Abstract
Adenovirus vectors are the most commonly employed vector for cancer gene therapy. They are also used for gene therapy and as vaccines to express foreign antigens. Adenovirus vectors can be replication-defective; certain essential viral genes are deleted and replaced by a cassette that expresses a foreign therapeutic gene. Such vectors are used for gene therapy, as vaccines, and for cancer therapy. Replication-competent (oncolytic) vectors are employed for cancer gene therapy. Oncolytic vectors are engineered to replicate preferentially in cancer cells and to destroy cancer cells through the natural process of lytic virus replication. Many clinical trials indicate that replication-defective and replication-competent adenovirus vectors are safe and have therapeutic activity.
Keywords: Adenovirus, replication-defective, oncolytic, cancer gene therapy, vaccines, safe and well-tolerated
1. HUMAN ADENOVIRUS BACKGROUND
Adenoviruses (Ads) have an icosahedral protein capsid that encompasses a linear duplex DNA genome of approximately 36,000 base pairs [1]. There are about a dozen capsid proteins and no lipid envelope. The genome encodes approximately 35 proteins that are expressed in two general phases, “early” which occurs prior to the initiation of viral DNA replication at about 7 hours post-infection, and “late” which occurs following the initiation of DNA replication. The ~20 early proteins have regulatory functions that allow the virus to take control of the cell and to carry out viral DNA replication. The late genes are mainly structural proteins of the virus [1] (see legend to Fig. 1). Virions assemble in the nucleus starting at about 1 day postinfection, and after several days the cell lyses to release infectious virus. About 10,000 progeny virions are produced in permissive cells.
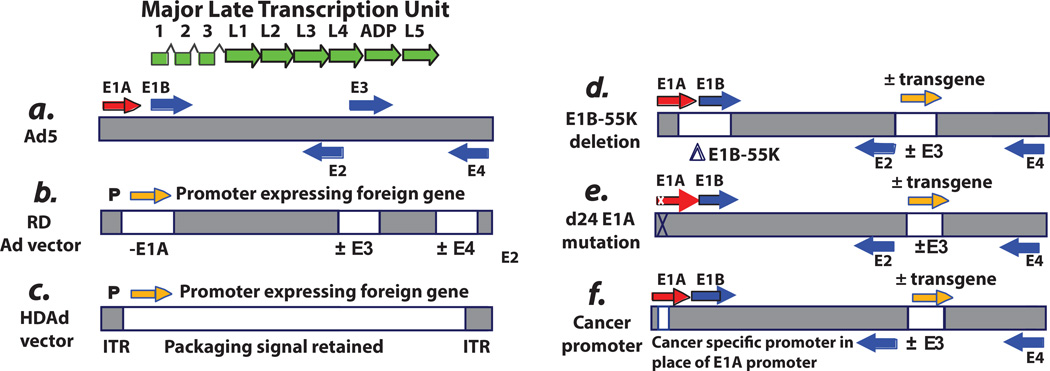
Fig. 1. Schematic of the genome of adenovirus type 5 (Ad5) and Ad5-based vectors.
A: Ad5. The horizontal bar indicates the duplex DNA genome of 36 kbp encoding ca. 35 genes. The arrows indicate transcription units. The “immediate early” E1A proteins derived from the E1A transcription unit (red arrow) induce expression of the “delayed early” proteins coded by the E1B, E2, E3, and E4 transcription units (blue arrows). Viral DNA begins to replicate at about 7 hours postinfection (p.i.), and then “late” proteins derived from the major late transcription unit (green arrows) are synthesized. The major late messenger RNAs (mRNAs) are formed by alternative splicing and polyadenylation of a large pre-mRNA initiated at the single major late promoter and extending to the right end of the genome. All late mRNAs have a tripartite leader (leaders 1, 2, and 3) at their 5′ termini that facilitates translation. Beginning at 20 to 24 hours p.i., virions begin to assemble in the cell nucleus, and then after 2 to 3 days the cells begin to lyse and release virions, with lysis complete by about 5 to 6 days. Efficient cell lysis is mediated by Adenovirus Death Protein (ADP). ADP is a late protein derived from the major late transcription unit. The vectors depicted are based on the Ad5 backbone. However, vectors based on serotypes other than Ad5 have also been developed. The reason for this is that Ad5 uses the Coxsackie-Adenovirus Receptor (CAR) receptor, whereas some other serotypes use different receptors (e.g., CD46 and sialic acid as receptors), and sometimes this is an advantage. Also, pre-existing antibody levels in humans are more prevalent for Ad5 than for certain other serotypes. B: Replication-defective vectors. The E1A and E1B regions (transcription units) (i.e., the E1 region) are deleted and replaced with an expression cassette with an exogenous promoter driving expression of the therapeutic gene. In gene therapy vectors, this gene can be one to correct a genetic defect. In vaccine vectors, the gene is the antigen. In cancer gene therapy vectors, the gene (e.g., p53) induces cell death. Usually the E3 region is deleted. Deletion of E3 does not affect the growth of the vector because the E3 genes are not required for Ad growth in cell culture. Also, deletion of E3 allows for larger inserts into the E1 region because only up to 105% of the genome can be packaged into virions. E1-deleted vectors are defective for replication because the E1A proteins, and in some cells the E1B proteins, are required for virus growth. The vectors are propagated in cell lines such as 293 or PER.C6, which retain and express the E1A and E1B proteins. Although the E1A proteins are required for expression of other Ad genes, these vectors can be leaky and express other Ad genes in an E1A-independent manner, especially at long periods following infection and if high multiplicities of infection are used. In animal model studies, this leakiness has led to elimination of vector-transduced cells by cells of the immune system. For this reason, second-generation vectors also lack the E4 region. E4 regions are essential for Ad replication, including late gene expression, so deletions of E4 eliminate the leakiness. Some vectors also lack the E2 region. These E4- and E2-deleted vectors must be grown on cell lines that complement the E1, E4, and E2 deletions. C: Helper-dependent vectors. These vectors lack all or most Ad genes but retain the cis-acting sequences such as the inverted terminal repeats (ITRs) at each end of the genome as well as the packaging sequence at the left end that are required for the genome to replicate and be packaged. These vectors are propagated in the presence of a helper Ad, which must be eliminated from the large-scale vector stocks. It is difficult to completely eliminate the vector. D: ONYX-015 and related vectors. ONYX-015 was the first replication-competent (oncolytic) Ad vector to be described. It lacks the E1B-55K gene as well as the rid and 14.7k genes in the E3B region. (ONYX-015 does not have a transgene in E3). Because of the E1B-55K deletion, this vector grows better in cancer cells than in noncancerous cells. However, in many cancer cells, the vector does not grow as well as Ad5. Many other oncolytic Ad vectors have a similar design (e.g., Oncorine), and some vectors have a therapeutic gene (orange arrow) (e.g. GM-CSF) incorporated into the E3 region and expressed from the E3 promoter, the major late promoter via splicing, or from a promoter inserted into E3. E: D24 vectors with an E1A CR2 deletion. Some vectors are targeted to cancer cells by virtue of deletions in the el a gene that knock out binding of the E1A proteins to the pRB family of tumor suppressors. Vectors with the CR2 deletion are able to replicate well in cancer cells with a defect in the pRB tumor suppressor pathway. The vectors replicate less well in normal cells. F: Vectors with a cancer-specific promoter replacing the E1A enhancer/promoter. Many vectors are targeted to cancer cells by replacement of the E1A promoter/enhancer by a cancer-specific promoter. These vectors grow in cancer cells in which the promoter is active. Many different promoters have been used. Some vectors have a therapeutic transgene in E3 and some vectors have an intact E3 region. In some vectors, the E4 promoter is replaced by a cancer-specific promoter.
There are at least 57 serotypes of human Ads, Ad1-Ad57, that form seven “species” A–G [2]. A serotype is defined by the ability of infection in cell culture to be neutralized by specific antisera. All serotypes are similar in general structure and the functions of most proteins, but certain unique protein functions contribute to the unique properties of the serotype and the species.
Human Ads are ubiquitous, and most people have been infected with one or more serotypes, leading to lifelong immunity. Infection can be asymptomatic or can lead to disease which is usually mild in immunocompetent individuals.
Serotypes in Species C (Ad1, 2, 5, 6) are the most common. They are usually acquired in early childhood and cause infections of the upper respiratory tract and to a lesser extent the gastrointestinal and urinary tracts. Approximately 5% of “common cold” cases are due to Ads. Species B Ads (Ad3, 7, 11, 14, 16, 21, 34, 35, 50) infect the respiratory tract, sometimes seriously, and also cause eye and gastrointestinal and urinary tract infections. Some serotypes in Species D (e.g. Ad8) cause epidemic keratoconjunctivitis. Ad40 and Ad41, in Species E, cause gastrointestinal infections. Ad4 (Species E) and Ad7 (Species B) cause acute respiratory disease among military recruits in the U.S.
Whereas most Ad infections are mild, Ads can be dangerous in immunosuppressed individuals, especially transplant patients in which Ads probably reactivate from latent or low grade persistent infections [2]. For example, in hematopoietic stem cell transplant patients, disease incidence with Ads ranges from 3% to 47%. With allogeneic stem cell transplants, the disease incidence is 9% to 14% with adults and 20% to 47% with children. In hematopoietic stem cell transplant patients, Ads can cause respiratory disease, colitis, hepatitis, hemorrhagic cystitis, keratoconjunctivitis, and disseminated infections. Disseminated infections and, commonly, lethality can occur in patients with severe lymphopenia (less than 300 cells/µl).
2. DRUGS TO TREAT ADENOVIRUS INFECTIONS
There are no drugs approved specifically to treat Ad infections. The relative lack of approved robust drugs to treat natural Ad infections as well as disseminated infections that may develop as a result of Ad vector therapy is an important issue. Cidofovir is used sometimes in the clinic, and although controlled clinical trials have not been conducted, it appears to have activity against Ad [2]. Cidofovir is an acyclic nucleoside monophosphate that is an analog of 2-deoxyquanosine. It is a substrate for the Ad DNA polymerase (but less so for the cellular DNA polymerases), and it functions, at least in part, as an Ad DNA synthesis chain terminator. Some clinics routinely use cidofovir in pediatric stem cell transplant patients when Ad DNA is detected by quantitative polymerase chain reaction (qPCR) in the plasma [3]. A problem with cidofovir is that it can lead to kidney toxicity. Recently, a lipid-linked derivative of cidofovir, named CMX001, has been developed that is more bioavailable and less toxic to the kidney than cidofovir [4]. CMX001 treatment probably saved the life of a pediatric stem cell transplant patient with a disseminated Ad2 infection that reached 109 Ad2 genome copies per ml [5]. Currently, CMX001 is being tested in a randomized, placebo-controlled, phase II clinical trial for prevention of Ad disease following hematopoietic stem cell transplantation.
3. INTRODUCTION TO ADENOVIRUS VECTORS
More than 400 gene therapy trials have been or are being conducted with human Ad vectors [2]. Most of these trials are for treatment of cancer, although some are for use of Ad vectors as vaccines in which the vector expresses a foreign antigenic protein or for gene therapy in which the vector expresses a non-mutant protein to correct a genetic defect. Also, millions of military recruits have been and are being immunized successfully against acute respiratory disease by gastrointestinal administration of live Ad serotype 4 (Ad4) and Ad7 vaccines in the form of enteric coated capsules [6]. Ad vectors have many advantages: Ads are well studied and Ad vectors can be grown into high titer stable stocks, they infect non-dividing and dividing cells of different types, and they are maintained in cells as an episome. As will be discussed in this article, nearly all clinical trials have indicated that Ad vectors are safe and well tolerated [2].
Most Ad vectors are genetically modified versions of Ad5, and they are of two types: replication-defective (RD) and replication-competent (RC) (Fig. 1). The RD vectors have the essential E1A and E1B genes deleted and replaced by an expression cassette with a high activity promoter such as the cytomegalovirus immediate early (CMV) promoter which drives expression of the foreign transgene (Fig. 1b). The immediate early E1A-coded proteins are essential for Ad replication; they induce expression of the ~20 delayed early genes in the E1B, E2, E3, and E4 transcription units and they alter expression of a multitude of cellular genes in order to facilitate Ad replication [1]. The E1B proteins in general inhibit the host cell apoptotic response to Ad infection. Most Ad vectors lack the E3 genes which in general prevent infected cells from elimination by the immune system and are not essential for Ad replication in cell culture or in vivo [2]. These E1A and E1B deleted vectors are usually constructed from plasmids or Ad DNA containing the genetically modified Ad genome, and the vectors are grown up on complementing cell lines such as HEK293, PER.C6, or N52.E6 which retain and express the E1A and E1B genes [7].
Vectors deleted for E1A, E1B, and E3 can exhibit leaky expression of other early as well as late Ad genes, and therefore transduced cells in animal models can be eliminated by a T cell response. Accordingly, many RD vectors also lack the E4 region, which encodes genes for double strand DNA repair as well as other essential functions [1] (Fig. 1b). These E1A, E1B, E3, and E4 deleted vectors are much less leaky. Complementing cell lines for these vectors express E1A, E1B, and E4 proteins.
Another type of RD Ad vector is the “helper-dependent” (HDAd) vector which has most of the genome deleted but retains the origins of DNA replication at each end of the genome as well as ~500 base pairs at the left end of the genome that are required to package the genome into virions (Fig. 1c) [7]. These vectors can have high cloning capacity (~37 ki-lobase pairs) and as such can retain multiple transgenes. HDAd vectors are constructed and propagated in the presence of a RC helper Ad which provides the required early and late proteins necessary for replication. Typically, the helper Ad has loxP sites flanking the packaging signal in the genome. The cell lines used to produce HDAd vectors conditionally express Cre recombinase which excises the loxP-flanked packaging signal from the helper Ad genome thereby allowing for preferential packaging of the HDAd genome (which lacks the loxP sites) [8]. The HDAd is separated from the helper by ultracentrifugation on cesium chloride density gradients. Multiple rounds of purification are required. For large scale purposes, the HDAd and the helper Ad are separated by anion exchange and size exclusion chromatography.
Figs. 1d, 1e, and 1f illustrate a variety of RC (oncolytic) Ad vectors that are used mostly for cancer gene therapy. These vectors will be discussed in later sections.
4. ADENOVIRUSES ARE STRONGLY IMMUNOGENIC
Ads are strongly immunogenic and this has consequences not only for Ad infection outcomes and prevention, but also for the use of Ads as vectors for gene therapy, for vaccines, and for cancer gene therapy. The most important antigens are the three major capsid proteins, the hexon, penton base, and fiber. Most neutralizing antibodies are directed against the most abundant capsid protein, hexon, and especially against so-called hypervariable loops (i.e. regions that differ among serotypes) that are exposed on the surface of hexon [9, 10]. Ad-specific CD4+ T cells have been found among peripheral blood lymphocytes of almost all individuals of all ages. Most of the CD4+ T cell epitopes are in hexon [11]. Although less common, hexon-specific CD8+ T cell epitopes are present in healthy donors [11]. There is evidence that cytotoxic T lymphocytes (CTL) specific to hexon are protective against Ad infections in humans [12].
Ads and Ad vectors induce a strong innate immune response that has been studied extensively in mouse models [13]. Understanding and appreciating these strong innate and adaptive immune responses are likely relevant to assessing the use and safety of Ad vectors. The innate response in the mouse, directed against capsid proteins, begins immediately after intravenous injection and is maximal by 6 hours. The response is lethal in mice at a dose of 1010 virus particles (vp) per mouse; it is also lethal in large animal models at high doses, such as >1013 vp per kg. The vectors induce biphasic synthesis of pro-inflammatory proteins including IL-6, TNFα, IFNγ, IL-1β, and IL-12 as well as a number of chemokines. The IL-6 comes from the spleen, and the other proteins are synthesized mainly by Kupffer cells and dendritic cells.
In mice, intravenously injected Ad5-based vectors interact mainly with Kupffer cells in the liver although they can interact with macrophages in the spleen as well as neutrophils, platelets, and red blood cells. The majority (>90%) of an Ad5-based vector injected into mice intravenously infects the liver, not directly via the primary receptor, Coxsackie Adenovirus Receptor (CAR), but rather by first binding to blood coagulation factor X, which mediates hepatocyte infection via heparan sulfate proteoglycans on hepatocytes. Kupffer cells are not involved in Factor X-mediated hepatocyte transduction. Scavenger receptor-A expressed on Kupffer cells plays a role in uptake of Ad by Kupffer cells [14]. Factor X binding to Ad5 also triggers an innate immune response: the Factor X-Ad5 complex is “sensed” by TLR4 on tissue macrophages in mice, leading to activation of a network of NFκB-dependent early response genes [15].
Ad5-based vectors also bind to proteins C4 and C4BP in the classical and alternative complement pathways resulting in complement activation, platelet aggregation, and induction of proinflammatory cytokines [16]. Anti-Ad antibodies can opsonize the virus particles, leading to Fc receptor-mediated uptake by macrophages and secretion of cytokines [17].
In lymphoid cells transduced in vitro by Ad5-based vectors, the capsid is reported to activate toll-like receptor 2 at the plasma membrane, and the DNA genome is sensed by TLR9 in endosomes [18]. Signaling through these TLR pathways leads to activation of NFκB and interferon regulatory factors and induction of type 1 interferon and cytokine and chemokine genes. Ad DNA is also sensed by TLR-independent mechanisms including the inflammasome which leads to release of IL-1β [19, 20].
5. CLINICAL EVALUATION OF REPLICATION-DEFECTIVE (RD) ADENOVIRUS VECTORS FOR SOMATIC CELL GENE THERAPY
As mentioned, Ads are strongly immunogenic, and considerable engineering is required to prevent leakiness of the gene that results in elimination of transduced cells by CTL in vivo. Also, there is much pre-existing immunity to Ad, especially to the most popular serotype (Ad5) used for gene therapy vectors. Pre-existing immunity can prevent the “take” of the Ad vector. While it became clear that Ads may not be optimal for long term complementation of faulty genes in monogenic diseases, clinical trials performed with RD Ad vectors were instrumental in advancing the field, inasmuch as a tremendous amount of data was collected in early clinical trials with RD Ads regarding to the safety of these vectors.
The results of several such studies, involving 90 patients receiving local administrations of RD Ads expressing cystic fibrosis transductance regulator (CFTR) (through intranasal drops or intrabronchial catheter), vascular endothelial growth factor (VEGF) (via intramyocardial or skeletal muscle injection), or E. coli cytosine deaminase (via direct intratumoral injection), were summarized in a two-paper series by Ronald Crystal and his coworkers [21, 22]. Altogether, 319 adverse events were observed with the study population; however, only one of these was deemed to be Ad-related, the rest were caused by various co-morbidities of the patients. To detect possible shedding, pharyngeal, rectal, and nasal swabs as well as urine and blood samples were analyzed for the presence of the vector at 1 and 7 days after vector injection. Shedding of the original RD vector was observed in one out of 1685 samples examined, and no RC virus (that might have been formed by a recombination event) was found in any of these samples. In another study in which an RD Ad vector was used to deliver CFTR either through bronchoscopy or by aerosol treatment, patients experienced relatively minor clinical signs including fever, myalgia, and arthralgia [23].
When RD Ad vectors were administered systemically, they caused more serious adverse events, including hepatic lesions as indicated by elevated transaminases, and thrombocytopenia and neutropenia [24]. The most severe adverse events were described for a patient with ornithine transcar-bamylase (OTC) deficiency, who received a very high dose, 6×1011 virus particles (vp) per kg, of an RD Ad vector expressing OTC into the right hepatic artery. The strong innate immune response and the resulting cytokine storm (see Section 4) resulted in multiple organ failure and eventually caused the death of the patient [25]. The nature and severity of these sequelae were unexpected, inasmuch as the same dose did not cause adverse events in nonhuman primates, and another patient dosed with the same dose of the vector developed only mild adverse events that were similar to those seen in a cohort of patients that received a lower dose of the vector. The reason why the innate immune response was disproportionately stronger in one of the patients than in others is unclear. The possible explanations include genetic predisposition and a strong memory response to the virus [26]. This death is the only one reported for Ad vectors of any kind. In one review it is stated that 16,000 patients have been administered various types of Ad vectors, mostly RC Ad vectors for cancer therapy [27].
6. CLINICAL USE OF REPLICATION-DEFECTIVE (RD) ADENOVIRUS VECTORS AS VACCINES
RD Ad vectors have been employed extensively as vaccines because they induce a strong humoral and especially a strong T cell response, tending to a T helper 1 type response, to the transgene expressed by the vector (see Fig. 1b) (reviewed by [28]). The most notable study was the so-called STEP study that was done using a mixture of three Ad5-based RD vectors expressing several human immunodeficiency virus proteins as a vaccine against HIV (reviewed in [29, 30]). This vaccine, manufactured by Merck, was employed in about 3000 volunteers in multiple countries, beginning in 2004. The vaccine was well tolerated, despite repeated intramuscular injections of as much as 1011 vp per injection. This STEP trial was stopped in 2007 because of the unexpected finding that volunteers who had been vaccinated had higher rates of HIV infection than those receiving placebo. The increased rate of HIV infection occurred in men-who-have-sex-with-men who were uncircumcised and/ or had pre-existing antibodies to Ad5. This phenomenon is still unexplained (see [2]).
More recently, an NIH-sponsored HIV vaccine clinical trial, named HVTN, was started in 2009. This phase II trial consisted of a prime with a DNA-based vaccine expressing HIV proteins followed by a boost with an RD Ad5 vector expressing the same HIV proteins. This trial was halted in 2013 because the vaccine regimen did not reduce the rate of HIV infection or mitigate the infection of those volunteers who had acquired HIV while on the trial [31].
In contrast to the RD Ad5-vectored HIV vaccines, an RD Ad5-based vaccine against tuberculosis seems very promising [32]. The vector, named AdHu5Ag85A, which expresses an immune-dominant antigen of Mycobacterium tuberculosis, was evaluated in a phase I study in 26 volunteers previously immunized with Bacille Calmette-Guerin (BCG) and patients naïve to BCG. Volunteers were given a single intramuscular injection of 3.2 × 109 vp (108 pfu). The authors concluded that the vaccine was safe and well tolerated in both volunteer groups, that it was immunogenic in both groups, and that it boosted CD4+ and CD8+ T cell responses in the previously BCG vaccinated group. Interestingly, preexisting immunity to Ad5 did not affect the safety or efficacy of the vaccine.
7. CLINICAL EXPERIENCE WITH ADVEXIN AND GENDICINE, TWO RD AD5-BASED VECTORS EXPRESSING THE TUMOR SUPPRESSOR P53, AND WITH SITIMAGENE CERADENOVEC, AN RD ADS-BASED VECTOR THAT EXPRESSES HSV THYMIDINE KINASE
A great deal of clinical data have been obtained with Ad-vexin and Gendicine. Advexin is an E1-minus E3-minus RD Ad5 vector expressing p53 from the CMV promoter in the deleted E1 region (Fig. 1b). Gendicine is very similar except that p53 is expressed from the Rous Sarcoma Virus promoter. About 50% of cancers lack functional p53, and nearly all cancers have a defect in the p53 tumor suppressor pathway. The rationale behind Advexin and Gendicine is that forced expression of p53 by the vectors in cancer cells will cause cell cycle arrest or apoptosis.
At least a dozen clinical trials have been conducted with Advexin at multiple testing sites for cancers including head and neck squamous cell carcinoma (HNSCC), colorectal cancer, hepatocellular carcinoma (HCC), non-small cell lung cancer, prostate cancer, breast cancer, ovary cancer, bladder cancer, and glioma (reviewed in [2, 33–35]). In phase I and II trials on HNSCC, the vector was injected as a monotherapy directly into tumors at doses up to 2.5 × 1011 vp/dose. The treatment was judged to be safe. Advexin was then evaluated in a phase III trial for advanced recurrent HNSCC that compared Advexin as monotherapy vs. Advexin in combination with methotrexate. Again, the vector was well tolerated, and evidence of anti-tumor activity was obtained [36].
In another trial with an Ad p53 vector (SCH 58500) similar to Advexin, patients receiving 7.5 × 1013 vp of vector intraarterially developed a strong innate and adaptive immune response to the vector, but no serious adverse events were reported [37].
Gendicine was approved in 2003 by the State Food and Drug Administration (SFDA) in China for intratumoral treatment of HNSCC in combination with chemotherapy (reviewed in [38–41]). Gendicine is the first gene therapy vector of any type approved for commercial use. One review published in 2009 states that more than 7,000 patients have been treated with Gendicine, with up to 1012 vp, injected intratumorally, with repeated injections, and often in combination with chemotherapy and radiation therapy [39]. Even repeated intraarterial injections of Gendicine produced only mild, transient symptoms including nausea, fever, fatigue, vomiting, leucopenia, and anemia [38]. Importantly, only 1 of 30 gargle samples, and only 1 of 23 urine samples were positive for vector DNA, indicating that vector shedding is extremely rare, even after intravascular administration [38]. The published reviews state that Gendicine is safe with side effects similar to those of Advexin.
Sitimagene ceradenovec is an E1-minus E3-minus Ad5-based vector that expresses herpes simplex virus (HSV) thymidine kinase (TK) from a CMV promoter-driven expression cassette inserted into the deleted E1 region. This vector is of interest because it has been evaluated in combination with intravenous ganciclovir for anti-cancer activity in a randomized, open-label, phase 3 trial (ASPECT) for patients with newly diagnosed operable high-grade glioma [42]. In the trial, the tumor was resected, then 1012 vp of the vector was injected in a volume of 10 ml into the wall of the resection cavity, 2 cm deep. Starting 5 days later, ganciclovir (5 mg/kg) was administered intravenously, twice per day, from day 5 to day 19. The rationale behind this therapy is that residual glioma cells remaining from the resection will be transduced by the vector, and the TK enzyme will convert ganciclovir to ganciclovir monophosphate, which after conversion to ganciclovir triphosphate, will be incorporated into tumor DNA and cause DNA chain termination and cellular apoptosis. Further, the ganciclovir phosphates are thought to enter (via gap junctions) neighboring tumor cells, again causing chain termination and apoptosis. Non-tumor neurons are expected to be spared because they are not synthesizing DNA.
In the trial, there were 124 patients in the vector treatment group and 126 patients in the standard care group [42]. Some patients in both groups were also treated with temo-zolomide, an alkylating agent. The authors concluded that the vector plus ganciclovir treatment increased the “time to death or re-intervention” (the primary end points of the trial), but not the overall survival. About one-third of patients had pre-existing immunity to Ad5; these patients as well as the naïve patients experienced a strong increase in neutralizing antibody to the vector. In a few patients, low amounts of vector were detected in the blood by qPCR for about a week following vector injection. Nearly all patients in the vector group and the standard care group experienced adverse events, but these were more common in the vector group (65%) vs. the standard care group (52%). The treatment-related adverse events were fever, increased frequency of seizures, hemiparesis, and hyponatraemia, which were transient in most cases. The authors concluded that “the risk-benefit ratio for this treatment seems positive” [42].
Taken together, the safety and environmental risks associated with RD Ad vectors are low. The vectors rarely cause severe adverse events, even when administered intravascularly or into the brain at high doses. Only minimal vector shedding was documented, and only in a very small portion of study subjects. Vector recombination events with wild-type Ad have not been reported; and even if they did happen, they would not generate an RC vector expressing the transgene (consider the recombination events that could occur between the vector genomes shown in Figs. 1a and 1b).
A possible exception to this would be RD vectors with the transgene in the E3 region, as follows. Consider the vector depicted in (Fig. 1d, 1e, or 1f). These vectors have deletions or promoter substitutions in the E1A and/or E1B regions that are designed to reduce replication in normal cells but to allow replication in cancer cells; these vectors also have a transgene in the E3 region. If one of these vectors were to infect a cell together with wild-type Ad5 (Fig. 1a), then recombination could occur in the DNA section between the E1 and E3 regions to yield a virus that is wild-type in the E1 region and with the transgene in the E3 region. This re-combinanant should be able to replicate and express the transgene in normal cells. The other product of this recombination event would be a vector that retains the genetic changes in the E1 region but is wild-type in E3; this recombinant should not pose a danger because it has the replication restriction features in the E1 region.
It is important to consider what risk, if any, is inherent in the transgenes expressed by Ad RD and RC vectors. In preclinical studies, hundreds of transgenes of very different types have been expressed by Ad RD vectors (almost always from the E1 region – see Fig. 1b), and a fairly large number also by Ad RC vectors. Often, these vectors have been characterized in animal models, nearly exclusively under Biosafety Level 2 conditions. We are not aware of any reports of significant adverse events associated with these various vectors.
Quite a large number of Ad RD vectors expressing various types of transgenes have also been examined in clinical trials (see ClinicalTrials.gov). Again, the transgene is almost always expressed from an expression cassette inserted into the deleted E1 region (Fig. 1b). In this article so far, we have discussed Ad RD vectors evaluated in clinical trials that express OTC, CFTR, VEGF, E.coli cytosine deaminase, HIV proteins (gag, pol, nef), an M.tuberculosis protein, p53, and TK. Clinical trials have also been conducted with Ad RD vectors that express other transgenes, including E.coli nitroreductase, granulocyte macrophage colony stimulating factor (GM-CSF), human interleukin (IL) 24 (also named MDA-7), human IL-2, human IL-12, human interferon (IFN)α, human IFNβ, human IFNγ, human fibroblast growth factor 4, heat shock protein 70, tumor necrosis factor α, prostate specific antigen (PSA), malaria circumsporzite protein and apical membrane antigen 1, Her-2, CD40 ligand, MART-1, and hepatitis C virus nonstructural proteins, among other transgenes. We are not aware of any reports describing serious and sustained adverse events associated with administration of these vectors.
It needs to be emphasized that, from an environmental risk aspect, the safety results from clinical trials with RD Ad vectors are best viewed as worst case scenario data. Natural infection with accidentally released vector would happen through the oral-enteric or nasal/oral-respiratory routes, and most likely with an incomparably lower dose than the one used in clinical trials. Thus, the safety data collected with high doses of RD Ad administered systemically probably reflect a much worse situation than what can be realistically expected from accidental infection with these vectors.
8. REPLICATION-COMPETENT (RC) ADENOVIRUS VECTORS WITH AN E1B-55K DELETION for USE IN CANCER GENE THERAPY
RC (oncolytic) Ad vectors are being studied intensely as therapeutics for cancer (reviewed in [2, 43, 44, 41, 45–48, 27, 49]). Oncolytic Ad vectors lyse cancer cells as the end result of their life cycle, with one infecting particle expected to yield 103 or even 104 progeny virus particles that can then infect and destroy neighboring tumor cells. Cancer cells are generally more permissive for Ad replication than quiescent or non-cancerous cells [50]. Among the reasons that Ads replicate better in cancer cells than in normal cells is that the entire pattern of gene expression in cancer cells is conducive for Ad replication, whereas in non-cancerous cells multiple Ad proteins must be expressed to force cells into a permissive state [51]. Nevertheless, essentially all oncolytic Ad vectors have genetic cell targeting features that permit efficient replication in cancer cells and that limit replication in non-cancerous cells. The following discussion will be restricted to those oncolytic Ad vectors that have been evaluated in clinical trials or, in the case of the vector named On-corine (H101), that is approved for cancer therapy in China.
The first oncolytic Ad vector to be examined in clinical trials is named ONYX-015. This vector lacks the gene coding for a protein named E1B-55K, a protein required for Ad replication in normal cells [52]. One of the functions of E1B-55K in normal cells is to facilitate export of Ad late mRNA from the nucleus; many cancer cell lines provide this function, thereby allowing the vector to replicate in cancer cells but not (well) in normal cells [53].
ONYX-015 has been examined in at least 300 patients [54] in more than 15 phase I and Phase II trials for various types of cancer [33, 55]. Many of the early trials were for HNSCC [56–60]. In these dose escalation trials, as much as 2 × 1013 vp were injected intratumorally, either as a monotherapy or together with chemotherapy. The vp to plaque forming unit (pfu) ratio is usually about 10:1 to 50:1, so perhaps 1010 to 1012 pfu were applied to these tumors. Transient low level replication of the vector occurred in tumors as evidenced by a peak of vector DNA in the blood as detected by qPCR. Tumor responses were modest, and were seen only with combination therapy. The toxicity was limited to transient fever and injection site pain that resolved in a day or two. No incidence of shedding or infection of untreated individuals was observed, as would be expected from such a crippled virus. Other cancers examined using ONYX-015 are prostate cancer [61, 49], HCC [62], pancreatic cancer [63, 64], colorectal cancer [65, 55, 66], and ovarian cancer [67, 60, 68]. These trials were all dose escalation trials, reaching at least 1011 vp/injection usually in combination therapy, with a course of multiple injections. Toxicities were flu-like symptoms, injection site pain, but with no serious adverse events. As with the HNSCC trials, not much vector replication was detected in tumors (qPCR of vector in blood), and there was no report of non-patient infection.
The safety and efficacy of ONYX-015 in HCC were tested in dose escalation trials designed for safety in patients who were not eligible for resection and had failed prior chemotherapy. ONYX-015 doses as high as ~3 × 1011 vp (~3 × 1010 pfu) [69, 62, 70] were administered for as many as three consecutive days. Vector injections were intratumoral, into the hepatic artery, or into the arm vein (vena mediana cubiti). The toxicities were mild and some patients had stable disease for a few months.
A series of studies was conducted by one research group that are especially instructive with regard to the anti-tumor activity and the toxicity and safety of ONYX-015 [71, 72, 65, 73, 66]. In a phase I/II study, 35 patients with colorectal cancer metastases to the liver were administered ONYX-015 by hepatic artery infusion in multiple cycles and in combination with 5-fluoruracil/leucovorin. Vector doses as high as ~2 × 1012 vp were administered directly to the hepatic artery on days 1 and 8 and then once per month for up to 6 months in some patients, for a total of 200 injections. Flu-like symptoms were observed transiently in most patients, no dose limiting toxicities were seen, no maximum tolerated dose was achieved, and there was no evidence of toxicity. The lack of hepatotoxicity is notable because 104–105 genome copies per ml of blood were detected at 3–10 days post infection of vector as determined by qPCR. It is noteworthy that millions of immunocompetent people experience Species C Ad with little evidence of hepatotoxicity [2].
In summary with ONYX-015, the vector has been tested in many types of cancers, and it has been administered in different routines including intratumoral, intravascular, intraperitoneal, intraesophageal, and as a mouthwash. In all studies, the authors concluded that the vector was safe and well tolerated.
A number of other E1B-55K-deleted vectors similar to ONYX-015 have been examined in clinical trials. By far the most data have been obtained with the vector Oncorine (also named H101), which was evaluated by intratumoral injection in phase I and II clinical trials in China for patients with >10 kinds of cancer including HNSCC, breast, and colon cancer and in a phase III trial for HNSCC [57, 74, 39, 27, 41, 75–77]. In those trials, the ideal dose was judged to be 5 × 1011 vp per day for 5 consecutive days, with more than one course of treatment. No remarkable adverse events were reported, and significant antitumor responses were observed. In 2005, this vector was approved by the Chinese SFDA for use in China for intratumoral treatment of nasopharyngeal carcinoma in combination with chemotherapy [78, 79]. Oncorine has been administered via the intraperitoneal route to patients with malignant ascites [80] and by transarterial injection into a patient with HCC [81]. The vector was well tolerated and no adverse events were observed. In 2009, a publication stated that over 600 patients had received Oncorine intratumorally in clinical trials [82].
Thorough studies have been done by Svend Freytag and colleagues on a series of E1B-55K-deleted vectors that carry therapeutic transgenes inserted into the deleted E1B-55K region (reviewed in [49]). One such vector, Ad5-CD/TKrep, expresses a cytosine deaminase (CD)-thymidine kinase (TK-from herpes simplex virus) fusion protein. This vector has been injected into prostate tumors at doses of 1010 to 1012 vp per injection in combination with 5-fluorocytosine plus valganciclovir [83] or 5-fluorocytosine plus ganciclovir [84] followed by radiation therapy. These drugs are converted by the CD-TK fusion enzyme into compounds that inhibit DNA replication in the tumor (and also of the vector) and sensitize the tumor cells to radiation therapy. This vector was further modified to express the Ad5-coded Adenovirus Death Protein (ADP) from the E3 region (ADP mediates efficient cell lysis) [85]. By 2007, 79 prostate cancer patients had been injected intratumorally with these vectors with no serious adverse events, no dose limiting toxicities, no maximum tolerated dose reached at two injections of 1012 vp, and improved survival of the patients compared to historical controls [49]. Another phase I trial for prostate cancer was conducted using a vector named Ad5-yCD/utTKSRrep-hNIS, which has the CD and TK suicide genes but also expresses the sodium iodide symporter (NIS). The goal was to examine the safety of introprostatic administration (up to 1012 vp) followed by noninvasive imaging of the prostate based on uptake of Na99mTc04 and use of photon emission-computed tomography (SPECT). The authors concluded that the treatment was safe, with mild (grade 1) to moderate (grade 2) toxicities, that the prostate could be imaged successfully, and that there was no evidence of extra-prostatic dissemination of the vector.
9. PROSTATE CANCER-SPECIFIC REPLICATION-COMPETENT (RC) ADENOVIRUS VECTORS WITH PROSTATE-SPECIFIC PROMOTERS REPLACING THE ADENOVIRUS E1A ENHANCER/PROMOTER
An oncolytic Ad vector named CV706 was evaluated by intraprostate injection in a phase I clinical trial for 20 patients with locally recurrent prostate cancer [86]. CV706 has the Ad E1A promoter/enhancer replaced with the prostate specific antigen (PSA) promoter-enhancer, which results in preferential replication in PSA-positive cancer cells. Again, there was low toxicity, no dose limiting toxicities, no maximum tolerated dose reached at vector doses up to 1013 vp, and encouraging PSA lowering responses (>50% in 5 patients). A vector named CG7870 has also been evaluated in a phase I trial [87]. Replication of CG7870 is restricted to prostate cancer because of substitution of the Ad E1A and E1B promoters with the prostate-specific rat probasin and PSA promoters, respectively. CG7870 was administered intravenously to 23 patients with hormone-refractory metastatic prostate cancer. The treatment was well tolerated up to a dose of 3 × 1012 vp. Five of 23 patients had a decline (25–49%) in PSA levels, most at the higher (>6 × 1011 vp) doses of vector.
10. REPLICATION-COMPETENT ADENOVIRUS VECTORS WITH AN E1A “CR2 DELETION” AND THAT ARE “INFECTIVITY-ENHANCED
Several laboratories have developed oncolytic Ad vectors that have a small deletion in a domain in the Ad5 E1A protein named “conserved region 2” (CR2) [88, 89]. This domain, which is conserved among most human Ad serotypes, functions in natural infections to bind to members of the retinoblastoma (pRB) family of tumor suppressors, causing the release of the E2F transcription factors from pRB/E2F transcription repression complexes and allowing free E2F to stimulate transcription of genes in the S-phase of the cell cycle [1]. Most cancers have a defect in the pRB pathway of tumor suppression. Ad vectors with a deletion in the E1A CR2 region are able to replicate in cancer cells (i.e. there is no need to disrupt pRB/E2F complexes); these vectors replicate less well in normal cells.
A CR2 deletion named A24 or D24 (deletion of 24 amino acids) has been employed in a number of so-called “infectivity-enhanced” oncolytic Ad vectors (reviewed in [27, 90, 91]). Infectivity-enhanced Ad5-based vectors have genetically-modified fiber genes such that the fiber knob (which interacts with the natural CAR receptor) is modified in some way. The reason for these modifications is that many tumors (especially of the ovary and bladder) express low levels of CAR and therefore are not infected well by vectors with the Ad5 fiber. Thus, in one modification, an arginine-glycine-aspartate (RGD) peptide has been incorporated (genetically) into the H1 loop of the Ad5 fiber knob, thereby enabling the vector to infect cells through the αvβ integrins that are present at high levels on the surface of many cancer cells [92]. Other infectivity-enhanced vectors have the Ad5 fiber knob completely replaced by the fiber knob of Ad3 [93]. The Ad3 knob binds to CD46 [94] or desmoglein 2 [95], which are present on the surface of many types of cancer cells [96]. A number of clinical studies and trials have been conducted with Δ24 and/or infectivity-enhanced vectors, namely ICOVIR-7 [97], Ad5Δ24-RGD [98], Ad5/3Cox2L-Δ24 [99] and other vectors discussed below. In these trials, the authors found evidence of vector replication (by qPCR of blood), observed no significant adverse events, and saw evidence of anticancer therapy. Pre-existing immunity to the vector did not correlate with adverse events or anti-tumor efficacy, and in all cases administration of the vector induced a robust neutralizing antibody response to the vector.
One research group has further engineered these Δ24 and infectivity-enhanced vectors to express granulocyte macrophage colony stimulating factor (GM-CSF) from the deleted E3 region of the vector genome. The rationale is that this cytokine will be secreted from infected tumor cells and stimulate differentiation and growth of myelo-monocytic lineage cells such as dendritic cells (DCs). The DCs will take up tumor antigens from the vector-infected tumor, migrate to lymph nodes, and activate anti-tumor responses. Clinical studies with oncolytic Ad vectors expressing GM-CSF have so far indicated that the vectors are well tolerated and may have anticancer activity [100–103].
In common with other oncolytic Ad vectors discussed previously, these vectors usually have been injected directly into tumors, but in some studies the vectors were injected intravenously, intracavitarily, or into the hepatic artery (reviewed by [27]) as discussed below.
In one clinical trial, a vector named Ad5-Δ24-RGD was injected intraperitoneally through a catheter at doses of 1 × 109 to 1 × 10 12 vp per day for three days into 21 patients with recurrent malignant gynecological disease [98]. (This vector has the 24 amino acid deletion [Δ24, or D24] in E1A CR2 and the RGD peptide insert into the Ad5 fiber knob). No serious adverse events were noted, there was evidence in some tumors of vector replication (as detected by immuno-histochemistry for hexon protein and by vector-specific PCR in the ascites) in 16 patients, and there were signs of clinical benefit. No vector dose limiting toxicity was obtained, and a maximum tolerated dose was not identified. Vector shedding was noted (by qPCR) in the serum, saliva, and urine in most of the high dose patients. In the serum, the genome copy levels were about 5 × 103 copies/ml, mostly at days 3 and 7.
This same research group has studied another vector, Ad5/3-Δ24, which has the D24 deletion in E1A and the Ad3 fiber knob. In a phase I trial for ovarian cancer, 9 patients were treated with intraperitoneal injection of vector for 3 consecutive days, at doses of 1010 to 1012 vp. Five patients experienced grade 1 or 2 adverse events including fever, chills, fatigue, nausea, and myalgia. There was suggestive evidence of antitumor activity.
A research group from Finland led by Akseli Hemminki has conducted a large number of clinical studies (to be de- scribed later) with a variety of D24-type infectivity-enhanced vectors, including some that express GM-CSF. These clinical studies are not controlled clinical trials. Rather, they are clinical studies conducted under the auspices of the Advanced Therapy Access Program (ATAP) in Finland. This program takes advantage of the European Union Advanced Therapy Directives (1394/2007/EC) which allows patients to be treated in an individually specific manner under the responsibility of the treating physician. The ATAP program has allowed patients with various types of advanced cancers to be treated by a variety of genetically engineered oncolytic Ad vectors and by a variety of treatment regimens [104]. While it is sometimes difficult to draw firm conclusions on anti-tumor efficacy from these studies, it seems clear that the vectors can be efficacious. Certain other lessons also have been learned. For example, the various vectors tested are remarkably safe, even when administered intravenously. Preexisting immunity to the vector is not a problem, and administration of the vectors induce strong immunity to the vector. It seems also that the vectors induce anti-tumor immunity.
The clinical studies conducted by the Finnish group are discussed below. In one study, the vector Ad5-D24-GMCSF was administered at a single dose of 1 × 1010 to 4 × 1011 vp into 20 patients with various refractory solid cancers [101]. The vector was administered by ultra-sound guided intratumoral or intracavitary (ovarian cancer, mesothelioma) injection, and one-fifth of the dose was given intravenously. Vector genomes were detected by qPCR in the serum in 15 of 18 patients, but with low genome copy number (<500 copies/ml) in most of these patients. One patient had 4.5 × 104 copies/ml at 1 day post-injection, but <500 copies at 21–40 days; this patient was unique in having no anti-Ad5 neutralizing antibodies at baseline and a minimal antibody response (1:64) at 4 weeks. The neutralizing antibodies were positive in 8 of 19 cases at baseline, and titers increased in nearly all patients. As is common in clinical studies with oncolytic Ad vectors, there was no correlation between neutralizing antibody titers, anti-tumor activity, or toxicity. The authors concluded that the treatment was well tolerated and it resulted in anti-tumor activity [101].
Another study of 43 similar patients with different types of advanced metastatic solid tumors was conducted with similar doses of several oncolytic vectors employed by the Finnish group administered intratumorally [100] in combination with low-dose cyclophosphamide to reduce regulatory T-cells. The cyclophosphamide treatment did not affect the typical development of neutralizing antibodies to the vector or the detection of the vector (measured by qPCR) in the blood. In most patients, the vector levels were in the range of 103 to 104 genome copies/ml, with peak levels at 2–7 days postinjection but persisting >21 days in a few patients. The vector and cyclophosphamide treatment were well tolerated, and evidence of anti-tumor immunity was seen as well as clinical benefit [100].
A similar study in 21 patients with different types of solid tumors was conducted in which the vector Ad5/3-D24-GMCSF was combined with low dose cyclophosphamide [103]. The vector doses ranged from 8 × 1010 vp to 4 × 1011 vp. The patients received a single round of intratumoral injection, and at least one-fifth of the dose was given intravenously. Intracavitary injection was performed with patients with intrapleural or intraperitoneal disease. The authors concluded that the vector was well tolerated and demonstrated evidence of anti-tumor immunity and clinical activity. At baseline, neutralizing antibodies to the Ad5/3-D24-GMCSF vector were absent in 6 of 21 patients and were low (1:64) in the remainder of patients. Neutralizing antibodies increased in all patients following treatment, reaching titers of 1:16,384 in 9 patients. At day 1, 17 of 19 patients had detectable vector in the serum (as measured by qPCR) with the highest amount being 2 × 103 genome copies/ml. Levels at 3–7 days ranged between zero or <500 to ~2 × 104 copies/ml; one patient had 3.3 × 105 copies/ml. In all patients, levels dropped to zero or <500 copies at days 8–14 and later time points.
In another study with the vectors named Ad5-D24-RGD and Ad5-RGD-D24-GMCSF, 16 patients with various types of solid advanced tumors were treated [105]. Patients received a single round of virus at doses from 2 × 1010 to 9 × 1011 vp. Four-fifth of the dose was given intratumorally (or intraperitoneally for patients with peritoneal disease) and one fifth was administered intravenously. The authors stated that the treatment was well tolerated, with typical side effects of grade 1–2 fatigue, fever, and injection site pain. Vector genome copies in the circulation ranged mostly from <500 to 3 × 105 copies/ml of serum at 2–10 days postinjection, then dropped to <500 copies/ml. One patient had 1.1 × 106 genome copies/ml at 6–10 days, but this patient expired after 58 days and no further vector circulation data were reported.
A study was conducted in 18 patients with various types of metastatic and refractory tumors using the vector Ad5/3-Cox-2L–D24 [99]. This vector has the cyclooxygenase 2 promoter driving E1A, the D24 deletion in E1A CR2, and the Ad3 fiber knob replacing the Ad5 knob. The patients were given a single round of vector at doses from 2 × 109 to 3 × 1011 vp, intratumorally or, with intraperitoneal disease, intraperitoneally. In some patients, some of the vector was administered intravenously. Flu-like symptoms, fever, and fatigue (grades 1–2) were observed in most patients, and thrombocytopenia and hepatic lesions as indicated by elevated transaminases were seen in some. One ovarian cancer patient had grade 3 ileus and grade 5 non-hematological side effects. The intraperitoneal route of vector administration correlated with vomiting and stomach pain, and the intravenous route with minor hypertension in 3 patients. Vector was detected (by qPCR) in the serum at 2–4 days postinjection and persisted at low levels in a few patients for up to 5 weeks. The authors concluded that the vector was safe and demonstrated some anti-tumor activity.
A study was conducted to evaluate the antivector and antitumor T-cell immunity as well as the safety in patients treated with oncolytic Ad vectors expressing GM-CSF [106]. Sixty patients with different types of solid tumors not treated previously with an oncolytic Ad vector were studied. The vectors used include three that express GM-CSF (Ad5/3-D24-GMCSF, Ad5-RGD-D24-GMCSF, Ad5-D24-GMCSF) and three that do not express GM-CSF (ICOVIR-7, Ad5/3-Cox-2L-D24, Ad3-hTERT-E1). Thirty-nine patients with various solid tumors were given either single or serial ad-ministration of these various vectors, and 115 other patients received either single or serial injections of Ad5/3-D24-GMCSF (this vector is also named CGTG-102). The authors concluded that safety was good, although some moderate adverse events were noted. Evidence for induction of antitumor immunity and antitumor efficacy were obtained.
A clinical study was conducted to test whether the combination of oncolytic Ad vector plus low dose temozolomide would be safe and effective in treating cancer [107]. Temozolomide is an alkylating agent used to treat certain tumors. Metronomic low-dose cyclophosphamide was employed as well to reduce regulatory T cells. Seventeen patients with different chemotherapy refractory tumors were given different combinations of vectors (described in previous paragraphs) as well as some other similar vectors plus 100 mg/day of temozolomide at different intervals before vector administration. Vector (1011 vp) was given intratumorally, intrapleurally (for intrapleural disease) and intravenously in some patients. Some patients were given more than one round of vector. All patients had an adverse reaction of some type, mostly grade 1, to lesser extent grade 2, a few had grade 3 (lymphopenia and leukopenia), and one patient had a grade 4 decrease in leukocyte counts. Most adverse events were self-limiting, but one patient had grade 3 ileus. A pediatric patient experienced grade 3 liver transaminase elevation and grade 2 hemoglobin decrease, and another patient had grade 3 abdominal pain and thrombocytopenia. Vector DNA was detected by qPCR at days 1–8 in 28 of 38 evaluable treatment cycles, with prolonged replication in a few patients. One patient had 611,235 vp/ml at days 3–8. The authors concluded that the combination of oncolytic Ad plus low dose temozolomide plus low dose metronomic cyclophosphamide is well tolerated, increased tumor cell autophagy, and elicited anti-tumor responses.
The studies by the Finnish group discussed above underscore the safety of the oncolytic viruses used. In most of their studies, vector is detected in the blood that peaks at quite modest levels within the first week or so, although there is prolonged replication for several weeks in a few patients. This systemic distribution of the vector does not seem to do much harm to the patients. A recent publication by the Finnish group addressed this issue more vigorously by using verapamil to increase the blood levels of vector in cancer patients [108]. Verapamil is a calcium channel blocker that has been reported to enhance oncolytic Ad vector release from tumor cells and to increase anti-tumor efficacy in animal models. In the clinical study [108], the vectors Ad5-RGD-D24-GMCSF (with 13 rounds of treatment), Ad5/3-D24-GMCSF (12 rounds of treatment), Ad5-D24-GMCSF (10 rounds of treatment), and ICOVIR-7 (1 treatment) were employed. The vector doses ranged from 2 × 1011 vp to 9 × 10 vp. Injections were intratumoral and at least one-fifth of the dose was given intravenously. There were two groups of 36 patients, all given multiple rounds of vector injection, with one group of patients receiving verapamil and the other group serving as a control. The authors found that the maximum titers at different time points were higher with the verapamil group, ~2.5 × 105 vp/ml at days 2–10 post vector injection (~46% of patients) and ~1 × 105 vp/ml at days 14–60 (~25% of patients). The levels for the control group were 10-fold less at days 2–10 (~35% of patients) and nearly 100- fold less at days 14–60 (~15% of patients). Despite these differences in blood levels of vector, the frequency or severity of adverse events, cytokine responses, and neutralizing antibody responses were not increased by verapamil. This study suggests that patients can tolerate quite high levels of vector in the blood (mean of 2.5 × 105 vp/ml for the verapamil group) for days or even weeks following vector injection.
An important point to emphasize regarding the safety of oncolytic Ad vectors is that in quite a large number of studies these D24-type vectors were administered systemically [101, 100, 103, 105, 108]. The research group in Finland has treated hundreds of cancer patients (various types of cancer) with several types of oncolytic Ad vectors. In a typical study by this group, from 1 × 1010 to 4 × 1011 virus particles were administered, with one-fifth of the dose given intravenously (i.e. 5 × 109 to 8 × 1010 vp). In some patients with intrapleural or intraperitoneal disease, the complete dose was given by intracavitary injection. In these various studies, the results were similar to those obtained using intratumoral injection: the safety profile was similar, vector levels and pharmacokinetics in the blood (assay by qPCR) were similar. The neutralizing antibody response developed within ~1 week (and pre-existing immunity to Ad5 was boosted). The E1A-D24 deletion is less attenuating than the E1B-55K deletion in the ONYX-015-type vectors, so the clinical data indicating that the D24-type vectors are quite safe are very encouraging.
The studies from the Finnish group described above have been done with Ad5-based vectors, even though some vectors have the Ad3 knob of fiber. This research group has also conducted a study with a vector named Ad3-hTERT-E1A [109]. This vector is fully Ad3, except the E1A promoter is replaced by the hTERT promoter. This vector is expected to infect cells via the Ad3 receptor, desmoglein 2 [95], or perhaps CD46 [94], both of which can be elevated on cancer cells. The hTERT promoter allows vector replication in most cancer cells because they express telomerase; most normal cells do not express telomerase and therefore vector replication should be limited in the cells. Twenty-five patients with different types of advanced solid progressing tumors were treated. Vector doses ranged from 1 × 1010 vp to 4 × 1012 vp. With most patients, the vector was injected into the tumor but some of the vector, ranging from 20% to 75%, was also given intravenously. With 5 patients, 100% of the vector was injected intravenously, at a dose of 7 × 10 vp (one patient) and 3 × 1012 vp (4 patients). The safety response of the patients to this vector was similar to other studies with Ad5-based vectors, and the only grade 3 adverse events were self-limiting cytopenias. Recalling that Ad5 vectors administered intravenously in high doses to animal models can invoke an early and strong innate response, it is noteworthy that the patients in the Ad3-hTERT-E1A study were able to tolerate the high dose of vector administered. The authors concluded that Ad3-hTERT-E1A seems safe for further testing and/or for arming with a therapeutic transgene.
11. CONCLUSION
In summary, many clinical trials and clinical studies with oncolytic Ad vectors have shown that these vectors have anti-tumor activity and are very well tolerated. Considering the large number of patients that have been treated, there have been only a few somewhat serious adverse events (e.g. grade 3 ileus), but even those resolved in a quite short period of time. We are not aware of any cases of extended systemic dissemination with Ad vectors that were known to be replication competent. Of further note, in nearly all the clinical studies discussed in this article, the presence of vector, usually in the serum, was detected and quantitated by qPCR. This method can detect a fragment of DNA, not necessarily complete genomes or, more importantly, infectious virus. Therefore, it is possible that the amount of infectious vector present in the blood of patients in these trials is less than is suggested by the qPCR data. If so, then these vectors may be even safer than we may think.
Considering the comment above about qPCR potentially quantitating DNA fragments that are not complete genomes, researchers in clinical trials could perform infectious virus assays in cell culture with vector isolated from blood, saliva, urine, or feces. However, such assays are rarely conducted.
Inactivation and Stability of Adenovirus
There are no reports that Ad vectors administered in clinical trials have been shed into the environment. Shedding would most likely be via feces, urine, or possibly the respiratory route, as can occur in naturally infected persons. Since shedding in theory could occur, it is prudent to consider whether such vectors would be stable and how they might be inactivated. Ads have a duplex DNA genome, a sturdy capsid, and no lipid membrane, and therefore Ads are expected to be stable. Many Ad serotypes have been detected in many types of environmental water (see [111]). Ads survive well in liquids or on solids in a desicated state [112]. Ads appear to be stable in surface and ground water for lengthy periods [113,114].
In one study in which PCR and DNA sequencing was used, Ad DNA was found in 5.5% (10 of 188) and 22.2% (10 of 45) samples of drinking water and river water, respectively [114]. These same authors also found Ad DNA in 11% to 22% of swimming pool water samples that had been treated to accepted specifications [115].
Although they are quite stable, Ads can be inactivated by a number of methods [2]. Ads including Ad5 are inactivated effectively by free chlorine in drinking water [116, 117]. In one extensive study with Ad8, which can cause epidemic keratoconjunctivitis, the authors found that environmental surfaces could be disinfected by rinsing with 1,900 parts per million chlorine plus a mixture of ethanol with quaternary ammonium compounds [118].
ACKNOWLEDGEMENTS
The authors thank Dawn Schwartz for help with the article. The author’s research on oncolytic adenovirus vectors was supported by grant NIH R01 CA11802.
Footnotes
CONFLICT OF INTEREST
The authors have a financial interest in an oncolytic adenovirus vector named VRX-007. VRX-007 is not discussed in this article.
PATIENT CONSENT
Declared none.
REFERENCES
- 1.Berk AJ. Adenoviridae. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. pp. 1704–1731. [Google Scholar]
- 2.Wold WSM, Ison MG. Adenoviruses. In: Knipe DM, How-ley PM, editors. Fields Virology. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. pp. 1732–1767. [Google Scholar]
- 3.Lindemans CA, Leen AM, Boelens JJ. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood. 2010;116:5476–5485. doi: 10.1182/blood-2010-04-259291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hostetler KY. Alkoxyalkyl prodrugs of acyclic nucleoside phos-phonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antiviral Res. 2009;82:A84–A98. doi: 10.1016/j.antiviral.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paolino K, Sande J, Perez E, et al. Eradication of disseminated adenovirus infection in a pediatric hematopoietic stem cell transplantation recipient using the novel antiviral agent CMX001. J Clin Virol. 2011;50:167–170. doi: 10.1016/j.jcv.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Deal C, Pekosz A, Ketner G. Prospects for oral replicating adenovi-rus-vectored vaccines. Vaccine. 2013;31:3236–3243. doi: 10.1016/j.vaccine.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunetti-Pierri N, Ng P. Helper-dependent adenoviral vectors for liver-directed gene therapy. Hum Mol Genet. 2011;20:7–13. doi: 10.1093/hmg/ddr143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parks RJ, Chen L, Anton M, et al. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts DM, Nanda A, Havenga MJ, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 10.Sumida SM, Truitt DM, Lemckert AA, et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol. 2005;174:7179–7185. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- 11.Serangeli C, Bicanic O, Scheible MH, et al. Ex vivo detection of adenovirus specific CD4+ T-cell responses to HLA-DR-epitopes of the Hexon protein show a contracted specificity of T(HELPER) cells following stem cell transplantation. Virology. 2010;397:277–284. doi: 10.1016/j.virol.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 12.Leen AM, Christin A, Khalil M, et al. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J Virol. 2008;82(1):546–554. doi: 10.1128/JVI.01689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- 14.Piccolo P, Vetrini F, Mithbaokar P, et al. SR-A and SREC-I are Kupffer and endothelial cell receptors for helper-dependent adenoviral vectors. Mol Ther. 2013;21:767–774. doi: 10.1038/mt.2012.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doronin K, Flatt JW, DiPaolo NC, et al. Coagulation factor X activates innate immunity to human species C adenovirus. Science. 2012;338:795–798. doi: 10.1126/science.1226625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khare R, May SM, Vetrini F, et al. Generation of a Kupffer cell-evading adenovirus for systemic and liver-directed gene transfer. Mol Ther. 2011;19:1254–1262. doi: 10.1038/mt.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaiss AK, Vilaysane A, Cotter MJ, et al. Antiviral antibodies target adenovirus to phagolysosomes and amplify the innate immune response. J Immunol. 2009;182:7058–7068. doi: 10.4049/jimmunol.0804269. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muruve DA, Petrilli V, Zaiss AK, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 20.Barlan AU, Griffin TM, McGuire KA, et al. Adenovirus membrane penetration activates the NLRP3 inflammasome. J Virol. 2011;85:146–155. doi: 10.1128/JVI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crystal RG, Harvey BG, Wisnivesky JP, et al. Analysis of risk factors for local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of comorbid conditions. Hum Gene Ther. 2002;13:65–100. doi: 10.1089/10430340152712647. [DOI] [PubMed] [Google Scholar]
- 22.Harvey BG, Maroni J, O’Donoghue KA, et al. Safety of local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions. Hum Gene Ther. 2002;13:15–63. doi: 10.1089/10430340152712638. [DOI] [PubMed] [Google Scholar]
- 23.Joseph PM, O’Sullivan BP, Lapey A, et al. Aerosol, lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. I. Methods, safety, and clinical implications. Hum Gene Ther. 2001;12:1369–1382. doi: 10.1089/104303401750298535. [DOI] [PubMed] [Google Scholar]
- 24.Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther. 2002;9:979–986. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in an ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Wilson JM. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol Genet Metab. 2009;96:151–157. doi: 10.1016/j.ymgme.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Pesonen S, Kangasniemi L, Hemminki A. Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol Pharm. 2011;8:12–28. doi: 10.1021/mp100219n. [DOI] [PubMed] [Google Scholar]
- 28.Lasaro MO, Ert1 HC. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray G, Buchbinder S, Duerr A. Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr Opin HIV AIDS. 2010;5:357–361. doi: 10.1097/COH.0b013e32833d2d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammer SM, Sobieszczyk ME, Janes H, et al. Efficacy Trial of a DNA/rAd5 HIV-1 Preventive Vaccine. N Engl J Med. 2013 doi: 10.1056/NEJMoa1310566. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smaill F, Jeyanathan M, Smieja M, et al. A Human Type 5 Adeno-virus-Based Tuberculosis Vaccine Induces Robust T Cell Responses in Humans Despite Preexisting Anti-Adenovirus Immunity. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006843. 205ra134. [DOI] [PubMed] [Google Scholar]
- 33.Nemunaitis J, Nemunaitis J. Head and neck cancer: response to p53-based therapeutics. HeadNeck. 2011;33:131–134. doi: 10.1002/hed.21364. [DOI] [PubMed] [Google Scholar]
- 34.Roth JA. Adenovirus p53 gene therapy. Expert Opin Biol Ther. 2006;6:55–61. doi: 10.1517/14712598.6.1.55. [DOI] [PubMed] [Google Scholar]
- 35.Senzer N, Nemunaitis J. A review of contusugene ladenovec (Ad-vexin)p53 therapy. Curr Opin Mol Ther. 2009;11:54–61. [PubMed] [Google Scholar]
- 36.Nemunaitis J, Clayman G, Agarwala SS, et al. Biomarkers Predict p53 Gene Therapy Efficacy in Recurrent Squamous Cell Carcinoma of the Head and Neck. Clin Cancer Res. 2009;15:7719–7725. doi: 10.1158/1078-0432.CCR-09-1044. [DOI] [PubMed] [Google Scholar]
- 37.Atencio IA, Grace M, Bordens R, et al. Biological activities of a recombinant adenovirus p53 (SCH 58500) administered by hepatic arterial infusion in a Phase 1 colorectal cancer trial. Cancer Gene Ther. 2006;13:169–181. doi: 10.1038/sj.cgt.7700870. [DOI] [PubMed] [Google Scholar]
- 38.Tian G, Liu J, Zhou JS, et al. Multiple hepatic arterial injections of recombinant adenovirus p53 and 5-fluorouracil after transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a pilot phase II trial. Anticancer Drugs. 2009;20:389–395. doi: 10.1097/CAD.0b013e32832a2df9. [DOI] [PubMed] [Google Scholar]
- 39.Ma G, Shimada H, Hiroshima K, et al. Gene medicine for cancer treatment: commercially available medicine and accumulated clinical data in China. Drug Des Devel Ther. 2009;2:115–122. doi: 10.2147/dddt.s3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng Z. Current status of Gendicine in China: Recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005;16:1016–1027. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- 41.Shi J, Zheng D. An update on gene therapy in China. Curr Opin Mol Ther. 2009;11:547–553. [PubMed] [Google Scholar]
- 42.Westphal M, Yla-Herttuala S, Martin J, et al. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:823–833. doi: 10.1016/S1470-2045(13)70274-2. [DOI] [PubMed] [Google Scholar]
- 43.Eager RM, Nemunaitis J. Clinical development directions in oncolytic viral therapy. Cancer Gene Ther. 2011;18:305–317. doi: 10.1038/cgt.2011.7. [DOI] [PubMed] [Google Scholar]
- 44.Gomez-Manzano C, Fueyo J. Oncolytic adenoviruses for the treatment of brain tumors. Curr Opin Mol Ther. 2010;12:530–537. [PubMed] [Google Scholar]
- 45.Strauss R, Lieber A. Anatomical and physical barriers to tumor targeting with oncolytic adenoviruses in vivo. Curr Opin Mol Ther. 2009;11:513–522. [PubMed] [Google Scholar]
- 46.Toth K, Wold WSM. Increasing the efficacy of oncolytic adenovirus vectors. Viruses. 2010;2:1844–1866. doi: 10.3390/v2091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toth K, Dhar D, Wold WSM. Oncolytic (replication-competent) adenoviruses as anticancer agents. Expert Opin Biol Ther. 2010;10:353–368. doi: 10.1517/14712590903559822. [DOI] [PubMed] [Google Scholar]
- 48.Schenk E, Essand M, Bangma CH, et al. Clinical adenoviral gene therapy for prostate cancer. Hum Gene Ther. 2010;21:807–813. doi: 10.1089/hum.2009.206. [DOI] [PubMed] [Google Scholar]
- 49.Freytag SO, Stricker H, Movsas B, et al. Prostate cancer gene therapy clinical trials. Mol Ther. 2007;15:1042–1052. doi: 10.1038/sj.mt.6300162. [DOI] [PubMed] [Google Scholar]
- 50.Vaillancourt M, Atencio I, Quijano E, et al. Inefficient killing of quiescent human epithelial cells by replicating adenoviruses: potential implications for their use as oncolytic agents. Cancer Gene Ther. 2005;12:691–698. doi: 10.1038/sj.cgt.7700840. [DOI] [PubMed] [Google Scholar]
- 51.Ferrari R, Pellegrini M, Horwitz GA, et al. Epigenetic reprogram-ming by adenovirus e1a. Science. 2008;321:1086–1088. doi: 10.1126/science.1155546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heise C, Sampson-Johannes A, Williams A, et al. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard che-motherapeutic agents. Nature Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 53.O’Shea CC, Johnson L, Bagus B, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Lin E, Nemunaitis J. Oncolytic viral therapies. Cancer Gene Ther. 2004;11:643–664. doi: 10.1038/sj.cgt.7700733. [DOI] [PubMed] [Google Scholar]
- 55.Reid TR, Freeman S, Post L, et al. Effects of Onyx-015 among metastatic colorectal cancer patients that have failed prior treatment with 5-FU/leucovorin. Cancer Gene Ther. 2005;12:673–681. doi: 10.1038/sj.cgt.7700819. [DOI] [PubMed] [Google Scholar]
- 56.Ganly I, Kirn D, Eckhardt G, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 57.Huang PI, Chang JF, Kirn DH, et al. Targeted genetic and viral therapy for advanced head and neck cancers. Drug Discov Today. 2009;14:570–578. doi: 10.1016/j.drudis.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Khuri FR, Nemunaitis J, Ganly I, et al. A controlled trial of intra-tumoral ONYX-015, a selectively replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 59.Morley S, MacDonald G, Kirn D, et al. The d11520 virus is found preferentially in tumor tissue after direct intratumoral injection in oral carcinoma. Clin Cancer Res. 2004;10:4357–4362. doi: 10.1158/1078-0432.CCR-03-0443. [DOI] [PubMed] [Google Scholar]
- 60.Nemunaitis J, Cunningham C, Buchanan A, et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 2001;8:746–759. doi: 10.1038/sj.gt.3301424. [DOI] [PubMed] [Google Scholar]
- 61.Demers GW, Johnson DE, Tsai V, et al. Pharmacologic indicators of antitumor efficacy for oncolytic virotherapy. Cancer Res. 2003;63:4003–4008. [PubMed] [Google Scholar]
- 62.Habib NA, Sarraf CE, Mitry RR, et al. E1B–deleted adenovirus (d11 520) gene therapy for patients with primary and secondary liver tumors. Hum Gene Ther. 2001;12:219–226. doi: 10.1089/10430340150218369. [DOI] [PubMed] [Google Scholar]
- 63.Hecht JR, Bedford R, Abbruzzese JL, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–561. [PubMed] [Google Scholar]
- 64.Mulvihill S, Warren R, Venook A, et al. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther. 2001;8:308–315. doi: 10.1038/sj.gt.3301398. [DOI] [PubMed] [Google Scholar]
- 65.Reid T, Galanis E, Abbruzzese J, et al. Intra-arterial administration of a replication-selective adenovirus (d11520) in patients with colorectal carcinoma metastatic to the liver: a phase I trial. Gene Ther. 2001;8:1618–1626. doi: 10.1038/sj.gt.3301512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sze DY, Freeman SM, Slonim SM, et al. Dr. Gary J. Becker Young Investigator Award: intraarterial adenovirus for metastatic gastrointestinal cancer: activity, radiographic response, and survival. J Vasc Interv Radiol. 2003;14:279–290. doi: 10.1097/01.rvi.0000058422.01661.1e. [DOI] [PubMed] [Google Scholar]
- 67.Vasey PA, Shulman LN, Campos S, et al. Phase I trial of intraperitoneal injection of the ElB-55-kd-gene-deleted adenovirus ONYX-015 (d11520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol. 2002;20:1562–1569. doi: 10.1200/JCO.2002.20.6.1562. [DOI] [PubMed] [Google Scholar]
- 68.Nemunaitis J, Cunningham C, Tong AW, et al. Pilot trial of intravenous infusion of a replication-selective adenovirus (ONYX-015) in combination with chemotherapy or IL-2 treatment in refractory cancer patients. Cancer Gene Ther. 2003;10:341–352. doi: 10.1038/sj.cgt.7700585. [DOI] [PubMed] [Google Scholar]
- 69.Makower D, Rozenblit A, Kaufman H, et al. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin Cancer Res. 2003;9:693–702. [PubMed] [Google Scholar]
- 70.Habib N, Salama H, et al. Abd E1 Latif Abu Median . Clinical trial of ElB-deleted adenovirus (dl1520) gene therapy for hepatocellular carcinoma. Cancer Gene Ther. 2002;9:254–259. doi: 10.1038/sj.cgt.7700431. [DOI] [PubMed] [Google Scholar]
- 71.Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: Lessons from clinical trials. Cancer Gene Ther. 2002;9:979–986. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Au T, Thorne S, Korn WM, et al. Minimal hepatic toxicity of Onyx-015: spatial restriction of coxsackie-adenoviral receptor in normal liver. Cancer Gene Ther. 2007;14:139–150. doi: 10.1038/sj.cgt.7700988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reid T, Galanis E, Abbruzzese J, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dll 520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62:6070–6079. [PubMed] [Google Scholar]
- 74.Lu W, Zheng S, Li XF, et al. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: a pilot phase II clinical trial. World J Gastroenterol. 2004;10:3634–3638. doi: 10.3748/wjg.v10.i24.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia ZJ, Chang JH, Zhang L, et al. [Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus.] Ai Zheng. 2004;23:1666–1670. [PubMed] [Google Scholar]
- 76.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 77.Yuan ZY, Zhang L, Li S, et al. [Safety of an E1B deleted adenovirus administered intratumorally to patients with cancer] Ai Zheng. 2003;22:310–313. [PubMed] [Google Scholar]
- 78.Jia H, Kling J. China offers alternative gateway for experimental drugs. Nat Biotechnol. 2006;24:117–118. doi: 10.1038/nbt0206-117. [DOI] [PubMed] [Google Scholar]
- 79.Liang M. Clinical development of oncolytic viruses in China. Curr Pharm Biotechnol. 2012;13:1852–1857. doi: 10.2174/138920112800958760. [DOI] [PubMed] [Google Scholar]
- 80.Liu HL, Chen J. Oncolytic virus as an agent for the treatment of malignant ascites. Cancer Biother Radiopharm. 2009;24:99–102. doi: 10.1089/cbr.2008.0559. [DOI] [PubMed] [Google Scholar]
- 81.He Q, Liu Y, Zou Q, et al. Transarterial injection of H101 in combination with chemoembolization overcomes recurrent hepatocellular carcinoma. World J Gastroenterol. 2011;17:2353–2355. doi: 10.3748/wjg.v17.i18.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li JL, Liu HL, Zhang XR, et al. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther. 2009;16:376–382. doi: 10.1038/gt.2008.179. [DOI] [PubMed] [Google Scholar]
- 83.Freytag SO, Stricker H, Pegg J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional con-formal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497–7506. [PubMed] [Google Scholar]
- 84.Freytag SO, Khil M, Stricker H, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 2002;62:4968–4976. [PubMed] [Google Scholar]
- 85.Barton KN, Paielli D, Zhang Y, et al. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol Ther. 2006;13:347–356. doi: 10.1016/j.ymthe.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 86.DeWeese TL, van der Poel H, Li S, et al. A Phase I Trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- 87.Small EJ, Carducci MA, Burke JM, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14:107–117. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 88.Fueyo J, Gomez-Manzano C, Alemany R, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 89.Heise C, Hermiston T, Johnson L, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto M, Curiel DT. Current issues and future directions of oncolytic adenoviruses. Mol Ther. 2010;18:243–250. doi: 10.1038/mt.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cerullo V, Koski A, Vaha-Koskela M, et al. Chapter eight-Oncolytic adenoviruses for cancer immunotherapy: data from mice, hamsters, and humans. Adv Cancer Res. 2012;115:265–318. doi: 10.1016/B978-0-12-398342-8.00008-2. [DOI] [PubMed] [Google Scholar]
- 92.Krasnykh V, Dmitriev I, Navarro JG, et al. Advanced generation adenoviral vectors possess augmented gene transfer efficiency based upon coxsackie adenovirus receptor-independent cellular entry capacity. Cancer Res. 2000;60:6784–6787. [PubMed] [Google Scholar]
- 93.Krasnykh VN, Mikheeva GV, Douglas JT, et al. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trinh HV, Lesage G, Chennamparampil V, et al. Avidity binding of human adenovirus serotypes 3 and 7 to the membrane cofactor CD46 triggers infection. J Virol. 2012;86:1623–1637. doi: 10.1128/JVI.06181-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang H, Li ZY, Liu Y, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tuve S, Wang H, Ware C, et al. A new group B adenovirus receptor is expressed at high levels on human stem and tumor cells. J Virol. 2006;80:12109–12120. doi: 10.1128/JVI.01370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nokisalmi P, Pesonen S, Escutenaire S, et al. Oncolytic Adenovirus ICOVIR-7 in Patients with Advanced and Refractory Solid Tumors. Clin Cancer Res. 2010;16:3035–3043. doi: 10.1158/1078-0432.CCR-09-3167. [DOI] [PubMed] [Google Scholar]
- 98.Kimball KJ, Preuss MA, Barnes MN, et al. A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases. Clin Cancer Res. 2010;16:5277–5287. doi: 10.1158/1078-0432.CCR-10-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pesonen S, Nokisalmi P, Escutenaire S, et al. Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L–D24 in patients with metastatic and refractory solid tumors. Gene Ther. 2010;17:892–904. doi: 10.1038/gt.2010.17. [DOI] [PubMed] [Google Scholar]
- 100.Cerullo V, Diaconu I, Kangasniemi L, et al. Immunological Effects of Low-dose Cyclophosphamide in Cancer Patients Treated With Oncolytic Adenovirus. Mol Ther. 2011;19:1737–1746. doi: 10.1038/mt.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cerullo V, Pesonen S, Diaconu I, et al. Oncolytic Adenovirus Coding for Granulocyte Macrophage Colony-Stimulating Factor Induces Antitumoral Immunity in Cancer Patients. Cancer Res. 2010;70:4297–4309. doi: 10.1158/0008-5472.CAN-09-3567. [DOI] [PubMed] [Google Scholar]
- 102.Chang J, Zhao X, Wu X, et al. A Phase I study of KH901, a conditionally replicating granulocyte-macrophage colony-stimulating factor: armed oncolytic adenovirus for the treatment of head and neck cancers. Cancer Biol Ther. 2009;8:676–682. doi: 10.4161/cbt.8.8.7913. [DOI] [PubMed] [Google Scholar]
- 103.Koski A, Kangasniemi L, Escutenaire S, et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther. 2010;18:1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hemminki A, Oksanen M, Merisalo-Soikkeli M. Oncolytic vi-rotherapy trials-letter. Clin Cancer Res. 2013;19:4541–4542. doi: 10.1158/1078-0432.CCR-13-1471. [DOI] [PubMed] [Google Scholar]
- 105.Pesonen S, Diaconu I, Cerullo V, et al. Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors. Int J Cancer. 2012;130:1937–1947. doi: 10.1002/ijc.26216. [DOI] [PubMed] [Google Scholar]
- 106.Kanerva A, Nokisalmi P, Diaconu I, et al. Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res. 2013;19:2734–2744. doi: 10.1158/1078-0432.CCR-12-2546. [DOI] [PubMed] [Google Scholar]
- 107.Liikanen I, Ahtiainen L, Hirvinen ML, et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther. 2013;21:1212–1223. doi: 10.1038/mt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koski A, Raki M, Nokisalmi P, et al. Verapamil results in increased blood levels of oncolytic adenovirus in treatment of patients with advanced cancer. Mol Ther. 2012;20:221–229. doi: 10.1038/mt.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hemminki O, Diaconu I, Cerullo V, et al. Ad3-hTERT-ElA, a fully serotype 3 oncolytic adenovirus, in patients with chemotherapy refractory cancer. Mol Ther. 2012;20:1821–1830. doi: 10.1038/mt.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sirikanchana K, Shisler JL, Marinas BJ. Effect of exposure to UV-C irradiation and monochloramine on adenovirus serotype 2 early protein expression and DNA replication. Appl Environ Microbiol. 2008;74:3774–3782. doi: 10.1128/AEM.02049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gordon YJ, Aoki K, Kinchington PR. Adenovirus keratoconjunctivitis. In: Pepose JS, Holland GN, Wilhelmus KR, editors. Ocular infectin and immunity. St. Louis: Mosby; 1996. [Google Scholar]
- 112.Rigotto C, Hanley K, Rochelle PA, et al. Survival of adenovirus types 2 and 41 in surface and ground waters measured by a plaque assay. Environ Sci Technol. 2011;45:4145–4150. doi: 10.1021/es103922r. [DOI] [PubMed] [Google Scholar]
- 113.van Heerden J, Ehlers MM, Heim A, et al. Prevalence, quantification and typing of adenoviruses detected in river and treated drinking water in South Africa. J Appl Microbiol. 2005;99:234–242. doi: 10.1111/j.1365-2672.2005.02617.x. [DOI] [PubMed] [Google Scholar]
- 114.van Heerden J, Ehlers MM, van Zyl WB, et al. Prevalence of human adenoviruses in raw and treated water. Water Sci Technol. 2004;50:39–43. [PubMed] [Google Scholar]
- 115.Baxter CS, Hofmann R, Templeton MR, et al. Inactivation of adenovirus types 2, 5, and 41 in drinking water by UV light, free chlorine, and monochloramine. J Environ Eng. 2007;133:95–103. [Google Scholar]
- 116.Page MA, Shisler JL, Marinas BJ. Mechanistic aspects of adenovirus serotype 2 inactivation with free chlorine. Appl Environ Microbiol. 2010;76:2946–2954. doi: 10.1128/AEM.02267-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rutala WA, Peacock JE, Gergen MF, et al. Efficacy of hospital germicides against adenovirus 8, a common cause of epidemic keratoconjunctivitis in health care facilities. Antimicrob Agents Chemother. 2006;50:1419–1424. doi: 10.1128/AAC.50.4.1419-1424.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]