Highlight
Screening revealed that the action of miR319/TCP4 in serving as a systemic defensive responder and regulator that modulated the RKN systemic defensive response was mediated via JA.
Key words: Deep sequencing, jasmonic acid, miRNAs, miR319/TCP4, root-knot nematode, tomato.
Abstract
MicroRNAs (miRNAs) are important transcriptional and post-transcriptional modulators of gene expression that play crucial roles in the responses to diverse stresses. To explore jasmonic acid (JA)-dependent miRNA-mediated regulatory networks that are responsive to root-knot nematode (RKN), two small RNA libraries were constructed from wild-type (WT) and JA mutant (spr2) plants. A total of 263 known miRNAs and 441 novel miRNAs were significantly regulated under RKN stress in the two libraries. The spatio-temporal expression of candidate miRNAs and their corresponding targets were analysed by qRT-PCR under RKN stress. A clear negative correlation was observed between miR319 and its target TEOSINTE BRANCHED1/CYCLOIDEA/PRO-LIFERATING CELL FACTOR 4 (TCP4) in leaf, stem, and root under RKN stress, implying that the miR319/TCP4 module is involved in the systemic defensive response. Reverse genetics demonstrated that the miR319/TCP4 module affected JA synthetic genes and the endogenous JA level in leaves, thereby mediating RKN resistance. These results suggested that the action of miR319 in serving as a systemic signal responder and regulator that modulated the RKN systemic defensive response was mediated via JA. The potential cross-talk between miR319/TCP4 and miR396/GRF (GROWTH RESPONDING FACTOR) in roots under RKN invasion is discussed, and a predictive model regarding miR319/TCP4-mediated RKN resistance is proposed.
Introduction
Small RNAs (sRNAs) are a class of short (20–30 nucleotide), endogenous, non-coding RNAs that play important roles in plant growth, development, and adaptation to environmental stress (Lu et al., 2008; Ruth et al., 2010). sRNAs primarily include microRNAs (miRNAs) and small interfering RNAs (siRNAs). In plants, miRNAs have been shown to regulate plant growth and development under various biotic and abiotic stresses, such as drought stress in Oryza sativa and Populus euphratica (Lu et al., 2008; Zhou et al., 2010; Li et al., 2011), heat stress in Brassica rapa (Yu et al., 2012), and heavy metal stress from copper (Waters et al., 2012), aluminium (Chen et al., 2012), and cadmium (Ding et al., 2011). miRNAs were also reported to be involved in plant–parasite interactions. In plants, miR393 was first reported to play a role in plant antibacterial PTI (pattern-triggered immunity) by regulating the auxin signalling pathway (Navarro et al., 2006). High-throughput sequencing identified a range of miRNAs, such as miR156, miR159, miR172, miR319, miR393, and miR396, in response to Pseudomonas syringae (Zhang et al., 2011). Recently, a study of tomato infection by Cucumber mosaic virus (CMV) revealed 79 miRNAs and 40 predicted candidate miRNAs that were responsive to CMV infection, including miR156, miR159, miR172, miR319, mi393, and miR396 (Feng et al., 2014). Shen et al. (2014) revealed that 62 miRNAs are responsive to Verticillium longisporum infection, including the conserved miR319 family. This growing body of evidence strongly suggests that miRNAs play roles in plant defences against viruses (Kasschau et al., 2003; Bazzini et al., 2007; Navarro et al., 2008), fungi (Lu et al., 2007), and bacteria (Jagadeeswaran et al., 2009; Xin et al., 2010; Zhang et al., 2011). In addition, a negative correlation between miRNA abundance and their targets was observed in Arabidopsis roots after infection with cyst nematode, leading to the down-regulation of miR156, miR159, miR172, and miR396 (Hewezi et al., 2008). Further studies have shown that the miR396/GROWTH RESPONDING FACTOR 1 (GRF1)/GRF3 regulatory module acts as a developmental regulator in the formation of syncytia during cyst nematode infection (Hewezi et al., 2012). Since cyst nematodes and root-knot nematodes (RKNs) feed on syncytia and giant cells, respectively, the potential mechanisms of formation of feeding sites are distinct (De Almeida Engler et al., 2011; Kyndt et al., 2013). To date, however, studies of the link between RKN invasion and miRNA-modulated defence responses are extremely limited.
Jasmonic acid (JA) as a systemic signalling molecule is effective against tomato RKN disease and can reduce the number of root knots from nematode invasion (Cooper et al., 2005; Fujimoto et al., 2011; Nahar et al., 2011; Fan et al., 2014). JA is synthesized via the octadecanoid pathway in leaves, which involves the translocation of lipid intermediates from chloroplast membranes to the cytoplasm and later into peroxisomes (León, 2013). However, JA-mediated RKN resistance occurs in roots. Therefore, synthetic JA and methyl jasmonate (MeJA), which are considered to be long-distance signalling compounds, can be transported from shoots to roots via the vasculature to activate a series of defence responses against RKN invasion (Heil and Ton, 2008). JA has been shown to be transported in the phloem in response to pathogen infection (Schilmiller and Howe, 2005; Lough and Lucas, 2006; Thorpe et al., 2007; Truman et al., 2007). Therefore, the phloem is considered to be a hub that connects shoots and roots and in which miRNAs potentially play crucial roles.
Recent studies indicate that miRNAs are responsive to JA. For example, exogenous MeJA down-regulates miR156, miR168, miR169, miR172, miR172, miR396, miR480, and miR1310 and up-regulates miR164 and miR390 in Chinese yew (Qiu et al., 2009). In one study, 49 known miRNAs, 15 novel miRNAs, and three tasiRNA (trans-acting small interfering RNA) families were induced in JA-treated wild-type (WT) Arabidopsis, whereas one new miRNA, one tasiRNA family, and 22 known miRNAs were repressed in the JA-deficient aos mutant (Zhang et al., 2012). Additionally, in Nicotiana attenuata, insect herbivores have been shown to alter sRNA transcriptomes related to phytohormone (JA and JA-Ile) signalling (Pandey et al., 2008), which implies that sRNAs probably serve as regulators in JA-mediated biotic stress responses.
The present work was planned to identify RKN-responsive JA-mediated miRNAs and interpret the roles of candidate miRNA. To the authors’ knowledge, it is the first exploration of the responsive miRNAs under RKN stress. Sequencing of sRNAs was performed on phloem tissue after RKN inoculation in WT and spr2 (suppressor of prosystemin-mediated response 2, JA-deficient) tomatoes. A total of 263 known and 441 novel differentially expressed miRNAs were identified, and the roles of the JA-mediated miR319/TEOSINTE BRANCHED1/CYCLOIDEA / PRO-LIFERATING CELL FACTOR 4 (TCP4) module in the RKN-defensive response were demonstrated. Previous evidence has demonstrated that miR319s and their targets participate in abiotic stress responses and play positive roles in high salinity and drought stress resistances (Sunkar and Zhu, 2004; Liu et al., 2008; Lv et al., 2010; Zhou et al., 2010; Thiebaut et al., 2012). The outcomes of this study would provide novel insight into the functions of the miR319/TCP module in plants.
Materials and methods
Plant materials and biotic stress treatment
Tomato cultivar CM (Solanum lycopersicum var Castlemart, WT) and its mutant spr2 (deficient in JA biosynthesis because Spr2 encodes a chloroplast fatty acid desaturase involved in JA biosynthesis; Li et al., 2003) were grown in a greenhouse with a day/night temperature of 25/18 °C, an air relative humidity (RH) of 60/70%, and a photosynthetic photon flux density (PPFD) of 500 μmol–2 s–1 for 10h d–1. RKN isolates were clonal populations established from a single female. They were maintained on tomato UC82 plants. When the seedlings had a total of four spread true leaves, nematodes were inoculated at 5000 per plant near the root. Leaves, phloem (stem), and roots were harvested from the WT and spr2 at 6, 12, 24, and 72h after inoculation. For hormone treatment, leaves were collected from the WT after JA treatment at 0h and 6h. These samples were used for quantitative reverse transcription–PCR (qRT–PCR) analysis of miRNAs and their targets. The infected seedlings were harvested after 20 d for high-throughput sequencing, and the primary stems were cut 10cm above the bottom of the root. The phloem tissue was quickly peeled off with forceps and a blade treated with DEPC (diethylpyrocarbonate), and the phloem tissue was placed in RNA-free tubes, flash-frozen in liquid nitrogen, and stored at –80 °C.
To investigate the role of miR319 in response to RKN (Meloidogyne incognita) stress, PCR was performed to confirm that the genomes of the plants under study did not contain Mi-1 (Supplementary Fig. S1 availbale at JXB online), which confers effective resistance against RKNs in tomato (S. lycopersicum L.) (Seah et al., 2004). The transgenic tomatoes overexpressing Arabidopsis pre-miR319a or tomato TCP4 (LA) were in the M82 background. To overexpress miR319 or LA, the op:gene plants (opmiR319 or opLA m) were crossed with FIL:LhG4 (pFIL), and hybrid F1 seedlings were used for further study. The relative expression levels of JA biosynthetic genes in the WT and transgenic tomatoes (miR319-oe, LAm-oe) were analysed at 0, 6, 12, and 24h after inoculation using qRT–PCR.
Small RNA library construction and high-throughput sequencing
Total RNA was extracted with the TRIzol reagent according to the manufacturer’s instructions. RNA quality was assessed with an Agilent Technologies 2100 Bioanalyzer. The RIN (RNA integrity number) was 7.2 for the WT and 6.2 for the spr2 mutant. Total RNA from WT and spr2 plants was prepared for sRNA sequencing-by-synthesis according to the protocols and standards for Illumina preparation at the Beijing Genomics Institute (BGI; Shenzhen, Guangdong, China). Briefly, RNA fragments of 18–30 nucleotides were isolated from the total RNA by electrophoretic separation via 15% TBE–urea denaturing PAGE. The 5ʹ RNA adaptor (5ʹ-GUUCAGAGUUCUACAGUCCGACGAUC-3ʹ) and 3ʹ RNA adaptor (5ʹ-pUCGUAUGCCGUCUUCUGCUUGUidT-3ʹ; p, phosphate; and idT, inverted deoxythymidine) were ligated to the isolated sRNAs using T4 RNA ligase (TaKaRa). The products were separated and purified at each step by TBE–urea PAGE. The 62–75 nucleotide ligated products were subsequently transcribed into cDNA and amplified by PCR, followed by purification and separation. The purified DNA fragments were used for sequencing with an Illumina 1G Genome Analyzer, an sRNA digital analysis system.
Small RNA annotation
Raw sequence reads were processed into clean full-length reads by discarding low-quality reads and corrupted adaptor sequences [reads <18 nucleotides, reads >30 nucleotides, reads with a poly(A), 3ʹ adaptor, or 5ʹ adaptor contaminants, and reads without a ligated adaptor]. Adaptors were then trimmed from the remaining high-quality sequences. The distribution of small RNA lengths was analysed, and the two samples were compared to identify common and genotype-specific transcripts. The Short Oligonucleotide Analysis Package (SOAP; http://soap.genomics.org.cn) was used to annotate the sRNA sequences, which were then mapped to tomato TIGR reference sequences. After comparing the unique sequences with the Rfam 9.1 (http://ftp.selab.janelia.org/pub/Rfam) and NCBI GenBank databases (http://ftp.ncbi.nlm.nih.gov), all rRNAs, scRNAs, snoRNAs, snRNAs, and tRNAs were discarded. The NCBI GenBank annotation has priority over Rfam (9.1). The sRNAs were then classified and annotated. The total rRNA content served as a quality standard.
Identification of known and novel differentially expressed miRNAs
The remaining sequences were analysed by BLAST searches of miRBase16.0 (http://www.mirbase.org/index.shtml) for matches to precursors or mature miRNAs; these known miRNAs came from all the plants. To evaluate the expression patterns of known miRNAs, a statistical analysis was used to determine the significance of known differences in miRNA expression between the WT and spr2 strains. Log2 ratios and scatter plots were used to compare miRNA expression, and fold change=log2 (treatment/control). Novel miRNAs were predicted according to the precursor hairpin secondary structure in Mireap (https://sourceforge.net/projects/mireap/). Briefly, potential novel miRNAs in the tomato genome were predicted using the following criteria: (i) the sequences of miRNA precursors folded into stem–loop structures that contain an ~21 nucleotide mature miRNA sequence on one arm and the miRNA* derived from the opposite arm to form a duplex with two nucleotides for the 3ʹ overhang; (ii) the Dicer PAZ structural domain, which binds the end of double-stranded RNA, recognizes the two-nucleotide overhang of the stem–loop structure, and cuts it into miRNAs; and (ii) the miRNAs have lower folding free energies than random sequences with the same nucleotide content, so the stem–loop structures have a folding free energy of at most –18 kcal mol–1 (the lowest free energy is Mfe ≤ –18 kcal mol–1). χ2 tests were used to determine the statistical significance of the differences between the two libraries. Compared with the WT group, the known and novel differentially expressed miRNAs with fold changes >2.0 and a P<0.01 were selected.
Identification and KEGG enrichment analysis of miRNA target genes
The targets of known and novel miRNAs were predicted using psRNATarget (Dai and Zhao, 2011). All the predictive targets were assigned KEGG terms (Moriya et al., 2007), and a search was carried out for significantly enriched KEGG terms compared with the entire transcriptome background.
Real-time PCR assay
A time-dependent expression analysis of selected miRNAs and their targets was performed by stem–loop RT–PCR and qRT–PCR. For stem–loop RT–PCR, total RNA was reverse-transcribed into cDNA using the Super-Script first-strand synthesis system (Invitrogen) according to the manufacturer’s instructions. The cDNA was used as a template to perform real-time PCR with gene-specific primers and SYBR Green Mix (TaKaRa). Target expression levels were normalized by using tomato U6 as an internal reference. Stem–loop reverse transcription was performed according to a previous protocol (Varkonyi-Gasic et al., 2007). For qRT–PCR, reverse transcription was performed using TransScript First-Strand cDNA Synthesis SuperMix (TransGen) following the manufacturer’s instructions. The resulting cDNA was analysed by relative quantitative PCR in the presence of SYBR Premix Ex Taq II (TaKaRa) in a Bio-Rad iCycler, with β-actin as the internal control. After the PCR, a melting curve was generated by gradually increasing the temperature to 95 °C to test the amplicon specificity. The primers for stem–loop RT–PCR and qRT–PCR are listed in Supplementary Table S1 at JXB online. All reactions were performed in triplicate.
Acid fuchsin staining
The root systems of each plant were harvested at 35 d after RKN infection and then submerged in a 15% solution of McCormick’s red food colour (Thies et al., 2002) for 15–20min to stain the egg masses. The root systems were carefully rinsed under running tap water and evaluated for gall severity and egg mass production.
JA level determination
Plant tissue was homogenized in liquid nitrogen, and ~100mg of fresh leaves was sealed in 2ml tubes (if >100mg, in 5ml tubes). A 0.5ml aliquot of extraction buffer (2:1:0.005, isopropanol:water:concentrated HCl) was added to each sample with d5-JA (40ng CDN Isotopes) as internal standards. The samples were homogenized for 45 s, followed by agitation for 30min at 4 °C. CH2Cl2 (1ml) was added to the samples, followed by agitation for another 30min (the CH2Cl2 extraction step was performed on a vortexer with a 30 sample attachment at room temperature) and then centrifuged at 13 000g for 5min. After centrifugation, two phases formed, and the plant debris was in the middle of the two layers. The aqueous phase was discarded, and the lower layer was collected, concentrated in a drying machine, and re-solubilized in MeOH+water. If the solution contained precipitates, it was transferred to a glass tube and centrifuged at 13 000 g for 30min. Afterwards, 5–10 μl was injected into the column for analysis (Pan and Wang, 2009).
Results
An overview of sRNA sequencing
To identify the miRNAs involved in JA-mediated RKN resistance in tomato, two sRNA libraries from WT and spr2 mutant tomatoes were constructed. Solexa sequencing technology was used to investigate the expression abundance of sRNA in the two libraries, which generated a total of 17 753 883 and 11 251 489 reads in the WT and spr2, respectively. After removing the adaptors and low-quality tags, 16 694 025 (representing 6 195 892 unique reads) and 10 439 048 (representing 2 520 926 unique reads) clean reads remained for the WT and spr2 libraries, respectively. In total, 5 019 256 (WT) and 1 921 123 (spr2) reads mapped perfectly to the S. lycopersicum genome. Thereafter, the non-coding RNAs, including rRNAs, tRNAs, snRNAs, and snoRNAs, were annotated and removed. Querying the remaining sequences against miRBase 16.0 identified 38 738 (WT) and 17 546 (spr2) unique reads that matched known miRNAs (Table 1).
Table 1.
A statistical analysis of sequencing reads from WT and spr2 sRNA libraries
| Library type | WT | spr2 |
|---|---|---|
| Total raw reads | 17 753 883 | 11 251 489 |
| Total clean reads | 16 694 025 | 10 439 048 |
| Unique clean reads | 6 195 892 | 2 520 926 |
| Total clean reads mapped to genome | 14 700 657 | 8 944 510 |
| Unique clean reads mapped to genome | 5 019 256 | 1 921 123 |
| Total miRNA reads | 38 738 | 17 546 |
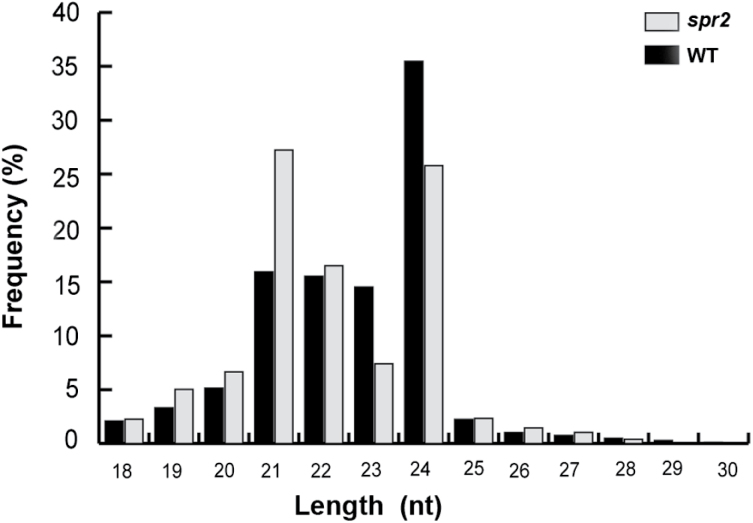
The 21 and 24 nucleotide RNAs were the most abundant sRNA species (Fig. 1). The sRNAs ranged from 21 to 24 nucleotides in length and comprised 81.46% of the WT and 76.94% of the spr2 transcripts, which was consistent with evidence found in the conifers Pinus contorta and Taxus chinensis, for which >50% of the sequenced sRNAs were 21 or 24 nucleotides (Qiu et al., 2009). In contrast to the results found in T. chinensis (Qiu et al., 2009), the percentage of 24 nucleotide sRNAs was much higher than the percentage of 21 nucleotide sRNAs in the WT in this study. The sRNAs had a strong bias for 21 nucleotide lengths in the spr2 strain, almost twice the rate in the WT strain. In addition, 24 nucleotide sRNAs dominated the WT strain, which was consistent with previous deep sequencing studies in which Hi-JA Arabidopsis exhibited a bias for 24 nucleotide sRNA populations (Zhang et al., 2012).
Fig. 1.
The length distribution and abundance of sRNAs in the libraries of WT (blank bars) and spr2 (grey bars).
Identification of differentially expressed known and novel miRNAs
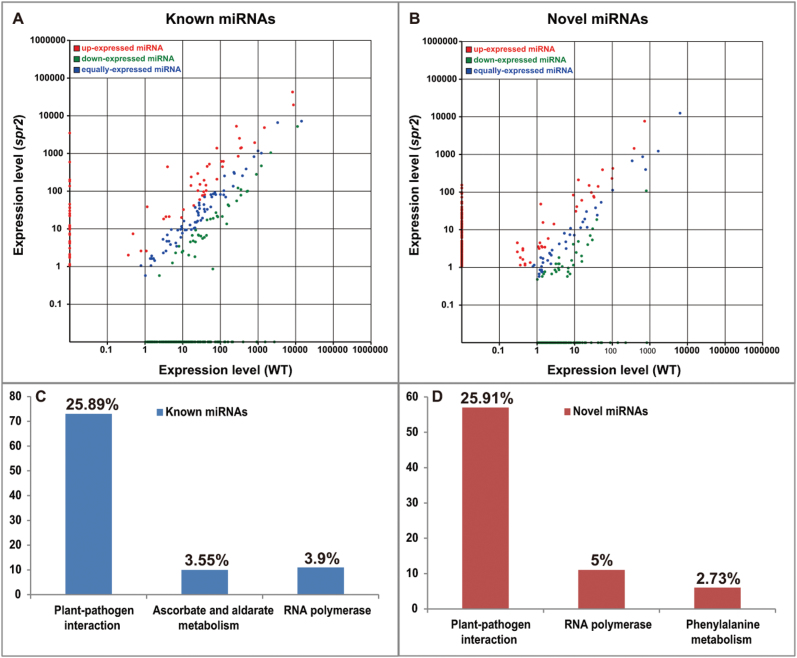
To identify the differential expression of known miRNAs from the two libraries for the WT and spr2 strain, clean reads were used to compare known plant miRNA precursors or mature miRNA sequences using miRBase 16.0. Novel miRNAs were predicted according to the precursors’ hairpin secondary structure in Mireap and by using the miRNA/miRNA* criteria. The miRNA expression was considered to be significantly up-regulated or down-regulated with fold changes >2.0 or fold changes < –2.0, respectively, with P≤0.001. In total, 263 known and 441 novel miRNAs were differentially expressed (Supplementary Tables S2, S3 at JXB online). Scatter plots were used to compare the expression of known and novel miRNAs (Fig. 2A, B).
Fig. 2.
A scatter plot of differentially expressed known (A) and novel (B) miRNAs. The abscissa represents the expression level of miRNAs in the WT; the ordinate represents the expression level of miRNAs in spr2. Dots appearing in the shaded sections represent differential expression miRNAs. Top left, increased expression; bottom right: reduced expression. (C, D) The number of known and novel differentially expressed miRNAs in KEGG pathways. The corresponding percentage is shown above the column. (This figure is available in colour at JXB online.)
Target prediction and KEGG enrichment of differentially expressed miRNAs
miRNAs regulate the expression of specific genes via hybridization to mRNA transcripts to promote RNA degradation, inhibit translation, or both (Krol et al., 2010). Known and novel miRNA sequences were searched against tomato genomic sequences on the psRNATarget webserver. Among the 263 known miRNAs, 131 could be searched for their potential targets, yielding 473 genes (Supplementary Table S4 at JXB online). Of the 441 novel miRNAs, 161 miRNAs had 359 predicted targets (Supplementary Table S5). KEGG pathway analysis indicated that the significantly enriched pathways were ‘plant–pathogen interaction’ (ko04626) and ‘ascorbate and aldarate metabolism’ (ko00053) for known miRNAs and ‘plant–pathogen interaction’ (ko04626) and ‘RNA polymerase’ (ko03020) for novel miRNAs (Fig. 2C, D; Supplementary Table S6). Therefore, the focus of further study was on miRNAs in the ‘plant–pathogen interaction’ category (Table 2).
Table 2.
The corresponding miRNAs in the plant–pathogen interaction KEGG pathway
| Fold change (log2 spr2/WT) | Predictive targets related to plant–pathogen interaction | |
|---|---|---|
| Known miRNA | ||
| miR1222a | –8.73233707 | Solyc01g005440.2.1 |
| miR2111a-5p | –10.16755622 | Solyc06g083390.2.1 |
| miR2593e | –7.90454432 | Solyc04g051540.2.1 |
| miR319b | 1.29821124 | Solyc03g115010.1.1 |
| miR396b | –16.49819511 | Solyc07g041640.2.1, Solyc10g083510.1.1, Solyc08g083230.1.1, Solyc08g075950.1.1, Solyc03g082430.1.1, Solyc08g005430.2.1, Solyc12g096070.1.1 |
| miR476b | –8.31052191 | Solyc11g064770.1.1, Solyc11g069620.1.1, Solyc11g071420.1.1 |
| miR482c | –10.79691559 | Solyc04g009110.1.1, Solyc04g009130.2.1, Solyc04g009290.1.1, Solyc04g026110.2.1, Solyc07g009180.1.1, Solyc08g075630.2.1, Solyc08g076000.2.1, Solyc09g098100.2.1 |
| miR482d-3p | 3.34641851 | Solyc02g036270.2.1, Solyc04g009070.1.1, Solyc11g065780.1.1, Solyc12g016220.1.1 |
| miR5081 | –1.35827709 | Solyc02g037540.1.1 |
| miR5139 | 1.0785291 | Solyc05g006620.2.1 |
| miR5185l-5p | –10.43434683 | Solyc01g106630.2.1, Solyc04g074000.2.1 |
| miR5658 | –10.71188213 | Solyc06g068930.1.1 |
| miR6020a-5p | –2.05955615 | Solyc01g066020.1.1 |
| miR6022 | –1.05886924 | Solyc01g005720.2.1, Solyc01g005730.2.1, Solyc01g005760.2.1, Solyc01g005780.1.1, Solyc01g005870.1.1, Solyc01g006550.2.1, Solyc01g008390.1.1, Solyc01g008410.1.1, Solyc01g009690.1.1, Solyc01g009700.1.1, Solyc03g082780.1.1, Solyc12g100020.1.1 |
| miR6023 | –1.2612632 | Solyc01g005780.1.1, Solyc01g009690.1.1, Solyc03g082780.1.1, Solyc01g005710.2.1, Solyc01g014160.1.1, Solyc01g014930.1.1 |
| miR6024 | –3.44754718 | Solyc02g070410.1.1, Solyc02g084890.1.1, Solyc03g006750.1.1, Solyc04g015220.2.1, Solyc05g005330.2.1, Solyc05g008070.2.1, Solyc11g006640.1.1, Solyc11g020100.1.1, Solyc11g069020.1.1, Solyc12g005970.1.1, Solyc12g006040.1.1, Solyc12g017800.1.1 |
| miR6025a | –8.04204236 | Solyc05g012890.1.1, Solyc05g012910.2.1, Solyc08g076050.2.1, Solyc08g076060.2.1 |
| miR6027 | 1.71280122 | Solyc04g007070.2.1, Solyc04g009090.1.1, Solyc04g009150.1.1 |
| miR6426a | 2.53010865 | Solyc10g009210.2.1 |
| miR6472 | –1.97800646 | Solyc05g044490.2.1 |
| miR7819 | –12.8364427 | Solyc01g113620.1.1 |
| miR900–3p | 11.55914762 | Solyc11g011880.1.1 |
| Novel miRNA | ||
| novel_mir_1138 | 9.8746433 | Solyc04g056570.2.1 |
| novel_mir_1167 | 8.21412481 | Solyc07g047910.1.1 |
| novel_mir_1248 | 8.25993153 | Solyc07g049180.2.1 |
| novel_mir_1297 | 7.58187845 | Solyc05g007170.2.1, Solyc09g092000.2.1 |
| novel_mir_1365 | 9.71937171 | Solyc07g047910.1.1 |
| novel_mir_17 | –6.83048352 | Solyc07g047910.1.1 |
| novel_mir_607 | 1.56364113 | Solyc05g007170.2.1, Solyc09g092000.2.1 |
| novel_mir_1310 | 11.79131757 | Solyc04g009110.1.1, Solyc04g009130.2.1, Solyc04g009290.1.1, Solyc04g026110.2.1, Solyc07g009180.1.1, Solyc08g075630.2.1, Solyc08g076000.2.1, Solyc09g098100.2.1 |
| novel_mir_1379 | 8.0148016 | Solyc03g113980.2.1 |
| novel_mir_1432 | 11.49834063 | Solyc10g047490.1.1, Solyc12g009510.1.1, Solyc12g009520.1.1, Solyc12g013680.1.1 |
| novel_mir_181 | 5.25660878 | Solyc01g087200.2.1, Solyc04g007320.1.1 |
| novel_mir_288 | –10.94453626 | Solyc06g008270.2.1, Solyc12g006020.1.1, Solyc12g099870.1.1, Solyc12g100010.1.1, Solyc12g100030.1.1 |
| novel_mir_313 | –8.44057854 | Solyc06g068870.2.1 |
| novel_mir_318 | –7.58263127 | Solyc07g039570.2.1 |
| novel_mir_366 | –9.07446263 | Solyc05g008070.2.1, Solyc07g049700.1.1, Solyc11g006530.1.1, Solyc11g006630.1.1, Solyc11g020100.1.1, Solyc12g017800.1.1 |
| novel_mir_453 | –10.12175366 | Solyc07g053220.1.1 |
| novel_mir_549 | –6.97487343 | Solyc09g092280.1.1 |
| novel_mir_553 | –9.42808795 | Solyc05g021140.1.1, Solyc10g085460.1.1, Solyc10g086590.1.1 |
| novel_mir_633 | –13.11884318 | Solyc03g078300.1.1, Solyc08g007630.1.1, Solyc11g043070.1.1, Solyc11g071430.1.1 |
| novel_mir_657 | –6.83048352 | Solyc11g071420.1.1 |
| novel_mir_683 | –3.49249358 | Solyc03g063650.1.1 |
| novel_mir_71 | 2.91441758 | Solyc01g005710.2.1, Solyc01g005730.2.1, Solyc01g005760.2.1, Solyc01g005780.1.1, Solyc01g005870.1.1, Solyc01g006550.2.1, Solyc01g009700.1.1, Solyc01g016370.1.1, Solyc03g082780.1.1, Solyc10g007210.1.1 |
| novel_mir_906 | 8.50787391 | Solyc07g018190.2.1, Solyc10g085120.1.1, Solyc12g044840.1.1 |
Validation and characterization of selected miRNAs responsive to RKN infection in the WT and spr2
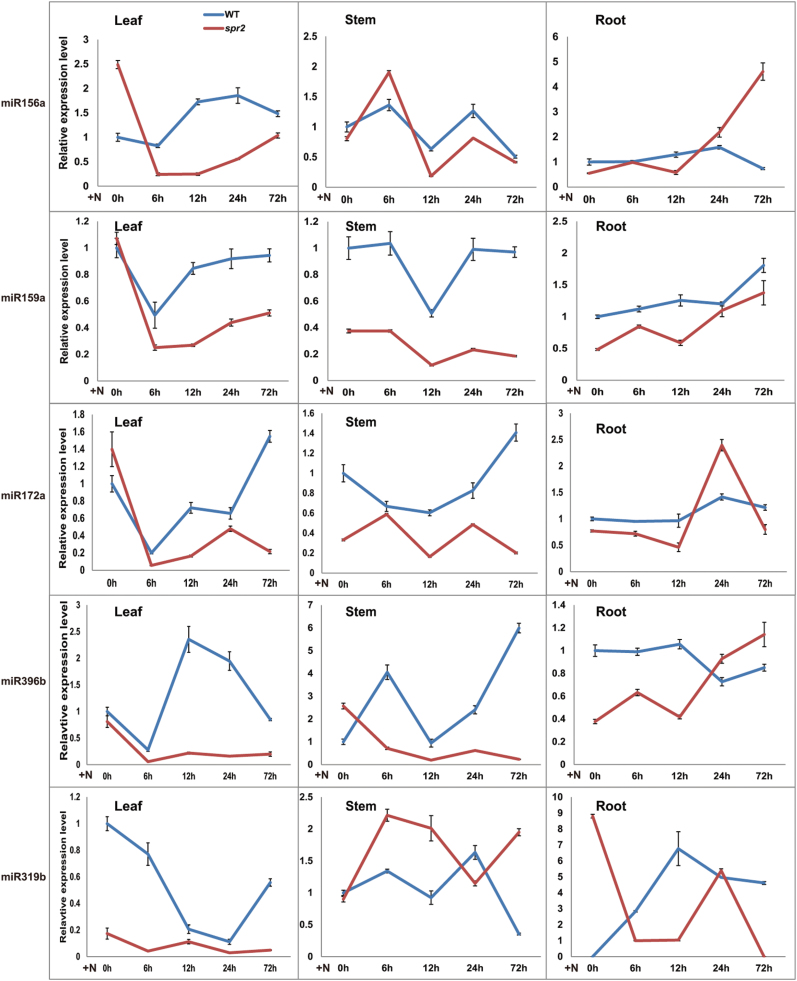
miR156 (Kasschau et al., 2003; Bazzini et al., 2007; Lu et al., 2007; Navarro et al., 2008; Xin et al., 2010; Zhang et al., 2011), miR159 (Subramanian et al., 2008; Xin et al., 2010; Zhang et al., 2011), miR172 (Subramanian et al., 2008; Wang et al., 2009; Zhang et al., 2011), miR396 (Hewezi et al., 2008, 2012; Navarro et al., 2008; Wang et al., 2009; Xin et al., 2010; Zhang et al., 2011), and miR319 (Subramanian et al., 2008; Zhang et al., 2011; Feng et al., 2014; Shen et al., 2014) have all been shown to respond to biotic stress in plants. Therefore, miRNA156a, miRNA159a, miRNA172a, miR319b, and miR396b were selected to validate the sRNA sequencing results and further characterize the spatio-temporal expression patterns in the WT and spr2. After RKN inoculation of the WT, dynamic transcription fluctuations in selected miRNAs appeared in the leaves, stems, and roots (Fig. 3), indicating that those miRNAs were responsive to RKN invasion. Using pre-RKN inoculation (0h) in the WT as the control, the expression levels of selected miRNAs in leaves, stems, and roots at 0, 6, 12, 24, and 72h after inoculation were analysed in the WT and spr2. Significant differences in miRNA expression between the WT and spr2 were observed (Fig. 3), which was consistent with the sRNA sequencing results and suggested that JA signal deficiency affects the response of selected miRNAs under RKN infection.
Fig. 3.
Time-course expression profiling of miRNAs (miR156a, miR159a, miR172a, miR396b, and miR319b) from leaves, stems, and roots at 0, 6, 12, 24, and 72h after RKN inoculation (N) in the WT and spr2. At least 10 plants were included in each pool, and three technical replicates were determined for each time point. Error bars indicate ±SE. The primary axis corresponds to the solid line, and the secondary axis corresponds to the dashed line. (This figure is available in colour at JXB online.)
Expression profiling of selected miRNAs and their targets under RKN invasion
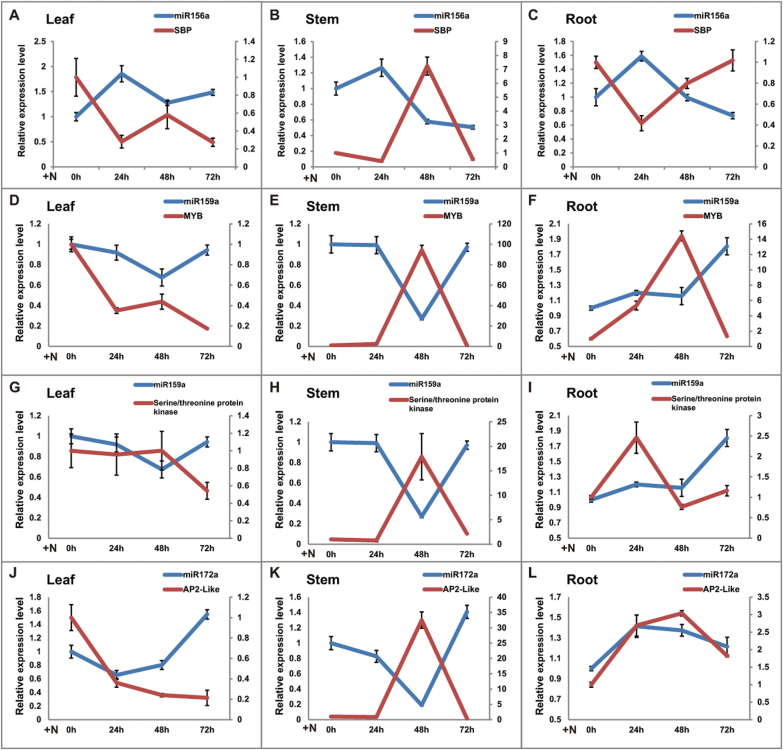
To investigate the potential role of selected miRNAs in response to RKN, the spatio-temporal expression profiling of predicted targets of miR156a, miR159a, and miR172a was also examined in the WT after RKN infection. The squamosa promoter-binding protein gene (Solyc10g078700.1.1, SPL) is the target of miR156 (Lu et al., 2005, 2008; Bazzini et al., 2007). An opposite expression pattern between miR156a and SPL was observed in leaf (0–24h), stem (0–48h), and root (0–72h) (Fig. 4A–C). The MYB transcription factor is reportedly targeted by miR159 (Achard et al., 2004; Sunkar et al., 2004; Reyes and Chua, 2007; Liu et al., 2008), and a serine/threonine protein kinase (Solyc06g008320.2.1) was also predicted to be targeted by miR159a in the present analysis. Compared with miR159a, the MYB transcription factor (Solyc01g009070.2.1) showed a reverse expression pattern after 48h in leaf and root (Fig. 4D, F), and from 24h to 72h in stem (Fig. 4E). A negative correlation was found between the expression of the serine/threonine protein kinase gene and miR159a from 24h to 72h in stem (Fig. 4H), and no negative correlation was observed in leaf and root (Fig. 4G, I). The AP2-like ethylene-responsive transcription factor is the target of miR172 (Aukerman and Sakai, 2003; Chen et al., 2004; Schwab et al., 2005), and miR172a and AP2-like transcription factor gene (Solyc04g049800.2.1) expression showed a reverse pattern from 0h to 72h in leaf and from 24h to 72h in stem (Fig. 4J, K), and analogous patterns occurred in root (Fig. 4L). The fact that these miRNA–target pairs exhibited inverse expression at particular times and places suggests that miRNAs potentially play roles in self-adaption to RKN invasion through the regulation of their target genes.
Fig. 4.
Time-course expression profiling of miRNAs and target genes from leaves, stems, and roots at 0, 24, 48, and 72h after RKN inoculation (N) in the WT. At least 10 plants were included in each pool, and three technical replicates were determined for each time point. Error bars indicate ±SE. The primary axis corresponds to the solid line, and the secondary axis corresponds to the dashed line. (This figure is available in colour at JXB online.)
miR319- and LA m-overexpressing transgenic tomatoes
In the present study, miR319b was responsive to RKN invasion (Fig. 3) and was predicted to be involved in plant–pathogen interaction (Table 2). TCP4, the target of miR319 (Palatnik et al., 2003; Koyama et al., 2007; Ori et al., 2007; Efroni et al., 2008), showed a significant up-regulation in leaf, though no obvious expression changes in miR319b were observed from 0h to 48h; however, miR319b expression dramatically declined from 0h to 24h (Fig. 5A). The reverse expression trends between TCP4 and miR319b were observed in stem and root (Fig. 5B, C). Although the fold change of miR319b expression in sRNA sequencing was not the highest, the plausible miR319b-targeted inhibition of TCP4 occurred in both shoots and roots. For JA treatment, the expression of miR319b declined, and TCP4 increased (Fig. 5D, E). Based on all the above, an attempt was made to identify the function of miR319 during RKN invasion. LA (LANCEOLATE) encodes a TCP-family transcription factor (TCP4) that contains a miR319-binding site in tomato, which could be cleaved and down-regulated by ath-miR319a from Arabidopsis (Ori et al., 2007). A sequence comparison of ath-miR319a (Arabidopsis) and sly-miR319s (tomato) showed that ath-miR319a and sly-miR319b are identical (Fig. 5F), indicating that ath-miR319a could function in place of sly-miR319b in tomato. To study the potential function of miR319b in response to RKN stress in tomato, the transactivation system was used to express ath-miR319a and TCP4 (LA) under the regulation of the FILAMENTOUS FLOWER (FIL) promoter in the transgenic M82 tomato line. The FIL promoter is active primarily in tomato young primordia and later in initiating leaflets (Lifschitz et al., 2006). The driver line expressed LhG4 under the regulation of the FIL promoter (FILpro:LhG4) and the responder line expressed miR319 or TCP4 under the control of an operator (OP) array. FILpro:LhG4 (pFIL) plants were crossed with the op:gene plants (op-miR319 or opLA m) to obtain miR319 or LA m overexpression plants (FIL>>miR319, miR319-oe; FIL>>LA m, LAm-oe). The LA m contains a mutation in the LA miRNA-binding site that is complementary to miR319 and is thus a miR319-resistant version of LA (Fig. 5G). Finally, three miR319 overexpression types were obtained, and the expression levels of miR319 and TCP4 in hybrid F1 were analysed by qRT–PCR (Fig. 5H). Based on the significance of the expression level, type 3 was chosen for the following tests.
Fig. 5.
(A–C) Changes in miR319b and TCP4 expression in leaves, stems, and roots after RKN inoculation (N) in the WT. The primary axis corresponds to the solid line, and the secondary axis corresponds to the dashed line. The mini line chart shows the dynamic changes of miR319b from 0h to 24h after RKN inoculation. (D, E) Changes in miR319b and TCP4 expression in leaves after JA treatment in the WT. The asterisk represents a significant difference as determined by Student’s t-test (P≤0.01). (F) The sequence similarity alignment between ath-miR319a in Arabidopsis and sly-miR319a, b, c in tomato. (G) Sequence of the miR319 recognition site in the LA (TCP4) mRNA of the WT and LAm. The box and asterisk represent G–U wobbles and mismatches, respectively. (H) Relative expression levels of miR319 and LA in the leaves of the WT and miR319-oe, LAm-oe plants. At least 10 plants were included in each pool, and three technical replicates were determined for each time point. Error bars indicate ±SE. (This figure is available in colour at JXB online.)
miR319 overexpression reduces resistance to RKN
Previous studies reported that the overexpression of miR319 plays a positive role in various abiotic stress responses. For example, miR319 overexpression leads to enhanced cold tolerance in rice (Yang et al., 2013). Zhou et al. (2013) revealed that miR319 overexpression enhances salt and drought tolerance in transgenic creeping bentgrass. In this study, WT and transgenic tomatoes were examined for their resistance to RKN infection. Tomato roots were inoculated with RKN and then stained with acid fuchsin at 35 d after inoculation, producing obvious red staining of the galls, and the number of galls per plant was then evaluated. The results indicated that the number of galls on miR319-oe roots was much higher than on WT roots; however, the LAm-oe plants had few galls (Fig. 6). This finding indicated that miR319 and TCP4 had opposite effects on resistance to RKN infection.
Fig. 6.
Representative root systems at 35 d after RKN infection, as well as the number of galls in WT, miR319-oe, and LAm-oe tomatoes. Three independent experiments were conducted, and 10 plants for each type were determined. Error bars indicate ±SD. (This figure is available in colour at JXB online.)
miR319 overexpression reduces endogenous JA levels
JA is a plant signalling compound that induces resistance to pathogens (Cooper et al., 2005). Schommer et al. (2008) showed that JA biosynthesis is associated with the miR319/TCP regulatory module in Arabidopsis. Moreover, the JA biosynthesis and signalling pathways play key roles in the RKN resistance of the rice root system (Cooper et al., 2005; Fujimoto et al., 2011; Nahar et al., 2011). In the present study, the expression levels of the pivotal genes involved in JA biosynthesis, including LOXD, AOS1, AOC1, and OPR3, were monitored in WT and transgenic tomatoes at 0, 6, 12, and 24h after RKN inoculation. LOXD expression in the WT line peaked at 12h after inoculation, but no obvious change was observed in miR319-oe (Fig. 7A). In the LAm-oe plants, LOXD expression peaked at 6h after inoculation (Fig. 7B). Moreover, similar expression patterns for AOS1, AOC1, and OPR3 were found in the WT; they first decreased and then peaked at 12h after inoculation. However, no obvious changes were observed in the miR319-oe plants (Fig. 7C, E, G). In LAm-oe, similar to LOXD, the expression levels of AOS1 and AOC1 peaked at 6h after inoculation (Fig. 7D, F), and the expression level of OPR3 peaked at 12h after inoculation (Fig. 7H). These results showed that the expression levels of JA biosynthetic genes in miR319-oe were low, and no obvious change was observed after RKN inoculation. However, those genes accumulated earlier and to a greater extent in LAm-oe plants, suggesting that the miR319/TCP4 module plays crucial roles in modulating JA biosynthesis induced by RKN invasion. In addition, the co-expression of LOXD, AOS1, AOC1, and OPR3 in WT and LAm-oe suggested that those genes belonging to the LOX pathway are probably under the control of the same regulatory mechanism.
Fig. 7.
(A–H) Relative expression levels of JA biosynthetic genes in WT and transgenic tomatoes (miR319-oe and LAm-oe) at 0, 6, 12, and 24h after inoculation. The expression patterns of LOXD (A, B), AOS1 (C, D), AOC1 (E, F), and OPR3 (G, H) are shown. At least 10 plants were included in each pool, and three technical replicates were determined for each time point. Error bars indicate ±SE. (I) Endogenous JA levels in the WT, miR319-oe, and LAm-oe lines. The JA concentration in leaves was measured in triplicate at 0h and 12h after inoculation. At least 10 plants were included in each pool, and three biological replicates were performed. (This figure is available in colour at JXB online.)
The levels of endogenous JA in WT and transgenic tomatoes were further detected at 0h and 12h after inoculation. The results showed that RNK invasion increased the endogenous JA levels in the WT but not in miR319-oe plants. LA m overexpression led to a remarkable increase in basal and induced JA levels (Fig. 7I).
Changes in expression of miR396 in roots under RKN stress
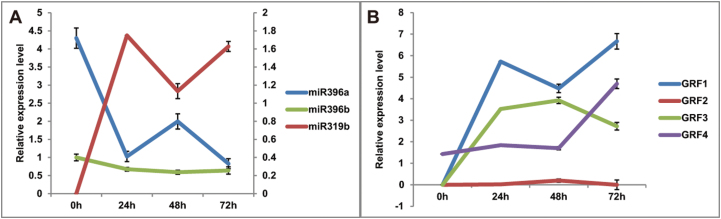
Previous studies reported that miR396/GRF functioned in nematode resistance in the roots of Arabidopsis (Hewezi et al., 2012). The sequencing data showed that both miR396a and miR396b were differentially expressed between the two libraries (Supplementary Table S2 at JXB online). miR396a expression levels declined in root; however, no obvious expression differences were observed for miR396b (Fig. 8A). The miR396 targets GRF1, GRF3, and GRF4, but not GRF2, were transcriptionally up-regulated (Fig. 8B), suggesting that the miR396/GRF module is responsive to RKN invasion in tomato.
Fig. 8.
The relative expression levels of miR396a, miR396b, miR319b (A), and GRF (B) genes in roots at different time points after RKN inoculation in the WT. The primary axis corresponds to the lines with diamonds and triangles and the secondary axis corresponds to the line with squares. At least 10 plants were included in each pool, and three technical replicates were determined for each time point. Error bars indicate ±SE. (This figure is available in colour at JXB online.)
Discussion
miR319 negatively regulates RKN resistance by affecting the JA level in plants
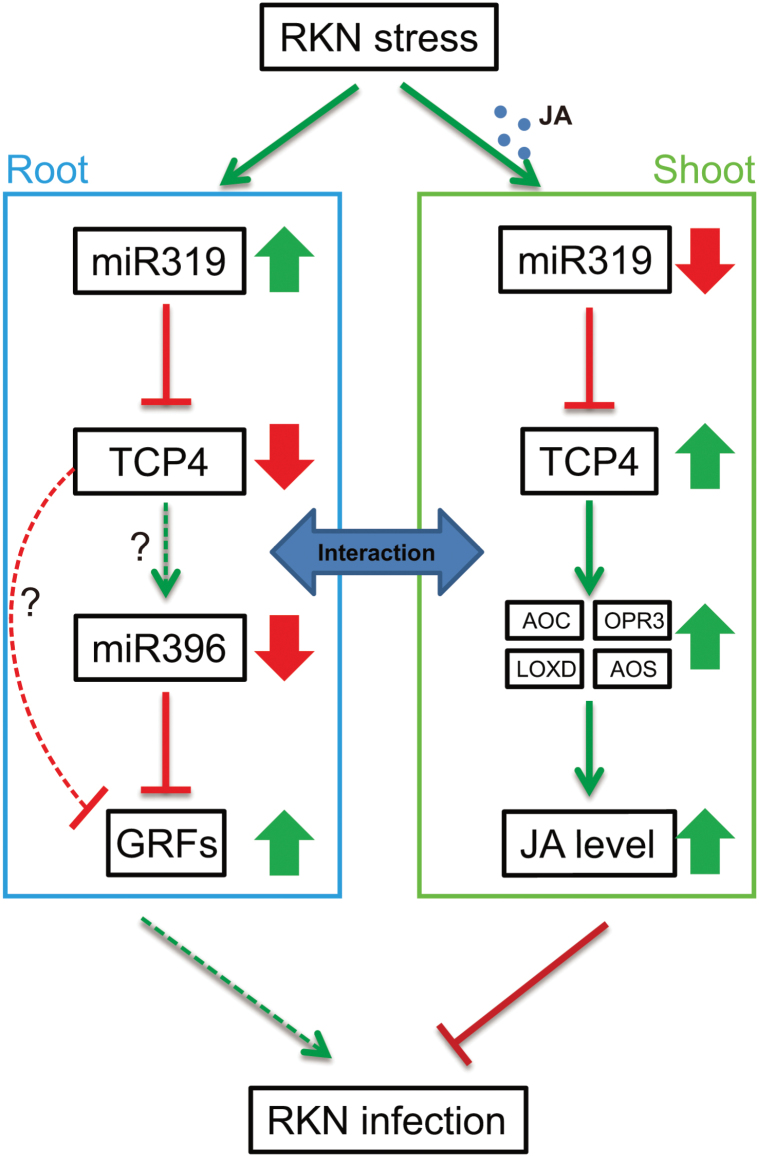
miR319 is one of the most highly conserved miRNAs in plants and was also one of the first to be identified (Weigel et al. 2000; Palatnik et al. 2003; Schommer et al. 2012). Previous studies revealed that the miR319 family is involved in biotic stress response (Zhang et al., 2011; Feng et al., 2014; Shen et al., 2014). In the present work, miR319 was identified between two libraries, WT+N and spr2+N (Supplemetnary Table S2 at JXB online), and the expression analysis indicated that the response of miR319/TCP4 to RKN invasion occurred in both shoots and roots (Fig. 5A–C). miR319 has been reported to target TCP genes, which encode plant-specific transcription factors (Palatnik et al., 2003, 2007; Schwab et al., 2005; Koyama et al., 2007, 2010; Ori et al., 2007; Schommer et al., 2008, 2012; Nag et al., 2009) that are involved in JA biosynthesis and senescence (Schommer et al., 2008). Microarray experiments comparing the shoot apical meristem transcriptomes of WT and miR319-oe plants have revealed a clear decrease in the levels of all miR319-targeted TCP genes (Palatnik et al., 2003; Efroni et al., 2008; Schommer et al., 2008). Consistent with these studies, miR319 overexpression led to a decrease in the transcriptional level of TCP4 (Fig. 5H). Schommer et al. (2008) and Hao et al. (2012) demonstrated that TCP positively regulates the JA level in plants. Similarly, it was also observed here that LA m overexpression resulted in an increase in the basal and RKN-induced levels of JA-synthetic gene expression and endogenous JA; however, these levels were decreased in the miR319-oe plants (Fig. 7). Previous studies revealed that the JA pathway is a crucial player in maintaining and defending RKN in plants (Cooper et al., 2005; Fujimoto et al., 2011; Nahar et al., 2011; Fan et al., 2014). In the present study, RKN resistance increased in LAm-oe but decreased in the miR319-oe plants (Fig. 6). Taken together, these results suggested that overexpressing miR319 inhibited TCP4, which in turn regulated JA biosynthetic genes (Fig. 7A–H), resulting in a lower endogenous JA level (Fig. 7I). Consequently, the resistance to RKN infection was affected in miR319-oe tomatoes (Fig. 6).
In the WT background, JA treatment led to the decrease of expression of miR319b and increase of TCP4 (Fig. 5D, E) in leaves, which is consistent with the observations in RKN stress (Fig. 5A). Combined with the results in spr2 (Fig. 3), these findings implied that JA acts as the regulator of miR319b in early RKN response (Fig. 9). miR319b was responsive to RKN invasion and was down-regulated in shoot, with the expression of TCP4 subsequently increasing (Fig. 5A, B), such that JA synthesis in shoots may become activated (Fig. 7A, C, E, G, I), leading to JA-mediated systemic resistance (Fig. 9). Combined with the results in miR319-oe and LAm-oe, this suggested that JA mediated miR319 serving as a systemic defensive responder and modulator that functioned at least partially via TCP4 under RKN stress (Fig. 9).
Fig. 9.
Model of miR319/TCP-mediated regulation of RKN resistance in the WT background. Small filled circles indicate JA molecules. Solid lines represent regulatory links observed in tomato, and dashed lines represent regulatory links observed in Arabidopsis. Arrows indicate positive regulation, and blunt-ended bars indicate inhibition. A line does not necessarily represent unique or direct regulation. A question mark refers to unverified regulation under RKN stress. Broad arrows represent the up- or down-regulation of genes and hormone levels. (This figure is available in colour at JXB online.)
miR396 probably participates in RKN resistance in tomato roots
miR396 is a negative regulator of mitotic cell division that acts through the down-regulation of GRF genes in shoot meristems, leaves, and roots (Liu et al., 2008; Rodriguez et al., 2010; Wang et al., 2011; Hewezi et al., 2012). The miR396/GRF module was reported to be involved in cyst nematode resistance in Arabidopsis (Hewezi et al., 2008, 2012). The accumulated evidence demonstrates that miR319 and miR396 are the miRNAs with the most molecular connections to phytohormone signalling pathways (Curaba et al., 2014). The overexpression of an miRNA-resistant form of AtTCP4, a target of miR319, results in increased miR396 accumulation and a corresponding decrease in AtGRF gene expression, and AtTCP4 also regulates GRF activity independently of miR396 (Rodriguez et al., 2010; Fig. 9). Recently, Schommer et al. (2014) reported that miR319-regulated TCP4 positively regulates miR396, thereby repressing GRFs and cell proliferation in Arabidopsis. In the present study, the expression patterns between miR319b and miR396a were diametrically opposite (Fig.5A), hence a similar regulation between miR396a and miR319b/TCP4 appeared to occur in tomato roots under RKN stress (Figs 8A, 9). Previous studies demonstrated that miR396 was significantly down-regulated in response to cyst nematodes, which is consistent with the present results (Fig. 8A; Hewezi et al., 2008), and miR396 overexpression reduced the syncytium size and arrested cyst nematode development by repressing GRFs in Arabidopsis roots (Fig. 9; Hewezi et al., 2012).
Based on the above data, a model is proposed whereby the miR319/TCP module influences RKN resistance via two pathways in tomato (Fig. 9): TCP-regulated JA biosynthesis in shoots and cell proliferation regulated by the miR396/GRF module in roots.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Mi-1 gene test of plant materials used in this study.
Table S1. Primers used for stem–loop RT–-PCR and qRT–PCR.
Table S2. Differentially expressed known miRNAs between the WT and spr2 after RKN inoculation.
Table S3. Differentially expressed novel miRNAs between the WT and spr2 after RKN inoculation.
Table S4. Predicted targets of differentially expressed known miRNAs.
Table S5. Predicted targets of differentially expressed novel miRNAs.
Table S6. KEGG enrichment of known and novel miRNA targets.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 31171952) and the Project of Great Wall Scholar, Beijing Municipal Commission of Education (CIT&TCD20130323). We are grateful to Professor V.M. Williamson for the gift of tomato UC82 seeds, Professor Chuanyou Li for tomato spr2 seeds, and Professor O. Naomi for the op:gene and FIL:LhG4 tomato seeds.
References
- Achard P, Herr A, Baulcombe DC, Harberd NP. 2004. Modulation of floral development by a gibberellin-regulated microRNA. Development 131, 3357–3365. [DOI] [PubMed] [Google Scholar]
- Aukerman MJ, Sakai H. 2003. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. The Plant Cell 15, 2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzini AA, Hopp HE, Beachy RN, Asurmendi S. 2007. Infection and coaccumulation of tobacco mosaic virus proteins alter microRNA levels, correlating with symptom and plant development. Proceedings of the National Academy of Sciences, USA 104, 12157–12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang T, Zhao M, Tian Q, Zhang WH. 2012. Identification of aluminum-responsive microRNAs in Medicago truncatula by genome-wide high-throughput sequencing. Planta 235, 375–386. [DOI] [PubMed] [Google Scholar]
- Chen X. 2004. A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303, 2022–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WR, Jia L, Goggin L. 2005. Effects of jasmonate-induced defenses on root-knot nematode infection of resistant and susceptible tomato cultivars. Journal of Chemical Ecology 31, 1953–1967. [DOI] [PubMed] [Google Scholar]
- Curaba J, Singh MB, Bhalla PL. 2014. miRNAs in the crosstalk between phytohormone signalling pathways. Journal of Experimental Botany 65, 1425–1438. [DOI] [PubMed] [Google Scholar]
- Dai X, Zhao PX. 2011. psRNATarget: a plant small RNA target analysis server. Nucleic Acids Research 39, W155–W159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida Engler J, Engler G, Gheysen G. 2011. Unravelling the plant cell cycle in nematode induced feeding sites. In: Jones J, Gheysen G, Fenoll C, eds. Genomics and molecular genetics of plant–nematode interactions. Dordrecht, The Netherlands: Springer Science + Business Media, 349–368. [Google Scholar]
- Ding Y, Chen Z, Zhu C. 2011. Microarray-based analysis of cadmium-responsive microRNAs in rice (Oryza sativa). Journal of Experimental Botany 62, 3563–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efroni I, Blum E, Goldshmidt A, Eshed Y. 2008. A protracted and dynamic maturation schedule underlies Arabidopsis leaf development. The Plant Cell 20, 2293–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JW, Hu CL, Zhang LN, Li ZL, Zhao FK, Wang SH. 2014. Jasmonic acid mediates tomato’s response to root knot nematodes. Journal of Plant Growth Regulation 34, 196–205. [Google Scholar]
- Feng JL, Liu SS, Wang MN, Lang QL, Jin CZ. 2014. Identification of microRNAs and their targets in tomato infected with Cucumber mosaic virus based on deep sequencing. Planta 240, 1335–1352. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Tomitaka Y, Abe H, Tsuda S, Futai K, Mizukubo T. 2011. Expression profile of jasmonic acid-induced genes and the induced resistance against the root-knot nematode (Meloidogyne incognita) in tomato plants (Solanum lycopersicum) after foliar treatment with methyl jasmonate. Journal of Plant Physiology 168, 1084–1097. [DOI] [PubMed] [Google Scholar]
- Hao J, Tu LL, Hu HY, Tan JF, Deng FL, Tang WX, Nie YC, Zhang XL. 2012. GbTCP, a cotton TCP transcription factor, confers fibre elongation and root hair development by a complex regulating system. Journal of Experimental Botany 63, 6267–6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewezi T, Howe P, Maier TR, Baum TJ. 2008. Arabidopsis small RNAs and their targets during Cyst nematode parasitism. Molecular Plant-Microbe Interactions 12, 1622–1634. [DOI] [PubMed] [Google Scholar]
- Hewezi T, Maier TR, Nettleton D, Baum TJ. 2012. The Arabidopsis microRNA396–GRF1/GRF3 regulatory module acts as a developmental regulator in the reprogramming of root cells during cyst nematode infection. Plant Physiology 159, 321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Ton J. 2008. Long-distance signalling in plant defence. Trends in Plant Science 13, 264–272. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran G, Saini A, Sunkar R. 2009. Biotic and abiotic stress down-regulate miR398 expression in Arabidopsis . Planta 229, 1009–1014. [DOI] [PubMed] [Google Scholar]
- Kasschau KD, Xie Z, Allen E, Llave C, Chapman EJ, Krizan KA, Carrington JC. 2003. P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA function. Developmental Cell 4, 205–217. [DOI] [PubMed] [Google Scholar]
- Koyama T, Furutani M, Tasaka M, Ohme-Takagi M. 2007. TCP transcription factors control the morphology of shoot lateral organs via negative regulation of the expression of boundary-specific genes in Arabidopsis . The Plant Cell 19, 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M. 2010. TCP transcription factors regulate the activities of ASYMMETRIC LEAVES1 and miR164, as well as the auxin response, during differentiation of leaves in Arabidopsis . The Plant Cell 22, 3574–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J, Loedige I, Filipowicz W. 2010. The widespread regulation of microRNA biogenesis, function and decay. Nature Reviews Genetics 11, 597–610. [DOI] [PubMed] [Google Scholar]
- Kyndt T, Vieira P, Gheysen G, de Almeida-Engler J. 2013. Nematode feeding sites: unique organs in plant roots. Planta 238, 807–818. [DOI] [PubMed] [Google Scholar]
- León J. 2013. Role of plant peroxisomes in the production of jasmonic acid-based signals. Subcellular Biochemistry 69, 299–313. [DOI] [PubMed] [Google Scholar]
- Li B, Qin Y, Duan H, Yin W, Xia X. 2011. Genome-wide characterization of new and drought stress responsive microRNAs in Populus euphratica . Journal of Experimental Botany 62, 3765–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Liu GH, Xu CC, Lee GI, Bauer P, Ling HQ, Ganal MW, Howe GA. 2003. The tomato suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. The Plant Cell 15, 1646–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschitz E, Eviatar T, Rozman A, Shalit A, Goldshmidt A, Amsellem Z. 2006. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proceedings of the National Academy of Sciences, USA 103, 6398–6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HH, Tian X, Li YJ, Wu CA, Zheng CC. 2008. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana . RNA 14, 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough TJ, Lucas WJ. 2006. Integrative plant biology: role of phloem long-distance macromolecular trafficking. Annual Review of Plant Biology 57, 203–232. [DOI] [PubMed] [Google Scholar]
- Lu SF, Sun YH, Amerson H, Chiang VL. 2007. MicroRNAs in loblolly pine (Pinus taeda L.) and their association with fusiform rust gall development. The Plant Journal 51, 1077–1098. [DOI] [PubMed] [Google Scholar]
- Lu SF, Sun YH, Chiang VL. 2008. Stress-responsive microRNAs in Populus. The Plant Journal 55, 131–151. [DOI] [PubMed] [Google Scholar]
- Lu SF, Sun YH, Shi R, Clark C, Li LG, Chiang VL. 2005. Novel and mechanical stress responsive microRNAs in Populus trichocarpa that are absent from Arabidopsis . The Plant Cell 17, 2186–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv DK, Bai X, Li Y, Ding XD, Ge Y, Cai H, Ji W, Wu N, Zhu YM. 2010. Profiling of cold-stress-responsive miRNAs in rice by microarrays. Gene 459, 39–47. [DOI] [PubMed] [Google Scholar]
- Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. 2007. KEEG: an automatic genome annotation and pathway reconstruction server. Nucleic Acid Research 35, w182–w185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag A, King S, Jack T. 2009. miR319a targeting of TCP4 is critical for petal growth and development in Arabidopsis. Proceedings of the National Academy of Sciences, USA 106, 22534–22539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar K, Kyndt T, De Vleesschauwer D, Höfte M, Gheysen G. 2011. The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice. Plant Physiology 157, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JD. 2006. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312, 436–439. [DOI] [PubMed] [Google Scholar]
- Navarro L, Jay F, Nomura K, He SY, Voinnet O. 2008. Suppression of the microRNA pathway by bacterial effector proteins. Science 321, 964–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori N, Cohen AR, Etzioni A, et al. 2007. Regulation of LANCEOLATE by miR319 is required for compound-leaf development in tomato. Nature Genetics 39, 787–791. [DOI] [PubMed] [Google Scholar]
- Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, Weigel D. 2003. Control of leaf morphogenesis by microRNAs. Nature 425, 257–263. [DOI] [PubMed] [Google Scholar]
- Palatnik JF, Wollmann H, Schommer C, et al. 2007. Sequence and expression differences underlie functional specialization of Arabidopsis microRNAs miR159 and miR319. Developmental Cell 13, 115–125. [DOI] [PubMed] [Google Scholar]
- Pandey SP, Shahi P, Gase K, Baldwin IT. 2008. Herbivory-induced changes in the small-RNA transcriptome and phytohormone signaling in Nicotiana attenuata . Proceedings of the National Academy of Sciences, USA 105, 4559–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan XQ, Wang XM. 2009. Profiling of plant hormones by mass spectrometry. Journal of Chromatography B 877, 2806–2813. [DOI] [PubMed] [Google Scholar]
- Qiu DY, Pan XP, Wilson IW, Li FL, Liu M, Teng WJ, Zhang BH. 2009. High throughput sequencing technology reveals that the taxoid elicitor methyl jasmonate regulates microRNA expression in Chinese yew (Taxus chinensis). Gene 436, 37–44. [DOI] [PubMed] [Google Scholar]
- Reyes JL, Chua NH. 2007. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. The Plant Journal 49, 592–606. [DOI] [PubMed] [Google Scholar]
- Rodriguez RE, Mecchia MA, Debernardi JM, Schommer C, Weigel D, Palatnik JF. 2010. Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development 137, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth CM, Liu PP, Natalya AG, Hiroyuki N. 2010. microRNA, seeds, and Darwin?: diverse function of miRNA in seed biology and plant responses to stress. Journal of Experimental Botany 61, 2229–2234. [DOI] [PubMed] [Google Scholar]
- Schilmiller AL, Howe GA. 2005. Systemic signaling in the wound response. Current Opinion in Plant Biology 8, 369–377. [DOI] [PubMed] [Google Scholar]
- Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D. 2005. Specific effects of microRNAs on the plant transcriptome. Developmental Cell 8, 517–527. [DOI] [PubMed] [Google Scholar]
- Schommer C, Bresso EG, Spinelli SV, Palatnik JF. 2012. Role of microRNA miR319 in plant development. MicroRNAs in Plant Development and Stress Responses. Signaling and Communication in Plants 15, 29–47. [Google Scholar]
- Schommer C, Juan M., Debernardi JM, Bresso1 EG, Rodriguez RE, Palatnik JF. 2014. Repression of cell proliferation by miR319-regulated TCP4. Molecular Plant 10, 1533–1544. [DOI] [PubMed] [Google Scholar]
- Schommer C, Palatnik JF, Aggarwal P, Chetelat A, Cubas P, Farmer EE, Nath U, Weigel D. 2008. Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biology 6, e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seah S, Yaghoobi J, Rossi M, Gleason CA, Williamson VM. 2004. The nematode-resistance gene, Mi-1, is associated with an inverted chromosomal segment in susceptible compared to resistant tomato. Theoretical and Applied Genetics 108, 1635–1642. [DOI] [PubMed] [Google Scholar]
- Shen D, Suhrkamp Ina, Wang Y, Liu SY, Menkhaus J, Verreet JA, Fan LJ, Cai DG. 2014. Identification and characterization of microRNAs in oilseed rape (Brassica napus) responsive to infection with the pathogenic fungus Verticillium longisporum using Brassica AA (Brassica rapa) and CC (Brassica oleracea) as reference genomes. New Phytologist 204, 577–594. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Fu Y, Sunkar R, Barbazuk WB, Zhu JK, Yu O. 2008. Novel and nodulation-regulated microRNAs in soybean roots. BMC Genomics 9, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkar R, Zhu JK. 2004. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis . The Plant Cell 16, 2001–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut F, Rojas CA, Almeida KL, Grativol C, Domiciano GC, Lamb CR, Engler Jde A, Hemerly AS, Ferreira PC. 2012. Regulation of miR319 during cold stress in sugarcane. Plant, Cell and Environment 35, 502–512. [DOI] [PubMed] [Google Scholar]
- Thies JA, Merrill SB, Corley EL. 2002. Red food coloring stain: new, safer procedures for staining nematodes in roots and egg masses on root surfaces. Journal of Nematology 34, 179–181. [PMC free article] [PubMed] [Google Scholar]
- Thorpe MR, Ferrieri AP, Herth MM, Ferrieri RA. 2007. 11C-imaging: methyl jasmonate moves in both phloem and xylem, promotes transport of jasmonate, and of photoassimilate even after proton transport is decoupled. Planta 226, 541–551. [DOI] [PubMed] [Google Scholar]
- Truman W, Bennett MH, Kubigsteltig I, Turnbull C, Grant M. 2007. Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proceedings of the National Academy of Sciences, USA 104, 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP. 2007. Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gu X, Xu D, Wang W, Wang H, Zeng M, Chang Z, Huang H, Cui X. 2011. miR396-targeted AtGRF transcription factors are required for coordination of cell division and differentiation during leaf development in Arabidopsis . Journal of Experimental Botany 62, 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li P, Cao X, Wang X, Zhang A, Li X. 2009. Identification and expression analysis of miRNAs from nitrogen-fixing soybean nodules. Biochemical and Biophysical Research Communications 378, 799–803. [DOI] [PubMed] [Google Scholar]
- Waters BM, McInturf SA, Stein RJ. 2012. Rosette iron deficiency transcript and microRNA profiling reveals links between copper and iron homeostasis in Arabidopsis thaliana . Journal of Experimental Botany 63, 5903–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Ahn JH, Blazquez MA, et al. 2000. Activation tagging in Arabidopsis . Plant Physiology 122, 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Wang Y, Yao Y, Xie C, Peng H, Ni Z, Sun Q. 2010. Diverse set of microRNAs are responsive to powdery mildew infection and heat stress in wheat (Triticum aestivum L.). BMC Plant Biology 10, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Li D, Mao D, et al. 2013. Overexpression of microRNA319 impacts leaf morphogenesis and leads to enhanced cold tolerance in rice (Oryza sativa L.). Plant, Cell and Environment 36, 2207–2218. [DOI] [PubMed] [Google Scholar]
- Yu X, Wang H, Lu Y, de Ruiter M, Cariaso M, Prins M, van Tunen A, He Y. 2012. Identification of conserved and novel microRNAs that are responsive to heat stress in Brassica rapa . Journal of Experimental Botany 63, 1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Xie D, Jin Z. 2012. Global analysis of non-coding small RNAs in Arabidopsis in response to jasmonate treatment by deep sequencing technology. Journal of Integrative Plant Biology 54, 73–86. [DOI] [PubMed] [Google Scholar]
- Zhang WX, Gao S, Zhou X, et al. 2011. Bacteria-responsive microRNAs regulate plant innate immunity by modulating plant hormone networks. Plant Molecular Biology 75, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Liu Y, Liu Z, Kong D, Duan M, Luo L. 2010. Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa . Journal of Experimental Botany 61, 4157–4168. [DOI] [PubMed] [Google Scholar]
- Zhou M, Li D, Li Z, Hu Q, Yang C, Zhu L, Luo H. 2013. Constitutive expression of a miR319 gene alters plant development and enhances salt and drought tolerance in transgenic creeping bentgrass. Plant Physiology 161, 1375–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.