Abstract
Cytosolic lipid storage droplets are primary functional organelles that regulate cellular lipid metabolism and homeostasis. Paradoxically, excess lipid stores are linked to both adaptive (fasting and chronic exercise) and mal-adaptive (obesity and related health complications) conditions. Thus, collective metabolic and physiological processes must balance lipid storage and utilization with prevention of lipocytotoxicity and compounding tissue dysfunctions, urging the need to further define the connection of mammalian lipid droplet function and lipid homeostasis. The perilipins are a multi-protein family that targets lipid droplet surfaces and regulates lipid storage and hydrolysis. Study of perilipin functions has provided insight into the physiological roles of cytosolic lipid droplets and their relationship with obesity-related pathologies. Here, we review the current knowledge of the multiple perilipin proteins in regulating tissue-specific lipid droplets and associations with tissue and systemic energetics.
Keywords: cytosolic lipid droplets, ectopic fat, metabolic disease, lipotoxicity, energy homeostasis
1. Complex relationships among CLDs, obesity, and health; more than meet the eyes
The worldwide pandemic of obesity has serious health consequences, including increased risks for hypertension, insulin resistance, diabetes, and coronary heart disease, and constitutes serious challenges to both biomedical research and treatment [1]. A general consensus is that we become “fat" because our intake of energy (food) exceeds our caloric expenditure. Our bodies first adapt to this chronic state of positive energy, using the unique high capability of white adipose tissue (WAT) to store surplus energy as neutral lipids [e.g. triacylglycerols (TAG)] in large unilocular cytosolic lipid droplets (CLDs). With time, and for reasons not yet fully determined, adipose tissue becomes limited in its lipid storage capacity. Lipids “spill over” to non-adipose tissue, such as skeletal and heart muscles, liver, and pancreas, which increase its own CLD number and size, to entrap these excess lipids, commonly termed “ectopic fat”. Chronic excess lipid flux in non-adipose tissues is thought to cause and/or potentate tissue insulin resistance, lipotoxicity, and eventually tissue dysfunction [2]. The pathophysiological consequences of deficient or ill-distributed adipose lipid storage and the importance of adipose CLD function to maintain systemic glucose and lipid homeostasis are best illustrated in lipodystrophies, which are often associated with monogenic mutations that affect adipose CLD growth and function [3].
While the presence of ectopic fat is highly correlated with insulin resistance, dyslipidemia, diabetes type 2 (T2D), and cardiovascular diseases, the relationships among ectopic fat and insulin resistance in skeletal and cardiac muscles and liver are complex, and indeed puzzling [4–6]. Skeletal muscle from exercised-trained subjects can display high insulin sensitivity, despite intramuscular TAG levels that exceed those of obese and diabetic individuals, a phenomenon described as the “athlete paradox” [4,5]. This dissociation between insulin resistance and accumulated CLDs is not restricted to muscle tissue, but is also described for liver, as evidenced by several studies in mouse models and humans [6].
Genetic manipulations that promote muscle TAG storage through enhanced esterification of fatty acids (FA) or inhibition of TAG hydrolysis protect muscle to insulin resistance. Transgenic mice with skeletal muscle over-expression of diacylglycerol acyltransferase-1 (DGAT1), an enzyme that catalyzes the last step in TAG synthesis, replicate the “athlete paradox” [7]. Although these mice have comparable levels of intramuscular CLDs observed in mouse models with fat-induced insulin resistance, they are insulin sensitive, with reduced levels of DAG and ceramides and increased FA oxidation [7]. Inhibition of TAG hydrolysis may also improve systemic insulin sensitivity; mice lacking ATGL (adipose tissue triglyceride lipase), a key ubiquitous lipolytic enzyme, are insulin sensitive, although exhibiting an ectopic fat phenotype [8] and other pathologies (see below).
While CLD content in non-adipose tissues is a reliable marker of altered lipid homeostasis, it does not indicate if dysfunction is in lipid storage or utilization. Non-adipose lipotoxicity and lipodystrophy are not due to the mere presence of TAG, but to a defect in CLD function(s), urging more focused studies for fuller mechanistic understandings.
2. Cytosolic lipid droplets: warehouses of fuel, signaling molecules, and lipid “detox” units
The CLD compartment is an essential storage organelle for multifaceted functions of lipid homeostasis [9]. CLD biogenesis is a fundamental and evolutionary conserved cellular function. Most cells have the ability to store free FA and sterols in the chemical form of lipid esters (neutral lipids) in CLDs. As storage depots, CLDs are sources of essential substrates for energy metabolism, membranes, signaling molecules, and steroid hormones. The diverse developmental- and cell-specific lipid requirements indicate that CLD compartments are highly dynamic and must interact closely with other cellular compartments, balancing TAG storage (e.g. ER) and utilization (e.g. mitochondria, endosomes) [10–12].
However, CLDs are also protective from lipotoxicity and serve to sequester (buffer) excess cytoplasmic FA or cholesterol to suppress their damaging effects on cellular function. By their biochemical nature, FA and cholesterol can incorporate in membrane bilayers and other cellular compartments, modifying membrane fluidity and charge and affecting key functions such as transport and receptor signaling. FAs and their metabolites can also trigger tissue insulin resistance and stimulate reactive oxygen species (ROS), which can induce inflammatory response and apoptosis. Still the connection of CLD storage to cytoprotection is not clear. The TAG synthetic pathway, and not the ATGL-mediated hydrolytic pathway, may be a source for the 1,2 diacylglycerols (1,2 DAGs) that activate atypical protein kinase C (aPKC), which is mechanistically linked to the development of tissue insulin resistance [13]. Such studies highlight the significance of a “lipid detoxification” role for CLDs by cycling potential “toxic” 1,2 DAGs into non-signaling 1,3 and 2,3 DAG isomers.
CLD function is dependent upon gene expression that is induced by the several nuclear transcription factors of the PPAR family [14–17], and, in turn, PPARs are activated by ligands produced through the catabolic functions of the CLDs [18–21], a feedforward loop. In addition, PPARs drive expression of FA oxidative genes. Thus, CLDs may play a significant protective role against lipid induced cytotoxicity, by regulating PPAR nuclear activities that promote an increased FA flux thru the mitochondrial oxidative pathway [22]. Strikingly, patients with defective ATGL function suffer myopathy and cardiomyopathy, illustrating the importance of CLD hydrolytic regulation and the essential interplay between TAG storage and FA availability for tissue-specific energetic function and adaptive cytoprotection [23]. Further, while mice lacking ATGL exhibit cardiac steatosis, severe cardiomyopathy, and premature death, treatment with exogenous PPARα ligands can reverse the cardiac steatosis, indicating a critical contribution of cardiac CLD hydrolysis for production of lipid moieties for nuclear signaling [19], apart from energy supply.
3. Perilipins, major CLD coat proteins
CLDs are comprised of a neutral lipid [TAG and/or cholesteryl ester (CE)] core surrounded by a single phospholipid/protein layer. The CLD proteome has been extensively studied across organisms and cell types. Studies performed in vertebrates and flies identified a proteome “signature” for CLDs that consistently includes at least one member of the perilipin multi-protein family [24,25]. Perilipin (as derived from Greek peri lipos, meaning surrounding lipid) proteins are defined by N-terminal sequence similarity within and across species [26,27] and quantitatively represent the most abundant signature of the CLD machinery. The mammalian genome encodes five perilipin (Plin) genes, and additional mRNA splice variants, with individual tissue-dependent expression patterns [26,27].
Perilipin 1 (Plin1) is expressed in WAT, brown adipose tissue (BAT), and steroidogenic tissue. Perilipin 2 (Plin2; previously ADRP, adipophilin) and perilipin 3 (Plin3; previously TIP47) are ubiquitously expressed. Perilipin 4 (Plin4; previously S3–12) is highly expressed in adipocytes, and perilipin 5 (Plin5; previously LSDP5, OXPAT, MLDP, PAT1) is expressed in oxidative tissue including heart, liver, BAT, and skeletal muscle [28]. Plins can differ in their preferential sub-cellular patterns [29]. Plin1 and Plin2 mostly localize to CLDs [24], whereas Plins3,4,5 may also be cytosolic or ER enriched [30,31]. Finally, Plins display differential targeting to TAG or CE cargos [32]. Thus, the Plins may regulate distinct metabolic pathways and control CLD tissue-specific adaptation to lipid utilization. Here, we review studies on recent mouse models toward understanding CLD association with tissue energetics and their impact on systemic glucose and lipid homeostasis (Table 1, Figure 1).
Table 1.
Main Phenotypes of Existing Genetic Mouse Models of the Perilipins
| Gene | Mouse Genetic Manipulation |
Phenotypes, Compared to WT controls |
Refs. |
|---|---|---|---|
| Plin1 | plin1−/− | Severe decrease in WAT mass, but no change in body weight (BW); Increased basal adipose lipolysis, but attenuated stimulated lipolysis; Increased β-oxidation; Mild hypertriglyceridemia and insulin resistance with aging. |
(33,34) |
|
plin1−/− fed a high-fat diet (HFD) |
Resistance to obesity. | (33,34) | |
|
plin1−/− in db/db (leptin- receptor deficient) |
Decreased WAT mass and BW; Improved insulin sensitivity. |
(33) | |
| Adipose-specific Plin1 over-expression on HFD |
Increased whole-body energy expenditure; Increased WAT browning; Resistance to obesity and improved insulin sensitivity. |
(53) | |
| Plin2 | plin2Δ2,3/Δ2,3 | Reduced hepatic CLDs (hepatic steatosis); Increased VLDL secretion; No change in adipogenesis; No change in fasting serum insulin, glucose, and lipids. |
(62) |
|
plin2Δ2,3/Δ2,3 in ob/ob (leptin deficient) |
Reduced hepatic steatosis; Improved insulin sensitivity. |
(65) | |
| Plin2Δ5/Δ5 on HFD | Reduced hepatic steatosis; Resistant to obesity; Increased WAT browning; Decreased food consumption and increased physical activity. |
(63) | |
| ASO on HFD or in ob/ob |
Reduced hepatic steatosis; Improved insulin sensitivity; Decreased VLDL secretion; Decreased hypertriglyceridemia; Decreased WAT mass, |
(66,67) | |
| Liver-specific Plin2 over-expression |
Increased hepatic steatosis; Increased systemic insulin sensitivity; No change in BW. |
(69) | |
| Plin3 | ASO on HFD | Reduced hepatic steatosis; Improved insulin sensitivity; Improved glucose tolerance; Improved liver, adipose, and muscle insulin resistance; Decreased VLDL secretion; Decreased WAT mass; Decreased hypertriglyceridemia. |
(82) |
| Plin4 | plin4−/− | No change in systemic glucose and insulin; Decreased cardiac CLDs; No change in adipogenesis or WAT mass. |
(84) |
| Plin5 | plin5−/− | Decreased cardiac CLDs; Increased cardiac β- oxidation and ROS, and dysfunction with aging; No change in systemic glucose and insulin. |
(77) |
| Cardiac-specific Plin5 over-expression |
Severe cardiac steatosis; Mild mitochondrial dysfunction; Increased ROS; Increased Nrf2 signaling; Cardiac hypertrophia, but no cardiac dysfunction. |
(78,79) |
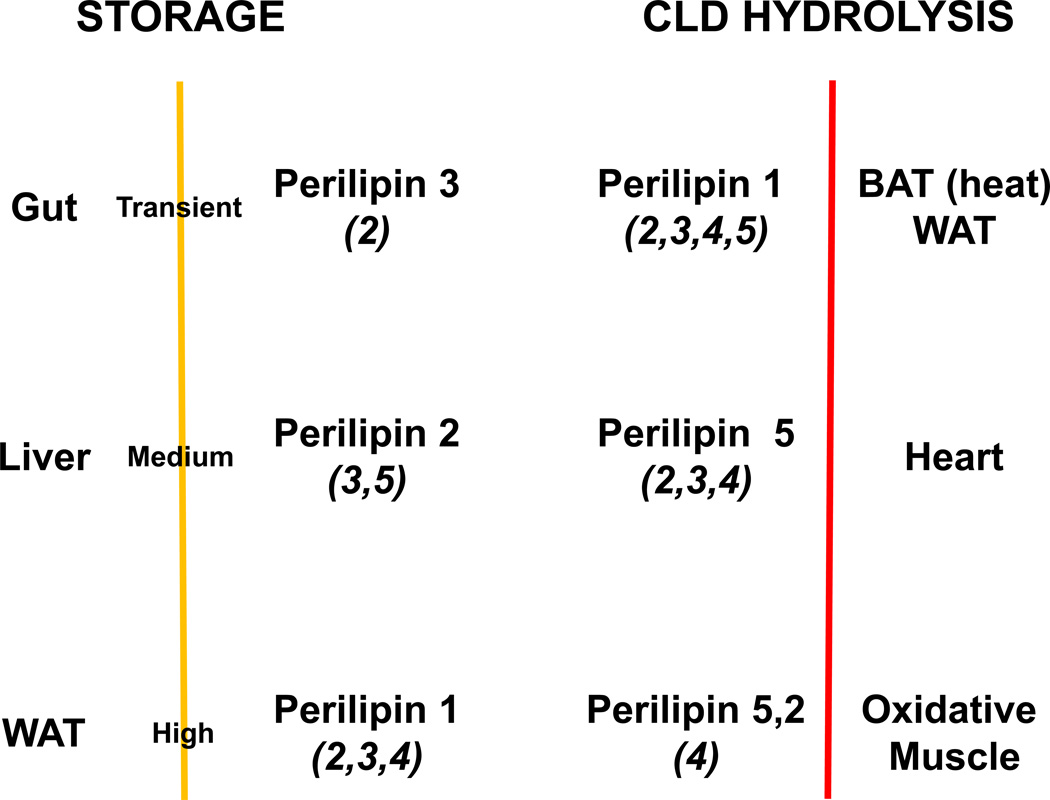
Figure 1. The lipid droplet coat is adapted to cellular and systemic energy needs.
Storage: Dietary lipids are absorbed in the gut, where Plin3 (and Plin2) may play a significant function. Plin3 coats CLDs of the small intestine, an organ that balances transitional storage and maximal transport of lipids for circulation. Perilipin 2 coats the CLDs of liver, an organ with the second greatest capacity for lipid storage. Plin1 coats the CLDs of the “professional” lipid storage unit, the adipose cell. Most abundant (and additional) perilipins for each tissue are indicated.
CLD Hydrolysis: BAT has thermogenic activity; WAT releases energy for whole-body function. Plin5 coats CLDs in energy-requiring, oxidative muscles. Most abundant (and additional) perilipins for each tissue are indicated.
3.1. Perilipin 1, a WAT CLD coat protein with demonstrated functions in systemic glucose and lipid homeostasis
Studies of Plin1, the founding member of the perilipin family [25], in WAT were the first to indicate a regulatory function involving global Plin function in lipid storage and hydrolysis [33,34]. Under fed or high circulating insulin conditions, WAT has low lipolytic activity. Protein kinase A (PKA) activation, via β-adrenergic stimulation, raises lipolytic rates ~50x, with Plin1 regulating substrate/CLD access of adipose lipolytic enzymes to coordinate TAG and DAG hydrolysis [35].
Under basal lipolytic conditions, lipases ATGL and HSL (hormone sensitive lipase) are cytosolic, sequestered from their lipid substrates [36–40]. In contrast, CGI-58 (comparative gene identification-58; ABHD5), the causative factor for Chanarin-Dorfman lipodystophy syndrome and a co-activator of ATGL, is localized at the CLD in association with unphosphorylated Plin1 [41,42], the major CLD coat protein of WAT. Plin1 also interacts with an A-kinase anchor protein (AKAP) to tether and regulate PKA type 1 and type 2 subunits at the CLD surface [43]. Upon β-adrenergic stimulation, Plin1 and HSL are phosphorylated by PKA, and the CLD scaffold structure is re-organized. pHSL binds pPlin1 at the CLD surface [35–40,44,45], CGI-58 dissociates from pPlin1 and recruits ATGL to the CLD, and lipolysis is activated [46]. Thus, unphosphorylated Plin1 serves a barrier to lipases, whereas pPlin1 participates in their recruitment; basal lipolytic rates in WAT of plin1−/− mice are elevated compared to WT, but absolute lipolytic activity in stimulated plin1−/− adipocytes was less than in WT, an indication that Plin1 is also required for maximal lipolytic activity [34].
Plin1 interacts with other proteins to balance lipid storage and hydrolysis. Cav-1 (caveolin 1), a component of caveolae, is highly enriched in adipose cells and is proposed to regulate FA trafficking and accumulation in WAT [47,48]; CIDE-C/Fsp27 can promote CLD growth via lipid transfer and exchange among CLDs [49–52]. Cav-1 and CIDE-C interaction with Plin1 on CLDs may facilitate FA flux through CLDs and be crucial to protect the adipose cell against lipotoxicity [47,48,51–53].
Adaptive signaling during adipose CLD hydrolysis can trigger other protections to lipocytotoxicity. AMPK (AMP-dependent protein kinase) is activated in response to acute FA release that is mediated by Plin1 phosphorylation. Increased AMPK activity may sustain FA-oxidative functions of mitochondria to suppress ROS production [54]. Loss of function mutations of Plin1 and CIDE-C and specific over-expression of ATGL in WAT lead to a chronic increase in adipose CLD hydrolysis and a concurrent adaptive response that enhances mitochondrial biogenesis and β-oxidation, possibly via enhanced production of PPAR ligands [55,56]. By contrast, the genetic loss of HSL in mice can reduce adipocyte differentiation and maturation [57,58]. Thus, CLD hydrolysis may stimulate the accumulation of lipid mediators that signal to cytosolic and nuclear compartments, promoting adaptive and compensatory effects on lipid storage or disposal [59].
The importance of Plin1 to regulate adipose LD stores, the largest mammalian lipid storage of the body, is highlighted by the serious metabolic consequences of Plin1 absence in mice and deficiency in humans [33,34,55,60]. The plin1−/− mice have very reduced WAT stores and enhanced ectopic fat, and develop insulin resistance with aging [33,34,55]. Humans, heterozygote for a truncated form of Plin1, display partial lipodystrophy, severe dyslipidemia, and insulin-resistant diabetes [60]. Thus, Plin1 is a central regulator of adipose CLD hydrolysis, adipose tissue function, and consequent systemic glucose and lipid homeostasis.
3.2. Perilipins 2–5, protective CLD functions in non-adipose tissues
Results from Plin1 studies further suggest that all of the Plins may have a primary function to regulate CLD/lipase access. Thus, lipolytic rates in cells expressing either Plin1 or Plin2 have a distinct hierarchy [61]. Basal cells expressing Plin1 are less active than Plin2-expressing cells, which are less active than stimulated Plin1-cells; lipolysis in Plin2-cells is unchanged by PKA-activation [61]. It was suggested that CLDs coated with unphosphorylated Plin1 is more protective to lipases than those with Plin2, which is more protective than with pPlin1. These conclusions are additionally supported by comparative studies of lipolysis in plin1+/+ (WT) and plin1−/− WAT. Plin2 is the major CLD coat protein of plin1−/− WAT and, accordingly, plin1−/− adipocytes have elevated basal lipolytic rates compared to WT,but decreased rates of stimulated lipolysis [34]. Two targeted Plin2 mutation models [62,63] have been characterized, which support the basic tenet that Plin2 can protect CLDs to hydrolysis.
In one [62], exons 2 and 3 of Plin2 were deleted (plin2Δ2,3/Δ2,3); full-length Plin2 protein was absent in these mice, but a large C-terminal variant was expressed in some (non-hepatic) tissues, through an alternative AUG translational start [64,65]. In response to starvation or a short (4 week) high-fat diet, the plin2Δ2,3/Δ2,3 mice have decreased hepatic CLD content, but normal adipose tissue growth and metabolism [62]. When maintained on a high-fat diet for a longer duration, plin2Δ2,3/Δ2,3 mice gain less weight than their WT littermates [63]. Absence of Plin2 also confers protection to genetic obesity; PlinΔ2,3/Δ2,3 mice lacking leptin (ob−/−) have improved systemic glucose and lipid homeostasis and reduced hepatic steatosis compared to leptin-deficient controls [65].
A second Plin2 targeted-mutant mouse was deleted of exon 5 (plin2Δ5/Δ5) and absence of Plin2 expression was confirmed [63]. In response to a prolonged (8–12 week) high-fat diet, these mice were also protected to CLD accumulation and chronic inflammation in liver, but also in WAT. Careful examination of subcutaneous WAT revealed an increased presence of brown-type adipose cells and expression of uncoupling protein 1 (UCP1), which dissipates oxidative energy as heat in contrast to ATP synthesis. Resistance to the high-fat diet in plin2Δ5/Δ5 mice was attributed partly to decreased food intake and increased physical activity, not simply to increased metabolic rates and “browning” in subcutaneous adipose tissues [63].
Plin2 has also been successfully depleted in adult mice, by treatment with antisense oligonucleotides (ASO). A highly similar phenotype was observed to that of plin2Δ5/Δ5 mice, although changes in lipid homeostasis were more attributed to differences in metabolic rate [66,67].
Plin2 is the most abundant perilipin in liver, and the collective data clearly indicate a significant in vivo role for Plin2 in hepatic lipid sequestration [62,63,65–67]. Indeed, Plin2 is shown to reduce the association of ATGL with CLDs [68], and, thus, overexpression of Plin2 will enhance CLD accumulation in hepatic and other cells [69]. In the absence of Plin2, hepatic CLDs are coated with Plin3 and Plin5, which together seem less permissive to accumulate ectopic lipid in hepatic cells.
Despite the extreme differences in hepatic lipid levels with Plin2 deficiency or Plin2 over-expression, neither condition increases lipotoxicity or insulin resistance in response to a high-fat diet [62,63,69]. While this appears a seemingly contradictory relationship, both situations may involve adaptive cytoprotection. Plin2 excess may promote sequestration of excess bioactive lipids in CLDs, whereas the absence of Plin2 may enhance FFA utilization and flux via mitochondria. A recent study shows that an S251P missense polymorphism in Plin2 was associated with reduced plasma TAG in humans, hinting that Plin2 may be an important regulator of human systemic lipid homeostasis [70]. Since, Plin2 has near ubiquitous tissue expression, its impact on overall energy homeostasis likely involves multiple tissue-specific functions, crosstalk, and signaling.
Although Plin2 limits ATGL binding to CLDs, Plin5 binds ATGL, as well as, CGI-58 [71–74]. The enhanced accumulation of Plin5 on CLDs in liver cells of plin2−/− mice, may partly explain protection to hepatic steatosis. In addition, Plin5 has a unique ability to tether mitochondria and CLDs [75,76], perhaps optimizing oxidative efficiency to buffer excess FA. Plin5 may offer an important protective feedback mechanism at the CLD surface to regulate ATGL-mediated hydrolysis and a futile TAG/FA cycle in plin2−/− hepatocytes, and other cells.
Nonetheless, Plin5 has a protective role to CLDs in other tissues. plin5−/− mice have decreased cardiac CLD/TAG content, elevated lipolysis, activated PPARα-dependent gene expression, and enhanced mitochondrial β-oxidation [77]. Still, while plin5−/− mice are protected from cardiac steatosis, they exhibit age-related cardiac dysfunction, which is prevented by treatment with antioxidants [77]. Absence of Plin5 is not associated with changes in systemic lipid and glucose homeostasis in mice consuming low fat, chow diets [77].
Cardiac specific over-expression of Plin5 has certain cardiac phenotypes that are opposite to plin5−/− mice [78,79], including pronounced cardiac steatosis and decreased PPARα-regulated gene expression and β-oxidation, but without impaired cardiac function. Cardiac over-expression of Plin5 also strongly activates the Nrf2 anti-oxidative pathway and increases expression of gluthatione enzymes [78]. The precise relationships among Plin5, PPARα, and Nrf2 signaling pathways remained to be defined. Plin5 mouse models will be valuable to understand the impact of Plin5 in other oxidative tissues and on lipid homeostasis, under obesity, exercise, and cold challenge.
Similar to Plin2, Plin3 is very widely distributed, although Plin3 is most predominant in the mouse small intestine [80]. The small intestine is the primary site of dietary lipid absorption in mammals and Plin3 expression is highly increased in response to an acute bolus of dietary fat. In cell culture experiments, Plin3 seems to have the least ability of the perilipin family to protect CLDs against the action of lipases [81]. One might speculate that by facilitating CLD hydrolysis at the small intestine, Plin3 could allow efficient packaging and transport of dietary lipids. However, Plin3 function is more complex. Plin3 accumulates in hepatic cells during a high-fat diet and, perhaps surprisingly, although Plin2 is the major CLD species in these cells, depletion of Plin3 by ASO suppresses hepatic steatosis [82], as is observed in plin2−/− mice [62,63].
Plin4 [25–27,83] was first identified in adipocytes, is induced by PPARγ during adipogenesis, and has stronger affinity for CE-enriched CLDs than for TAG [32]. Plin4 is structurally the most diverged of the perilipins, with a very highly expanded 11-mer repeat region, which may facilitate interaction with CLDs. Plin 4 expression is limited to WAT, heart, and skeletal muscle [84]. Surprisingly, loss of Plin4 did not lead to perturbation in adipocyte differentiation or related metabolism [84]. By contrast, loss of Plin4 was associated with decreased cardiac Plin5 expression and reduced cardiac CLDs [84]. Since Plin4 and Plin5 are linked genes, further studies will determine the more global functions of Plin4 and if the physical targeting of Plin4 had an unexpected impact on transcriptional action at the Plin5 locus.
4. Perspectives
Results from cell culture, mouse models, and human studies indicate that the primary function of the collective Plin protein family is to sequester lipids into CLDs by protection to neutral lipase action. This may be achieved by orchestrating relative lipase activities at the CLDs, via protein/protein interactions or substrate competition to channel lipid flux. Exquisite regulation of energy and substrate release also suppresses lipocytotoxicity. Intriguingly, although all five Plin proteins are suggested to share an ability to inhibit the action of the same set of CLD lipases [85], their actions seem to involve very different mechanisms. The tissue-specific distributions of the different Plin variants may reflect the relative degree toward either lipid storage or utilization (Figure 1, Table 1). The rapidity of phosphorylation/dephosphorylation in WAT, facilitates Plin1-dependent energy storage, as well as, efficient energy release, but the mechanisms for Plin2–5 inhibition of CLD hydrolysis remain to be firmly established.
A thorough understanding of Plin functions may help resolve the conundrum involving obesity, insulin resistant conditions, ectopic fat, and lipocytotoxicity. An uncoupled balance of CLD lipid storage and FA utilization may direct signals that decrease insulin sensitivity. The protective function of the Plins on CLDs in WAT and critical non-adipose tissues, such as liver, skeletal and cardiac muscles, and pancreas, impact global energy homeostasis and metabolic disease.
Highlights.
CLDs are storage depots for energy, signaling molecules, and lipid “detox” units.
Lipid droplet coat perilipins (Plins) regulate lipid stores and hydrolysis.
The Plins are adapted for tissue-specific needs and systemic lipid homeostasis.
Plin 1 main function is to regulate lipase access to CLDs of WAT and BAT adipocytes
Plin 2–5 regulate lipid stores in non-adipose tissues, protect against lipotoxicity
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases (A.R.K.), career development award 1-05-CD-17 from the American Diabetes Association (to C.S.), a grant from NIH 1RO1 DK 075017 (to C.S.), grant in aid 11GRNT7600027 from the American Heart Association (to C.S.), and the Geriatric Research, Education and Clinical Center, Baltimore Veterans Affairs Health Care Center, the Clinical Nutrition Research Unit of Maryland (DK072488).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Carole Sztalryd, Email: csztalry@grecc.umaryland.edu.
Alan R. Kimmel, Email: alank@helix.nih.gov.
REFERENCES
- 1.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012;70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boden G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011;2:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vigouroux C, Caron-Debarle M, Le Dour C, Magré J, Capeau J. Molecular mechanisms of human lipodystrophies: from adipocyte lipid droplet to oxidative stress and lipotoxicity. Int. J. Biochem. Cell Biol. 2011;43:862–876. doi: 10.1016/j.biocel.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Coen PM, Goodpaster BH. Role of intramyocelluar lipids in human health. Trends Endocrinol. Metab. 2012;8:391–398. doi: 10.1016/j.tem.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muoio DM. Intramuscular triacylglycerol and insulin resistance: guilty as charged or wrongly accused? Biochim. Biophys. Acta. 2010;1801:281–288. doi: 10.1016/j.bbalip.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Z, Lazar MA. Dissociating fatty liver and diabetes. Trends Endocrinol. Metab. 2013;24:4–12. doi: 10.1016/j.tem.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Shi X, Choi CS, Shulman GI, Klaus K, Nair KS, Schwartz GJ, Zhang Y, Goldberg IJ, Yu YH. Paradoxical coupling of triglyceride synthesis and fatty acid oxidation in skeletal muscle overexpressing DGAT1. Diabetes. 2009;58:2516–2524. doi: 10.2337/db08-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoy AJ, Bruce CR, Turpin SM, Morris AJ, Febbraio MA, Watt MJ. Adipose triglyceride lipase-null mice are resistant to high-fat diet-induced insulin resistance despite reduced energy expenditure and ectopic lipid accumulation. Endocrinology. 2011;152:48–58. doi: 10.1210/en.2010-0661. [DOI] [PubMed] [Google Scholar]
- 9.Walther TC, Farese RV., Jr. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy DJ. The dynamic roles of intracellular lipid droplets: from archaea to mammals. Protoplasma. 2012;249:541–585. doi: 10.1007/s00709-011-0329-7. [DOI] [PubMed] [Google Scholar]
- 11.Beller M, Sztalryd C, Southall N, Bell M, Jäckle H, Auld DS, Oliver B. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 2008;6:2530–2549. doi: 10.1371/journal.pbio.0060292. e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV, Jr., Guo Y. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eichmann TO, Kumari M, Haas JT, Farese RV, Jr., Zimmermann R, Lass A, Zechner R. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J. Biol. Chem. 2012;287:41446–41457. doi: 10.1074/jbc.M112.400416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalen KT, Schoonjans K, Ulven SM, Weedon-Fekjaer MS, Bentzen TG, Koutnikova H, Auwerx J, Nebb HI. Adipose tissue expression of the lipid droplet-associating proteins S3-12 and perilipin is controlled by peroxisome proliferator-activated receptor-gamma. Diabetes. 2004;53:1243–1252. doi: 10.2337/diabetes.53.5.1243. [DOI] [PubMed] [Google Scholar]
- 15.Kershaw EE, Schupp M, Guan HP, Gardner NP, Lazar MA, Flier JS. PPARgamma regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1736–E1745. doi: 10.1152/ajpendo.00122.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am. J. Physiol. Endocrinol. Metab. 2005;288:E1195–E1205. doi: 10.1152/ajpendo.00513.2004. [DOI] [PubMed] [Google Scholar]
- 17.Bindesbøll C, Berg O, Arntsen B, Nebb HI, Dalen KT. Fatty acids regulate perilipin5 in muscle by activating PPARd. J. Lipid Res. 2013 doi: 10.1194/jlr.M038992. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mottillo EP, Bloch AE, Leff T. J.G. Granneman Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) a and d in brown adipocytes to match fatty acid oxidation with supply. J. Biol. Chem. 2012;287:25038–25048. doi: 10.1074/jbc.M112.374041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haemmerle G, Moustafa T, Woelkart G, Büttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, Schreiber R, Eichmann T, Kolb D, Kotzbeck P, Schweiger M, Kumari M, Eder S, Schoiswohl G, Wongsiriroj N, Pollak NM, Radner FP, Preiss-Landl K, Kolbe T, Rülicke T, Pieske B, Trauner M, Lass A, Zimmermann R, Hoefler G, Cinti S, Kershaw EE, Schrauwen P, Madeo F, Mayer B, Zechner R. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-a and PGC-1. Nat. Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sapiro JM, Mashek MT, Greenberg AS. D.G. Mashek. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J. Lipid Res. 2009;50:1621–1629. doi: 10.1194/jlr.M800614-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgiadi A, Kersten S. Mechanisms of gene regulation by fatty acids. Adv. Nutr. 2012;3:127–134. doi: 10.3945/an.111.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am. J. Physiol. Endocrinol. Metab. 2009;297:E289–E296. doi: 10.1152/ajpendo.00099.2009. [DOI] [PubMed] [Google Scholar]
- 24.Londos C, Sztalryd C, Tansey JT, Kimmel AR. Role of PAT proteins in lipid metabolism. Biochimie. 2005;87:45–49. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J. Lipid Res. 2010;51:468–471. doi: 10.1194/jlr.R000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu X, Gruia-Gray J, Copeland NG, Gilbert DJ, Jenkins NA, Londos C C, Kimmel AR. The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm. Genome. 2001;12:741–749. doi: 10.1007/s00335-01-2055-5. [DOI] [PubMed] [Google Scholar]
- 27.Miura S, Gan JW, Brzostowski J, Parisi MJ, Schultz CJ, Londos C, Oliver B, Kimmel AR. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and, Dictyostelium . J. Biol. Chem. 2002;277:32253–32257. doi: 10.1074/jbc.M204410200. [DOI] [PubMed] [Google Scholar]
- 28.Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim. Biophys. Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 30.Skinner JR, Shew TM, Schwartz DM, Tzekov A, Lepus CM, Abumrad NA, Wolins NE. Diacylglycerol enrichment of endoplasmic reticulum or lipid droplets recruits perilipin 3/TIP47 during lipid storage and mobilization. J. Biol. Chem. 2009;284:30941–30948. doi: 10.1074/jbc.M109.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartholomew SR, Bell EH, Summerfield T, Newman LC, Miller EL, B Patterson, Niday ZP, 4th Ackerman WE, Tansey JT. Distinct cellular pools of perilipin 5 point to roles in lipid trafficking. Biochim. Biophys. Acta. 2012;1821:268–278. doi: 10.1016/j.bbalip.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh K, Lee YK, Londos C, Raaka BM, Dalen KT, Kimmel AR. Perilipin family members preferentially sequester to either triacylglycerol-specific or cholesteryl-ester-specific intracellular lipid storage droplets. J. Cell Sci. 2012;125:4067–4076. doi: 10.1242/jcs.104943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, Quast MJ, Gorenstein D, Chen KH, Chan L. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat. Genet. 2000;4:474–479. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- 34.Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viswanadha S, Londos C. Determination of lipolysis in isolated primary adipocytes. Methods Mol. Biol. 2008;456:299–306. doi: 10.1007/978-1-59745-245-8_22. [DOI] [PubMed] [Google Scholar]
- 36.Egan JJ, Greenberg AS, Chang MK, Wek SA, Moos MC, Jr., Londos C. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc. Natl. Acad. Sci. U. S. A. 1992;89:8537–8541. doi: 10.1073/pnas.89.18.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brasaemle DL, Levin DM, Adler-Wailes DC, Londos C. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim. Biophys. Acta. 2000;1483:251–262. doi: 10.1016/s1388-1981(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 38.Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J. Cell Biol. 2003;161:1093–1103. doi: 10.1083/jcb.200210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su CL, Sztalryd C, Contreras JA, Holm C, Kimmel AR, Londos C. Mutational analysis of the hormone-sensitive lipase translocation reaction in adipocytes. J. Biol. Chem. 2003;278:43615–43619. doi: 10.1074/jbc.M301809200. [DOI] [PubMed] [Google Scholar]
- 40.Miyoshi H, 2nd Perfield JW, Souza SC, Shen WJ, Zhang HH, Stancheva ZS, Kraemer FB, Obin MS. A.S. Greenberg. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J. Biol. Chem. 2007;282:996–1002. doi: 10.1074/jbc.M605770200. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi T, Omatsu N, Matsushita S, Osumi T. CGI-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. J. Biol. Chem. 2004;279:30490–30497. doi: 10.1074/jbc.M403920200. [DOI] [PubMed] [Google Scholar]
- 42.Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, Brasaemle DL. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J. Biol. Chem. 2004;279:42062–42071. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 43.Pidoux G, Witczak O, Jarnæss E, Myrvold L, Urlaub H, Stokka AJ, Küntziger T, Taskén K. Optic atrophy 1 is an A-kinase anchoring protein on lipid droplets that mediates adrenergic control of lipolysis. EMBO J. 2011;30:4371–4386. doi: 10.1038/emboj.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyoshi H, Souza SC, Zhang HH, Strissel KJ, Christoffolete MA, Kovsan J, Rudich A, Kraemer FB, Bianco AC, Obin MS, Greenberg AS. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J. Biol. Chem. 2006;281:15837–15844. doi: 10.1074/jbc.M601097200. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Hu L, Dalen K, Dorward H, Marcinkiewicz A, Russell D, Gong D, Londos C, Yamaguchi T, Holm C, Rizzo MA, Brasaemle D, Sztalryd C. Activation of hormone-sensitive lipase requires two steps, protein phosphorylation and binding to the PAT-1 domain of lipid droplet coat proteins. J. Biol. Chem. 2009;284:32116–32125. doi: 10.1074/jbc.M109.006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J. Biol. Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen AW, Razani B, Schubert W, Williams TM, Wang XB, Iyengar P, Brasaemle DL, Scherer PE, Lisanti MP. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 2004;53:1261–1270. doi: 10.2337/diabetes.53.5.1261. [DOI] [PubMed] [Google Scholar]
- 48.Storey SM, McIntosh AL, Senthivinayagam S, Moon KC, Atshaves BP. The phospholipid monolayer associated with perilipin-enriched lipid droplets is a highly organized rigid membrane structure. Am. J. Physiol. Endocrinol. Metab. 2011;301:E991–E1003. doi: 10.1152/ajpendo.00109.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong J, Sun Z, Wu L, Xu W, Schieber N, Xu D, Shui G, Yang H, Parton RG, Li P. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol. 2011;195:953–963. doi: 10.1083/jcb.201104142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puri V, Konda S, Ranjit S, Aouadi M, Chawla A, Chouinard M, Chakladar A, Czech MP, Fat-specific protein 27. a novel lipid droplet protein that enhances triglyceride storage. J. Biol. Chem. 2007;282:34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 51.Grahn TH, Zhang Y, Lee MJ, Sommer AG, Mostoslavsky G, Fried SK, Greenberg AS, Puri V. FSP27 and PLIN1 interaction promotes the formation of large lipid droplets in human adipocytes. Biochem. Biophys. Res. Commun. 2013;432:296–301. doi: 10.1016/j.bbrc.2013.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Z, Gong J, Wu H, Xu W, Wu L, Xu D, Gao J, Wu JW, Yang H, Yang M, Li P. Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Nat. Commun. 2013;4:1594. doi: 10.1038/ncomms2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyoshi H, Souza SC, Endo M, Sawada T, 2nd Perfield JW, Shimizu C, Stancheva Z, Nagai S, Strissel KJ, Yoshioka N, Obin MS, Koike T, Greenberg AS. Perilipin overexpression in mice protects against diet-induced obesity. J. Lipid Res. 2010;51:975–982. doi: 10.1194/jlr.M002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J. Biol. Chem. 2008;283:16514–16524. doi: 10.1074/jbc.M708177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castro-Chavez VK, Yechoor PK, Saha J, Martinez-Botas EC, Wooten S, Sharma P, O'Connell H, Taegtmeyer L, Chan Coordinated upregulation of oxidative pathways and downregulation of lipid biosynthesis underlie obesity resistance in perilipin knockout mice: a microarray gene expression profile. Diabetes. 2003;52:2666–2674. doi: 10.2337/diabetes.52.11.2666. [DOI] [PubMed] [Google Scholar]
- 56.Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, Inoue K, Kitazawa R, Kitazawa S, Matsuki Y, Hiramatsu R, Masubuchi S, Omachi A, Kimura K, Saito M, Amo T, Ohta S, Yamaguchi T, Osumi T, Cheng J, Fujimoto T, Nakao H, Nakao K, Aiba A, Okamura H, Fushiki T, Kasuga M. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J. Clin. Invest. 2008;118:2808–2821. doi: 10.1172/JCI34090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen WJ, Yu Z, Patel S, Jue D, Liu LF, Kraemer FB. Hormone-sensitive lipase modulates adipose metabolism through PPARγ. Biochim. Biophys. Acta. 2011;1811:9–16. doi: 10.1016/j.bbalip.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ström K, Gundersen TE, Hansson. O, Lucas S, Fernandez C, Blomhoff R, Holm C. Hormone-sensitive lipase (HSL) is also a retinyl ester hydrolase: evidence from mice lacking HSL. F.A.S.E.B. J. 2009;7:2307–2316. doi: 10.1096/fj.08-120923. [DOI] [PubMed] [Google Scholar]
- 59.Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gandotra FS, Le Dour C, Bottomley W, Cervera P, Giral P, Reznik Y, Charpentier G, Auclair M, Delépine M, Barroso I, Semple RK, Lathrop M, Lascols O, Capeau J, O’Rahilly S, Magré J, Savage DB, Vigouroux C. Perilipin deficiency and autosomal dominant partial lipodystrophy. N. Engl. J. Med. 2011;364:740–748. doi: 10.1056/NEJMoa1007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tansey JT, Huml AM, Vogt R, Davis KE, Jones JM, Fraser KA, Brasaemle DL, Kimmel AR, Londos C. Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. J. Biol. Chem. 2003;278:8401–8406. doi: 10.1074/jbc.M211005200. [DOI] [PubMed] [Google Scholar]
- 62.Chang BH, Li L, Paul A, Taniguchi S, Nannegari V, Heird WC, Chan L. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol. Cell. Biol. 2006;3:1063–1076. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McManaman JL, Bales ES, Orlicky DJ, Jackman M, MacLean PS, Cain S, Crunk AE, Mansur A, Graham CE, Bowman TA, Greenberg AS. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J. Lipid Res. 2013;54:1346–1359. doi: 10.1194/jlr.M035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russell TD, Schaack J, Orlicky DJ, Palmer C, Chang BH, Chan L, McManaman JL. Adipophilin regulates maturation of cytoplasmic lipid droplets and alveolae in differentiating mammary glands. J. Cell Sci. 2011;124:3247–3253. doi: 10.1242/jcs.082974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang BH, Li L, Saha P, Chan L. Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis, and improves whole body insulin resistance in leptin-deficient mice. J. Lipid Res. 2010;51:2132–2142. doi: 10.1194/jlr.M004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imai Y, Varela GM, Jackson MB, Graham MJ, Crooke RM, Ahima RS. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology. 2007;132:1947–1954. doi: 10.1053/j.gastro.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 67.Varela GM, Antwi DA, Dhir R, Yin X, Singhal NS, Graham MJ, Crooke RM, Ahima RS. Inhibition of ADRP prevents diet-induced insulin resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G621–G628. doi: 10.1152/ajpgi.90204.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, Brown WJ, DA Brown. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J. Lipid Res. 2007;48:2751–2761. doi: 10.1194/jlr.M700359-JLR200. [DOI] [PubMed] [Google Scholar]
- 69.Sun Z, Miller RA, Patel RT, Chen J, Dhir R, Wang H, Zhang D, Graham MJ, Unterman TG, Shulman GI, Sztalryd C, Bennett MJ, Ahima RS, Birnbaum MJ, Lazar MA. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat. Med. 2012;18:934–942. doi: 10.1038/nm.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Magné J, Aminoff A, Sundelin JP, Mannila MN, Gustafsson P, Hultenby K, Wernerson A, Bauer G, Listenberger L, Neville MJ, Karpe F, Borén J, Ehrenborg E. The minor allele of the missense polymorphism Ser251Pro in perilipin 2 (PLIN2) disrupts an α-helix, affects lipolysis, and is associated with reduced plasma triglyceride concentration in humans. F.A.S.E.B. J. 2013 doi: 10.1096/fj.13-228759. in press. [DOI] [PubMed] [Google Scholar]
- 71.Wang H, Bell M, Sreenivasan U, Hu H, Liu J, Dalen K, Londos C, Yamaguchi T, Rizzo MA, Coleman R, Gong D, Brasaemle D, Sztalryd C. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J. Biol. Chem. 2011;286:15707–15715. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Granneman JG, Moore HP, Mottillo EP, Zhu Z, Zhou L. Interactions of perilipin-5 (Plin5) with adipose triglyceride lipase. J. Biol. Chem. 2011;286:5126–5135. doi: 10.1074/jbc.M110.180711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Granneman JG, Moore HP, Mottillo EP, Zhu Z. Functional interactions between Mldp (LSDP5) and Abhd5 in the control of intracellular lipid accumulation. J. Biol. Chem. 2009;284:3049–3057. doi: 10.1074/jbc.M808251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacPherson RE, Ramos SV, Vandenboom R, Roy BD, Peters SJ. Skeletal muscle PLIN proteins, ATGL and CGI-58, interactions at rest and following stimulated contraction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304:R644–R650. doi: 10.1152/ajpregu.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H, Sreenevasan U, Hu H, Saladino A, Polster BM, Lund M, Gong DW, Stanley WC, Sztalryd C. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J. Lipid Res. 2011;52:2159–2168. doi: 10.1194/jlr.M017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bosma M, Minnaard R, Sparks LM, Schaart G, Losen M, de Baets MH, Duimel H, Kersten S, Bickel PE, Schrauwen P, Hesselink MK. The lipid droplet coat protein perilipin 5 also localizes to muscle mitochondria. Histochem. Cell Biol. 2012;137:205–216. doi: 10.1007/s00418-011-0888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuramoto K, Okamura T, Yamaguchi T, Nakamura TY, Wakabayashi S, Morinaga H, Nomura M, Yanase T, Otsu K, Usuda N, Matsumura S, Inoue K, Fushiki T, Kojima Y, Hashimoto T, Sakai F, Hirose F, Osumi T. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J. Biol. Chem. 2012;287:23852–23863. doi: 10.1074/jbc.M111.328708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H, Sreenivasan U, Gong DW, O’Connell KA, Dabkowski ER, Hecker PA, Ionica N, Konig M, Mahurkar A, Sun Y, Stanley WC, Sztalryd C. Cardiomyocyte-specific perilipin 5 overexpression leads to myocardial steatosis and modest cardiac dysfunction. J Lipid Res. 2013;54:953–965. doi: 10.1194/jlr.M032466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pollak NM, Schweiger M, Jaeger D, Kolb D, Kumari M, Schreiber R, Kolleritsch S, Markolin P, Grabner GF, Heier C, Zierler KA, Rülicke T, Zimmermann R, Lass A, Zechner R, Haemmerle G. Cardiac-specific overexpression of perilipin 5 provokes severe cardiac steatosis via the formation of a lipolytic barrier. J. Lipid Res. 2013;54:1092–1102. doi: 10.1194/jlr.M034710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee B, Zhu J, Wolins NE, Cheng JX, Buhman KK. Differential association of adipophilin and TIP47 proteins with cytoplasmic lipid droplets in mouse enterocytes during dietary fat absorption. Biochim. Biophys. Acta. 2009;1791:1173–1180. doi: 10.1016/j.bbalip.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Bell M, Wang H, Chen H, McLenithan JC, Gong DW, Yang RZ, Yu D, Fried SK, Quon MJ, Londos C, Sztalryd C. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 2008;57:2037–2045. doi: 10.2337/db07-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carr RM, Patel RT, Rao V, Dhir R, Graham MJ, Crooke RM, Ahima RS. Reduction of TIP47 improves hepatic steatosis and glucose homeostasis in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R996–R1003. doi: 10.1152/ajpregu.00177.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolins NE, Skinner JR, Schoenfish MJ, Tzekov A, Bensch KG, Bickel PE. Adipocyte protein S3–12 coats nascent lipid droplets. J. Biol. Chem. 2003;278:37713–37721. doi: 10.1074/jbc.M304025200. [DOI] [PubMed] [Google Scholar]
- 84.Chen W, Chang B, Wu X, Li L, Sleeman M, Chan L. Inactivation of Plin4 downregulates Plin5 and reduces cardiac lipid accumulation in mice. Am. J. Physiol. Endocrinol. Metab. 2013;304:E770–E779. doi: 10.1152/ajpendo.00523.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]