Abstract
Several studies highlight the role of inflammatory markers in thrombosis as well as in cancer. However, their combined role in cancer-associated deep vein thrombosis (DVT) and the molecular mechanisms, involved in its pathophysiology, needs further investigations. In the present study, C-reactive protein, interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1β), matrix metalloproteases-9 (MMP-9), vascular endothelial growth factor (VEGF), tissue factor (TF), fibrinogen and soluble P-selectin, were analyzed in plasma and in monocyte samples from 385 cancer patients, of whom 64 were concomitantly affected by DVT (+). All these markers were higher in cancer patients DVT+ than in those DVT-. Accordingly, significantly higher NF-kB activity was observed in cancer patients DVT+ than DVT-. Significant correlation between data obtained in plasma and monocyte samples was observed. NF-kB inhibition was associated with decreased levels of all molecules in both cancer DVT+ and DVT-. To further demonstrate the involvement of NF-kB activation by the above mentioned molecules, we treated monocyte derived from healthy donors with a pool of sera from cancer patients with and without DVT. These set of experiments further suggest the significant role played by some molecules, regulated by NF-kB, and detected in cancer patients with DVT. Our data support the notion that NF-kB may be considered as a therapeutic target for cancer patients, especially those complicated by DVT. Treatment with NF-kB inhibitors may represent a possible strategy to prevent or reduce the risk of DVT in cancer patients.
Introduction
The relative risk of developing deep venous thrombosis (DVT) is approximately seven times higher in patients with cancer [1,2] suggesting a bidirectional correlation between thrombosis and inflammation in cancer. Chemotherapy is one of the most important risk factors for increased risk of DVT [3,4]. Thrombosis and cancer are linked by numerous pathophysiological mechanisms that are generally related to the host response to cancer. These mechanisms include activation of the coagulation and fibrinolytic systems, acute phase reaction, inflammation, and cytokine production [5]. The systemic activation of coagulation that occurs in malignancy is well known and has been described under the name of Trousseau’s syndrome [6,7]. Systemic inflammation is a potent prothrombotic stimulus leading to an up-regulation of procoagulant factors, down regulation of anticoagulants and inhibition of fibrinolytic activity [8,9]. Chronic inflammation is often associated with increased risk of cancer [10,11]. Rudolf Virchow demonstrated the presence of leukocytes in tumors and suggested that tumors arise at sites of chronic inflammation and that inflammatory mediators, by enhancing cell proliferation, may serve as tumor promoters [12]. Inflammatory cells, cytokines in malignant tumors affect the stromal microenvironment, suggesting that inflammation and cancer may be interrelated through the angiogenic process [13–15]. During inflammation, angiogenesis often coincides with the infiltration of inflammatory cells such as neutrophils, monocytes/ macrophages, which secrete cytokines and growth factors [13,16,17]. It was shown that numerous mediators play a critical role in inflammation, cancer and thrombosis such as C-reactive protein, interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) (markers of inflammation), matrix metalloproteases-9 (MMP-9), vascular endothelial growth factor (VEGF) (reflecting angiogenesis), tissue factor (TF) and fibrinogen (coagulation markers) and soluble P-selectin (marking platelet activation). Inflammatory cytokines up-regulate various angiogenic factors, such as VEGF and MMP-9, in vascular endothelial cells, cancer cells, and monocytes/macrophages [18–35]. Monocytes participate in the pathological processes of inflammation and thrombosis through their ability to synthesize TF and expressing P-selectin upon stimulation [36–38]. Tissue factor (TF), which is the primary cellular initiator of blood coagulation, contributes to the tumor-related pathological processes, such as hypercoagulability, tumor growth, angiogenesis, and metastasis [39–40]. Intriguingly, all of these molecules are regulated by NF-kB [41,42]. This is an inducible transcription factor controlled by the signal activation cascades. NF-kB controls a number of genes involved in inflammatory responses, cell cycle progression, inhibition of apoptosis and cell adhesion, thus promoting tumor angiogenesis, carcinogenesis and cancer progression. Prevention and management of DVT in cancer patients can significantly affect patient treatment, prognosis, and quality of life. Therefore, there is a need to identify novel bio-molecular markers that can recognize cancer patients with high risk of DVT. Although several studies investigated on the role of different bio-markers and/or cytokines in cancer and/or thrombosis, no previous studies have analyzed altogether these NF-kB-regulated markers that in turn regulate NF-kB itself in cancer patients with and without thrombosis. The identification of these markers may recognize NF-kB as an appealing target for therapeutic intervention.
In the present study a fraction of NF-kB-regulated markers have been measured in peripheral blood from cancer patients with and without DVT. Moreover, the effects of dehydroxymethylepoxyquinomicin (DHMEQ), a NF-kB inhibitor [43,44], were evaluated to demonstrate the direct role of the NF-kB-regulated markers in thrombosis development among cancer patients. Furthermore, the identification of biomolecular markers of DVT in cancer patients may support the significance of pharmacological thromboprophylaxis.
Methods
Study population
Peripheral blood samples from three groups of subjects were collected in the last 10 years at the Department of Bio-medical Sciences, University of Catania, Catania, Italy. These groups included: 64 cancer patients with concomitant DVT (DVT+) (mean age 64 ±10 years); 321 cancer patients with no history of DVT (DVT-) (mean age 62 ± 9 years); 100 healthy controls (mean age 61± 12 years), matched by sex and age (Table 1). Patients were diagnosed as affected by the DVT based on the no compression of a deep vein of lower limbs by doppler probe and/or the by the presence of the echogenic pattern into a deep veins of the lower limbs. DVT patients were recruited at the time of the DVT occurrence. Peripheral blood samples from these patients were obtained before the assumption of any specific antithrombotic drug. Detailed information on the study were given to the patients. All patients gave a written informed consent prior to enrolment. Our study was approved by the University of Catania ethics committee. All procedures were conducted in accordance with the principles outlined in the Declaration of Helsinki. All the individuals in this manuscript have given written informed consent (as outlined in PLOS consent form) to participate in this study. Patients treated with anti-inflammatory drugs, statins and anticancer compounds such as, bevacizubam, thalidomide, lenalidomide and/or radiation therapy were excluded. Other exclusion criteria included: BMI > 35 kg/m2, severe systemic depression, diabetes, dyslipidemias, hypertension, chronic inflammatory diseases, heart failure at different stage, mild or severe renal failure (creatinine ≥ 2.0 mg/dl), pancytopenia and more recent surgical intervention (≤ 3 months). Venous blood samples were collected at least after 3 months of the last anticancer treatment for both cancer patients DVT+ and DVT-.
Table 1. Characteristics of controls and cancer patients with and without deep vein thrombosis.
Abbreviations: DVT, deep vein thrombosis; BMI, body mass index.
| Controls (n = 100) % | Cancer (n = 321) % | Cancer DVT+ (n = 64) % | |||||
|---|---|---|---|---|---|---|---|
| Age (Years, mean ± SD) | 60±10 | 62±9 | 64±10 | p = 0.07 $ | |||
| Sex | |||||||
| Male | 57 | (57) | 150 | (46.7) | 35 | (54.7) | p = 0.14 ^ |
| Female | 43 | (43) | 171 | (53.3) | 29 | (45.3) | |
| Smoking habits | |||||||
| Yes | 53 | (53) | 156 | (48.6) | 37 | (57.8) | p = 0.35 ^ |
| No | 47 | (47) | 165 | (51.4) | 27 | (42.2) | |
| BMI (kg/m2, mean ± SD) | 24.5±6.8 | 25.3±7.3 | 26.4±8.3 | p = 0.25 $ | |||
| Metastasis | |||||||
| No | 181 | (56.4) | 29 | (45.3) | p = 0.10 ^ | ||
| Yes | 140 | (43.6) | 35 | (54.7) | |||
| Cancer type or site | |||||||
| Lung | 47 | (14.6) | 7 | (10.9) | p = 0.14 ^ | ||
| Breast | 53 | (16.5) | 6 | (9.4) | |||
| Gastrointestinal | 101 | (31.5) | 31 | (48.4) | |||
| Genitourinary | 41 | (12.8) | 6 | (9.4) | |||
| Hematologic | 34 | (10.6) | 8 | (12.5) | |||
| Other | 45 | (14) | 6 | (9.4) | |||
$Evaluated through analysis of variance (ANOVA).
^Evaluated through χ2 test.
Blood collection and laboratory procedures
Blood was collected from each patient and healthy controls drawn in to pyrogen-free blood collection tubes with and without additives. Citrated platelet-poor plasma was made using two centrifuge steps: 5 min at 4000 r.p.m and 10 min at 11000 r.p.m. Multiple aliquots of serum and plasma were stored at—80°C. Blood samples were utilized for the following analyses: 1) CRP, Fibrinogen, IL-6, TNF-α, IL-1β, MMP-9, VEGF, TF antigen and sP-selectin plasma levels; 2) monocytes isolation. Plasma fibrinogen levels and high-sensitivity C-reactive protein were measured with standard techniques used in the Central Laboratory of Catania University Hospital. IL-6, TNF-α, IL-1β, MMP-9, VEGF and sP-selectin were measured by enzyme-linked immunosorbent assays (ELISAs; R&D Systems Europe, Abingdon, Oxfordshire, UK). Plasma TF antigen level was measured by ELISA using a commercially available kit, IMUBIND (American Diagnostica Inc, Stamford, CT). All assay procedures were performed according to the manufacturer’s protocol. Control specimens were analyzed simultaneously on each plate for every marker.
Reagents
Ficoll Histopaque 1077, RPMI 1640 medium, penicillin-streptomicin solution, L-glutamin, fetal bovin serum (FBS), phosphate-buffered saline solution (PBS), Percoll, lipopolysaccharide (LPS) from Escherichia coli serotype 0128:B12, MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; Thiazolyl blue) and Dimethylsulphoxide (DMSO) were purchased from Sigma Chemical (St. Louis, MO, USA). Iscove's medium and Polymixin B from Gibco, Life Technologies Inc (Milan, Italy).
DHMEQ (dehydroxymethylepoxyquinomicin), synthesized as previously described, [43] was dissolved in dimethylsulfoxide (DMSO) at a concentration of 10 μg/mL and stored at 20°C. This stock solution was diluted in culture medium to a final concentration of <0.1%. Agents. DHMEQ was kindly provided by Dr Kazuo Umezawa, Department of Applied Chemistry, Faculty of Science and Technology, Keio University, Yokohama, Japan.
Isolation of human monocytes
Peripheral blood mononuclear cells (PBMC) were isolated from citrated blood of cancer patients DVT+ and DVT- and healthy controls by Ficoll-Paque. For isolation of monocytes, PBMC were placed for 2 h in culture plate, and the non-adherent cells were removed with three changes of warm PBS. Pure monocytes were then positively selected by anti-CD14-coated magnetic microbeads (Mini MACS separation column; Milteny Biotec, Bergisch Gladbach, Germany) following manufacturer’s instructions. Monocyte purity was > 98%, as assessed by flow cytometry (data not shown). The cells were pooled and resuspended in a final concentration of 1x106 cells/ml in a freeze medium consisting of 30% autologous citrated plasma, 60% Iscove's and 10% DMSO. Next the cells were frozen in aliquots of 1 ml in sterile cryovials (Greiner, Germany) using a standard controlled freezing procedure and then stored in liquid nitrogen.
Treatment and culturing of human monocytes
After thawing at room temperature, the cryopreserved monocytes had a viability of 85%, as shown by trypan blue exclusion. Human monocytes were analyzed for NF-kB activation, for cytokines production and for TF activity assay (see succeeding text). Briefly, monocytes (5 x105/mL) were harvested, washed, and seeded onto wells in RPMI 1640 supplemented with 10% heat-inactivated FBS, L-glutamine (2 mM), penicillin (100 IU/mL), streptomycin (100 μg/mL), and pretreated with DHMEQ (10 μg/mL) for 2 hours and after which incubated for 24 hours. Next, cell culture supernatants were collected for quantification of cytokine protein level by an enzyme-linked immunosorbent assay (ELISA). Endotoxin contamination of cell cultures was routinely excluded with the chromogenic limulus amebocyte lysate assay (Sigma). Furthermore, in all cell cultures 10 μg/ml of polymixin B was added to neutralize any potential LPS contamination.
ELISA for active NF-kB p65 subunit
For measurement NF-kB p65 subunit activation, nuclear extracts were prepared from 5x105 monocytes, pretreated and not with DHMEQ (10 μg/mL) for 2 h and after which stimulated with 100 ng/ml LPS for the desired period of time, using a Nuclear Extract Kit (Active Motif, Rixensart, Belgium) according to the manufacturer’s protocol. Levels of nuclear p65 concentrations were determined by a sensitive ELISA assay (TRANS-AM, Active Motif, Rixensart, Belgium).
Detection of TF activity in monocytes by a chromogenic assay
In cell lysates the activity of TF was measured by actichrome1 TF activity kit (American diagnostic, Pfungstadt, Germany) according to manufacturer’s instructions. In brief. samples were coincubated with factor VII and spectrozyme fVIIa. TF-FVIIa complex cleaves spectrozyme fVIIa as a highly specific chromogenic substrate releasing a paranitroanilin-chromophore with a specific change of absorption at 405 nm. Results are expressed in picomoles per litre (pM) of peptidyl activity of lipidated TF cleaving the spectrozyme fVIIa complex.
Serum-dependent activation of cytokines and NF-kB in monocytes
Monocytes (5x105) from 25 healthy donors were cultured for 20 hours in medium (RPMI 1640) supplemented with either 40% serum from 64 cancer patients DVT+ and 257 DVT- with the highest cytokines plasma levels (≥ 75th percentile) or 40% serum from 100 healthy donors with the lowest cytokines values (≤ 25th percentile). For processing of serum, 80μl of serum from each cancer patient DVT+ and DVT- and from each healthy donor was added to the monocyte culture medium immediately after thawing. The cells were treated with DHMEQ (10 μg/mL) for 2 h, and after which stimulated with 100 ng/mL LPS for the desired period of time. Treatment of monocytes with LPS was used as positive control. In the LPS experiments no polymixin B was added. After incubation, cell culture supernatants were also collected for quantification of cytokine protein level and NF-kB activation by ELISA.
MTT assay
Effects of DHMEQ on cell viability were assayed by the 3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyl tetrasodium bromide (MTT) method [45]. The monocytes (3x105) were seeded in 96-well plates for 48 hours for cytostatic DHMEQ. Serial dilutions of DHMEQ (at 2 μg/mL, 5 μg/mL, 10 μg/mL) were generated and added to the cells. After incubation with DHMEQ or DMSO at the indicated concentrations and time points. Cells treated by MTT solution (1mg/mL) for 4 hours were measured by a microplate reader (Bio-Rad, Richmond, CA) at a reference wavelength of 630 nm and a test wavelength of 570 nm. The cell viability was expressed as a percentage of the DMSO-treated control samples.
Statistical analysis
For continuous variables, difference in mean between study groups was evaluated through the analysis of variance (ANOVA, in the case of three groups) or t-test (two groups). For discrete variables, the difference in distribution across study groups was evaluated through the test χ2 test. The correlation between continuous variables was evaluated through the Spearman rank correlation coefficient r.
Results
Baseline characteristics of healthy controls and cancer patients with and without DVT are shown in Table 1. No significant differences were observed among groups with respect to age, sex, current smoking, and body mass index. The frequency of localized cancers was similar among cancer patients with and without DVT, as well as the distribution of cancer type/site (Table 1).
Biomarker plasma levels of inflammation, angiogenesis and coagulation in cancer patients DVT+ and DVT-
Mean plasmatic levels of different biomarkers, including those of inflammation, angiogenesis and coagulation, analyzed in the control group and in cancer patients with and without DVT are reported in Table 2. Compared to control group, higher plasma levels of CRP, fibrinogen, IL-6, TNF-α, IL-1β, MMP-9, VEGF, TF antigen, and sP-selectin were observed in cancer patients with and without DVT (P<0.01). As expected, the concentration of all markers in DVT+ group was significantly higher than in DVT- (p<0.01) with the only exception of fibrinogen (p = 0.35). Correlation of all markers with each other are reported in (S1 Table). Positive correlations (|r|>0.45) among CRP, IL-6, TNF-α, IL-1β, MMP-9, VEGF, and TF antigen were found in both groups of cancer patients, whereas the fibrinogen correlated only in DVT+ group. No correlation among markers were observed in healthy controls (S1 Table).
Table 2. Plasma levels of inflammation, angiogenic and coagulation markers from controls and cancer with and without deep vein thrombosis.
Abbreviations: DVT, deep vein thrombosis; IL-6, interleukin-6; TNF- α, tumor necrosis factor-α; IL-1β, interleukin-1β; CRP, C-reactive protein; MMP-9, matrix metalloproteinase-9; VEGF, vascular endothelial growth factor; TF, tissue factor; soluble P-selectin (sP-selectin). Significant differences among inflammatory, angiogenic and coagulation markers were evident in cancer patients with and without DVT compared to controls with further increments in DVT cancer patients. P value are given using analysis of variance (ANOVA test, in the case of three groups) or t-test (two groups).
| Controls (n = 100) | Cancers (n = 321) | Cancers DVT+ (n = 64) | Controls vs Cancers vs Cancer DVT+ | Cancers vs Cancer+DVT | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| Inflammation markers | |||||
| IL-6 | 3.7 ± 1.5 | 11.5 ± 3.9 | 16.7 ± 4.9 | p<0.01 | p<0.01 |
| TNF-α | 2.6 ±1.3 | 8.5 ± 3.7 | 10.6 ± 3.4 | p<0.01 | p<0.01 |
| IL-1β | 1.5 ± 0.7 | 5.5 ± 1.8 | 9.4 ± 2.5 | p<0.01 | p<0.01 |
| CRP | 0.1 ± 0.1 | 0.8 ± 0.7 | 1.6 ± 1.1 | p<0.01 | p<0.01 |
| Angiogenesis markers | |||||
| MMP-9 | 43.0 ± 16.1 | 153.1 ± 50.5 | 204.3 ± 60.4 | p<0.01 | p<0.01 |
| VEGF | 60.1 ± 33.2 | 322.7 ± 125.1 | 439.3 ± 126.8 | p<0.01 | p<0.01 |
| Procoagulant markers | |||||
| Fibrinogen | 246.4 ± 47.2 | 404.2 ± 71.1 | 413.7 ± 87.7 | p<0.01 | p = 0.35 |
| TF | 30.4 ± 7.8 | 141.1 ± 34.0 | 184.5 ± 41.4 | p<0.01 | p<0.01 |
| sP-selectin | 36.4 ± 11.5 | 55.4 ± 22.5 | 75.8 ± 32.3 | p<0.01 | p<0.01 |
Cytokines and MMP-9 secretion in human monocytes
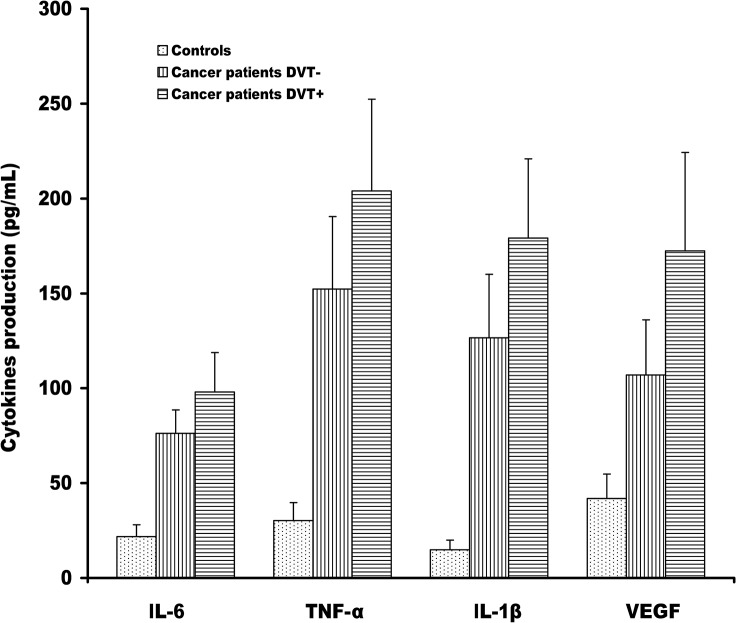
The secretion of these markers analyzed in monocytes supernatant showed similar trend to that observed in plasma. Spontaneous production of cytokines, such as IL-6, TNF-α, Il-1β and VEGF were significantly higher in both groups of cancer patients with and without DVT than those from healthy controls (p<0.0001) (Fig 1). Compared to DVT- cancer patients, DVT+ showed an increased fold change of 1.28 (95% CI: 1.20–1.37; p<0.0001), 1.34 (95% CI: 1.23–1.46; p<0.0001), 1.41 (95% CI: 1.30–1.54; p<0.0001), and 1.61 (95% CI: 1.45–1.78; p<0.0001) for IL-6, TNF-α, Il-1β, and VEGF, respectively. In addition to cytokines, also MMP-9 production and TF activity were higher in DVT+ cancer patients than in those DVT- and in healthy controls (mean ± SD, 103 ± 23, 73 ± 24, 8.8 ± 4, for MMP-9 and mean ± SD, 35.6 ± 6.4, 28 ± 5, 3.3 ± 0.7 for TF activity, respectively). As expected, strong correlations between levels of IL-6, TNF-α, IL-1β, VEGF, MMP-9 and TF in plasma from cancer patients DVT+ and DVT- and those released from monocytes of the same patients were observed (Table 3).
Fig 1. Cytokines secretion in monocytes from cancer patients with and without DVT.
Levels of IL-6, TNF-α, IL-1β, and VEGF were measured in supernatants of purified monocytes from cancer patients with and without DVT by a sensitive enzyme-linked immunosorbent assay (ELISA). The results are shown as the means ± SD.
Table 3. Spearman correlation coefficients between in vivo and in vitro marker concentrations.
DVT, deep vein thrombosis. A positive strong correlation between the plasma cytokines, angiogenic and coagulation markers and the secretion in vitro of the same markers in two groups of cancer patients with and without DVT.
| Cancer patients (n = 321) | Cancer patients +DVT (n = 64) | |
|---|---|---|
| IL-6 vs IL-6 monocytes | 0.746 | 0.758 |
| TNF-α vs TNF-α monocytes | 0.684 | 0.708 |
| IL-1β vs IL-1β monocytes | 0.750 | 0.772 |
| MMP-9 vs MMP-9 monocytes | 0.728 | 0.697 |
| VEGF vs VEGF monocytes | 0.805 | 0.740 |
| TF antigen vs TF activity monocytes | 0.52 | 0.61 |
Effect of DHMEQ on NF-kB p65 subunit activity in monocytes
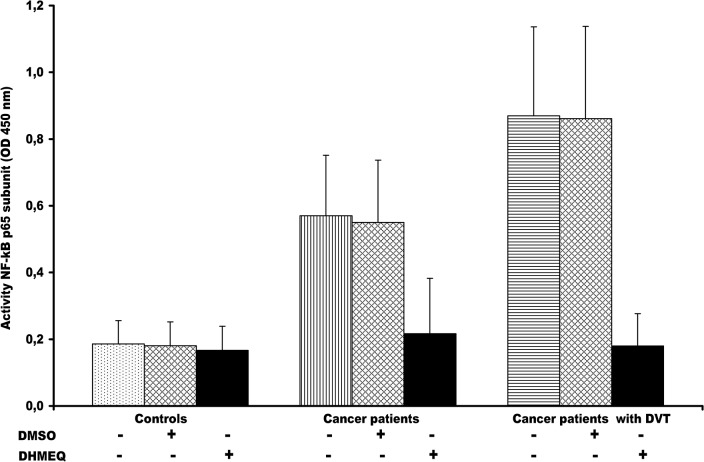
Activation of NF-kB p65 subunit in unstimulated monocytes, from cancer patients DVT+ and DVT- and from healthy control, was analyzed by a sensitive ELISA assay. This assay has the advantage of being 10-fold more sensitive than electrophoresis mobility shift assay (EMSA) and allows greater flexibility in the experimental step. As shown in Fig 2, NF-kB p65 subunit activity in monocytes varied in all examined groups. Higher NF-kB p65 subunit activity was observed in cancer patients DVT+ and DVT- than in healthy controls (p< 0.0001). Significant differences of NF-kB p65 activity were also detected between cancer patients DVT+ and DVT- (p< 0.0001). It was already shown that NF-kB regulates several molecules including those analyzed in the present study [41,42]. Accordingly, all these markers positively correlated with NF-kB activity (Table 4). The identification of these markers may recognize NF-kB as an attractive target for therapeutic intervention. Therefore, we thought to inhibit NF-kB by DHMEQ, a known NF-kB inhibitor [46,47]. The effect of DHMEQ, at the dose of 10 μg/ml, on NF-kB p65 subunit activity inhibition in monocytes from the two groups of cancer patients, was explored (Fig 2). Control experiments with DMSO have been included (Fig 2). Notably, DHMEQ was not effective in monocytes from healthy controls at different dose and time (S1 Fig). Although, DHMQ caused the reduction of cell viability of monocytes derived from cancer patients DVT+ and DVT-, this effect was not observed in monocytes from healthy controls (S2 Fig).
Fig 2. Nuclear factor (NF)–kB p65 subunit activity in monocytes from cancer patients with and without DVT and effect of DHMEQ.
The activated NF-kB p65 subunit was significantly higher in cancer patients DVT+ and DVT- than in healthy controls (P< 0.0001). (ANOVA test). NF-kB p65 subunit was significantly higher in cancer patients DVT+ than in those DVT- (P< 0.001) (t-test). To examine the effects of DHMEQ, monocytes were treated with 10 μg/mL DHMEQ. As control experiments, DMSO was used instead of DHMEQ. Monocytes from cancer patients DVT+ were more responsive to DHMEQ than those from DVT-. Intriguingly, no effect of DHMEQ there was in healthy monocytes. The results are shown as the means ± SD. OD, optical density; DVT, Deep Vein thrombosis; DMSO, dimethyl sulfoxide; DHEMQ, dehydroxymethylepoxyquinomicin.
Table 4. Correlation between NF-kB p65 activity and inflammation, angiogenetic and thrombotic molecules in monocytes of cancer patients with and without deep vein thrombosis.
Abbreviations: DVT, deep vein thrombosis. A positive correlation was found between NF-kB p65 subunit activity and all molecules in cancer patients with and without DVT. Spearman Rank correlation analysis was used.
| Cancer patients (n = 64) | Cancer patients+ DVT (n = 64) | |
|---|---|---|
| NF-kB vs IL-6 | 0.66 | 0.73 |
| NF-kB vs TNF-α | 0.58 | 0.63 |
| NF-kB vs IL-1 beta | 0.54 | 0.57 |
| NF-kB vs MMP-9 | 0.43 | 0.64 |
| NF-kB vs VEGF | 0.51 | 0.65 |
| NF-kB vs TF | 0.59 | 0.67 |
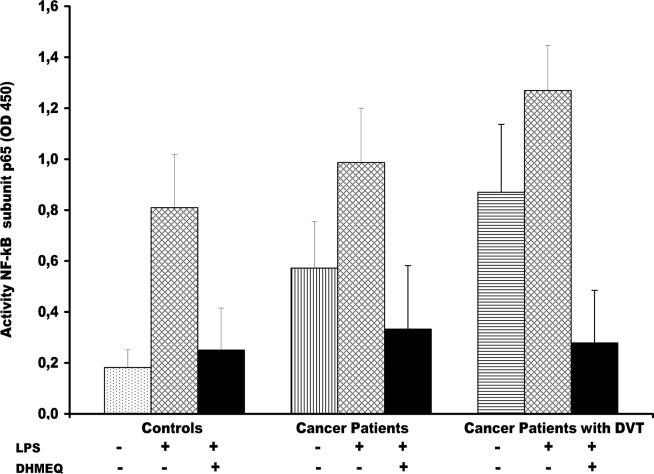
Effect of LPS and DHMEQ on nuclear factor-kB p65 in monocytes
As shown in Fig 3, treatment of monocytes with LPS at 100 ng/mL strongly activated NF-kB p65 of 46%, 73% and 35% over the basal value compared to unstimulated cells in cancer patients DVT+, DVT- and in healthy controls, respectively (p<0.0001). Preincubation with DHMEQ, prior to LPS stimulation, strongly decreased NF-kB p65 subunit activity of 4.5-fold, 3-fold and 3.2-fold in cancer DVT+ and DVT- and in healthy controls (p<0,0001), respectively, compared with LPS alone. To reinforce our findings on NF-kBp65 down-regulation by DHMEQ in LPS-activated monocytes, the amount of NF-kB p65 subunit activity in nuclear extracts from the same experiments were subsequently measured by ELISA. As expected, treatment with DHMEQ in LPS stimulated monocyte induced the decrease of NF-kB activity among the three groups analyzed (Fig 3).
Fig 3. Effect of dehydroxymethylepoxyquinomicin (DHMEQ) on lipopolysaccharide (LPS)-induced nuclear factor (NF)–kB p65 activation in monocytes from cancer patients with and without DVT.
Nuclear extracts were prepared from monocytes, pretreated and not with DHMEQ (10 μg/mL) for 2 h and after which stimulated with 100 ng/ml LPS for 24 hour, using a Nuclear Extract Kit. The stimulation of monocytes with LPS at 100 ng/mL induce un significant increase of the nuclear NF-kB p65 protein level in all groups or cancer patients DVT+, DVT- and in healthy controls, compared to unstimulated cells, (P<0.0001). NF-kB p65 subunit was significantly higher in cancer patients DVT+ than in those DVT- (P< 0.0001) (t-test). DVT, Deep Vein thrombosis.
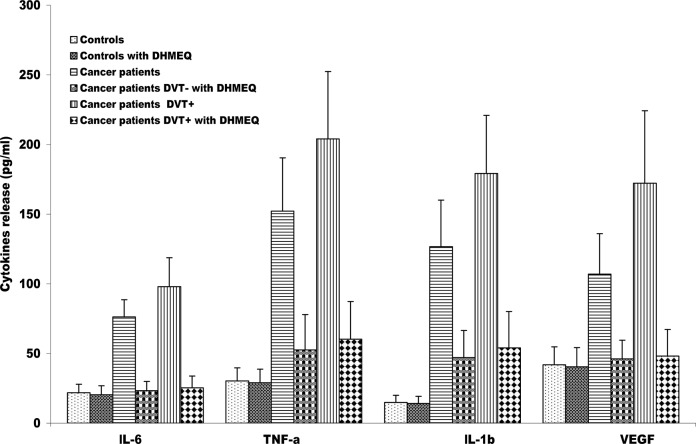
Effects of DHMEQ on markers release by monocytes
DHMEQ treatment was used to asses if NF-kB is directly associated with the release of all markers, detected above, from monocytes of cancer patients DVT+ and DVT-. The Fig 4 shows that treatment with DHMQ dramatically decreases the levels of IL-6, TNF-alpha, IL-1beta and VEGF in both groups of cancer patients compared to untreated cells. Similar trend was observed for MMP-9 and TF. In contrast, no differences were observed in the control group in which constitutive NF-kB activation was absent.
Fig 4. Effects of dehydroxymethylepoxyquinomicin (DHMEQ) on cytokines release by monocytes from cancer patients with and without DVT.
Monocytes were treated or not with 10 μg/mL DHMEQ, after which the amounts of interleukins (IL)-6, tumor necrosis factor alpha (TNF-α), IL-1β and vascular endothelial growth factor (VEGF) secreted were measured by enzyme-linked immunosorbent assay (ELISA). Monocytes were incubated for 24 hr. The treatment with DHMEQ induces the decrease of all molecules in both groups of cancer patients with and without DVT compared to untreated cells (P<0.0001), (t-test). The results are shown as the means ± SD. DVT, Deep Vein thrombosis;
Serum-dependent activation of NF-kB in healthy monocytes
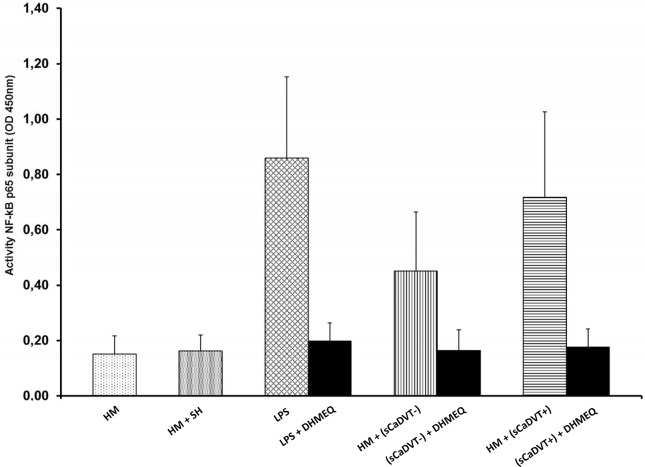
As shown in Fig 5, the incubation of healthy monocytes with pooled sera, derived from the cancer patients DVT+ or DVT- with the highest values of cytokines, induced a significant increase of NF-kB activation compared with pooled sera, derived from the healthy controls with the lowest values of the same cytokines, (p<0.0001). In addition, a higher NF-kB activation was observed in monocytes stimulated by sera derived from cancer patients DVT+ compared to that stimulated by sera derived from DVT- (p<0.001) (Fig 5). In order to further exclude the possibility that some LPS contamination might contribute to the NF-kB activation, 10 μg/ml of polymixin B was added in all cell cultures to neutralize any potential LPS contamination. Instead, as positive control of NF-kB activation, LPS was considered. LPS-stimulated cultures induced an higher NF-kB activity statistically significant compared to cancer derived sera-stimulated cultures (p<0.001), while no significant difference was evident between LPS-stimulated cultures and DVT cancer derived sera- stimulated cultures. In the LPS experiments no polymixin B was added. The NF-kB activation, stimulated with the sera derived from cancer patients with and without DVT or LPS, was partially blocked by DHMEQ (10 μg/mL) when added in both cultures and LPS-stimulated cultures.
Fig 5. Serum-dependent activation of nuclear factor (NF)–NF-kB p65 subunit in healthy monocytes.
Monocytes from 25 healthy controls were evaluated for NF-kB activation after they were cultured for 20 hours in medium supplemented with either 40% serum from three groups. Monocytes (5x105) from 25 healthy donors were cultured for 20 hours in medium (RPMI 1640) supplemented with either 40% serum derived from 64 cancer patients DVT+ and 257 DVT- with the highest cytokines plasma levels (> 75th percentile) or 40% serum derived from 100 healthy donors with the lowest cytokines values (< 25th percentile). The incubation of healthy monocytes with pooled sera derived from cancer patients DVT+ (sCADVT+) or DVT- (sCADVT-) induced a significant increase of NF-kB activity compared with that derived from healthy controls (HM) after treatment with sera from healthy controls (SH) (P<0.0001). An higher NF-kB p65 subunit activation was observed in monocytes stimulated by sera from cancer patients DVT+ compared to that stimulated by sera derived from DVT- (P<0.001) (t-test). No NF-kB p65 subunit activation was observed in monocytes stimulated with sera derived from healthy controls.
Serum-dependent activation of cytokines production in healthy monocytes
In addition to NF-kB activity, we explored the activation of cytokines in healthy monocytes treated with pooled sera obtained from cancer patients DVT+ and DVT- and from healthy controls. Higher mean levels of IL-6, TNF-α, IL-1β, VEGF, MMP-9 and TF were secreted from healthy monocytes treated with the sera derived from cancer patients DVT compared to those from DVT- (p<0.01). On the contrary, healthy monocyte treated with the sera from healthy controls, showed no significant effects (Table 5). When healthy monocytes were incubated with the sera derived from patients with and without DVT in the presence of DHMEQ, the cytokines production strongly decreased compared to untreated cells (p<0.0001) (Table 6).
Table 5. Serum-depend activation of NF-kB regulated markers production from healthy monocytes.
HM, Monocytes from healthy subjects; sH, sera from healthy subjects; LPS, lipopolysaccharide; sCaDVT-, serum from cancer patients without deep venous thrombosis; sCADVT+, serum from cancer patients with deep venous thrombosis; IL-6, Interleukin-6; TNF- α, Tumor necrosis factor alpha; IL-1β, Interleukin-1 beta; VEGF, Vascular endothelial growth factor; MMP-9, matrix metalloproteinase-9; TF, Tissue factor. All p values were calculated by Wilcoxon Matched-Pairs Signed-Ranks Test.
| HM | HM + sH | P value° | HM + sCaDVT- | HM + sCaDVT+ | P value* | |
|---|---|---|---|---|---|---|
| IL-6 | 21.1 ± 6.1 | 21 ± 7.1 | ns | 66.4 ± 25.4 | 82.3 ± 25.9 | 0.01 |
| TNF-α | 30.3 ± 9.4 | 29.2 ± 9.2 | ns | 121 ± 44.2 | 143 ± 43.6 | 0.01 |
| IL-1β | 15 ± 5.1 | 14 ± 5.9 | ns | 80.2 ± 27.2 | 111 ± 34.9 | 0.01 |
| VEGF | 42 ± 12.8 | 36 ± 11.6 | ns | 98.8 ± 17.6 | 121 ± 28.7 | 0.01 |
| MMP-9 | 8.8 ± 3.7 | 9.4 ± 4.9 | ns | 58.4 ± 12.4 | 70.9 ± 17 | 0.01 |
| TF | 2.7 ± 0.8 | 2.5 ± 0.5 | ns | 57.2 ± 14.7 | 85.4 ± 18.6 | 0.01 |
(°) p value calculated between HM and (HM+sH)
(*) p value calculated between (HM + sCaDVT-) and (HM + sCaDVT+).
Table 6. Serum-depend activation of NF-kB regulated markers production from healthy monocytes treated with DHMEQ.
HM, Monocytes from healthy subjects; sH, sera from healthy subjects; LPS, lipopolysaccharide; sCaDVT-, serum from cancer patients without deep venous thrombosis; sCADVT+, serum from cancer patients with deep venous thrombosis; IL-6, Interleukin-6; TNF- α, Tumor necrosis factor alpha; IL-1β, Interleukin-1 beta; VEGF, Vascular endothelial growth factor; MMP-9, matrix metalloproteinase-9; TF, Tissue factor. All p values were calculated by Wilcoxon Matched-Pairs Signed-Ranks Test.
| HM + sCaDVT- | HM + sCa DVT-+ DHMEQ | P value $ | HM + sCaDVT+ | HM + sCaDVT+ DHMEQ | P value £ | |
|---|---|---|---|---|---|---|
| IL-6 | 66.4 ± 25.4 | 19.4 ± 9 | 0.0001 | 82.3 ± 25.9 | 22 ± 7.1 | 0.0001 |
| TNF-α | 121 ± 44.2 | 30.7 ± 9.2 | 0.0001 | 143 ± 43.6 | 32 ± 9.7 | 0.0001 |
| IL-1β | 80.2 ± 27.2 | 15.2 ± 6.4 | 0.0001 | 111 ± 34.9 | 16.2 ± 7.3 | 0.0001 |
| VEGF | 98.8 ± 17.6 | 35 ± 10 | 0.0001 | 121 ± 28.7 | 37 ± 11 | 0.0001 |
| MMP-9 | 58.4 ± 12.4 | 10.2 ± 5.5 | 0.0001 | 70.9 ± 17 | 11 ± 5.1 | 0.0001 |
| TF | 57.2 ± 14.7 | 2.8 ± 0.8 | 0.0001 | 85.4 ± 18.6 | 3.1 ± 1.2 | 0.0001 |
($) p value calculated between (HM + sCaDVT-) and (HM + sCa DVT-+ DHMEQ)
(£) (HM + sCaDVT+) and (HM + sCaDVT+ DHMEQ)
Control experiments, conducted with LPS, indicated that a remarkable significant enhancement of all markers production, compared to those stimulated with the sera derived from cancer patients DVT- (p<0.0001). As expected, no significant differences were observed in the production of all markers between the group of healthy monocytes treated with LPS and that treated with the sera derived from cancer patients DVT+ (S2 Table).
Discussion
Patients with cancer can experience complications including thrombosis, bleeding, and disseminated intravascular coagulation. The identification of novel therapeutic targets in cancer patients with high risk of DVT may encourage further protective studies to improve the management of these patients. To our knowledge, this is the first report that analyzes multiple NF-kB-dependent markers in cancer patients with and without DVT.
In addition, protein secretion was also tested in order to find out whether the changes in the cytokine plasma levels were associated with changes in cytokines production. As expected, the plasma levels of markers analyzed were upregulated in the cancer patients with and without DVT compared to healthy controls, although they were statistically higher in DVT+, with the exception of fibrinogen that was similar in both groups. The cytokines measured in our experiments are largely involved in the upregulation of inflammatory reactions and thus in numerous malignancies. When we have investigated the protein's secretion, the same plasma trend was also observed in monocytes of patients. We have found an increasing release of inflammatory cytokines, MMP-9 and VEGF in monocytes from two groups of cancer patients with the highest release levels in the group with DVT. As expected, the involvement of tumor microenvironment in cancer and in its complications, such as DVT, is emphasized in the correlation between plasma levels of IL-6,TNF-α, IL-1β, VEGF and MMP-9 from cancer patients with and without DVT and those released from monocytes. In addition, mTF activity was very high in cancer patients with DVT. It is an important coagulation factor that has been reported in many types of cancers [48,49]. Furthermore, mTF activity in our patients linearly correlated with TF antigen and other inflammatory and angiogenic markers in vivo and in vitro. Cytokines such as, IL-6, TNF-α and IL-1β have been shown to be able to increase tumor cell pro-coagulant activity [50–52], enhancing clotting activation in cancer patients.
Therefore, increased production of all these markers, found in the present study, may result as activation of host cells, such as monocytes, present in tumor environment, and/or of cytokines released by tumor cells themselves. Malignant cells can directly activate blood coagulation by regulating fibrinogen, tissue factor, cancer procoagulant activity, inflammatory reactions and cytokines [53]. These findings are in agreement with previous studies, demonstrating increased plasmatic or cellular release in patients with different tumor types [21,23,54–60], and in those with DVT [28,61–64].
As previously mentioned, monocytes of two groups of cancer patients produced high levels of CRP, TNF-α and IL-1β, that may stimulate NF-kB activity by an autocrine mechanism [42]. A growing body of studies suggests that NF-kB activation mediates up-regulation of inflammatory cytokines, angiogenic factors (e.g. CRP, IL-6, TNF-α, IL-1β, VEGF, MMP-9) in cancer [41,65–69]. Administration of selective inhibitors of the NF-kB pathway can sensitize tumor cells to reduce the release of cytokines [47,70,71]. We have, first, observed that the activity on NFkB p65 subunit in our patient groups examined was higher when compared with controls. Next, we evaluated the effect of the DHMEQ, a specific inhibitor of NF-kB, to determine whether NF-kB inhibition was associated with the decrease of IL-6,TNF-a, IL-1β, VEGF, MMP-9 and mTF activity. As expected, all inflammatory, angiogenic and thrombotic cytokine, were significantly reduced when the monocytes of patients were treated with DHMEQ. These observation are in agreement with recent in vitro studies [72,73].
Tumor microenvironment, in our patient groups, may be responsible of NFkB activation [41,67,74]. To further investigate the inhibitory mechanism of DHMEQ action, we examined its effect on monocytes LPS-mediated NF-kB activation. A precise monitoring of NF-kB activation in cells of cancer patients with and without DVT is essential for signal transduction pathway analysis. In this study, we also showed that a constitutive activation of NF-kB p65 subunit was more elevated in cancer patients with DVT than in cancer patients without DVT; NF-kB activation was not evident in healthy controls. In addition, we have observed that LPS-induced NF-kB activation was significantly reduced in DHMEQ pretreated monocytes from cancer patients with and without DVT and in healthy controls.
A correlation among NF-kB and inflammatory, angiogenic and thrombotic markers, here observed, suggest that NF-kB activation is considered an amplifying and perpetuating mechanism of the inflammation, angiogenic and thrombotic process. Accordingly, it was shown that NF-kB regulates the expression of many genes producing, cytokines, chemokines and adhesion molecules involved in the cascade of both inflammation and coagulation [51,75–77]. Tumor cells themselves can induce the expression of TF by host cells such as endothelial cells or monocytes/macrophages [78]. Thus, TF on tumor or host cell surface represent a major mediator of clotting activation at the tumor-host interface.
To further demonstrated if NF-kB activation was mediated by the several molecules involved in both cancer and DVT development, we used a pool of sera from cancer patients with and without DVT to treat the monocyte derived from healthy donors. The results suggest the significant role played by all molecules, detected in cancer patients, that are able to activate NF-kB pathway that in turn enhance the production of inflammatory, angiogenic and thrombotic markers.
Overall, the results of the present study suggest that the inhibition of NF-kB by DHMEQ reduces the positive inflammatory, angiogenic and thrombotic loop generated by multiple markers detected in both cancer and DVT. Therefore, our data support the notion that NF-kB transcription factor may be considered as an excellent therapeutic target for cancer patients, especially those complicated by DVT and treatment with NF-kB inhibitors may corroborate the efficacy of chemotherapeutic agents.
Supporting Information
Dose and time dependent manner. (A) Dose-dependent reduction of NF-kB p65 subunit activity was observed in monocytes treated with DHMEQ. Monocytes from cancer patients DVT+ and DVT- and those from healthy volunteers were treated at the indicated concentrations of DHMEQ and time points. (B) Time course analyses of NF-kB p65 subunit activity in monocytes from three groups analyzed treated with DHMEQ. NF-kB p65 subunit activity was measured by ELISA. Data represent the mean ± SD in the 3 groups of individuals.
(TIF)
(A) Dose-dependent reduction of cell viability of monocytes treated with DHMEQ. Monocytes from cancer patients with and without DVT and those of healthy volunteers, used as controls, were treated at the indicated concentrations of DHMEQ and time points. Cell viability were determined by MTT assay. (B) Time course analyses of cell viabilities of monocytes from three groups analyzed treated with DHMEQ. Data represent the mean ± SD of relative viabilities of 3 independent experiments.
(TIF)
Note: CRP, C Reactive Protein, IL-1β, Interleukin-1 beta; IL-6, Interleukin-6; TNF- α, Tumor necrosis factor-alpha; MMP-9, matrix metalloproteinase-9; VEGF, Vascular endothelial growth factor; TF, Tissue factor; sP, Spearman correlation.
(DOC)
Note: HM, Monocytes from healthy subjects; sH, sera from healthy subjects; LPS, lipopolysaccharide; sCaDVT-, serum from cancer patients without deep venous thrombosis; sCADVT+, serum from cancer patients with deep venous thrombosis; IL-6, Interleukin-6; TNF- α, Tumor necrosis factor alpha; IL-1β, Interleukin-1 beta; VEGF, Vascular endothelial growth factor; MMP-9, matrix metalloproteinase-9; TF, Tissue factor. All p values were calculated by Wilcoxon Matched-Pairs Signed-Ranks Test. (§) p value calculated between (HM + LPS) and (HM + sCaDVT-); (^) p value was calculated between (HM + LPS) and (HM + sCaDVT+).
(DOCX)
Data Availability
All relevant data are freely available in the paper and in Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost. 2002; 7: 575–579. [PubMed] [Google Scholar]
- 2. Wun T, White RH. Venous thromboembolism (VTE) in patients with cancer: epidemiology and risk factors. Cancer Invest. 2009; 27: S63–S74. [DOI] [PubMed] [Google Scholar]
- 3. Haddad TC, Greeno EW. Chemotherapy-induced thrombosis. Thromb Res 2006; 118: 555–568. [DOI] [PubMed] [Google Scholar]
- 4. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007; 5: 632–634. [DOI] [PubMed] [Google Scholar]
- 5. Rickles FR, Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb Res. 2001; 102: V215–224. [DOI] [PubMed] [Google Scholar]
- 6. Rickles FR, Edward RL. Activation of blood coagulation in cancer: Trousseau’s syndrome revisited. Blood. 1983; 62: 14–17. [PubMed] [Google Scholar]
- 7. Sorensen HT, Mellemkjaer L, Steffensen FH, Olsen JH, Nielsen GL. The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N Engl J Med. 1998; 338: 1169–1173. [DOI] [PubMed] [Google Scholar]
- 8. Esmon CT. Does inflammation contribute to thrombotic events? Haemostasis. 2000; 30 (Suppl 2): S34–S40. [DOI] [PubMed] [Google Scholar]
- 9. Szaba FM, Smiley ST. Roles for thrombin and fibrinogen in cytokine/chemokine production and macrophage adhesion in vivo. Blood. 2002; 99: 1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140: 883–899. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009; 9: 351–369 10.1016/j.coph.2009.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001; 357: 539–545. [DOI] [PubMed] [Google Scholar]
- 13. Ono Mayumi. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008; 99: 1501–1506. 10.1111/j.1349-7006.2008.00853.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merogi AJ, Marrogi AJ, Ramesh R, Robinson WR, Fermin CD, Freeman SM, et al. Tumor-host interaction: analysis of cytokines, growth factors, and tumor-infiltrating lymphocytes in ovarian carcinomas. Hum Pathol. 1997; 28: 321–331. [DOI] [PubMed] [Google Scholar]
- 15. Signorelli SS, Mazzarino MC, Di Pino L, Malaponte G, Porto C, Pennisi G, et al. High circulating levels of cytokines (IL-6 and TNFalpha), adhesion molecules (VCAM-1 and ICAM-1) and selectins in patients with peripheral arterial disease at rest and after a treadmill test. Vasc Med. 2003; 8: 15–19. [DOI] [PubMed] [Google Scholar]
- 16. Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007; 10: 149–166. [DOI] [PubMed] [Google Scholar]
- 17. Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004; 4: 71–78. [DOI] [PubMed] [Google Scholar]
- 18. Hopkins MH, Flanders WD, Bostick RM. Associations of circulating inflammatory biomarkers with risk factors for colorectal cancer in colorectal adenoma patients. Biomark Insights. 2012; 7: 143–150. 10.4137/BMI.S10092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dima SO, Tanase C, Albulescu R, Herlea V, Chivu-Economescu M, Purnichescu-Purtan R, et al. An exploratory study of inflammatory cytokines as prognostic biomarkers in patients with ductal pancreatic adenocarcinoma. Pancreas. 2012; 41: 1001–1007. 10.1097/MPA.0b013e3182546e13 [DOI] [PubMed] [Google Scholar]
- 20. Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr Drug Targets. 2010; 11: 1133–1146. [DOI] [PubMed] [Google Scholar]
- 21. Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith CW, et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999; 5: 1369–1379. [PubMed] [Google Scholar]
- 22. Goldenberg N, Kahn SR, Solymoss S. Markers of coagulation and angiogenesis in cancer-associated venous thromboembolism. J Clin Oncol. 2003; 21: 4194–4199. [DOI] [PubMed] [Google Scholar]
- 23. Yoshida N, Ikemoto S, Narita K, Sugimura K, Wada S, Yasumoto R, et al. Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. Br J Cancer. 2002; 86: 1396–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Culig Z. Cytokine disbalance in common human cancers. Biochim Biophys Acta. 2011; 1813: 308–314. 10.1016/j.bbamcr.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 25. Macrì A, Versaci A, Loddo S, Scuderi G, Travagliante M, Trimarchi G, et al. Serum levels of interleukin 1beta, interleukin 8 and tumour necrosis factor alpha as markers of gastric cancer. Biomarkers. 2006; 11: 184–193. [DOI] [PubMed] [Google Scholar]
- 26. Kwon KA, Kim SH, Oh SY, Lee S, Han JY, Kim KH, et al. Clinical significance of preoperative serum vascular endothelial growth factor, interleukin-6, and C-reactive protein level in colorectal cancer. BMC Cancer. 2010; 10: 203 10.1186/1471-2407-10-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Castellano G, Malaponte G, Mazzarino MC, Figini M, Marchese F, Gangemi P, et al. Activation of the osteopontin/matrix metalloproteinase-9 pathway correlates with prostate cancer progression. Clin Cancer Res. 2008; 14: 7470–7480. 10.1158/1078-0432.CCR-08-0870 [DOI] [PubMed] [Google Scholar]
- 28. Ten Cate JW, van der Poll T, Levi M, ten Cate H, van Deventer SJ. Cytokines: triggers of clinical thrombotic disease. Thromb Haemost. 1997; 78: 415–419. [PubMed] [Google Scholar]
- 29. Christiansen SC, Naess IA, Cannegieter SC, Hammerstrøm J, Rosendaal FR, Reitsma PH, et al. Inflammatory cytokines as risk factors for a first venous thrombosis: a prospective population-based study. PLoS Med. 2006; 3: e334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ay C, Simanek R, Vormittag R, Dunkler D, Alguel G, Kornek G, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood. 2008; 112: 2703–2708. 10.1182/blood-2008-02-142422 [DOI] [PubMed] [Google Scholar]
- 31. Song CJ, Nakagomi A, Chandar S, Cai H, Lim IGS, McNeil HP, et al. C-reactive protein contributes to the hypercoagulable state in coronary artery disease. J Thromb Haemost. 2006; 4: 98–106. [DOI] [PubMed] [Google Scholar]
- 32. Khorana AA (2012) Cancer-associated thrombosis: updates and controversies. Hematology Am Soc Hematol Educ Program. 2012: 626–630. 10.1182/asheducation-2012.1.626 [DOI] [PubMed] [Google Scholar]
- 33. Ryuto M, Ono M, Izumi H, Yoshida S, Weich HA, Kohno K, et al. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J Biol Chem. 1996; 271: 28220–28228. [DOI] [PubMed] [Google Scholar]
- 34. Cohen T, Nahari D, Weiss Cerem L, Neufeld G, Levi B. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996; 271: 736–741. [DOI] [PubMed] [Google Scholar]
- 35. Roselli M, Mineo TC, Basili S, Mariotti S, Martini F, Bellotti A, et al. Vascular endothelial growth factor (VEGF-A) plasma levels in non-small cell lung cancer: relationship with coagulation and platelet activation markers. Thromb Haemost. 2003; 89: 177–184. [PubMed] [Google Scholar]
- 36. Edwards RL, Rickles FR, Bobrove AM. Mononuclear cell tissue factor: cell of origin and requirements for activation. Blood. 1979; 54: 359–370. [PubMed] [Google Scholar]
- 37. Osterud B. Tissue factor expression by monocytes: regulation and pathophysiological roles. Blood Coagul Fibrinolysis. 1998; 9: S9–14. [PubMed] [Google Scholar]
- 38. Celi A, Pellegrini G, Lorenzet R, De Blasi A, Ready N, Furie BC, et al. P-selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci USA. 1994; 91: 8767–8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rak J, Milsom C, May L, Klement P, Yu J. Tissue factor in cancer and angiogenesis: the molecular link between genetic tumor progression, tumor neovascularization, and cancer coagulopathy. Semin Thromb Hemost. 2006; 32: 54–70. [DOI] [PubMed] [Google Scholar]
- 40. Han X, Guo B, Li Y, Zhu B. Tissue factor in tumor microenvironment: a systematic review. J Hematol Oncol. 2014; 1: 7–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappa B functions as a tumour promoter in inflammation-associated cancer. Nature. 2004; 431: 461–466. [DOI] [PubMed] [Google Scholar]
- 42. Mercurio F, Manning AM. Multiple signals converging on NF-kappa B. Curr Opin Cell Biol. 1999; 11: 226–232. [DOI] [PubMed] [Google Scholar]
- 43. Matsumoto N, Ariga A, To-e S, Nakamura H, Agata N, Hirano S, et al. Synthesis of NF-kappa B activation inhibitors derived from epoxyquinomicin C. Bioorg Med Chem Lett. 2000; 10: 865–869. [DOI] [PubMed] [Google Scholar]
- 44. Umezawa K, Chaicharoenpong C. Molecular design and biological activities of NF-kappaB inhibitors. Mol Cells. 2002; 14: 163–167. [PubMed] [Google Scholar]
- 45. Cardile V, Frasca G, Libra M, Caggia S, Umezawa K, Stivala F, et al. Dehydroxymethylepoxyquinomicin inhibits expression and production of inflammatory mediators in interleukin-1beta-induced human chondrocytes. Cell Physiol Biochem. 2010; 25: 543–50. 10.1159/000303058 [DOI] [PubMed] [Google Scholar]
- 46. Ariga A, Namekawa J, Matsumoto N, Inoue J, Umezawa K. Inhibition of tumor necrosis factor-alpha-induced nuclear translocation and activation of NF-kappa B by dehydroxymethylepoxyquinomicin. J Biol Chem. 2002; 277: 24625–24630. [DOI] [PubMed] [Google Scholar]
- 47. Matsumoto G, Namekawa J, Muta M, Nakamura T, Bando H, Tohyama K, et al. Targeting of nuclear factor kappaB Pathways by dehydroxymethylepoxyquinomicin, a novel inhibitor of breast carcinomas: antitumor and antiangiogenic potential in vivo. Clin Cancer Res. 2005; 11: 1287–1293. [PubMed] [Google Scholar]
- 48. Rickles FR, Hair GA, Zeff RA, Lee E, Bona RD. Tissue factor expression in human leukocytes and tumor cells. Thromb Haemost. 1995; 74: 391–395. [PubMed] [Google Scholar]
- 49. Lwaleed BA, Chisholm M, Francis JL. The significance of measuring monocyte tissue factor activity in patients with breast and colorectal cancer. Br J Cancer. 1999; 80: 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hu T, Bach RR, Horton R, Konigsberg WH, Todd MB. Procoagulant activity in cancer cells is dependent on tissue factor expression. Oncol Res. 1994; 6: 321–327. [PubMed] [Google Scholar]
- 51. Szotowski B, Antoniak S, Poller W, Schultheiss HP, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005; 96: 1233–1239. [DOI] [PubMed] [Google Scholar]
- 52. Grignani G, Maiolo A, Meloni F. Procoagulant activity of tumor cells and its modulation by different cytokines. Haematologica. 1998; 83: S20–S22. [Google Scholar]
- 53. Falanga A, Donati MB. Pathogenesis of thrombosis in patients with malignancy. Int J Hematol. 2001; 73: 137–144. [DOI] [PubMed] [Google Scholar]
- 54. Grignani G, Maiolo A. Cytokines and hemostasis. Haematologica. 2000; 85: 967–792. [PubMed] [Google Scholar]
- 55. Malaponte G, Polesel J, Candido S, Sambataro D, Bevelacqua V, Anzaldi M, et al. IL-6-174 G > C and MMP-9-1562 C > T polymorphisms are associated with increased risk of deep vein thrombosis in cancer patients. Cytokine. 2013; 62: 64–69. 10.1016/j.cyto.2013.02.017 [DOI] [PubMed] [Google Scholar]
- 56. Förster Y, Meye A, Albrecht S, Kotzsch M, Füssel S, Wirth MP, et al. Tissue specific expression and serum levels of human tissue factor in patients with urological cancer. Cancer Lett. 2003; 193: 65–73. [DOI] [PubMed] [Google Scholar]
- 57. Vieira LM, Dusse LM, Fernandes AP, Martins-Filho OA, de Bastos M, Ferreira MF, et al. Monocytes and plasma tissue factor levels in normal individuals and patients with deep venous thrombosis of the lower limbs: potential diagnostic tools? Thromb Res. 2007; 119: 157–165. [DOI] [PubMed] [Google Scholar]
- 58. Lwaleed BA, Francis JL, Chisholm M. Monocyte tissue factor levels in cancer patients. Saudi Med J 2000; 21: 722–729. [PubMed] [Google Scholar]
- 59. Nowak M, Klink M, Glowacka E, Sulowska Z, Kulig A, Szyllo K, et al. Production of cytokines during interaction of peripheral blood mononuclear cells with autologous ovarian cancer cells or benign ovarian tumour cells. Scand J Immunol. 2010; 71: 91–98. 10.1111/j.1365-3083.2009.02350.x [DOI] [PubMed] [Google Scholar]
- 60. Economou JS, Colquhoun SD, Anderson TM, McBride WW, Golub S Holmes EC, et al. Interleukin-1 and tumor necrosis factor production by tumor-associated mononuclear leukocytes and peripheral mononuclear leukocytes in cancer patients. Int J Cancer. 1988; 42: 712–714 [DOI] [PubMed] [Google Scholar]
- 61. Nakashima J, Tachibana M, Ueno M, Baba S, Tazaki H. Tumor necrosis factor and coagulopathy in patients with prostate cancer. Cancer Res. 1995; 55: 4881–4885. [PubMed] [Google Scholar]
- 62. Makin AJ, Chung NA, Silverman SH, Lip GY. Vascular endothelial growth factor and tissue factor in patients with established peripheral artery disease: a link between angiogenesis and thrombogenesis? Clin Sci (Lond). 2003; 104: 397–404. [DOI] [PubMed] [Google Scholar]
- 63. Signorelli SS, Fiore V, Malaponte G. Inflammation and peripheral arterial disease: the value of circulating biomarkers. Int J Mol Med. 2014; 33: 777–783. 10.3892/ijmm.2014.1657 [DOI] [PubMed] [Google Scholar]
- 64. Moses AG, Maingay J, Sangster K, Fearon KC, Ross JA. Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: relationship to acute phase response and survival. Oncol Rep. 2009; 21: 1091–1095. [DOI] [PubMed] [Google Scholar]
- 65. Karin M. Nuclear factor-kappa B in cancer development and progression. Nature. 2006; 441: 431–436. [DOI] [PubMed] [Google Scholar]
- 66. Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci. 2008; 99: 836–842. 10.1111/j.1349-7006.2008.00763.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Adzić M, Nićiforović A, Vucić V, Nesković-Konstantinović Z, Spasić SD, Jones DR, et al. Systemic NF-kappaB activation in blood cells of breast cancer patients. Redox Rep. 2006; 11: 39–44. [DOI] [PubMed] [Google Scholar]
- 68. Setia S, Nehru B, Sanyal SN. Activation of NF-κB: bridging the gap between inflammation and cancer in colitis-mediated colon carcinogenesis. Biomed Pharmacother. 2014; 68: 119–128. 10.1016/j.biopha.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 69. Schauer IG, Zhang J, Xing Z, Guo X, Mercado-Uribe I, Sood AK, et al. Interleukin-1β promotes ovarian tumorigenesis through a p53/NF-κB-mediated inflammatory response in stromal fibroblasts. Neoplasia. 2013; 15: 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Duffey DC, Chen Z, Dong G, Ondrey FG, Wolf JS, Brown K, et al. Expression of a dominant-negative mutant inhibitor-kappaBalpha of nuclear factor-kappaB in human head and neck squamous cell carcinoma inhibits survival, proinflammatory cytokine expression, and tumor growth in vivo. Cancer Res. 1999; 59: 3468–3474. [PubMed] [Google Scholar]
- 71. Lampiasi N, Azzolina A, D'Alessandro N, Umezawa K, McCubrey JA, et al. Antitumor effects of dehydroxymethylepoxyquinomicin, a novel nuclear factor-kappaB inhibitor, in human liver cancer cells are mediated through a reactive oxygen species-dependent mechanism. Mol Pharmacol. 2009; 76: 290–300. 10.1124/mol.109.055418 [DOI] [PubMed] [Google Scholar]
- 72. Kimura YN, Watari K, Fotovati A, Hosoi F, Yasumoto K, Izumi HK, et al. Inflammatory stimuli from macrophages and cancer cells synergistically promote tumor growth and angiogenesis. Cancer Sci. 2007; 98: 2009–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Suzuki E, Umezawa K. Inhibition of macrophage activation and phagocytosis by a novel NF-kappaB inhibitor, dehydroxymethylepoxyquinomicin. Biomed Pharmacother. 2006; 60: 578–586. [DOI] [PubMed] [Google Scholar]
- 74. Dong G, Chen Z, Kato T, Van Waes C. The host environment promotes theconstitutive activation of nuclear factor-kappaB and proinflammatory cytokine expression during metastatic tumor progression of murine squamous cell carcinoma. Cancer Res. 1999; 59: 3495–3504. [PubMed] [Google Scholar]
- 75. Conkling PR, Greenberg CS, Weinberg JB. Tumor necrosis factor induces tissue factor-like activity in human leukemia cell line U937 and peripheral blood monocytes. Blood. 1988; 72: 128–133. [PubMed] [Google Scholar]
- 76. Cermak J, Key NS, Bach RR, Balla J, Jacob HS, Vercellotti GM, et al. , C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993; 82: 513–520. [PubMed] [Google Scholar]
- 77. Mechtcheriakova D, Wlachos A, Holzmuller H, Binder BR, Hofer E. Vascular endothelial cell growth factor-induced tissue factor expression in endothelial cells is mediated by EGR-1. Blood. 1999; 93: 3811–38. [PubMed] [Google Scholar]
- 78. Morgan D, Edwards RL, Rickles FR. Monocyte procoagulant activity as a peripheral marker of clotting activation in cancer patients. Haemostasis. 1988; 18: 55–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dose and time dependent manner. (A) Dose-dependent reduction of NF-kB p65 subunit activity was observed in monocytes treated with DHMEQ. Monocytes from cancer patients DVT+ and DVT- and those from healthy volunteers were treated at the indicated concentrations of DHMEQ and time points. (B) Time course analyses of NF-kB p65 subunit activity in monocytes from three groups analyzed treated with DHMEQ. NF-kB p65 subunit activity was measured by ELISA. Data represent the mean ± SD in the 3 groups of individuals.
(TIF)
(A) Dose-dependent reduction of cell viability of monocytes treated with DHMEQ. Monocytes from cancer patients with and without DVT and those of healthy volunteers, used as controls, were treated at the indicated concentrations of DHMEQ and time points. Cell viability were determined by MTT assay. (B) Time course analyses of cell viabilities of monocytes from three groups analyzed treated with DHMEQ. Data represent the mean ± SD of relative viabilities of 3 independent experiments.
(TIF)
Note: CRP, C Reactive Protein, IL-1β, Interleukin-1 beta; IL-6, Interleukin-6; TNF- α, Tumor necrosis factor-alpha; MMP-9, matrix metalloproteinase-9; VEGF, Vascular endothelial growth factor; TF, Tissue factor; sP, Spearman correlation.
(DOC)
Note: HM, Monocytes from healthy subjects; sH, sera from healthy subjects; LPS, lipopolysaccharide; sCaDVT-, serum from cancer patients without deep venous thrombosis; sCADVT+, serum from cancer patients with deep venous thrombosis; IL-6, Interleukin-6; TNF- α, Tumor necrosis factor alpha; IL-1β, Interleukin-1 beta; VEGF, Vascular endothelial growth factor; MMP-9, matrix metalloproteinase-9; TF, Tissue factor. All p values were calculated by Wilcoxon Matched-Pairs Signed-Ranks Test. (§) p value calculated between (HM + LPS) and (HM + sCaDVT-); (^) p value was calculated between (HM + LPS) and (HM + sCaDVT+).
(DOCX)
Data Availability Statement
All relevant data are freely available in the paper and in Supporting Information files.