Highlight
The elp2 mutant affected the expression of key transcription factors and CYCB1 through either acetylation or methylation, and also altered auxin polar transport and reduced auxin content in root.
Key words: Auxin ELP2, epigenetics, root.
Abstract
The elongator complex subunit 2 (ELP2) protein, one subunit of an evolutionarily conserved histone acetyltransferase complex, has been shown to participate in leaf patterning, plant immune and abiotic stress responses in Arabidopsis thaliana. Here, its role in root development was explored. Compared to the wild type, the elp2 mutant exhibited an accelerated differentiation of its root stem cells and cell division was more active in its quiescent centre (QC). The key transcription factors responsible for maintaining root stem cell and QC identity, such as AP2 transcription factors PLT1 (PLETHORA1) and PLT2 (PLETHORA2), GRAS transcription factors such as SCR (SCARECROW) and SHR (SHORT ROOT) and WUSCHEL-RELATED HOMEOBOX5 transcription factor WOX5, were all strongly down-regulated in the mutant. On the other hand, expression of the G2/M transition activator CYCB1 was substantially induced in elp2. The auxin efflux transporters PIN1 and PIN2 showed decreased protein levels and PIN1 also displayed mild polarity alterations in elp2, which resulted in a reduced auxin content in the root tip. Either the acetylation or methylation level of each of these genes differed between the mutant and the wild type, suggesting that the ELP2 regulation of root development involves the epigenetic modification of a range of transcription factors and other developmental regulators.
Introduction
The maintenance of a functional root system makes an important contribution to a plant’s capacity to adapt to instability in its growing environment. At the root apical meristem, root stem cells deliver the cells that differentiate to form the various tissue types found in the root (Dolan et al., 1993). Stem cell identity within the root meristem is maintained by signals generated from cells in the quiescent centre (QC) (Vernoux and Benfey, 2005). The mitotically less active QC, together with its surrounding stem cells, constitutes the ‘root stem cell niche’, a structure that is essential for the maintenance of root growth (Sabatini et al., 2003). The specification of cell identity within the root stem cell niche is determined by the activity of a number of transcription factors: these include members of both the AP2 (PLTs) and GRAS (SCR and SHR) transcription factor families (Helariutta et al., 2000; Sabatini et al., 2003; Aida et al., 2004; Galinha et al., 2007). PLT proteins are not only involved in QC specification during embryogenesis but also have a more general role in the maintenance of root stem cell niche identity. In loss-of-function mutants, the embryonic root does not form, while the expression of PLT in the shoot is sufficient to specify root identity in shoot (Aida et al., 2004; Galinha et al., 2007). Both SHR and SCR affect radial patterning in the root, and are also required for QC specification and root stem cell niche maintenance (Helariutta et al., 2000; Sabatini et al., 2003). The homeodomain transcription factor WOX5 is specifically expressed in the QC in the wild-type (WT) plant. Its loss-of-function results in the formation of enlarged QC cells and promoted differentiation of the distal stem cells, but has no observable effect on either root growth or the size of the more proximal meristem cells; its over-expression blocks the differentiation of distal stem cells, thereby inducing the formation of multiple layers of distal stem cells. The primary function of WOX5 appears to be to maintain distal stem cell identity (Sarkar et al., 2007).
Root stem cell niche identity is also regulated by auxin and cytokinin (Dello Ioio et al., 2007; Ding and Friml, 2010). Auxin initiates, organizes and maintains the root stem cell niche (Benjamins and Scheres, 2008), while the effect of cytokinin is to modulate the auxin pathway or polar auxin transport (Dello Ioio et al., 2008; Ruzicka et al., 2009). Auxin and cytokinin act antagonistically to define root apical meristem size by promoting, respectively, cell division and differentiation (Dello Ioio et al., 2007). The maintenance of the root stem cell niche depends on the establishment of an auxin concentration gradient, which peaks at the stem cell niche. The stability of this gradient relies on both PIN-mediated distribution and its local synthesis (Blilou et al., 2005; Grieneisen et al., 2007; Tian et al., 2013, 2014).
The eukaryotic elongator complex consists of an ELP1, ELP2 and ELP3 core and an accessory ELP4, ELP5 and ELP6 subcomplex (Wittschieben et al., 1999; Kim et al., 2002; Nelissen et al., 2010; Versees et al., 2010). It participates in acetylation, methylation/demethylation, exocytosis and tRNA modification (Kim et al., 2002; Creppe et al., 2009; Chen et al., 2011; Defraia et al., 2013; Wang et al., 2013). In Arabidopsis thaliana, it mediates part of the response to biotic and abiotic stress, as well as being involved in the determination of leaf patterning (Nelissen et al., 2005, 2010; Zhou et al., 2009; Xu et al., 2011; Defraia et al., 2013). Previous studies also showed that the elp1, elp3, elp4 mutants have defected root growth, however, the detailed mechanism was not well studied (Nelissen et al., 2005). Here, we mapped elp2 mutant according to its root stem cell defective phenotype and thoroughly investigated the role of ELP2 on root development.
Materials and methods
Plant materials and growth conditions
The transgenic A. thaliana lines used were WOX5:GFP (Sarkar et al., 2007); CYCB1;1:GUS (Colon-Carmona et al., 1999); DR5:GUS (Ulmasov et al., 1997); DR5rev:GFP (Benkova et al., 2003); PIN1:PIN1-GFP (Benkova et al., 2003); PIN2:PIN2-GFP (Blilou et al., 2005); PLT1 pro :CFP, PLT1 pro :PLT1-YFP, PLT2 pro :CFP, and PLT2 pro :PLT2-YFP (Kornet and Scheres, 2009); QC25 (Sabatini et al., 2003); SCR pro :SCR-GFP (Di Laurenzio et al., 1996); SHR pro :SHR-GFP (Nakajima et al., 2001).The mutant elp2, and elp6 are from an ethyl methanesulfonate mutagenized populations, elp1 (SALK_004690) and elp4 (SALK_079193) are from the Arabidopsis Stock Center, which are all in the Columbia (Col-0) background (Nelissen et al., 2005; Zhou et al., 2009;). Seeds were surface-sterilized by exposure to chlorine gas, sown on solidified Murashige and Skoog (1962) medium (MS medium), held for 2 d at 4ºC, then raised under a 16h photoperiod at 19ºC for a further 5 d.
TAIL-PCR
DNA was extracted from leaves of WT ecotype Col-0 and the elp2 mutant using a CTAB-based method. The primary PCR was primed with the T-DNA specific LBa1 and a degenerate AD primer (sequences given in Supplementary Table S1). The secondary PCR was based on LBb1.3 and an AD primer, using a diluted aliquot of the primary PCR as the template. The tertiary PCR was based on LBb1 and an AD primer, using a diluted aliquot of the secondary PCR as the template. The amplicons were separated by agarose gel electrophoresis and the dsr1 specific band compared to the WT (Col) was sequenced to find the T-DNA boundary sequence.
GUS, EdU and lugol staining
Staining of seedling roots for β-glucuronidase (GUS) activity was carried out by incubation at 37°C in 0.05M NaPO4 buffer (pH 7.0), 5mM K3Fe(CN)6, 5mM K4Fe(CN)6 and 2mM X-glucuronide. Once the colour had developed, the material was passed through an ethanol series (70%, 50% and 20%) before mounting in 70% chloral hydrate plus 10% glycerol. EdU staining was performed following the protocol supplied with the Click-iT® EdU Alexa Fluor® 555 Imaging kit (Invitrogen). Detection of starch granules in the root tip followed staining in Lugol’s solution for 1–2min, after which the material was mounted in chloral hydrate as above.
RNA analysis
Seedlings were grown on MS medium for 5 d, after which the distal 2mm of the roots were harvested for RNA extraction. Total RNA was isolated using a RNeasy® Mini Kit (Qiagen) and the first strand of cDNA was synthesized from a 2 µg aliquot using a Transcriptor First Strand cDNA Synthesis kit (Roche) following the manufacturer’s protocol. Quantitative real-time PCRs (qRT-PCRs) were based on the CFX ConnectTM Real-Time System (Bio-Rad) and FastStart Universal SYBR Green Master mix (Roche). Three biological replicates were included, each of which was represented by three technical replicates. AtACTIN2 was used as the reference sequence.
Chromatin immunoprecipitation (ChIP) assay
ChIP was used to quantify the acetylation level of the set of selected genes. The assay was based on an ~3g sample of two-week-old seedlings, following the Wang et al., (2013) protocol. After in vivo cross-linking and tissue lysis, DNA was sheared by sonication and evaluated by agarose gel electrophoresis. The extracted nucleosomes were precleared using 40 µl protein A agarose beads (Invitrogen), after which was added 2–3 µg Ac-Histone H3 (Lys-9/14) antibody (sc-8655-R; Santa Cruz Biotechnology) before incubation at 4ºC overnight. After de-cross-linking, DNA was extracted and precipitated using triple volumes of ethanol, NaAc, and glycogen. The resulting DNA was used as the template for a qRT-PCR, based on primers detailed in Supplementary Table S1. The acetylation level in each sample was normalized to both input DNA and AtACTIN2 as described elsewhere (Mosher et al., 2006). The Chromatin immunoprecipitation (ChIP) assay was done three times independently. The data represent mean values with their associated SD (n=3), P <0.05.
Bisulfite sequencing
Genomic DNA was extracted with a CTAB protocol from ~1g seedlings harvested from plants raised for two weeks on MS medium. About 2 µg DNA was treated with an EpiTect® Bisulfite kit (Qiagen). Strand-specific and bisulfite-specific primers (see Supplementary Table S1) were designed using MethPrimer software (http://www.urogene.org/methprimer/). The amplicons were introduced into a pEASY-T1 simple vector (Tiangen) for sequencing, and the resulting sequences were analysed using DNAMAN software. Each sample was represented by three biological replicates.
Immunolocalization assay
PIN1 and PIN2 were immunolocalized as described elsewhere (Dai et al., 2012).
Phenotypic analysis, microscopy, statistics
Seedlings grown on MS were scanned using EPSON PERFECTION V700 PHOTO, and root length was measured by Image J. Root meristems were analysed on seedlings mounted in HCG solution (Chloral hydrate:water:glycerol=8:3:1). Root meristem size was assessed as the cell number from the QC to the first elongating cell in the cortex cell file. Root micrographs were photographed using OLYMPUS BX53. Confocal imaging was obtained using an LSM-700 laser-scanning confocal microscope (Zeiss). Five-day-old seedling root tips were stained in propidium iodide.
Data presented are mean values of at least three biological repeats with SD. The statistical significance was analysed by Student’s t-test analysis.
Results
The elp2 mutant shows defective root stem cell niche maintenance
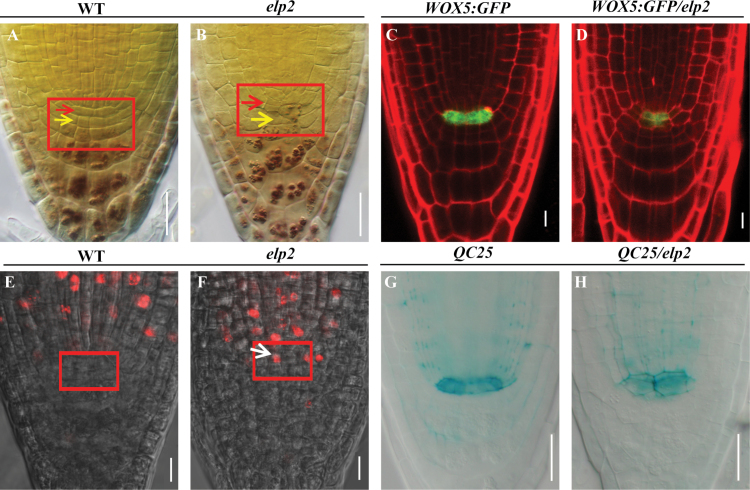
A search for T-DNA mutants displaying a defective root stem cell niche maintenance phenotype identified drs1, a mutant that exhibited enhanced root distal stem cell differentiation and increased mitotic activity in its QC (Fig. 1). When five-day-old seedlings were exposed for 24h to EdU [a thymidine analogue used to mark S-phase progression (Vanstraelen et al., 2009)], a stronger level of fluorescence was observed in the nuclei of mitotically active cells in the mutant than in WT, due to the coupling of EdU with Aexa Fluor 555. This assay indicated that mitotic activity was enhanced in elp2 QC cells (Fig. 1E, F). The QC specific transcription factor WOX5 was also strongly down-regulated (Fig. 1C, D), as was the signal produced by the QC marker QC25 (Fig. 1G, H).
Fig. 1.
The elp2 mutant is defective with respect to root stem cell niche maintenance. Lugol stained (A) WT and (B) elp2 roots. Cell division in the mutant QC is enhanced (red arrowheads), while its distal stem cells are deficient in starch (yellow arrowheads). The root stem cell niche is shown boxed. Confocal micrographs of (C) WT and (D) elp2 plants expressing the transgene WOX5:GFP; the level of expression in the mutant is lower than in WT. Confocal micrographs illustrating the incorporation of EdU into (E) WT and (F) elp2 QC cells. The fluorescent signals show that the mutant QC is in a state of active division (white arrowheads). The QC is shown boxed. QC25 is expressed at a higher level in (G) the WT than in (H) the elp2 mutant. Bars: 20 µm (A, B, G, H); 10 µm (C–F). (This figure is available in colour at JXB online.)
A TAIL-PCR analysis identified that in the mutant, the ELP2 locus (At1g49540) had been interrupted by the insertion of a T-DNA element within the second intron (Supplementary Figs S1, S2). The hybrid between drs1 and elp2 [the latter differs from WT by a G to A transition at the acceptor splice site of the fifth intron (Zhou et al., 2009)], just like the parental mutant lines, displayed both enhanced root distal stem cell differentiation and increased QC division, confirming that the defective root phenotype expressed by drs1 was caused by a lesion in ELP2. We renamed the elp2 mutant from EMS populations (Zhou et al., 2009) as the elp2-6, and renamed the drs1 as the elp2-7 following the elp2-2, elp2-3, elp2-4, elp2-5 indentified previously (Nelissen et al., 2005; DeFraia et al., 2010). The elp2-7 was used for most of the analysis and termed elp2 afterwards in this paper. Mutations affecting other subunits of the elongation complex exhibited similar defective root stem cell niche maintenance (Supplementary Fig. S3).
The elp2 mutant’s root meristem is reduced in size
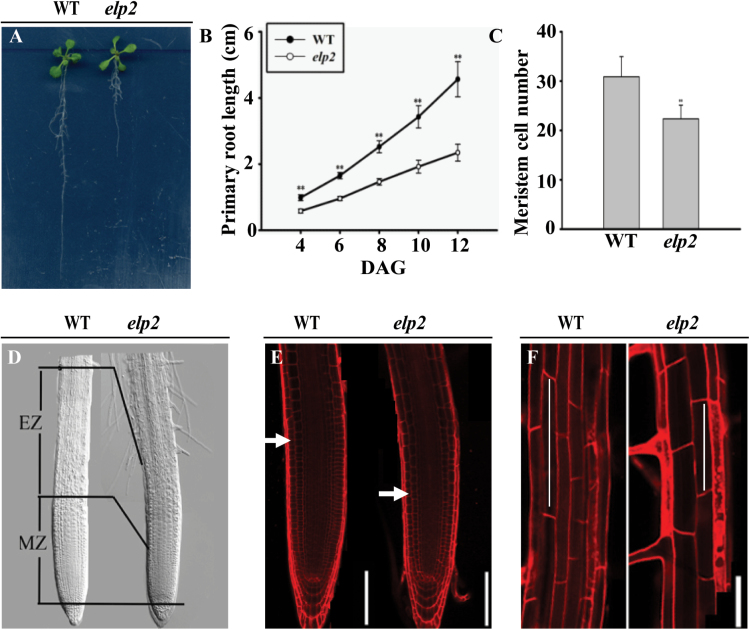
In addition to the root stem cell niche identity defect, the elp2 mutant also produced shorter roots than the WT seedlings (Fig. 2A, B, Supplementary Fig. S4). This shortening was a result of smaller elongation (EZ) and meristem (MZ) zones (Fig. 2D, E). Cell number in the MZ and cell length in the EZ were both reduced compared to the WT (Fig. 2C, F), suggesting that ELP2 affects both cell proliferation and cell elongation.
Fig. 2.
The foreshortening of the roots produced by the elp2 mutant reflects a reduced level of cell proliferation and cell elongation. (A) Roots of 12-day-old seedlings of WT and elp2. (B) Primary root growth of WT and elp2 seedlings 4 d post germination. The data represent the mean and SD (n=40) derived from at least three independent experiments. (C) Variation in root meristem cell number of five-day-old WT and elp2 seedlings. Cell numbers counted from the QC to the TZ. The data represent the mean and SD (n=20) derived from at least three independent experiments. (D) Representative five-day-old WT and elp2 seedlings illustrating that the latter develop a reduced MZ and EZ. (E) Five-day-old WT and elp2 seedlings. The cortex TZ is indicated by white arrowheads. (F) Epidermal cell length of the DZ in six-day-old WT and elp2 seedlings. Bars, 50µm (E, F). **, P<0.001 (This figure is available in colour at JXB online.)
The down-regulation of transcription factors in the elp2 mutant
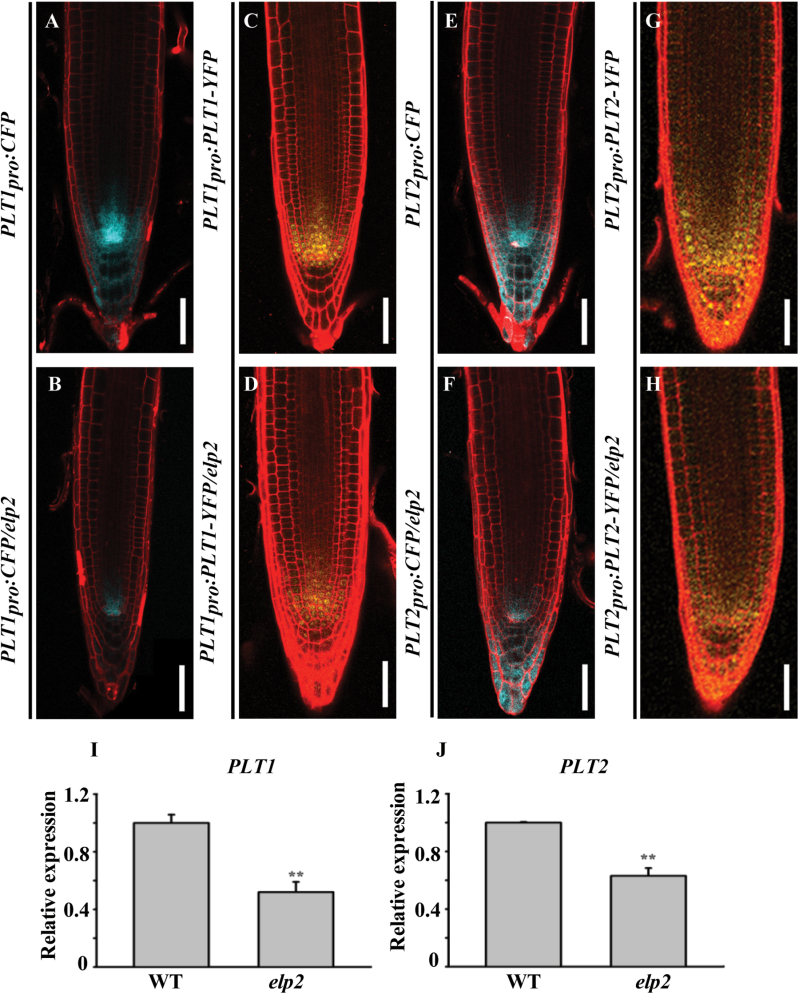
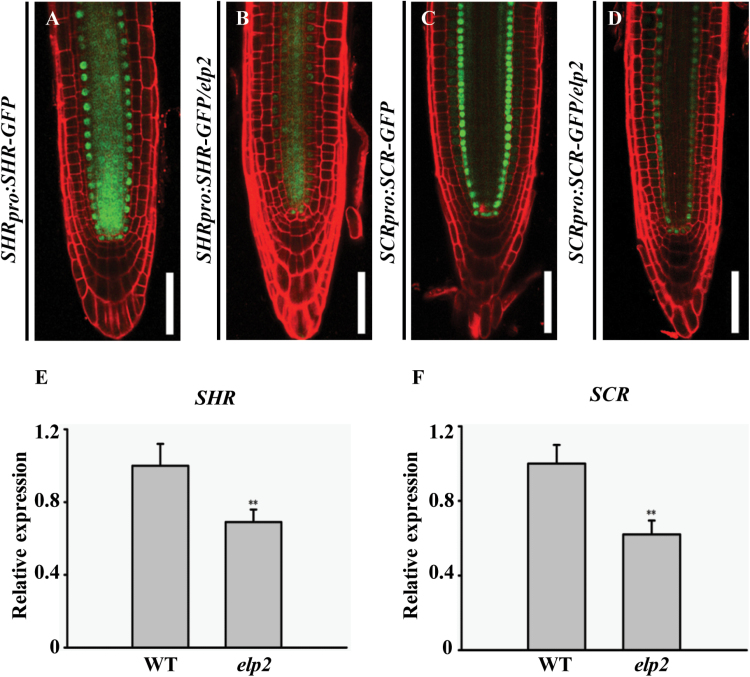
To determine whether the effects of ELP2 on root stem cell niche maintenance is dependent on the well characterized root stem cell niche-defining transcription factors, we examined the expression of AP2 transcription factors such as PLT1 and PLT2 and GRAS transcription factors such as SCR and SHR (Helariutta et al., 2000; Sabatini et al., 2003; Aida et al., 2004; Galinha et al., 2007) in elp2. The expression of both PLT1 pro :PLT1-YFP (Fig. 3C, D) and PLT2 pro:PLT2-YFP (Fig. 3G, H) was strongly reduced in elp2 compared to the levels achieved in the WT, and the PLT1 pro :CFP and PLT2 pro:CFP behaved similarly (Fig. 3A, B, E, F). Consistently, a qRT-PCR assay showed that the expression level of PLT1 and PLT2 was lower in the elp2 mutant than in the WT (Fig. 3I, J). Thus the evidence was that ELP2 affected the transcription of both PLT1 and PLT2. The expression of SHR and SCR was investigated by comparing the expression of both SCR pro :SCR-GFP and SHR pro :SHR-GFP in both elp2 and WT. Both transgenes were strongly down-regulated in the mutant (Fig. 4A–D), a result that was confirmed by a qRT-PCR assay (Fig. 4E, F).
Fig. 3.
PLT1 and PLT2 expression is reduced in the elp2 mutant. Expression of the transgenes (A, B) PLT1 pro :CFP, (C, D) PLT1 pro :PLT1-YFP, (E, F) PLT2 pro :CFP, (G, H) PLT2 pro :PLT2-YFP. qRT-PCR assay of the transcription of (I) PLT1 and (J) PLT2. The data represent mean values with their associated SD (n=3); **, P<0.001. Bars, 50 µm (A–H). (This figure is available in colour at JXB online.)
Fig. 4.
SHR and SCR expression is reduced in the elp2 mutant. Expression of the transgenes (A, B) SHR pro :SHR-GFP, (C, D) SCR pro :SCR-GFP. qRT-PCR assay of the transcription of (E) SHR and (F) SCR. The data represent mean values with their associated SD (n=3); **, P<0.001. Bars, 50 µm (A–D). (This figure is available in colour at JXB online.)
ELP2 is required for cell-cycle progression in the root
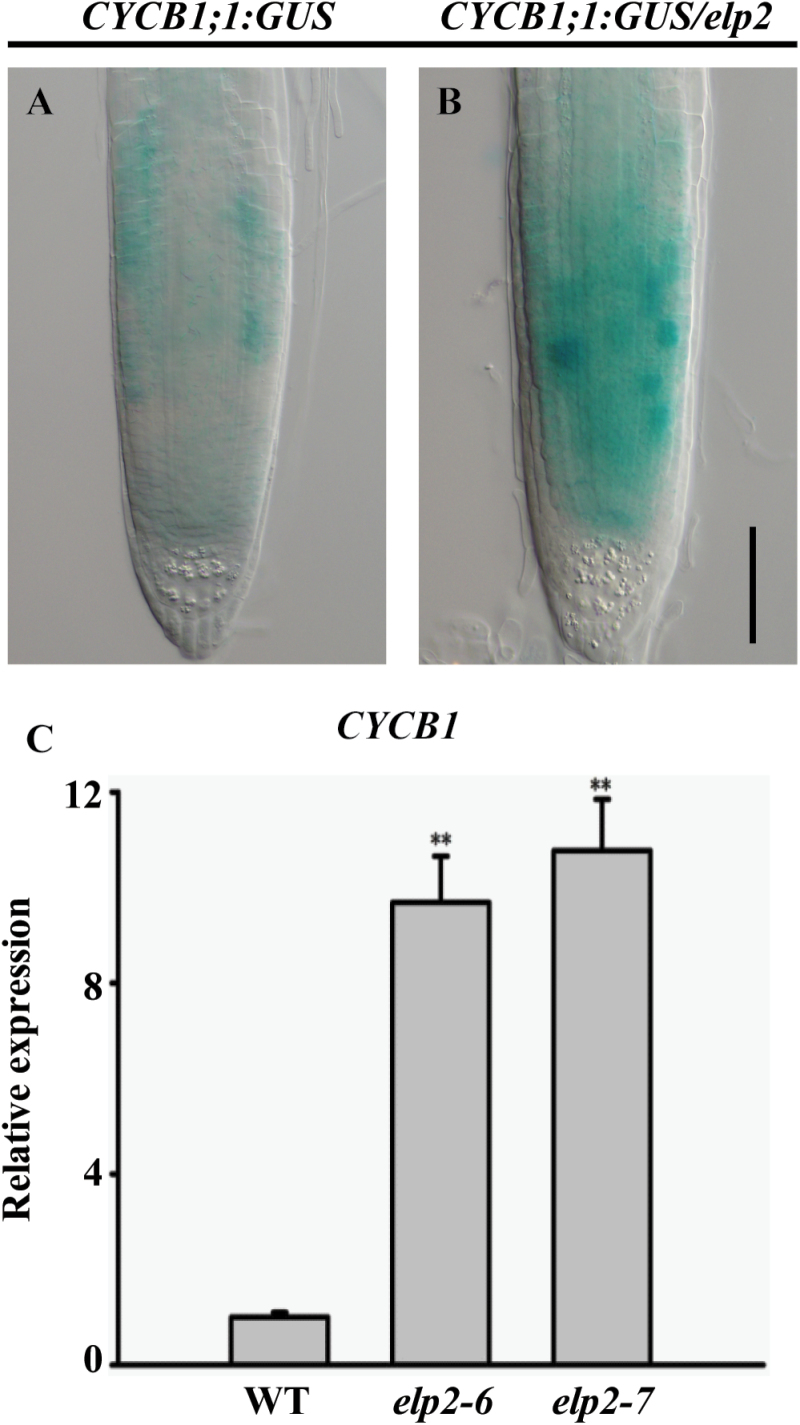
The increased QC division and reduced root meristem cell numbers in elp2 suggest that ELP2 is required for proper cell-cycle progression in the root. Cells at the G2-M phase were visualized by the transgene CYCB1;1:GUS which reflects the cell cycle activity, however timely degradation of proteins is necesssary to ensure the access of M phase (Colon-Carmona et al., 1999). In tobacco, the nondegradable Cyclin B1 results in abnormal cytokinesis and endomitosis (Weingartner et al., 2004). We observed that accumulation of the CYCB1;1:GUS signal in root meristem of elp2 was much stronger than that in the WT (Fig. 5A, B). In addition, stronger staining in the root QC of elp2 is consistent with the enhanced cell division in QC (Fig. 5A, B). Next, using qRT-PCR assays, we compared the CYCB1;1 expression level in the elp2 mutation and WT. In line with GUS staining, the elp2 mutant has high CYCB1;1 expression in the root (Fig. 5C). Therefore, our results showed that ELP2 is required for cell-cycle progression in the root.
Fig. 5.
CYCB1 expression is increased in the elp2 mutant. (A, B) Expression of the CYCB1;1:GUS transgene, (C) transcription of CYCB1 assayed by qRT-PCR. The data represent mean values with their associated SD, (n=3); **, P<0.001. Bars, 50 µm (A, B). (This figure is available in colour at JXB online.)
The elp2 mutant has a reduced auxin content in root tip
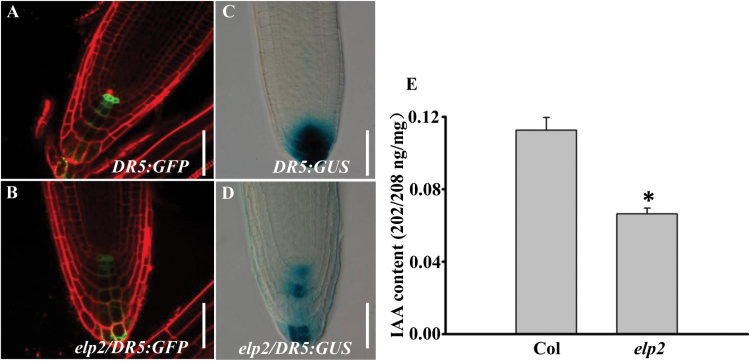
Auxin gradients (Vanneste and Friml, 2009) are central to the robust development of the primary root in Arabidopsis, and the auxin gradient in the root tip could be instructive for the patterning of root distal stem cell (DSC) niches (Tian et al., 2013, 2014). To substantiate if the root defective phenotypes in elp2 are resulting from the changed auxin signalling in root tips, DR5rev:GFP and DR5:GUS synthetic auxin response reporters (Friml et al., 2003) were used to assay auxin signalling. The signal from both reporters was markedly lower in the mutant than in the WT root tip (Fig. 6A–D). The GUS enzyme activity assay using the MUG as the substrate further confirmed this difference (data not shown). Furthermore, we measured the free IAA content in roots of the elp2 mutant. Consistently, the elp2 mutant has reduced IAA content in roots compared to the wild-type control (Fig. 6E).
Fig. 6.
The elp2 mutant exhibits reduced auxin contents in roots. (A, B) The mutant shows a reduced level of DR5rev:GFP expression in the root than the WT. (C, D) The mutant shows a reduced level of DR5:GUS expression in the root than the WT. (E) IAA content measurement of roots on 14-day-old seedlings of col and elp2. The data represent mean values of three independent biological repeats with their associated SD (n=3) *, P<0.05. Bars, 50 µm (A–D). (This figure is available in colour at JXB online.)
Auxin polar transport was affected in elp2
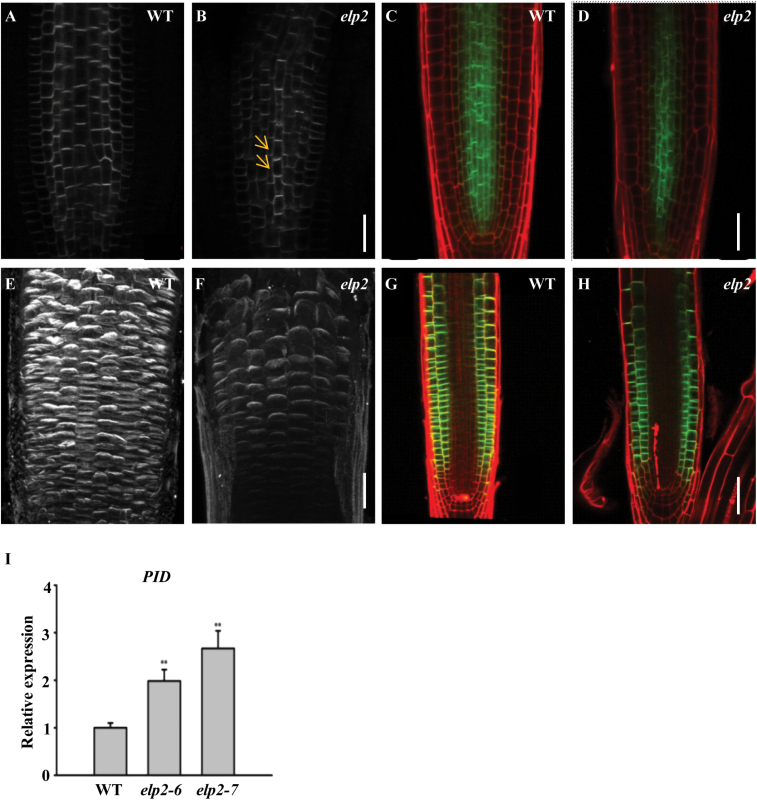
To address if the reduced auxin signalling in root tips of elp2 was a result of the affected polar auxin transport, we examined the polar localization of auxin efflux carriers such as PIN1 and PIN2 (Petrasek et al., 2006), which mediate auxin distribution in plants, including to the roots (Blilou et al., 2005; Wisniewska et al., 2006). Basal localized PIN1 in the stele and basal localized PIN2 in the cortex contribute to auxin transportation from shoot to root, thus contributing to maximum auxin formation in root tips and regulating root growth (Galweiler et al., 1998; Friml et al., 2003; Blilou et al., 2005; Wisniewska et al., 2006). However, in elp2, basal localization of PIN1 is less polar, and the increased lateral localization of PIN1 was observed from both our immunolocalization examinations with the anti-PIN1 antibody and confocal examinations with the PIN1-GFP line (Fig. 7A–D). Though apical PIN2 in the epidermis displays WT polarity in elp2 (Fig. 7E–H), both the protein expression levels of PIN1 and PIN2 were reduced in elp2 (Fig. 7C, D, G, H).
Fig. 7.
PIN1 and PIN2 localization and expression in the elp2 mutant. (A, B) PIN1 immunolocalization in five-day-old roots. (C, D) Expression and polarity of PIN1:PIN1-GFP in the WT and mutant root. (E, F) PIN2 immunolocalization in five-day old roots. (G, H) Expression and polarity of PIN2:PIN2-GFP in the WT and mutant root. (I) PID transcription assayed by qRT-PCR. The data represent mean values with their associated SD (n=3); **, P<0.001. Bars, 50 µm (A–H). (This figure is available in colour at JXB online.)
Previous studies have already demonstrated that PP2A and PINOID partially colocalize with PINs and act antagonistically on the phosphorylation state of PINs hydrophilic loop (Benjamins et al., 2001; Michniewicz et al., 2007; Huang et al., 2010). 35S:PID seedlings have basal to apical localization of PIN1 (Michniewicz et al., 2007). Thus, we examined the PINOID expression in the elp2 mutant. We found the elp2 mutant to have a consistently higher expression level of PINOID compared with the WT control(Fig. 7I).
ELP2 affects the histone acetylation levels of several key transcription factor genes
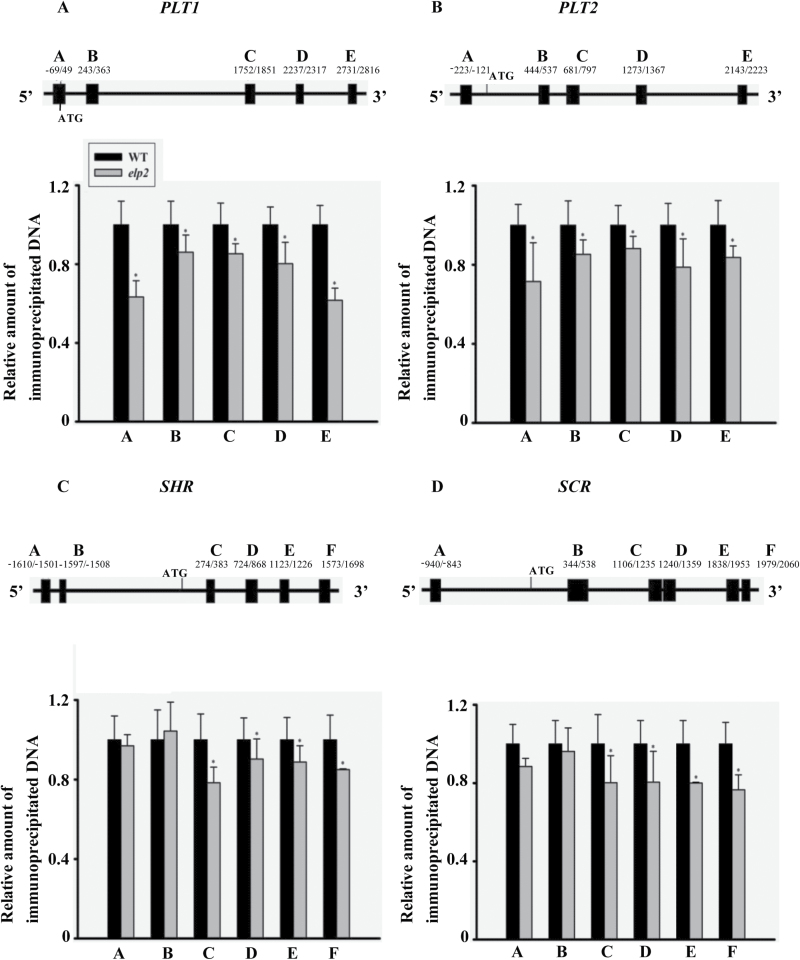
The histone acetyltransferase of ELP3 has been confirmed both in yeast and plants (Winkler et al., 2002; Nelissen et al., 2010). To address whether the reduced expression level of several key transcription factors encoding genes such as PLT1, PLT2, SCR and SHR in elp2 may be associated with the reduced acetylation levels, we performed CHIP-PCR using an antibody specific for histone H3 acetylated at Lys-9 and -14 (H3K9/14ac). CHIP-PCR was performed to verify whether the reduced expression of PLT1, PLT2, SCR and SHR in the mutant was correlated with the altered acetylation level. The assay based on primers targeting the coding region and the 5ʹ-UTR of PLT1 and PLT2 showed that acetylation in this part of the gene was markedly reduced in the mutant (Fig. 8), while the acetylation level of the internal reference gene ACTIN2 was not changed (Supplementary Fig. S5A). A similar result was obtained with respect to the SHR and SCR coding regions which show reduced acetylation, but there was no difference between the WT and the mutant within promoter regions of both genes (Fig. 8). In addition, we also found a reduced acetylation level in 3ʹ coding region of PIN1, consistent with the reduced expression level of PIN1 (Supplementary Fig. S5B).
Fig. 8.
Histone H3 acetylation levels in (A) PLT1, (B) PLT2, (C) SHR and (D) SCR. The placement of the primers is indicated. The relative amount of immunoprecipitated chromatin fragments in the elp2 mutant, as determined by qRT-PCR, was compared to that produced in the WT. The data represent mean values with their associated SD (n=3); *, P<0.05.
ELP2 is required for the DNA methylation of CYCB1 but not of PID, SHR or SCR
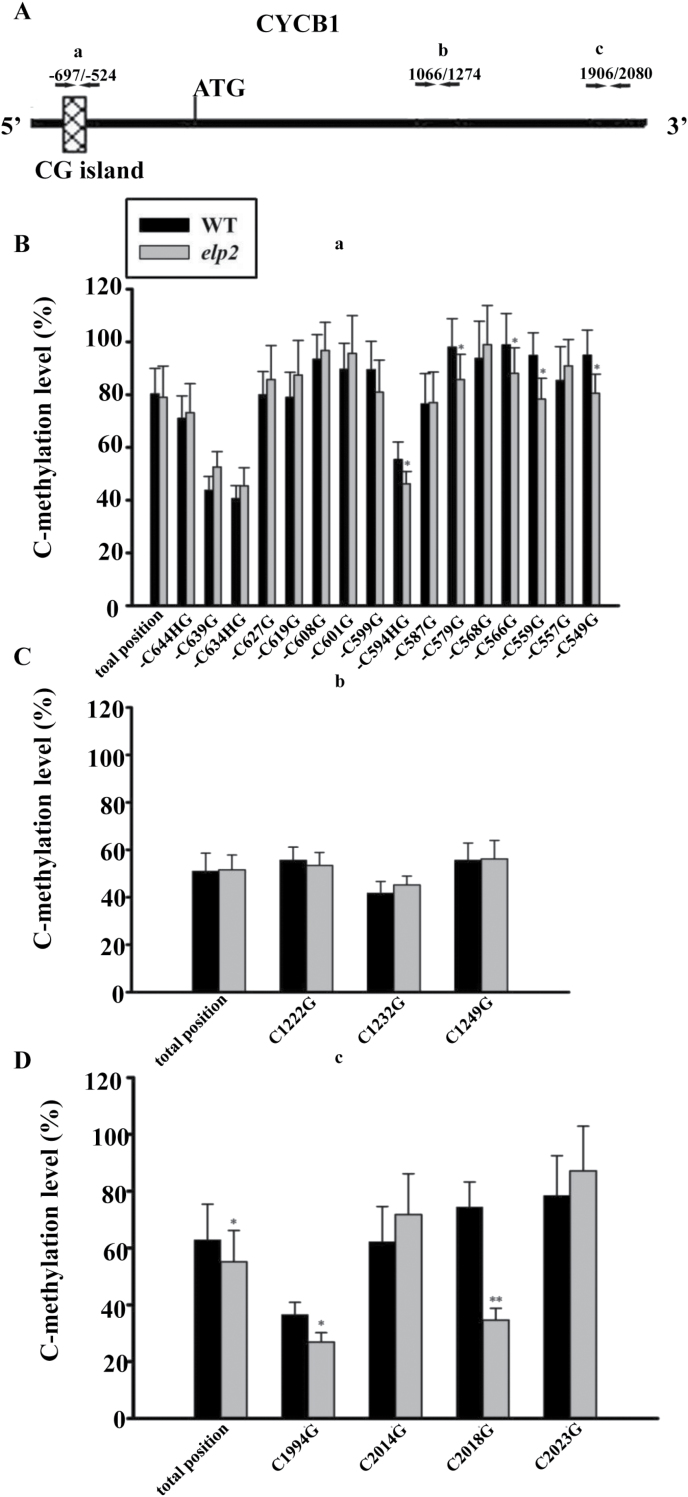
ELP2 has recently been reported to be involved in somatic DNA demethylation/methylation, thus regulating pathogen-induced transcriptome reprogramming and plant immune responses (Wang et al., 2013). To address the reduced expression levels of transcription factors such as SCR and SHR, the increased expression of PID, and whether the increased expression of the cell cycle gene CYCB1 in elp2 are associated with the altered methylation levels, DNA methylation levels in CYCB1, PID, SHR and SCR were estimated by bisulfite sequencing. The level of methylation throughout both the promoter and coding regions of PID, SHR and SCR was low (Supplementary Figs S6, S7), indicating that these genes are not under the control of DNA demethylation/methylation. In the CYCB1 promoter, five cytosines showed a reduced frequency of methylation in the elp2 mutant compared to the WT. The mutant sequence was less methylated at the 3ʹ end of its coding region (Fig. 9). The observed reduced level of methylation in this gene was consistent with its up-regulation in the mutant.
Fig. 9.
DNA methylation level is altered in the elp2 mutant. (A) Placement of the primers is indicated. The rectangular box represents a CG island. (B–D) The methylation level of amplified fragments a (B), b (C) and c (D). The DNA was extracted from three biological replicates of both WT and elp2. Three replicates of 60 clones derived from each of WT and elp2 were bisulfite-sequenced to assess their methylation level. The data represent mean values with their associated SD (n=3); *, P<0.05, **, P<0.001.
Discussion
ELP2 is one of a six-subunit (ELP1 to 6) protein complex first isolated from yeast (Wittschieben et al., 1999), and later termed ‘elongator’ on the basis that it was co-purified with the hyper-phosphorylated form of RNA polymerase II. The complex has been shown to support transcription via the regulation of DNA methylation, histone acetylation or tRNA modification (Winkler et al., 2002; Chen et al., 2009; Wang et al., 2013). In plants, its activity has been associated with growth and development, pathogen defence, and responses to abiotic stress. elp1, elp3 and elp4 have been reported to regulate root growth (Nelissen et al., 2005), although the underlying mechanism of this control has not yet been well elucidated. Here, we demonstrated that ELP2 also acts as a regulator of root growth, via its effect on the maintenance of the root stem cell niche. The basis of the regulation was the epigenetic modification of the transcription factors SCR, SHR, PLT1 and PLT2, and the G2/M transition activator CYCB1.
The role of ELP2 in root development
A systematic contrast in root growth and development between WT and the elp2 loss-of-function mutant showed that the absence of ELP2 caused root foreshortening as a result of a reduction in cell size in the EZ and in cell number in the MZ. In addition, the mutant was also defective with respect to root stem cell niche maintenance, exhibiting a much increased rate of cell division in the QC and an accelerated differentiation of the root distal stem cells. The mutants scr, shr and plt1plt2 were not only compromised with respect to their root growth, but also experienced increased cell division in the QC and accelerated differentiation of their root distal stem cells (Helariutta et al., 2000; Sabatini et al., 2003; Aida et al., 2004; Galinha et al., 2007). A clear hypothesis therefore was that the loss of ELP interfered with the functioning of SCR, SHR, PLT1 and PLT2. The knockdown of WOX5, the product of which is specifically deposited in the QC (Sarkar et al., 2007; Forzani et al., 2014), may also have contributed to the defective root stem cell niche maintenance shown by the elp2 mutant. It has been established that an optimized level of auxin signalling in the QC is required to ensure root stem cell identity. QC-centered auxin gradients are formed by polar auxin transport and local auxin synthesis (Grieneisen et al., 2007; Tian et al., 2013). Disturbing the optimal level favours accelerated differentiation of the root distal stem cells (Tian et al., 2013, 2014), so a reduction in auxin supply to the mutants’ root tips may also have made a contribution to its root phenotype.
ELP2 epigenetically affects the transcription of genes involved in root meristem maintenance
The elongator complex acts to acetylate both core histones and nucleosomal substrates (Winkler et al., 2002). In A. thaliana elp3 mutant, histone H3 lysine 14 acetylation level is reduced in the coding region of both SHORT HYPOCOTYL 2 (auxin repressor) and LAX2 (auxin influx carrier), resulting in a reduction in their expression and hence the mutant’s phenotype (Nelissen et al., 2010). The loss of ELP2 similarly reduces acetylation level in the coding region of the GRAS family transcription factors SCR and SHR, and that of the AP2 transcription factors PLT1 and PLT2, thereby down-regulating them all. The result underlines the requirement that each of the elongator complex components be present if complex assembly, integrity and enzymatic activity are to proceed normally (Glatt and Muller, 2013). DNA methylation is a central process regulating many of the genes involved in growth and the response to the plant’s environment (Chinnusamy and Zhu, 2009; He et al., 2011; Li et al., 2012; Wang et al., 2013). ELP2 has been recently shown to contribute to DNA methylation (Wang et al., 2013). Consistent with the generally observed negative correlation between DNA methylation and gene expression, the up-regulation of CYCB1 in the elp2 mutant may reflect its reduced level of methylation within both the promoter and/or coding region.
ELP2 affects auxin signal in root tip
An optimal auxin maximum is required to maintain root stem cell niche identity and root growth (Grieneisen et al., 2007; Tian et al., 2013, 2014). The elp2 mutant has reduced IAA content in roots, indicating that the ELP2 interferes with the auxin homeostasis regulation. The basal auxin efflux carrier PIN1 drives the flow of auxin from the stele towards the root tip (Galweiler et al., 1998; Blilou et al., 2005; Wisniewska et al., 2006; Grieneisen et al., 2007), while PIN2 controls its flow from the cortex (Blilou et al., 2005; Wisniewska et al., 2006). In the elp2 mutant, the less polarized PIN1 and decreased levels of both PIN1 and PIN2 reduced the supply of auxin to root tip. Therefore, the elp2 mutant has reduced auxin signal in the root, which could break the stem cell niche maintenance.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Figure S1. Agarose gel analysis of TAIL-PCR products amplified from WT and drs1 (elp2) genomic DNA
Supplementary Figure S2. The structure of elp2.
Supplementary Figure S3. Microgaphs of the root stem cell niche in (A) WT, (B) elp2-6, (C), elp2-7, (D) elp1, (E) elp4 and (F) elp6.
Supplementary Figure S4. elp mutants have short primary roots.
Supplementary Figure S5. Histone H3 acetylation levels in (A) ACTIN2, (B) PIN1.
Supplementary Figure S6. Levels of methylation in (A) SHR and (B) SCR.
Supplementary Figure S7. DNA methylation level assay of the PINOID.
Supplementary Table S1. Summary of primers used in this study.
Acknowledgements
ZD is supported by grants from the Ministry of Science and Technology of China (2015CB942901) and the National Natural Science Foundation of China (Projects 31470371, 31270327 and 31222005). HT is supported by the National Natural Science Foundation of Shandong (ZR2013CQ042). The authors acknowledge B. Scheres, T. Laux, Z.-H. Gong, C.-Y. Li, Z.-L. Mou and P Benfey for published materials. We also like to give our special thanks to Prof. Chuanyou Li for the helpful discussions and suggestions in this study.
References
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B. 2004. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119, 109–120. [DOI] [PubMed] [Google Scholar]
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R. 2001. The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128, 4057–4067. [DOI] [PubMed] [Google Scholar]
- Benjamins R, Scheres B. 2008. Auxin: the looping star in plant development. Annual Review of Plant Biology 59, 443–465. [DOI] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J. 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602. [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. 2005. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433, 39–44. [DOI] [PubMed] [Google Scholar]
- Chen C, Huang B, Eliasson M, Ryden P, Bystrom AS. 2011. Elongator complex influences telomeric gene silencing and DNA damage response by its role in wobble uridine tRNA modification. PLoS Genetics 7, e1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Tuck S, Bystrom AS. 2009. Defects in tRNA modification associated with neurological and developmental dysfunctions in Caenorhabditis elegans elongator mutants. PLoS Genetics 5, e1000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu JK. 2009. Epigenetic regulation of stress responses in plants. Current Opinion in Plant Biology 12, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Carmona A, You R, Haimovitch-Gal T, Doerner P. 1999. Technical advance: spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. The Plant Journal 20, 503–538. [DOI] [PubMed] [Google Scholar]
- Creppe C, Malinouskaya L, Volvert ML, et al. 2009. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell 136, 551–564. [DOI] [PubMed] [Google Scholar]
- Dai M, Zhang C, Kania U, et al. 2012. A PP6-type phosphatase holoenzyme directly regulates PIN phosphorylation and auxin efflux in Arabidopsis . The Plant Cell 24, 2497–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defraia CT, Wang Y, Yao J, Mou Z. 2013. Elongator subunit 3 positively regulates plant immunity through its histone acetyltransferase and radical S-adenosylmethionine domains. BMC Plant Biology 13, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFraia CT, Zhang X, Mou Z. 2010. Elongator subunit 2 is an accelerator of immune responses in Arabidopsis thaliana . The Plant Journal 64, 511–523. [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, Costantino P, Sabatini S. 2007. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Current Biology 17, 678–682. [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S. 2008. A genetic framework for the control of cell division and differentiation in the root meristem. Science 322, 1380–1384. [DOI] [PubMed] [Google Scholar]
- Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn M G, Feldmann KA, Benfey PN. 1996. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86, 423–433. [DOI] [PubMed] [Google Scholar]
- Ding Z, Friml J. 2010. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proceedings of the National Academy of Sciences, USA 107, 12046–12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. 1993. Cellular organisation of the Arabidopsis thaliana root. Development 119, 71–84. [DOI] [PubMed] [Google Scholar]
- Forzani C, Aichinger E, Sornay E, Willemsen V, Laux T, Dewitte W, Murray JA. 2014. WOX5 suppresses CYCLIN D activity to establish quiescence at the center of the root stem cell niche. Current Biology 24, 1939–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jurgens G. 2003. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis . Nature 426, 147–153. [DOI] [PubMed] [Google Scholar]
- Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B. 2007. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449, 1053–1057. [DOI] [PubMed] [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K. 1998. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230. [DOI] [PubMed] [Google Scholar]
- Glatt S, Muller CW. 2013. Structural insights into elongator function. Current Opinion in Structural Biology 23, 235–242. [DOI] [PubMed] [Google Scholar]
- Grieneisen VA, Xu J, Maree AF, Hogeweg P, Scheres B. 2007. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449, 1008–1013. [DOI] [PubMed] [Google Scholar]
- He XJ, Chen T, Zhu JK. 2011. Regulation and function of DNA methylation in plants and animals. Cell Research 21, 442–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser MT, Benfey PN. 2000. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101, 555–567. [DOI] [PubMed] [Google Scholar]
- Huang F, Zago MK, Abas L, van Marion A, Galvan-Ampudia CS, Offringa R. 2010. Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport. The Plant Cell 22, 1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lane WS, Reinberg D. 2002. Human elongator facilitates RNA polymerase II transcription through chromatin. Proceedings of the National Academy of Sciences, USA 99, 1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornet N, Scheres B. 2009. Members of the GCN5 histone acetyltransferase complex regulate PLETHORA-mediated root stem cell niche maintenance and transit amplifying cell proliferation in Arabidopsis . The Plant Cell 21, 1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qian W, Zhao Y, Wang C, Shen J, Zhu JK, Gong Z. 2012. Antisilencing role of the RNA-directed DNA methylation pathway and a histone acetyltransferase in Arabidopsis . Proceedings of the National Academy of Sciences, USA 109, 11425–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michniewicz M, Zago MK, Abas L, et al. 2007. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130, 1044–1056. [DOI] [PubMed] [Google Scholar]
- Mosher RA, Durrant WE, Wang D, Song J, Dong X. 2006. A comprehensive structure-function analysis of Arabidopsis SNI1 defines essential regions and transcriptional repressor activity. The Plant Cell 18, 1750–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Sena G, Nawy T, Benfey PN. 2001. Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413, 307–311. [DOI] [PubMed] [Google Scholar]
- Nelissen H, De Groeve S, Fleury D, et al. 2010. Plant Elongator regulates auxin–related genes during RNA polymerase II transcription elongation. Proceedings of the National Academy of Sciences, USA 107, 1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen H, Fleury D, Bruno L, Robles P, De Veylder L, Traas J, Micol JL, Van Montagu M, Inze D, Van Lijsebettens M. 2005. The elongata mutants identify a functional elongator complex in plants with a role in cell proliferation during organ growth. Proceedings of the National Academy of Sciences, USA 102, 7754–7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek J, Mravec J, Bouchard R, et al. 2006. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312, 914–8. [DOI] [PubMed] [Google Scholar]
- Rahl PB, Chen CZ, Collins RN. 2005. Elp1p, the yeast homolog of the FD disease syndrome protein, negatively regulates exocytosis independently of transcriptional elongation. Molecular Cell 17, 841–53. [DOI] [PubMed] [Google Scholar]
- Ruzicka K, Simaskova M, Duclercq J, Petrasek J, Zazimalova E, Simon S, Friml J, Van Montagu MC, Benkova E. 2009. Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proceedings of the National Academy of Sciences, USA 106, 4284–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Heidstra R, Wildwater M, Scheres B. 2003. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes and Development 17, 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T. 2007. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446, 811–814. [DOI] [PubMed] [Google Scholar]
- Tian H, Niu T, Yu Q, Quan T, Ding Z. 2013. Auxin gradient is crucial for the maintenance of root distal stem cell identity in Arabidopsis . Plant signaling & Behavior 8, e26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Wabnik K, Niu T, et al. 2014. WOX5-IAA17 feedback circuit-mediated cellular auxin response is crucial for the patterning of root stem cell niches in Arabidopsis . Molecular Plant 7, 277–289. [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. 1997. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell 9, 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Friml J. 2009. Auxin: a trigger for change in plant development. Cell 136, 1005–1016. [DOI] [PubMed] [Google Scholar]
- Vanstraelen M, Baloban M, Da Ines O, Cultrone A, Lammens T, Boudolf V, Brown SC, De Veylder L, Mergaert P, Kondorosi E. 2009. APC/C-CCS52A complexes control meristem maintenance in the Arabidopsis root. Proceedings of the National Academy of Sciences, USA 106, 11806–11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Benfey PN. 2005. Signals that regulate stem cell activity during plant development. Current Opinion in Genetics & Development 15, 388–394. [DOI] [PubMed] [Google Scholar]
- Versees W, De Groeve S, Van Lijsebettens M. 2010. Elongator, a conserved multitasking complex? Molecular Microbiology 76, 1065–1069. [DOI] [PubMed] [Google Scholar]
- Wang Y, An C, Zhang X, Yao J, Zhang Y, Sun Y, Yu F, Amador D M, Mou Z. 2013. The Arabidopsis elongator complex subunit2 epigenetically regulates plant immune responses. The Plant Cell 25, 762–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartner M, Criqui MC, Meszaros T, Binarova P, Schmit AC, Helfer A, Derevier A, Erhardt M, Bogre L, Genschik P. 2004. Expression of a nondegradable cyclin B1 affects plant development and leads to endomitosis by inhibiting the formation of a phragmoplast. The Plant Cell 16, 643–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler GS, Kristjuhan A, Erdjument-Bromage H, Tempst P, Svejstrup JQ. 2002. Elongator is a histone H3 and H4 acetyltransferase important for normal histone acetylation levels in vivo. Proceedings of the National Academy of Sciences, USA 99, 3517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska J, Xu J, Seifertova D, Brewer PB, Ruzicka K, Blilou I, Rouquie D, Benkova E, Scheres B, Friml J. 2006. Polar PIN localization directs auxin flow in plants. Science 312, 883. [DOI] [PubMed] [Google Scholar]
- Wittschieben BO, Otero G, de Bizemont T, Fellows J, Erdjument-Bromage H, Ohba R, Li Y, Allis CD, Tempst P, Svejstrup JQ. 1999. A novel histone acetyltransferase is an integral subunit of elongating RNA polymerase II holoenzyme. Molecular Cell 4, 123–128. [DOI] [PubMed] [Google Scholar]
- Xu D, Huang W, Li Y, Wang H, Huang H, Cui X. 2011. Elongator complex is critical for cell cycle progression and leaf patterning in Arabidopsis . The Plant Journal 69, 792–808. [DOI] [PubMed] [Google Scholar]
- Zhou X, Hua D, Chen Z, Zhou Z, Gong Z. 2009. Elongator mediates ABA responses, oxidative stress resistance and anthocyanin biosynthesis in Arabidopsis . The Plant Journal 60, 79–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.