Abstract
It has been shown that during the planning of a voluntary movement the transmission of cutaneous afferent inputs to the somatosensory cortex is attenuated shortly before the motor output as well as during movement execution. However, it is not known whether the sensory suppression observed during the planning phase (i.e., before any movement execution) is a systemic phenomenon or whether it is dependent on movement context. For example, movements such as step initiation are controlled based on information received from cutaneous receptors in the feet. Because afferent information emerging from these receptors is critical for movement initiation, we hypothesized that suppression of these inputs may not occur during the planning phase prior to gait initiation. To examine this hypothesis we measured the cortical response to somatosensory stimulation during the planning phase of step initiation and during movement execution. Sensitivity to cutaneous stimulation was assessed by measuring the amplitude of the cortical somatosensory-evoked potential (SEP, over the Cz electrode) following electrical stimulations of the plantar sole of one foot. Two stimulations were provided during the planning phase of a step movement and two stimulations during movement execution. It was found that the P50-N80 SEP was facilitated in the early planning phase (−700 ms before motor execution) compared with when participants remained still (control standing task). This mechanism might contribute to an enhanced perception of cutaneous input leading to a more accurate setting of the forces to be exerted onto the ground to shift the body's weight toward the supporting side prior to foot-off.
Keywords: EEG, gait planning, cutaneous inputs
contemporary models of motor control propose that the transmission of afferent input can be attenuated if the internal prediction of the action's sensory consequences (corollary discharge) is similar to the sensory input obtained during movement execution (Wolpert et al. 1995; see for review Angelaki and Cullen 2008). This suppression of sensory information has been observed at both peripheral (e.g., afferent fibers; Seki et al. 2003) and central (Blakemore et al. 1998, 1999a, 1999b; Haggard and Whitford 2004) levels, and many studies have shown that this attenuation is most pronounced during the actual performance of voluntary actions (Seki et al. 2003; Voss et al. 2006; Williams and Chapman 2002). Recent investigations have also revealed that this sensory attenuation occurs, albeit to a slightly lesser extent, shortly before movement execution. For example, in studies of the cortical transmission of somatosensory inputs prior to movement onset, researchers have found a downmodulation ∼100 ms prior to finger movements (Voss et al. 2006) and 60 ms prior to ankle joint movements (Morita et al. 1998). In addition to the observation that afferent sensory information can be suppressed prior to action onset, there has also been evidence implicating the role of the movement characteristics in modulating sensory processing. Collins et al. (1998), for example, noted that attenuation of proprioceptive afferents during wrist flexion-extension was stronger when the velocity of the movement was faster.
In studies examining locomotion, researchers observed that the amount of sensory gating can also be dynamically modulated during the different phases of locomotion. For instance, after lower limb nerve stimulation, Altenmüller et al. (1995) and Duysens et al. (1995) observed a smaller attenuation, both at the end of the swing phase, just prior to foot contact, and during the early stance phase, than during the other phases of locomotion. From a functional point of view, recovering access to somatosensory input just before and at the onset of foot contact would benefit the control of equilibrium. Indeed, Duysens et al. (1995) have shown an increase of the perception of tactile stimuli when sensory transmission increased.
Current literature outlines a modulation of tactile transmission during gait execution. It remains to be determined, however, whether these context-dependent modulations during locomotion also occur well before the first step of gait (i.e., during the planning phase of gait initiation). Indeed, the stepping sequence is known to be progressively assembled and stored up to 1.5 s before step initiation (MacKinnon et al. 2007). One of the main characteristics of gait initiation is that it is preceded by anticipatory postural adjustments (APAs) whose aim is to initiate the body's weight transfer toward the supporting foot prior to lifting off. The APAs are considered to be controlled through both feedforward mechanisms (Lyon and Day 1997) and online mechanisms (Mouchnino et al. 2012). The scaling of these APAs relies on the ability to use sensory information to determine the body orientation relative to the support surface prior to step execution (Inglis et al. 1994; Mouchnino and Blouin 2013; Timmann and Horak 2001). For example, in Timmann and Horak (2001) the participants stood on a mobile platform and were asked to step forward as soon as they felt the platform was moving backward (“step to cue” paradigm). The backward platform displacement would result in a forward body displacement assisting step initiation. The kinematics of the platform displacement were carefully set to evoke reliable somatosensory cues without eliciting lower limb reflex responses. The results of this study revealed a reduction in the amplitude of the APAs responsible for moving forward over the stance limb: the larger the platform displacement, i.e., the greater the assistance to moving the body forward, the smaller the APAs.
In a recent study, we found evidence that the APAs can be properly scaled based on the cutaneous input arising from the translation of a support platform (Mouchnino and Blouin 2013). In this study, we used a “step to cue” paradigm similar to that used by Timmann and Horak (2001) in which the participants were instructed to produce a step as soon as they felt the platform start to move. The platform first slowly accelerated (between 0.14 m/s2 and 0.30 m/s2) in the lateral direction up to a constant velocity of 0.02 m/s. This translation resulted in a change in the somatosensory cues from the soles of the feet without modifying vestibular inputs (head acceleration remained below the 0.048 m/s2 vestibular threshold reported by Gianna et al. 1996). We found that transient changes in the contact forces between the feet and the support, occurring ∼250 ms before the APAs onset, triggered consistent changes in the subsequent APAs regardless of visual (i.e., participants had their eyes closed) and vestibular feedback information about the support surface translation. These results support the idea that the central nervous system (CNS) uses plantar sole cutaneous cues to determine body orientation relative to the environment during the planning phase of gait initiation. In light of these results, and the previously mentioned literature on context-dependent sensory processing, we sought to determine, in the present study, whether the transmission of sensory input to the cortex is modulated during the time course of step planning and execution.
We compared the cortical somatosensory-evoked potentials (SEPs) following the electric stimulation of plantar sole cutaneous receptors while participants stood still to the SEPs recorded when participants planned or executed a step. We expected the SEPs to be greatest (i.e., sensory facilitation) when the stimulation occurred during the planning phase of the stepping movement to enhance tactile perception.
METHODS
Twelve healthy participants performed the experiment (mean age: 36 ± 9 yr; mean height: 166 ± 9 cm; mean weight: 66 ± 7 kg). Informed consent was obtained from all participants, all procedures were approved by the local Ethics Committee (Sud Méditerranée 1, ID RCB: 2010-A00074-35), and all protocols and procedures were in accordance with the ethical standards set out in the Declaration of Helsinki.
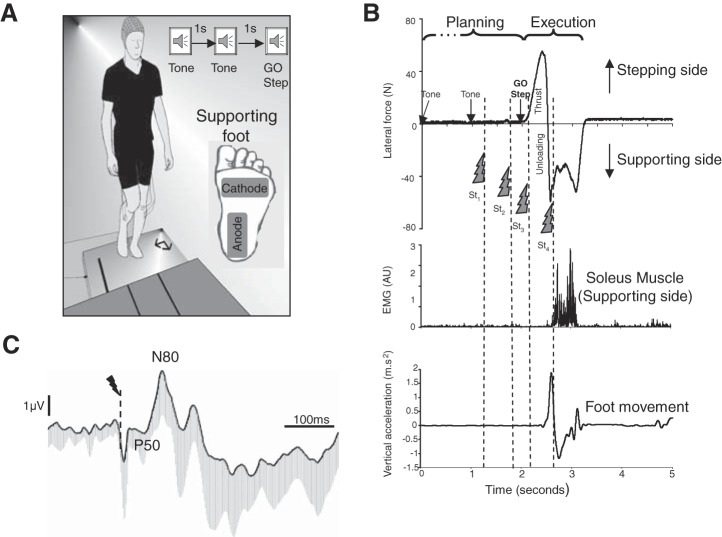
Participants stood barefoot on a 60 × 120-cm AMTI (Watertown, MA) force platform with their arms alongside their bodies and their eyes closed (Fig. 1A). They were requested to self-select a side-by-side foot position (feet approximately shoulder width apart). In the task hereafter referred to as the Stepping task, participants were instructed to step forward with the right leg in response to an imperative auditory signal (a 100-ms tone), keeping their eyes closed. This imperative GO signal corresponded to the third (and last) 1-s-interspaced tone (Fig. 1, A and B). The first two tones thus served as warning stimuli, allowing participants to have a period of preparation (i.e. planning phase) (MacKinnnon et al. 2007).
Fig. 1.
A: experimental setup. Speakers represent the 3-sound signal with 1-s interval. Inset: position of stimulation electrodes underneath left supporting foot. B: behavioral recordings during the Stepping condition for 1 representative participant: mediolateral ground reaction forces, left soleus muscle activity [arbitrary units (AU)] of supporting leg, and right foot (stepping leg) vertical acceleration. Dashed lines indicate the delivery of the electric stimulations during motor planning and execution. C: grand average somatosensory-evoked potential (SEP) of St1 of all participants recorded at Cz (n = 12) in the supine task. Note that this stimulation (i.e., St1) was not preceded by any stimulation. All the early (i.e., P50-N80) and later components are shown with 1 SD. Dashed line indicates the moment of the stimulation.
The plantar sole of the supporting foot was stimulated twice during the planning phase of the step, that is 600 ms (St1) and 100 ms (St2) before the GO step signal (see below for the stimulation technique). Particular attention is paid to the fact that the participants wait for the GO signal to initiate their step. Afterwards, two more stimulations (St3 and St4) were delivered during the execution phase. St3 was designed to test the transmission of cutaneous inputs during the initial phase of the APA and was triggered when the mediolateral force (i.e., APAs) exceeded 16.7 N (on average 389 ms after St2). This value was found during pretesting to occur during the second third of the thrust duration for all participants. This time frame was selected on the basis of a previous study showing that the thrust can be controlled online (i.e., modulated) if perturbations (e.g., mechanical) occur at this time but not when they occur later during the thrust (Mouchnino et al. 2012). St4 was used to test the sensory transmission during the one-foot support phase and was triggered 500 ms after St3. To prevent asymmetric weight bearing (a possible strategy before step execution), three to five catch trials were performed randomly during the experimental session. For these trials, the participants were asked, immediately after the first tone, to stand still instead of stepping. Each participant performed 40 stepping movements.
Two control tasks were performed with a similar design (i.e., 3 tones and 4 electrical stimulations), with the exception that all stimulations after St1 were separated by 500 ms. This constant 500-ms interval between each electrical stimulus was designed to avoid the “interference phenomenon” (Burke and Gandevia 1988) (i.e., depressed SEPs when stimulations are too close in time, i.e., <300 ms according to Morita et al. 1998). In these control tasks, participants adopted either an upright static standing position (Standing) or a semisupine position (Supine) seated in a reclining chair. In the latter control task, participants' plantar soles were not in contact with a support surface. Each participant performed 20 trials in each control task (i.e., 80 stimulations). The three tasks (Stepping, Standing, and Supine) were presented in block, and the order of the blocks was counterbalanced between participants.
Stimulation procedure.
The electrical stimulation was applied on the plantar sole of the supporting foot (Fig. 1A). The electrical stimulus was delivered by a DS5 isolated bipolar constant-current stimulator (Digitimer, Welwyn Garden City, UK). The cathode was located under the metatarsal region, and the anode was positioned underneath the heel of the supporting foot (5 × 9-cm electrodes, Platinium Foam Electrodes). The stimulation consisted of a single rectangular 10-ms pulse applied under the supporting foot. We used the technique of Sayenko et al. (2009), who showed that plantar skin stimulation above the perceptual threshold and below the motor threshold of five 1-ms pulses triggered different cutaneous reflexes of the plantar foot depending on the location of the electrodes. In the study of Sayenko et al. both electrodes were located under either the heel or the metatarsal regions. Taking into account the signature of the cutaneous reflexes reported in their study, we carefully selected both the position of the electrodes to stimulate the plantar sole as a whole without targeting a specific portion of the foot and the amplitude of the stimulation to avoid cutaneous reflexes. The stimulation intensity was set as follows: for each participant while in a quiet standing position, we first found the lowest intensity that led to constant perception of the stimulations (mean amplitude 6.3 ± 1.7 mA). This stimulation was determined as the baseline value. The stimulation intensity for the subject was set at 25% higher than this baseline value (i.e., well below the motor threshold).
Behavioral recordings and analyses.
Ground reaction forces and moments were recorded at a sampling rate of 1,000 Hz and used to compute the center of pressure (CoP) displacement. APAs were measured by computing lateral thrust defined from the CoP displacement as shown in Fig. 1B. First, the CoP shifts toward the movement side: this CoP shift corresponds to a vigorous lateral force onto the ground (“thrust”) exerted mainly by the forthcoming moving leg while still on the ground. The CoP then moves toward the supporting side, unloading the leg to be moved. During “unloading,” the leg movement (foot-off) is initiated. On average the thrust onset occurred 202 ± 160 ms after the GO Step signal. The duration of the thrust was defined as the time between the initial change in lateral force (Fig. 1B) and the peak in the force toward the stepping side (517 ± 162 ms relative to the GO Step signal).
Bipolar surface electromyography (EMG) signals of ankle joint antagonist muscles [tibialis anterior (TA) and soleus muscle (Sol) of the left supporting leg (i.e., stimulated side)] were recorded with a Bortec AMT-8 system (Bortec Biomedical, Calgary, AB, Canada). The EMG signals were preamplified and sampled at 1,000 Hz (band-pass filtered 20–250 Hz). To quantify TA and Sol muscle activities, for each trial we computed the integral of the full-wave rectified EMG (iEMG) activity during a time window of 200 ms immediately following each electrical stimulation.
The kinematics of the stepping foot was recorded with a triaxial accelerometer (Entran, model 4630, Hampton, VA) placed on the top of the right foot. The vertical acceleration of the foot movement was analyzed to determine foot-off onset, which occurred 622 ± 147 ms after the GO step signal (Fig. 1B). The stepping foot movement lasted 909 ± 153 ms.
Electroencephalography recordings and analyses.
Electroencephalographic (EEG) activity was recorded continuously from 64 Ag-AgCl surface electrodes embedded on an elastic cap (ActiveTwo system, BioSemi). According to the specifications of the BioSemi system, “ground” electrodes were replaced by Common Mode Sense active and Driven Right Leg passive electrodes. These two electrodes, located near Pz and POz electrodes, form a feedback loop, which drives the average potential of the subject (the Common Mode voltage) as close as possible to the analog-to-digital converter (ADC) reference voltage in the AD box. The signals were preamplified at the electrode sites and postamplified with DC amplifiers, filtered online with a 0.16-Hz high-pass filter (Actiview acquisition program), and digitized at a sampling rate of 1,024 Hz (24-bit resolution). Signals from each channel were referenced using the average of the 64 scalp electrodes. The signals were further filtered off-line with 50-Hz notch, 48-Hz (high cutoff) filters (digital filters, 24 dB/octave) and 0.1-Hz (low cutoff) filters (digital filters, 12 dB/octave; BrainVision Analyzer 2, Brain Products). Vertical electrooculograms were recorded bipolarly with electrodes placed above and below the left eye; horizontal electrooculograms were recorded bipolarly with electrodes positioned near the outer canthus of each eye. EEG signals were corrected for eyeblinks according to the statistical method of Gratton et al. (1983), as implemented in BrainVision Analyzer 2 software.
SEPs were obtained by averaging, for each participant and task, all synchronized epochs relative to the electrical stimulus. The average amplitude of the 50-ms prestimulus epoch served as baseline. We examined the SEPs over the Cz electrode as this electrode overlies the sensorimotor cortices and on the homunculus the feet are located on the inner surface of the longitudinal fissure. The earliest discernible positive (P50; 54 ± 12 ms) and negative (N80; 87 ± 17 ms) peaks after each stimulus were identified. Such peak latencies are comparable to latencies observed by Duysens et al. (1995) and Altenmüller et al. (1995) evoked by stimulating the sural nerve. The fact that the sural nerve is a primarily/exclusively cutaneous nerve (Burke et al. 1981) lends an argument for the P50-N80 originating from cutaneous input. The amplitude of the P50-N80 waveform was measured peak to peak (Fig. 1C).
In addition, we used low-resolution brain electromagnetic tomography (LORETA), implemented in the Brainstorm software (Tadel et al. 2011; freely available at http://neuroimage.usc.edu/brainstorm), to estimate the neural sources of the SEPs. LORETA calculates the current density at each of 2,394 voxels in the gray matter and the hippocampus of a reference brain (MNI 305, Brain Imaging Centre, Montreal Neurological Institute) based on the linear, weighted sum of the scalp electric potentials. LORETA selects the smoothest of all possible current density configurations throughout the brain volume by minimizing the total squared Laplacian of source strengths. This procedure only assumes that neighboring voxels should have a maximally similar electrical activity; no other assumptions are made. The applied version of LORETA uses a three-shell spherical head model registered to the Talairach space. The sources were reconstructed using the waves obtained from the grand average of monopolar recordings of each participant and task (i.e., Supine, Standing, and Stepping tasks) during the planning phase. Topographic maps were computed at peak negativity (N80) with the grand average of the waves (monopolar recordings) obtained for all participants.
Statistical analyses.
The amplitudes and latencies of the SEPs (recorded at Cz) were submitted to repeated-measures analysis of variance (ANOVA) with Epochs (St1, St2, St3, St4) as within-subject factors (more details are given below). Task-specific data were also submitted to ANOVA designed with Epochs and Task (Standing, Supine, and/or Stepping). Significant effects were further analyzed with Newman-Keuls post hoc tests. We also conducted paired t-tests when necessary (Standing and Supine tasks). The level of significance was set at 5% for all analyses. All dependent variables (EEG and behavioral data) showed normal distributions (i.e., P > 0.05, Kolmogorov-Smirnov test).
RESULTS
Control tasks (Standing vs. Supine).
To confirm the absence of the sensory interference phenomenon between two consecutive electrical stimulations, we compared the amplitudes and latencies of the P50-N80 SEP resulting from each epoch (St1, St2, St3, St4) in the two control tasks (Standing and Supine). The ANOVA did not reveal a significant main effect of epoch on the amplitude of the SEPs recorded in either the Supine (F3,33 = 1.60, P = 0.21) or the Standing (F3,33 = 1.07, P = 0.37) task. Furthermore, the latencies of P50 (F3,33 = 0.80, P = 0.50 and F3,33 = 1.80, P = 0.17 for Standing and Supine, respectively) and N80 (F3,33 = 0.67, P = 0.58 and F3,33 = 0.22, P = 0.88 for Standing and Supine, respectively) were not significantly different between the different stimulations in both tasks. Overall, these results indicate that the 500-ms interval used in the present study between two electrical stimuli was sufficiently long to avoid the sensory interference phenomenon (see methods).
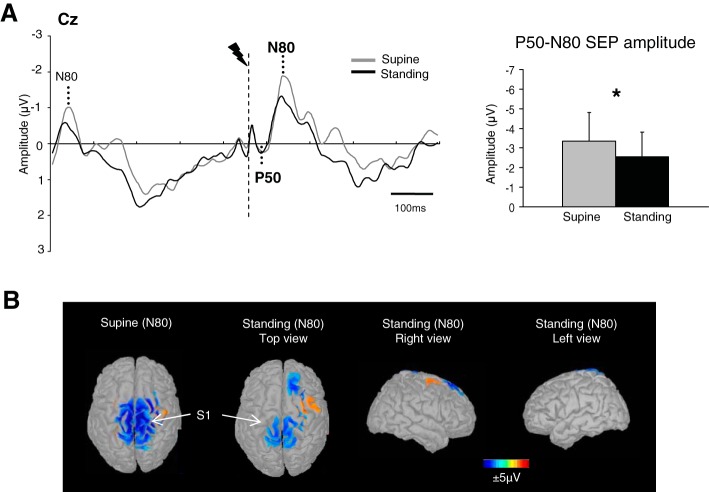
As no interference phenomenon was observed, we pooled the data from the four stimulations (total of 80 synchronized epochs) and compared the mean SEP amplitude between the Standing and Supine tasks (Fig. 2A). The t-test revealed a significant difference (t11 = −2.54, P = 0.03): the P50-N80 amplitude in the Supine task (−3.3 ± 1.5 μV) was greater than the P50-N80 amplitude in the Standing task (−2.5 ± 1.2 μV). Nonetheless, there was no difference between the P50 (t11 = 1.13, P = 0.27) and N80 (t11 = 0.96, P = 0.35) latencies in the two tasks (55 ± 14 ms and 90 ± 17 ms for P50 and N80 in the Supine task and 54 ± 12 ms and 87 ± 17 ms for P50 and N80 in the Standing task).
Fig. 2.
Cortical activity during the control conditions. A, left: grand average SEP of all participants recorded at Cz in both control conditions (Supine and Standing). The SEP variation before the P50-N80 SEP was due to the choice of the time window before and after the synchronized signal. Indeed, the evoked potentials were extracted from averaging epochs of raw EEG beginning 450 ms before electrical stimulus and continuing for 450 ms after stimulus. This large time window exhibited the SEP of the preceding stimulus that occurred 500 ms before the stimulus of interest. Right: mean for the 80 stimulations of the P50-N80 SEP amplitudes (error bars are SD across participants). *P < 0.05. B: topographic maps of voltage distribution [low-resolution brain electromagnetic tomography (LORETA)] computed with the grand average of the waves (monopolar recordings) obtained by all participants. The cortical maps are shown at the latency of the peak negativity (i.e., N80). The scale of the maps was chosen to maximize identification of the sources localized in the primary somatosensory region, and the sources were given a marked threshold to only show source activity that was 18% above minimal activation. Note that the primary somatosensory cortex (S1) was activated in both control tasks (i.e., Supine and Standing).
The difference in P50-N80 mean amplitude between the two control tasks was indeed surprising. The spread of activity in the Supine task (see scalp topography, Fig. 2B) is the likely cause. However, the mechanisms leading to the greater SEP in the supine task cannot be formally elucidated here. We can speculate that it may have resulted from an increased sensitivity of the cutaneous receptors or indeed from changes in the physical properties of the stimulations in the absence of foot loading. Therefore, only the data in the Standing task (i.e., the control task with foot loading) are used as reference for the Stepping task.
Facilitation of sensory input during step planning.
The Standing task was included as a level along with St1, St2, St3, and St4 in a one-way ANOVA after recomputing an average SEP calculated from 40 randomized synchronized epochs (out of 80 epochs) to have the same sample size and therefore a similar signal-to-noise ratio as for each Stepping stimulation epoch (Fig. 3A). To test for homogeneity of variance (essential assumption to meet to perform ANOVAs), we used the Bartlett test of the null hypothesis that the columns of data vector x come from normal distributions with the same variance. The alternative hypothesis is that not all columns of data have the same variance. The result of the test indicated that the variances were homogeneous (Barlett's statistic = 6.54, P = 0.16) across the epochs (i.e., Standing and Stepping St1, St2, St3, and St4).
Fig. 3.
Cz cortical activities during step planning and motor execution. A: group mean of the P50-N80 SEP amplitudes in the Standing and the 4 Stepping epochs (error bars are SD across participants). Bottom: topographic maps (LORETA) computed from all participants' grand average of the waves (monopolar recordings). The maps are shown for the Stepping St1 epoch at the latency of the peak negativity (i.e., N80). Note that the extrastriate body area (EBA), the dorsal premotor cortex (PMd), the pre-supplementary motor area (pre-SMA), and S1 are activated simultaneously. B: grand average of St1 and St2 SEPs for the Stepping condition. Traces were aligned to their electrical stimuli. The large positivity before the stimulation (St2) represents a preparatory processes and corresponds to the contingent negative variation (CNV) because the stimulation precedes the GO step signal by 100 ms. Note that for St1 the stimulation was triggered −600 ms before the GO step. *P < 0.05. C: TA and Sol muscle activity [integral of the full-wave rectified EMG (iEMG)] following the stimulation for the 3 epochs before weight shift (Standing, Stepping St1, and Stepping St2). ns, P > 0.05.
For the amplitude of the P50-N80 SEPs, the ANOVA revealed a main effect of epoch (F4,44 = 5.92, P < 0.05). Post hoc analysis revealed that the SEP of the St1 epoch (−3.8 ± 2.4 μV) had a greater amplitude than that in the Standing task (−2.3 ± 1.1 μV) and in all other epochs of the stepping task (St2, St3, St4). The amplitude of the SEPs recorded in the other stimulation epochs of the Stepping and Standing tasks were not significantly different (St2 = −2.6 ± 1.5 μV, St3 = −1.8 ± 1.4 μV, St4 = −1.3 ± 1.7 μV). To verify whether this result was due to the inability to detect differences, the mean SEP magnitudes were converted into z scores on a per-subject basis. z Scores were obtained using the mean and standard deviation of all conditions for each subject's five SEP epochs. A repeated-measures ANOVA followed by Newman-Keuls post hoc tests revealed significant change in SEP for the St1 epoch (F4,44 = 4.92, P = 0.002); the z score of the SEP for the St1 epoch had a greater amplitude than in the Standing task and in all other epochs of the Stepping task (St2, St3, St4).
For the latencies of the P50 and N80 SEPs, the ANOVA did not show any effect on the P50 (F4,44 = 1.04, P = 0.39) or N80 (F4,44 = 0.90, P = 0.46) latencies (overall mean for P50: 56 ± 12 ms and for N80: 89 ± 16 ms).
To determine whether the SEP facilitation observed after St1 could be due to a change in the pressure exerted on the foot and thus on the electrodes, we computed the integral of the CoP oscillations (i.e., iCoP) 200 ms before the stimulation at St1 and 200 ms after St1 in both the Standing and Stepping tasks. These behavioral data were submitted to repeated ANOVAs combining two Tasks (Standing, Stepping) with two Periods (before St1, after St1) and two Directions (mediolateral and anteroposterior). All dependent variables showed normal distributions (i.e., all P > 0.05, Kolmogorov-Smirnov test). The ANOVAs revealed no significant effect of Task (F1,11 = 0.4, P = 0.53), Period (F1,11 = 0.01, P = 0.89), or Direction (F1,11 = 0.48, P = 0.50) on the iCoP and no significant interaction between these factors. These results indicate that the pressures under the feet were similar in the Standing task and in the first epoch of the stepping task (i.e., step planning, St1) where an increased response to the stimulation was observed at a cortical level.
In the Standing and Stepping St1 epochs, although the whole body was motionless (i.e., as revealed by ground force analyses; Fig. 1B), the central processes diverged. To confirm that the facilitation of the cutaneous input occurred during the planning of the stepping movement, we computed the topographic maps of voltage distribution (LORETA) at N80 latency (St1 epoch; Fig. 3A). In addition to the activation of the primary somatosensory cortex (S1), LORETA revealed simultaneous activation of the extrastriate body area (EBA) in both the right and left lateral occipitotemporal cortex (Fig. 3A, top view) and in the right hemisphere on the dorsal premotor cortex (PMd) and the supplementary motor area (pre-SMA) (Fig. 3A; see for comparison right and left views). These additional activations, which were not observed in the Standing task (see scalp topography, Fig. 2B, right and left views), attest that the participants were engaged in the planning process of the stepping task.
Furthermore, the planning process is evidenced in the St2 epoch (i.e., 100 ms before the GO step signal) by the large slow increase of negativity that predominantly developed at the vertex (Cz electrode) (because of baseline choice, this appears as a decreased positivity in Fig. 3B). This negative wave is known to emerge after participants receive a warning stimulus (e.g., first tone) and before they receive the GO step cue to initiate their movement (Walter et al. 1964). The increased negativity (−5.8 ± 2.6 μV) started 272 ± 66 ms before the GO step signal. This general preparatory process is known as the movement-related contingent negative variation (CNV; Bonnet et al. 1998; Caldara et al. 2004; Cunnington et al. 1996; Stippich et al. 2002).
To verify that the difference in the SEP amplitude between Standing, Stepping St1, and Stepping St2 was not due to a difference of motor activity, the activities in the TA and Sol ankle muscles (i.e., iEMG) were compared across conditions with separate one-way ANOVAs (3 epochs: Standing, Stepping St1, and Stepping St2; Fig. 3C). The analyses revealed no main effect of condition (F2,20 = 0.92, P = 0.41 and F2,20 = 1.67, P = 0.21 for TA and Sol muscle activity, respectively).
Overall, these results (SEPs, iCoP, and muscle activity) suggest that the increased neural activity observed during the early phase of motor planning (Stepping St1, 600 ms before the GO step signal) was related to somatosensory processing and did not result from a change of motor activity (i.e., motor-evoked potential).
DISCUSSION
The aim of this study was to determine whether sensory transmission from the plantar sole tactile receptors is modulated during the planning phase of a voluntary step movement. It was hypothesized that a facilitation of the sensory transmission would be observed to enhance tactile perception during the planning phase.
Here we used electrical stimulation to probe the state of cutaneous plantar sole input transmission from the periphery to the cortical level. Therefore we did not expect any changes in the APAs, as no information was contained in the electrical stimulation.
The results showed an increase in activity over the somatosensory cortex (i.e., P50-N80, 709 ms before APA onset). This increased activity points to a facilitation of sensory input during the early planning phase of step initiation and supports our hypothesis that cutaneous afferent inputs are monitored and processed by the CNS to determine the actual standing position prior to motor execution. Furthermore, this sensory facilitation was released in the later process (i.e., ∼300 ms before motor execution and during the APAs) without giving rise to a sensory gating (i.e., no decreased activity with respect to the Standing control condition). Together, these results revealed the dynamic nature of the sensory modulation most likely to comply with the balance requirements of the stepping task.
Somatosensory facilitation during early planning process.
Our results indicate that transmission of sensory information from cutaneous receptors is facilitated at an early stage during the planning process. One possible explanation for this finding is that the early sensory facilitation observed in this phase is indicative of the construction of an accurate representation of the equilibrium body position. This assertion is also in line with existing research demonstrating that an increase in tactile sensory transmission is accompanied by an improvement in perception of tactile stimuli (Duysens et al. 1995). In addition, results from a recent study revealed balance-dependent somatosensory facilitation (Bolton et al. 2011). In that study, the authors examined how tactile cues from the finger contribute to balance control. The authors noted that a light fingertip touch (i.e., <1 N) onto a stable surface improved balance control compared with no touch. Interestingly, this improvement was reflected by an increase in the amplitude of SEPs (Bolton et al. 2011). The short latency observed in the P50 could be associated with early somatosensory processes and generated by S1 (Chapin and Woodward 1982; Hämäläinen et al. 1990). For example, Chapin and Woodward (1982) reported after electrical stimulation of the paw of the rat (single pulse) a modulation in the discharge of the S1 neurons 7–45 ms after the stimulation. The N80 has been described as reflecting the early processes involved in multisensory integration performed by the secondary somatosensory cortex (S2). For example, Ferrè et al. (2012) observed a facilitation of the N80 when vestibular and electrical median nerve stimulations were associated. In our study, the P50-N80 SEP facilitation could be related to the processing of somatosensory information from the plantar sole mechanoreceptors and other sensory information (i.e., proprioceptive; Saradjian et al. 2013, 2014). This information could help construct a representation of body position in space with respect to stability region. Furthermore, this enhancement of central sensory processing might suggest a link between directed attention and tactile sensitivity and discrimination. Eimer and Forster (2003) observed a N80 contralateral increase when a tactile stimulation on the index finger was delivered in a sustained attention task. Their results show that when participants were instructed to direct attention on the target hand, the discrimination of the stimulus was improved. In light of both our present results and the above studies, we suggest that the amplitude of the SEP would correspond to the sensitivity of neuronal populations to inputs. In other words, attention (for example) could modulate the background activity of neurons and augment stimulus-induced dynamics. The suggestion is that modulation of baseline activity may reflect a top-down signal that gives a competitive advantage to a stimulus when relevant for the task. This has been extensively studied for the neuronal populations in primary visual cortex (Chawla et al. 1999; Chen et al. 2008).
Although in the present study the latencies were not statistically different between epochs, we might have expected a shift in latency between the standing and stepping epochs. Indeed, it was found by Duysens et al. (1995) that latencies of the P50 component were significantly longer during gait (55.3 ± 5 ms during midswing and 61.4 ± 6.3 ms after footfall) than during standing (49 ± 5.2 ms), while there was no significant shift in N80 latencies. One interpretation was that the shift in latencies was consistent with a gating effect on cortical level associated with movement performance. It appears difficult to pinpoint the reasons for the (statistical) difference between the results obtained by Duysens et al. (1995) and those reported in the present study (54 ± 12 ms during standing, 56 ± 12 ms for the St1 epoch, and 57 ± 11 ms for the St3 epoch, for example). The difference between the studies could be due to the expression of the cortical gating when participants were really engaged in a locomotor task (Duysens et al. 1995) compared with step initiation as in the present study.
The timing of the larger SEP amplitude (St1, −709 ms before motor execution) could reflect an optimal period for facilitating sensory information. This is in agreement with Cuisinier et al.'s (2005) behavioral experiment reporting that the shortest reaction times to produce APAs in the lower limb associated with arm movements are observed when the foreperiod (i.e., the time elapsed between a preparatory signal and the imperative stimulus) is 700 ms. Moreover, the activation of the EBA in both the left and right lateral occipitotemporal cortex suggests that the participants were engaged in the planning process of their stepping movements (i.e., 600 ms before the GO step signal). Indeed, it has been suggested that EBA is part of a network that includes premotor and motor areas potentially involved in self-processing (Jeannerod 2004). For instance, Astafiev et al. (2004) showed that the EBA was specifically involved in the visual processing of body and goal-directed action even when vision of the moving limb was occluded. In addition, Blanke et al. (2005) and Arzy et al. (2006) reported activation of the EBA as part of a network involved in the “sense of agency” (i.e., recognition of oneself as the body owner or as the agent of the action). In addition, the right pre-SMA activity is related to processing of relevant information mainly originating from the left stimulated foot together with the rostral portion of the dorsal part of the lateral premotor cortex (PMd) which is specifically related to sensorimotor association (see for review Picard and Strick 2001). Although these data do not shed light on the functional interactions between these regions (S1, EBA and pre-SMA and PMd), their simultaneous activities at the peak negativity (N80) suggest that these regions are involved in the facilitated sensorimotor processes during the planning of a stepping movement.
Absence of sensory suppression during a later stage of planning process and step execution.
The absence of gating mechanism in the later stage of step planning (i.e., St2, 300 ms before motor execution, APA onset) might suggest that the plantar sole cutaneous information is still processed in order to rapidly correct suboptimally planned APAs (MacKinnon et al. 2007). This time frame corresponds closely to a motor preparation process as indicated by the presence of a CNV that corresponds to a general preparatory cortical activity preceding the St2 stimulation (here the CNV preceded the St2 by 170 ms). The suggestion that cutaneous afferences monitor the balance is supported by recent results demonstrating that, in the absence of vestibular or visual information, APAs could be modulated as early as 300 ms before their onset based on information (i.e., shear force) from plantar sole mechanoreceptors (Mouchnino and Blouin 2013).
During motor execution the SEP amplitude was not attenuated in St3 and St4 compared with the quiet standing control task. The absence of gating in the transmission of cutaneous inputs to the cortex is consistent with the idea that gait initiation requires online control of the forces exerted onto the ground to ascertain proper weight shift. Indeed, a behavioral study (Mouchnino et al. 2012) has shown that APAs were modified online (increase in both ground pressure and muscle activity) to compensate for perturbations occurring during thrust execution. In addition, it has already been demonstrated that attenuation of cutaneous information could pose problems for performance during challenging balance control tasks (McIlroy et al. 2003) and for tactile discrimination (Cybulska-Klosowicz et al. 2011; Staines et al. 2002; Ziat et al. 2010). Cybulska-Klosowicz et al. (2011) have shown that, for the hand, the sensory gating observed during the movement was alleviated for a fast movement promoting a tactile exploration. In our study, this mechanism would enable online modulation of the ground reaction forces to trigger the foot-off at the right time. Such mechanisms have been observed in other animals (rats) by Chapin and Woodward (1992), who found that touch stimulation during forepaw exploration (i.e., when the animal walked on an irregular surface) led to greater S1 activity than when the touch stimulation was not associated with an exploratory behavior (i.e., walking on a regular surface). A neuronal population in the S1 cortical forepaw area has been found to be activated during footfall and inhibited for all other locomotor step cycle phases (Chapin and Woodward 1992). This type of neuron could have a supplementary role in the adjustment of the paw contact time.
Conclusions.
In conclusion, our results show a sensory transmission facilitation in an early phase of step planning (−700 ms before APA onset) that could be involved in the setting of the forces initiating the body weight transfer toward the supporting side. As a result, these afferents play a critical role for feedforward mechanisms. Surprisingly, this facilitation is transient and does not give rise to any sensory gating either at the end of the planning phase (−300 ms before the APAs) or during step execution. This highlights the crucial role of somatosensory processes in monitoring the balance constraints during the entire motor task.
GRANTS
This study was funded by the Centre National d'Etudes Spatiales, the Defisens grant program, the Natural Sciences and Engineering Research Council of Canada discovery grant program, and the Institut Méditerranéen de Recherche Avancées.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.M., A.F., C.T., J.P., J.B., and M.S. conception and design of research; L.M., A.F., C.T., J.P., A.H.S., J.B., and M.S. performed experiments; L.M., A.F., J.P., A.H.S., and M.S. analyzed data; L.M., A.F., C.T., J.P., A.H.S., J.B., and M.S. interpreted results of experiments; L.M. prepared figures; L.M., A.F., C.T., J.P., J.B., and M.S. drafted manuscript; L.M., J.B., and M.S. edited and revised manuscript; L.M., A.F., C.T., J.P., A.H.S., J.B., and M.S. approved final version of manuscript.
REFERENCES
- Altenmüller E, Berger W, Prokop T, Trippel M, Dietz V. Modulation of sural nerve somatosensory evoked potentials during stance and different phases of the step-cycle. Electroencephalogr Clin Neurophysiol 96: 516–525, 1995. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci 31: 125–150, 2008. [DOI] [PubMed] [Google Scholar]
- Arzy S, Thut G, Mohr C, Michel CM, Blanke O. Neural basis of embodiment: distinct contributions of temporoparietal junction and extrastriate body area. J Neurosci 26: 8074–8081, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev SV, Stanley CM, Shulman GL, Corbetta M. Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci 7: 542–548, 2004. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Frith CD, Wolpert DM. Spatio-temporal prediction modulates the perception of self-produced stimuli. J Cogn Neurosci 11: 551–559, 1999a. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD. The cerebellum contributes to somatosensory cortical activity during self-produced tactile stimulation. Neuroimage 10: 448–459, 1999b. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat Neurosci 1: 635–640, 1998. [DOI] [PubMed] [Google Scholar]
- Blanke O, Mohr C, Michel CM, Pascual-Leone A, Brugger P, Seeck M, Landis T, Thut G. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. J Neurosci 25: 550–557, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton DA, McIlroy WE, Staines WR. The impact of light fingertip touch on haptic cortical processing during a standing balance task. Exp Brain Res 212: 279–291, 2011. [DOI] [PubMed] [Google Scholar]
- Bonnet M, Chiambretto M, Decety J, Vidal F. Laplacian ERPs for planning to imagine a learned motor sequence. Curr Psychol Cogn 17: 685–698, 1998. [Google Scholar]
- Burke D, Gandevia SC. Interfering cutaneous stimuli and the muscle afferent contribution to cortical potentials. Electroencephalogr Clin Neurophysiol 70: 118–125, 1988. [DOI] [PubMed] [Google Scholar]
- Burke D, Skuse NF, Lethlean AK. Cutaneous and muscle afferent components of the cerebral potential evoked by electrical stimulation of human peripheral nerves. Electroencephalogr Clin Neurophysiol 51: 579–588, 1981. [DOI] [PubMed] [Google Scholar]
- Caldara R, Deiber MP, Andrey C, Michel CM, Thut G, Hauert CA. Actual and mental motor planning and execution: a spatiotemporal ERP study. Exp Brain Res 159: 389–399, 2004. [DOI] [PubMed] [Google Scholar]
- Chapin JK, Woodward DJ. Somatic sensory transmission to the cortex during movement: phasic modulation over the locomotor step cycle. Exp Neurol 78: 670–684, 1982. [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci 2: 671–676, 1999. [DOI] [PubMed] [Google Scholar]
- Chen Y, Martinez-Conde S, Macknik SL, Bereshpolova Y, Swadlow HA, Alonso JM. Task difficulty modulates the activity of specific neuronal populations in primary visual cortex. Nat Neurosci 11: 974–982, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Cameron T, Gillard DM, Prochazka A. Muscular sense is attenuated when human moves. J Physiol 508: 635–643, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuisinier R, Olivier I, Nougier V. Effects of foreperiod duration on anticipatory postural adjustments: determination of an optimal preparation in standing and sitting for a raising arm movement. Brain Res Bull 66: 163–170, 2005. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Bradshaw JL, Phillips JG. Movement-related potentials associated with movement planning and motor imagery. Exp Brain Res 111: 429–436, 1996. [DOI] [PubMed] [Google Scholar]
- Cybulska-Klosowicz A, Meftah el-M, Raby M, Lemieux ML, Chapman CE. A critical speed for gating of tactile detection during voluntary movement. Exp Brain Res 210: 291–301, 2011. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AA, Nawijn S, Berger W, Prokop T, Altenmüller E. Gating of sensation and evoked potentials following foot stimulation during human gait. Exp Brain Res 105: 423–431, 1995. [DOI] [PubMed] [Google Scholar]
- Eimer M, Forster B. Modulations of early somatosensory ERP components by transient and sustained spatial attention. Exp Brain Res 151: 24–31, 2003. [DOI] [PubMed] [Google Scholar]
- Ferrè ER, Bottini G, Haggard P. Vestibular inputs modulate somatosensory cortical processing. Brain Struct Funct 217: 859–864, 2012. [DOI] [PubMed] [Google Scholar]
- Gianna C, Heimbrand S, Gresty M. Thresholds for detection of motion direction during passive lateral whole-body acceleration in normal subjects and patients with bilateral loss of labyrinthine function. Brain Res Bull 40: 443–447, 1996. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Clin Neurophysiol 55: 468–484, 1983. [DOI] [PubMed] [Google Scholar]
- Haggard P, Whitford B. Supplementary motor area provides an efferent signal for sensory suppression. Cogn Brain Res 19: 52–58, 2004. [DOI] [PubMed] [Google Scholar]
- Hämäläinen H, Kekoni J, Sams M, Reinikainen K, Näätänen R. Human somatosensory evoked potentials to mechanical pulses and vibration: contributions of SI and SII somatosensory cortices to P50 and P100 components. Electroencephalogr Clin Neurophysiol 75: 13–21, 1990. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Horak FB, Shupert CL, Jones-Rycewicz C. The importance of somatosensory information in triggering and scaling automatic postural responses in humans. Exp Brain Res 101: 159–164, 1994. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Visual and action cues contribute to the self-other distinction. Nat Neurosci 7: 422–423, 2004. [DOI] [PubMed] [Google Scholar]
- Lyon IN, Day BL. Control of frontal plane body motion in human stepping. Exp Brain Res 115: 345–356, 1997. [DOI] [PubMed] [Google Scholar]
- MacKinnon D, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, Zhang Y, Mille ML, Rogers MW. Planning of anticipatory postural adjustments prior to stepping. J Neurophysiol 97: 4368–4379, 2007. [DOI] [PubMed] [Google Scholar]
- McIlroy WE, Bishop DC, Staines WR, Nelson AJ, Maki BE, Brooke JD. Modulation of afferent inflow during the control of balancing tasks using the lower limbs. Brain Res 961: 73–80, 2003. [DOI] [PubMed] [Google Scholar]
- Morita H, Petersen N, Nielsen J. Gating of somatosensory evoked potentials during voluntary movement of the lower limb in man. Exp Brain Res 120: 143–152, 1998. [DOI] [PubMed] [Google Scholar]
- Mouchnino L, Blouin J. When standing on a moving support, cutaneous inputs provide sufficient information to plan the anticipatory postural adjustments for gait initiation. PloS One 8: e55081, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchnino L, Robert G, Ruget H, Blouin J, Simoneau M. Online control of anticipated postural adjustments in step initiation: evidence from behavioral and computational approaches. Gait Posture 35: 616–620, 2012. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672, 2001. [DOI] [PubMed] [Google Scholar]
- Saradjian AH, Paleressompoulle D, Louber D, Coyle T, Blouin J, Mouchnino L. Do gravity-related sensory information enable the enhancement of cortical proprioceptive inputs when planning a step in microgravity? PloS One 9: e108636, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saradjian AH, Tremblay L, Perrier J, Blouin J, Mouchnino L. Cortical facilitation of proprioceptive inputs related to gravitational balance constraints during step planning. J Neurophysiol 110: 397–407, 2013. [DOI] [PubMed] [Google Scholar]
- Sayenko DG, Vette AH, Obata H, Alekhina MI, Akai M, Nakazawa K. Differential effects of plantar cutaneous afferent excitation on soleus stretch and H-reflex. Muscle Nerve 39: 761–769, 2009. [DOI] [PubMed] [Google Scholar]
- Seki K, Perlmutter SI, Fetz EE. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci 6: 1309–1316, 2003. [DOI] [PubMed] [Google Scholar]
- Staines WR, Graham SJ, Black SE, McIlroy WE. Task-relevant modulation of contralateral and ipsilateral primary somatosensory cortex and the role of a prefrontal-cortical sensory gating system. Neuroimage 15: 190–199, 2002. [DOI] [PubMed] [Google Scholar]
- Stippich C, Ochmann H, Sartor K. Somatotopic mapping of the human primary sensorimotor cortex during motor imagery and motor execution by functional magnetic resonance imaging. Neurosci Lett 331: 50–54, 2002. [DOI] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011: 879716, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmann D, Horak FB. Perturbed step initiation in cerebellar subjects. 2. Modification of anticipatory postural adjustments. Exp Brain Res 141: 110–120, 2001. [DOI] [PubMed] [Google Scholar]
- Voss M, Ingram JN, Haggard P, Wolpert DM. Sensorimotor attenuation by central motor command signals in the absence of movement. Nat Neurosci 9: 26–27, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent negative variation: an electric sign of sensorimotor association and expectancy in the human brain. Nature 203: 380–384, 1964. [DOI] [PubMed] [Google Scholar]
- Williams SR, Chapman CE. Time course and magnitude of movement-related gating of tactile detection in humans. III. Effect of motor tasks. J Neurophysiol 88: 1968–1979, 2002. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science 269: 1880–1882, 1995. [DOI] [PubMed] [Google Scholar]
- Ziat M, Hayward V, Chapman CE, Ernst MO, Lenay C. Tactile suppression of displacement. Exp Brain Res 206: 299–310, 2010. [DOI] [PubMed] [Google Scholar]