Abstract
Blast quantification by Flow Cytometry (FCM) may become essential in situations where morphologic evaluation is difficult or unavailable. As hemodilution invariably occurs, a means of determining Bone Marrow Purity (BMP) and normalizing FCM blast counts is essential, especially when blast percentages are diagnostically critical as in Acute Myeloid Leukemia (AML) and Myelodysplasia (MDS). By evaluating different leukocyte populations in eight initial patients, a formula to predict BMP was developed and compared to the actual BMP determined by manual counts. Performance of the formula was then validated in 86 AML/MDS patients by comparing normalized FCM blast counts to those determined by the reference manual method. A BMP formula was empirically developed, primarily based on changes in lymphocytes which reliably correlated with the actual BMP (R2 = 0.8955). Components of the formula were derived entirely from automated lymphocyte and total leukocyte counts from the peripheral blood and FCM analyses. BMP formula was then validated in 86 AML/MDS patients. When used to normalize blast counts, the formula showed accurate correction when BMP fell between 40%-90%. In this group, correlation of normalized FCM and manual blast counts was acceptable (R2 = 0.8335), being greatest at lower blast percentages. Normalization of the FCM blast count appropriately reclassified disease in 26.8% of cases. We identified a practical means of estimating hemodilution and allowing FCM blast normalization in the evaluation of AML and MDS. BMP assessment by this simple method improves the quality of the FCM data and facilitates accurate diagnosis and patient management.
Keywords: Acute myeloid leukemia, Myelodysplastic syndrome, Blast normalization, Flow cytometry, Peripheral blood dilution, Bone marrow purity
Introduction
Flow Cytometry (FCM) analysis has become standard of care in the evaluation of hematopoietic malignancies, offering the ability to rapidly identify, enumerate, and phenotypically characterize blast populations in such way that diagnosis can be established within hours affer bone marrow procedures [1]. In conjunction with morphologic, immunophenotypic and genetic data, the classification of the Myelodysplastic Syndromes (MDS) and Acute Myeloid Leukemia (AML), in particular, requires accurate determination of the bone marrow myeloblast percentage to establish a diagnosis and to allow for appropriate patient management. Manual differential cell count of the bone marrow aspirate continues to be the gold standard for quantifying blasts, allowing classification of MDS and AML based on stepwise blast thresholds established by the World Health Organization (WHO) [2]. While obtaining an accurate blast count is typically straightforward, FCM enumeration may become critical in certain situations, such as when morphologic evaluation is difficult to interpret or not possible, increasing the reliance on other techniques to acquire this information. Moreover, in the reference laboratory setting, the bone marrow aspirate may not be available for correlation, resulting in FCM data that is essentially blinded from the morphologic impression. When performing FCM in these situations, methods to estimate inevitable hemodilution [3] and possibly normalize cell counts to prevent misleading or conflicting information are desirable.
The admixture of peripheral blood during the bone marrow aspiration process, depending on the technique used, may result in highly variable myeloblast counts as increasing numbers of mature cells from the peripheral blood contaminate the immature elements derived from the bone marrow space. This phenomenon has been well-documented in experiments performed on normal bone marrow specimens in which peripheral blood contamination was mathematically accounted for by a normalization equation [4]. Earlier, Holdrinet et al. performed a series of experiments using radioactive-labeled red blood cells and albumin to demonstrate and quantify peripheral blood dilution of bone marrow aspirate material [5]. While precise blast quantification is typically not a concern in the evaluation of normal bone marrow and the routine use of radioactive-labeled blood elements is not practical in the clinical laboratory, these studies importantly documented the effects of hemodilution during bone marrow aspiration and suggested methods in which to overcome this technical obstacle.
Earlier approaches aimed at addressing and correcting for peripheral blood contamination of bone marrow aspirate material used DNA S-phase analysis [6-8]. These studies compared DNA S-phase with percentage of lymphocytes and monocytes within the bone marrow aspirate specimen, since these latter cellular components are indicative of peripheral blood contamination. Based on the negative correlation observed between erythroid and myeloid bone marrow cells in S-phase and the percentage of lymphocytes and monocytes in the aspirates, a formula to correct for peripheral blood dilution was developed. Other studies have addressed peripheral blood contamination by evaluating the numbers of immature elements in bone marrow aspirate material compared to what is typically expected [9]. A similar approach was devised by Loken et al. who exploited the phenotypic variability of granulocytes to determine the proportion of immature neutrophils using a specific FCM gating strategy [10] and then applying these data to normalize bone marrow blast count. While promising, these methodologies were not tested in the setting of bone marrow abnormality, such as in MDS or AML, where anticipated cellular maturation rates and phenotypic profiles are inherently atypical.
Outside the setting of normal hematopoiesis, the issue of peripheral blood contamination becomes more complicated as the cellular components of the peripheral blood are offen abnormal. Patients with high-grade myeloid neoplasms frequently have immature leukocytes and nucleated red blood cells within the peripheral circulation. In addition, typical antigen expression profiles in the setting of leukemia and dysplasia are offen aberrant [11], and thus, relying on specific antigenic maturation patterns may not always be suitable in the setting of disease. Moreover, while data has been variable on the subject, one study showed the greatest degree of discordant results between morphological and FCM quantification of blasts around the 5% cut-off, a threshold which is critical for determining remission status of acute leukemia [12]. Given the vital role in which blast quantification plays in the diagnosis as well as monitoring of myeloid neoplasms, in addition to widespread use of FCM analysis as a part of the diagnostic process [13-16], a straightforward, reliable method for determining the degree of hemodilution in bone marrow aspirates of AML and MDS patients is necessary. While many of the previously proposed methodologies for determining Bone Marrow Purity (BMP) have offered foundational knowledge of this phenomenon, a practical approach to specifically overcome the morphologic and phenotypic abnormalities seen in these myeloid diseases, where precise blast counts become clinically relevant, is required to allow for timely and appropriate management of these patients.
Materials and Methods
Study design
Approval was obtained from the Institutional Review Board prior to commencing this study. Patients diagnosed with AML or MDS were identified through a search of the surgical pathology database at our institution between the years of 2010 and 2014. Retrospective review of the electronic medical records was conducted of the selected patients to verify the diagnosis and obtain clinical history. In order to focus on cases with elevated blast counts and the effects of hemodilution on disease classification, original diagnostic biopsies as well as cases of AML with residual/relapsed disease were included in this study. In addition, patients were excluded if archival bone marrow aspirate and biopsy slides, FCM scatterplots, Complete Blood Count (CBC) and peripheral blood smears were not available for review. Cases that met inclusion criteria were then reviewed to classify each case according to the current WHO classification system [2]. In particular, total white blood cell and lymphocyte counts from the CBC analyzer were collected and 500-cell manual differential counts of the bone marrow aspirate and peripheral blood specimens were performed on all cases in this study.
The study was conducted in two phases. First, a formula to assess BMP was developed, and second, the clinical utility of the BMP formula in determining blast percentages in MDS and AML was tested. In the initial portion of the study, a BMP gold standard was first created by performing 500-cell manual differential counts on the Peripheral Blood (PB), Bone Marrow Aspirate (BM) and Flow Cytometry (FCM) specimens on eight patients with AML or MDS. Based on the blast percentages determined from each of these differentials, the gold standard BMP (i.e., Actual BMP) was then calculated using a ratio equation as follows:
Next, a formula to predict BMP was developed based on the principle that the specimen used for flow cytometric analysis is composed of a mixture of bone marrow aspirate material and peripheral blood in unknown proportions. Using manual differential counts from the eight initial patients, several different leukocyte populations, such as lymphocytes, monocytes and neutrophils, were tested to determine which population(s) best predicted the amount of peripheral blood dilution present in the bone marrow aspirate material. From these experiments, a BMP formula was empirically derived using only values obtained from the peripheral and FCM specimens. Where possible, automated leukocyte counts were then used, in place of manually-derived counts, to provide a more straightforward and objective means of implementing the formula. The accuracy of the equation was ultimately assessed by comparing the predicted BMP to the actual BMP, determined from the above-described ratio.
In the second phase of the study, the BMP formula was validated by studying its performance in 86 patients with AML or MDS. The predicted BMP was used to normalize the myeloblast percentage determined by FCM and compared to the actual blast count determined from the 500-cell manual differential. Cases that challenged the diagnostic blast thresholds for AML and MDS were utilized. The limitations of the BMP formula to accurately normalize the blast count were also investigated. Finally, the cases were reviewed to assess the impact of blast normalization in disease classification, thereby determining its clinical utility in the diagnosis and monitoring of AML and MDS [17].
Bone marrow aspirates
Wright's-Giemsa stained smears of bone marrow aspirates from eligible patients were examined by light microscopy and 500-cell manual differentials were performed. Only areas of the smear near the bone marrow particle were evaluated to decrease the effect of peripheral blood dilution and obtain the most accurate bone marrow blast count possible. Differential counts consisted of 11 components including blasts, promyelocytes, myelocytes, metamyelocytes, bands/segmented neutrophils, eosinophils, basophils, monocytes, lymphocytes, plasma cells and erythroid precursors. For the initial portion of the study (8 patients), a smear made from the FCM specimen, offen obtained from a second aspirate pull, was evaluated in a similar manner including performing a 500-cell manual differential. In the latter phase of the study (86 patients), total leukocyte counts were obtained on the FCM specimens using a CELL-DYN Sapphire Automated Hematology Analyzer (Abbott Diagnostics, Abbott Park, IL).
Peripheral blood specimens and CBC analysis
Peripheral blood from each patient, obtained on the same day of the bone marrow procedure, was examined via Giemsa-stained smears. A 500-cell manual differential count was performed, recording the same cell types as those determined for the bone marrow aspirate specimens. In addition, CBC analysis with automated differential was performed using a CELL-DYN Sapphire Automated Hematology Analyzer (Abbott Diagnostics).
Multiparameter flow cytometry
Ten-color flow cytometry was performed using a FACSCanto flow cytometer (Becton Dickinson Biosciences, San Jose, CA) in conjunction with a 4-tube antibody panel designed to characterize blasts as detailed in Table 1. Briefly, 100 μl of bone marrow aspirate specimen was prepared according to standard whole-blood lysis procedure, with the use of Becton Dickinson Lyse-Wash method. Cell solutions were stained with fluorophore-conjugated monoclonal antibodies and isotype matched controls were used to exclude nonspecific binding. Primary CD45 versus side scatter (SSC) gating was employed to discriminate blast, lymphocyte, monocyte, granulocyte and debris/erythroid populations [18]. Data was acquired via FACS Diva soffware (Becton Dickinson Biosciences, San Jose, CA) and analyzed using FCS Express soffware program (De Novo Soffware, Los Angeles, CA).
Table 1.
Flow Cytometry Antibody Panel for Evaluating AML and MDS.
| V421 | V500 | BV605 | FITC | PE | PerCP-Cy5.5 | PE-Cy7 | APC | AlexaFluor-700 | APC-Cy7 | |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | CD33 | CD45 | CD38 | CD61 | CD13 | CD117 | CD34 | CD15 | HLA-DR | CD14 |
| #2 | CD4 | CD45 | CD56 | CD71 | CD11b | CD36 | CD34 | CD33 | CD64 | CD14 |
| #3 | CD5 | CD45 | CD38 | CD58 | CD22 | CD20 | CD34 | CD10 | HLA-DR | CD19 |
| #4 | CD5 | CD45 | CD3 | CD1a | CD8 | CD2 | CD34 | CD10 | CD7 | CD4 |
Statistical analysis
Data were entered into an Excel database and correlated by linear regression analysis for the purpose of comparing predicted values (i.e., BMP, blast percentage, etc.) to the reference method. Where appropriate, polynomial regression analysis was used in a similar manner to assess trends.
Results
The dilutional effects of peripheral blood on bone marrow aspirate material were investigated in two phases. The primary aim of the first phase was to develop a formula which would mathematically predict BMP without utilizing data from the aspirate differential, and while only using values derived from the peripheral blood CBC and FCM. The second phase of the study sought to assess the utility of the BMP formula in the setting of AML and MDS. In this phase, the validity of the formula was tested by comparing normalized blast counts, based on the predicted BMP, to those determined by the gold standard manual method.
Manual determination of bone marrow dilution (8 Initial Specimens)
Eight bone marrow and accompanying peripheral blood specimens were initially studied by examining populations of leukocytes in the bone marrow in the setting of hemodilution. Specimens were chosen to represent a range of myeloblast percentages (2-66%) and included three cases of high-grade MDS, four cases of AML with myelodysplasia-related changes and one remission pattern marrow. For each case, 500-cell manual differential counts were performed on the peripheral blood, the bone marrow aspirate spicule and the accompanying FCM specimens. In all cases used in this phase of the study, the number of blasts in the FCM material fell between the peripheral blood and bone marrow aspirate percentages. The actual BMP was then calculated from the blast percentages derived from these differential counts and used as the reference (gold standard) method using the following equation:
In a similar manner, percentages of neutrophils, monocytes and lymphocytes were then compared from each specimen part to determine the suitability of these populations to assess BMP. This was accomplished utilizing essentially the same equation as above in which percentages of each type of leukocyte were substituted in place of blasts to determine the “predicted” bone marrow purity as follows:
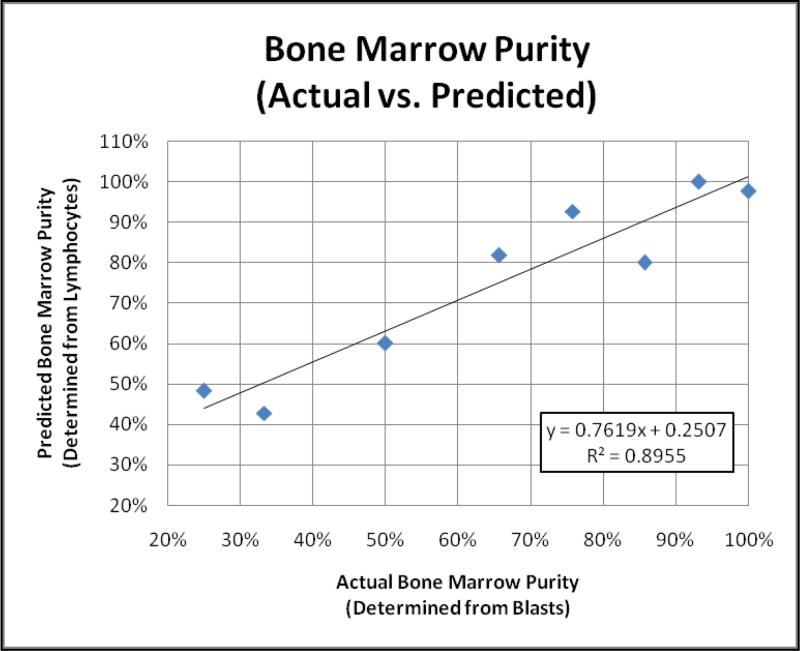
where X represents neutrophils, monocytes or lymphocytes. The lymphocyte populations demonstrated the greatest correlation in estimating the actual BMP (R2 = 0.8955), suggesting the admixture of peripheral blood and bone marrow lymphocytes provide a reasonable estimate of the same phenomenon observed with the blast population of interest (Figure 1). In contrast, correlations with neutrophil and monocyte populations with respect to the actual BMP were poor (R2 = 0.4114 and R2 = 0.6770, respectively; data not shown).
Figure 1.
Actual versus Predicted Bone Marrow Purity. Reasonable correlation was seen between the actual and predicted BMP suggesting that lymphocytes may serve as a suitable population to estimate bone marrow dilution. “Actual” BMP was based on comparing the blast populations in peripheral blood, bone marrow aspirate and flow cytometry specimens, while the “predicted” BMP utilized lymphocyte populations to estimate the degree of peripheral blood dilution.
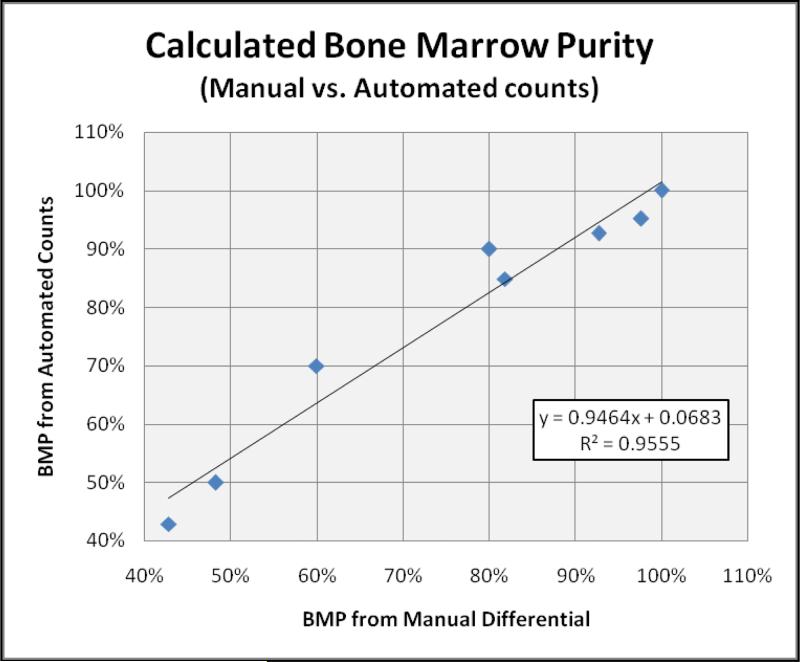
Lymphocyte percentages for each case were determined by an automated cell counter and then utilized to calculate BMP. While some variation due to the difference in methodology was noted, BMP determined from these automated counts strongly correlated with those determined by the rigorous manual method (R2 = 0.9555). These findings permitted the use of automated data, in place of the manually-derived values, in the BMP calculation for the remainder of the study (Figure 2).
Figure 2.
Comparison of BMP determined from Manual vs. Automated Lymphocyte counts. BMP calculation using lymphocyte populations were repeated on the same eight cases using counts determined by CBC analyzer. A high degree of correlation was seen between manual and automated-derived counts, allowing a more practical means of calculating BMP.
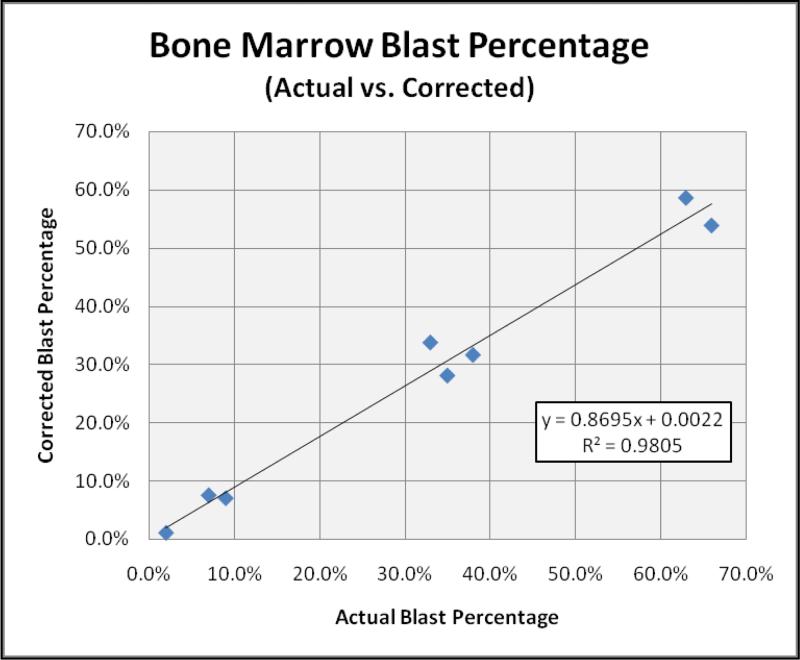
Finally, the BMP determined from the automated lymphocyte counts (BMPLymph) was used to adjust the bone marrow blast percentage in an attempt to correct for the degree of peripheral blood dilution. The calculated blast percentage (Blastcalculated), determined from the equation below, was then compared to the actual bone marrow blast percentage as determined by 500-cell manual differential.
The high degree of correlation observed in these initial eight cases (R2 = 0.9805) formulated the basis for utilizing lymphocyte populations as a reliable means of calculating BMP and adjust blast percentages in the setting of hemodilution (Figure 3).
Figure 3.
Using lymphocytes as a surrogate population to calculate BMP, blast percentages were adjusted and compared to the actual blast count as determined by 500-cell manual differential in the initial eight patients. The strong correlation observed further supports the concept that lymphocyte populations provide a reliable means of calculating BMP and thereby allow reasonable correction of the bone marrow blast percentage.
Development of bone marrow purity calculation
Using data from the initial study, a BMP calculation was formulated using ratios of FCM and peripheral blood lymphocyte percentages as well as FCM and peripheral blood total leukocyte counts. Total leukocyte counts were determined by CBC analyzer for both the peripheral blood (LeukocytesPB) and flow cytometry specimens (LeukocytesFCM). The absolute lymphocyte count for the peripheral blood (LymphocytesPB) was determined by CBC analysis while the absolute lymphocyte count of the FCM specimen (LymphocytesFCM) was derived by multiplying the FCM total leukocyte count by the lymphocyte percentage determined from the CD45 vs. SSC scatterplot. The predicted BMP formula, which was then used in the second phase of the study, is as follows:
Validation of bone marrow purity formula (86 AML/MDS Cases)
Using the predicted BMP formula, BMP was calculated in 86 sequential bone marrow specimens that had elevated blast counts and a history of MDS or AML with myelodysplasia-related changes. A history of myelodysplasia was required to test the clinical utility of the BMP calculation in situations where blast percentage is critical for diagnostic classification. The specimens included 13 patients with MDS and 73 patients with AML (34 new diagnoses, 30 status post chemotherapy with residual disease, 9 in relapse). Bone marrow blast percentage ranged from 2 to 90% (median 16%) as determined from 500-cell manual differential.
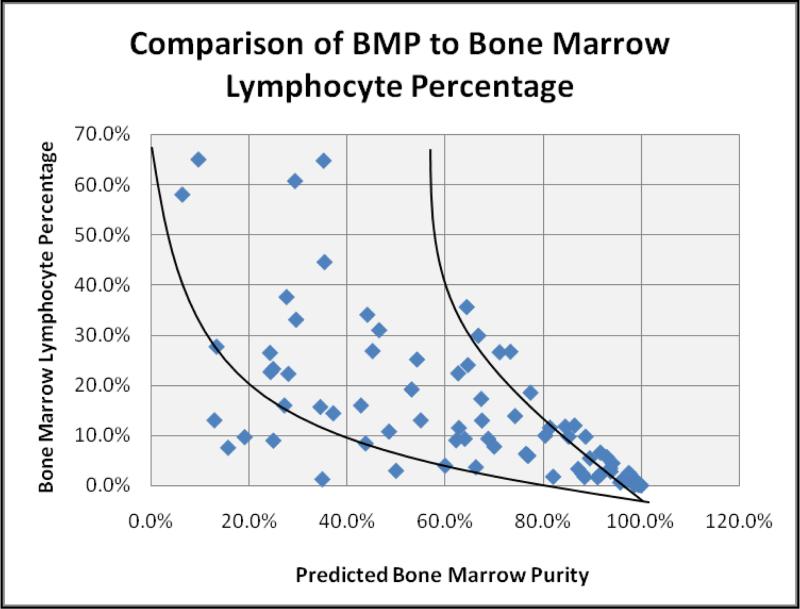
When comparing predicted BMP with the percentage of lymphocytes in the flow specimens, an inverse relationship was observed, reflecting an increasing contribution of lymphocytes from the peripheral blood as dilution of the aspirate specimen occurred (Figure 4A). Predicted BMP (BMPpredicted) ranged from 6.5–99.8% in the 86 cases analyzed. As this general trend suggested inherent limitations to the BMP calculation, such as when the lymphocyte count was very high or very low, predicted blast counts were then calculated and compared to the actual blast percentages as determined by manual differential, according to the following equation:
Figure 4A.
Comparison of predicted BMP to lymphocyte percentage in 86 bone marrow aspirate specimens. In general, BMP decreases as the percentage of bone marrow lymphocytes increases. This general trend is consistent with peripheral blood contributing lymphocytes to the bone marrow aspirate as hemodilution increases.
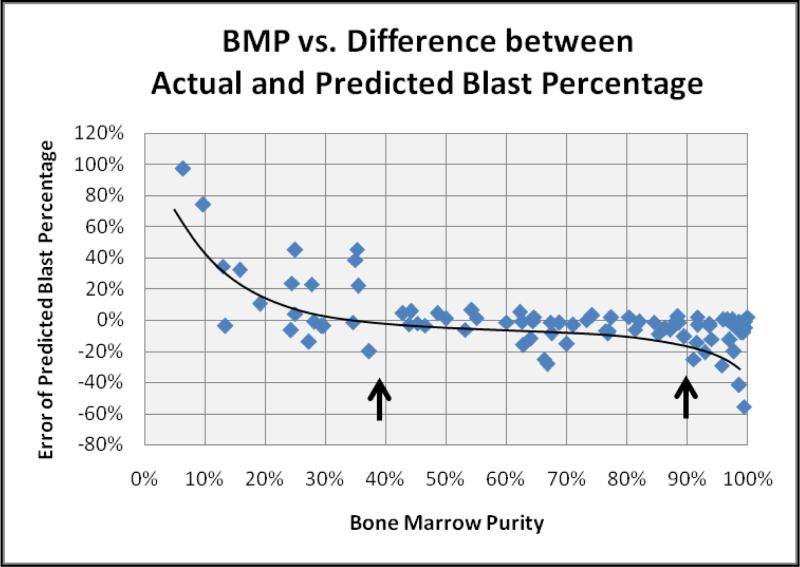
Based on BMP, the predicted blast percentage showed acceptable correlation with the actual blast count in 56 (65.1%) of the 86 specimens analyzed. However, the ability of the equation to accurately adjust the bone marrow blast percentage declined rapidly when the BMP fell below 40% (Figure 4B). Based on the raw data, these cases clearly showed hemodilution, as the FCM analysis significantly underestimated the true blast count. As noted previously, these cases had significantly elevated lymphocyte percentages in the FCM specimen, nearly reaching those of the peripheral blood. A similar phenomenon was observed at the opposite extreme (>90% BMP) where the lymphocyte counts in the FCM specimen were extremely low.
Figure 4B.
Comparison of BMP to error in predicted blast percentage. At the extremes of the BMP calculation, the ability to accurately predict the blast percentage in the bone marrow decreases. Deviation from the polynomial trend line demonstrates reliable prediction of the bone marrow blast percentage in the range of 40 – 90% purity (arrows).
Correction of bone marrow blast percentage & impact on evaluation of MDS and AML
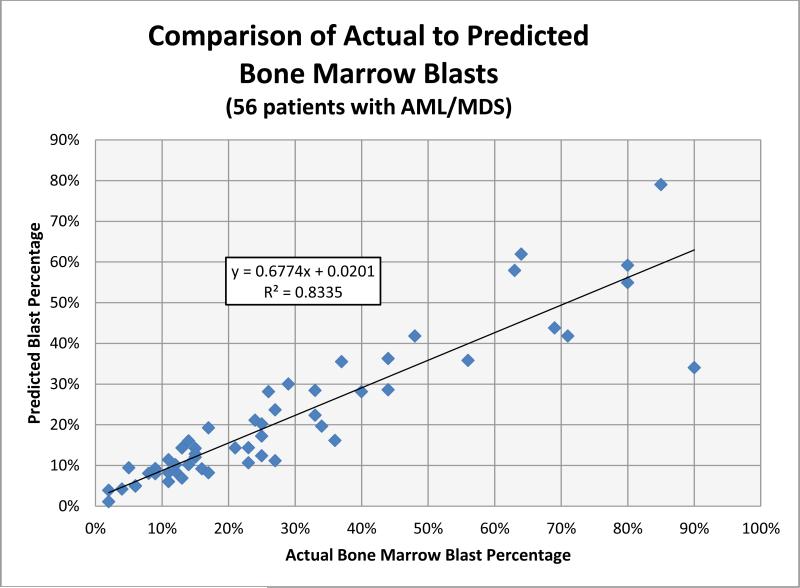
To establish the clinical utility of estimating BMP in the setting of MDS and AML, the 56 remaining cases which demonstrated BMP within the acceptable range were then studied to determine the effect on pathologic diagnosis. This group consisted of 31 cases of AML (≥20% blasts), 16 cases of refractory anemia with excess blast-2 (RAEB-2) (≥10% - 19% blasts), 5 cases of refractory anemia with excess blast-1 (RAEB-1) (≥5% - 9% blasts) and 4 cases of low-grade MDS (< 5% blasts). Using the BMP calculation to predict blast percentage, the overall correlation across all disease categories was acceptable (R2 = 0.8335), with the greatest correlation identified within the lower blast percentages (Figure 5). This observation is of clinical significance as the MDS and AML diagnostic thresholds reside in this blast range (i.e., ≤ 20%). At higher blast percentages, greater variation was seen; however, underestimating the blast population in the setting of established acute leukemia is of lesser clinical importance.
Figure 5.
Comparison of actual to predicted bone marrow blast percentage based on BMP in 56 patients with MDS or AML. Correlation was greatest at lower blast percentages, where diagnostic thresholds become an important consideration. More variability was noted at higher blast percentages.
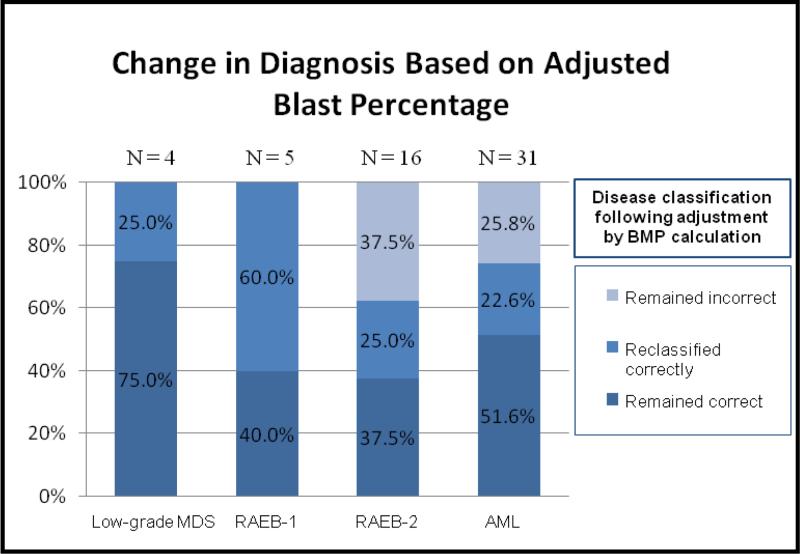
In order to evaluate the impact of correcting for peripheral blood dilution, disease classification was performed by way of flow cytometric determination of blast percentage before and affer adjusting for BMP (Figure 6). Of the 56 cases studied, 27 (48.2%) remained in the appropriate diagnostic category affer adjusting for hemodilution. These cases generally consisted of specimens that showed either high BMP or had high blast counts and were already in the diagnostic category of AML. An additional 15 cases (26.8%) were appropriately reclassified following blast adjustment for BMP. These cases were spread across all diagnostic categories and, in effect, showed stepwise movement from lower to higher grade diagnoses once the blast percentage was corrected. Finally, 14 cases (25.0%) remained incorrectly classified following application of the BMP calculation. All of these cases were in the RAEB-2 and AML categories and, for the most part, consisted of cases which were close to the 20% blast diagnostic threshold for AML. Further analysis of these cases showed that either insufficient elevation or slight overcorrection of the blast percentage failed to appropriately reclassify these cases when compared to the diagnosis established by the 500-cell manual differential.
Figure 6.
Comparison of bone marrow diagnosis before and after applying BMP calculation. When classified according to the blast percentage determined by manual differential, the majority of cases either appropriately remained in the same diagnostic category (48.2%) or were correctly reclassified (26.8%) following adjustment via the BMP calculation.
Discussion
AML and MDS represent a complex group of hematopoietic malignancies that require bone marrow evaluation as well as flow cytometric immunophenotyping for accurate diagnosis and monitoring. In conjunction with clinical and molecular genetic data, the classification of myeloid disorders is highly reliant on bone marrow blast quantification, where stepwise thresholds have been established to delineate these disorders and stratify prognosis [2]. While imperfect, manual differential of the bone marrow aspirate is considered the gold standard in ascertaining the blast count and typically consists of evaluating a direct smear preparation of the bone marrow particle. Although technique plays a role, hemodilution inevitably occurs during the aspiration process. In fact, Holdrinet et al. demonstrated that approximately 97% of the measured hemoglobin in the aspirate specimen is derived from the peripheral blood using 51Cr-labeled red blood cells and 125I-labeled albumin [5]. Peripheral blood contamination is therefore a factor affecting the blast count determined by manual differential as well; however, in well-prepared smears, evaluation of cells near the spicule may help minimize this artifact.
Flow cytometric analysis is typically used as an ancillary tool in the evaluation of myeloid neoplasms to primarily establish the immunophenotypic profile of the blast population. Less frequently, the FCM study may additionally be necessary to quantify blasts, especially in circumstances when morphologic evaluation is difficult to interpret or not possible. The reference laboratory setting, in particular, sets up a challenging situation as the bone marrow aspirate may not be available for correlation, thus creating the potential for misleading information should the FCM-determined blast count differ significantly from that of the aspirate differential. Recognizing this potential diagnostic role in the evaluation of myeloid disorders and as a quality assurance measure, quantifying the degree of hemodilution in bone marrow aspirate material is an important consideration in the flow cytometry laboratory. With these objectives in mind, our study set out to establish a practical means of estimating bone marrow purity and normalizes blast counts in the setting of AML and MDS, using automated data derived only from the peripheral blood analysis and flow cytometry study.
Although there is presently no consensus on how to address hemodilution of bone marrow specimens [19,20], a number of investigators have studied this phenomenon, primarily in normal bone marrows. Early studies in this field used DNA S-phase analysis to differentiate immature (dividing) cells from mature (non-dividing) elements in the bone marrow aspirate [6,7]. This technique estimated the proportion of lymphocytes and monocytes, primarily derived from the peripheral blood, and thus provided a means of estimating hemodilution and correcting bone marrow cell counts [8]. Unfortunately, this methodology may not be available or would be cumbersome for many clinical laboratories to perform and, in these studies, was only reliable when the amount of peripheral blood contamination was < 30%.
A similar approach, but instead using phenotypic data, was performed by Loken et al. in which the expression pattern of CD16 was used to estimate proportions of mature and immature granulocytes [10]. CD16 shows dim expression in immature granulocytes and characteristically shows bright expression in mature neutrophils, providing a straightforward means of differentiating these elements. In a series of FCM experiments conducted on normal patients, the average percentage of dim CD16 expression in the bone marrow was 80%, allowing for the development of a simple equation to normalize the bone marrow cell counts for hemodilution. In contrast, the current study sought to assess BMP in disease states where phenotypic profiles are inherently abnormal. As anticipated, erroneous blast percentages were obtained in a significant number of cases normalized by the Loken equation in our group of AML and MDS patients due to aberrant CD16 expression within the granulocyte population (data not shown).
Although the literature is limited, correcting for bone marrow hemodilution in abnormal hematopoiesis, specifically myelodysplasia, was addressed in an elegant study by Lee et al. in which a “partial nucleated differential count” was used to essentially eliminate cellular elements contributed by the peripheral blood [9]. Affer establishing a reference range, this methodology was applied to 66 bone marrow aspirates with MDS. As expected, a significant number of cases showed reclassification to a higher grade disorder. Although the focus of our study was to normalize the FCM count to that determined by the aspirate differential, our results similarly showed reclassification of disease in approximately one-fourth of patients with MDS affer correction for hemodilution. However, the advantage of normalizing the FCM results, as in our study, is that it can be performed in the absence of the aspirate smear evaluation but will increase the confidence in the blast count derived from the manual differential, the accepted standard for disease classification. In any case, reducing the effects of hemodilution is an important step in improving the accuracy of diagnosis and facilitating the appropriate level of treatment for these patients.
In the development of our BMP formula, we initially studied eight patients and found an inverse relationship between the numbers of lymphocytes present and degree of BMP. These data suggested that lymphocytes, primarily derived from the peripheral blood, correlated with hemodilution and could therefore be used as a surrogate means to estimate the proportions of pure bone marrow and peripheral blood constituents in the specimen submitted for flow cytometry. An additional advantage to utilizing lymphocytes as a major component in the BMP calculation is the relatively consistent and reliable identification of this cell type by automated methods, preserving the practical application of the formula. Moreover, as the morphologic, phenotypic and light scatter characteristics of lymphocytes are essentially unaffected in myeloid disorders, isolation and quantification of these cells by flow cytometry is unhindered as compared to other bone marrow elements.
Finally, the utility of the BMP formula was validated in series of patients with AML and MDS by comparing the normalized FCM results to the manual differential blast counts. While not all cases were successfully reclassified, the corrected blast percentages decreased the disparity and were reasonably close to the manual differential in most cases to definitively improve the quality of the flow cytometry data. To this end, further in-depth analysis of the 56 cases of MDS or AML which demonstrated acceptable BMP levels, was performed. An average bias of approximately 15% was noted whereby the predicted blast count underestimated the actual blast percentage, suggesting an overestimation in the BMP prediction. Despite the relative accuracy of the equation at lower blast percentages (< 20% blasts) and lack of clinical significance at higher blast percentages (≥20% blasts), this issue was addressed by attempting to add a correction factor to the equation. Following this modification, differences between actual and predicted blast percentages did, in fact, decrease especially in the higher blast percentage cases. However, this adjustment was at the expense of overcorrecting four cases in the lower blast percentage group, resulting in incorrectly upgrading the diagnoses of 4 patients (one RAEB-1, two RAEB-2 & one AML); a single case was appropriately upgraded to AML using this modified formula (data not shown). Considering these outcomes, a correction factor was not included in the final BMP formula as the clinical implications of over-diagnosing myeloid neoplasms outweigh the apparent improvement in overall mathematical accuracy.
The formula developed in our study showed limitation at the extremes of hemodilution, where small variations in lymphocyte numbers, being either extremely high or low, resulted in significant changes in the BMP percentage. Although the mathematical reliability of the blast correction was diminished in these situations, the cases with low BMP clearly showed evidence of hemodilution, demonstrated by the significant underestimation of blasts determined by the FCM study. Conversely, specimens with extremely high BMP, typically cases with high blast counts that already met criteria for AML, demonstrated erroneous results when attempting to normalize the blast count. This limitation is of little clinical significance as the high BMP precludes the need for blast normalization in these cases. Finally, while the focus of this study was to assess BMP and normalize blast counts in myeloid disorders, this methodology would not be suitable in the evaluation of lymphoid malignancies.
Conclusion
Peripheral blood contamination frequently results in inaccurate quantification of blasts during the FCM analysis of bone marrow specimens. This phenomenon is of greatest concern in the evaluation of high-grade myeloid neoplasms where these dilutional effects may underestimate the severity and classification of the patient's disease. Based on the results of this study, BMP may be calculated with relative ease and accuracy by using automated total leukocyte and lymphocyte counts derived solely from the peripheral blood CBC and flow cytometric data. Therefore, inclusion of peripheral blood dilution/BMP data in flow cytometry reporting is an appropriate quality assurance measure in cases where blast percentage is critical. This is especially applicable in the reference laboratory setting where correlation with other components of the bone marrow evaluation may not be available. In the evaluation of AML and MDS, BMP assessment and blast normalization by this simple method improves the quality of the data that ultimately contributes to accurate diagnosis and optimal patient management.
Acknowledgment
The authors would like to thank David Howell, M.D. Ph.D., for his assistance with the data analysis. This study was, in part, supported by National Institutes of Health (NHLBI) R01 HL056888 (LFG), P30 GM103488 (LFG), WV CTR-IDEA NIH 1U54 GM104942, the Alexander B. Osborn Hematopoietic Malignancy and Transplantation Program of the MBRCC, and the WV Research Trust Fund.
Abbreviations
- AML
Acute Myeloid Leukemia
- BMP
Bone Marrow Purity
- CBC
Complete Blood Count
- FCM
Flow Cytometry
- MDS
Myelodysplastic syndromes
References
- 1.Craig FE, Foon KA. Flow Cytometric Immunophenotyping for Hematologic Neoplasms. Blood. 2008;111:3941–3967. doi: 10.1182/blood-2007-11-120535. [DOI] [PubMed] [Google Scholar]
- 2.Swerdllow S, Campo E, Harris NL, Pileri Stefano, Stein Harald, Jaffe Elaine S. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th edn. IARC Press; France: 2008. [Google Scholar]
- 3.Vladimirskaya EB. Peripheral Blood Admixture in Bone Marrow Aspirates. Folia Haematol Int Mag Klin Morphol Blutforsch. 1987;114:542–548. [PubMed] [Google Scholar]
- 4.Brooimans R, Kraan J, Van Putten W, Cornelissen J, Löwenberg B, Gratama J. Flow Cytometric Differential of Leukocyte Populations in Normal Bone Marrow: Influence of Peripheral Blood Contamination 1. Cytometry Part B: Clinical Cytometry. 2009;76:18–26. doi: 10.1002/cyto.b.20439. [DOI] [PubMed] [Google Scholar]
- 5.Holdrinet RS, von Egmond J, Wessels JM, Haanen C. A Method for Quantification of Peripheral Blood Admixture in Bone Marrow Aspirates. Exp Hematol. 1980;8:103–107. [PubMed] [Google Scholar]
- 6.Colly LP, Peters WG, Hermans J, Arentsen-Honders W, Willemze R. Percentage of S-phase Cells in Bone Marrow Aspirates, Biopsy Specimens and Bone Marrow Aspirates Corrected for Blood Dilution from Patients with Acute Leukemia. Leuk Res. 1987;11:209–213. doi: 10.1016/0145-2126(87)90044-0. [DOI] [PubMed] [Google Scholar]
- 7.Abrahamsen JF, Lund Johansen F, Laerum OD, Schem BC, Sletvold O, Smaaland R. Flow Cytometric Assessment of Peripheral Blood Contamination and Proliferative Activity of Human Bone Marrow Cell Populations. Cytometry. 1995;19:77–85. doi: 10.1002/cyto.990190110. [DOI] [PubMed] [Google Scholar]
- 8.Abrahamsen JF, Smaaland R, Skjærven R, Laerum OD, Schem BC, Sletvold O, et al. Flow Cytometric Measurement of DNA S phase in Human Bone Marrow Cells: Correcting for Peripheral Blood Contamination. Eur J Haematol. 1996;56:138–147. doi: 10.1111/j.1600-0609.1996.tb01332.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Ho S, Thomas D, Giri P, Lee H, Sia H, et al. A Partial Nucleated Differential Cell Count of the Bone Marrow Aspirate that is Independent of Peripheral Blood Dilution. International Journal of Laboratory Hematology. 2008;30:473–479. doi: 10.1111/j.1751-553X.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- 10.Loken MR, Chu S, Fritschle W, Kalnoski M, Wells DA. Normalization of Bone Marrow Aspirates for Hemodilution in Flow Cytometric Analyses. Cytometry Part B: Clinical Cytometry. 2009;76:27–36. doi: 10.1002/cyto.b.20429. [DOI] [PubMed] [Google Scholar]
- 11.Ossenkoppele GJ, Loosdrecht Van De, Arjan A, Schuurhuis GJ. Review of the Relevance of Aberrant Antigen Expression by Flow Cytometry in Myeloid Neoplasms. Br J Haematol. 2011;153:421–436. doi: 10.1111/j.1365-2141.2011.08595.x. [DOI] [PubMed] [Google Scholar]
- 12.Wongprajun S, Auewarakul CU. A Method Comparison Study of Flow Cytometry and Cytomorphology to Determine the Percentages of Blasts in Patients with Acute Leukemia after Induction and Consolidation Chemotherapy. J Med Association Thai. 2010;93:157–164. [PubMed] [Google Scholar]
- 13.d'Onofrio G, Zini G. Analysis of Bone Marrow Aspiration Fluid Using Automated Blood Cell Counters. ClinLab Med. 2015;35:25–42. doi: 10.1016/j.cll.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Loosdrecht Van De, Arjan A, Ireland R, Kern W, Della Porta MG, Alhan C, et al. Rationale for the Clinical Application of Flow Cytometry in Patients with Myelodysplastic Syndromes: Position Paper of an International Consortium and the European LeukemiaNet Working Group. Leuk Lymphoma. 2013;54:472–475. doi: 10.3109/10428194.2012.718341. [DOI] [PubMed] [Google Scholar]
- 15.Garcia Manero G. Myelodysplastic Syndromes: 2014 Update on Diagnosis, Risk Stratification, and Management. Am J Hematol. 2014;89:97–108. doi: 10.1002/ajh.23642. [DOI] [PubMed] [Google Scholar]
- 16.Porwit A, Loosdrecht Van De, Bettelheim P, Brodersen LE, Burbury K, Cremers E, et al. Revisiting Guidelines for Integration of Flow Cytometry Results in the WHO Classification of Myelodysplastic Syndromes—Proposal from the International/European LeukemiaNet Working Group for Flow Cytometry in MDS. Leukemia. 2014;28:1793–1798. doi: 10.1038/leu.2014.191. [DOI] [PubMed] [Google Scholar]
- 17.Roug AS, Hansen MC, Nederby L, Hokland P. Diagnosing and Following Adult Patients with Acute Myeloid Leukemia in the Genomic Age. Br J Haematol. 2014;167:162–176. doi: 10.1111/bjh.13048. [DOI] [PubMed] [Google Scholar]
- 18.Lacombe F, Durrieu F, Briais A, Dumain P, Belloc F, Bascans E, et al. Flow Cytometry CD45 Gating for Immunophenotyping of Acute Myeloid Leukemia. Leukemia. 1997;11:1878–1886. doi: 10.1038/sj.leu.2400847. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz Argüelles A, Rivadeneyra Espinoza L, Duque RE, Orfao A. Report on the Second Latin American Consensus Conference for Flow Cytometric Immunophenotyping of Hematological Malignancies. Cytometry Part B: Clinical Cytometry. 2006;70:39–44. doi: 10.1002/cyto.b.20083. [DOI] [PubMed] [Google Scholar]
- 20.Orfao A, Ortuno F, de Santiago M, Lopez A, San Miguel J. Immunophenotyping of Acute Leukemias and Myelodysplastic Syndromes. Cytometry Part A. 2004;58:62–71. doi: 10.1002/cyto.a.10104. [DOI] [PubMed] [Google Scholar]