Highlight
Plants carefully control where and when flowers are made through activators and repressors. We show that spatially the shoot meristem is key in responding to the repressors of flowering TFL1.
Key words: Architecture, expression, flowering, identity, meristem, TFL1.
Abstract
Models for the control of above-ground plant architectures show how meristems can be programmed to be either shoots or flowers. Molecular, genetic, transgenic, and mathematical studies have greatly refined these models, suggesting that the phase of the shoot reflects different genes contributing to its repression of flowering, its vegetativeness (‘veg’), before activators promote flower development. Key elements of how the repressor of flowering and shoot meristem gene TFL1 acts have now been tested, by changing its spatiotemporal pattern. It is shown that TFL1 can act outside of its normal expression domain in leaf primordia or floral meristems to repress flower identity. These data show how the timing and spatial pattern of TFL1 expression affect overall plant architecture. This reveals that the underlying pattern of TFL1 interactors is complex and that they may be spatially more widespread than TFL1 itself, which is confined to shoots. However, the data show that while TFL1 and floral genes can both act and compete in the same meristem, it appears that the main shoot meristem is more sensitive to TFL1 rather than floral genes. This spatial analysis therefore reveals how a difference in response helps maintain the ‘veg’ state of the shoot meristem.
Introduction
The development of plants with different architectures reflects variation in underlying molecular patterns (Sussex and Kerk, 2001). These patterns are complex interactions of gene, protein, and metabolite systems (Kaufmann et al., 2010a; Sparks et al., 2013; Park et al., 2014). Analysis of these systems has identified many of the genes that control the formation of parts, their identity, position, and complexity (Benlloch et al., 2007; Studer et al., 2011; Alonso-Blanco and Mendez-Vigo, 2014). Such phenotypic traits have been selected during evolution and characterize different species, but, for any gene, what elements of its pattern are key in giving rise to a particular architecture?
Pattern elements include the level of gene expression, its timing, and in which cells it is expressed. For example, as plants pass through various developmental stages, different genes are expressed at appropriate levels and times, such as those maintaining the vegetative state of Arabidopsis (Poethig, 2010; Andres and Coupland, 2012). Other genes are expressed in specific domains to direct formation of organs in particular places, such as petals in flowers (O’Maoileidgh et al., 2014). The diversity of forms amongst species is the result of the evolution of these complex patterns. In addition to representing where, when, or how much of a gene is expressed, these patterns also determine potential new interacting genetic networks. Analysis of gene interactions, expression patterns, and loss- or gain-of-function phenotypes give us models for how these systems might operate. However, central to any models is the need to test how any pattern element contributes to generating a particular form. What is the effect of changing an element so a gene is expressed in novel domains or with different timing or levels? These questions have now been addressed for TFL1, a controller of plant architecture (Shannon and Meeks-Wagner, 1991; Alvarez et al., 1992; Schultz and Haughn, 1993; Ohshima et al., 1997).
TFL1 functions as a repressor of flowering and belongs to a small family of six genes in Arabidopsis (Kim et al., 2013). FT is a member of this family and is a key promoter of flowering, a florigen (Kardailsky et al., 1999; Kobayashi et al., 1999; Abe et al., 2005; Wigge et al., 2005; Shalit et al., 2009). The antagonism, different levels, and different expression patterns of these very similar proteins affect overall plant architecture (Hanzawa et al., 2005; Wigge et al., 2005: Shalit et al., 2009; Jaeger et al., 2013; Ho and Weigel, 2014). TFL1 can act through transcription to repress floral genes, and can modulate protein cellular protein trafficking patterns (Sohn et al., 2007; Hanano and Goto, 2011). Controlling the pattern and levels of TFL1 interactions in relation to floral meristem genes is predicted to be crucial (Prusinkiewicz et al., 2007; Koes, 2008; Jaeger et al., 2013; Park et al., 2014).
In wild-type (WT) Arabidopsis, TFL1 expression is limited to shoots (Simon et al., 1996; Bradley et al., 1997; Ratcliffe et al., 1999). During the vegetative phase, TFL1 is weakly expressed in the centre of the shoot meristem. This vegetative shoot meristem generates leaf primordia from its flanks to form a compact rosette. Upon integration of developmental and environmental signals, the shoot meristem makes cauline leaves (CLs; bearing shoot meristems in their axils) and the shoot elongates (bolts) (Poethig, 2010; Andres and Coupland, 2012; O’Maoileidgh et al., 2014). The level of TFL1 expression is up-regulated at this stage. TFL1 expression remains high in the shoot meristem as it generates floral meristems from its flanks, and TFL1 becomes strong in the stem.
This pattern of TFL1 expression appears to reflect its function. In tfl1 mutants, the shoot meristem makes fewer rosette leaves (RLs; see Table 1 for abbreviations) and plants bolt early compared with the WT (Shannon and Meeks-Wagner, 1991; Schultz and Haughn, 1993). Also, tfl1 mutants make fewer CLs and only a few flowers (Fs) before the shoot meristem converts to a floral meristem to give a terminal flower (Shannon and Meeks-Wagner, 1991; Alvarez et al., 1992; Schultz and Haughn, 1993). Thus TFL1 is needed to maintain and regulate shoot identity throughout the different phases of the plant life cycle to generate a particular architecture.
Table 1.
Abbreviations used for growth phases and plant organs scored
| Phase | Phase abbreviation | Lateral organs made by shoot meristem | Organ abbreviation |
|---|---|---|---|
| Vegetative rosette | V | Leaves | L |
| Inflorescence bolting and bearing cauline leaves | I1 | Cauline leaves | CL |
| Inflorescence/ap1-like structures without subtending leaves | I1* | Shoots or ap1-like flowers without subtending cauline leaves | I1* shoots, ap1-like F |
| Inflorescence with flowers | I2 | Flowers | F |
Models suggest how the TFL1 expression pattern controls Arabidopsis architecture (Ratcliffe et al., 1999; Liljegren et al., 1999; Ferrandiz et al., 2000; Prusinkiewicz et al., 2007). TFL1 delays the action of floral signals at the shoot meristem that promotes bolting, and TFL1 prevents their activity in the shoot meristem so that floral genes are not expressed in the shoot but only in lateral meristems. This maintains shoot identity and prevents the shoot meristem converting to a flower. An integrated model summarizes these interactions as acting upon the vegetativeness character (‘veg’) of the shoot apex (Prusinkiewicz et al., 2007). TFL1 contributes to ‘veg’ to delay flowering. Models also show how genes affecting floral meristem development, such as LFY, AP1, CAL, or FUL, prevent TFL1 expression in floral meristems. For example, both LFY and AP1 repress TFL1 by direct binding to its promoter (Kaufmann et al., 2010b; Winter et al., 2011). Also, a series of MADS box transcription factors promoting floral meristem identity suppress TFL1 in emerging floral meristems in Arabidopsis, and similarly in other species (Liu et al., 2013). This mutual inhibition results in clear domains of expression and activity, and a shoot architecture of leaves and branches at the base and an elongated stem with flowers on its sides. These models are consistent with mutant phenotypes and the expression patterns of these genes in various backgrounds (Blazquez et al., 2006). However, how do these myriad of interactions tie in with the spatial network of TFL1 action?
These models are supported by the phenotypes of plants ectopically expressing floral genes or TFL1 (Weigel and Nilsson, 1995; Mandel and Yanofsky, 1995; Ratcliffe et al., 1998, 1999; Liljegren et al., 1999; Parcy et al., 2002). Most of these ectopic studies have used the p35S promoter which is expressed constitutively, in most tissues, though patterns can vary (van Leeuwen et al., 2001). In p35S::LFY or p35S::AP1 plants, all phases are shorter (like tfl1 mutants), with plants bolting early and shoot meristems converting to flowers (Mandel and Yanofsky, 1995; Weigel and Nilsson, 1995). In p35S::TFL1 plants, all phases are longer, with more RLs and CLs. These plants also make a novel I1* phase of shoots without subtending CLs and ap1-like structures (Ratcliffe et al., 1998). In double transgenics such as p35S::pAP1;35S::TFL1, intermediate phenotypes occur, again suggesting that TFL1 and floral genes are antagonistic in their effects on meristem identity (Liljegren et al., 1999; Ratcliffe et al., 1999). However, the p35S promoter does not tell us where these genes act; is it in the same or different tissues? Neither does p35S tell us how genes may have quantitative effects or whether their expression at different times has different effects on architecture.
In this study, tests were carried out to determine how different elements of the TFL1 expression pattern contribute to plant architecture. Three different promoters were used to change the regulation of TFL1 expression, and when and where it can act. Further, by using floral promoters to express TFL1, it was directly tested how floral genes and TFL1 compete in the same tissues, and at the same time. By using tfl1 and WT backgrounds, the action of TFL1 in the shoot meristem could also be directly compared with its effects in lateral meristems, using the same constructs. It is shown that TFL1 can act outside of the shoot meristem, affecting the fate of lateral primordia. Therefore, TFL1 interactors and signalling components to affect ‘veg’ are not restricted to the shoot meristem. TFL1 prevented leaves from becoming flowers and delayed floral gene action. However, floral genes eventually overcame TFL1 action in lateral meristems. Despite ectopic expression, plants can tolerate quite different TFL1 expression patterns and yet still generate a raceme. The use of different spatial promoters has allowed the suggestion that TFL1 is expressed in its specific pattern to engineer sharp transitions from shoots to flowers, with the main and lateral meristems having different responses at the flowering transition.
Materials and methods
Plant materials and analyses
Arabidopsis ecotype Col (WT) and tfl1-1 (Shannon and Meeks-Wagner, 1991) were used as controls and hosts for transformation of promoter::TFL1 constructs. Lines were compared with p35S::TFL1 and grown in the greenhouse under controlled temperatures of 20–28 °C and long-day (LD) photoperiods supplemented with light as necessary [400W Philips HDK/400 HPI (R) (N) or cool light from fluorescent tubes at an intensity of 90–120 mmol m–2 s–1] to give 16h light/8h dark as described (Ratcliffe et al., 1998).
The WT and tfl1 mutants were transformed with pLFY::TFL1 or pAP1::TFL1, and 14–33 transformants were obtained. These transformants were analysed and 7–12 single insertion locus lines were identified for each construct. For pANT::TFL1, only WT plants were directly transformed. Subsequently, lines were used to introduce pANT::TFL1 into tfl1 mutants by crossing. Five to seven lines for all constructs were preliminarily analysed to show consistent results (Supplementary Fig. S1 available at JXB online). Data from two representative strong lines for each construct were collected when all lines were grown at the same time and under the same LD greenhouse conditions, allowing a direct, quantitative comparison of phenotypes. Lines were sown and analysed in 3–5 independent experiments, and the results obtained showed that the phenotypes of all lines were highly consistent relative to controls. Abbreviations used for phenotypes of growth phases and plant organs scored are given in Table 1.
Promoter::TFL1 constructs
The ANT promoter was a 4.2kb 5’ region (pYM-94-1) kindly provided by Yukiko Mizukami (Grandjean et al., 2004). The LFY promoter was a 2.3kb 5’ region (pDW132) kindly provided by Detlef Weigel (Blazquez et al., 1997). The AP1 promoter was a 1.7kb 5’ region (pKY72) kindly provided by Marty Yanofsky (Hempel et al., 1997). The TFL1 cDNA (Hanzawa et al, 2005) was amplified with primers Y34 (AGTGGATCCATGGAGAATATGGGAACT) and Y37 (ATGGAATTCCTAGCGTTTGCGTGCAG) to add XhoI and BglII sites 5’ of ATG and 3’ of the stop codon, respectively, and cloned into pGEM-T (Stratagene). This XhoI–BglII fragment was cloned into a vector with a multiple cloning site and the p35S terminator to give pK6. The different promoter fragments were cloned into this vector as XhoI–BglII (pANT), SalI–BamHI (pLFY), or HindIII–BamHI (pAP1), respectively. The full promoter::TFL1 fragments were then transferred as XhoI–BamHI (pANT::TFL1), XhoI–BamHI (pLFY::TFL1), or HindIII–BamHI (pAP1::TFL1) to the binary vector pGreen0229 (Basta resistant; Hellens et al., 2000). This gave pK31 (pANT::TFL1), pK30 (pLFY::TFL1), and pK26 (pAP1::TFL1), which were transformed into Agrobacterium GV3101 with pSOUP and used to transform Arabidopsis plants by dipping as described (Clough and Bent, 1998).
RNA in situ hybridization
RNA in situ hybridization experiments with TFL1, LFY, AP1, and ANT antisense and sense probes were carried out as described (Ferrandiz et al., 2000). Note that quantification of in situ signals is not possible. Signal from probes cannot be compared to say if one is at different levels of expression, even though all probes are made at the same time, in the same way, as their base sequences. Also, the same probe on two different plants is still difficult to compare as tissue fixation and tissues vary.
Results
Three promoters, pANT, pLFY, and pAP1, were used to express TFL1 in novel patterns during Arabidopsis development. These allowed TFL1, normally expressed only in the centre of shoot meristems, to be expressed ectopically in leaf primordia and floral meristems. In the WT, pANT is expressed in leaf primordia on the flanks of the shoot during all phases of growth (Elliott et al., 1996; Klucher et al., 1996; Long and Barton, 2000; Mizukami and Fischer, 2000; Grandjean et al., 2004). pLFY is weakly expressed in leaf primordia, but strongly in young floral meristems (Blazquez et al., 1997; Hempel et al., 1997). pAP1 is only expressed in floral meristems, from stage 1 (Mandel et al., 1992; Hempel et al., 1997). The promoter fragments used have been shown largely to direct these expression patterns (Hempel et al., 1997; Krizek 1999; Benlloch et al., 2011). Further, the data below also showed that these promoters could drive expression of TFL1 ectopically in such patterns. In the tfl1 mutant background, both pLFY and pAP1 become ectopically active in the shoot meristem (Weigel et al., 1992; Bowman et al., 1993; Gustafson-Brown et al., 1994; Bradley at al., 1997; Liljegren et al., 1999). The 35S promoter (p35S) was also used as a control to drive general, constitutive TFL1 expression in the WT during all phases and in most tissues as described (Ratcliffe et al., 1988). This promoter is considered strong and general, but can be variable, especially due to position effects (van Leeuwen et al., 2001).
Abbreviations were used in scoring phenotypes in terms of growth phases and types of organs generated from lateral primordia and meristems (Table 1).
Different promoters complement the TFL1 vegetative phase defect
The effects of the promoter::TFL1 constructs on vegetative development were investigated in terms of whether they extend the vegetative phase so that more RLs were generated.
WT plants made ~11 RLs in long days (Fig. 1). No significant changes in leaf number were observed in any lines carrying any of the three constructs (Fig. 1). The range of leaf numbers was greater in plants carrying pANT::TFL1 (9–16 leaves compared with 10–13 for the WT), but the averages were not statistically significant. This variability for pANT::TFL1 was seen in different experiments. In contrast, plants carrying p35S::TFL1 (which is strongly expressed in all tissues, including primordia and the shoot meristem) had an extension of the vegetative rosette (V) phase to 19 RLs, as previously shown (Fig. 1; Ratcliffe et al., 1998).
Fig. 1.
Ectopic TFL1 affects plant organ numbers. The number of rosette leaves (RLs), cauline leaves (CLs), I1* structures (shoots without subtending CLs or ap1-like structures), and flowers (Fs) made by the main shoot were recorded for wild-type (WT) Arabidopsis or tfl1-1 mutants containing pANT::TFL1, pLFY::TFL1, or pAP1::TFL1. WT plants containing p35S::TFL1 and ap1-12 mutants were also analysed. Numbers represent the average of 23–54 plants with standard deviations as shown. The solid black bars in (F) in the tfl1 background represent termination of the main shoot by conversion to a flower.
TFL1 extended the V phase in the WT, but only when expressed via p35S. There are a number of differences between p35S and the other promoters, including the timing and expression domain. To determine which aspect of p35S was important, either other promoters that had each of these features could be sought or these same promoters could be used but the genetic background could be altered to change their pattern. It was possible to change the pattern of these tested promoters by putting them into the tfl1 mutant background. In tfl1, pLFY and pAP1 express TFL1 in primordia and throughout the shoot meristem (a change in domain), and earlier than in the WT (change in timing).
Analysis of pANT::TFL1 or pLFY::TFL1 in the tfl1 mutant showed that the V phase was extended compared with tfl1 (Fig. 1). The tfl1 mutant had a shorter V phase compared with transformants (Fig. 2E, F). After ~16–20 d, the tfl1 mutant had already flowered and made seed pods (siliques). At this time point, tfl1 lines carrying pLFY::TFL1 or pAP1::TFL1 had just started to bolt and were making flowers that had not yet matured. The common effect in all lines was to restore the V phase to WT (Fig. 1). Interestingly, pAP1::TFL1 could also restore the V phase to WT in the strongest examples, and weaker lines always made significantly more RLs than tfl1. Unlike p35S, all other promoter lines made WT numbers of RLs, not more.
Fig. 2.
Plant architectures due to TFL1 expression. (A–D) Mature plants of Arabidopsis WT (A) or WT containing pANT::TFL1 (B), pLFY::TFL1 (C), or pAP1::TFL1 (D). In (A), the WT phases V, I1, and I2 are indicated. (E, F) Young tfl1-1 mutant plants already bolted with terminal flowers (E) compared with tfl1 containing pAP1::TFL1 at the same age of 16 d (F). (G–I) Mature plants showing tfl1-1 (G) or tfl1-1 containing pLFY::TFL1 (H) or pAP1::TFL1 (I). the insert in (H) shows that these plants eventually make normal flowers and terminate. Insert in (I) shows CLs with axillary ap1-like structures. Scale bars=1cm.
The expression patterns of TFL1 were analysed in the different lines by RNA in situ hybridization to see how they related to the plant phenotypes. In the vegetative phase of all WT lines, no early endogenous or transgenic TFL1 expression was clearly seen, except for general, constitutive p35S::TFL1 (Fig. 3A–E). Thus the lack of any TFL1 effect on the vegetative phase was most probably simply a lack of detectable expression. This may reflect the use of promoter fragments, sensitivity of detection, or even mRNA stability at early stages.
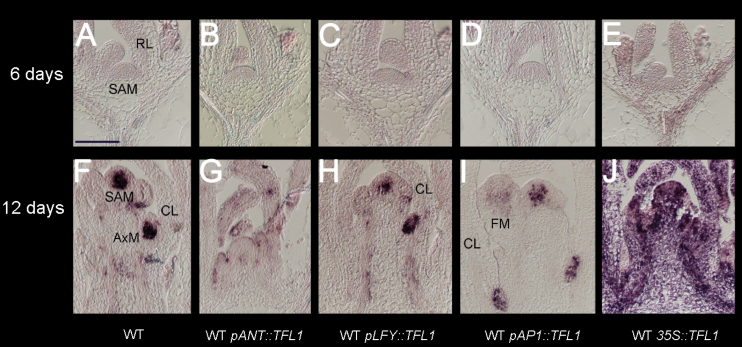
Fig. 3.
Early TFL1 expression patterns in the WT background. (A–E) TFL1 expression in the vegetative phase (6-day-old plants) of the WT (A) or the WT containing pANT::TFL1 (B), pLFY::TFL1 (C), pAP1::TFL1 (D), or p35S::TFL1 (E). For example, in (A), the shoot apical meristem (SAM) and rosette leaves (RL) generated by this meristem are indicated. (F–J) TFL1 expression in early to late I1 phase (10- to 12-day-old plants) of the WT (F) or the WT containing pANT::TFL1 (G), pLFY::TFL1 (H), pAP1::TFL1 (I), or p35S::TFL1 (J). Examples of inflorescence SAM, axillary meristems (AxM) in axils of cauline leaves (CL) and floral meristems (FM) are highlighted. All images were obtained with the same probes and signals developed for the same time. Signal is seen as a purple stain on a pale/pink background. Scale bar=100 μm.
In the tfl1 mutant background, no endogenous mutant tfl1-1 mRNA was seen at the early phase (Fig. 4A). Similarly, no transgenic TFL1 or tfl1-1 signal (together referred to as TFL1/tfl1-1) was seen at early time points, suggesting that it was below the detection limit (Fig. 4A–D). Thus, despite undetectable expression, all promoter::TFL1 lines in tfl1 had V phases restored to WT. Therefore, tfl1 may be easily complemented by different promoters, but the length of the V phase may be generally robust due to many flowering pathways controlling the ‘veg’ character of this growth phase (Prusinkiewicz et al., 2007).
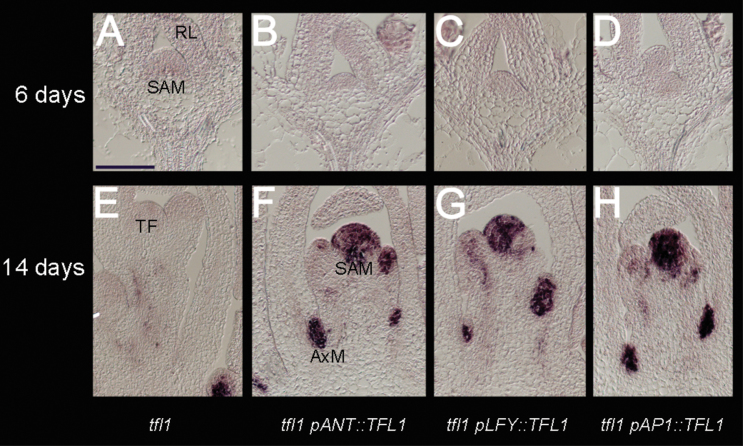
Fig. 4.
Early TFL1/tfl1-1 expression patterns in the tfl1-1 background. (A–D) TFL1/tfl1-1 expression at 6 d in tfl1-1 (A) or tfl1-1 containing pANT::TFL1 (B), pLFY::TFL1 (C), or pAP1::TFL1 (D). The shoot apical meristem is generating rosette leaves (RL). (E–H) TFL1/tfl1-1 expression at 12–14 d in the I1–I2 phase of tfl1-1 (E) or tfl11-1 containing pANT::TFL1 (F), pLFY::TFL1 (G), or pAP1::TFL1 (H). In tfl1, the SAM has already converted to a terminal flower (TF) while the other lines have TFL1/tfl1-1 mRNA in the shoot apical meristems but are still not terminating at this stage. All images were obtained with the same probes and signals developed for the same time. Signal is seen as a purple stain on a pale/pink background. Scale bar=100 μm.
Increased cauline leaf numbers by ectopic TFL1
After the V phase, the Arabidopsis shoot enters the first inflorescence (I1) phase, making CLs that have secondary shoots in their axils, on an elongated stem (bolt). WT plants made about three CLs on the main stem (Figs 1, 2). In the WT background, ectopic expression of TFL1 during this phase increased the number of CLs (Fig. 1). The strongest lines carrying pANT::TFL1 and pLFY::TFL1 showed an increase of 1–2 CLs compared with the WT. The range of CL numbers was only 2–4 for the WT, but 2–7 for pANT::TFL1. Therefore, these transformed plants displayed a more branching architecture compared with the WT (Fig. 2A–C). In contrast, WT lines containing pAP1::TFL1 had no change in their numbers of CLs and appeared as WT in I1 (Figs 1, 2D). Plants carrying p35S::TFL1 had a dramatic increase in the length of this phase (Fig. 1).
The tfl1 mutants made only one CL compared with three in the WT (Figs 1, 2). Also, in tfl1 mutants, all CLs had single flowers in their axils, while WT plants had shoots (Fig. 2A, E, G). In the tfl1 background, pANT::TFL1 and most pLFY::TFL1 lines had CL numbers similar to the WT (Fig. 1). Also, these lines had shoots in their CL axils (Fig. 2H). In tfl1, pAP1::TFL1 did not restore CL numbers to WT, but their numbers were significantly increased in the strongest lines compared with tfl1 (Fig. 1). Also, for the stronger lines, CLs usually had shoots in their axils (Fig. 2I). There was only one exception (of a flower) in 146 individuals scored. For the weaker lines, CLs often had axillary shoots, but flowers or AP1-like flowers (see below) were found at frequencies of 25–45% (Fig. 2I insert).
For plants in the I1 phase, endogenous WT TFL1 expression was seen for all lines in the shoot meristems (Fig. 3F–I). In the WT, TFL1 mRNA was observed in the main shoot meristem and stem tissues, and in the axillary shoot meristems of CLs (Fig. 3F). A similar pattern was seen for the different lines, but each line had a different pattern of ectopic TFL1 expression superimposed on the endogenous TFL1 mRNA pattern. For pANT::TFL1, ectopic TFL1 expression was seen in CLs (Fig. 3G). In pLFY::TFL1 there was also TFL1 expression in CLs, while pAP1::TFL1 lines had no TFL1 mRNA in leaves, only hints of ectopic expression in the first floral meristems as plants entered I2 (Fig. 3H, I). The p35S::TFL1 lines showed expression throughout most tissues (Fig. 3J). Therefore, these expression patterns appeared to correlate with small effects on the I1 phase for pANT and pLFY, as ectopic TFL1 mRNA appeared in CLs. No I1 expression was observed for pAP1, in agreement with no phenotypic effect in this phase. In contrast, the general expression of p35S must account for its strong I1 phenotype. It is difficult to comment on levels of expression as plant tissues differ; however, in situ hybridizations do reveal the distribution, and this is clearly more extensive in p35S. Thus making more CLs and branching architecture is dependent upon the pattern of TFL1 expression. This must then contribute to maintaining the ‘veg’ character to delay phase transitions (Prusinkiewicz et al., 2007). It suggests that repressors such as TFL1 can contribute to ‘veg’ even when expressed ectopically outside of the shoot meristem.
During this I1 phase in tfl1 mutants, endogenous mutant tfl1-1 mRNA was absent from the main shoot as it had already converted to a terminal flower by 12–14 d (Fig. 4E). Signal was restricted to young axillary meristems in the axils of RLs that had not yet converted to terminal flowers (Fig. 4E). For the different promoter lines, TFL1/tfl1-1 mRNA was seen for much longer in the main shoot meristems (Fig. 4F–H). Also, signal was strong in lateral meristems of CLs, correlating with their conversion from axillary flowers to shoots in these lines. It was also seen that TFL1/tfl1-1 signal appeared to be more extensive throughout the shoot meristem, not as restricted to the centre as in the WT. This novel pattern may reflect the partial floral nature of the main meristem to allow these promoters to be expressed beyond the normal central domain of TFL1. How each domain within the meristem contributes to the effect of TFL1 on ‘veg’ and delaying flowering cannot be resolved here.
TFL1 inhibits floral meristem development
After the I1 phase of making CLs, WT plants enter a second inflorescence phase (I2) and the shoot meristem generates floral meristems (FMs) from its flanks. These FMs proceed through various stages of development until forming siliques (Fig. 2A; Smyth et al., 1990). Expression of LFY from stage 0, and AP1 from stage 1, reflecting low ‘veg’, ensures suppression of shoot identity and production of flowers.
In all the transgenic lines tested here, expression of TFL1 from pANT, pLFY, or pAP1 inhibited floral meristem development, delaying the production of flowers and causing the production of an I1* phase. This phase was also seen in p35S::TFL1 lines, and consisted of shoot-like structures without any subtending CL, or ap1-like flowers that resulted in multiple siliques arising from a common floral stem, the pedicel (e.g. Fig. 2 insert). Although numbers were variable, these structures were found in all transgenic lines (Fig. 1). In contrast, these structures were rarely seen in control WT or tfl1 plants (Fig. 1; zero in this experiment). Although clear in the WT background, the number of novel structures generated was small (0.1–2) for any of the promoters used (Fig. 1). Stronger effects appeared in the tfl1 mutant background, where both pLFY::TFL1 and pAP1::TFL1 gave up to 4–8 structures.
The length of I1* seen in pAP1::TFL1 and pLFY::TFL1 lines was sometimes the same as in p35S::TFL1 (Fig. 1). Also, pLFY::TFL1 usually gave I1* shoots before I1* ap1-like floral structures, while pAP1::TFL1 rarely gave I1* shoots and more usually gave only ap1-like floral structures. ap1 mutants were examined, and it was found that, as in previous reports, an early, strong phenotype could be distinguished where mutant plants made structures similar to the ap1-like floral structures recorded in the transgenic lines, with flowers within flowers (Figs 1, 2I insert; Bowman et al., 1993). Later, weak phenotypes were observed where flowers were abnormal (e.g. reduced petals), but did not have flowers within flowers. These later weak phenotypes were called ap1-like ‘flowers’ for this comparative analysis, as the final siliques were singular, as in the WT, rather than having multiple siliques on one pedicel. Thus the strongest pAP1::TFL1 lines could have an I1* phase similar to ap1 mutants (Fig. 1).
The I1* phase of pANT::TFL1 lines consisted of both I1* shoots and ap1-like flowers, but this phase in tfl1 was short as in the WT (Fig. 1). Therefore, unlike pLFY or pAP1, the pANT::TFL1 lines never appeared to have a strong effect on flower development.
Since all lines had significant delays in making the transition from CL production (I1) to normal flowers (I2) and made a I1* phase, TFL1 expression was analysed in comparison with the floral genes LFY and AP1.
In the WT, TFL1 was expressed in the centre of the shoot meristem (and weakly in inflorescence stems), but not in lateral primordia or floral meristems (Fig. 5A). In a complementary manner, LFY and AP1 were not expressed in the shoot meristem of the WT, but only in the lateral meristems of I2, the floral meristems (Fig. 5B).
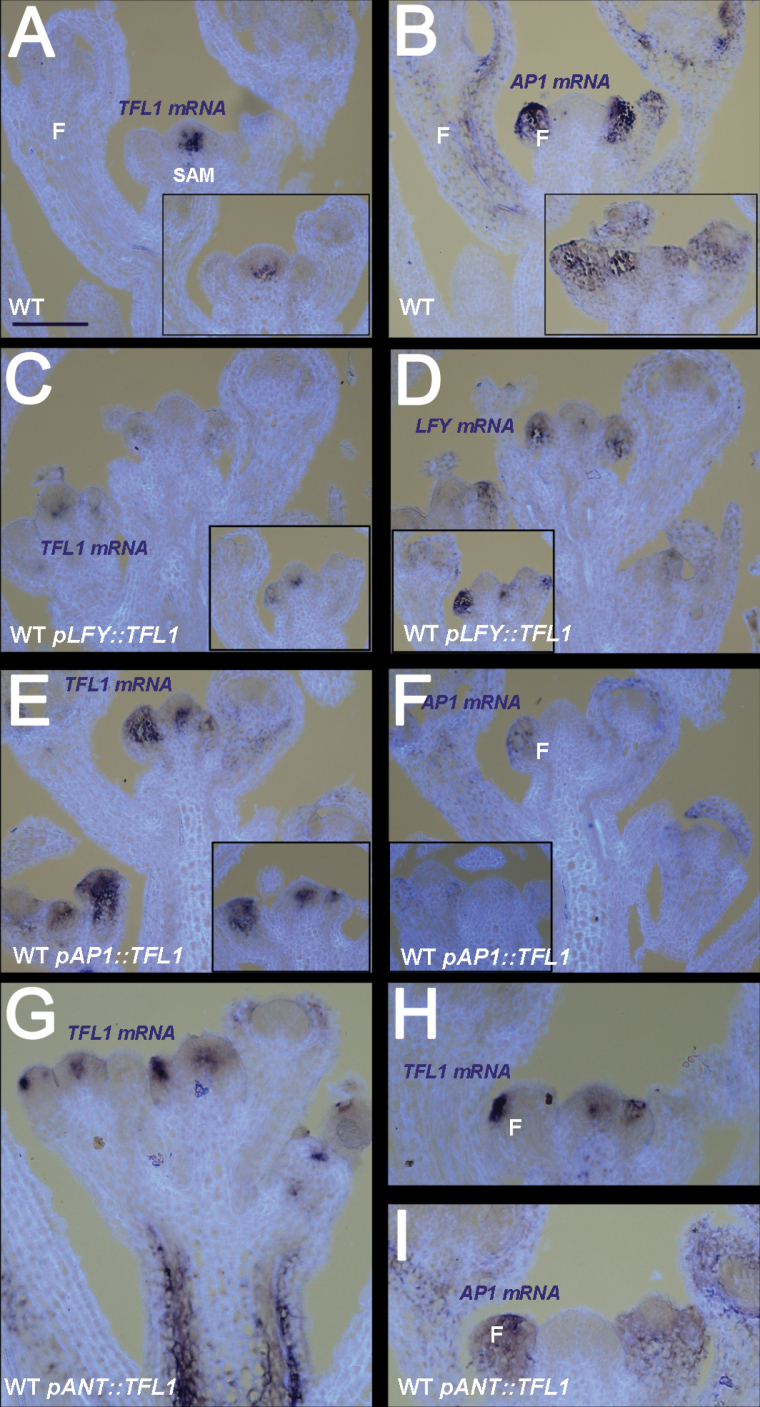
Fig. 5.
TFL1 and floral gene expression patterns in inflorescences in the WT background. (A, B) WT flowering shoots in the I2 phase at 21 d showing expression of TFL1 (A) or AP1 (B). Inserts in TFL1 (A) and LFY (B) at 17 d. (C, D) WT plants containing pLFY::TFL1 at 16 d showing TFL1 (C) and LFY (D) expression. Inserts show another transgenic line at 21 d. (E, F) WT plants containing pAP1::TFL1 at 21 d showing TFL1 (E) and (AP1) expression. Inserts show another line. (G–I) WT plants containing pANT::TFL1. Expression of TFL1 in a young tertiary shoot (G). Expression of TFL1 in an older secondary shoot (H) and AP1 expression (I). Shoot apical meristem (SAM), flower (F), and corresponding mRNA signals seen as a purple stain on pale blue/white tissue background. Scale bar=100 μm.
In WT lines with pLFY::TFL1, TFL1 was expressed ectopically and overlapping with LFY (Fig. 5C, D). Note that although LFY signal appeared stronger than TFL1, it could not be concluded that TFL1 was expressed less or was less stable than LFY, as the probes were different. WT lines containing pAP1::TFL1 also had clear ectopic expression of TFL1 in lateral meristems (Fig. 5E). Endogenous AP1 expression overlapped with TFL1 (Fig. 5F). These patterns for pLFY and pAP1 lines were consistent for many different lines, while lines with the weakest phenotypes had undetectable ectopic TFL1 expression. Also, the patterns were generally consistent over 16–22 d of growth, during which time some I1* shoot or ap1-like structures would have been made, as well as normal flowers.
WT lines containing pANT::TFL1 showed ectopic TFL1 in the young developing lateral meristems and their primordia (Fig. 5G). Comparison of TFL1 with AP1 in these lines showed that ectopic TFL1 occurred in lateral meristems that probably gave rise to flowers (Fig. 5H, I).
The TFL1/tfl1-1 expression patterns were compared with those of LFY and AP1. Due to having common promoters, the pattern of LFY mRNA reflected the pattern of TFL1 in pLFY::TFL1 expression. In contrast, tfl1-1 mRNA reflected its own promoter and this was highest in shoot-like structures. As tfl1 mutant plants were just bolting, tfl1-1 expression was seen in the main shoot (in the centre below the dome) at the same time as LFY also became ectopically expressed there (Fig. 6A, B). In pLFY::TFL1, the TFL1/tfl1-1 RNA was also seen at bolting, but no clear expression was seen of LFY at this time, reflecting the delay in flowering (Fig. 6C, D). After bolting, pLFY::TFL1 lines had TFL1/tfl11-1 expression that partially overlapped with that of LFY (Fig. 6E, F). Ectopic LFY in the shoot meristem was weak compared with LFY in lateral meristems (Fig. 6F). Ectopic expression of TFL1/tfl1-1 in the shoot meristem, beyond the central cells, was again seen. Some structures appeared ap1-like in tissue sections, and had ectopic TFL1/tfl11-1 (Fig. 6E left insert). In contrast, in ap1 mutant plants, ap1 mutant flowers did not appear to have strong ectopic TFL1 expression (Fig. 6E right insert).
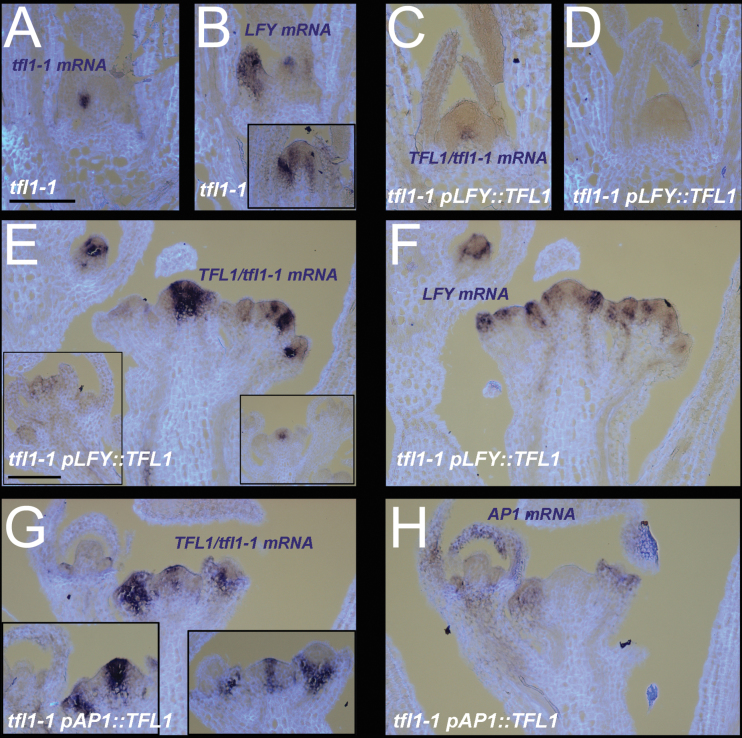
Fig. 6.
Expression patterns in tfl1 mutant backgrounds. (A, B) Young 10-day-old tfl1 mutants showing tfl1-1 (A) and LFY (B) expression. The insert in (B) shows plants just starting to bolt and ectopically expressing LFY in the shoot. (C, D) Ten-day-old day tfl1 plants containing pLFY::TFL1 showing TFL1/tfl1-1 (C) and absence of LFY (D) expression. (E, F) Older tfl1 plants containing pLFY::TFL1 at 17 d showing TFL1/tfl1-1 (E) and LFY (F) expression. The left insert in (E) shows expression at 20 d in another line in an ap1-like structure. The right insert shows that TFL1 expression is largely limited to the shoot meristem in ap1 mutants. (G, H) At 21 d, tfl1 mutants containing pAP1::TFL1, showing TFL1/tfl1-1 (G) and AP1 (H) expression. Inserts in (G) show other examples. Corresponding mRNA signals seen as a purple stain on pale blue/white tissue background. Scale bars=100 μm.
For pAP1::TFLl lines in tfl1, ectopic expression of TFL1 often appeared as clear as endogenous tfl1-1 seen in the tfl1 mutant itself (Fig. 6A, G). This expression was found at different time points, even when the shoot was making apparently normal flowers (Fig. 6G, H). Expression of AP1 partially overlapped with ectopic TFL1 in flowers in these lines (Fig. 6H). Interestingly, AP1 was often undetectable in the shoot meristem, compared with lateral AP1 (Fig. 6H). This suggested that pAP1::TFL1 was also undetectable in the shoot meristem, yet no terminal flower was evident at any of these time points (13–21 d).
Flowers are produced despite TFL1 expression
For all of the lines in the WT background, normal-looking, fertile flowers (Fs) were produced after the I1* phase, typical of a wild-type I2 phase (Fig. 2A–D). Occasionally, ap1-like structures were found later on the shoot in I2. The inflorescences of WT and transgenic lines generated a similar number of flowers before normal senescence (Fig. 1). However, in one pLFY::TFL1 line and one pANT::TFL1 line, both of which had very weak V-I1* phenotypes, terminal flowers were made in I2 after ~25–30 flowers. This suggested problems in late TFL1 function in these lines.
The I2 flowering phase of tfl1 mutants is very short, with very few lateral Fs being made before the shoot meristem itself is converted to a terminal flower (Fig. 1). This gave tfl1 mutant plants their characteristic short stature (Fig. 2E, G). In the tfl1 mutant background, pLFY::TFL1 and pAP1::TFL1 lines made many more lateral Fs (up to 30 times) compared with tfl1 (Fig. 1). These Fs also generally appeared normal, as in the WT, indicating that LFY and AP1 were largely unaffected by co-expression of TFL1 (Fig. 2H, I). However, during this same growth phase the conversion of the shoot meristem to terminal flowers was strongly delayed, indicating that TFL1 strongly inhibited LFY and AP1 action in the main shoot meristem, promoting the ‘veg’ character of these plants.
The conversion of the shoot meristem to a terminal flower results in a typical architecture of siliques clustered at the apex, with the central silique either normal or a bit smaller and distorted (Shannon and Meeks-Wagner, 1991; Schultz and Haughn, 1993). However, in ~5% of pANT and 20–25% of pLFY or pAP1 lines, the apex appeared fasciated and bent as it terminated. This phenotype can often be seen in lfy mutants when they terminate in a carpel-like structure (Weigel et al., 1992). This may reflect TFL1 inhibiting LFY even at very late stages, still promoting ‘veg’.
Discussion
Use of three independent promoters revealed how the timing and level of TFL1 expression is important in affecting plant architecture. It was shown that TFL1 is able to act outside of the shoot meristem. Ectopic expression of TFL1 is sufficient to convert lateral meristems to leaves and shoots, by delaying the activity of co-expressed floral meristem identity genes. By expressing TFL1 in different domains (using WT and tfl1 backgrounds), it was also revealed that the main shoot appears more responsive to TFL1 than lateral meristems, as more extensive phase effects were seen in the tfl1 background where the promoters used became active not just in the lateral meristems but also in the shoot meristem. Therefore, the underlying spatiotemporal patterns of interactors for TFL1 probably differ between shoot and lateral meristems. Thus TFL1 promotes ‘veg’ most probably through the shoot meristem. These data should help in understanding which elements of the TFL1 expression pattern are important in establishing and maintaining particular plant architectures.
TFL1 can function outside of the shoot meristem
Ectopic TFL1 prevents lateral meristems from undergoing a floral fate. This change in pattern leads to increased branching and plant size, resulting in an altered architecture. In the WT, ectopic expression of TFL1 (via pANT or pLFY) in lateral meristems resulted in more cauline leaves being made. The normal actions of LFY, and probably other factors such as LIM1, were inhibited by TFL1 and so these factors were unable to act in their normal role to suppress leaf and shoot formation (Weigel et al., 1992; Huala and Sussex, 1992; Saddic et al., 2006). Leaf and shoot development occurred despite LFY being expressed at the same time and in the same place as TFL1. Further, when LFY action appeared to be partially restored (as cauline leaves were suppressed), TFL1 still inhibited flower development in lateral meristems. The strongest effects gave rise to abnormal shoots similar to ap1;lfy double mutants, but more often to ap1-like structures (Bowman et al., 1993; Weigel and Meyerowitz, 1993; Mandel and Yanofsky, 1995; Parcy et al., 1998; Wagner et al., 1999). Thus the action of both LFY and AP1, and probably other genes such as FUL or CAL, was inhibited by TFL1 when co-expressed in the same primordia or meristems (Mandel and Yanofsky, 1995; Liljegren et al., 1999; Ratcliffe et al., 1999; Ferrandiz et al., 2000).
TFL1 may also act ectopically in the vegetative phase of Arabidopsis development. Of the promoters used, only p35S had a significant effect on RL number. As the other promoters are known to have only weak expression in early phases, which aspect of p35S, its high expression or expression in the shoot meristem and in leaves, was critical in affecting V phase, cannot be resolved.
A clear effect on the vegetative phase was found when TFL1 was ectopic in both the lateral primordia and shoot meristem. In the tfl1 mutant, both pLFY::TFL1 and pAP1::TFL1 increased the number of rosette leaves and delayed bolting. Both complemented the tfl1 flowering time effect, and restored the number of RLs to WT, but not greater. There are two possibilities to explain why TFL1 was now active in the vegetative phase. Either earlier expression of TFL1 in the shoot is more effective in the vegetative phase, or a few lateral primordia are more sensitive to TFL1 action, and so TFL1 can act in lateral primordia which may be a characteristic of different subphases (Kersetter and Poethig, 1998; Hempel et al., 1998; Suh et al., 2003). The first possibility is suggested to be more likely. First, the complementation of RL number in these lines was the same as when TFL1 was only expressed in the shoot (as in WT plants). Secondly, TFL1 has stronger effects (to inhibit floral genes) when expressed in the shoot meristem compared with lateral meristems (see below).
Shoot and lateral meristems have different responses to TFL1
TFL1 is more effective in inhibiting floral meristem genes when expressed in the main shoot. By introducing pLFY::TFL1 or pAP1::TFL1 into tfl1, TFL1 became expressed in both lateral meristems and the shoot meristem, in direct competition with LFY and AP1. In the tfl1 mutant, only one or two lateral flowers are made before the shoot itself is converted to a terminal flower. In tfl1 carrying pLFY::TFL1 or pAP1::TFL1, up to 30 lateral flowers were generated before LFY and AP1 could finally overcome inhibition by TFL1 and convert the shoot meristem into a terminal flower. Therefore, the competence of the shoot and lateral meristems differs for TFL1 and LFY/AP1 action, as, in these lines, all three genes are expressed together at the same level and with the same timing in the two types of meristem, lateral or shoot. This competence may reflect an underlying pattern of interactors needed for TFL1, or LFY and AP1 action, to specify shoot or floral meristem identity. Potential interactors include bZIP transcription factors, one of which, FD, is expressed both in the shoot meristem and on its flanks in leaf and floral anlage (Abe et al., 2005; Wigge et al., 2005; Jaeger et al., 2006).
The effects of ectopic TFL1 on lateral meristem development were enhanced in the tfl1 background. More I1*/ap1-like structures were made when pLFY::TFL1 or pAP1::TFL1 were active in tfl1 compared with the WT. This may reflect earlier TFL1 expression from these promoters in the shoot meristem so that TFL1 affects the fate of the earliest cells (anlagen) destined to form the primordia. By establishing some TFL1 in these cells, this might lead to greater inhibition of LFY and AP1 when these cells emerge on the flanks of the shoot meristem. Therefore, even if TFL1 is not expressed any more strongly than LFY or AP1 in these lateral meristems, earlier expression (overlapping with key interactors) may be an important factor. The movement of TFL1 protein throughout the shoot meristem (which includes the anlagen) could restrict early floral gene effects (Conti and Bradley, 2007).
The present study also raises the important question of where or when TFL1 cannot act. If TFL1 was expressed later than the floral genes, what would happen? For example, TFL1 expressed only in the later stages of FM development (via pAG) did not promote shoot development (Parcy et al., 2002). Thus expression at the same time as LFY or AP1 may be required to affect meristem identity. This is supported by studies on ATC, a TFL1 homologue. ATC is expressed in the hypocotyl, and an atc mutant has no effects on meristem identity (Mimida et al., 2001). However, if expressed via p35S, ATC can act as TFL1.
One idea of this work was to test if the indeterminate shoot architecture of Arabidopsis (a raceme) would be altered significantly. If the main shoot terminated in tfl1, but TFL1 was expressed in the lateral meristem by pANT, for example, then maybe the lateral meristem would have grown and generated a new lateral meristem before terminating. If this occurred, then a branching determinate architecture could be formed, equivalent to the other major form of architecture, a determinate cyme. However, this did not happen by simply placing TFL1 under the control of lateral/floral promoters in a tfl1 mutant background. Rather, the data support models that predict it to be necessary to change both the pattern of TFL1 and floral genes reciprocally (Prusinkiewicz et al., 2007; Koes, 2008: Jaeger et al., 2013). By changing promoters and thus gene regulation, interactions necessary to generate cyme architectures may result (Souer et al., 2008; Kurokura et al., 2013; Park et al., 2014).
Studies in tomato on TFL1 and FT homologues have used p35S and mutant backgrounds to highlight the differences in primary and axillary meristems in this species with a cymose architecture (Shalit et al., 2009). In this case, it is suggested that balancing the levels of these factors has key effects on the fate of different meristem types.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Independent lines show that ectopic TFL1 affects plant organ numbers.
Acknowledgements
We thank Antonio Serrano for critical reading of the manuscript and stimulating discussions, and Enrico Coen for support and discussions. Two anonymous reviewers greatly helped in clarifying the text. This work was supported by the Biotechnology and Biological Sciences Research Council [grant no. G18134] and the Spanish Minesterio de Ciencia e Innovacion [grant no. BIO2009-10876]. The authors declare no conflict of interest associated with this work.
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. 2005. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309, 1052–1056. [DOI] [PubMed] [Google Scholar]
- Alonso-Blanco C, Mendez-Vigo D. 2014. Genetic architecture of naturally occurring quantitative traits in plants: and updated synthesis. Current Opinion in Plant Biology 18, 37–43. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Guli CL, Yu X-H, Smyth DR. 1992. terminal flower: a gene affecting inflorescence development in Arabidopsis thaliana . The Plant Journal 2, 103–116. [Google Scholar]
- Andres F, Coupland G. 2012. The genetic basis of flowering responses to seasonal cues. Nature Reviews Genetics 13, 627–639. [DOI] [PubMed] [Google Scholar]
- Benlloch R, Berbel A, Serrano-Mislata A, Madueno F. 2007. Floral initiation and inflorescence architecture: a comparative view. Annals of Botany 100, 659–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlloch R, Kim MC, Sayou C, Thevenon E, Parcy F, Nilsson O. 2011. Integrating long-day flowering signals: a LEAFY binding site is essential for proper photoperiodic activation of APETALA1 . The Plant Journal 67, 1094–1102. [DOI] [PubMed] [Google Scholar]
- Blazquez MA, Ferrandiz C, Madueno F, Parcy F. 2006. How floral meristems are built. Plant Molecular Biology 60, 855–870. [DOI] [PubMed] [Google Scholar]
- Blazquez MA, Soowal LN, Lee I, Weigel D. 1997. LEAFY expression and flower initiation in Arabidopsis . Development 124, 3835–3844. [DOI] [PubMed] [Google Scholar]
- Bowman JL, Alvarez J, Weigel D, Meyerowitz EM, Smyth DR. 1993. Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development 119, 721–743. [Google Scholar]
- Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E. 1997. Inflorescence commitment and architecture in Arabidopsis . Science 275, 80–83. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Conti L, Bradley D. 2007. TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. The Plant Cell 19, 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQJ, Gerentes D, Perez P, Smyth DR. 1996. AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. The Plant Cell 8, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandiz C, Gu Q, Martienssen R, Yanofsky MF. 2000. Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER . Development 127, 725–734. [DOI] [PubMed] [Google Scholar]
- Grandjean O, Vernoux T, Laufs P, Belcram K, Mizukami Y, Traas J. 2004. In vivo analysis of cell division, cell growth, and differentiation at the shoot apical meristem in Arabidopsis. The Plant Cell 16, 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson-Brown C, Savidge B, Yanofsky MF. 1994. Regulation of the arabidopsis floral homeotic gene APETALA1 . Cell 76, 131–143. [DOI] [PubMed] [Google Scholar]
- Hanano S, Goto K. 2011. Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. The Plant Cell 23, 3172–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzawa Y, Money T, Bradley D. 2005. A single amino acid converts a repressor to an activator of flowering. Proceedings of the National Academy of Sciences, USA 102, 7748–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. 2000. pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Molecular Biology 42, 819–832. [DOI] [PubMed] [Google Scholar]
- Hempel FD, Weigel D, Mandel MA, Gitta G, Zambryski PC, Feldman LJ, Yanofsky MF. 1997. Floral determination and expression of floral regulatory genes in Arabidopsis . Development 124, 3845–3853. [DOI] [PubMed] [Google Scholar]
- Hempel FD, Zambryski PC, Feldman LJ. 1998. Photoinduction of flower identity in vegetatively biased primordia. The Plant Cell 10, 1663–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WWH, Weigel D. 2014, Structural features determining flower-promoting activity of Arabidopsis FLOWERING LOCUS T. The Plant Cell 26, 552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huala E, Sussex IM. 1992. LEAFY interacts with floral homeotic genes to regulate Arabidopsis floral development. The Plant Cell 4, 901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger KE, Graf A, Wigge PA. 2006. The control of flowering in time and space. Journal of Experimental Botany 57, 3415–3418. [DOI] [PubMed] [Google Scholar]
- Jaeger KE, Pullen N, Lamzin S, Morris R, Wigge PA. 2013. Interlocking feedback loops govern the dynamic behavior of the floral transition in Arabidopsis. The Plant Cell 25, 820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I, Shukla V, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. 1999. Activation tagging of the floral inducer FT . Science 286, 1962–1965. [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Pajoro A, Angenent GC. 2010. a Regulation of transcription in plants: mechanisms controlling developmental switches. Nature Reviews Genetics 11, 830–842. [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Wellmer F, Muino JM, et al. 2010. b Orchestration of floral initiation by APETALA1. Science 328, 85–89. [DOI] [PubMed] [Google Scholar]
- Kerstetter RA, Poethig RS. 1998. The specification of leaf identity during shoot development. Annual Review of Cell and Developmental Biology 14, 373–398. [DOI] [PubMed] [Google Scholar]
- Kim W, Park TI, Yoo SJ, Jun AR, Ahn JH. 2013. Generation and analysis of a complete mutant set for the Arabidopsis FT/TFL1 family shows specific effects on thermo-sensitive flowering regulation. Journal of Experimental Botany 64, 1715–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucher KM, Chow H, Reiser L, Fischer RL. 1996. The AINTEGUMENTA gene of Arabidopsis required for ovule and female gametophyte development is related to the floral homeotic gene APETALA2. The Plant Cell 8, 137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. 1999. A pair of related genes with antagonistic roles in mediating flowering signals. Science 286, 1960–1962. [DOI] [PubMed] [Google Scholar]
- Koes R. 2008. Evolution and development of virtual inflorescences. Trends in Plant Science 13, 1–3. [DOI] [PubMed] [Google Scholar]
- Krizek BA. 1999. Ectopic expression of AINTEGUMENTA in Arabidopsis plants results in increased growth of floral organs. Developmental Genetics 25, 224–236. [DOI] [PubMed] [Google Scholar]
- Kurokura T, Mimida N, Battey NH, Hytonen T. 2013. The regulation of seasonal flowering in the Rosaceae. Journal of Experimental Botany 64, 4131–4141. [DOI] [PubMed] [Google Scholar]
- Liljegren SJ, Gustafason-Brown C, Pinyopich A, Ditta GS, Yanofsky MF. 1999. Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. The Plant Cell 11, 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Teo ZWN, Bi Y, Song S, Xi W, Yang X, Yin Z, Yu H. 2013. A conserved genetic pathway determines inflorescence architecture in Arabidopsis and rice. Developmental Cell 24, 612–622. [DOI] [PubMed] [Google Scholar]
- Long J, Barton MK. 2000. Initiation of axillary and floral meristems in Arabidopsis. Developmental Biology 218, 341–353. [DOI] [PubMed] [Google Scholar]
- Mandel MA, Yanofsky MF. 1995. A gene triggering flower formation in Arabidopsis. Nature 377, 522–524. [DOI] [PubMed] [Google Scholar]
- Mimida N, Goto K, Kobayashi Y, Araki T, Ahn JH, Weigel D, Murata M, Motoyoshi F, Sakamoto W. 2001. Functional divergence of the TFL1-like gene family in Arabidopsis revealed by characterization of a novel homologue. Genes to Cells 6, 327–336. [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Fischer RL. 2000. Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proceedings of the National Academy of Sciences, USA 97, 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima S, Murata M, Sakamoto W, Ogura Y, Motoyoshi F. 1997. Cloning and molecular analysis of the Arabidopsis gene Terminal Flower 1 . Molecular and General Genetics 254, 186–194. [DOI] [PubMed] [Google Scholar]
- O’Maoileidigh DS, Graciet E, Wellmer F. 2014. Gene networks controlling Arabidopsis thaliana flower development. New Phytologist 201, 16–30 [DOI] [PubMed] [Google Scholar]
- Parcy F, Bomblies K, Weigel D. 2002. Interaction of LEAFY, AGAMOUS and TERMINAL FLOWER1 in maintaining floral meristem identity in Arabidopsis. Development 129, 2519–2527. [DOI] [PubMed] [Google Scholar]
- Parcy F, Nilsson O, Busch MA, Lee I, Weigel D. 1998. A genetic framework for floral patterning. Nature 395, 561–566. [DOI] [PubMed] [Google Scholar]
- Park SJ, Eshed Y, Lippman ZB. 2014. Meristem maturation and inflorescence architecture—lessons from the Solonaceae. Current Opinion in Plant Biology 17, 70–77. [DOI] [PubMed] [Google Scholar]
- Poethig RS. 2010. The past, present, and future of vegetative phase change. Plant Physiology 154, 541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusinkiewicz P, Erasmus Y, Lane B, Harder LD, Coen E. 2007. Evolution and development of inflorescence architectures. Science 316, 1452–1456. [DOI] [PubMed] [Google Scholar]
- Ratcliffe OJ, Amaya I, Vincent CA, Rothstein S, Carpenter R, Coen ES, Bradley DJ. 1998. A common mechanism controls the life cycle and architecture of plants. Development 125, 1609–1615. [DOI] [PubMed] [Google Scholar]
- Ratcliffe OJ, Bradley DJ, Coen ES. 1999. Separation of shoot and floral identity in Arabidopsis . Development 126, 1109–1120. [DOI] [PubMed] [Google Scholar]
- Saddic LA, Huvermann B, Bezhani S, Su Y, Winter CM, Kwon CS, Collum RP, Wagner D. 2006. The LEAFY target LMI1 is a meristem identity regulator and acts together with LEAFY to regulate expression of CAULIFLOWER. Development 133, 1673–1682. [DOI] [PubMed] [Google Scholar]
- Schultz EA, Haughn GW. 1993. Genetic analysis of the floral initiation process (FLIP) in Arabidopsis . Development 119, 745–765. [Google Scholar]
- Shalit A, Rozman A, Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y, Lifschitz E. 2009. The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proceedings of the National Academy of Sciences, USA 106, 8392–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S, Meeks-Wagner DR. 1991. A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. The Plant Cell 3, 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Igeno MI, Coupland G. 1996. Activation of floral meristem identity genes in Arabidopsis . Nature 384, 59–62. [DOI] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM. 1990. Early flower development in Arabidopsis. The Plant Cell 2, 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn EJ, Rojas-Pierce M, Pan S, Carter C, Serrano-Mislata A, Madueño F, Rojo E, Surpin M, Raikhel NV. 2007. The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. Proceedings of the National Academy of Sciences, USA 104, 18801–18806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souer E, Rebocho AB, Bliek M, Kusters E, de Bruin RA, Koes R. 2008. Patterning of inflorescences and flowers by the F-Box protein DOUBLE TOP and the LEAFY homolog ABERRANT LEAF AND FLOWER of petunia. The Plant Cell 20, 2033–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks E, Wachsman G, Benfey PN. 2013. Spatiotemporal signalling in plant development. Nature Reviews Genetics 14, 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer A, Zhao Q, Ross-Ibarra J, Doebley J. 2011. Identification of a functional transposon insertion in the maize domestication gene tb1. Nature Genetics 43, 1160–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh S-S, Choi K-R, Lee I. 2003. Revisiting phase transition during flowering in Arabidopsis. Plant and Cell Physiology 44, 836–843. [DOI] [PubMed] [Google Scholar]
- Sussex IM, Kerk NM. 2001. The evolution of plant architecture. Current Opinion in Plant Biology 4, 33–37. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen W, Ruttink T, Borst-Vrenssen AWM, van der Plas LHW, van der Krol A. 2001. Characterisation of position-induced spatial and temporal regulation of transgene promoter activity in plants. Journal of Experimental Botany 52, 949–959. [DOI] [PubMed] [Google Scholar]
- Wagner D, Sablowski RW, Meyerowitz EM. 1999. Transcriptional activation of APETALA1 by LEAFY. Science 285, 882–584. [DOI] [PubMed] [Google Scholar]
- Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM. 1992. LEAFY controls floral meristem identity in Arabidopsis. Cell 69, 843–859. [DOI] [PubMed] [Google Scholar]
- Weigel D, Meyerowitz EM. 1993. Activation of floral homeotic genes in Arabidopsis. Science 261, 1723–1726. [DOI] [PubMed] [Google Scholar]
- Weigel D, Nilsson O. 1995. A developmental switch sufficient for flower initiation in diverse plants. Nature 377, 495–500. [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D. 2005. Integration of spatial and temporal information during floral induction in Arabidopsis . Science 309, 1056–1059. [DOI] [PubMed] [Google Scholar]
- Winter CM, Austin RS, Blanvillain-Baufume S, et al. 2011. LEAFY target genes reveal floral regulatory logic, cis motifs, and a link to biotic stimulus response. Developmental Cell 20, 430–443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.