I) in the OsHrd3 KD seeds. Indirect immunohistochemical analysis of rice endosperm (18 DAF) using antibodies against OsTIP3 (a–f), RM1 (g–l), and calnexin (m–r). Signals were detected using a secondary antibody conjugated with Alexa488, which emits green fluorescence (a, d, g, j, m, p). PB-I was stained with rhodamine B (b, e, h, k, n, q). Right panels (c, f, i, l, o, r) are merged images of the left (green fluorescence) and the middle (red fluorescence) panels. Scale bar=5 μm.
RM1 was detected in the periphery of PB-I in WT seeds (Fig. 5g, i). In contrast, in OsHrd3 KD seeds, RM1 was detected not only around the periphery of PB-I but also on mesh-like structures that were connected to PB-I (Fig. 5j, l). These mesh-like structures were likely to be ER lumen because the staining pattern of an ER marker, obtained using an antibody against calnexin (CNX; Fig. 5m, o, p, r), was very similar to the RM1 staining pattern observed in OsHrd3 KD seeds. It should be noted that CNX was seldom detected around PB-I in WT seeds (Fig. 5m, o), whereas CNX was observed around PB-I in OsHrd3 KD seeds (Fig. 5p, r). These results demonstrate the aberrant distribution of RM1 and the deformation of PB-I in OsHrd3 KD seeds.
OsHrd3 is required for ubiquitination of RM1
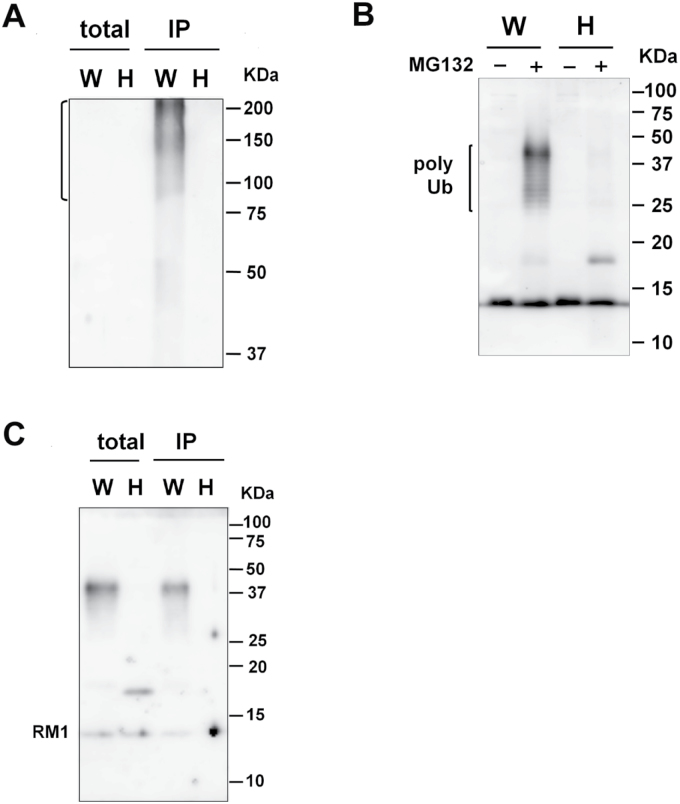
The results reveal that RM1 formed aberrant aggregations in OsHrd3 KD seeds (Fig. 4). However, other cysteine-rich prolamins [such as 10kDa prolamin, 13kDa prolamin (RM9), and 16kDa prolamin] did not form such aberrant aggregations in OsHrd3 KD seeds (Fig. 4). Since the polyubiquitination of unfolded proteins was reduced in OsHrd3 KD seeds (Figs 2B, 4), RM1 is the most plausible candidate for a protein that is mislocated into the cytoplasm as an unfolded/misfolded protein and ubiquitinated by the Hrd1 ubiquitin ligase complex containing OsHrd3. To explore this possibility, the polyubiquitination of RM1 was examined (Fig. 6). When the dehulled seeds (14 DAF) were treated with MG132 and then with a deubiquitinase inhibitor, PR-619 (Seiberlich et al., 2012), RM1 with a higher molecular weight (ranging from 30kDa to 50kDa) was detected in MG132-treated samples from WT seeds but not from OsHrd3 KD seeds (Fig. 6A). To confirm whether the higher molecular weight RM1 was ubiquitinated, an immunoprecipitation experiment was carried out using anti-ubiquitinated proteins. RM1 is usually deposited in insoluble PB-I. To carry out immunoprecipitation experiments, it was important first to examine whether RM1 could be solubilized in extraction buffer. Some RM1 was detected in the soluble fraction (S3) from the WT, developing seeds (Supplementary Fig. S3A at JXB online). Furthermore, RM1 was detected in the microsomal fraction, which could be solubilized by 1% Triton X-100 (Supplementary Fig. S3B). Therefore, seed proteins were extracted with buffer containing Triton X-100. Total proteins were extracted from the MG132-treated seeds with a buffer containing 0.5% Triton X-100. Consistent with Fig. 2B, the levels of polyubiquitinated proteins in WT seeds were higher than those in OsHrd3 KD seeds (Fig. 6B). RM1 was detected in total extracts before immunoprecipitation, indicating that RM1 from both WT and OsHrd3 KD seeds was extracted with buffer containing 0.5% Triton X-100. As shown in Fig. 6C, the higher molecular weight RM1 was immunoprecipitated by the anti-ubiquitinaed protein only from the WT seeds but not from OsHrd3 KD seeds (Fig. 6C). Mono RM1 that had not been modified by ubiquitin was also found only in WT immunoprecipitated samples. Since mature RM1 does not have lysine residues, which are authentic acceptor sites of ubiquitin, other residues such as cysteine and serine are likely to serve as ubiquitin acceptor sites. The mono RM1 observed in the WT may have been derived from polyubiquitinated RM1 by partial cleavage of the disulphide bond between cysteine and ubiquitin during denaturation under reducing conditions. Thus, these results demonstrate that OsHrd3 is involved in the ubiquitination of RM1 in rice endosperm.
Fig. 6.
OsHrd3 is required for polyubiquitination of RM1. Seeds (14 DAF) from wild-type (WT) and OsHrd3 KD plants were dehulled and treated with 100 μM MG132 for 24h, and then with 20 μM PR-619 for 1h. (A) Total proteins were extracted with SDS–urea buffer supplemented with 2-mercaptoethanol and separated by SDS–PAGE, followed by immunoblot analyses using antibodies against RM1. Total proteins were extracted from the seeds and then immunoprecipitated with antibody against ubiquitin–protein conjugates. Total proteins (2%) and immunoprecipitated proteins (IP) were separated by SDS–PAGE, followed by immunoblot analyses using antibodies against the ubiquitin–protein conjugates (B) and RM1 (C). W and H represent samples from WT and OsHrd3 KD seeds, respectively.
Discussion
Hrd3/EBS5 was first identified as a suppressor of the Arabidopsis brassinosteroid-insensitive mutant bri1-9 (Su et al., 2011). Salt stress induces the UPR in Arabidopsis, and ebs5-1/hd3a-1 also exhibits increased salt sensitivity (Liu et al., 2011). Interestingly, the levels of polyubiquitinated proteins are higher in ebs5-1/hd3a-1 than in WT plants, raising the possibility that unfolded proteins produced during salt stress are ubiquitinated by other ERAD ubiquitin ligases such as Arabidopsis homologues of Doa1/TEB4 and gp78 (Liu et al., 2011). The current study identified an SSP, RM1, as another substrate of Hrd3 in plants. In contrast to Arabidopsis plants, the level of polyubiquitinated proteins was dramatically reduced in rice OsHrd3 KD seeds (Figs 2B, 6A). Thus, the Hrd1 ubiquitin ligase complex is likely to be a major ERAD ubiquitin ligase in rice endosperm.
Unfolded proteins are produced in seeds in several different ways, and they affect the formation and accumulation of SSPs. For example, the expression of recombinant proteins such as human β-amyloid and human interleukin 7 (hIL-7) induces the UPR in transgenic rice seeds, and accumulation of SSPs is reduced in these transgenic seeds (Oono et al., 2010; Kudo et al., 2013). Moreover, genetic mutations in zeins, the major SSPs of maize, stimulate UPRs in maize endosperm (Coleman et al., 1997; Kim et al., 2004, 2006). These studies reveal the importance of a protein quality control system to remove proteins that are unfolded due to the fact that they are foreign or mutant proteins. In contrast, as demonstrated in the current study, WT proteins can become unfolded even under normal conditions, since the UPR was induced in OsHrd3 KD seeds and unfolded proteins formed aggregations in these seeds. The results reveal that protein quality control is important for maintaining healthy conditions in rice endosperm even under normal conditions.
It was found that the expression of OsHrd3 is essential for polyubiquitination of RM1. This result suggests that unfolded RM1 is mislocated into the cytosol and ubiquitinated by Hrd1 ubiquitin ligase due to the loss of OsHrd3 function. The exact mechanism of how OsHrd3 affects the ubiquitin ligase activity of OsHrd1 is unknown. One possibility is that Hrd3 affects the stability of Hrd1, as shown in yeast (Plemper et al., 1999; Gardner et al., 2000). Consistent with the yeast Hrd1p complex, Hrd1-HA was detected in rice protoplasts only when Hrd3-FLAG was co-transfected with Hrd1-HA (Supplementary Fig. S4 at JXB online). Rice Hrd1 ubiquitin ligase may be destabilized in OsHrd3 KD seeds and, consequently, the level of polyubiquitinated proteins is reduced in these seeds (Figs 2B, 6A). Another possibility is that substrate recognition is impaired in OsHrd3 KD seeds, as Hrd3/SEL1L is involved in substrate recognition in yeast and mammalian cells (Lilley and Ploegh, 2005; Carvalho et al., 2006; Denic et al., 2006).
The formation of multiprotein complexes potentially leads to aggregation because subunits are likely to have exposed hydrophobic aggregation-prone surfaces and long unstructured regions, which mediate protein–protein interactions among the subunits (Tsai et al., 2009). If the level of one subunit exceeds that of the other subunits, the excess unassembled subunit may bind to other proteins, thereby interfering with their functions. Therefore, the excess subunits must be quickly removed by a cellular protein control system. For example, the ζ subunits of the T-cell receptor complex are a limiting factor for assembly, and >70% of the other subunits remain unassembled and degraded without reaching the cell surface (Minami et al., 1987; Sussman et al., 1988). Thus, stoichiometric co-ordination of subunit abundance and protein quality control of excess subunits are essential for the proper formation of multiple subunit complexes. RM1 is localized in the middle layer of PB-I between the central core cysteine-rich 10kDa prolamin and the peripheral cysteine-depleted 13kDa prolamin (Saito et al., 2012), suggesting that RM1 can interact with multiple proteins and form aggregations through disulphide bonds between these proteins. Unfolded proteins are cross-linked by interchain disulphides in mammalian cells (Machamer and Rose, 1988). Indeed, OsHrd3 KD seeds accumulated aberrant aggregations of RM1 and induced the UPR (Figs 2, 3,d 5). Thus, it is proposed that the Hrd1 ERAD system is required for removing unassembled RM1 from the ER, and that protein quality control by the Hrd1 ERAD system provides a mechanism for co-ordinating the abundance of prolamins in PB-I. It has been proposed that the concentric layered structure of rice prolamins is formed by temporal regulation of prolamin genes during seed development (Saito et al., 2012). The present proposal provides insights into the importance of protein quality control in the formation of PB-I in rice endosperm.
In addition to RM1, rice cysteine-rich prolamins also include 10kDa prolamin, 13kDa prolamin (RM9), and 16kDa prolamin. The 10kDa prolamin initially accumulates and forms a core inside PB-I, and other cysteine-rich prolamins are subsequently synthesized to cover the core 10kDa prolamin (Saito et al., 2012). This differential expression of 10kDa and other prolamins enables free cysteine residues of 10kDa prolamin to be masked by other cysteine-rich prolamins through proper disulphide bond formation. Consequently, the risk of formation of aberrant S–S bonds between the 10kDa prolamin and other non-relevant proteins may be reduced. Thus, it is speculated that 10kDa prolamin levels are not necessarily regulated by the Hrd1 ubiquitin ligase complex. In contrast to 10kDa prolamin, RM9 and 16kDa prolamin may have similar properties to RM1 and may therefore be ubiquitinated by the Hrd1 ubiquitin ligase complex if these prolamins overaccumulate in rice endosperm. However, the possibility cannot be ruled out that RM9 and 16kDa prolamin are ubiquitinated by other ubiquitin ligases. This possibility was examined; however, it was difficult to analyse the ubiquitination of RM9 and 16kDa prolamin because RM9 or 16kDa prolamin could not be solubilizes, even with SDS (Fig. 4). Developing methods to analyse the ubiquitination of insoluble proteins may help shed light on the protein quality control system in rice endosperm.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Subcellular localization and membrane topology of OsHrd3 in rice protoplasts.
Figure S2. OsHrd3 KD seeds show unfolded protein responses.
Figure S3. Fractionation of RM1 from maturing seeds.
Figure S4. OsHrd3 is necessary for the accumulation of OsHrd1.
Table S1. A list of primers used in this study
Acknowledgements
We thank Ms Y. Ikemoto, K. Miyashita, M. Utsuno, and Y. Yajima for technical assistance. This work was supported by Grants-in-Aid for Scientific Research to FT (# 25292220) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Glossary
Abbreviations:
- BiP
immunoglobulin binding protein
- Co-IP
co-immunoprecipitation
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- Hsp70
heat shock protein
- IL
interleukin
- PB
protein body.
References
- Braun S, Matuschewski K, Rape M, Thoms S, Jentsch S. 2002. Role of the ubiquitin-selective CDC48 (UFD1/NPL4) chaperone (segregase) in ERAD of OLE1 and other substrates. EMBO Journal 21, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL. 2012. Cleaning up: ER-associated degradation to the rescue. Cell 151, 1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho P, Goder V, Rapoport TA. 2006. Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell 126, 361–373. [DOI] [PubMed] [Google Scholar]
- Coleman CE, Clore AM, Ranch JP, Higgins R, Lopes MA, Larkins BA. 1997. Expression of a mutant alpha-zein creates the floury2 phenotype in transgenic maize. Proceedings of the National Academy of Sciences, USA 94, 7094–7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F, Liu L, Zhao Q, Zhang Z, Li Q, Lin B, Wu Y, Tang S, Xie Q. 2012. Arabidopsis ubiquitin conjugase UBC32 is an ERAD component that functions in brassinosteroid-mediated salt stress tolerance. The Plant Cell 24, 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denic V, Quan EM, Weissman JS. 2006. A luminal surveillance complex that selects misfolded glycoproteins for ER-associated degradation. Cell 126, 349–359. [DOI] [PubMed] [Google Scholar]
- Gardner RG, Swarbrick GM, Bays NW, Cronin SR, Wilhovsky S, Seelig L, Kim LC, Hampton RY. 2000. Endoplasmic reticulum degradation requires lumen to cytosol signaling: transmembrane control of Hrd1p by Hrd3p. Journal of Cell Biology 151, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenson A, Gierasch LM. 2011. Protein folding in the cell: challenges and progress. Current Opinion in Structural Biology 21, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F. 1999. Iron fortification of rice seed by the soybean ferritin gene. Nature Biotechnology 17, 282–286. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. 2011. Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Wakasa Y, Takahashi H, Kawakatsu T, Takaiwa F. 2012. Signal transduction by IRE1-mediated splicing of bZIP50 and other stress sensors in the endoplasmic reticulum stress response of rice. The Plant Journal 69, 946–956. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. 1998. The ubiquitin system. Annual Review of Biochemistry 67, 425–479. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. 1996. Ubiquitin-dependent protein degradation. Annual Review of Genetics 30, 405–439. [DOI] [PubMed] [Google Scholar]
- Hong Z, Jin H, Tzfira T, Li J. 2008. Multiple mechanism-mediated retention of a defective brassinosteroid receptor in the endoplasmic reticulum of Arabidopsis. The Plant Cell 20, 3418–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosch E, Taxis C, Volkwein C, Bordallo J, Finley D, Wolf DH, Sommer T. 2002. Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nature Cell Biology 4, 134–139. [DOI] [PubMed] [Google Scholar]
- Jin H, Hong Z, Su W, Li J. 2008. A plant-specific calreticulin is a key retention factor for a defective brassinosteroid receptor in the endoplasmic reticulum. Proceedings of the National Academy of Sciences, USA 106, 13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Yan Z, Nam KH, Li J. 2007. Allele-specific suppression of a defective brassinosteroid receptor reveals a physiological role of UGGT in ER quality control. Molecular Cell 26, 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakatsu T, Yamamoto MP, Touno SM, Yasuda H, Takaiwa F. 2009. Compensation and interaction between RISBZ1 and RPBF during grain filling in rice. The Plant Journal 59, 908–920. [DOI] [PubMed] [Google Scholar]
- Kim CS, Gibbon BC, Gillikin JW, Larkins BA, Boston RS, Jung R. 2006. The maize Mucronate mutation is a deletion in the 16-kDa gamma-zein gene that induces the unfolded protein response. The Plant Journal 48, 440–451. [DOI] [PubMed] [Google Scholar]
- Kim CS, Hunter BG, Kraft J, Boston RS, Yans S, Jung R, Larkins BA. 2004. A defective signal peptide in a 19-kD alpha-zein protein causes the unfolded protein response and an opaque endosperm phenotype in the maize De*-B30 mutant. Plant Physiology 134, 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan HB, Franceschi VR, Okita TW. 1986. Immunochemical studies on the role of the Golgi complex in protein-body formation in rice seeds. Planta 169, 471–480. [DOI] [PubMed] [Google Scholar]
- Kudo K, Ohta M, Yang L, Wakasa Y, Takahashi S, Takaiwa F. 2013. ER stress response induced by the production of human IL-7 in rice endosperm cells. Plant Molecular Biology 81, 461–475. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Kimizu M, Mikami C. 2010. A simple set of plasmids for the production of transgenic plants. Bioscience Biotechnology Biochemistry 74, 2348–2351. [DOI] [PubMed] [Google Scholar]
- Lilley BN, Ploegh HL. 2005. Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane. Proceedings of the National Academy of Sciences, USA 102, 14296–14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cui F, Li Q, Yin B, Zhang H, Lin B, Wu Y, Xia R, Tang S, Xie Q. 2011. The endoplasmic reticulum-associated degradation is necessary for plant salt tolerance. Cell Research 21, 957–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer CE, Rose JK. 1988. Vesicular stomatitis virus G proteins with altered glycosylation sites display temperature-sensitive intracellular transport and are subject to aberrant intermolecular disulfide bonding. Journal of Biological Chemistry 263, 5955–5960. [PubMed] [Google Scholar]
- Marshall RS, Jolliffe NA, Ceriotti A, Snowden CJ, Lord JM, Frigerio L, Roberts LM. 2008. The role of CDC48 in the retro-translocation of non-ubiquitinated toxin substrates in plant cells. Journal of Biological Chemistry 283, 15869–15877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y, Weissman AM, Samelson LE, Klausner RD. 1987. Building a multichain receptor: synthesis, degradation, and assembly of the T-cell antigen receptor. Proceedings of the National Academy of Sciences, USA 84, 2688–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Piffanelli P, Devoto A, Miklis M, Elliott C, Ortmann B, Schulze-Lefert P, Panstruga R. 2005. Conserved ERAD-like quality control of a plant polytopic membrane protein. The Plant Cell 17, 149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Kumamaru T, Satoh H, Iwata N, Omura T, Kasai Z, Tanaka K. 1987. Purification of protein body-I of rice seed and its polypeptide composition. Plant and Cell Physiology 28, 1517–1527. [Google Scholar]
- Ohta M, Wakasa Y, Takahashi H, Hayashi S, Kudo K, Takaiwa F. 2013. Analysis of rice ER-resident J-proteins reveals diversity and functional differentiation of the ER-resident Hsp70 system in plants. Journal of Experimental Botany 64, 5429–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oono Y, Wakasa Y, Hirose S, Yang L, Sakuta C, Takaiwa F. 2010. Analysis of ER stress in developing rice endosperm accumulating beta-amyloid peptide. Plant Biotechnology Journal 8, 691–718. [DOI] [PubMed] [Google Scholar]
- Plemper RK, Bordallo J, Deak PM, Taxis C, Hitt R, Wolf DH. 1999. Genetic interactions of Hrd3p and Der3p/Hrd1p with Sec61p suggest a retro-translocation complex mediating protein transport for ER degradation. Journal of Cell Science 112, 4123–4134. [DOI] [PubMed] [Google Scholar]
- Saito T, Saito Y, Shigemitsu T, et al. 2012. Formation mechanism of the internal structure of type I protein bodies in rice endosperm: relationship between the localization of prolamin species and the expression of individual genes. The Plant Journal 70, 1043–1055. [DOI] [PubMed] [Google Scholar]
- Seiberlich V, Goldbaum O, Zhukareva V, Richter-Landsberg C. 2012. The small molecule inhibitor PR-619 of deubiquitinating enzymes affects the microtubule network and causes protein aggregate formation in neural cells: implications for neurodegenerative diseases. Biochimica et Biophysica Acta 1823, 2057–2068. [DOI] [PubMed] [Google Scholar]
- Su W, Liu Y, Xia Y, Hong Z, Li J. 2011. Conserved endoplasmic reticulum-associated degradation system to eliminate mutated receptor-like kinases in Arabidopsis. Proceedings of the National Academy of Sciences, USA 108, 870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman JJ, Bonifacino JS, Lippincott-Schwartz J, Weissman AM, Saito T, Klausner RD, Ashwell JD. 1988. Failure to synthesize the T cell CD3-zeta chain: structure and function of a partial T cell receptor complex. Cell 52, 85–95. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Rai M, Kitagawa T, Morita S, Masumura T, Tanaka K. 2004. Differential localization of tonoplast intrinsic proteins on the membrane of protein body Type II and aleurone grain in rice seeds. Bioscience Biotechnology Biochemistry 68, 1728–1736. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Saito Y, Kitagawa T, Morita S, Masumura T, Tanaka K. 2005. A novel vesicle derived directly from endoplasmic reticulum is involved in the transport of vacuolar storage proteins in rice endosperm. Plant and Cell Physiology 46, 245–249. [DOI] [PubMed] [Google Scholar]
- Takaiwa F, Kikuchi S, Oono K. 1987. A rice glutelin gene family—a major type of glutelin mRNAs can be divided into two classes. Molecular Genetics and Genomics 208, 15–22. [Google Scholar]
- Tanaka K, Sugimoto T, Ogawa M, Kasai Z. 1980. Isolation and characterization of two types of protein bodies in rice endosperm. Agricultural and Biological Chemistry 44, 1633–1639. [Google Scholar]
- Tsai CJ, Ma B, Nussinov R. 2009. Protein–protein interaction networks: how can a hub protein bind so many different partners? Trends in Biochemical Sciences 34, 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasa Y, Yasuda H, Oono Y, Kawakatsu T, Hirose S, Takahashi H, Hayashi S, Yang L, Takaiwa F. 2011. Expression of ER quality control-related genes in response to changes in BiP1 levels in developing rice endosperm. The Plant Journal 65, 675–689. [DOI] [PubMed] [Google Scholar]
- Wakasa Y, Hayashi S, Takaiwa F. 2012. Expression of OsBiP4 and OsBiP5 is highly correlated with the endoplasmic reticulum stress response in rice. Planta 236, 1519–1527. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Hayashi Y, Jomori T, Takaiwa F. 2006. The correlation between expression and localization of a foreign gene product in rice endosperm. Plant and Cell Physiology 247, 756–763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.