Highlight
RNA sequencing of mutants defective in the expression of three paralogous MYB transcription factors revealed pleiotropic roles and dynamic shifts in the function of the proteins upon exposure to phosphate starvation.
Key words: Gene regulation, genetic redundancy, phosphate starvation, RNA-seq, root hairs, transcriptional profiling.
Abstract
Phosphate (Pi) deficiency alters root hair length and frequency as a means of increasing the absorptive surface area of roots. Three partly redundant single R3 MYB proteins, CAPRICE (CPC), ENHANCER OF TRY AND CPC1 (ETC1) and TRIPTYCHON (TRY), positively regulate the root hair cell fate by participating in a lateral inhibition mechanism. To identify putative targets and processes that are controlled by these three transcription factors (TFs), we conducted transcriptional profiling of roots from Arabidopsis thaliana wild-type plants, and cpc, etc1 and try mutants grown under Pi-replete and Pi-deficient conditions using RNA-seq. The data show that in an intricate interplay between the three MYBs regulate several developmental, physiological and metabolic processes that are putatively located in different tissues. When grown on media with a low Pi concentration, all three TFs acquire additional functions that are related to the Pi starvation response, including transition metal transport, membrane lipid remodelling, and the acquisition, uptake and storage of Pi. Control of gene activity is partly mediated through the regulation of potential antisense transcripts. The current dataset extends the known functions of R3 MYB proteins, provides a suite of novel candidates with critical function in root hair development under both control and Pi-deficient conditions, and challenges the definition of genetic redundancy by demonstrating that environmental perturbations may confer specific functions to orthologous proteins that could have similar roles under control conditions.
Introduction
Deviations from intrinsic developmental patterns, dictated by environmental signals, confer phenotypic plasticity to plants and allow for an efficient acclimation to prevailing conditions. Root hairs, specialized epidermal cells that play critical roles in the absorption of water and nutrients, provide an excellent model for studying such changes. While genetically determined, the development of root epidermal cells is highly responsive to the environment, resulting in alterations in both the density and length of root hairs. This response is specifically triggered by suboptimal phytoavailability of mineral nutrients with low mobility such as phosphate (Pi), iron and manganese (Perry et al., 2007). In Arabidopsis, root hairs develop from files of epidermal cells that are positioned over the clefts of two underlying cortical cells (H position), triggered by a yet unidentified positional signal. This ‘cortical bias’ is perceived by the leucine-rich receptor kinase SCRAMBLED (SCM) (Kwak et al., 2005). SCM activity is regulated in turn by the zinc finger protein JACKDAW in a non-cell autonomous manner through a signal from the underlying cortex cell layer. This signal is presumably stronger in H-positioned cells than in epidermal cells adjacent to periclinal cortical cell walls (Hassan et al., 2010). SCM represses the expression of the R2R3 MYB proteins WEREWOLF (WER) and MYB23 in future hair cells (trichoblasts). In non-hair cells, WER forms a complex with the WD repeat protein TTG, the basic helix-loop-helix protein GLABRA3 (GL3), and its paralogue ENHANCER OF GLABRA3 (EGL3). This complex supports the expression of the homeodomain-leucine zipper protein GLABRA2 (GL2), which represses the root hair cell fate. Four R3 MYB proteins with putatively redundant function, CAPRICE (CPC), ENHANCER OF TRY AND CPC 1 (ETC1), ENHANCER OF TRY AND CPC3 (ETC3), and TRIPTYCHON (TRY), compete with WER for binding to the TTG-GL3/EGL3 complex in a lateral inhibition mechanism. In this mechanism, R3 MYB proteins migrate, possibly via plasmodesmata, from non-hair cells to hairs cells where they are trapped by EGL3 (Kang et al., 2013). The movement of R3 MYBs from non-hair cells to hair cells and the reduced expression of WER and MYB23 repress the non-hair pathway and force cells in the H-position into the hair fate.
CPC and TRY act redundantly in trichome and root hair patterning (Schellmann et al., 2007), but TRY has a specific role in regulating the expression of SCM (Kwak et al., 2005; Kwak and Schiefelbein, 2014), indicating specific, partly non-redundant functions of the R3 MYB proteins. Three other R3 MYBs, ETC2, TCL1 and TCL2, are not expressed in roots and play a role in trichome formation (Wang et al., 2007; Kirik et al., 2004b ). ETC1, ETC2 and ETC3 possess similar biochemical properties and can substitute for CPC function by acting in a mechanistically similar manner (Kirik et al., 2004a ; Simon et al., 2007; Tominaga et al., 2008). In support of this proposition, the number of root hairs is significantly decreased in etc3 mutants (Tominaga et al., 2008; Wester et al., 2009).
GL3 and EGL3 are preferentially expressed in root hair cells, but the proteins can migrate to non-hair cells to reinforce the cell fate decisions (Bernhardt et al., 2005). In cells that develop into root hairs, the CPC/ETC1/TRY-GL3/EGL3-TGG complex represses the expression of GL2, and cells enter the hair cell fate (Wada et al., 2002). GL2 negatively regulates the expression of the bHLH-type transcription factor (TF) ROOT HAIR DEFECTIVE6 (RHD6), which is essential for the assembly of the root hair initiation site (Masucci and Schiefelbein, 1994). Root hair initiation starts with the formation of a dome-shaped structure at the basal end of the trichoblast. ROOT HAIR DEFECTIVE6-LIKE4 (RSL4) is a direct transcriptional target of RHD6 and controls a suite of genes involved in the elongation of the hairs (Yi et al. 2010).
Pi-deficient plants form longer and more frequent root hairs than plants grown under Pi-replete conditions (Ma et al., 2001; Müller and Schmidt, 2004). This increase in root hair number is primarily caused by a decrease of the longitudinal length of epidermal cells (Ma et al., 2003; Savage et al., 2013). In addition, in roots of Pi-deficient plants a small fraction of root hairs are formed in positions normally occupied by non-hair cells (Ma et al., 2001; Müller and Schmidt, 2004; Savage et al., 2013). The restricted elongation of root epidermal cells under conditions of Pi deficiency is caused by reduced strength of the positional signal that determines root hair patterning via SCM. This scenario was inferred from the observation that scm mutants develop short, trichoblast-like epidermal cells (Savage et al., 2013). RSL4 controls the duration of root hair elongation and is thus critical for later phases of root hair development (Yi et al., 2010; Wada et al., 2015). Expression of RSL4 is responsive to Pi availability. Increased activity of ETC1 upon Pi starvation has been proposed as a mechanism for conferring additional root hair cell fate assignment. Overexpression of ETC1 causes the formation of excessive root hairs (Kirik et al. 2004a ), supporting the assumption that ETC1 and CPC have similar functions. In cpc etc1 double mutants the formation of extra root hairs in response to Pi deficiency was abolished (Savage et al., 2013), and ETC1 was identified as a putative target of the homeodomain protein ALFIN LIKE6 (AL6), a PHD finger-containing ‘histone reader’ that is critical for the elongation of Pi deficiency-induced root hairs (Chandrika et al., 2013). AL6 binds to trimethylated lysine 4 on histone 3 (Lee et al., 2009) and probably controls gene activity by recruiting additional factors that are required for transcriptional elongation and pre-mRNA processing as observed for other PHD finger proteins (Sims et al., 2007).
Interestingly, several genes that are required for the formation of root hairs typical of Pi-deficient plants are not essential for the development of root hairs under Pi-replete conditions. For example, mutations in AL6 cause a visible root hair phenotype only under Pi-deficient conditions. A similar Pi-dependent root hair phenotype was observed for per1 mutants that harbour a synonymous substitution in UBIQUITIN SPECIFIC PROTEASE14 (Li and Schmidt, 2010). This suggests that activation of a Pi-specific subset of root hair genes is required for inducing the Pi-deficiency root hair phenotype. The signalling cascade that dictates this phenotype is supposedly interlinked with Pi sensing, and its elucidation will yield valuable information on the regulation of cellular Pi homeostasis.
In the present study, we set out to dissect the role of the paralogous R3 MYB proteins CPC, ETC1 and TRY in the Pi starvation response using a transcriptional profiling approach. It is shown that the three TFs control various aspects of root hair development and Pi homeostasis in a partly non-redundant manner and are important for the coordination of cell wall-modifying enzymes, the regulation of lipid metabolism, and the orchestration of mineral nutrient homeostasis. Inferred from the function of the proteins regulated by CPC, ETC1 and TRY, we propose that these three TFs sophisticatedly control not only root hair formation, but also root-hair-unrelated processes in other types of tissues.
Materials and methods
Plant growth conditions
Arabidopsis thaliana plants were grown in a growth chamber on a solidified medium as described by Estelle and Somerville (1987). Seeds of the accession Columbia (Col-0) and cpc (CS6399), etc1 (Salk_071734C) and try (CS6518) mutants were obtained from the Arabidopsis Biological Resource Center (Ohio State University). Seeds were surface-sterilized by immersing them in 5% (v/v) NaOCl for 5min and 70% ethanol for 7min, followed by four rinses in sterile water. Seeds were placed onto Petri dishes and kept for 1 d at 4°C in the dark, before the plates were transferred to a growth chamber and grown at 21°C under continuous illumination (50 µmol m-2 s-1; Phillips TL lamps). The medium was solidified with 0.4% Gelrite pure (Kelco), the pH was adjusted to 5.5. Low Pi conditions were obtained by growing plants on media containing 2.5 µM KH2PO4, the Pi concentration of control medium was 2.5mM KH2PO4. The lower concentration of potassium due to the reduced KH2PO4 concentration was compensated for by the addition of KCl.
RNA-seq
Total RNA was extracted from roots using the RNeasy Plant Mini Kit (Qiagen), following the manufacturer’s instructions. For analysis, equal amounts of total RNA were collected and cDNA libraries for sequencing were prepared from total RNA following the manufacturer’s protocol (Illumina). The cDNA libraries were subsequently enriched by PCR amplification. The resulting cDNA libraries were subjected to sequencing on a single lane of an Illumina Genome Analyzer II. RNA-seq and data collection were done following the protocol of Mortazavi et al. (2008). The length of the cDNA library varied from 250 to 300bp with a 5′-adapter of 20bp and a 3′-adapter of 33bp at both ends.
To quantify gene expression levels, 75-mers sequences were aligned to the genomic sequence annotated in TAIR10 using the BLAT program (Kent, 2002), and RPKM (reads per kbp per million reads) values were computed using RACKJ (Read Analysis & Comparison Kit in Java, http://rackj.sourceforge.net/) software. Only those genes whose expression level in RPKM was over the square root of the mean expression value of the whole dataset (~4.5 RPKM) were considered as relevant for further analyses. Relevant differentially expressed genes were selected based on Student’s t-test (P<0.05) and 1.5-fold change in expression level between treatments.
Bioinformatics
For gene clustering, we used the MACCU software (http://maccu.sourceforge.net/) to build co-expression networks based on co-expression relationships with a Pearson’s coefficient greater than or equal to 0.60. In order to capture the tissue-specific co-expression relationships, Pearson’s coefficients were computed based on robust multi-array averaged array data derived from leaf- and root-specific experiments for each tissue downloaded from NASCArrays (http://affymetrix.arabidopsis.info/). Visualization of the networks was performed with the Cytoscape software version 3.2.0 (http://www.cytoscape.org/).
Real-time RT-PCR
For real-time RT-PCR, total RNA was extracted from the roots using the RNeasy Plant Mini Kit (Qiagen) and DNase treated with the Turbo DNA-free Kit (Ambion) following the manufacturer’s instructions. cDNA was synthesized using SuperScript III reverse transcription kits (Invitrogen) following the manufacturer’s instructions. Real-time PCR was performed using Power SYBR Green PCR Master Mix (Applied Biosystems) on an Applied Biosystems 7500 Fast Real-Time PCR System with programs recommended by the manufacturer. Samples were normalized first to an endogenous reference (AtTUA) and then the relative target gene was determined by performing a comparative ΔΔCt. The following primers were used: AtTUA (At5g19770) forward, GTGCTGAAGGTGGAGACGAT; reverse, AACACGAAGACCGAACGAAT.
Measurement of root hair length and density
Confocal images with a scale bar of 100 µm at 10× resolution were used for measuring the root hair length. A ZEISS DISCOVERY V.12 microscope equipped with an ocular scale bar was used for measuring root hair density. Root hair density was measured from 2 to 6mm from the tip of the primary root. Statistical significant deviations from the wild type were determined by Student’s t-test. Micrographs were taken between 0 and 5mm from the tips of primary roots.
Confocal microscopy
Roots were mounted on a glass slide with a drop of water and a coverslip, and observed with a Zeiss Axio Imager microscope equipped with a Zeiss Axiocam MRc CCD camera to capture root hair pictures. Roots were dipped in 10mg ml-1 propidiumiodide (PI) solution for 10 s and gently rinsed in water for 1min. Roots were then mounted on a glass slide as above and scanned using a confocal laser scanning microscope (Zeiss LSM510 Meta). The wavelength for excitation and emission of PI was 536 and 620nm, respectively.
GUS staining
Root samples were fixed, dehydrated, and then embedded in Technovit 7100 (Heraeus Kulzer, Wehrheim) resin in gelatine capsules. Transverse sections (30 μm) were cut using a RM 2255 Leica microtome (Leica, Nussloch, Germany). Histochemical GUS staining was performed as described in Schmidt et al. (2007). Sections were dried and examined using brightfield on an Imager Z1 microscope (Zeiss, Jena, Germany).
ICP-OES analysis
Mineral nutrient analysis was determined by inductively coupled plasma optical emission spectrometry (ICP-OES) as described in Rodríguez-Celma et al. (2013). Five plants were harvested per treatment and genotype.
Results and discussion
The root hair phenotype of cpc, etc1 and try mutants is responsive to the Pi supply
Mutants defective in the expression of CPC, ETC1 and TRY were analysed for their root hair phenotypes under control (Pi-replete) and low Pi conditions. All mutants have been described before (cpc, Wada et al., 1997; etc1, Kirik et al., 2004a ; try, Wang et al., 2013).
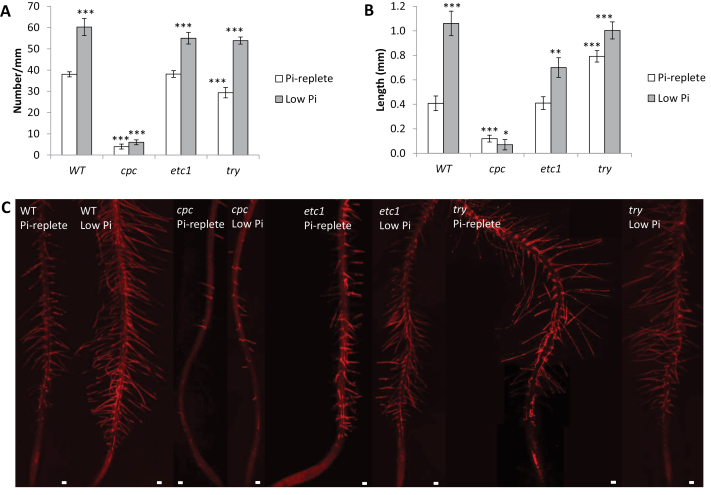
As described previously, homozygous mutations in CPC caused a dramatic reduction in root hair density (Fig. 1A, C; Kirik et al., 2004a ). In the present investigation, root hair frequency in cpc was reduced by 89% and also the root hair length was strongly decreased (Fig. 1A, B). Under control (Pi-replete) conditions, no root hair phenotype was observed for etc1 plants. In roots of try, the number of root hairs was reduced by 23% relative to the wild type, but root hairs were ~2-fold longer than those of wild-type plants.
Fig 1.
Root hair phenotypes of the wild type and mutants. (A) Root hair number. (B) Root hair length. (C) Compiled confocal micrographs of the various genotypes. The number of asterisks denotes statistically significant differences to Pi-replete wild-type plants based on Student’s t-test (* P<0.05, ** P<0.01, *** P<0.001). Bar, 100 μM.
In the wild type, growth on low Pi media was correlated both with increased root hair length and density (Fig. 1). An increased frequency of root hairs is a hallmark of Pi-deficient plants and has been well documented for Arabidopsis (Ma et al., 2001; Müller and Schmidt, 2004). In agreement with previous results, the increase in root hair density in response to Pi deficiency was much less pronounced in cpc plants than in the wild type (Fig. 1A, C; Savage et al., 2013). No increase in root hair length upon Pi starvation was observed in cpc plants. Roots of etc1 did not show altered root hair density under control conditions. When grown on low Pi media, root hairs were slightly less frequent but significantly shorter than those of the wild type. Roots of try showed 23 and 10% reduced root hair frequency under control and low Pi conditions respectively (Fig. 1A). Under control conditions, root hairs of try mutants were ~2-fold longer than those of the wild type; no difference in root hair length was observed when plants were grown on low Pi media (Fig. 1B). The difference in the root hair phenotypes between the mutants in response to Pi starvation reflects the relative importance of the three R3 MYBs for inducing root hairs typical of Pi-deficient plants.
To gain further insights into the role of the three TFs under investigation, we investigated the Pi deficiency-induced changes in the root hair phenotypes of cpc etc1 double mutants and TRY overexpressing (TRY OE) lines, which were not analysed by RNA-seq. As reported previously (Savage et al., 2013), the cpc etc1 double mutant did not respond to Pi starvation with the formation of extra root hairs (Supplementary Fig. S1). In contrast, TRY OE plants showed extremely dense and long root hairs when grown on low Pi media (Supplementary Fig. S1). An independent allele of try (Hülskamp et al., 1994) showed a behaviou similar to the allele used in the RNA-seq experiments (CS6518). Together these results support a role of all three R3 MYBs as positive regulators of the long and dense root hair phenotype of Pi-deficient plants.
Mutations in CPC, ETC1 and TRY led to dramatic changes in the transcriptome
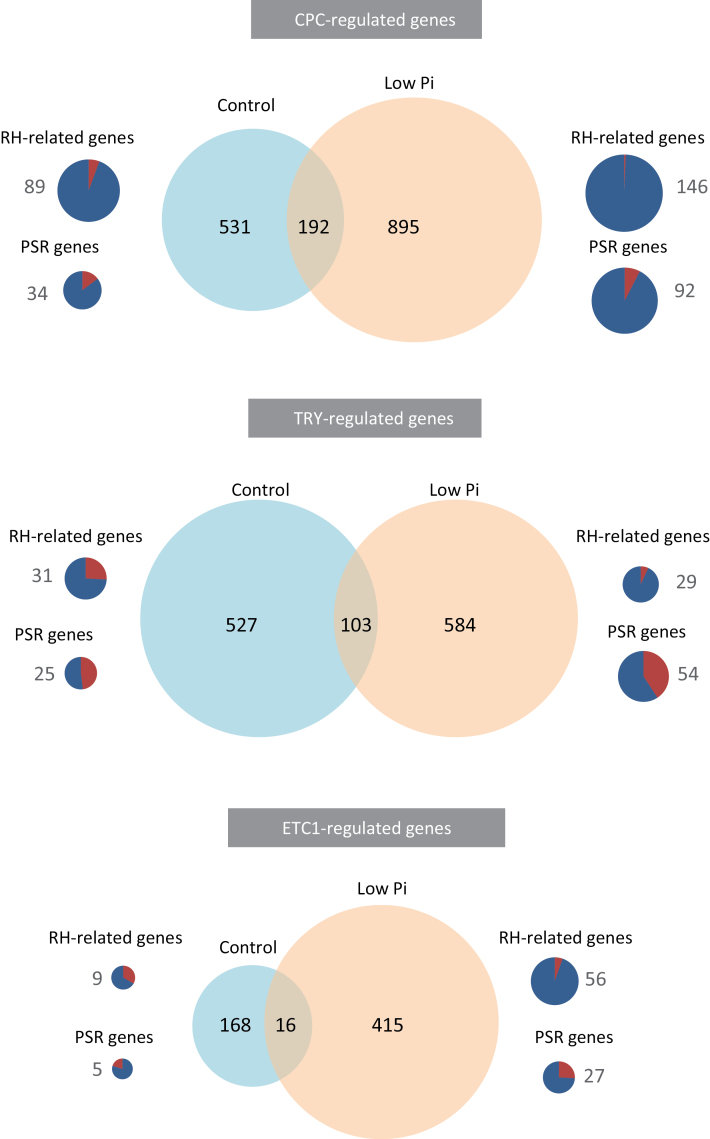
To identify genes that are regulated by one or more of the TFs under investigation, we surveyed the transcriptome of roots from wild-type and cpc, etc1 and try plants using RNA-seq. Sequencing was performed in triplicates with on average ten million reads per library (Supplementary Table S1), and genes that showed a deviation in transcript abundance from the wild type greater than or equal to 1.5-fold with P<0.05 were defined as differentially expressed. Validation of a subset of the differentially expressed genes by qRT-PCR showed a generally good agreement between the two methods (Supplementary Fig. S2, Supplementary Table S4). For a relatively large suite of genes at least one of the three R3 MYBs was required for wild type-like expression. Under control conditions, the largest subset was dependent on CPC expression (723 genes), followed by TRY (630) and ETC1 (184) (Fig. 2, Supplementary Table S2).
Fig. 2.
Genes differentially expressed between cpc, etc1 and try mutants and the wild-type plants. Pie charts show genes that are preferentially expressed in root hairs (RH; Lan et al., 2013) and in the phosphate starvation response (PSR) genes. Numbers represent gene counts. In the pie charts, red and blue colour represents the percentage of up- and down-regulated genes, respectively. (This figure is available in colour at JXB online.)
Regulation of root hair genes by CPC, ETC1 and TRY
Consistent with their role in promoting the non-hair cell fate, the expression of WER and MYB23 was higher in cpc and etc1 than in wild-type plants. This increase was less pronounced in try mutants. In all three mutants, the negative regulator of the root hair cell fate GL2 showed higher expression than the wild type. Also, the expression of ROOT HAIR DEFECTIVE 6-LIKE2 (RSL2), encoding a functional paralogue of RSL4 (Yi et al., 2010) was strongly decreased in cpc and etc1, but less so in try mutants.
For 120 of the 635 genes that we previously defined as preferentially expressed in root hairs (Lan et al., 2013), 2-fold or greater changes in expression were observed in cpc plants when compared with the wild type (Supplementary Table S3). The vast majority of root hair-specific genes had lower transcript levels in the mutants than in the wild-type plants. A subset comprising 51 of these genes has been previously defined as root hair core genes (Bruex et al., 2012). In etc1 and try, only 12 and 15 of the differentially expressed genes, respectively, are preferentially expressed in root hairs.
In cpc, the expression of a suite of genes with highly root hair-specific expression (PRP3, EXP7, EXP18, RHS2, RHS5, RHS7, RHS9, RHS10, RHS12-RHS14, RHS16-RHS19) (Won et al., 2009; Lan et al., 2013) was repressed. Also, genes that encode proteins with experimentally validated roles in root hair formation such as the Zn2+ transporter ZIP3 (Lan et al., 2013), RSL4 (Yi et al., 2010), the phosphatidylinositol transfer protein COW1 (Grierson et al., 1997), the xyloglucan endotransglycosylases XTH14 and XTH26 (Maris et al., 2009), the ATPase AHA7 (Santi and Schmidt, 2009), MRH6 (Jones et al., 2006), and the serine/threonine kinase IRE (Oyama et al., 2002), had expression levels that were 2-fold lower in the mutant than in the wild type. The most pronounced decrease in transcripts was observed for the genes encoding the proline-rich extensin-like family protein At4g08410 and the NAD(P)-binding Rossmann-fold superfamily protein At2g30670. Also, the expression of several unknown proteins was dependent on functional CPC. Only three of these proteins (At2g34910, At3g12540 and At3g49960) have been associated with root hair development (Deal and Henikoff, 2010; Bruex et al., 2012), indicating that a large number of uncharacterized proteins still await placement into the surprisingly complex puzzle of processes that control and mediate the development of root hairs. No genes with confirmed roles in root hair formation were differentially expressed in roots of etc1 and try.
Changes in R3 MYB expression upon Pi starvation
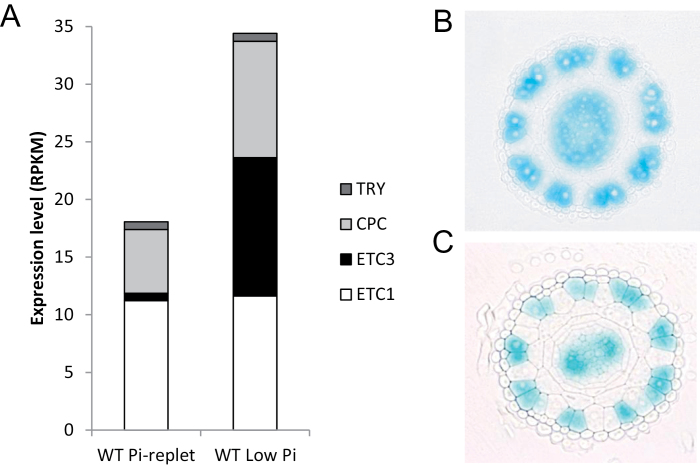
A previous RNA-seq study revealed that of the genes that determine root epidermal cell fate only ETC1 and ETC3 were Pi-responsive, being significantly up-regulated upon Pi starvation (Lan et al., 2012). In the present study, a relative small, non-significant increase in ETC1 expression was observed (Fig. 3A). This difference is likely due to differences in growth conditions between the two studies [transfer to Pi-free media in Lan et al. (2012) versus growth on low Pi media in this study]. The expression of CPC increased ~2-fold when plants were grown on low Pi media, TRY transcript levels were not affected by the Pi regime. Notably, ETC3 expression was low under Pi-replete conditions but was strongly induced by low Pi, resulting in a robust change in the relative abundance of the R3 MYB transcripts (Fig. 3A). Another homologue of ETC1, ETC2 was not expressed in roots. Lack of detectable ETC2 and ETC3 transcript in (Pi-replete) roots was reported previously (Kirik et al., 2004b ; Simon et al., 2007).
Fig. 3.
Effect of Pi deficiency on the expression of R3 MYB protein. (A) Abundance changes in the transcripts of CPC, ETC1, ETC3 and TRY determined by RNA-seq analysis. Values are given in RPKM. (B, C) CPC promoter activity. Cross-sections are from the meristematic region of pCPC-GUS plants grown under (B) control and (C) Pi-deficient conditions. (This figure is available in colour at JXB online.)
To investigate whether Pi deficiency alters the spatial expression pattern of CPC, cross-sections in the late meristematic zone of pCPC-GUS reporter lines grown under Pi-replete and low Pi conditions were analysed. In the epidermis, GUS expression was restricted to atrichoblasts (Fig. 3B). Similar to what have been reported previously (Wada et al., 2002), in pCPC-GUS plants GUS staining was also prominent in the stele (Fig. 3B). No changes in the spatial distribution of CPC promoter activity were observed when plants were grown on low Pi media (Fig. 3B).
Pi starvation induces alterations in the genes that are controlled by CPC, ETC1 and TRY
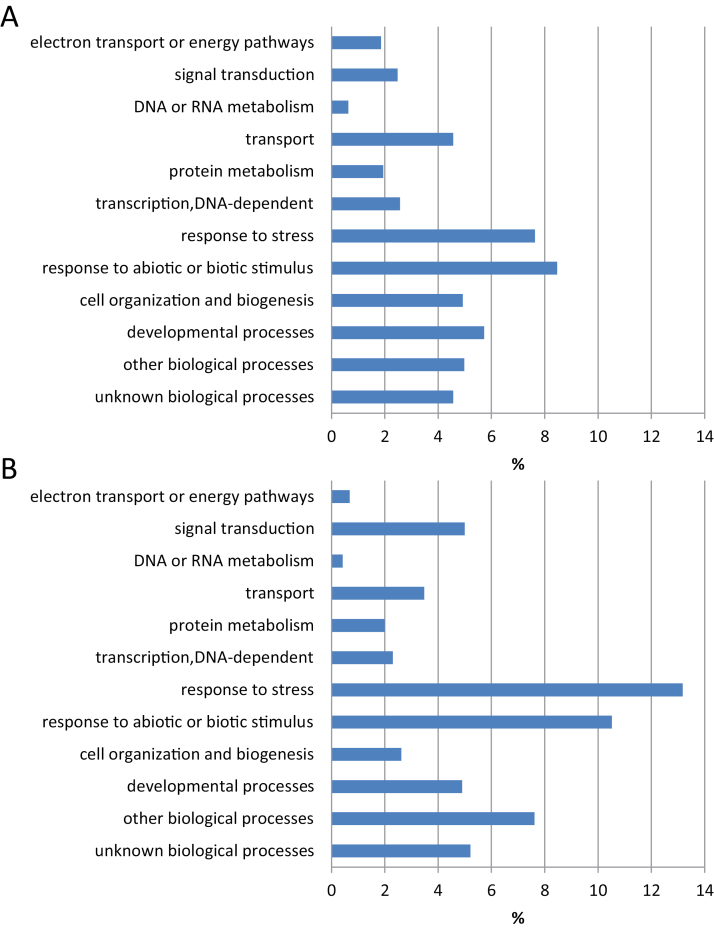
Gene ontology (GO) enrichment analysis of genes dependent on CPC, ETC1 or TRY reveals that the biological function categories ‘cell organization and biogenesis’, ‘developmental process’, ‘DNA/RNA metabolism’ and ‘electron transport or energy pathways’ were less prominent in plants grown on low Pi, while genes belonging to the GOs ‘response to biotic or abiotic stimulus’, ‘response to stress’ and ‘signal transduction’ showed increased abundance (Fig. 4). This suggests that a dynamic shift in the roles of the three TFs under study is induced by subjecting the plants to Pi starvation.
Fig. 4.
Over-represented GO categories (biological function) of genes that were differentially expressed in roots of cpc, etc1 or try under (A) control and (B) Pi-deficient conditions. (This figure is available in colour at JXB online.)
Under low Pi conditions, the number of genes that was affected by mutations in R3 MYBs was increased by 50% for CPC, by 9% for TRY-controlled genes and by 134% for genes that are dependent of ETC1 (Fig. 2). This indicates that in particular ETC1 acquires additional functions under Pi-limited conditions. Consistent with the strong root hair phenotype of cpc plants under low Pi conditions, RSL4 showed lower expression in cpc plants relative to the wild type under low Pi conditions (Fig. 1). RSL4 expression was not significantly reduced under control conditions in cpc plants, indicating that up-regulation of RSL4 by Pi deficiency (Yi et al., 2010) is dependent on CPC and critically required for the Pi deficiency root hair phenotype. Several genes that are preferentially expressed in root hairs had message levels that were 5-fold or more lower in one or more of the mutants compared to the wild type, including the peroxidase PRX2, the cytochrome P450 CYP94B3, the squalene monooxygenase SQP2, and the mannose-binding lectin superfamily protein At5g49870. CYP94B3 has been associated with the oxidative catabolism of jasmonate (Kitaoka et al., 2011), establishing a connection between Pi-induced root hair formation and jasmonate signalling. Transcripts of the proline-rich extensin-like protein At4g08410 and the NAD(P)-binding protein At2g30670 were also strongly down-regulated in cpc under both Pi-replete and low Pi conditions. The expression patterns of these genes and their regulation invite speculation on their roles in root hair elongation in particular under Pi-deficient conditions.
Regulation of Pi-responsive Genes by CPC, ETC1 and TRY
Out of the 475 genes that were defined as being Pi-responsive (expression changes of greater than or equal to 1.5-fold with P<0.05), a subset of 165 was either positively or negatively affected by the loss of function of one or more of the R3 MYBs investigated. The group of Pi-responsive genes with increased expression in cpc, etc1 or try mutants was substantially smaller than that of negatively affected genes (Fig. 2). This effect was less pronounced in roots of try, where almost half of the Pi-responsive genes had higher expression levels than the wild type.
Several genes encoding purple acid phosphatases (PAPs) were regulated by CPC, ETC1 or TRY (Supplementary Table S2). PAPs can release Pi from organic P due to their hydrolytic activity, thereby contributing to the acquisition of Pi from the rhizosphere. Most, but not all, of these PAPs were responsive to Pi. Interestingly, PAP27, which was down-regulated upon Pi starvation in the wild type, was co-expressed with the Zn2+ transporters ZIP4, HMA2 and IRT3, all of which showed reduced transcript abundance relative to Pi-deficient wild-type plants (atted.jp). This stands in contrast to other PAPs (e.g. PAP23, PAP25 and PAP14) that were highly up-regulated in Pi-deficient plants and co-expressed with genes involved in lipid metabolism that were strongly induced upon Pi starvation such as PEPC1 and MGD3. The regulatory pattern is indicative of functional diversity of Pi-responsive PAPs, a finding that corresponds to their variable molecular and biochemical properties (Li et al., 2002). The physiological functions of the R3 MYB-regulated PAPs have yet to be resolved.
Co-expression analysis reveals functional diversity of R3 MYB-regulated genes
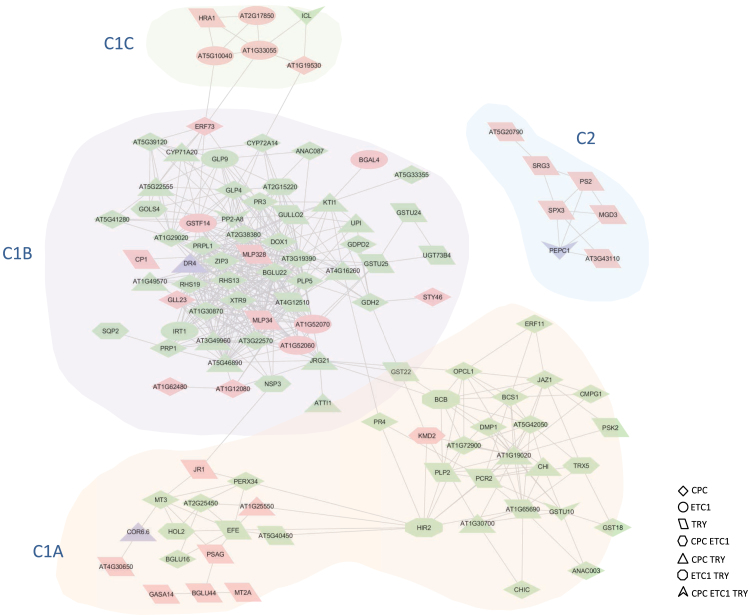
A custom-made co-expression network of the Pi-responsive genes that are dependent on at least one of the TFs under study was constructed using the MACCU toolbox, computed based on robust multi-array averaged array data derived from root-specific experiments for each tissue downloaded from NASCArrays (http://affymetrix.arabidopsis.info/; Lin et al., 2011) with a Pearson correlation coefficient cutoff P≤0.6. This procedure yielded one large cluster (C1) that could be subdivided into three sub-clusters (C1A-C1C) and one smaller cluster (C2) (Fig. 5).
Fig. 5.
Co-expression network of PSR genes that were differentially expressed in roots of cpc, etc1 and try. Genes were clustered based on their co-expression relationships with a Pearson’s coefficient of ≥0.60 using the MACCU software package. Pink nodes denote genes that were up-regulated in the mutants, green nodes indicate genes with decreased transcript abundance and purple nodes represent genes that are regulated in opposite directions in different mutants.
Sub-cluster 1 (C1A) contains several genes involved in redox homeostasis, with specific roles in cell death (e.g. HIR2, At5g42050, At1g72900), response to oxidative stress (GASA14, PCR2, ATBCB, At1g19020), oxidative burst (PERX34, GMPG1), and prevention of oxidative damage (MT3, MT2A). This group of genes was mostly down-regulated in cpc plants under low Pi conditions. KISS ME DEADLY2 (KMD2), encoding a subunit of the SCF ubiquitin ligase complex that negatively regulates the cytokinin response, had increased transcript levels in cpc and etc1 under low Pi conditions, indicative of a role of cytokinin in the Pi starvation response. Crosstalk between cytokinin, sugar and Pi starvation signalling has been reported (Martín et al., 2000; Franco-Zorilla et al., 2005; Rouached et al., 2010). Pi starvation decreases cytokinin levels (Horgan and Wareing, 1980) by repressing the expression of cytokinin-related genes (Hammond et al., 2003; Misson et al., 2005; Morcuende et al., 2007). KMD2 was down-regulated in the wild type upon Pi starvation but had higher expression levels in cpc and etc1 plants. These results are indicative of an involvement of the R3 MYBs under study in the interaction between cytokinin and Pi starvation signalling.
Sub-cluster C1B includes several genes related to the response to biotic and abiotic stress, with an overrepresentation of proteins with predicted extracellular localization. Several of these genes are preferentially expressed in root hairs and encode proteins that are involved in cell elongation (XTR9, PRP1, PRPL1, RHS13, RHS19 and the peroxidase At1g30870). One of these genes, the proline-rich protein-like PRPL1, had lower transcript abundance in cpc mutants both under Pi-replete and low Pi conditions. Similar to PRP1 and PRP3, two key players in root hair differentiation that cross-connect cell wall components (Bernhardt and Tierney, 2000), the expression of PRPL1 is restricted to trichoblasts and has been related to the elongation of root hairs (Boron et al., 2014). Also the Zn2+/Fe2+ transporters IRT1 and ZIP3 are within this cluster. Most of the genes were down-regulated in the mutants, in particular in cpc and were more so under low Pi conditions.
Another group of genes in this cluster is related to lipid metabolism, comprising the patatin-related phospholipase A-encoding gene PLP5, two lipid-transfer proteins with undefined function (At3g22570 and At5g46890), a GDSL-like lipase (GLL23) and the glycerophosphodiester phosphodiesterase GDPD2, indicative of a link between lipid metabolism and root hair elongation. The expression of most of these genes is dependent on functional CPC. Also for these genes, the difference in transcript levels was more pronounced when plants were grown on low Pi media. A smaller sub-cluster of C1, C1C, contains five hypoxia-related genes that were all up-regulated in the mutants.
An unconnected cluster (C3) is composed of seven genes involved in lipid metabolism that were all strongly up-regulated upon Pi starvation in the wild type. The expression of several of these genes was increased in try mutants. A similar pattern was observed for two other genes in this cluster, encoding the TF SPX3 and two unknown proteins (At5g20790 and At3g43110), suggesting that these three genes also participate in Pi starvation-induced reprogramming of the lipid metabolism. Interestingly, one of these genes, the pyrophosphate-dependent phosphatase PHOSPHATE STARVATION-INDUCED GENE2 (PS2) PS2 was identified as a putative target of AL6 indicating that root hair elongation is co-regulated with other Pi starvation responses (PSRs) (Chandrika et al., 2013).
A lipid metabolic pathway partly controlled by CPC, ETC1 and TRY
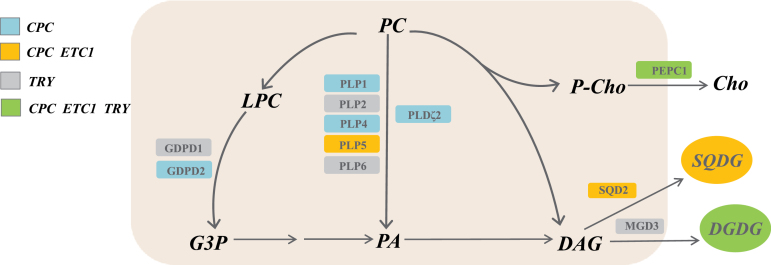
A large percentage of Pi is recycled during Pi starvation by replacing PLs in membranes with the galactolipid digalactosyldiacylglycerol (DGDG) and a sulfolipid, sulfoquinovosyldiacylglycerol (SQDG) (‘membrane lipid remodelling’; Nakamura et al., 2009; Nakamura et al. 2014). The current analysis suggests that membrane lipid remodelling is partly or chiefly controlled by the three R3 MYBs (see above). R3 MYB-controlled steps in a putative metabolic pathway that ultimately leads to the liberation of Pi from phospholipids (PLs) and substitution of PLs by DGDG and SQDG are depicted in Fig. 6.
Fig. 6.
Scheme depicting the regulation and putative roles of genes involved in membrane lipid remodelling by CPC, ETC1 and TRY in Pi-deficient Arabidopsis roots. PC, phosphatidylcholine; LPC, lysophosphatidylcholine; G3P, glycerol-3-phosphate; PA, phosphatidic acid; P-Cho, phosphocholine; Cho, cholin; DAG, diacylglycerol. (This figure is available in colour at JXB online.)
PHOSPHOLIPASE D ZETA 2 (PLDζ2) has been implicated in the hydrolysis of PLs using phosphatidylcholine (PC) and phosphatidylethanolamine (PE) as substrates to produce phosphatidic acid (PA) (Cruz-Ramirez et al., 2006). Full induction of PLDζ2 upon Pi starvation was dependent on functional CPC. Interestingly, PLDζ2 is also required for root hair elongation in response to Pi starvation (Li et al. 2006). PA could then be converted to diacylglycerol (DAG) and used as substrate for DGDG synthesis via MGD3 and for the synthesis of SQDG via SQD2. Both enzymes are strongly induced in response to Pi starvation. PEPC1 is supposedly involved in the hydrolization of phosphocholine (PCho) (May et al., 2012). PEPC1 transcript abundance was also increased in plants grown on low Pi media.
Patatin-related phospholipases A (PLPs) have been implicated in cell elongation and auxin signalling (Ryu, 2004; Rietz et al., 2010), but have not been assigned a clearly defined role in the PSR. Five of the ten Arabidopsis PLP family members were partly regulated either by CPC, ETC1 or TRY (Fig. 5). Interestingly, alterations in PLP4 expression affected several traits that are also responsive to Pi starvation, including changes in lipid levels and composition, primary root length and root hair elongation (Rietz et al., 2010), suggesting an involvement of PLP4 and possibly other PLPs in the PSR. Out of the five PLPs that were de-regulated by mutations in CPC, ETC1 or TRY, four were up-regulated upon Pi starvation. PLPs could hydrolyse PC to form lysophosphatidylcholine (LPC), which can then be converted to glycerol-3-phosphate (G3P) by GDPD1 and GDPD2 (Fig. 6).
Interestingly, PLD-derived PA has been shown to interact with WER, promoting its nuclear localization (Yao et al., 2013). This finding establishes a solid mechanistic connection between lipid metabolism and root hair cell fate. Competition between different MYBs for PA binding and controlled nuclear localization of TFs represents a possible mechanism for the modulation of root hair formation and elongation in response to environmental signals.
With the exception of PLP6, all genes shown in Fig. 6 are only dependent on CPC, ETC1 or TRY only when grown on low Pi media. Also of note and in contrast to what was observed in cpc and etc1, several TRY-regulated genes showed a higher transcript abundance in the mutant, indicative of a negative regulation of these genes by TRY. Thus TRY on one hand and CPC and ETC1 on the other appear to play antagonistic roles in membrane lipid remodelling.
CPC, ETC1 and TRY may regulate the activity of Pi-responsive genes partly via natural antisense transcripts
In cpc, etc1 and try plants, several potential natural antisense transcripts (NATs) accumulated differentially between the mutants and the wild type (Table 1). Natural antisense genes are transcribed from the opposite strand of protein-coding genes and overlap in part with sense RNA, regulating sense gene expression, transcript processing, translation or degradation of sense RNA via their expression. Expression of NATs can ‘rewire’ regulatory networks by changing positive regulators into repressors through the expression of the respective antisense RNA (Pelechano and Steinmetz, 2013).
Table 1.
Putative antisense genes controlled by CPC, ETC1 and TRY
| Antisense gene | Overlaps with | Description | Fold-change |
|---|---|---|---|
| etc1 Pi-replete | |||
| At2g35738 | At2g35740 | INOSITOL TRANSPORTER 3 (INT3) | 0.52 |
| etc1 low Pi | |||
| At1g74205 | At1g74210 | GLYCEROPHOSPHODIESTER PHOSPHODIESTERASE 5 (GDPD5) | 1.69 |
| At4g26795 | At4g26790 | GDSL-like lipase/acylhydrolase superfamily protein | 2.18 |
| At1g60505 | At1g60510 | DYNAMIN RELATED PROTEIN 4D (DRP4D) | 1.90 |
| cpc Pi-replete | |||
| At1g72852 | At1g72850 | Disease resistance protein | 0.42 |
| At1g67365 | At1g67370 | ASYNAPTIC 1 (ASY1) | 0.33 |
| At2g35738 | At2g35740 | INOSITOL TRANSPORTER 3 (INT3) | 0.35 |
| At5g07152 | At5g07150 | Leucine-rich repeat protein kinase family protein | 2.42 |
| cpc low Pi | |||
| At4g27852 | At4g27850; At4g27860 | Glycine-rich protein family; MEMBRANE OF ER BODY 1 (MEB1) | 0.35 |
| At1g03545 | At1g03550 | SECRETORY CARRIER MEMBRANE PROTEIN 4 (SCAMP4) | 2.98 |
| At1g07128 | At1g07130 | STN1 | 0.18 |
| At3g22072 | At3g22070 | Proline-rich family protein | ∞ |
| At1g28685 | At1g28680 | HXXXD-type acyl-transferase family protein | 0.14 |
| At3g21755 | At3g21760 | HYPOSTATIN RESISTANCE 1 (HYR1) | 7.12 |
| At3g48115 | At3g48120 | Unknown protein | 0.56 |
| At2g22821 | At2g22820 | Unknown protein | 0.22 |
| try low Pi | |||
| At1g60505 | At1g60510 | DYNAMIN RELATED PROTEIN 4D (DRP4D) | 1.88 |
| At4g26795 | At4g26790 | GDSL-like lipase/acylhydrolase superfamily protein | 2.99 |
| At4g31398 | At4g31400 | CHROMOSOME TRANSMISSION FIDELITY 7 (CTF7) | 0.14 |
| At3g21755 | At3g21760 | HYPOSTATIN RESISTANCE 1 (HYR1) | 5.46 |
| At4g13700 | At1g44120 | CELLULOSE SYNTHASE INTERACTIVE 2 (CSI2) | 1.60 |
| At1g56165 | At1g56160 | MYB72 | 0.35 |
| try Pi-replete | |||
| At2g46572 | At2g46570 | LACCASE 6 (LAC6) | 2.62 |
| At3g24518 | At3g24520 | HEAT SHOCK TRANSCRIPTION FACTOR C1 (HSFC1) | 0.40 |
| At5g01175 | At5g01180 | PEPTIDE TRANSPORTER 5 (PTR5) | 1.59 |
| At1g28685 | At1g28680 | HXXXD-type acyl-transferase family protein | 0.38 |
| At2g35738 | At2g35740 | INOSITOL TRANSPORTER 3 (INT3) | 0.49 |
| At2g35637 | At2g35640 | Homeodomain-like superfamily protein | 0.43 |
| At1g60525 | At1g60530 | DYNAMIN RELATED PROTEIN 4A (DRP4D) | 1.61 |
In etc1 and cpc but not in try plants, differential expression of antisense genes was more frequent when plants were grown under low Pi conditions. Among the putatively regulated genes, two are encoding proteins involved in lipid metabolism (GDPD5 and the GDSL-like lipase At4g26790); the respective antisense genes were regulated by ETC1 and TRY. A potential antisense gene that is regulated by CPC under low Pi conditions overlaps with the proline-rich protein At3g22070. At3g22070 is co-expressed with several important genes in root hair formation such as LRX2, LRX3 and JKD (atted.jp) and may thus be involved in root hair elongation in response to Pi starvation. Another CPC-regulated NAT overlaps with the secretory carrier membrane protein SCAMP4, a node of a gene regulatory network that controls root hair development (Bruex et al., 2012). The antisense gene that potentially regulates the inositol transporter INT3 was controlled by all TFs under study. Inositol can bind Pi to and store Pi as phytate (InsP6). Inositol polyphosphate kinases have been implicated in root hair elongation and Pi sensing (Stevensen-Paulik et al., 2005). Thus, the control of INT3 by NATs may have impact on cellular Pi homeostasis and may indirectly influence root hair elongation via alterations of the plant’s Pi status.
The most pronounced effect of mutations in R3 MYBs was observed for At3g21755, a putative antisense gene of HYR1. HYR1 was highly up-regulated in cpc and try mutants in both growth types (Pi-replete and low Pi), but was more so in Pi starved plants (more than 7-fold up in cpc and more than 5-fold up in try). HYR1 encodes a UDP glycosyltransferase that confers resistance against the cell expansion inhibitor hypostatin via glucosylation (Zhao et al., 2007). The role of HYR1 in Pi homeostasis in roots and its regulation awaits further characterization.
CPC and ETC1 participate in the regulation of cellular zinc and Pi homeostasis
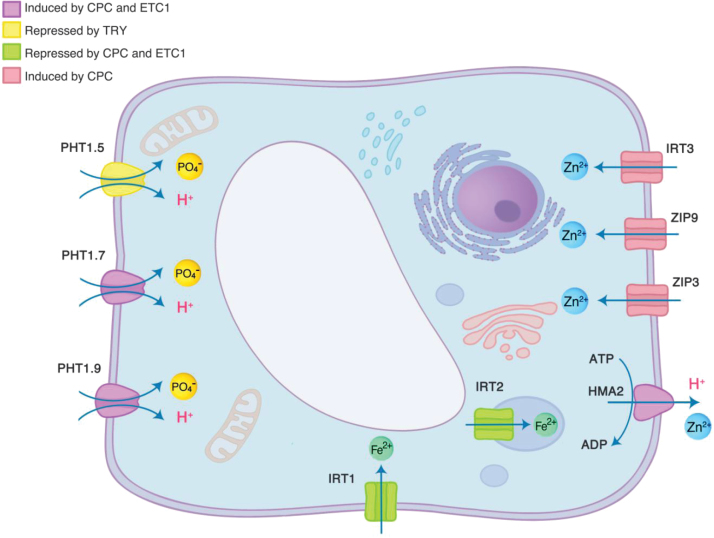
To further decipher processes that are regulated by the three TFs under control and/or low Pi conditions, we considered genes in which the ratio (wild type low Pi/wild type control)/(mutant low Pi/mutant control) was more than 2-fold changed. Interestingly, several genes encoding transition metal and Pi transporters regulated by CPC and ETC1 fulfilled this criterion (Fig. 7). In particular, genes encoding plasma membrane (PM)-bound Fe2+/Zn2+ transporters were positively regulated either by CPC alone (ZIP3, ZIP9), or by both CPC and ETC1 (HMA2). In the wild type, the expression of Zn2+ transporters was strongly reduced upon Pi starvation, a response that was less pronounced in cpc and etc1 mutants. In addition, under control conditions, expression levels of these genes were 2–3-fold lower in the mutants than in the wild type. In contrast, the expression of the Fe2+ transporters IRT1 and IRT2 was higher in cpc and etc1 mutants than in the wild type when grown on Pi-replete media. Pi starvation leads to an increase in Fe concentrations for reasons that are still under debate, causing a pronounced down-regulation of the Fe2+ transporters (Misson et al., 2005; Lan et al., 2012). This down-regulation was more pronounced in cpc and etc1 mutants. It thus appears that under Pi-replete conditions the expression of Zn2+ transporters is reduced in the mutants relative to the wild type, while that of Fe2+ transporters was higher. Under low Pi conditions, this relationship is reversed, i.e. a slightly higher (but statistically insignificant) expression of Zn2+ transporters and a lower expression of Fe2+transporters in the mutants. In support of an altered Zn2+ homeostasis in response to Pi starvation, the PM-bound Zn2+ exporter PCR2 was highly induced upon Pi starvation in roots of wild-type plants. In all three mutants, the induction of PCR2 was lower than in the wild type, but the difference did not reach the 2-fold threshold used here.
Fig. 7.
Regulation of transition metal transporters by CPC, ETC1 and TRY. The transport proteins are likely localized in different cell types and tissues.
The plasma membrane (PM)-localized Pi transporters PHT1;5, PHT1;7 and PHT1;9 were strongly induced in the wild type upon Pi starvation, but this increase was much less pronounced in cpc and etc1 mutants. In try roots, the (wild type low Pi/wild type control)/(mutant low Pi/mutant control) ratio of PHT1;5 expression was decreased, due to a lower expression of PHT1;5 (0.36-fold) under control conditions and a slightly increased expression under low Pi conditions when compared with the wild type.
The transporters depicted in Fig. 7 may not be localized in the same cell type and are summarized here in a single cell for simplicity. With the exception of IRT2, which is expressed in internal membranes (Vert et al., 2009), all transporters have a predicted or experimentally validated localization on the PM. IRT1 is preferentially expressed in root epidermal cells (Vert et al., 2002). Governed by its low substrate specificity, IRT1 transports other metal ions such as Zn2+ and Mn2+ beside Fe2+ (Korshunova et al., 1999). The primary active Zn2+ pump HMA2 catalyses the loading of Zn2+ into the xylem and is predominantly expressed in the vasculature (Hussain et al., 2004). Based on a similar expression pattern, IRT3 has been associated with xylem unloading and/or phloem loading (Lin et al., 2009). While a role of transition metal homeostasis in root hair formation remains to be demonstrated, the results make it tempting to speculate that the concentration, distribution or relative concentration in relation of Zn to other transition metals is critical for the differentiation of trichoblasts. This assumption is supported by the root hair phenotype of zip3 mutants (Lan et al., 2013). The sophisticated, Pi-responsive control of cellular Zn levels is dependent on CPC and partly on ETC1. Whether the regulation of these transporters by R3 MYBs is direct or indirect remains elusive.
Also the Pi transporters that were affected by the mutations in CPC, ETC1 and TRY are most likely expressed in different root tissues. While PHT1;9 was implicated in Pi acquisition from the soil-root interphase under conditions of Pi starvation (Remy et al., 2012), PHT1;5 was shown to be involved in P allocation to shoots (Nagarajan et al., 2011), indicative of a localization in the stele. The regulation of genes primarily expressed in the epidermis and the stele is in line with the expression pattern of CPC (Fig. 3). Interestingly, ectopic expression of PHT1;5 resulted in increased root hair formation and reduced primary root growth. PHT1;5 transcripts were decreased in WRKY75 RNAi lines (Nagarajan et al., 2011). WRKY75 non-autonomously regulates the expression of CPC and indirectly that of GL2, thereby altering the number of hairs in non-hair cell files (Rishmawi et al., 2014). WRKY75 is induced upon Pi starvation; suppression of WRKY75 significantly reduced root hair density both under control and Pi-deficient conditions (Devaiah et al., 2007). Together these results suggest that WRKY75 might affect root hair formation and Pi homeostasis via controlling the expression of CPC.
Conclusions
The current study shows that the three TFs under study directly or indirectly control a plethora of processes mediated by >2000 genes, including cell wall remodelling, Pi transport and storage, transition metal homeostasis, hormone signalling and lipid metabolism. This diversity is surprising since, as positive regulators of the root hair cell fate, CPC, ETC1 and TRY supposedly have targets that are chiefly associated with processes involved in root hair differentiation. It should also be noted that the number of genes that are regulated by CPC, ETC1 and TRY might be underestimated by the current approach since genes that are regulated in a fully fashioned manner are not detected.
The genes that are affected by mutations in the TFs investigated shifted in response to Pi starvation towards GO categories that are related to stress response and signal transduction, indicating an extension of their role to confer phenotypic plasticity. This shift may be caused by changes in R3 MYB expression levels, interaction with other proteins, competition for promoter binding sites or other factors. A tempting scenario implies regulation of the nuclear localization of R3 MYBs and WER/MYB23 via differential binding to PA (or other lipids), which would positively or negatively regulate root hair cell fate and also control several processes that adapt plants to low Pi availability. Dynamic shifts in the nuclear localization of particular MYBs would not affect the steady-state abundance of their transcripts, but may have strong impacts on downstream transcription.
The current dataset is also indicative of a sophisticated interplay of the three TFs, contrary to an understanding of a largely redundant functional relationship. This interplay becomes apparent particularly during Pi starvation, where the relative abundance of the three R3 MYBs is massively altered to favour ETC1, ETC3 and CPC while TRY abundance remains un-responsive to the Pi supply. A possible regulatory mechanism implies that membrane lipid remodelling is calibrated by the relative abundance of ETC3 and CPC as positive regulators and the negative regulator TRY; a concept that awaits experimental validation.
Our data further reveal that root hair development under both control and Pi-deficient conditions may be intertwined with various processes located in different tissues, which may directly or indirectly affect the differentiation of epidermal cells in a feedback mechanism. For example, the regulation of Pi and Zn2+ distribution among different tissues or organs may alter cellular nutrient homeostasis, which in turn has consequences for epidermal cell differentiation. Another example is membrane lipid remodelling that may also affect root hair development indirectly by altering the level of free Pi and thereby the overall Pi status of the plant and/or by its impact on Pi signalling. Also, changes in the storage of Pi by the regulation of inositol transport may influence root hair formation. Similar to the plant hormones auxin and ethylene that act downstream of the cell specification cascade (Masucci and Schiefelbein, 1996), other factors such as the internal concentration of transition metals may have direct consequences for the phenotype. In contrast to hormones, which act on the cell fate via a route that is independent of the decisions made upstream, our data reveal that R3 MYBs control a variety of physiological processes, which may in turn affect epidermal patterning.
In summary, the current survey reveals several novel candidates that may play important yet undiscovered roles in root hair formation. The data obtained here also challenge the generalization of genetic redundancy of paralogous genes by demonstrating that such apparent redundancy, while true under controlled conditions, may turn into a complex interplay of gene control when environmental factors dictate adaptive alterations of metabolic and developmental programmes.
Supplementary data
Supplementary data are available at JBX online.
Supplementary Figure S1. Root hair phenotypes of the cpc etc1 double mutant, try, and an TRY overexpressing line.
Supplementary Figure S2. Validation of differentially expressed genes by qRT-PCR. Supplementary Table S1. Number of reads of the various libraries.
Supplementary Table S2. Ratios [low Pi (LP)/Pi-replete (ES)] of differentially expressed genes in the wild type and the mutants investigated.
Supplementary Table S3. Genes that are more than 2-fold differentially expressed between wild-type plants and cpc, etc1 and try mutants.
Supplementary Table S4. Primers used for the qRT-PCR experiments shown in Figure S2.
Acknowledgements
We thank John Schiefelbein (University of Michigan) for pCPC-GUS plants and Martin Hülskamp (University of Cologne) for TRY OE lines. We further thank Yuki Nakamura (IPMB, Academia Sinica) and Thomas J Buckhout (Humboldt University Berlin) for valuable suggestions and critical comments on the manuscript, and Paula Perry for preparing cross sections. We also thank Ally Kuo and Robert Potter for figure artwork. Experiments and data analysis were performed in part through the use of the inductively coupled plasma optical emission spectrometer at Agricultural Biotechnology Research Center of Academia Sinica and with the assistance of Mr Yan-Chen Huang. Work in the Schmidt lab is supported by grants from Academia Sinica and MoST.
References
- Bernhardt C, Tierney ML. 2000. Expression of AtPRP3, a proline-rich structural cell wall protein from Arabidopsis, is regulated by cell-type-specific developmental pathways involved in root hair formation. Plant Physiology 122, 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt C, Zhao M, Gonzalez A, Lloyd A, Schiefelbein J. 2005. The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development 132, 291–298. [DOI] [PubMed] [Google Scholar]
- Boron AK, Van Orden J, Nektarios Markakis M, Mouille G, Adriaensen D, Verbelen JP, Hofte H, Vissenberg K. 2014. Proline-rich protein-like PRPL1 controls elongation of root hairs in Arabidopsis thaliana . Journal of Experimental Botany 65, 5485–5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruex A, Kainkaryam RM, Wieckowski Y, Kang YH, Bernhardt C, Xia Y, Zheng X, Wang JY, Lee MM, Benfey P, Woolf PJ, Schiefelbein J. 2012. A gene regulatory network for root epidermis cell differentiation in Arabidopsis . PLOS Genetics 8, e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrika NN, Sundaravelpandian K, Yu SM, Schmidt W. 2013. ALFIN-LIKE 6 is involved in root hair elongation during phosphate deficiency in Arabidopsis . New Phytologist 198, 709–720. [DOI] [PubMed] [Google Scholar]
- Cruz-Ramírez A, Oropeza-Aburto A, Razo-Hernández F, Ramírez-Chávez E, Herrera-Estrella L. 2006. Phospholipase DZ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proceedings of the National Academy of Sciences, USA 103, 6765–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal RB, Henikoff S. 2010. A simple method for gene expression and chromatin profiling of individual cell types within a tissue. Developmental Cell 18, 1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah BN, Karthikeyan AS, Raghothama KG. 2007. WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis . Plant Physiology 143, 1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estelle MA, Somerville C. 1987. Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Molecular and General Genetics 206, 200–206. [Google Scholar]
- Franco-Zorrilla JM, Martin AC, Leyva A, Par-Ares JP. 2005. Interaction between phosphate-starvation, sugar, and cytokinin signaling in Arabidopsis and the roles of cytokinin receptors CRE1/AHK4 and AHK3. Plant Physiology 138, 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson CS, Roberts K, Feldmann KA, Dolan L. 1997. The COW1 locus of Arabidopsis acts after RHD2, and in parallel with RHD3 and TIP1, to determine the shape, rate of elongation, and number of root hairs produced from each site of hair formation. Plant Physiology 115, 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JP, Bennett MJ, Bowen HC, Broadley MR, Eastwood DC, May ST, Rahn C, Swarup R, Woolaway KE, White PJ. 2003. Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiology 132, 578–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H, Scheres B, Blilou I. 2010. JACKDAW controls epidermal patterning in the Arabidopsis root meristem through a non-cell-autonomous mechanism. Development 137, 1523–1529. [DOI] [PubMed] [Google Scholar]
- Horgan JM, Wareing PF. 1980. Cytokinins and the growth-responses of seedlings of Betula pendula Roth. and Acer pseudoplatanus L. to nitrogen and phosphorus deficiency. Journal Experimental Botany 31, 525–532. [Google Scholar]
- Hülskamp M, Misra S, Jürgens G. 1994. Genetic dissection of trichome cell development in Arabidopsis . Cell 76, 555–566. [DOI] [PubMed] [Google Scholar]
- Hussain D, Haydon MJ, Wang Y, Wong E, Sherson SM, Young J, Camakaris J, Harper JF, Cobbett CS. 2004. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis . Plant Cell 16, 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MA, Raymond MJ, Smirnoff N. 2006. Analysis of the root-hair morphogenesis transcriptome reveals the molecular identity of six genes with roles in root-hair development in Arabidopsis . Plant Journal 45, 83–100. [DOI] [PubMed] [Google Scholar]
- Kang YH, Song SK, Schiefelbein J, Lee MM. 2013. Nuclear trapping controls the position-dependent localization of CAPRICE in the root epidermis of Arabidopsis . Plant Physiology 163, 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas B, Amyot L, Johansen C, Sato S, Tabata S, Kawaguchi M, Szczyglowski K. 2009. Conservation of Lotus and Arabidopsis basic helix-loop-helix proteins reveals new players in root hair development. Plant Physiology 151, 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik V, Simon M, Hülskamp M, Schiefelbein J. 2004. a The ENHANCER OF TRY AND CPC1 gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis . Developmental Biology 268, 506–513. [DOI] [PubMed] [Google Scholar]
- Kirik V, Simon M, Wester K, Schiefelbein J, Hülskamp M. 2004. b ENHANCER of TRY and CPC 2 (ETC2) reveals redundancy in the region-specific control of trichome development of Arabidopsis . Plant Molecular Biology 55, 389–398. [DOI] [PubMed] [Google Scholar]
- Kitaoka N, Matsubara T, Sato M, Takahashi K, Wakuta S, Kawaide H, Matsui H, Nabeta K, Matsuura H. 2011. Arabidopsis CYP94B3 encodes jasmonyl-l-isoleucine 12-hydroxylase, a key enzyme in the oxidative catabolism of jasmonate. Plant and Cell Physiology 52, 1757–1765. [DOI] [PubMed] [Google Scholar]
- Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB. 1999. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Molecular Biology 40, 37–44. [DOI] [PubMed] [Google Scholar]
- Kwak SH, Schiefelbein J. 2014. TRIPTYCHON, not CAPRICE, participates in feedback regulation of SCM expression in the Arabidopsis root epidermis. Plant Signaling and Behavior 9, e973815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak SH, Shen R, Schiefelbein J. 2005. Positional signaling mediated by a receptor-like kinase in Arabidopsis . Science 307, 1111–1113. [DOI] [PubMed] [Google Scholar]
- Lan P, Li W, Lin WD, Santi S, Schmidt W. 2013. Mapping gene activity of Arabidopsis root hairs. Genome Biology 14, R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P, Li W, Schmidt W. 2012. Complementary proteome and transcriptome profiling in phosphate-deficient Arabidopsis roots reveals multiple levels of gene regulation. Molecular and Cellular Proteomics 11, 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Lee D, Chung WI, Kwon CS. 2009. Arabidopsis ING and Alfin1-like protein families localize to the nucleus and bind to H3K4me3/2 via plant homeodomain fingers. The Plant Journal 58, 511–524. [DOI] [PubMed] [Google Scholar]
- Li D, Zhu H, Liu K, Liu X, Leggewie G, Udvardi M, Wang D. 2002. Purple acid phosphatases of Arabidopsis thaliana. Comparative analysis and differential regulation by phosphate deprivation. Journal of Biological Chemistry 277, 27772–27781. [DOI] [PubMed] [Google Scholar]
- Li MY, Welti R, Wang XM. 2006. Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation. Roles of Phospholipases D zeta 1 and D zeta 2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiology 142, 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Schmidt W. 2010. A lysine-63-linked ubiquitin chain-forming conjugase, UBC13, promotes the developmental responses to iron deficiency in Arabidopsis roots. Plant Journal 62, 330–343. [DOI] [PubMed] [Google Scholar]
- Lin WD, Liao YY, Yang TJ, Pan CY, Buckhout TJ, Schmidt W. 2011. Coexpression-based clustering of Arabidopsis root genes predicts functional modules in early phosphate deficiency signaling. Plant Physiology 155, 1383–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YF, Liang HM, Yang SY, Boch A, Clemens S, Chen CC, Wu JF, Huang JL, Yeh KC. 2009. Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytologist 182, 392–404. [DOI] [PubMed] [Google Scholar]
- Ma Z, Baskin TI, Brown KM, Lynch JP. 2003. Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiology 131, 1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Walk TC, Marcus A, Lynch JP. 2001. Morphological synergism in root hair length, density, initiation and geometry for phosphorus acquisition in Arabidopsis thaliana: a modeling approach. Plant and Soil 236, 221–235. [Google Scholar]
- Maris A, Suslov D, Fry SC, Verbelen JP, Vissenberg K. 2009. Enzymic characterization of two recombinant xyloglucan endotransglucosylase/hydrolase (XTH) proteins of Arabidopsis and their effect on root growth and cell wall extension. Journal of Experimental Botany 60, 3959–3972. [DOI] [PubMed] [Google Scholar]
- Martín AC, del Pozo JC, Iglesias J, Rubio V, Solano R, de La Pena A, Leyva A, Paz-Ares J. 2000. Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis . Plant Journal 24, 559–567. [DOI] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW. 1994. The rhd6 Mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiology 106, 1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW. 1996. Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root. Plant Cell 8, 1505–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, Spinka M, Kock M. 2012. Arabidopsis thaliana PECP1-Enzymatic characterization and structural organization of the first plant phosphoethanolamine/phosphocholine phosphatase. Biochimica et Biophysica Acta 1824, 319–325. [DOI] [PubMed] [Google Scholar]
- Misson J, Raghothama KG, Jain A, et al. 2005. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proceedings of the National Academy of Sciences, USA 102, 11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcuende R, Bari R, Gibon Y, et al. 2007. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant, Cell and Environment 30, 85–112. [DOI] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature Methods 5, 621–628. [DOI] [PubMed] [Google Scholar]
- Müller M, Schmidt W. 2004. Environmentally induced plasticity of root hair development in Arabidopsis . Plant Physiology 134, 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan VK, Jain A, Poling MD, Lewis AJ, Raghothama KG, Smith AP. 2011. Arabidopsis Pht1;5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiology 156, 1149–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Koizumi R, Shui G, Shimojima M, Wenk MR, Ito T, Ohta H. 2009. Arabidopsis lipins mediate eukaryotic pathway of lipid metabolism and cope critically with phosphate starvation. Proceedings of the National Academy of Sciences, USA 106, 20978–20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Teo NZ, Shui G, Chua CH, Cheong WF, Parameswaran S, Koizumi R, Ohta H, Wenk MR, Ito T. 2014. Transcriptomic and lipidomic profiles of glycerolipids during Arabidopsis flower development. New Phytologist 203, 310–322. [DOI] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K. 2002. The IRE gene encodes a protein kinase homologue and modulates root hair growth in Arabidopsis . Plant Journal 30, 289–299. [DOI] [PubMed] [Google Scholar]
- Pelechano V, Steinmetz LM. 2013. Gene regulation by antisense transcription. Nature Reviews Genetics 14, 880–893. [DOI] [PubMed] [Google Scholar]
- Perry P, Linke B, Schmidt W. 2007. Reprogramming of root epidermal cells in response to nutrient deficiency. Biochemical Society Transactions 35, 161–163. [DOI] [PubMed] [Google Scholar]
- Remy E, Cabrito TR, Batista RA, Teixeira MC, Sa-Correia I, Duque P. 2012. The Pht1;9 and Pht1;8 transporters mediate inorganic phosphate acquisition by the Arabidopsis thaliana root during phosphorus starvation. New Phytologist 195, 356–371. [DOI] [PubMed] [Google Scholar]
- Rietz S, Dermendjiev G, Oppermann E, Tafesse FG, Effendi Y, Holk A, Parker JE, Teige M, Scherer GFE. 2010. Roles of Arabidopsis patatin-related phospholipases A in root development are related to auxin responses and phosphate deficiency. Molecular Plant 3, 524–538. [DOI] [PubMed] [Google Scholar]
- Rishmawi L, Pesch M, Juengst C, Schauss AC, Schrader A, Hülskamp M. 2014. Non-cell-autonomous regulation of root hair patterning genes by WRKY75 in Arabidopsis . Plant Physiology 165, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Celma J, Lin W-D, Fu G-M, Abadía J, López-Millán A-F, Schmidt W. 2013. Mutually exclusive alterations in secondary metabolism are critical for the uptake of insoluble iron compounds by Arabidopsis and Medicago truncatula . Plant Physiology 162, 1473–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouached H, Arpat AB, Poirier Y. 2010. Regulation of phosphate starvation responses in plants: Signaling players and cross-talks. Molecular Plant 3, 288–299. [DOI] [PubMed] [Google Scholar]
- Ryu SB. 2004. Phospholipid-derived signaling mediated by phospholipase A in plants. Trends in Plant Science 9, 229–235. [DOI] [PubMed] [Google Scholar]
- Santi S, Schmidt W. 2009. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytologist 183, 1072–1084. [DOI] [PubMed] [Google Scholar]
- Savage N, Yang TJW, Chen CY, Lin KL, Monk NAM, Schmidt W. 2013. Positional signaling and expression of ENHANCER OF TRY AND CPC1 are tuned to increase root hair density in response phosphate deficiency in Arabidopsis thaliana . Plos One 8 e75452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellmann S, Hülskamp M, Uhrig J. 2007. Epidermal pattern formation in the root and shoot of Arabidopsis . Biochemical Society Transactions 35, 146–148. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Santi S, Pinton R, Varanini Z. 2007. Water-extractable humic substances alter root development and epidermal cell pattern in Arabidopsis . Plant and Soil 300, 259–267. [Google Scholar]
- Simon M, Lee MM, Lin Y, Gish L, Schiefelbein J. 2007. Distinct and overlapping roles of single-repeat MYB genes in root epidermal patterning. Developmental Biology 311, 566–578. [DOI] [PubMed] [Google Scholar]
- Sims RJ, III, Millhouse S, Chen CF, Lewis BA, Erdjument-Bromage H, Tempst P, Manley JL, Reinberg D. 2007. Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Molecular Cell 28, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson-Paulik J, Bastidas RJ, Chiou ST, Frye RA, York JD. 2005. Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. Proceedings of the National Academy of Sciences, USA 102, 12612–12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga R, Iwata M, Sano R, Inoue K, Okada K, Wada T. 2008. Arabidopsis CAPRICE-LIKE MYB 3 (CPL3) controls endoreduplication and flowering development in addition to trichome and root hair formation. Development 135, 1335–1345. [DOI] [PubMed] [Google Scholar]
- Vert G, Barberon M, Zelazny E, Seguela M, Briat JF, Curie C. 2009. Arabidopsis IRT2 cooperates with the high-affinity iron uptake system to maintain iron homeostasis in root epidermal cells. Planta 229, 1171–1179. [DOI] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. 2002. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14, 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Kurata T, Tominaga R, Koshino-Kimura Y, Tachibana T, Goto K, Marks MD, Shimura Y, Okada K. 2002. Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development 129, 5409–5419. [DOI] [PubMed] [Google Scholar]
- Wada Y, Kusano H, Tsuge T, Aoyama T. 2015. Phosphatidylinositol phosphate 5-kinase genes respond to phosphate deficiency for root hair elongation in Arabidopsis thaliana . The Plant Journal 81, 426–437. [DOI] [PubMed] [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K. 1997. Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 277, 1113–1116. [DOI] [PubMed] [Google Scholar]
- Wang S, Kwak SH, Zeng Q, Ellis BE, Chen XY, Schiefelbein J, Chen JG. 2007. TRICHOMELESS1 regulates trichome patterning by suppressing GLABRA1 in Arabidopsis . Development 134, 3873–3882. [DOI] [PubMed] [Google Scholar]
- Wang G Zhao GH Jia YH and Du XM. 2013. Identification and characterization of cotton genes involved in fuzz‐fiber development. Journal of Integrative Plant Biology 55, 619–630. [DOI] [PubMed] [Google Scholar]
- Wester K, Digiuni S, Geier F, Timmer J, Fleck C, Hülskamp M. 2009. Functional diversity of R3 single-repeat genes in trichome development. Development 136, 1487–1496. [DOI] [PubMed] [Google Scholar]
- Won SK, Lee YJ, Lee HY, Heo YK, Cho M, Cho HT. 2009. Cis-element- and transcriptome-based screening of root hair-specific genes and their functional characterization in Arabidopsis . Plant Physiology 150, 1459–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HY, Wang GL, Guo L, Wang XM. 2013. Phosphatidic acid interacts with a MYB transcription factor and regulates its nuclear localization and function in Arabidopsis . Plant Cell 25, 5030–5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Menand B, Bell E, Dolan L. 2010. A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nature Genetics 42, 264–267. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chow TF, Puckrin RS, Alfred SE, Korir AK, Larive CK, Cutler SR. 2007. Chemical genetic interrogation of natural variation uncovers a molecule that is glycoactivated. Nature Chemical Biology 3, 716–721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.