Abstract
Background
Some patients with COPD may share characteristics of asthma; this is the so-called asthma–COPD overlap syndrome (ACOS). There are no universally accepted criteria for ACOS, and most treatments for asthma and COPD have not been adequately tested in this population.
Materials and methods
We performed a survey among pulmonology specialists in asthma and COPD aimed at collecting their opinions about ACOS and their attitudes in regard to some case scenarios of ACOS patients. The participants answered a structured questionnaire and attended a face-to-face meeting with the Metaplan methodology to discuss different aspects of ACOS.
Results
A total of 26 pulmonologists with a mean age of 49.7 years participated in the survey (13 specialists in asthma and 13 in COPD). Among these, 84.6% recognized the existence of ACOS and stated that a mean of 12.6% of their patients might have this syndrome. In addition, 80.8% agreed that the diagnostic criteria for ACOS are not yet well defined. The most frequently mentioned characteristics of ACOS were a history of asthma (88.5%), significant smoking exposure (73.1%), and postbronchodilator forced expiratory volume in 1 second/forced vital capacity <0.7 (69.2%). The most accepted diagnostic criteria were eosinophilia in sputum (80.8%), a very positive bronchodilator test (69.2%), and a history of asthma before 40 years of age (65.4%). Up to 96.2% agreed that first-line treatment for ACOS was the combination of a long-acting β2-agonist and inhaled steroid, with a long-acting antimuscarinic agent (triple therapy) for severe ACOS.
Conclusion
Most Spanish specialists in asthma and COPD agree that ACOS exists, but the diagnostic criteria are not yet well defined. A previous history of asthma, smoking, and not fully reversible airflow limitation are considered the main characteristics of ACOS, with the most accepted first-line treatment being long-acting β2-agonist/inhaled corticosteroids.
Keywords: asthma, COPD, ACOS, survey, guidelines
Introduction
Asthma and COPD are two of the most important public health care problems, due to their elevated prevalence and high socioeconomic cost.1–4 Although COPD and bronchial asthma are chronic inflammatory diseases affecting the airway and essentially characterized by the presence of bronchial obstruction, they are separate nosological entities with different etiopathogenic bases, diagnostics, and therapeutic and prognostic characteristics.5
Nonetheless, these two diseases may coexist. The mixed asthma–COPD phenotype (asthma–COPD overlap syndrome [ACOS]) has been defined as symptoms of increased variability of airflow in association with incompletely reversible airflow obstruction.6 These patients are of special interest, since they are usually excluded from clinical trials with medications and also represent a clinically very important population with particular characteristics: more respiratory symptoms, more frequent exacerbations, and worse health-related quality of life.7,8 They are also characterized by an increase in comorbidity9 and a greater consumption of health care resources compared with patients with only asthma or COPD.10
The frequency of ACOS in patients with COPD ranges from 10% to 55% according to the type of population studies, and particularly the diagnostic criteria applied. In the epidemiological EPI-SCAN study, 17% of subjects with COPD were classified as ACOS.8 In another study, Soriano et al11 estimated that approximately 23% of patients with COPD 50–59 years of age could have ACOS, with this value rising to 52% in subjects 70–79 years of age.
There are currently no universally accepted, validated criteria for the diagnosis of ACOS. The Spanish consensus document on ACOS in COPD was published in 2012,12 and included the consensus of a series of diagnostic criteria that were later adopted in part or modified by the Spanish guidelines for COPD,13 as well as other national guidelines.14,15 The recent publication of the GINA (Global Initiative for Asthma) and GOLD (Global initiative for chronic Obstructive Lung Disease) guidelines in 2014 also included recommendations for the identification of these patients.16
Although different studies recognize the presence of ACOS, the detection, diagnosis, and treatment of these patients in clinical practice is not always simple and involves different interpretations. In this context, following the publication of the Spanish consensus on ACOS and in light of the new GINA and GOLD guidelines, the aim of the present study was to determine the opinion of Spanish pulmonologists who are experts in COPD or asthma in relation to ACOS in clinical practice and know what they consider to be the diagnostic criteria and management strategies to be undertaken in these patients. For this study, a representative sample of specialists in asthma and COPD from all regions of Spain were invited to participate.
Materials and methods
A process of debate was carried out in two phases in a group of pulmonologists who are specialists in asthma or COPD. The participating physicians were selected based on their participation in the Spanish working groups and in the Spanish guidelines of asthma or COPD. In the first phase, the participants were sent a structured questionnaire developed by two coordinators of the study (MM and VP), which was composed of 22 items on the profile of the specialists and their opinion on different aspects of ACOS: epidemiology, diagnosis, and treatment. The data obtained in the questionnaire were analyzed by means of descriptive statistics (percentages and mean values) before undergoing the second phase in order to have, a priori, some orientation as to the opinion of the participants in the debate.
In the second phase, a work meeting was held following the methodology of brainstorming based on the Metaplan® technique.17 This technique allows for structuring the knowledge of the attendees, who write their answers on cards. A facilitator then presents each card to the group and moderates the debate generated. The main advantages compared to a conventional meeting are that it promotes equal participation of all the attendees and facilitates an orderly debate, which in turn allows the determination of points of consensus and dissent.
The participants gave their opinion and orderly debate about the aspects included in the questionnaire in the first phase:
The new definition of patients with ACOS provided by the GINA–GOLD guidelines:16 ACOS is defined by a limitation of airflow with different characteristics generally associated with asthma and COPD. ACOS is therefore identified with characteristics shared by the two diseases asthma and COPD.
The definition of ACOS proposed by a Spanish consensus group:12 the diagnosis is made when two major criteria or one major and two minor criteria are met. The major criteria include a very positive bronchodilator test (increase in forced expiratory volume in 1 second [FEV1] ≥15% and ≥400 mL), eosinophilia in sputum, and personal history of asthma. Minor criteria include high total IgE, personal history of atopy, and positive bronchodilator test (increase in FEV1 ≥12% and ≥200 mL) on two or more occasions.
The diagnostic criteria that the participants consider should be taken into account in these patients. These criteria were classified according to their clinical relevance and applicability in respiratory clinics and primary care practices.
The treatment to use, according to the presentation of three clinical situations of patients with a possible diagnosis of ACOS.
Future actions to be carried out to consolidate the concept of ACOS.
Statistical analysis
For analysis of the answers to the questionnaire, the categorical variables are expressed as absolute frequencies and percentages, and the quantitative variables are expressed as mean, median, and minimum and maximum standard deviation. To analyze the data obtained in the debate session, the answers were transferred to an Office Excel calculation sheet (Microsoft Corporation, Redmond, WA, USA), calculating the percentage of votes for each of the blocks established by grouping the main ideas developed in the meeting. Statistical analysis was performed with SPSS version 17.0 (SPSS Inc, Chicago, IL, USA).
Results
A total of 26 pulmonologists participated in the study: 13 specialists in asthma and 13 in COPD. Of these, 20 (77%) were men and six women (23%), with a mean age of 49.7 years (standard deviation 7.4 years).
Phase 1: predebate questionnaire
All the participants answered the questionnaire from 2 weeks to 2 months before the meeting. Responses obtained are presented in Table 1. Among the most relevant results, it was of note that 84.6% of the participants agreed in recognizing ACOS and declared that a mean of 12.6% (standard deviation 7.0%) of COPD patients attended in their office presented with a combination of asthma and COPD. According to 80.8% of the participants, the diagnostic criteria for ACOS were still not well defined, and according to 61.5% an agreement among experts was essential with respect to the possible coexistence of asthma and COPD criteria in the same patient, due to possible future therapeutic consequences. In this sense, 76.9% were of the opinion that the ACOS criteria defined by a group of Spanish experts in 2012 should be validated, and 34.8% and 30.8% partially agreed with this or were equidistant between agreement and disagreement, respectively. Up to 57.7% considered that diagnostic assessment of the component “asthma” was insufficient with these criteria, since it did not include nonallergic patients with asthma, and 61.5% had the opinion that the criteria should be redefined in depth to include this aspect.
Table 1.
Responses to the questionnaire about ACOS administered before the debate
| Epidemiology | n (%) |
| Of all the patients you see, give an approximation of the percentage with ACOS (mean [SD]) | 12.6 (7.0) |
| Do some patients have a mixed asthma–COPD phenotype? Yes | 22 (84.6) |
| Diagnosis | |
| Are the diagnostic criteria of ACOS well defined? Yes | 5 (19.2) |
| Regarding the debate on the possible coexistence of asthma and COPD in the same patient, do you think it is: | |
| Unnecessary. They do not coexist. | 0 |
| Inadmissible. This is an academic more than a practical question. In the end, the patients receive the same treatment. | 1 (3.8) |
| Futile. The opinions among professionals are very conflicting, irreconcilable. | 1 (3.8) |
| Admissible, but it is not well defined. | 8 (30.8) |
| Essential. The experts should reach some agreement. It could have specific future therapeutic consequences. | 16 (61.5) |
| In your opinion, the combination of criteria of asthma and COPD in the same patient is: | |
| A specific phenotype of a disease. These are patients with COPD who have a history and criteria of allergic asthma. | 8 (30.8) |
| A coincidence of the two diseases in the same patient. It is the overlapping of two prevalent diseases, not the phenotype of one of these diseases. | 14 (53.8) |
| An invention. This does not exist. One disease always predominates another. The other characteristics act as diagnostic confounders. | 2 (7.7) |
| None of the above. | 2 (7.7) |
| What is your opinion of the ACOS criteria of the Spanish consensus? (multiple choice: more than one answer allowed) | |
| They are adequate. | 1 (3.8) |
| They are too complex. | 9 (34.6) |
| They should be validated. | 20 (76.9) |
| They are incomplete. | 7 (26.9) |
| They are too many. | 2 (7.7) |
| To what degree do you agree with the definition and diagnostic criteria of ACOS drawn up in the Spanish consensus? | |
| I fully agree. | 0 |
| Partially, but in the main I agree. | 9 (34.6) |
| Mid-position between agreement and disagreement. | 8 (30.8) |
| Partially, but in the main I disagree. | 6 (23.1) |
| I completely disagree. | 3 (11.5) |
| I have no definite opinion. | 0 |
| Should there be main, major, and minor diagnostic criteria for ACOS? | |
| Yes. | 9 (34.6) |
| No. | 5 (19.2) |
| I don’t know. | 12 (46.2) |
| Which of the following criteria of ACOS do you consider to be adequate? (multiple choice: more than one answer allowed) | |
| Very positive bronchodilator test. | 13 (50.0) |
| At least two positive bronchodilator tests. | 9 (34.6) |
| Elevated IgE. | 11 (42.3) |
| History of atopy. | 14 (53.8) |
| Previous history of asthma. | 23 (88.5) |
| History of smoking. | 19 (73.1) |
| Postbronchodilator FEV1/FVC <0.7 | 18 (69.2) |
| Elevated FENO. | 14 (53.8) |
| Eosinophilia in sputum. | 12 (46.2) |
| Eosinophilia in blood. | 11 (42.3) |
| The diagnostic evaluation of the asthma component of ACOS in the Spanish consensus is: | |
| Perfect, and well adjusted to the phenotype. | 0 |
| Partial, but sufficient for clinical practice. | 7 (26.9) |
| Insufficient, since it does not include patients with nonallergic asthma. | 15 (57.7) |
| Inadmissible, as there is no such phenotype. | 3 (11.5) |
| Diagnostic evaluation of the asthma component of ACOS in the Spanish consensus should be: | |
| Eliminated. There is no mixed asthma–COPD phenotype. | 1 (3.8) |
| Redefined in depth, including patients with nonallergic asthma. | 16 (61.5) |
| Partially modified, perhaps being simplified. | 9 (34.6) |
| Fully maintained as defined by the consensus of experts. | 0 |
| The role of smoking in the pathogenesis of the combination of asthma and COPD in the same patient: | |
| Is irrelevant. The symptoms and pulmonary function are important. | 0 |
| Is overevaluated. These patients may exist without having previously smoked. Requiring the presence of smoking undeservedly excludes patients with the syndrome. | 3 (11.5) |
| Important, since it actually involves smoker or ex-smoker asthma patients. | 18 (69.2) |
| Is essential. This phenomenon cannot exist without its presence or history. | 5 (19.2) |
| Do you think the ACOS criteria of the Spanish consensus have contributed to the diffusion of the mixed asthma–COPD phenotype? Yes | 21 (80.8) |
| Can ACOS change over time? | |
| Yes. | 14 (53.8) |
| No. | 4 (15.4) |
| I don’t know. | 8 (30.8) |
| Treatment | |
| Does ACOS require differentiated treatment? Yes | 23 (88.5) |
| From the following, select the first choice of pharmacological treatment for a patient with severe poorly controlled asthma and chronic airflow obstruction. | |
| Combination of LABA/ICS + montelukast. | 6 (23.1) |
| Combination of LABA/ICS + LAMA. | 16 (61.5) |
| Combination of LABA/ICS + theophylline. | 1 (3.8) |
| Combination of LABA/ICS + omalizumab. | 3 (11.5) |
| From the following, select the first choice of pharmacological treatment for a patient with very severe ACOS. | |
| Combination of LABA/ICS + montelukast. | 1 (3.8) |
| Combination of LABA/ICS + LAMA. | 25 (96.2) |
| Combination of LABA/ICS + theophylline. | 0 |
| Combination of LABA/ICS + roflumilast. | 0 |
| The commercial impact of the new bronchodilators for COPD and the greater risk of respiratory infections associated with inhaled steroids may make anti-inflammatory treatment in ACOS patients: | |
| Absolutely underused, particularly in primary care. | 1 (3.8) |
| Less used than it should be on being consigned to the characteristic treatment of COPD. | 12 (46.2) |
| Minor. There is a great tradition of prescribing LABA + ICS in severe COPD. | 8 (30.8) |
| Null. ICS continue to be the treatment of choice in asthma as well as ACOS. | 5 (19.2) |
| Therapeutic management by specialties (pulmonology/primary care) | |
| Should the criteria of ACOS be different for pulmonology and primary care? | |
| Yes. | 3 (11.5) |
| No. | 19 (73.1) |
| I don’t know. | 4 (15.4) |
| Appropriate diagnostic and therapeutic evaluation of patients with ACOS should: | |
| Always be done by a primary care physician. | 0 |
| Always be done by a pulmonologist. | 7 (26.9) |
| Be done according to the severity of the patient, with mild cases evaluated by primary care physicians and severe cases by pulmonologists. | 6 (23.1) |
| Be done according to the quality of care and resources available in primary care of a determined health care area. If these are low, it should be done by a pulmonologist. | 13 (50.0) |
| I don’t know. | 0 |
Abbreviations: ACOS, asthma–COPD overlap syndrome; SD, standard deviation; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FENO, fraction of exhaled nitric oxide in exhaled air; LABA, long-acting β2-agonist; ICS, inhaled corticosteroids; LAMA, long-acting muscarinic antagonist.
The criteria considered to be the most adequate for the diagnosis of ACOS were a previous history of asthma (88.5%), a history of smoking (73.1%), and postbronchodilator FEV1/forced vital capacity (FVC) <0.7 (69.2%) (Figure 1). Elevated Fraction of nitric oxide in exhaled air (FeNO) and a history of atopy were not considered to be adequate criteria by 53.8% (both). Of the participants, 69.2% considered smoking to be important in that ACOS is basically composed of smoking asthmatic patients, and 80.8% agreed that the Spanish ACOS criteria had contributed to disseminating the recognition of ACOS.
Figure 1.
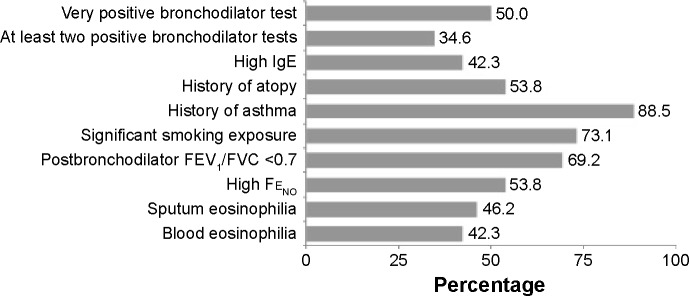
Criteria preferred by the participants for the diagnosis of asthma–COPD overlap syndrome.
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FENO, fraction of nitric oxide in exhaled air.
With regard to treatment, 88.5% stated that ACOS required differentiated treatment, with 96.2% considering that first-line treatment of very severe ACOS should include long-acting β2-agonists (LABAs)/inhaled corticosteroids (ICS) + long-acting muscarinic antagonists (LAMAs). Only 61.5% described this treatment as the first choice in the case of severe poorly controlled asthma and chronic airway obstruction.
Up to 73.1% said they agreed that the criteria of mixed phenotype should not differ for pneumology and primary care, and according to 50% the diagnostic and therapeutic evaluation of patients with ACOS should be made by pulmonologists. The remaining questions and responses to the questionnaire are shown in Table 1.
Phase 2: structured debate
During the phase 2 meeting, some of the statements included in the questionnaire were introduced into the structured discussion. The main points of discussion were as follows.
Definition of ACOS in the GINA–GOLD document
With regard to the definition of ACOS included in the GINA–GOLD document,16 the participants concluded that this definition was very general and not very precise, although it did recognize ACOS and could be used as a starting point.
Diagnostic criteria of ACOS
The classification of importance and applicability of diagnostic criteria for ACOS in respiratory clinics and primary care are presented in Table 2. The criteria considered most important were first eosinophilia in sputum (80.8%), followed by a very positive bronchodilator test (increase in FEV1 ≥15% and ≥400 mL compared to basal values) (69.2%) and a history of asthma before the age of 40 years (65.4%). FENO values were considered important by 53.8% of the participants.
Table 2.
Classification of the importance of the criteria and applicability in specialized and primary care
| Very important criteria | Important criteria | Less important criteria | |
|---|---|---|---|
| Specialized care | |||
| Very applicable | Very positive bronchodilator test (increase in FEV1 ≥15% and ≥400 mL compared to basal value) Previous history of asthma before 40 years of age |
Positive bronchodilator test on two or more occasions Blood eosinophilia |
Positive bronchodilator test Family history of atopy and/or asthma |
| Quite applicable | Elevated FeNO | Total IgE Positive methacholine test History of atopy Rhinitis of any type |
Positive skin tests |
| Not very applicable | Eosinophilia in sputum Positive oral corticosteroid test |
Increased or seasonal variability of symptoms Variability of peak flow >20% |
|
| Primary care | |||
| Very applicable | Previous history of asthma before 40 years of age | Eosinophilia in blood | |
| Quite applicable | Very positive bronchodilator test | Rhinitis of any type Elevated total IgE Personal history of atopy |
Family history of asthma and/or atopy |
| Not very applicable | Eosinophilia in sputum Elevated FeNO Positive oral corticosteroid test |
Positive bronchodilator test on two or more occasions Positive methacholine test |
Increased or seasonal variability of symptoms Positive skin tests Variability in peak flow >20% Positive bronchodilator test |
Notes: The “very applicable” criteria were considered as such by more than 65% of the panel. The “quite applicable” criteria were considered as such by 50%–65%, and the “not very applicable” were considered as such by less than 50% of the panel. Very positive bronchodilator test means an increase in FEV1≥15% and ≥400 mL compared to basal value. Positive bronchodilator test means an increase in FEV1 ≥12% and ≥200 mL compared to basal value.
Abbreviations: FEV1, forced expiratory volume in 1 second; FENO, fraction of nitric oxide in exhaled air.
More than 65% of the specialists thought the most applicable criterion was a very positive bronchodilator test, while a history of asthma and eosinophilia in blood were considered the more applicable criteria in primary care. The least applicable tests in primary care were eosinophilia in sputum, elevated FENO levels, and positive skin and methacholine tests. The criteria least assessed were seasonal or increased variability of symptoms, positive skin tests, variability in maximum expiratory flow >20%, reversibility in the current bronchodilator test, and a family history of asthma and/or atopy. The presence of rhinitis was considered important and applicable both in primary care and pulmonology clinics.
Treatment of ACOS
The debate about treatment was centered around three clinical scenarios (Table 3). The first case was a 62-year-old female ex-smoker with dyspnea, postbronchodilator FEV1/FVC =0.61, a positive bronchodilator test, and without exacerbations. The most frequent diagnosis was asthma followed by ACOS. The main recommendation for therapy was LABA/ICS, although some participants suggested LAMA alone or combined with LABA, considering that she was a nonexacerbator with moderate COPD. For second-line therapy, the panel agreed on triple-therapy LAMA + LABA/ICS.
Table 3.
Case scenarios of patients and suggested diagnosis and treatment
| Case definition | Diagnosis | Treatment
|
|
|---|---|---|---|
| First choice | Second choice | ||
| Case 1: A 62-year-old woman, smoker of 14 pack-years up to 10 years ago. She consulted for breathlessness with little effort. She had a history of frequent bronchitis during childhood and youth with unknown diagnosis. She did not report recent episodes of bronchitis or the use of antibiotics. Postbronchodilator spirometry: FEV1 (%) =67%; FEV1/FVC =0.61. Positive bronchodilator test with +14% and 320 mL increase in FEV1. | Asthma (or ACOS) | LABA/ICS | LABA/ICS + LAMA |
| Case 2: A 71-year-old man, smoker of one pack/day and accumulated consumption of 44 pack-years. He had a history of cough with daily expectoration since many months ago. A history of diagnosis of asthma at 21 years of age. He followed irregular treatment with theophyllines and salbutamol until the age of 45. He reported frequent exacerbations with an increase in dyspnea and mucopurulent expectoration treated with oral corticosteroids and antibiotics. He had never required hospitalization. Postbronchodilator spirometry: FEV1(%) =41%; FEV1/FVC =0.52. Negative bronchodilator test with +10% and 180 mL increase in FEV1. | ACOS (or COPD) | LABA/ICS ± LAMA | LABA/ICS + LAMA ± PDE4 inhibitor |
| Case 3: A 68-year-old man, smoker of ten cigarettes/day (accumulated consumption of 20 pack-years) who consulted for an acute episode of 2 days of evolution of an increase in dyspnea with purulent expectoration, without fever. He had not taken any treatment for his respiratory disease. In the stable phase, he presented with dyspnea with intermediate effort (he did not climb stairs) and spirometry results from 3 months previously with postbronchodilator values FEV1(%) =63%, FEV1/FVC =0.68, and positive postbronchodilator test with increase in FEV1 of 14% and 312 mL. | ACOS (or asthma) | LABA/ICS ± antibiotic | LABA/ICS + LAMA |
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ACOS, asthma–COPD overlap syndrome; LABA, long-acting β2-agonist; ICS, inhaled corticosteroids; LAMA, long-acting muscarinic antagonist.
The second case was a 71-year-old male heavy smoker with a previous diagnosis of asthma, frequent exacerbations, postbronchodilator FEV1/FVC =0.52, and a negative bronchodilator test. The majority of the panel agreed on the diagnosis of ACOS, although the possibility of COPD was also considered. The main treatment option was LABA/ICS, and some participants suggested triple therapy as the initial treatment. The second option was triple therapy with the possible addition of roflumilast.
The third case was a 68-year-old male ten-cigarettes/day smoker, diagnosed with asthma but without any respiratory treatment, who presented an exacerbation with purulent sputum. Postbronchodilator FEV1/FVC was 0.68, and a positive bronchodilator test showed an increase in FEV1 of 312 mL and 14%. The main diagnosis was ACOS, with some participants suggesting a diagnosis of asthma. The first choice of treatment was LABA/ICS with an antibiotic for the exacerbation, while the second choice was triple therapy with LAMA + LABA/ICS.
Future investigation
Of all the proposals made, those with the greatest consensus were actions to be carried out within the setting of investigation using longitudinal, population studies, and clinical trials (73%) to allow a more robust definition of ACOS to be made, as well as the possibility of more precisely identifying these patients with biological markers (54%).
With regard to diffusion, more than half of the participants in the session agreed that a specific chapter on ACOS should be included in the guidelines of asthma and COPD. In addition, activities should be conducted with the clinicians involved in their diagnosis and treatment, whether these be primary care physicians or not, with the aim of detecting ACOS or specialists elaborating a useful consensus for primary and specialized care.
Discussion
The existence of patients who fulfill the criteria for COPD – adult smokers with respiratory symptoms and postbronchodilator FEV1/FVC <0.7 – and who present characteristics of asthma, such as high reversibility of airflow, signs of bronchial and systemic eosinophilic inflammation, history of atopy, or even a previous diagnosis of asthma before the age of 40 years, is widely recognized. In a study in primary care, the participating physicians had difficulties in diagnosing asthma or COPD in 19% of adult patients with respiratory symptoms taking respiratory medications, because they shared characteristics of both diseases.18 Recognition of the existence of patients who share characteristics of more than one chronic obstructive respiratory disease is not new. In 1958, the Ciba Symposium had already described individuals with asthma and chronic bronchitis or emphysema,19 and the American Thoracic Society COPD guidelines published in 1995 included a Venn diagram with the overlap of asthma and chronic bronchitis, emphysema, or both.20 However, during the last 15 years and until very recently, the treatment of COPD has been managed exclusively by the degree of airflow obstruction and the presence of exacerbations.21 The concepts of phenotypes and endotypes in asthma22 and the results of large studies in COPD, such as ECLIPSE,23,24 have stimulated the interest in the concept of phenotypes in COPD.25 Among these phenotypes, ACOS has attracted increasing interest, due to its prevalence, prognosis, and specific treatment.26 In our survey, more than 80% of asthma and COPD specialists accepted the existence of ACOS as a particular subgroup of adult patients with chronic airflow limitation.
Despite the interest in ACOS, there are no universally accepted criteria for diagnosis. Up to 81% of the participants in our survey agreed that diagnostic criteria for ACOS were not yet well defined. In 2012, a group of COPD experts proposed diagnostic criteria for ACOS in COPD.12 These criteria were 1) major criteria of very positive bronchodilator response (>400 mL and >15% in FEV1), sputum eosinophilia, or previous diagnosis of asthma, and 2) minor criteria of increased total serum IgE, previous history of atopy, or positive bronchodilator test (>200 mL and >12% in FEV1) on at least two occasions. To be diagnosed with ACOS, a patient must fulfill two major or one major and two minor criteria. The new Finnish guidelines for the treatment of COPD proposed the same criteria for the diagnosis of ACOS as the Spanish guidelines, with the addition of an elevated FeNO higher than 50 parts per billion as a major criterion and a peak flow follow-up typical for asthma as an additional minor criterion.15 The latest Czech Republic guidelines, published in 2013, also include ACOS with its own diagnostic criteria, similar to the Spanish recommendations.14 These are quite restrictive criteria, and represent a very conservative approach until more evidence about the characterization of ACOS becomes available from large clinical trials or prospective studies. In fact, two recent studies in Spain using the previous criteria identified only between 5% and 6% of patients fulfilling the criteria for ACOS in patients with smoking-related COPD.27,28 This percentage is clearly below the expected number of individuals sharing the characteristics of asthma and COPD, according to epidemiological data. In fact, only 34.6% of the specialists surveyed were in agreement with the Spanish criteria, and 30.8% were in an intermediate position between agreement and disagreement. The main aspect highlighted by 76.9% of the specialists was that these criteria had to be validated in prospective studies.
In 2014, GINA and GOLD published a joint document on ACOS.16 ACOS was defined as the presence of persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD. The document presents the characteristics of asthma and COPD listed separately and suggests that ACOS may be the diagnosis when a similar number of features of asthma and COPD are identified in a given patient. The opinion of most of the group was that this definition was imprecise. In particular, the GINA–GOLD criteria included postbronchodilator FEV1/FVC <0.7 and significant exposure to smoking as two more on the list of possible criteria. However, a history of smoking exposure and airflow obstruction were considered necessary for the diagnosis of ACOS by 73.1% and 69.2% of the participants. A recent population-based study on phenotypes of airway disease performed in New Zealand identified a group of ACOS individuals by cluster analysis, and all were smokers or ex-smokers with chronic airflow obstruction.29 In fact, without smoking exposure and/or chronic airflow obstruction, the diagnosis can only be asthma, but ACOS cannot be accepted without evidence of COPD.
Regarding the diagnostic criteria, in a patient with COPD, those considered to be more important for the diagnosis of ACOS were a very positive bronchodilator test, a previous history of asthma before the age of 40 years, sputum eosinophilia, high FENO, and a positive test for oral corticosteroids. This last test consists of the administration of 0.5 mg/kg of prednisone during 2–3 weeks, and is considered positive if there is an increase in FEV1 superior to 200 mL and 12%. Among those considered important were a positive bronchodilator test on at least two occasions, increased blood eosinophilia, high total IgE, a positive methacholine-challenge test, a personal history of atopy, and any type of rhinitis. Most of these criteria had already been described in the previous Spanish consensus, except for rhinitis, the methacholine-challenge test, elevated FENO, and the positive oral corticosteroid test.12 However, the participants could not agree on any particular requirement in terms of number or preference of criteria for the diagnosis of ACOS. Another important aspect was that some of the criteria were considered not feasible for routine clinical practice, as is the case with sputum eosinophilia, the oral corticosteroid test, and FeNO determination in the context of primary care. It was agreed that until validation studies are developed, the clinician should consider ACOS in a patient with COPD and the presence of some of the aforementioned criteria. In particular, 88.5% considered a previous history of asthma before the age of 40 years in a patient with COPD to be very indicative of ACOS. With only this criterion, in the COPDGene study, Hardin et al found that 13% of subjects with COPD had ACOS.7 In the PLATINO study performed in Latin America, a prevalence of 11.6% was described using a post-bronchodilator FEV1/FVC <0.7 and the diagnosis of asthma as ACOS criteria.30 In the Spanish EPISCAN epidemiological study, including patients with COPD and a previous diagnosis of asthma before the age of 40 years, the prevalence of ACOS observed among the COPD population was 17.4%.8 Similarly, in a large study on 3,125 COPD patients from primary and secondary care, 15.9% were classified as ACOS due to a previous diagnosis of asthma.31 It would be very relevant to investigate if the previous diagnosis of asthma by itself could identify the population with ACOS in COPD.
Patients with ACOS have been systematically excluded from both COPD and asthma pharmacological trials for not being “pure” subjects. As a consequence, there is no clear information about the response of these patients to most of the current pharmacological therapies. The only clinical trial performed to date in patients with ACOS studied the spirometric effects of tiotropium in individuals with concomitant COPD and asthma. Improvements in lung function and a reduction in rescue medication were observed with tiotropium.32 However, the main interest in differentiating ACOS from COPD lies in the different response to ICS. In the three cases presented to the panel, the first option of treatment chosen was the combination of LABA and ICS. This is the first option described in the Spanish guidelines for COPD,13 as in those of the Canadians,33 Japanese,34 Finns,15 and Czechs.14 The GINA–GOLD document also indicates that the default position in the case of ACOS should be to start treatment accordingly for asthma, and recommends the LABA/ICS combination with special attention to avoid the use of LABAs in monotherapy.16 In our survey, 88.5% of the participants agreed that ACOS requires a different treatment compared to COPD, starting with LABA/ICS and stepping up to triple therapy (LABA/ICS + LAMA) in severe cases.
Finally, the majority of the participants were of the opinion that the diagnosis and assessment of ACOS should be done by the pulmonologist, but the follow-up can be shared with the primary care physician, and in particular 73.1% thought that the criteria for ACOS must be the same in primary and secondary care. Among the suggestions for future research, it was agreed that longitudinal multicenter studies are required to validate the diagnostic criteria and identify biomarkers of the disease. In addition, clinical trials are necessary to verify the response to treatments of this group of patients.
Acknowledgments
The authors want to thank Montserrat Falgueras, Maria Fontanals, Laura Molina, Rosa Palomino, and Sonia Pisa (GOC Networking, Barcelona, Spain) for their help in the organization of the study, data entry and analysis of the questionnaires, and the facilitation of the Metaplan session. This study was funded by Chiesi Spain.
Footnotes
Disclosure
Marc Miravitlles has received speaker fees from Almirall, Boehringer Ingelheim, Pfizer, AstraZeneca, Chiesi, Esteve, GlaxoSmithKline, Menarini, Grifols, and Novartis, and consulting fees from Almirall, Boehringer Ingelheim, Cipla, Pfizer, GlaxoSmithKline, Gebro Pharma, CLS Behring, Medi-Immune, Takeda, Teva, Novartis, and Grifols. Bernardino Alcázar reports personal fees from Novartis AG, Boehringer Ingelheim, GSK, Almirall, AstraZeneca, and grants and personal fees from Menarini. Teresa Bazús has received honoraria or funding for attending scientific meetings from GSK, Chiesi, and Teva. Ciro Casanova has received speaker fees from AstraZeneca, GSK, Novartis, and Almirall y Gebro Pharma and consulting fees from Almirall, Novartis, and GSK. Carolina Cisneros has received speaker or consulting fees from AstraZeneca, GSK, Boehringer Ingelheim, Novartis, Takeda, Vifor Pharma, Chiesi, Orion, and Mundipharma. Luis M Entrenas has in the last 3 years received honoraria for speaking at sponsored meetings from Alter, AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GSK, Menarini, MSD, Mundipharma, Novartis, Pfizer, and Teva, as a consultant for Chiesi, Mundipharma, and Novartis, and has received funding/grant support for research projects from not-for-profit foundations, as well as Novartis. Patricia García-Sidro has received fees for scientific meetings, scientific advice, and participating in clinical studies or writing for publications from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, Gebro, GlaxoSmithKline, Menarini, MSD, Mundipharma, Novartis, Pfizer, Rovi, Takeda-Nycomed, and Teva. Borja G Cosio has in the last 3 years received honoraria for speaking at sponsored meetings from Chiesi, GSK, Boehringer Ingelheim, Novartis, and Pfizer, consultant fees from Pfizer, Chiesi, and GSK, travel grants from Boehringer Ingelheim, Novartis, and Chiesi, and received unrestricted funding/grant support for research projects from a variety of government agencies and not-for-profit foundations, as well as Boehringer Ingelheim, Chiesi, and Menarini. Arturo Huerta has received personal fees from GSK, Novartis, Takeda, Menarini, and Boehringer Ingelheim. José Luis Izquierdo reports personal fees for consulting and lectures from Almirall, AstraZeneca, Bayer, Boehringer Ingelheim, Takeda-Nycomed, Pfizer, and Menarini. Antolin López-Viña has in the last 3 years received honoraria for speaking at sponsored meetings from Chiesi, GSK, Boehringer Ingelheim, Novartis, and Pfizer, as a consultant for Pfizer, Boehringer Ingelheim, and Novartis, has received assistance to meet travel costs from Boehringer Ingelheim, Novartis, Teva, and Chiesi, and has received funding/grant support for research projects from a variety of government agencies and not-for-profit foundations, as well as Chiesi and Menarini. Eva Martínez-Moragón has in the last 3 years received honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mundipharma, Teva, and Pfizer. Luis Pérez de Llano has in the last 3 years received honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer Ingelheim, Menarini, Chiesi, GlaxoSmithKline, Teva, Almirall, Mundipharma, Esteve, Novartis, and Pfizer, as a consultant for Mundipharma, Pfizer, Ferrer, and Novartis, has received assistance to meet travel costs from GSK and Novartis, and has received funding/grant support for research projects from SEPAR and SERGAS. Juan José Soler-Cataluña has received speaker fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GSK, Merck, Menarini, Sharp & Dohme, Novartis, Takeda, and Pfizer, and consulting fees from Boehringer Ingelheim, GSK, AstraZeneca, Ferrer, Novartis, Almirall, Merck, Sharp & Dohme, and Takeda. Vicente Plaza has in the last 3 years received honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Merck, Mundipharma, and Pfizer, as a consultant for Mundipharma, Orion and Teva, received assistance to meet travel costs from Boehringer Ingelheim and Chiesi, and received funding/grant support for research projects from a variety of government agencies and not-for-profit foundations, as well as Chiesi, Menarini, and Merck. The authors report no other conflicts of interest in this work.
References
- 1.Bahadori K, Doyle-Waters MM, Marra C, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24. doi: 10.1186/1471-2466-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Marco R, Cappa V, Accordini S, et al. Trends in the prevalence of asthma and allergic rhinitis in Italy between 1991 and 2010. Eur Respir J. 2012;39:883–892. doi: 10.1183/09031936.00061611. [DOI] [PubMed] [Google Scholar]
- 3.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Campos JL, Ruiz-Ramos M, Soriano JB. Mortality trends in chronic obstructive pulmonary disease in Europe, 1994–2010: a join-point regression analysis. Lancet Respir Med. 2014;2:54–62. doi: 10.1016/S2213-2600(13)70232-7. [DOI] [PubMed] [Google Scholar]
- 5.Fabbri LM, Romagnoli M, Corbetta L, et al. Differences in airway inflammation in patients with chronic fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:418–424. doi: 10.1164/rccm.200203-183OC. [DOI] [PubMed] [Google Scholar]
- 6.Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64:728–735. doi: 10.1136/thx.2008.108027. [DOI] [PubMed] [Google Scholar]
- 7.Hardin M, Silverman EK, Barr RG, et al. The clinical features of overlap between COPD and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miravitlles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107:1053–1060. doi: 10.1016/j.rmed.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Pleasants RA, Ohar JA, Croft JB, et al. Chronic obstructive pulmonary disease and asthma – patient characteristics and health impairment. COPD. 2014;11:256–266. doi: 10.3109/15412555.2013.840571. [DOI] [PubMed] [Google Scholar]
- 10.Rhee CK, Yoon HK, Yoo KH, et al. Medical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthma. COPD. 2014;11:163–170. doi: 10.3109/15412555.2013.831061. [DOI] [PubMed] [Google Scholar]
- 11.Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest. 2003;124:474–481. doi: 10.1378/chest.124.2.474. [DOI] [PubMed] [Google Scholar]
- 12.Soler-Cataluña JJ, Cosío B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012;48:331–337. doi: 10.1016/j.arbres.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish guideline for COPD (GesEPOC). Update 2014. Arch Bronconeumol. 2014;50(Suppl 1):1–16. doi: 10.1016/S0300-2896(14)70070-5. [DOI] [PubMed] [Google Scholar]
- 14.Kovlizek V, Chlumsky J, Zindr V, et al. Chronic obstructive pulmonary disease: official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented care. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:189–201. doi: 10.5507/bp.2013.039. [DOI] [PubMed] [Google Scholar]
- 15.Kankaanranta H, Harju T, Kilpeläinen M, et al. Diagnosis and pharmacotherapy of stable chronic obstructive pulmonary disease: the Finish guidelines. Basic Clin Pharmacol Toxicol. 2015;116:291–307. doi: 10.1111/bcpt.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Global Initiative for Asthma, Global initiative for chronic Obstructive Lung Disease (GOLD) Asthma, COPD, and asthma-COPD overlap syndrome. 2014. [Accessed August 5, 2014]. Available from: http://www.goldcopd.org/asthma-copd-overlap.html.
- 17.UNICEF . Visualisation in Participatory Programme: A Manual for Facilitators and Trainers Involved in Participatory Group Events. Dhaka: UNICEF; 1993. [Google Scholar]
- 18.Miravitlles M, Andreu I, Romero Y, Sitjar S, Altés A, Anton E. Difficulties in differential diagnosis of COPD and asthma in primary care. Br J Gen Pract. 2012;62:e68–e75. doi: 10.3399/bjgp12X625111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terminology, definitions, and classification of chronic pulmonary emphysema and related conditions. A report of the conclusions of a Ciba guest symposium. Thorax. 1959;14:286–299. No authors listed. [Google Scholar]
- 20.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:S77–S120. No authors listed. [PubMed] [Google Scholar]
- 21.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global initiative for chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 22.Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127:355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 23.Agusti A, Calverley PMA, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miravitlles M, Calle M, Soler-Cataluña JJ. Clinical phenotypes of COPD. Identification, definition and implications for guidelines. Arch Bronconeumol. 2012;48:86–98. doi: 10.1016/j.arbres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Barrecheguren M, Esquinas C, Miravitlles M. The asthma-COPD overlap syndrome (ACOS). Opportunities and challenges. Curr Opin Pulm Med. 2015;21:74–79. doi: 10.1097/MCP.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 27.Golpe R, Sanjuán López P, Cano Jiménez E, et al. Distribution of clinical phenotypes in patients with chronic obstructive pulmonary disease caused by biomass and tobacco smoke. Arch Bronconeumol. 2014;50:318–324. doi: 10.1016/j.arbres.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Miravitlles M, Huerta A, Fernández-Villar JA, et al. Generic utilities in chronic obstructive pulmonary disease patients stratified according to different staging systems. Health Qual Life Outcomes. 2014;12:120. doi: 10.1186/s12955-014-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fingleton J, Travers J, Williams M, et al. Treatment responsiveness of phenotypes of symptomatic airways obstruction in adults. J Allergy Clin Immunol. 2015 Mar 4; doi: 10.1016/j.jaci.2015.01.013. Epub. [DOI] [PubMed] [Google Scholar]
- 30.Menezes AM, de Oca MM, Pérez-Padilla R, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype COPD-asthma. Chest. 2014;145:297–304. doi: 10.1378/chest.13-0622. [DOI] [PubMed] [Google Scholar]
- 31.Miravitlles M, Barrecheguren M, Roman-Rodriguez M. Frequency and characteristics of different clinical phenotypes of COPD. Int J Tub Lung Dis. 2015;19:992–998. doi: 10.5588/ijtld.15.0021. [DOI] [PubMed] [Google Scholar]
- 32.Magnussen H, Bugnas B, van Noord J, Schmidt P, Gerken F, Kesten S. Improvements with tiotropium in COPD patients with concomitant asthma. Respir Med. 2008;102:50–56. doi: 10.1016/j.rmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 33.O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2007 update. Can Respir J. 2007;14(Suppl B):5B–32B. doi: 10.1155/2007/830570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagai A, Aizawa H, Aoshiba K, et al. Guidelines for the Diagnosis and Treatment of COPD. 3rd ed. Tokyo: Japanese Respiratory Society; 2009. [Google Scholar]


