Abstract
We know a great deal about the biochemistry of cells because they can be isolated and studied. The biochemistry of the much more complex in vivo environment is more difficult to study because the only ways to quantitate concentrations is to sacrifice the animal or biopsy the tissue. Either method disrupts the environment profoundly and neither method allows longitudinal studies on the same individual. Methods of measuring chemical concentrations in vivo are very valuable alternatives to sacrificing groups of animals. We are developing microscopic magnetic nanoparticle (mNP) probes to measure the concentration of a selected molecule in vivo. The mNPs are targeted to bind the selected molecule and the resulting reduction in rotational freedom can be quantified remotely using magnetic spectroscopy. The mNPs must be contained in micrometer sized porous shells to keep them from migrating and to protect them from clearance by the immune system. There are two key issues in the development of the probes. First, we demonstrate the ability to measure concentrations in the porous walled alginate probes both in phosphate buffered saline and in blood, which is an excellent surrogate for the complex and challenging in vivo environment. Second, sensitivity is critical because it allows microscopic probes to measure very small concentrations very far away. We report sensitivity measurements on recently introduced technology that has allowed us to improve the sensitivity by two orders of magnitude, a factor of 200 so far.
Index Terms: Magnetic particle imaging, perpendicular magnetic particle imaging, sMSB
I. Introduction
There are a host of applications where the concentration of a given chemical agent or metabolite is needed for patient care [1]. Blood sugar, lactate reveal energy metabolism for diabetics [2]. Hormones tell us about the patient’s physiological state. Drug concentrations [3] and signaling molecules reveal a great deal about how well a given therapy is performing [4]. In most cases, a local concentration is needed to isolate the response of one organ from another. Currently, physicians can only measure concentrations in blood or using biopsy. Those are suboptimal for a great many applications. Biopsy needles are large enough to alter signaling by themselves [5]. Blood levels are not useful in many circumstances because hormones and signaling molecules are not specific to disease. For example, VEGF stimulates blood vessel development in cancer and heart disease, but also around a paper cut or even a needle stick [5]. Or the inflammation markers distinguishing successful from failed cancer immune checkpoint therapy [6]–[8] are also produced by a common cold or a bacterial infection around a simple cut. There is a tremendous need to get the local concentrations of specific metabolites in vivo.
We are developing microscopic probes to measure the concentration of agents locally over time. Placing the probes anywhere in the body would be minimally invasive, and once placed, they could be read out over time noninvasively using magnetic fields. The read out would be based on magnetic spectroscopy of nanoparticle Brownian motion (MSB) [9]–[12]. The probes described here are porous shells encapsulating magnetic nanoparticles (mNPs) conjugated with aptamers that specifically bind the desired molecule. The shells protect the mNPs from immune absorption and keep them localized allowing longitudinal measurements. The targeted agent biomarker diffuses into the shell and binds two mNPs together. The number of bound mNPs can be determined from their spectroscopic signal [9], [10], enabling the concentration to be estimated from the spectra of the magnetization of the mNPs in an alternating magnetic field.
The MSB is important because it has the potential for very high sensitivity. Nanoparticle binding has been demonstrated previously using methods not amenable to in vivo use [13], [14] or limited to superficial applications [15], [16]. The sensitivity achieved by measuring the harmonics of the magnetization of mNPs should allow microscopic probes to be envisioned; the previous method of measuring biomarker concentration in vivo at depth required centimeter size probes to achieve sufficient signal [17]. Size is critical because the tissue damage associated with probe insertion is much larger for biopsy needles or large probes. In addition, lesions have to be larger than the probes for proper measurements so large probes are limited in usefulness. In consequence, the probes in [17] were described as measuring concentrations in blood where microscopic probes should be able to measure concentrations in interstitial fluid. The reduction in tissue damage resulting from reduced probe size should not be underestimated in an in vivo application.
We have showed that the methods work well in vitro [12] and have been working toward in vivo capabilities. We are showing three important things that are needed for high impact in vivo applications: we demonstrate that: 1) the chemistry functions well in the probes; 2) the probes function adequately within a complex and challenging environment very like the actual in vivo environment; and 3) the sensitivity can be improved to allow small probes to measure very low concentrations.
II. Methods
Alginate probes were prepared using extrusion methods [18], [19]. Briefly, 1% sodium alginate in water functioned as the anionic solution and 2% calcium chloride containing 1% carboxymethylcellulose functioned as the cationic solution. Functionalized mNPs [12] (Micromod, 100 nm hydrodynamic diameter, iron oxide, dextran coated) were added to the cationic solution to achieve 1 mg/ml concentration. Droplets of the cationic solution were injected into 100 ml of anionic solution under constant stirring to generate hollow core beads. After alginate beads formed, they were rinsed with 2% calcium chloride three times, and incubated in 0.01% calcium solution at 4 °C. The resulting probes proved stable for two months. For spectroscopic measurements, calcium-alginated beads containing around 300 μg mNP were used.
We used blood as a surrogate for the interstitial in vivo environment. Blood contains most of the chemical complexities of the in vivo environment: the antibodies, enzymes, and a host of other proteins large and small present in vivo are all present in blood. Other biological samples are generally less problematic: urea, saliva, tissue, and so on. Blood is also the most commonly collected biological sample and has the most complicated composition. It has been shown that mNPs were readily taken in by cells resulting in a lower MSB signal [11]. Whole blood was harvested in an eppendorf tube containing heparin from the vena cava of euthanized C57BL/6 mice using a 3 mL syringe and a 25G needle. The whole blood was spun at 4500 r/min for 20 min at 4 °C, and the supernatant was used for experiments as plasma.
The spectroscopic measurements for the probe data were acquired at 1270 Hz on our original apparatus [9]–[12]. The ratio of the fifth over the third harmonics of the mNP magnetization was used as a concentration-independent metric [9], [10].
In the second arm of this paper, we tested the sensitivity of a recently introduced spectrometer that measures the magnetization perpendicular to the oscillating applied field. The perpendicular magnetization is induced by applying a small static field perpendicular to the oscillating applied field [20].
III. Results
First, the probes’ ability to measure concentrations in biological samples that mimic the in vivo environment was demonstrated. Then, the improved sensitivity of new spectrometer designs was demonstrated. The combination addresses two critical areas in the development of microscopic in vivo probes.
1) ssDNA Detection in Different Biological Samples
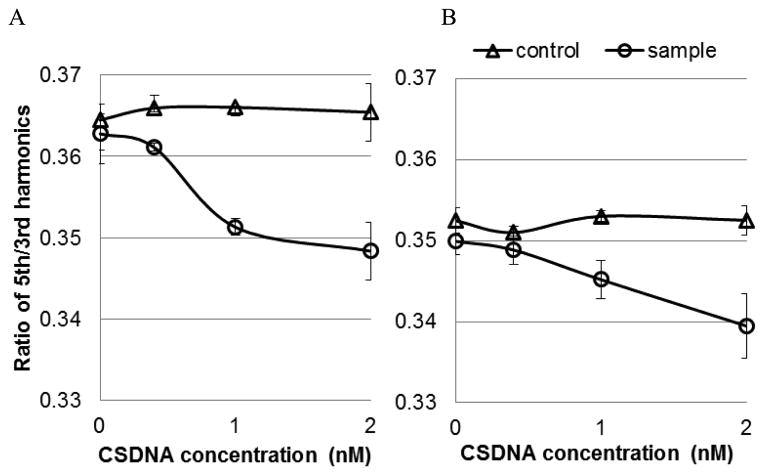
As previously reported, using properly functionalized mNP, MSB is capable of detecting proteins and DNA molecules with sub-picomole sensitivity [12]. Here, we demonstrate that the same chemistry functions in blood. The MSB signals from mNP show different offsets in different environmental conditions, Fig. 1. The high viscosity of plasma caused a lower MSB signal, Fig. 1(b). The addition of cell uptake caused even lower MSB signals, Fig. 1(c) and [11]. However, the MSB signal dropped in response to increasing concentration of the analyte in all of the conditions. In the presence of 2 nM complimentary single stranded DNA (CSDNA), the decrease in MSB signal in buffer and plasma are comparable (4.7% and 5.1%, compared with the control). This result confirmed that the protein and other molecules in plasma did not inhibit the binding between ligands on the mNP and analyte in samples. But, when the samples are blood, which contains blood cells, the mNP cell uptake process competes with the binding between analyte and ligands on mNPs, resulting in poorer sensitivity. This demonstrates the need for shells to protect the mNPs from immune system cells.
Fig. 1.
MSB signal generated by ssDNA functionalized mNP in response to increasing concentration of analyte (24 mer ssDNA). Detection was taking in different conditions (a) in PBS buffer, (b) in plasma, and (c) in whole blood. Control was added with PBS buffer, and sample was added with CSDNA.
2) Encapsulated mNP as Sensing Probes
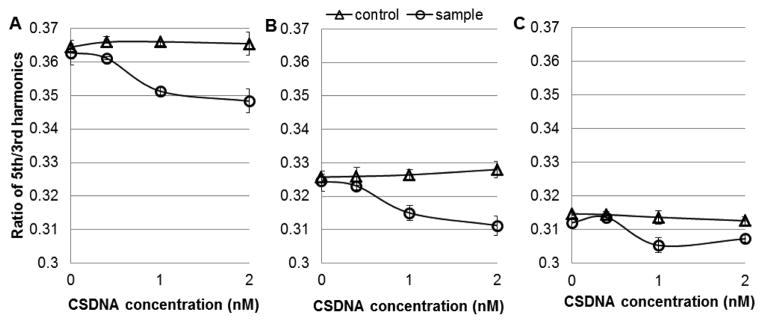
Alginate probes are attractive biodegradable and biocompatible material and have been used in humans for drug delivery applications for many years. It is applied in this paper to encapsulate the mNPs protecting them from absorption by phagocytes and other immune system cells. First, the MSB signals of mNPs with and without alginate encapsulation were compared in phosphate buffered saline (PBS) buffer, Fig. 2. The alginate shells were 1–2 mm in size for these experiments. These shells are already three orders of magnitude smaller volumes than those previously used for in vivo measurements [17] and they can be made much smaller [18], [19]. The capsulated mNPs produced reduced MSB signals partly because the shell reduces rotational freedom, but it might also be attributed to stray strands of alginate in the shell. The MSB signal dropping in the presence of 2 nM CSDNA was 4.7% and 3.7% without and with alginate encapsulation.
Fig. 2.
MSB signal generated by ssDNA functionalized mNP in response to increasing concentration of analyte (24 mer ssDNA). Detection was made in PBS buffer (a) without alginate shells and (b) with alginate shells. PBS buffer was added to the control, and CSDNA was added to the samples.
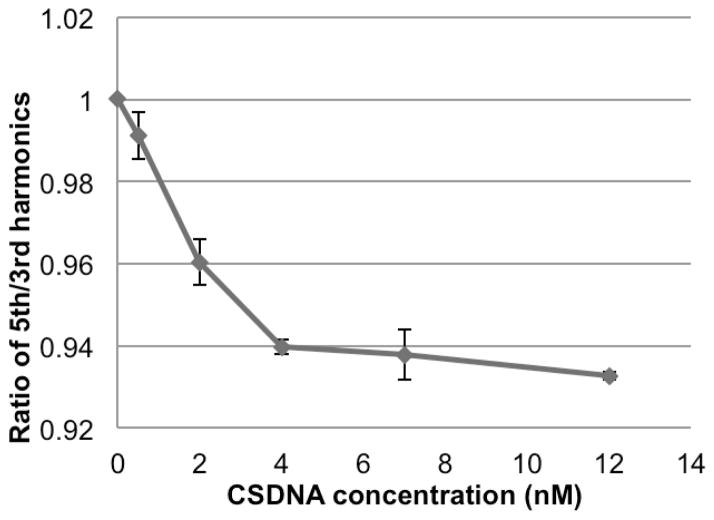
We further investigated the detection of alginate capsulated mNPs in whole blood the results of which are shown in Fig. 3. The linear detection range is from 0 to 4 nM CSDNA. For 2 nM CSDNA, the signal dropped 4.0%, which is comparable with the result from detection in buffer.
Fig. 3.
MSB signal of mNPs encapsulated in alginate beads in response to increasing concentration of CSDNA in blood.
The two highest concentration points suggest the system is reaching a less sensitive region and that needs to be further explored. We quantitate the analyte concentration via a calibration curve and that curve tends to be steep at low concentrations producing high sensitivity and less steep at higher concentrations where a significant proportion of the binding sites are occupied [21]. This produces high sensitivity at low concentrations and lower resolution at higher concentrations. The reduction in resolution at higher concentrations also extends the dynamic range. However, the shoulder shown in Fig. 3 might be at too low a concentration to represent competitive binding effects. The quantitation of analyte is still possible no matter what the genesis of the shoulder. We are primarily interested in the lowest concentrations where mNPs are more likely to be bound in pairs and the response is linear because that is most important physiologically. But, further exploration is needed.
This result also confirmed that mNP uptake by cells was eliminated by encapsulation.
We selected alginate shells partly because they are biodegradable. However, that also means their lifetime in biological environments is limited and our current probes have not yet been optimized for stability or size distribution. The current beads swelled and slowly degraded in biological conditions. For example, in one experiment, the MSB signal lost 30% in 1 h (data not shown). The harmonic ratios in Fig. 3 have been normalized for shell degradation using a control shell with unfunctionalized mNPs. By optimizing the formulation process, the stability of the alginate shells needs to be tuned to balance stability and biodegradability.
3) Improved Sensitivity Using sMSB
Previous spectroscopy used the harmonics of the magnetization induced in the magnetic mNPs by an alternating magnetic field [9]–[12]. We recently showed that spectroscopy can also be performed using a perpendicular component of the magnetization induced by a small static field in the direction perpendicular to the alternating magnetic field [20]. It was termed sMSB. One of the advantages of that geometry is that the pickup coil is geometrically isolated from the large alternating field.
We implemented a second prototype sMSB spectroscopy system. The system used the perpendicular geometry and epoxy was used to fix the drive and pickup coils. The feed-through from the alternating magnetic field was minimized by rotating the pickup coil. The feed-through was reduced enough to measure the Johnson noise of the pickup coil.
The sensitivity of the system was sufficient to measure 100 ng of Micromod mNPs. Our previous apparatus [9]–[12] could only measure 20 μg of the same mNPs so we achieved an improvement by a factor of roughly 200. Most of our data have been taken using 100 μg of mNPs.
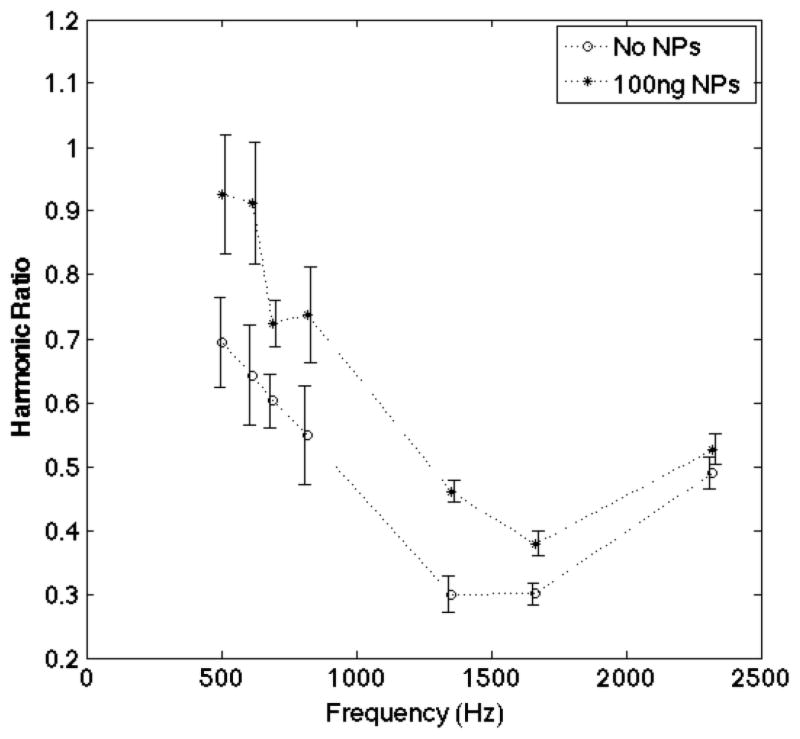
The difference between the 100 ng spectra and the empty baseline spectra in Fig. 4 is highly significant; the p-value for the null hypothesis that the 100 ng spectra is different from the empty spectra is 2 × 10−7. It is clear that lower quantities of mNPs would be significantly different from the empty spectra measurements so the sensitivity is actually higher. Using a linear model, these data suggest that concentrations of 40 ng would be the minimum that is significantly different from the empty spectra measurements taken without mNPs.
Fig. 4.
MSB signal of 100 ng of iron oxide (100 nm diameter) mNPs compared with the signal from no sample. The spectra are clearly separated by more than two standard deviations at each frequency.
There are several other steps that will be taken to further improve the sensitivity including: stabilizing the temperature of the coils and the power amplifier, optimized the design and fabrication of the pickup coil, including the number of turns, magnetically shielding the apparatus, and placing a smaller, low-noise preamp closer to the pickup coil in the read out circuit.
IV. Conclusion
We demonstrated two critical aspects of the proposed methods of measuring molecular concentrations in vivo that show that magnetic spectroscopy sensing techniques are capable of measuring diagnostic concentrations of biomarkers in vivo with microscopic probes. First, the probes formed of targeted mNPs encapsulated in porous alginate shells allowed concentrations to be measured in blood. Blood effectively mimics many aspects of the in vivo environment suggesting the system should be capable of in vivo use. The sensitivity loss was minimal, less than a factor of four.
Second, new spectrometer designs are able to improve the sensitivity a great deal; using the improved geometry produced an improvement by a factor of 200–400 in the sensitivity to nanoparticles reducing the amount of iron that has to be encapsulated, as well as increasing the sensitivity to small analyte concentration changes.
Acknowledgments
This work was supported in part by NIH-NCI under Grant 1U54CA151662-01 and in part by Norris Cotton Cancer Center Hopeman Fund.
References
- 1.Lee TMH. Over-the-counter biosensors: Past, present, and future. Sensors. 2008;8(9):5535–5559. doi: 10.3390/s8095535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henkin AH, et al. Real-time noninvasive imaging of fatty acid uptake in vivo. ACS Chem Biol. 2012;7(11):1884–1891. doi: 10.1021/cb300194b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nature Rev Cancer. 2006 Aug;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 4.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 5.Ozawa CR, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113(4):516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okazaki T, Honjo T. PD-1 and PD-1 ligands: From discovery to clinical application. Int Immunol. 2007;19(7):813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 7.Pardoll D. The blockade of immune checkpoints in cancer immunotherapy. Nature Rev Cancer. 2012 Apr;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer RJ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New England J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauwerdink AM, Weaver JB. Measurement of molecular binding using the Brownian motion of magnetic nanoparticle probes. Appl Phys Lett. 2010;96(3):033702. [Google Scholar]
- 10.Rauwerdink AM, Weaver JB. Concurrent quantification of multiple nanoparticle bound states. Med Phys. 2011;38(3):1136–1140. doi: 10.1118/1.3549762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giustini AJ, Perreard I, Rauwerdink AM, Hoopes PJ, Weaver JB. Noninvasive assessment of magnetic nanoparticle-cancer cell interactions. Integr Biol. 2012;4(10):1283–1288. doi: 10.1039/c2ib20130e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, et al. Molecular sensing with magnetic nanoparticles using magnetic spectroscopy of nanoparticle Brownian motion. Biosensors Bioelectron. 2013 Dec;50:441–446. doi: 10.1016/j.bios.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang CC, Yang SY, Chieh JJ, Horng HE, Hong CY, Yang HC. Universal behavior of biomolecule-concentration-dependent reduction in AC magnetic susceptibility of bioreagents. IEEE Magn Lett. 2012;3:1500104. [Google Scholar]
- 14.Heim E, Ludwig F, Schilling M. Binding assays with streptavidin-functionalized superparamagnetic nanoparticles and biotinylated analytes using fluxgate magnetorelaxometry. J Magn Magn Mater. 2009;321(10):1628–1631. [Google Scholar]
- 15.Berk DA, Yuan F, Leunig M, Jain RK. Direct in vivo measurement of targeted binding in a human tumor xenograft. Proc Nat Acad Sci United States Amer. 1997 Mar;94:1785–1790. doi: 10.1073/pnas.94.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choo-Smith LP, et al. Medical applications of Raman spectroscopy: From proof of principle to clinical implementation. Biopolymers. 2002;67(1):1–9. doi: 10.1002/bip.10064. [DOI] [PubMed] [Google Scholar]
- 17.Ling Y, Pong T, Vassiliou CC, Huang PL, Cima MJ. Implantable magnetic relaxation sensors measure cumulative exposure to cardiac biomarkers. Nature Biotechnol. 2011 Feb;29:273–277. doi: 10.1038/nbt.1780. [DOI] [PubMed] [Google Scholar]
- 18.Gimi B, et al. A nanoporous, transparent microcontainer for encapsulated islet therapy. J Diabetes Sci Technol. 2009;3(2):297–303. doi: 10.1901/jaba.2009.3-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimi B, et al. Cell encapsulation and oxygenation in nanoporous microcontainers. Biomed Microdevices. 2009;11(6):1205–1212. doi: 10.1007/s10544-009-9338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reeves DB, Weaver JB. Magnetic nanoparticle sensing: Decoupling the magnetization from the excitation field. J Phys D, Appl Phys. 2014;47(4):045002-1–045002-8. doi: 10.1088/0022-3727/47/4/045002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn YK, et al. Magnetophoretic immunoassay of allergen-specific IgE in an enhanced magnetic field gradient. Anal Chem. 2007;79(6):2214–2220. doi: 10.1021/ac061522l. [DOI] [PubMed] [Google Scholar]