Abstract
Rationale
Regulator of G-protein signaling 4 (RGS4) is a brain-enriched negative modulator of G-protein-coupled receptor signaling. Decreased availability of RGS4 in the frontal cortex and striatum has been described in animal models of schizophrenia and drug addiction. However, cellular and behavioral consequences of dysregulated RGS4-dependent receptor signaling in the brain remain poorly understood.
Objective
To investigate whether RGS4, through inhibiting function of mGluR5 receptors in the dorsal striatum (dSTR), regulates cellular and behavioral responses to acute amphetamine.
Methods
After HSV-RGS4 was infused into dSTR, RGS4 overexpression as well as binding of recombinant RGS4 to mGluR5 were assessed. The effect of RGS4 overexpression on behavioral activity induced by the intra-striatal mGluR5 agonist, DHPG, or amphetamine was recorded. Activation of extracellular signal-regulated kinase (ERK) and Akt (protein kinase B) was measured in the dSTR tissue at the end of each behavioral experiment.
Results
RGS4 overexpressed in the dSTR co-immunoprecipitated with mGluR5 receptors and suppressed both behavioral activity, as well as phospho-ERK levels induced by DHPG. RGS4 over-expression or the mGluR5 antagonist, MTEP attenuated amphetamine-induced phospho-ERK (but not phospho-Akt) levels. RGS4 suppressed amphetamine–induced vertical activity and augmented horizontal activity over 90 min. Similarly, MTEP augmented amphetamine-induced horizontal activity but did not affect vertical activity.
Conclusions
The present data demonstrate that RGS4 in the dSTR attenuates amphetamine-induced ERK signaling and decreases the behavioral efficacy of acute amphetamine likely by limiting mGluR5 function.
Keywords: Akt, amphetamine, dorsal striatum, ERK, locomotor activity, mGluR5, overexpression, RGS4
Introduction
Regulator of G-protein signaling 4 (RGS4) is a member of the large family of RGS proteins that function as negative modulators of G-protein-coupled receptor (GPCR)-mediated signaling pathways (Siderovski and Willard 2005). All RGS proteins bind directly to the GTP-bound Gαi or Gαq subunit of activated heterotrimeric G-proteins and increase the rate of GTP hydrolysis. This accelerated “turn-off” of activated G-proteins provides a cellular mechanism for limiting temporal and spatial resolution of GPCR-signaling and GPCR-mediated synaptic plasticity by RGS proteins (Abramow-Newerly et al. 2006; Kimple et al. 2011). A growing body of evidence indicates that disruption of this regulatory mechanism is a critical component of pathophysiologies underlying various human diseases (Emilsson et al. 2006; Nishiguchi et al. 2004; Talkowski et al. 2006; Tekumalla et al. 2001).
RGS4 is a small RGS protein enriched in the brain, with highest levels of RGS4 protein present in the frontal cortex, dorsal striatum (dSTR), amygdala and thalamus (Gold et al. 1997). As such, dysregulation of RGS4 has been linked to several neuropsychiatric disorders including schizophrenia (Ding and Hegde 2009; Mirnics et al. 2001), Parkinson's (Ding et al. 2006; Zhang et al. 2005), Alzheimer disease (Emilsson et al. 2006) and drug addiction (Hooks et al. 2008). With regard to addiction, our laboratory as well as others have documented that acute exposure to psychostimulants or opiates results in rapid downregulation of RGS4 mRNA and protein levels, predominately in the striatum (Chase et al. 2010; Gonzalez-Nicolini and McGinty 2002; Schwendt et al. 2006; Yuferov et al. 2003). Furthermore, chronic exposure to both experimenter- and self-administered cocaine resulted in a lasting RGS4 decrease in the prefrontal cortex and striatum, followed by a rapid up-regulation of RGS4 levels as a result of cue-induced drug-seeking (Schwendt et al. 2007). This suggests that RGS4 can play a role in cellular adaptations underlying acute dopaminergic activity (hyperlocomotion) as well as enduring drug-induced behaviors (such as relapse to drug-seeking). However, the identity of GPCRs and signaling pathways affected by fluctuation of RGS4 levels remains obscure.
Although a number of receptors regulated by RGS4 have been identified in heterologous cell-lines (as reviewed by (Bansal et al. 2007), few studies have addressed receptor-specificity and the regulatory function of RGS4 in native neuronal cultures or directly in the brain. In one of those studies (Saugstad et al. 1998), it was demonstrated that RGS4 is a potent inhibitor of mGluR1/5-induced signaling in hippocampal neurons. In the striatum, which contains high concentrations of both RGS4 and mGluR5 (but not mGluR1) receptors, this functional interaction is likely to occur via direct physical association of RGS4 with the mGluR5 receptor signaling complex (Schwendt and McGinty 2007). mGluR5 receptors in the brain are coupled to downstream signaling pathways either through a conventional Gαq-to-phospholipase C, subtype β1 (PLCβ1) pathway or through a pathway utilizing Homer scaffolding proteins (Conn and Pin 1997; Ribeiro et al. 2010). While the conventional pathway leads to a release of calcium from intracellular stores and activation of mitogen-activated protein kinase (MAPK) signaling pathways, the Homer-dependent pathway is calcium-independent and leads to an activation of both MAPK and PI3K-Akt-mTOR pathways (Mao et al. 2005; Ronesi and Huber 2008). In agreement, local administration of the mGluR1/5 agonist, DHPG, into the dSTR dose-dependently increased phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 MAPK, that was blocked by pretreatment with an mGluR1/5 antagonist (Choe and Wang 2001). Activation of mGluR5 receptors in the striatum is also necessary for induction of ERK1/2 signaling by Amph (Choe et al. 2002) and it is likely that this signaling mechanism is also involved in regulating mGluR5-dependent synaptic plasticity after exposure to psychostimulants (Fourgeaud et al. 2004; Grueter et al. 2006). On the other hand, regulation of PI3K-Akt-mTOR signaling in the STR by mGluR5 receptors has not been investigated. In addition to cellular signaling, striatal mGluR5s can regulate behavior in experimental animals, as demonstrated by the finding that intra-striatal administration of DHPG induced complex locomotor and stereotypical behaviors (Wang and Mao 2000). However, despite a number of studies investigating the cellular and behavioral significance of striatal mGluR5 receptors, there are considerable gaps in our understanding of their role in psychostimulant addiction.
Therefore, in the present study, we hypothesized that RGS4 limits mGluR1/5 signaling in the dSTR and further, that the mGluR5-RGS4 functional relationship is manifested by altered behavioral responses to an mGluR5 agonist and/or to Amph. Since psychostimulants decrease the levels of RGS4 in the dSTR, we employed a technique of viral-mediated gene transfer to over-express RGS4 within the dSTR in order to study the effects of elevated RGS4 protein levels on cellular signaling and behavior.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (275-300 g) (Charles River Laboratories, Wilmington, MA, USA) were single-housed in clear plastic cages and maintained on a 12 h light/dark cycle with food and water available ad libitum. All animals were acclimated to their home cage environment for a minimum of 3 days prior to surgery and handled for an additional 5 days prior to drug administration to minimize effects of stress on behavioral and neurochemical parameters. Every animal procedure in this study was approved by the Institutional Animal Care and Use Committee and was performed in strict accordance with the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academy Press 1996).
Drugs
D-amphetamine sulfate (Amph) was purchased from Sigma-Aldrich (St. Louis, MO) or acquired from the NIDA Controlled Substances Program (Research Triangle Institute, NC). The mGluR1/5 agonist, (S)-3,5-DHPG, was purchased from Tocris Bioscience (Ellisville, Missouri) and the selective mGluR5 antagonist, MTEP hydrochloride, was purchased from Ascent Scientific (Bristol, UK). All drugs were freshly prepared on the day of the experiment. Amph was dissolved in physiological saline (Sal) and injected intraperitoneally (i.p.) in a volume of 1 ml/kg. For intrastriatal infusions, DHPG was first dissolved in 25% dimethylsulfoxide (DMSO) and then diluted in artificial cerebrospinal fluid (aCSF) (in mM: NaCl 123, CaCl2 0.86, KCl 3.0, MgCl2 0.89, NaH2PO4 0.50 and Na2HPO4 0.25, pH 7.4) to a final concentration of 125 or 250 nmol/μl. MTEP was dissolved in 1% Tween 80 in aCSF to a final concentration of 5μg/μl. DMSO in combination with aCSF or Tween80 in combination with aCSF was therefore used as vehicle control for the respective agents. Solutions of all drugs were neutralized to pH 7.2–7.4 with 1N NaOH, if necessary. The concentrations of the drugs used were determined based on the results of previously published studies (Choe and Wang 2001; Gass and Olive 2009; Gonzalez-Nicolini and McGinty 2002; Molina-Hernandez et al. 2006), as well as our preliminary data.
Stereotaxic surgery
On the day of surgery, rats were anesthetized with ketamine/xylazine (66 mg/kg and 1.33 mg/kg i.p.), followed by equithesin (0.5 ml/kg i.p.) and ketorolac (2.0 mg/kg, i.p.). Rats were mounted onto a stereotaxic device (Stoelting, Wood Dale, IL) and bilateral stainless steel guide cannulae precut (24-gauge, Plastics One, Roanoke, VA, USA) were implanted 2 mm above the dSTR infusion target (+1.2 mm antero-posterior, ±3.4 mm medio-lateral and −3.4 mm dorso-ventral relative to bregma according to Paxinos and Watson, 2007). Animals were allowed to recover for 5 days prior to the beginning of behavioral experiments.
HSV-Mediated Gene Transfer
Recombinant herpes simplex virus (HSV)-LacZ, and HSV-RGS4 constructs were gifts from Drs. Stephen Gold and David Self (UT Southwestern, Dallas, TX). They were generated in the laboratory of Dr. Rachel Neve (McGovern Institute for Brain Research, MIT, Cambridge, MA) and were previously described (Neve et al. 1997; Rahman et al. 2003). The average titer of the purified virus stocks was >108 infectious units/μl. Two microliters of HSV vectors were bilaterally infused into dSTR at the rate of 0.2 μl/min using a 33-gauge injector cannula inserted to a depth of 2 mm below the tip of the guide cannula. The injector was connected via polyethylene tubing to a 10 μl gas-tight Hamilton syringe mounted in an infusion pump (PHD 2000, Harvard Apparatus, Holliston, MA). The injector remained in place for 5 min after the infusion to prevent backflow along the cannula track. HSV-mediated transgene expression was confirmed by β-galactosidase stain for HSV-LacZ or by immunohistochemistry and immunoblot for the HSV-RGS4. All behavioral experiments were performed on day 3 after HSV injection, at the peak of HSV-driven transgene expression (Rahman et al. 2003).
Drug administration and locomotor activity
DHPG (125, 250 nmols), MTEP (5 μg), or the appropriate vehicle were bilaterally infused into dSTR (1 μl/side) through a 33-gauge injector over a period of 4 min using an infusion pump (as described in detail for HSV microinfusions). After the infusions, the injectors were left in place for an additional 1 min. Amph (2.5-3 mg/kg, i.p.) or Sal was administered to rats 15 min later. This dose of Amph has been shown to reliably stimulate horizontal and vertical activity in rats (Wang and McGinty 1995). To measure baseline and drug-induced behavioral activity, animals were placed in automated photocell beam activity chambers (Accuscan Instruments, Columbus, OH, USA) and horizontal (total distance traveled) as well as vertical activity (rearing) was recorded for 15 min - 3h as described previously (Schwendt et al. 2006). At the end of each behavioral experiment, whole brains or selected brain tissues were harvested for protein analysis as described below.
Immunohistochemistry
For the immunohistochemical analysis of HSV-driven LacZ or RGS4 expression, rats were anesthetized and perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS) (in mM: NaCl 137, KCl 2.7, Na2HPO4 4.3, KH2PO4 1.47, pH 7.4). Brains were removed and post-fixed for 4 h and saturated in 30% sucrose/PBS solution. After embedding and freezing, 30 μm sections were cut on a freezing microtome and used for histology (cannulae placements) or for confirmation of transgene expression. Detection of β-galactosidase expression (LacZ-staining) was performed as described previously (Neve et al. 1997). Viral over-expression of RGS4 was detected via immunohistochemistry using a specific antibody against FLAG-tagged RGS4, anti-FLAG M2 HRP-conjugated antibody (1:1000, Sigma,-Aldrich). The staining was visualized using VIP substrate (Vector Labs, Burlingame, CA). The sections were viewed and photographed with an Olympus microscope equipped with a digital camera.
Co-immunoprecipitation
For immunoprecipitation of FLAG-tagged RGS4, dSTR tissue was homogenized by a brief sonication followed by solubilization of striatal membrane proteins in a mild Triton-based lysis buffer (50 mM Tris HCl, pH 7.4, with 150 mM NaCl, 1 mM EDTA, 1% Triton X-100) containing the following inhibitors: Complete Mini protease inhibitor (Roche Diagnostics, Indianapolis, IN, USA), Halt phosphatase inhibitor (Pierce Chemical, Rockford, IL, USA) and 5 mM MG-132 proteasome inhibitor (Tocris). Insoluble proteins were sedimented at 15,000g for 10 min and supernatants (500 μg of protein at 1μg/μl concentration) were used for immunoprecipitation of FLAG-tagged RGS4 with anti-FLAG M2 agarose resin according to the manufacturer's instructions (Sigma-Aldrich). After a series of washes, immunocomplexes were dissociated from the beads under native conditions by a competition with a 3×FLAG peptide. Proteins were then resolved by SDS-PAGE and analyzed by immunoblotting as described below. Co-immunoprecipitation experiments included a positive control (immunoprecipitation of FLAG-BAP fusion protein; Sigma-Aldrich) as well as negative controls (omission of lysate, FLAG M2 beads or no over-expression of FLAG-tagged RGS4).
Immunoblotting
For immunoblotting analysis, 5 mm-thick forebrain coronal slices containing dSTR were collected using a Precision brain slicer (Braintree Scientific, Braintree, MA) and rapidly frozen in isopentane on dry ice. Frozen coronal slices were then trimmed in the cryostat to a 2 mm thickness corresponding to ∼ 2.7-0.7 mm anterior to bregma (Paxinos and Watson 2007) and the dSTR was bilaterally dissected using a 2 mm tissue micropuncher and stored at -80°C until processed. This approach allowed for monitoring of cannulae placement and potential tissue damage. Total tissue protein was extracted from dSTR by sonication in 1% SDS/PBS buffer with inhibitors (as described above), denatured for 10 min at 85°C and centrifuged for 10 min at 12,000g to pellet insoluble proteins. Protein concentrations were measured with the Micro-Bicinchoninic Acid assay kit (Pierce Chemical, Rockford, IL, USA). Equal protein amounts (15 μg) were separated by SDS-PAGE (4–15% polyacrylamide) and transferred onto polyvinylidene difluoride membranes. Membranes were blocked for 1 h in 5% milk/Tris-buffered saline and probed overnight at 4°C with a primary antibody diluted in 3 or 5% milk/Tris-buffered saline with 0.1% Tween 20. The following primary antisera were used: rabbit phospho-ERK (1:2500), rabbit tERK (1:15000), rabbit phospho-Akt-Thr308 (1:1000), rabbit phospho-Akt-Ser473 (1:2000), rabbit tAkt (1:7500); all from Cell Signalling Technology (Danvers, MA), rabbit RGS4 (1:5000), rabbit mGluR5 (1:5000; Millipore, Billerica, MA), mouse FLAG M2 (1:1000, Sigma-Aldrich), mouse β-galactosidase (1:2500, Promega, Madison, WI), mouse D1 receptor (1:250; Millipore), rabbit D2 receptor (1:1000; Abcam, Cambridge, MA) and calnexin (1:10000; Enzo Life Sciences, Farmingdale, NY). After the incubation with an appropriate HRP-conjugated secondary antiserum (Jackson Immuno Research, West Grove, PA), immunoreactive bands on the membranes were detected by ECL+ chemiluminescence reagents on an X-ray film (GE Healthcare, Piscataway, NJ). Equal loading and transfer of proteins were confirmed by stripping and re-probing of the same membranes either for proteins independent of their phosphorylation state (tERK, tAkt) or for calnexin, an intracellular protein that was not altered by any experimental treatment. The integrated band density of each protein sample was measured using Image J software (U. S. National Institutes of Health, Bethesda, Maryland).
Statistical Analysis
Results are expressed as Mean ± Standard Error of the Mean (S.E.M.). Behavioral data were analyzed by calculating the area under the curve (AUC) for the total distance traveled and vertical activity plotted against time starting at time zero. For the immunoblotting data, the ratios of phospho-to-total protein levels (ERK and Akt), or ratios of protein-to-calnexin levels are presented. Because of the less abundant levels of phospho-ERK1 in the dSTR and because p-ERK2 has been identified as the isoform that mediates the neurochemical and behavioral effects of psychostimulants (Girault et al. 2007), only the phospho-ERK2/tERK2 ratio was analyzed. Behavioral and immunoblotting data were evaluated using a one-way ANOVA followed by Student–Newman–Keuls (SNK) multiple comparison tests. Sigma Stat (Systat Software, Chicago, IL, USA) software was used for all statistical analyses.
Results
Infusion of HSV-RGS4 into the dSTR resulted in a robust over-expression of recombinant RGS4 protein and binding to mGluR5 receptors
In order to study the role of RGS4 in the dSTR in mGluR5- and psychostimulant-induced behaviors and signaling, we over-expressed the protein specifically in the dSTR using HSV vectors previously demonstrated to be effective for in vivo gene transfer (Carlezon et al. 2000; Han et al. 2010; Rahman et al. 2003). A single bilateral microinfusion of HSV-RGS4 or HSV-LacZ directed into dSTR (as depicted on Fig 1A, top panel) resulted in expression of FLAG-tagged RGS4 or β-galactosidase restricted to an area of ∼1-1.5 mm in diameter as detected 3 days post-infection by immunohistochemical staining (Fig 1A, middle panels). Microinfusion of HSV vectors was associated with minimal tissue damage, comparable to the damage seen after HSV vehicle (10% sucrose) as demonstrated by a Nissl stain of dSTR sections collected at the site of infusion (Fig 1A, lower panel). Over-expression of RGS4 was also confirmed by immunoblotting, again at 3 days post-infection (Fig.1B). Microinfusion of HSV-RGS4 (but not HSV-LacZ) resulted in a robust expression of FLAG-tagged RGS4 as detected by antibody against the FLAG tag as well as antibody against the endogenous RGS4 protein (Fig 1B). Anti-RGS4 antibody recognized both endogenous RGS4 and over-expressed FLAG-tagged RGS4 which has a higher molecular weight due to the presence of the FLAG tag.
Fig 1. Infusion of HSV-RGS4 into the dSTR results in over-expression of RGS4 protein as well as binding of recombinant RGS4 to mGluR5 receptors.
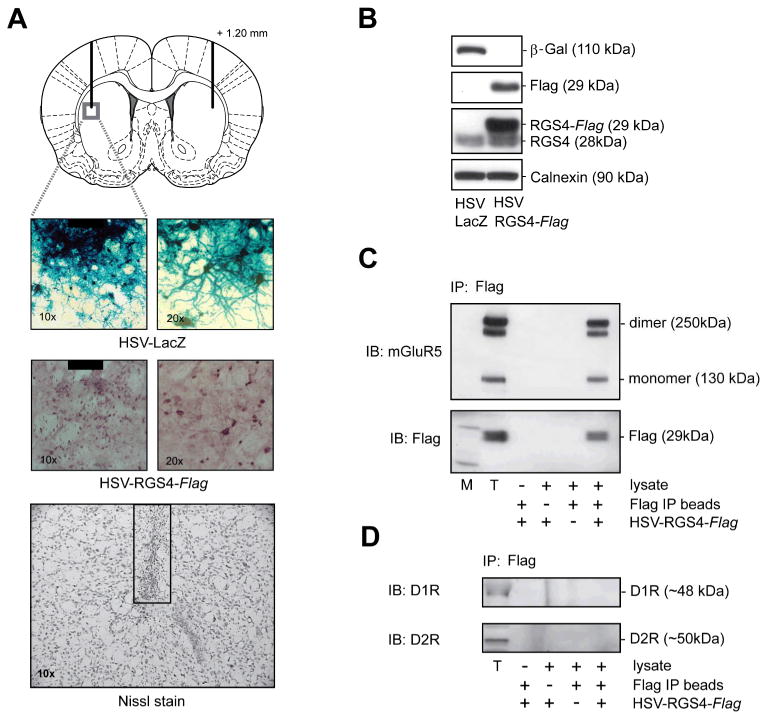
Animals received bilateral intra-dSTR infusion of HSV-RGS4-FLAG or a control vector HSV-LacZ (2μl/side) and brains were analyzed 3 days later. A, Upper panel: Outline of the rat brain coronal section depicting a representative placement of HSV infusions; adapted from Paxinos and Watson (2005). Lower panels: Immunohistochemical detection of RGS4 and β-galactosidase over-expression in the dSTR near the infusion site (black box represents a tip of the injector). Representative image of Nissl-stained striatal section showing no necrosis or abnormal cytoarchitecture in the proximity of the infusion site. B, Protein levels of β-galactosidase, RGS4, RGS4-FLAG and calnexin as measured by immunoblotting in tissue lysates prepared from the dSTR. C, FLAG-tagged RGS4 co-immunoprecipitates with mGluR5, but not with, dopamine D1 or D2 receptors in the dSTR (D). Immunoprecipitates (IP) were analyzed by immunoblotting (IB) using antibodies against FLAG-tag, mGluR5 or D1 and D2 receptors. T - total lysate used as an immunoprecipitation input. co-IP analysis included negative controls in which lysate, FLAG IP beads or RGS4-FLAG over-expression was omitted.
We have previously shown that endogenous RGS4 protein co-immunoprecipitates with mGluR5 receptors in rat dSTR (Schwendt and McGinty 2007). In order to assess the behavioral and neurochemical significance of this interaction, we first had to examine whether FLAG-tagged RGS4 over-expressed in the dSTR also interacts with mGluR5 receptors. To that effect, a specific anti-FLAG antibody was used to immunoprecipitate the RGS4-FLAG protein fraction from dSTR lysates obtained 3 days after HSV-RGS4-FLAG microinfusion. As illustrated in Fig. 1C (top panel), probing the immunoprecipitated sample (+++) and total (T) striatal lysates with anti-mGluR5 antibody revealed 130- and 250-kDa bands corresponding to monomer and dimer isoforms of mGluR5 receptors. In contrast, no bands were detected in negative control samples in which lysate or Flag-IP beads were omitted, as well as in immunoprecipitates from the dSTR with no RGS4-FLAG over-expression. To confirm the efficiency and specificity of immunoprecipitation, samples were also probed with antibody against the FLAG tag itself. As demonstrated in a middle panel (Fig.1C), FLAG signal was only detected in total dSTR lysate (T) and complete FLAG-tag immunoprecipitates (+++), but not in a series of negative controls. In addition to mGluR5, other Gαi- and Gαq-coupled GPCRs have been shown to interact with RGS4 protein when co-expressed in various cell lines (Jaen and Doupnik 2006; Ruiz de Azua et al. 2010; Wang et al. 2009). In this study, we tested the ability of FLAG-tagged RGS4 to interact with D1 and D2 dopamine receptors, which are both abundant in the STR and critical for psychostimulant-induced behaviors (Berke and Hyman 2000). However, co-immunoprecipitation experiments revealed no interaction of RGS4-FLAG with D1 or D2 receptors in dSTR lysates (Fig. 1D). These results suggest a specific interaction of over-expressed RGS4 with native mGluR5 (but not dopamine) receptors in the rat dSTR under these experimental conditions.
Over-expression of RGS4 in the dSTR attenuated mGluR5-dependent behavior and phospho-ERK signaling
In the next series of experiments, we sought to determine whether increasing levels of RGS4 in the dSTR interferes with mGluR5 function, assessed as a suppression of mGluR5-dependent behavioral activation and phosphoprotein signaling.
In agreement with previous observations (Choe and Wang 2001), intrastriatal infusion of DHPG (125 and 250 nmol) dose-dependently increased phospho-ERK2 levels 30 minutes post infusion (Fig. 2A left panel). A one-way ANOVA (F(2, 13) = 14.92, P < 0.001) followed by SNK multiple comparisons revealed not only significantly higher phospho-ERK2 levels in both DHPG-treated groups vs. the vehicle-treated group (p<0.01), but also a significant dose-dependent effect (DHPG250 vs. DHPG125, p<0.05). Similarly, there was a dose-dependent effect of DHPG on phospho-Akt-Thr308 levels in the dSTR (Fig.2A right panel). However in the latter case, DHPG induced a dose-dependent de-phosphorylation of Akt-Thr308 as demonstrated by one-way ANOVA (F(2, 13) = 12.85, P = 0.001) followed by SNK multiple comparison test (DHPG250 vs. DHPG125, p<0.05). Because phosphorylation of both Thr308 and Ser473 is thought to be required for full kinase activity (Alessi et al. 1996; Manning and Cantley 2007), the level of Akt phosphorylation at Ser 473 was also examined throughout this study. However, no changes in phospho-Akt-Ser473 were found among any groups in all the experiments (data not shown). It should be noted, that the two doses of DHPG used in this study did not alter total levels of ERK2 or Akt in the dSTR. In addition, microinfusion of 125 or 250 nmols DHPG had no effect on protein levels of endogenous RGS4 in the dSTR as measured 2 hrs post-infusion (Fig. 2B). This was not due to the inability of DHPG to induce mGluR5-dependent protein expression because 250 nmol significantly induced Arc protein levels in the dSTR (data not shown).
Fig.2. Intrastriatal infusion of mGluR5 agonist DHPG dose-dependently regulates phospho-ERK, and phospho-Akt-Thr308, but not RGS4 protein levels in the dSTR.

Animals received bilateral infusion of DHPG (125 or 250 nmol) and brain were analyzed 30 min or 2hr post-infusion. A, Levels of phospho-ERK and phospho-Akt-Thr308 in the dSTR as analyzed by immunoblotting 30 min after intrastriatal infusion of DHPG or vehicle. B, RGS4 protein levels in the dSTR as analyzed by immunoblotting 2h after intrastriatal infusion of DHPG or vehicle. Data are expressed as mean ± S.E.M. of phospho-ERK2/tERK2, phospho Akt/tAkt and RGS4/calnexin integrated density ratio (n=4-6 samples/group). *p<0.05 vs. Vehicle, **p<0.01 vs. Veh,ˆp<0.05 vs. DHPG 125 nmol dose.
In a subsequent study, the effect of RGS4 over-expression on DHPG-induced behavior and signaling was investigated. Rats received bilateral microinfusions of HSV-RGS4 into dSTR in order to over-express RGS4 or they were infused with HSV-LacZ. Three days later, 250 nmol DHPG (or vehicle) was delivered into dSTR and behavior was analyzed for the first 30 min after the infusion. As shown in Fig. 3A, DHPG-infused rats demonstrated a robust increase in locomotor activity (total distance traveled) compared to vehicle-infused rats 20-30 min post-infusion. Quantitative analysis of total distance traveled over the last 10 min of the test (20-30 min post-infusion) revealed a significant difference in AUC between treatment groups as detected by ANOVA (F(3,14) =19.60, P<0.01). Pairwise comparisons showed that in control animals over-expressing β-galactosidase in the dSTR, DHPG microinfusion induced a significant increase of total distance traveled (LacZ-DHPG vs. LacZ-Veh, p<0.01). Interestingly, behavioral activation by DHPG was blunted in animals over-expressing RGS4 in the dSTR (RGS4-DHPG vs. LacZ-DHPG, p<0.01). The effects of DHPG on vertical activity (rearing) were not significantly different in any treatment group (data not shown).
Fig. 3. Over-expression of RGS4 in the dSTR attenuates DHPG-induced behavior and ERK phosphorylation.
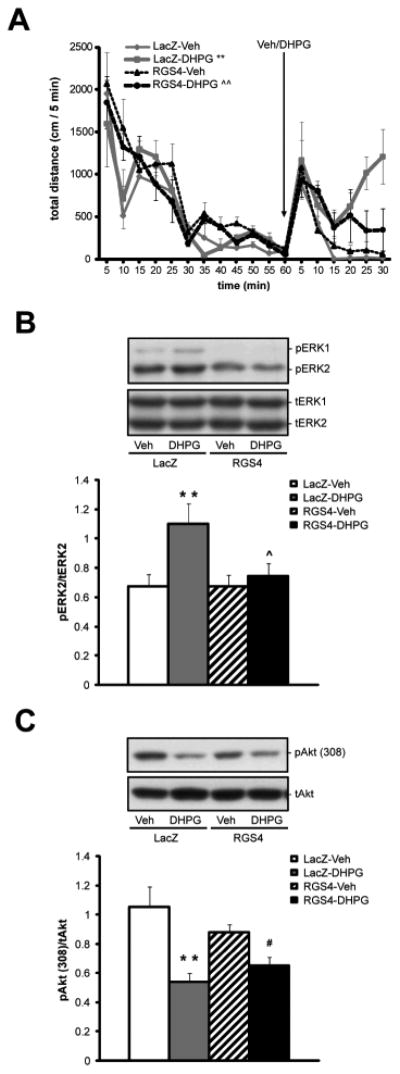
Animals received bilateral infusion of HSV-RGS4-FLAG or a control vector HSV-LacZ (2μl/side) followed by the bilateral infusion of either DHPG (250 nmol) or vehicle into dorsolateral STR 3 days later. A, Locomotor activity (total distance traveled) as recorded for 60 min pre- and 30 min post-infusion of DHPG. Mean total distance traveled (per 5 mins) ± S.E.M. plotted over time and analyzed as area under the curve (AUC). *p<0.05 vs. LacZ-Vehicle, ˆp<0.05 RGS4-DHPG vs. LacZ-DHPG. B and C, Immunoblotting analysis of phospho-ERK (B) and phospho-Akt-Thr308 (C) levels in the dSTR 30 min after the infusion of DHPG or Veh. Mean integrated density ratio ± S.E.M. of phospho-ERK2/tERK2 and phospho-Akt-Thr308/tAkt (n=4-5 samples/group). *p<0.05 vs. LacZ-Vehicle, **p<0.01 vs. LacZ-Vehicle, ˆp<0.05 vs. LacZ-DHPG, #p<0.05 vs. RGS4-Vehicle.
At the end of the experiment (30 min post Veh/DHPG microinfusion), the dSTR was harvested for phosphoprotein analysis (Fig. 3B and C). In accordance with the previous experiment, DHPG induced ERK phosphorylation, but to a different degree across treatment groups (Fig 3B, F(3,14) =7.84, P<0.01). SNK pairwise comparison tests revealed a significantly greater increase in phospho-ERK2 levels in the dSTR of LacZ-DHPG animals than in LacZ-Veh rats (p<0.01). In addition, RGS4 over-expression suppressed DHPG-induced phospho-ERK2 levels (RGS4-DHPG vs. LacZ-DHPG, p<0.01). On the other hand, DHPG-induced dephosphorylation of Akt-Thr308 was not prevented by RGS4 over-expression (Fig 3C). One-way ANOVA (F(3,14) =10.29, P<0.01) followed by SNK pairwise comparisons revealed that phospho-Akt-Thr308 levels were downregulated by DHPG regardless of β-galactosidase or RGS4 over-expression (LacZ-DHPG vs. LacZ-Veh, p<0.01 and RGS4-DHPG vs. RGS4-LacZ, p<0.05).
The effects of RGS4 over-expression in the dSTR on early-onset Amph-induced locomotion and phosphoprotein signaling: comparison to intra-dSTR mGluR5 receptor blockade
The preceding experiments suggested that RGS4 regulates mGluR5-mediated locomotor behavior and ERK phosphorylation in the dSTR. Follow-up experiments sought to determine whether RGS4-mGluR5 functional interactions play a role in psychostimulant-induced behaviors and protein phosphorylation in the dSTR. Therefore, the effects of RGS4 over-expression were compared to a site-specific blockade of mGluR5s in the dSTR after acute Amph exposure.
Animals received microinfusion of HSV-LacZ or HSV-RGS4 into dSTR and 3 days post infection, locomotor and phosphorylation responses to Amph were measured (Fig. 4). A single injection of Amph (2.5 mg/kg i.p.) induced significant locomotor hyperactivity (F(3,19) =4.57, P<0.05) in both LacZ-Amph as well as RGS4-Amph groups when compared to their saline counterparts (Fig.4A; LacZ-Amph vs. LacZ-Sal, p<0.05 and RGS4-AMPH vs RGS4-Sal, p<0.05). Phosphoprotein analysis revealed that Amph increased ERK2 phosphorylation as previously reported (Shi and McGinty 2007), although to a different degree across treatment groups (Fig.4B; F(3,20) = 6.64, P<0.05). There was a significant increase in phospho-ERK2 levels in the LacZ-Amph group (p<0.01, vs. LacZ-Sal), which was attenuated in animals over-expressing RGS4 (RGS4-Amph vs, LacZ-Amph, p<0.05; Fig 4B). Amph treatment also induced phospho-Akt-Thr308 levels (F(3,21) =4.24, P<0.05) as previously reported (Shi and McGinty 2007). SNK tests revealed that phospho-Akt-Thr308 levels were significantly greater in the LacZ-Amph group than in the LacZ-Sal group (p<0.05). RGS4 over-expression did not attenuate Amph-induced phosphorylation of Akt-Thr308 when compared to LacZ-Sal animals (p=0.4); nevertheless, it prevented the Amph-induced rise in phospho-Akt308 levels (RGS4-Amph vs. RGS4-Sal, p=0.21; Fig.4C).
Fig. 4. The effects of RGS4 (or blockade of mGluR5 receptors) on early onset Amph-induced behavior and phosphoprotein levels in the dlSTR.

Left column (A-C): Animals received bilateral infusion of HSV-RGS4-FLAG or a control vector HSV-LacZ (2μl/side). Three days later animals received Amph (2.5 mgs/kg, i.p) or saline injection and behavioral activity and phosphoprotein levels in the dlSTR were analyzed. A, Total distance traveled as recorded for 60 min pre- and 15 min post- Sal or Amph administration. Mean total distance traveled (per 5 mins) ± S.E.M. plotted over time and analyzed as area under the curve (AUC). *p<0.05 vs. LacZ-Sal, ˆp<0.05 RGS4-Amph vs. LacZ-Amph. B and C, Upper panels: Representative immunoblot images for each treatment group. Lower panels: Immunoblotting analysis of pERK2 and tERK2 (B), phospho-Akt-Thr308 and tAkt (C) levels in the dlSTR 20min after the injection Sal or Amph. Mean integrated density ratio ± S.E.M. of phospho-ERK2/tERK2 and phospho-Akt-Thr308/tAkt (n=5-7 samples/group). *p<0.05, **p<0.01 vs. LacZ-Sal, ˆp<0.01 vs. LacZ-Amph. Right column (D-F): Animals received bilateral infusion of Veh or MTEP (5μg/side). Fifteen minutes later animals received Amph (2.5 mgs/kg, i.p) or saline injection and behavioral activity and phosphoprotein levels in the dSTR were analyzed. D, Total distance traveled as recorded for 60 min pre- and 15 min post- Sal or Amph administration. Mean total distance traveled (per 5 mins) ± S.E.M. plotted over time and analyzed as area under the curve (AUC). E and F, Upper panels: Representative immunoblot images for each treatment group. Lower panels: Immunoblotting analysis of phospho-ERK2 and tERK2 (E), pAkt-Thr308 and tAkt (F) levels in the dlSTR 20min after the injection Sal or Amph. Mean integrated density ratio ± S.E.M. of pERK2/tERK2 and pAkt-Thr308/tAkt (n=5-7 samples/group). *p<0.05, *p<0.01 vs. Veh-Sal, ˆˆp<0.01 vs. Veh-Amph.
In the next experiment, the selective mGluR5 antagonist, MTEP, was bilaterally infused into dSTR 15 mins before Amph (2.5 mg/kg i.p.) or Sal injection. Amph induced significant locomotor hyperactivity (total distance traveled) when compared to Sal rats (t(21) =2.77, P<0.05) during the 15 min period following i.p. injections (Fig. 4D). However, no group-specific effects of Veh or MTEP pretreatment on Amph-induced locomotor activity emerged during this short time interval. As expected, acute Amph induced an increase in phospho-ERK2 levels (Fig 4E; F(3,19) =10.96, P<0.01). SNK multiple comparison tests showed that elevated phospho-ERK2 levels were present in the Veh-Amph group when compared to the Veh-Sal group (p<0.01) whereas phospho-ERK2 was significantly suppressed in the Amph group pretreated with MTEP (MTEP-Amph vs. Veh-Amph; p<0.01). Amph also induced phospho-Akt-Thr308 levels (F(3,19) =3.44, P<0.05), specifically in Veh-pretreated animals (SNK test: p<0.05 Veh-Sal vs. Veh-Amph). MTEP microinfusion showed a trend toward partial inhibition of Amph-induced phosphorylation of Akt-Thr308 (p=0.06, Fig.4F).
The effects of RGS4 over-expression in the dSTR or intra-dSTR mGluR5 receptor blockade on extended behavioral activity after acute Amph
The previous experiments suggested that RGS4 and mGluR5 may share a common mechanism in regulating Amph-induced phosphoprotein signaling in the dSTR. However, due to the short time period analyzed, it remained unclear whether local manipulation of RGS4 (and/or mGluR5s) in the dSTR alters the overall pattern of post-Amph behavioral activation. Therefore, in the following experiment, the effects of RGS4 over-expression or mGluR5 inhibition in the dSTR on Amph-induced behaviors were measured over a 2 hr period.
First, HSV–LacZ or HSV-RGS4 was microinfused into dSTR as illustrated by the distribution of cannulae placement sites on Fig. 5A. Three days later, rats were injected i.p. with Sal or Amph (2.5 mg/kg) and behavioral activity was recorded for the following 2 hours. Quantitative analysis of total distance traveled (over the period of peak activity, 15-90 min post injection) revealed significant differences between AUC values among the treatment groups as detected by ANOVA (F(3,13) =14.06, P<0.01; Fig. 5B). Pairwise SNK comparisons showed that in LacZ animals, Amph induced a significant increase in total distance traveled (LacZ-Amph vs. LacZ-Sal, p<0.01). Interestingly, Amph-induced distance traveled was augmented in animals over-expressing RGS4 in the dSTR (RGS4-Amph vs. LacZ-Amph, p<0.05). Similarly, Amph significantly increased the peak vertical activity of animals (F(3,13) =17.93, P<0.01; Fig. 5C). Further, pairwise comparisons revealed that Amph induced a significant increase in vertical activity of LacZ animals (LacZ-Amph vs. LacZ-Sal, p<0.01). In contrast to horizontal activity, Amph-induced vertical activity was blunted in animals over-expressing RGS4 in the dSTR (RGS4-Amph vs. LacZ-Amph, p<0.05; Fig. 5C).
Fig. 5. The effects of RGS4 overexpression (or a blockade of mGluR5 receptors) in the dSTR on extended Amph-induced behavioral activity.
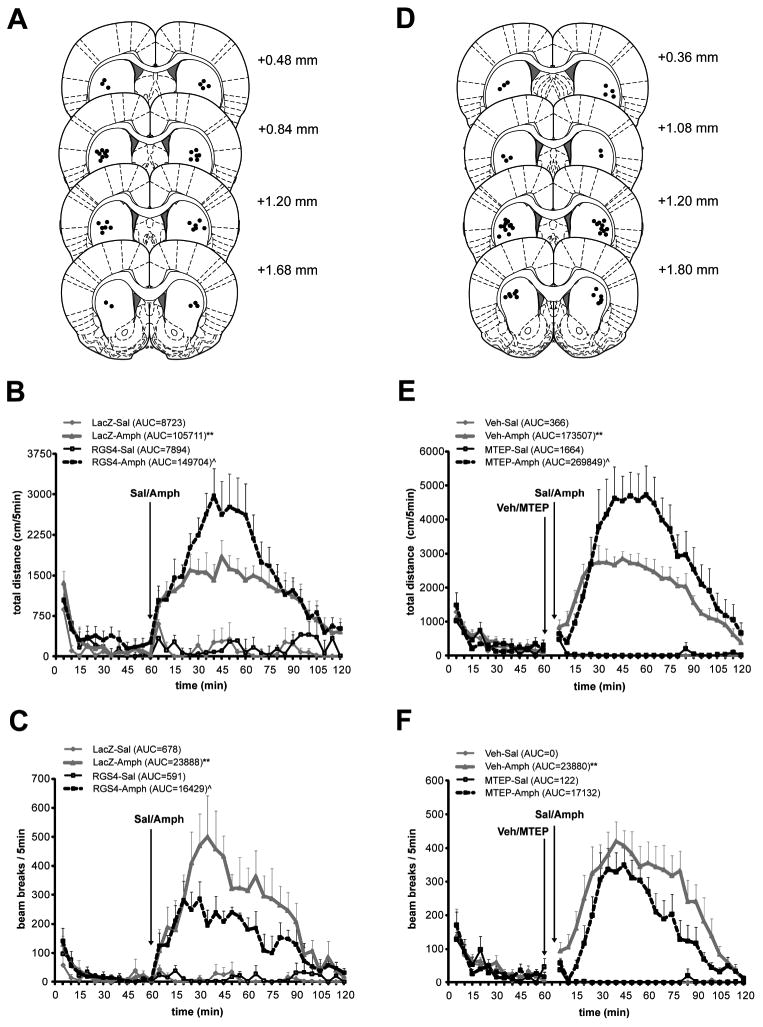
Left column (A-C): Animals received bilateral infusion of HSV-RGS4-FLAG or a control vector HSV-LacZ (2μl/side) into dSTR. Three days later animals received Amph (2.5 mgs/kg, i.p) or saline injection and behavioral activity was recorded for 2hrs. A, Outline of the rat brain coronal section depicting distribution of cannulae placements adapted from Paxinos and Watson (2005). B, Total distance traveled as recorded for 60 min pre- and 120 min post- drug administration. Mean total distance traveled (per 5 mins) ± S.E.M. plotted over time and analyzed as area under the curve (AUC). **p<0.01 vs. LacZ-Sal, ˆp<0.05 RGS4-Amph vs. LacZ-Amph. C, Vertical activity (rearing) is shown as mean number of beam breaks (per 5 mins) ± S.E.M. plotted over time and analyzed as area under the curve. **p<0.01 vs. LacZ-Sal, ˆp<0.05 RGS4-Amph vs. LacZ-Amph. Right column (D-F): Animals received bilateral infusion of Veh or MTEP (5μg/side). Fifteen minutes later animals received Amph (2.5 mgs/kg, i.p) or saline injection and behavioral activity was recorded for 2hrs. D, Outline of the rat brain coronal section depicting distribution of cannulae placements adapted from Paxinos and Watson (2005). E, Total distance traveled as recorded for 60 min pre- and 120 min post- Sal or Amph administration. Mean total distance traveled (per 5 mins) ± S.E.M. plotted over time and analyzed as area under the curve (AUC). *p<0.05 vs. LacZ-Sal, ˆp<0.05 RGS4-Amph vs. LacZ-Amph. F, Vertical activity (rearing) is shown as mean number of beam breaks (per 5 mins) ± S.E.M. plotted over time and analyzed as area under the curve. **p<0.01 vs. LacZ-Sal.
In the next experiment, MTEP was microinfused into dSTR 15 mins before an i.p. Sal or Amph (2.5 mg/kg) injection and behavior was again monitored for 2 hours. Distribution of individual cannulae placement sites is shown on Fig. 5D. Quantitative analysis of the total distance traveled (over the period of the peak Amph-induced activity, 15-90 min post injection) revealed significant differences between AUC values among the treatment groups as detected by ANOVA (F(3,13) =18.48, P<0.01; Fig. 5E). Pairwise SNK comparisons showed that in Veh-treated rats, Amph induced a significant increase in total distance traveled (Veh-Amph vs. Veh-Sal, p<0.01, Fig. 5E). In a striking similarity to the effect of RGS4 over-expression, Amph-induced distance traveled was augmented in animals that received intrastriatal MTEP infusion (MTEP-Amph vs. Veh-Amph, p<0.05). As expected, Amph significantly increased the peak vertical activity of rats (F(3,13) =11.55, P<0.01; Fig. 5F). Pairwise comparisons revealed that Amph induced a significant increase in vertical activity of Veh-treated rats (Veh-Amph vs. Veh-Sal, p<0.01). In contrast to RGS4 over-expression, Amph-induced vertical activity was not blunted by intra-striatal administration of MTEP (MTEP-Amph vs. Veh-Amph, p=0.15).
Discussion
The present study investigated the role of striatal RGS4 protein in regulating mGluR5- and Amph-dependent cellular signaling and behavioral output. First, infusion of HSV-RGS4 into the dSTR resulted in a robust over-expression of RGS4 protein, which co-immunoprecipitated with mGluR5 receptors. Second, over-expression of RGS4 in the dSTR suppressed both early locomotor activation as well as increased phospho-ERK2 levels induced by local administration of the mGluR5 agonist, DHPG. However, RGS4 over-expression did not reverse DHPG-induced dephosphorylation of Akt-Thr308 in the dSTR. Third, HSV-driven RGS4 over-expression attenuated Amph-induced phospho-ERK2 levels in a manner similar to the mGluR5 antagonist, MTEP. In contrast, RGS4 (and MTEP) only partially inhibited phospho-Akt-Thr308 upregulation by Amph. Fourth, although neither RGS4 nor MTEP showed an effect on early onset Amph-induced behaviors, analysis of the extended 3h behavioral profile of acute Amph revealed that RGS4 over-expression caused a bi-directional regulation of behavior, augmenting peak Amph-induced horizontal activity while suppressing peak vertical activity. Similarly, microinfusion of MTEP into dSTR also augmented peak Amph-induced horizontal locomotion.
A growing body of evidence suggests that group I mGluRs play an important role in the behavioral and neurochemical actions of Amph as well as other abused drugs (for review see: Bird and Lawrence 2009). As such, systemic administration of mGluR5 antagonists inhibited Amph-induced hyperactivity (Gormley and Rompre 2011; McGeehan et al. 2004; Pietraszek et al. 2004) and expression of Amph-induced conditioned place preference (Herzig et al. 2005). However, some studies provided contradictory evidence regarding the role of mGluR5 in regulating rewarding and locomotor-stimulating effects of psychostimulants (Gormley and Rompre 2011; McGeehan and Olive 2003; Veeneman et al. 2011). Discrepancies could stem from the fact that whereas different drugs exert region-specific effects on the brain, a population of mGluR1/5 receptors in the whole brain was typically blocked in these studies. Furthermore, there is only limited information regarding brain region-specific effects of mGluR1/5 antagonists on psychostimulant-induced behavior and cellular signaling. In one study, intra-dSTR blockade of group I mGluRs suppressed Amph-induced ERK and CREB phosphorylation within this brain region (Choe et al. 2002). This finding was not surprising considering that: (1) STR receives major excitatory inputs from all regions of the cortex and from the thalamus (McGeorge and Faull 1989; Mengual et al. 1999; Smith et al. 2004), (2) striatal medium spiny neurons are densely populated with glutamate receptors, including group I mGluRs, and (3), Amph-induced glutamate release plays an important role in controlling Amph-induced behaviors and activation of immediate early genes (Ferguson and Robinson 2004; Gray et al. 1999; Mao and Wang 1999). The present study was the first one to investigate the effects of intra-dSTR delivery of an mGluR5 antagonist on both cellular signaling and behavior after acute Amph. The suppressive effect of MTEP on ERK phosphorylation is an agreement with previous findings (Choe and Wang 2001) and is likely mediated by a suppression of both conventional Gαq/PLCβ1- and Homer-dependent pathways (Mao et al. 2005). The precise mechanism of mGluR5-mediated regulation of Akt activity is not clear, but it seems to be of a transient character. In the hippocampus, administration of DHPG resulted in a rapid increase in phospho-Akt308 at 5 minutes, returning back to baseline levels within 20 minutes post-administration (Hou and Klann 2004). Further, the current study suggests that DHPG treatment actually produces delayed dephosphorylation of Akt-Thr308 in the dSTR 30 minutes after the treatment. Surprisingly, manipulating mGluR5 function (by RGS4 or MTEP) in combination with DHPG or Amph treatment revealed none or very limited control of this receptor over the phosphorylation/dephosphorylation equilibrium of Akt-Thr308 in the dSTR. In agreement, delayed dephosphorylation of phospho-Akt308 in the STR after Amph treatment has been found to depend on activation of D2 receptors (Beaulieu et al. 2007; Shi and McGinty 2011), a GPCR likely not regulated by RGS4 (Ghavami et al. 2004; Ding et al. 2006).
A unique observation of the current study was the striking resemblance between the effects of an mGluR5 antagonist and those of RGS4 over-expression, supporting the hypothesis that RGS4 negatively regulates mGluR5 signaling in the dSTR. Indeed, over-expression of RGS4 in non-neuronal cell lines inhibited Gαq-coupled GPCRs and attenuated MAPK activation (Yan et al. 1997). It is also significant that both the long-form Homer protein and RGS4 are necessary to fine-tune calcium oscillations downstream from Gαq-coupled receptors (Shin et al. 2003). Taken together, these findings suggest that aberrant regulation of RGS4 levels has direct consequences for the cellular signaling downstream of mGluR5 receptors. Since abused drugs (for the most part) downregulate RGS4 levels in the striatum, it could be hypothesized that this leads to hypersensitivity of mGluR5 receptors manifested as augmented ERK phosphorylation during re-exposure to the drug or drug-paired cues (Valjent et al. 2006; Schwendt et al. 2010).
Besides cellular signaling, over-expression of RGS4 and mGluR5 inhibition altered Amph-induced behavioral profiles in a similar, but complex, manner. It is possible that both the mGluR5 antagonist and RGS4 decrease the behavioral efficacy of Amph, since increased time spent in horizontal activity at the expense of vertical activity corresponds to a behavioral repertoire characteristic of lower doses of Amph (Antoniou and Kafetzopoulos 1991; Kuczenski and Segal 1989; Wang and McGinty 1995). It is likely that cellular mechanisms of this complex behavioral shift are not limited to a single signaling pathway or exclusive to inhibition of mGluR5 receptors in the dSTR. For example, direct inhibition of ERK activity results in suppression of both horizontal and vertical post-Amph hyperactivity (Shi and McGinty 2006; Sutton et al. 2000), whereas selective inhibition of PI3K/Akt activity in the dSTR results in a behavioral profile similar to one observed in animals with RGS4 overexpression (Shi et al. 2009). However, we observed that both MTEP administration and RGS4 overexpression result in greater inhibition of ERK than Akt activity in the STR. It is possible that in our study, manipulating mGluR5 function has different outcomes depending on the cell-type-specific localization of the receptor. Although mGluR5s are abundantly expressed in both D1- and D2- positive populations of striatal medium spiny neurons (Tallaksen-Greene et al. 1998), evidence suggests that mGluR5 receptors are co-localized and closely interact with D1 receptors to regulate striatal neurotransmission (Paolillo et al. 1998; Schotanus and Chergui 2008; Voulalas et al. 2005) and many of the long-term effects of addictive drugs (Novak et al. 2010). On the other hand, mGluR5s can dimerize with D2 receptors resulting in suppressed function and cellular signaling of D2 receptors present in this heterodimer (Fuxe et al. 2009). Thus inhibition of mGluR5 function (by MTEP or RGS4) could also alter Amph-induced activation of D2 receptors and behaviors mediated by the indirect (striato-pallidal) pathway. Due to a complex character of signaling and behavioral consequences of mGluR5 (and RGS4) manipulations in the dSTR, future studies will have to examine the functional mGluR5/RGS4 relationship in a cell-type specific manner.
In conclusion, the data presented in this study show novel evidence for the significance of mGluR5 receptors and mGluR5-RGS4 interactions in the dSTR in modulating psychostimulant-induced behaviors and signaling. Genetic disruption of either mGluR5 (Chiamulera et al. 2001) or mGluR5-interacting proteins (Atkinson et al. 2006; Chuang et al. 2001; Kammermeier et al. 2000; Swanson et al. 2001) produces profound alterations in responses to psychomotor stimulants, similar to direct pharmacological inhibition of group I mGluRs (Herzig and Schmidt 2004; McGeehan and Olive 2003). Since, our data suggest that RGS4 tightly regulates mGluR5 signaling, dynamic regulation of striatal RGS4 levels by psychostimulants could have a dramatic effect on the behavioral and neural functions of mGluR5 receptors. Therefore, modulating RGS4-mGluR5 interactions may represent a novel mechanism suitable for therapeutic interventions for psychostimulant addictions as well as for other psychiatric disorders (Sjogren et al. 2010).
Acknowledgments
Authors thank Amena Smith, Adrian Gomez and John Yang for their excellent technical assistance. This work was supported by the National Institutes of Health Grants R01 DA03982 (JFM), R21 DA025846 (MS) and CO6 RR01 5155 from the Extramural Research Facilities Program of the National Center for Research Resources.
References
- Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;18:579–91. doi: 10.1016/j.cellsig.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 1996;15:6541–51. [PMC free article] [PubMed] [Google Scholar]
- Antoniou K, Kafetzopoulos E. A comparative study of the behavioral effects of d-amphetamine and apomorphine in the rat. Pharmacol Biochem Behav. 1991;39:61–70. doi: 10.1016/0091-3057(91)90398-l. [DOI] [PubMed] [Google Scholar]
- Atkinson PJ, Young KW, Ennion SJ, Kew JN, Nahorski SR, Challiss RA. Altered expression of G(q/11alpha) protein shapes mGlu1 and mGlu5 receptor-mediated single cell inositol 1,4,5-trisphosphate and Ca(2+) signaling. Mol Pharmacol. 2006;69:174–84. doi: 10.1124/mol.105.014258. [DOI] [PubMed] [Google Scholar]
- Bansal G, Druey KM, Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol Ther. 2007;116:473–95. doi: 10.1016/j.pharmthera.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–5. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–32. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bird MK, Lawrence AJ. Group I metabotropic glutamate receptors: involvement in drug-seeking and drug-induced plasticity. Curr Mol Pharmacol. 2009;2:83–94. doi: 10.2174/1874467210902010083. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Nestler EJ, Neve RL. Herpes simplex virus-mediated gene transfer as a tool for neuropsychiatric research. Crit Rev Neurobiol. 2000;14:47–67. doi: 10.1080/08913810008443546. [DOI] [PubMed] [Google Scholar]
- Chase SL, Taussig RL, Neve DW, Self DW. The role of RGS4 in cocaine and D2 sensitization (Neuroscience Meeting Planner Online) Society for Neuroscience; San Diego, CA, USA: 2010. p. 770.25. [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nature neuroscience. 2001;4:873–4. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Choe ES, Chung KT, Mao L, Wang JQ. Amphetamine increases phosphorylation of extracellular signal-regulated kinase and transcription factors in the rat striatum via group I metabotropic glutamate receptors. Neuropsychopharmacology. 2002;27:565–75. doi: 10.1016/S0893-133X(02)00341-X. [DOI] [PubMed] [Google Scholar]
- Choe ES, Wang JQ. Group I metabotropic glutamate receptor activation increases phosphorylation of cAMP response element-binding protein, Elk-1, and extracellular signal-regulated kinases in rat dorsal striatum. Brain Res Mol Brain Res. 2001;94:75–84. doi: 10.1016/s0169-328x(01)00217-0. [DOI] [PubMed] [Google Scholar]
- Chuang SC, Bianchi R, Kim D, Shin HS, Wong RK. Group I metabotropic glutamate receptors elicit epileptiform discharges in the hippocampus through PLCbeta1 signaling. J Neurosci. 2001;21:6387–94. doi: 10.1523/JNEUROSCI.21-16-06387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Ding J, Guzman JN, Tkatch T, Chen S, Goldberg JA, Ebert PJ, Levitt P, Wilson CJ, Hamm HE, Surmeier DJ. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nature neuroscience. 2006;9:832–42. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- Ding L, Hegde AN. Expression of RGS4 splice variants in dorsolateral prefrontal cortex of schizophrenic and bipolar disorder patients. Biol Psychiatry. 2009;65:541–5. doi: 10.1016/j.biopsych.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Emilsson L, Saetre P, Jazin E. Low mRNA levels of RGS4 splice variants in Alzheimer's disease: association between a rare haplotype and decreased mRNA expression. Synapse. 2006;59:173–6. doi: 10.1002/syn.20226. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Robinson TE. Amphetamine-evoked gene expression in striatopallidal neurons: regulation by corticostriatal afferents and the ERK/MAPK signaling cascade. Journal of neurochemistry. 2004;91:337–48. doi: 10.1111/j.1471-4159.2004.02712.x. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–45. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Woods AS, Giuseppina L, Antonelli T, Ferraro L, Tanganelli S, Agnati LF. Integrated signaling in heterodimers and receptor mosaics of different types of GPCRs of the forebrain: relevance for schizophrenia. J Neural Transm. 2009;116:923–39. doi: 10.1007/s00702-008-0174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Role of protein kinase C epsilon (PKCvarepsilon) in the reduction of ethanol reinforcement due to mGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology (Berl) 2009;204:587–97. doi: 10.1007/s00213-009-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavami A, Hunt RA, Olsen MA, Zhang J, Smith DL, Kalgaonkar S, Rahman Z, Young KH. Differential effects of regulator of G protein signaling (RGS) proteins on serotonin 5-HT1A, 5-HT2A, and dopamine D2 receptor-mediated signaling and adenylyl cyclase activity. Cell Signal. 2004;16:711–21. doi: 10.1016/j.cellsig.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997;17:8024–37. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Nicolini V, McGinty JF. Gene expression profile from the striatum of amphetamine-treated rats: a cDNA array and in situ hybridization histochemical study. Brain Res Gene Expr Patterns. 2002;1:193–8. doi: 10.1016/s1567-133x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Gormley S, Rompre PP. Blockade of mGLUR5 receptors differentially alters amphetamine-induced enhancement of locomotor activity and of brain stimulation reward. J Psychopharmacol. 2011;25:393–401. doi: 10.1177/0269881110367460. [DOI] [PubMed] [Google Scholar]
- Gray AM, Rawls SM, Shippenberg TS, McGinty JF. The kappa-opioid agonist, U-69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. Journal of neurochemistry. 1999;73:1066–74. doi: 10.1046/j.1471-4159.1999.0731066.x. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, Winder DG. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–9. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MH, Renthal W, Ring RH, Rahman Z, Psifogeorgou K, Howland D, Birnbaum S, Young K, Neve R, Nestler EJ, Zachariou V. Brain region specific actions of regulator of G protein signaling 4 oppose morphine reward and dependence but promote analgesia. Biol Psychiatry. 2010;67:761–9. doi: 10.1016/j.biopsych.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig V, Capuani EM, Kovar KA, Schmidt WJ. Effects of MPEP on expression of food-, MDMA- or amphetamine-conditioned place preference in rats. Addict Biol. 2005;10:243–9. doi: 10.1080/13556210500223272. [DOI] [PubMed] [Google Scholar]
- Herzig V, Schmidt WJ. Effects of MPEP on locomotion, sensitization and conditioned reward induced by cocaine or morphine. Neuropharmacology. 2004;47:973–84. doi: 10.1016/j.neuropharm.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Hooks SB, Martemyanov K, Zachariou V. A role of RGS proteins in drug addiction. Biochem Pharmacol. 2008;75:76–84. doi: 10.1016/j.bcp.2007.07.045. [DOI] [PubMed] [Google Scholar]
- Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–61. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaen C, Doupnik CA. RGS3 and RGS4 differentially associate with G protein-coupled receptor-Kir3 channel signaling complexes revealing two modes of RGS modulation. Precoupling and collision coupling. The Journal of biological chemistry. 2006;281:34549–60. doi: 10.1074/jbc.M603177200. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. J Neurosci. 2000;20:7238–45. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimple AJ, Bosch DE, Giguere PM, Siderovski DP. Regulators of G-protein signaling and their Galpha substrates: promises and challenges in their use as drug discovery targets. Pharmacol Rev. 2011;63:728–49. doi: 10.1124/pr.110.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal D. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–65. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Wang JQ. Protection against acute amphetamine-induced behavior by microinjection of a group II metabotropic glutamate receptor agonist into the dorsal striatum of rats. Neuroscience letters. 1999;270:103–6. doi: 10.1016/s0304-3940(99)00480-2. [DOI] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25:2741–52. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeehan AJ, Janak PH, Olive MF. Effect of the mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine (MPEP) on the acute locomotor stimulant properties of cocaine, D-amphetamine, and the dopamine reuptake inhibitor GBR12909 in mice. Psychopharmacology (Berl) 2004;174:266–73. doi: 10.1007/s00213-003-1733-2. [DOI] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–2. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–37. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Mengual E, de las Heras S, Erro E, Lanciego JL, Gimenez-Amaya JM. Thalamic interaction between the input and the output systems of the basal ganglia. J Chem Neuroanat. 1999;16:187–200. doi: 10.1016/s0891-0618(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry. 2001;6:293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP, Perez-Garcia J, Olivera-Lopez JI, Jaramillo MT. Antidepressant-like and anxiolytic-like actions of the mGlu5 receptor antagonist MTEP, microinjected into lateral septal nuclei of male Wistar rats. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1129–35. doi: 10.1016/j.pnpbp.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Neve RL, Howe JR, Hong S, Kalb RG. Introduction of the glutamate receptor subunit 1 into motor neurons in vitro and in vivo using a recombinant herpes simplex virus. Neuroscience. 1997;79:435–47. doi: 10.1016/s0306-4522(96)00645-8. [DOI] [PubMed] [Google Scholar]
- Nishiguchi KM, Sandberg MA, Kooijman AC, Martemyanov KA, Pott JW, Hagstrom SA, Arshavsky VY, Berson EL, Dryja TP. Defects in RGS9 or its anchor protein R9AP in patients with slow photoreceptor deactivation. Nature. 2004;427:75–8. doi: 10.1038/nature02170. [DOI] [PubMed] [Google Scholar]
- Novak M, Halbout B, O'Connor EC, Rodriguez Parkitna J, Su T, Chai M, Crombag HS, Bilbao A, Spanagel R, Stephens DN, Schutz G, Engblom D. Incentive learning underlying cocaine-seeking requires mGluR5 receptors located on dopamine D1 receptor-expressing neurons. J Neurosci. 2010;30:11973–82. doi: 10.1523/JNEUROSCI.2550-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolillo M, Montecucco A, Zanassi P, Schinelli S. Potentiation of dopamine-induced cAMP formation by group I metabotropic glutamate receptors via protein kinase C in cultured striatal neurons. Eur J Neurosci. 1998;10:1937–45. doi: 10.1046/j.1460-9568.1998.00203.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. 6th. Academic press; San Diego: 2007. [Google Scholar]
- Pietraszek M, Rogoz Z, Wolfarth S, Ossowska K. Opposite influence of MPEP, an mGluR5 antagonist, on the locomotor hyperactivity induced by PCP and amphetamine. J Physiol Pharmacol. 2004;55:587–93. [PubMed] [Google Scholar]
- Rahman Z, Schwarz J, Gold SJ, Zachariou V, Wein MN, Choi KH, Kovoor A, Chen CK, DiLeone RJ, Schwarz SC, Selley DE, Sim-Selley LJ, Barrot M, Luedtke RR, Self D, Neve RL, Lester HA, Simon MI, Nestler EJ. RGS9 modulates dopamine signaling in the basal ganglia. Neuron. 2003;38:941–52. doi: 10.1016/s0896-6273(03)00321-0. [DOI] [PubMed] [Google Scholar]
- Ribeiro FM, Paquet M, Cregan SP, Ferguson SS. Group I metabotropic glutamate receptor signalling and its implication in neurological disease. CNS Neurol Disord Drug Targets. 2010;9:574–95. doi: 10.2174/187152710793361612. [DOI] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28:543–7. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz de Azua I, Scarselli M, Rosemond E, Gautam D, Jou W, Gavrilova O, Ebert PJ, Levitt P, Wess J. RGS4 is a negative regulator of insulin release from pancreatic beta-cells in vitro and in vivo. Proc Natl Acad Sci U S A. 2010;107:7999–8004. doi: 10.1073/pnas.1003655107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad JA, Marino MJ, Folk JA, Hepler JR, Conn PJ. RGS4 inhibits signaling by group I metabotropic glutamate receptors. J Neurosci. 1998;18:905–13. doi: 10.1523/JNEUROSCI.18-03-00905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotanus SM, Chergui K. Dopamine D1 receptors and group I metabotropic glutamate receptors contribute to the induction of long-term potentiation in the nucleus accumbens. Neuropharmacology. 2008;54:837–44. doi: 10.1016/j.neuropharm.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Gold SJ, McGinty JF. Acute amphetamine down-regulates RGS4 mRNA and protein expression in rat forebrain: distinct roles of D1 and D2 dopamine receptors. Journal of neurochemistry. 2006;96:1606–15. doi: 10.1111/j.1471-4159.2006.03669.x. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Hearing MC, See RE, McGinty JF. Chronic cocaine reduces RGS4 mRNA in rat prefrontal cortex and dorsal striatum. Neuroreport. 2007;18:1261–5. doi: 10.1097/WNR.0b013e328240507a. [DOI] [PubMed] [Google Scholar]
- Schwendt M, McGinty JF. Regulator of G-protein signaling 4 interacts with metabotropic glutamate receptor subtype 5 in rat striatum: relevance to amphetamine behavioral sensitization. J Pharmacol Exp Ther. 2007;323:650–7. doi: 10.1124/jpet.107.128561. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Madell RL, McGinty JF. Relapse to cocaine-seeking after abstinence is associated with increased ERK1/2 phosphorylation in the dorsolateral striatum: the role of mGluR5 receptors (Neuroscience Meeting Planner Online) Society for Neuroscience; Chicago, IL, USA: 2010. p. 770.19. [Google Scholar]
- Shi X, Gomez A, McGinty JF. Inhibition of phosphoinositide 3-kinase decreases the activation of protein kinase B in rat striatum without altering the phosphorylation of extracellular signal-regulated kinase induced by amphetamine (Neuroscience Meeting Planner Online) Society for Neuroscience; Chicago, IL, USA: 2009. p. 66.25. [Google Scholar]
- Shi X, McGinty JF. Extracellular signal-regulated mitogen-activated protein kinase inhibitors decrease amphetamine-induced behavior and neuropeptide gene expression in the striatum. Neuroscience. 2006;138:1289–98. doi: 10.1016/j.neuroscience.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Shi X, McGinty JF. Repeated amphetamine treatment increases phosphorylation of extracellular signal-regulated kinase, protein kinase B, and cyclase response element-binding protein in the rat striatum. Journal of neurochemistry. 2007;103:706–13. doi: 10.1111/j.1471-4159.2007.04760.x. [DOI] [PubMed] [Google Scholar]
- Shi X, McGinty JF. D1 and D2 dopamine receptors differentially mediate the activation of phosphoproteins in the striatum of amphetamine-sensitized rats. Psychopharmacology (Berl) 2011;214:653–63. doi: 10.1007/s00213-010-2068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Dehoff M, Luo X, Kang SH, Tu J, Nayak SK, Ross EM, Worley PF, Muallem S. Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCbeta GAP activities. J Cell Biol. 2003;162:293–303. doi: 10.1083/jcb.200210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci. 2005;1:51–66. doi: 10.7150/ijbs.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren B, Blazer LL, Neubig RR. Regulators of G protein signaling proteins as targets for drug discovery. Prog Mol Biol Transl Sci. 2010;91:81–119. doi: 10.1016/S1877-1173(10)91004-1. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–7. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Sutton MA, McGibney K, Beninger RJ. Conditioned locomotion in rats following amphetamine infusion into the nucleus accumbens: blockade by coincident inhibition of protein kinase A. Behav Pharmacol. 2000;11:365–76. doi: 10.1097/00008877-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–52. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkowski ME, Chowdari K, Lewis DA, Nimgaonkar VL. Can RGS4 polymorphisms be viewed as credible risk factors for schizophrenia? A critical review of the evidence. Schizophr Bull. 2006;32:203–8. doi: 10.1093/schbul/sbj058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallaksen-Greene SJ, Kaatz KW, Romano C, Albin RL. Localization of mGluR1a-like immunoreactivity and mGluR5-like immunoreactivity in identified populations of striatal neurons. Brain research. 1998;780:210–7. doi: 10.1016/s0006-8993(97)01141-4. [DOI] [PubMed] [Google Scholar]
- Tekumalla PK, Calon F, Rahman Z, Birdi S, Rajput AH, Hornykiewicz O, Di Paolo T, Bedard PJ, Nestler EJ. Elevated levels of DeltaFosB and RGS9 in striatum in Parkinson's disease. Biol Psychiatry. 2001;50:813–6. doi: 10.1016/s0006-3223(01)01234-3. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corbillé AG, Bertran-Gonzalez J, Hervé D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A. 2006;103(8):2932–7. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeneman MM, Boleij H, Broekhoven MH, Snoeren EM, Guitart Masip M, Cousijn J, Spooren W, Vanderschuren LJ. Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacology (Berl) 2011;214:863–76. doi: 10.1007/s00213-010-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voulalas PJ, Holtzclaw L, Wolstenholme J, Russell JT, Hyman SE. Metabotropic glutamate receptors and dopamine receptors cooperate to enhance extracellular signal-regulated kinase phosphorylation in striatal neurons. J Neurosci. 2005;25:3763–73. doi: 10.1523/JNEUROSCI.4574-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Mao L. Sustained behavioral stimulation following selective activation of group I metabotropic glutamate receptors in rat striatum. Pharmacol Biochem Behav. 2000;65:439–47. doi: 10.1016/s0091-3057(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Dose-dependent alteration in zif/268 and preprodynorphin mRNA expression induced by amphetamine or methamphetamine in rat forebrain. J Pharmacol Exp Ther. 1995;273:909–17. [PubMed] [Google Scholar]
- Wang Q, Liu-Chen LY, Traynor JR. Differential modulation of mu- and delta-opioid receptor agonists by endogenous RGS4 protein in SH-SY5Y cells. The Journal of biological chemistry. 2009;284:18357–67. doi: 10.1074/jbc.M109.015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Chi PP, Bourne HR. RGS4 inhibits Gq-mediated activation of mitogen-activated protein kinase and phosphoinositide synthesis. The Journal of biological chemistry. 1997;272:11924–7. doi: 10.1074/jbc.272.18.11924. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Kroslak T, Laforge KS, Zhou Y, Ho A, Kreek MJ. Differential gene expression in the rat caudate putamen after “binge” cocaine administration: advantage of triplicate microarray analysis. Synapse. 2003;48:157–69. doi: 10.1002/syn.10198. [DOI] [PubMed] [Google Scholar]
- Zhang Y, James M, Middleton FA, Davis RL. Transcriptional analysis of multiple brain regions in Parkinson's disease supports the involvement of specific protein processing, energy metabolism, and signaling pathways, and suggests novel disease mechanisms. Am J Med Genet B Neuropsychiatr Genet. 2005;137B:5–16. doi: 10.1002/ajmg.b.30195. [DOI] [PubMed] [Google Scholar]


