Abstract
The neuronal processes that underlie visual searches can be divided into two stages: target discrimination and saccade preparation/generation. This predicts that the length of time of the prediscrimination stage varies according to the search difficulty across different stimulus conditions, whereas the length of the latter postdiscrimination stage is stimulus invariant. However, recent studies have suggested that the length of the postdiscrimination interval changes with different stimulus conditions. To address whether and how the visual stimulus affects determination of the postdiscrimination interval, we recorded single-neuron activity in the lateral intraparietal area (LIP) when monkeys (Macaca fuscata) performed a color-singleton search involving four stimulus conditions that differed regarding luminance (Bright vs. Dim) and target-distractor color similarity (Easy vs. Difficult). We specifically focused on comparing activities between the Bright-Difficult and Dim-Easy conditions, in which the visual stimuli were considerably different, but the mean reaction times were indistinguishable. This allowed us to examine the neuronal activity when the difference in the degree of search speed between different stimulus conditions was minimal. We found that not only prediscrimination but also postdiscrimination intervals varied across stimulus conditions: the postdiscrimination interval was longer in the Dim-Easy condition than in the Bright-Difficult condition. Further analysis revealed that the postdiscrimination interval might vary with stimulus luminance. A computer simulation using an accumulation-to-threshold model suggested that the luminance-related difference in visual response strength at discrimination time could be the cause of different postdiscrimination intervals.
Keywords: discrimination time, reaction time, stimulus luminance, visual search, LIP, monkey
in the real world, we search for a target object under a variety of stimulus conditions. First, the target is discriminated from distractors using stimulus feature signals; the target spatial information is then linked to the shift in covert attention/overt saccades (Schall and Thompson 1999). It is generally thought that the interval required for the former process (prediscrimination interval) varies according to the search difficulty across different stimulus conditions (e.g., Bichot et al. 2001; Sato et al. 2001), whereas the interval for the latter process (postdiscrimination interval) might be stimulus invariant, because stimulus feature signals are no longer necessary after the target location is specified. However, this has not been thoroughly assessed in previous neurophysiological studies.
The lateral intraparietal area (LIP) plays roles in visual-motor transformation (Bisley and Goldberg 2010; Gold and Shadlen 2007; Gottlieb and Snyder 2010) and is crucial for visual searching (Liu et al. 2010; Wardak et al. 2002). Changes in the saccadic reaction time in a given stimulus condition could be accounted for by comparable changes in the target-discrimination time in LIP activity, suggesting that the postdiscrimination interval is constant across trials within a condition (Ipata et al. 2006; Thomas and Paré 2007). Similar results have been reported in the frontal eye field (FEF) (Sato et al. 2001; but see Thompson et al. 1996) and in some neurons of the superior colliculus (SC) (McPeek and Keller 2002). However, the results of some studies have suggested that the mean length of the postdiscrimination interval might change when the stimulus condition is altered (Balan et al. 2008; Cohen et al. 2009; White and Munoz 2011; but see Bichot et al. 2001). It remains unclear whether and how the postdiscrimination interval varies across different stimulus conditions.
To assess this issue, we recorded neurons in the LIP when monkeys performed a color-singleton search task under four stimulus conditions comprising two luminance levels (Bright vs. Dim) and two target-distractor color similarities (Easy vs. Difficult) (Fig. 1). The four stimulus conditions were presented randomly across trials without any cue. A crucial problem here is that changes in the stimulus condition could produce differences not only in the physical properties of the stimulus but also in the saccadic reaction time. Differences in the latter variable may reflect differences in search speed (which is reciprocally related to search accuracy), and these changes might affect the postdiscrimination interval (Shen et al. 2011). To minimize this possibility, we specifically focused on neuronal activity from the Bright-Difficult and Dim-Easy conditions, in which the mean reaction times were indistinguishable, and therefore the search speed could be balanced between conditions. We found that neuronal target discrimination occurred at different times in the two stimulus conditions but was followed by similar reaction-time saccades. A further analysis using neuronal activity from all four stimulus conditions suggested that the postdiscrimination interval might vary with the stimulus luminance, and our computer simulations predicted that the luminance-dependent difference in the strength of visual responses could affect determination of the postdiscrimination interval.
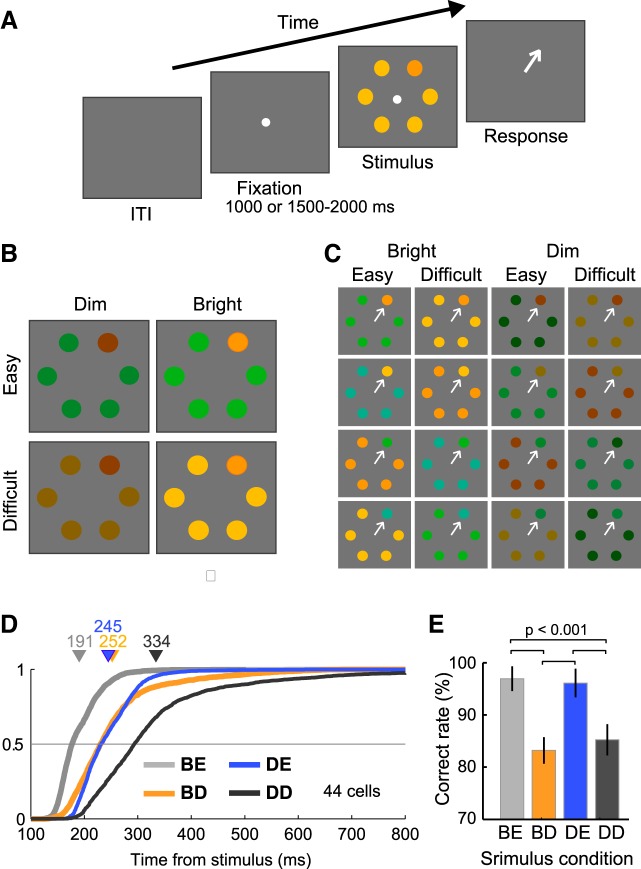
Fig. 1.
Visual stimuli, behavioral task, and behavioral performances. A: reaction-time visual search task. In the target-present trials, a search array consisting of a singleton uniquely colored element (target) and 5 identical elements (distractors) was presented after fixation, and the monkeys were required to make a saccade toward the target (arrow). When a saccadic eye movement was detected, the visual stimuli and fixation spot were immediately extinguished. B: configuration of the visual stimuli. Four different stimulus conditions were produced by manipulating the target-distractor color similarity (Easy vs. Difficult) and stimulus luminance (Bright vs. Dim). C: the stimulus set used in the current study. Each stimulus condition consisted of 4 target-distractor color pairs. All of the stimuli were presented quasirandomly with equal probability. D: cumulative plots of the saccadic reaction times obtained during the recording sessions (44 neurons). Triangles and numbers above data points indicate the mean saccadic reaction time in each condition. The target-distractor color similarity was manipulated so that the mean reaction times were comparable between the Bright-Difficult and Dim-Easy conditions. E: task performance of the 4 stimulus conditions. Error bars indicate ± SE. BE, Bright-Easy; BD, Bright-Difficult; DE, Dim-Easy; DD, Dim-Difficult.
MATERIALS AND METHODS
The single-neuron data (n = 122, 71 from monkey Y and 51 from monkey S) presented here were examined for other purposes in our previous studies (Nishida et al. 2013, 2014). The surgical procedure, stimulus presentation, data acquisition, and data analyses have been described elsewhere (Nishida et al. 2013, 2014; Tanaka et al. 2013).
Animal Preparation and Apparatus
Data were collected from two female macaque monkeys (Macaca fuscata; monkey Y, 4 yr old, 5.0 kg; monkey S, 10 yr old, 6.3 kg). Subconjunctival scleral search coils (Judge et al. 1980), head-restraining devices, and recording chambers were implanted during aseptic surgery that was performed under general anesthesia using ketamine and pentobarbital sodium. The recording chambers (22 mm inner diameter) were placed at stereotaxic coordinates (anterioposterior, P0-4 mm; mediolateral, L18-21.5 mm) above the intraparietal sulcus (IPS) guided by T1 magnetic resonance imaging (MRI). All of the animal care procedures and experimental protocols were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (1996) and were approved by the Animal Care and Use Committee of Kyoto University.
Visual stimuli were generated by a video-signal generator (ViSaGe; Cambridge Research Systems, Cambridge, UK) and were presented on a video monitor with a 100 Hz refresh rate and an 800 × 600-pixel resolution (RDF223H; Mitsubishi, Tokyo, Japan). Stimuli were viewed binocularly from a distance of 42 cm in a dark room and subtended a visual angle of 51.5 × 40.0°. Extracellular activity was recorded using an epoxylite-insulated tungsten electrode (Frederick Haer & Co, Bowdoinham, ME) with impedance > 2 MΩ (model IMP-1; Bak Electronics, Germantown, MD), and the signal was stored at a sampling rate of 50 kHz. The eye position was monitored with the scleral search-coil technique (Fuchs and Robinson 1966) (eye-position detector DSC-2001; Datel, Tokyo, Japan), and the signals were analyzed at a 1 kHz resolution.
Behavioral Paradigms
Reaction-time visual search task.
Figure 1A illustrates the time sequence for a reaction-time visual search task. In each trial, after sustained fixation within a window (± 1.2–1.8°) for 1,000 or 1,500–2,000 ms, a six-element search array was displayed on an imaginary circle (eccentricity = 8.5°). In the target-present trials, the monkeys were required to make a saccade toward a color singleton target after array presentation in the target-present trials. The target stimulus appeared with equal probability in the receptive field or in the location diametrically opposite the fixation spot; therefore, the target appeared at just two fixed locations in each recording session. Due to this design, the correct response rate was slightly higher than reported previously (e.g., Bichot et al. 2001; Thompson et al. 2005; White and Munoz 2011). When the computer detected a saccadic eye movement, the visual stimuli and fixation spot were extinguished immediately. If the monkeys made a single saccade that landed inside a square window (± 3.0 × 3.0°) centered on the target, another fixation point appeared at the target position. The monkey received a juice reward after 600 ms of fixation on this point. Target-absent trials were also interleaved with target-present trials. In the target-absent trials, all six elements had the same stimulus features, and the monkeys received a reward if they maintained their fixation (600-1,800 ms) until the end of the trial. The target-present and target-absent trials were presented pseudorandomly at a ratio of 4:1. Because the reaction-time trials were started without any cue that provided information regarding the stimulus condition or target location, the monkeys did not use any top-down bias for target selection (e.g., Bichot et al. 2005; Bichot and Schall 1999; Buschman and Miller 2007; Ogawa and Komatsu 2004, 2009).
Figure 1B shows the four stimulus conditions used for the target-present trials, which were created by manipulating the target-distractor color similarity and luminance of the array elements (Bright-Easy, Bright-Difficult, Dim-Easy, Dim-Difficult conditions). The luminance of each element was either 10 cd/m2 (Bright) or 1 cd/m2 (Dim) on a gray background (1 cd/m2). The color of each element was either orange (International Commission on Illumination chromaticity coordinate, x = 0.43, y = 0.47 for monkey Y; x = 0.44, y = 0.46 for monkey S), yellowish-orange (x = 0.41, y = 0.48 for monkey Y; x = 0.42–0.43, y = 0.47 for monkey S), green (x = 0.24, y = 0.41 for monkey Y; x = 0.23, y = 0.37–0.39 for monkey S), or bluish-green (x = 0.23, y = 0.38 for monkey Y; x = 0.22, y = 0.34 for monkey S). The target- and distractor-color pairs were selected so that their colors were either more different (Easy: target/distractor colors orange/green, green/orange, yellowish-orange/bluish-green, or bluish-green/yellowish-orange) or more similar (Difficult: yellowish-orange/orange, orange/yellowish-orange, bluish-green/green, or green/bluish-green) (Fig. 1C). Each color pair was presented with equal probability. The color differences between the target and distractor elements were carefully manipulated so that the two array stimuli of the Dim-Easy and Bright-Difficult conditions evoked similar mean reaction times. Therefore, the degree of search speed was balanced in terms of the saccadic reaction time between the Dim-Easy and Bright-Difficult conditions.
Memory-guided saccade task.
The memory-guided saccade task (Hikosaka and Wurtz 1983) was used to assess visual, motor, and delay activity and to estimate the receptive field of the cell being studied. A trial of this task was initiated by the appearance of a central fixation point (yellow circle/square or white square spot, luminance = 56 cd/m2 or 66 cd/m2), and a single circular stimulus (luminance = 10 cd/m2, size = 2.24°2) was flashed for 500–700 ms during fixation. After a delay (900-1,800 ms), the fixation point was removed, and the monkeys were required to make a saccade to the location of the target that flashed previously.
Data Collection
We specified the location of the IPS lateral bank that exhibits visual- and saccade-related responses (Barash et al. 1991a,b; Maimon and Assad 2006; Mountcastle et al. 1975) and regarded a cortical region as the LIP where neurons exhibit robust, spatially tuned responses during the delay period of a memory-guided saccade task (Barash et al. 1991a,b; Colby et al. 1996; Gnadt and Andersen 1988; Shadlen and Newsome 2001). Area 7a is known to play important roles in visual searches (e.g., Constantinidis and Steinmetz 2001). To ensure that the samples measured were in the LIP rather than in area 7a, neurons recorded at a depth shallower than 3 mm from the dura surface were excluded from the present analysis (Andersen et al. 1990; Linden et al. 1999; Gifford and Cohen, 2004). Neurons were typically recorded at a depth >5 mm from the dura surface (80.3%). Postoperative structural MRI images were acquired for both monkeys and were used to determine the x-y coordinates of the recording position.
Once a neuron was isolated during the recording sessions, the location of the receptive field was assessed in the memory-guided saccade task for all neurons studied (Tanaka et al. 2013). It should be noted that, because the target-location eccentricity was fixed at 8.5° in the present task, this would have induced suboptimal visual stimulation of each neuron (Schall et al. 2007). In previous studies, the target eccentricity was instead changed according to the location of a neuron's receptive field, and the size of the stimuli was scaled according to that eccentricity to provide equal visibility. Nevertheless, because the primary aim of the current study was to match the mean reaction times between the Bright-Difficult and Dim-Easy conditions, the use of a fixed eccentricity was a time-saving and pragmatic method for this purpose, and this also excluded the possibility that the difficulty of the task under these two conditions would be imbalanced when the eccentricity was changed (e.g., Gruber et al. 2014; Shive and Francis 2013).
Data Analysis
Unless indicated otherwise, only data from the successful trials were analyzed in this study.
Spike Density Function
The spike train was sampled at 1 kHz and was convolved with a Gaussian of sigma 10 ms to produce a spike density function (Richmond and Optican 1987). We calculated the normalized spike density function for each neuron by transforming the range between the baseline activity during a 200 ms interval before stimulus onset and maximum activity across stimulus conditions during the interval from stimulus onset to the mean saccadic reaction time in the range of 0–1. We constructed the normalized population responses by averaging the normalized spike density functions across neurons.
Target-discrimination Time
The target-discrimination time was defined as the first time point at which target activity (the activity when the target was within a neuron's receptive field) was significantly greater than distractor activity (the activity when the target was opposite the receptive field) for at least 10 ms (Wilcoxon rank-sum tests, P < 0.01). The length of time from stimulus onset to neuronal target discrimination was defined as the prediscrimination interval, and that from target discrimination to saccade onset was defined as the postdiscrimination interval. Neurons that exhibited target discrimination before both the mean reaction time in stimulus-aligned activity and the saccade onset in saccade-aligned activity were analyzed. The target-discrimination time of the normalized population response was defined as the earliest point at which the target and distractor activities differed significantly for at least 10 ms (Wilcoxon rank-sum tests, P < 0.01).
To calculate the target-distractor discrimination probability, we also performed receiver operating characteristic (ROC) analysis in a way that was similar to a method described previously (Thomas and Paré 2007; Thompson et al. 1996). At each millisecond time point, the separation of the distribution of target and distractor activity was quantified by calculating area under the ROC curves (AUCs) as a function of time. Next, the time course of discrimination probability (time sequence of AUCs) was fitted to the cumulative Weibull distribution function in the form W(t) = γ − (γ − δ)·exp[− (t/α)β], where t = the time from when the AUC reached its minimum value (δ) to its maximum value (γ), α = the time at which the area under the ROC curve reached the sum of 63.2% of its maximum, and β = the slope. The functions were terminated when the number of either target or distractor trials was <5.
Goal-discrimination Time in False-alarm Error Trials
Catch trials occasionally resulted in false-alarm errors in which the monkeys made a saccade to one of the array elements before the trial ended. We compared the activity when the saccade goal was located in the receptive field with the activity when the saccade goal was located diagonally opposite the fixation point from the receptive field. Using an analytic method that was similar to the aforementioned method employed to determine target-discrimination time, we evaluated the goal-discrimination time at which a neuron reliably predicted the goal location of an erroneous saccade response. We analyzed neurons in which the monkey made ≥7 false-alarm saccades to both the receptive field and the location opposite to the receptive field in either the Bright or Dim catch conditions.
Covariability between the Target-Discrimination Time and Saccadic Reaction Time
To assess the temporal relationship between the target-discrimination time and the saccadic reaction time, the covariability of these two variables was examined (Thompson et al. 1996). For each neuron, trials under each stimulus condition were divided into two groups according to the saccadic reaction time (short vs. long reaction-time groups), and the target-discrimination time was calculated for each reaction-time group. If there were a tight link between the target-discrimination time and the saccadic reaction time, the changes in the target-discrimination time might be equal to those in the saccadic reaction time, indicating that the ratio of a change in the target-discrimination time to a change in the saccadic reaction time was 1. In contrast, if there were no link, changes in the target-discrimination time might be zero regardless of changes in the saccadic reaction time, indicating that the ratio was 0.
Reaction-time Matching
The mean saccadic reaction times between the Bright-Difficult and Dim-Easy conditions were balanced by manipulating the properties of the stimulus (Fig. 1). Nevertheless, a small difference in the mean saccadic reaction time and a substantial difference in the variability remained. To eliminate any possible effects of these differences on the present results, we further performed reaction-time matching. A pair of trials from the Bright-Difficult and Dim-Easy trials were selected for each neuron such that the saccadic reaction-time difference did not exceed 5 ms, and this choice was repeated for as long as possible. This procedure ensured that the reaction-time distributions were statistically indistinguishable between the two stimulus conditions.
Leaky Integration of Visually Responsive LIP Neuronal Activities
Recent studies suggest that visually responsive neurons in the LIP and FEF provide signals that drive an accumulation process in downstream neurons (movement neurons in the FEF and SC), which then initiate a saccade response when their activity reaches a fixed response threshold (Heitz and Schall 2012; Purcell et al. 2010, 2012; Schall et al. 2011). To explore this possibility, the population-averaged raw histograms (1 ms resolution) for the trials when the target appeared in the receptive filed were convolved with a leaky integrator, which was modeled as a single exponential (a varying time constant, 1–140 ms) to produce the accumulated responses. The time constant of the leaky integrator was adjusted so that the sum of the differences in the accumulated responses of the four stimulus conditions was minimal at the time of saccade initiation.
RESULTS
Neuronal Database
The activity of 122 visually responsive neurons in the LIP of two macaques (71 from monkey Y, 51 from monkey S) performing the visual search task was recorded. The neurons that showed target discrimination before the mean reaction times in stimulus-aligned activity and before the saccade onset in saccade-aligned activity were selected and analyzed. Of the 122 cells, 93, 87, 95, and 81 fulfilled this criterion in the Bright-Easy, Bright-Difficult, Dim-Easy, and Dim-Difficult conditions, respectively. The variation in the number of neurons across conditions might be due to differences in the discrimination probability between the Easy (93–95 neurons) and Difficult (81–87 neurons) conditions (Nishida et al. 2013). Of these, 78 fulfilled the criterion in all four stimulus conditions, and 44 (23 from monkey Y and 21 from monkey S) still met the criterion, even when the trials were divided into short and long reaction-time groups in the Bright-Difficult and Dim-Easy conditions. These 44 cells were used for subsequent analyses.
Behavioral Performance
Figure 1D shows the saccadic reaction times obtained from the recordings of the 44 neurons studied. The cumulative reaction-time distributions for each neuron were calculated and then averaged across all neurons. The mean reaction time (triangles) was shortest in the Bright-Easy trials (gray) and longest in the Dim-Difficult trials (black). The reaction times largely overlapped and were indistinguishable between the Bright-Difficult (orange) and Dim-Easy (blue) conditions (Wilcoxon signed-rank test, P = 0.55), although the variability was larger in the Bright-Difficult condition compared with the Dim-Easy condition. Figure 1E shows the percentage of correct response rates across stimulus conditions. On average, the correct response rates in the Easy conditions (range, 96–97%) were significantly greater than were those in the Difficult conditions (83–85%; Wilcoxon signed-rank-test, P < 0.001). Thus, although the reaction times were indistinguishable, the correct response rates were significantly different between the Bright-Difficult and Dim-Easy conditions. The possible effects of the differences in the rate of correct responses are considered below.
Target-discrimination Time under Different Stimulus Conditions
Figure 2A shows the spike density functions of an example LIP neuron in the Bright-Difficult (Fig. 2A, orange traces, top) and Dim-Easy (Fig. 2A, blue traces, bottom) conditions aligned separately according to stimulus onset (Fig. 2A, left) and saccade onset (Fig. 2A, right). The visually responsive activity was quite different between the two stimulus conditions. The response onset latency (squares), defined as the time point at which the response was greater than the mean + 2 SDs of the baseline activity during a 200 ms period before stimulus onset, was 25 ms earlier in the Bright-Difficult condition (68 ms) than in the Dim-Easy condition (93 ms). In addition, the strength of the initial visual response 70–120 ms after stimulus onset for the Bright-Difficult condition (28 spikes/s) was more than twice that for the Dim-Easy condition (13 spikes/s). In spite of this difference in activity, there was no distinguishable difference in the mean reaction times between conditions (mean ± SE = 200 ± 5.6 ms vs. 199 ± 2.1 ms; two sample t-test, P = 0.86).
Fig. 2.
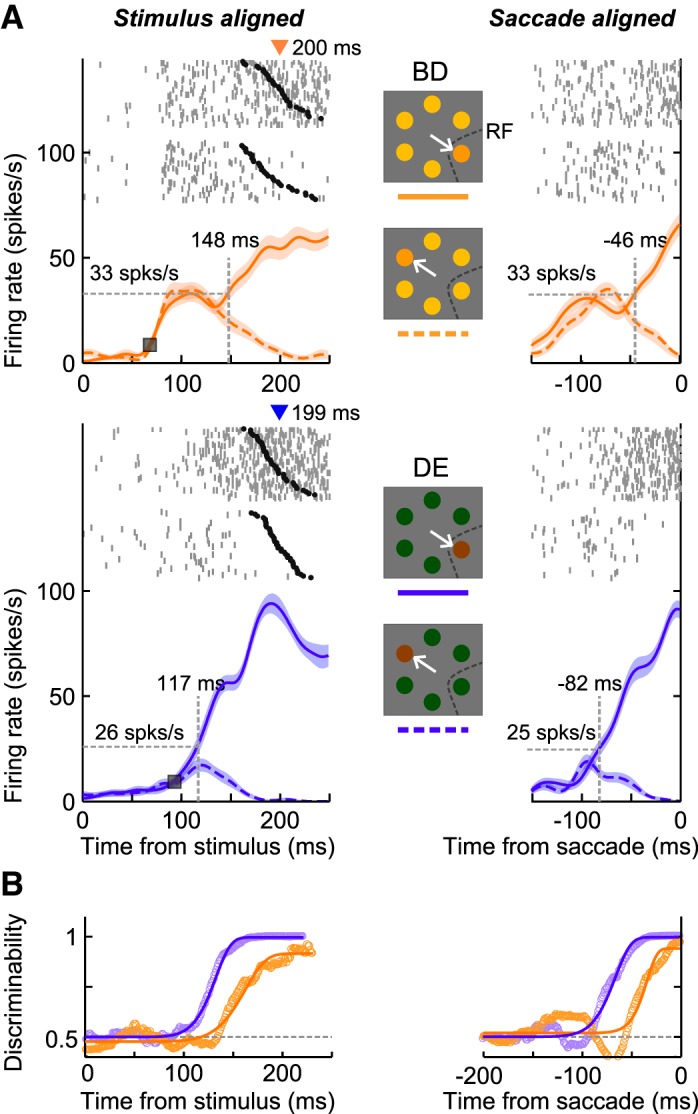
Activity of a single lateral intraparietal area (LIP) neuron in the Bright-Difficult and Dim-Easy conditions. A: spike density functions (mean ± SE) in the Bright-Difficult (orange traces) and Dim-Easy conditions (blue traces). Left and right panels indicate stimulus- and saccade-aligned activity, respectively. Solid and dashed traces of the spike density functions denote the activity when the target appeared in the receptive field and away from it, respectively. Vertical dashed lines with a number above indicate the target-discrimination times, and horizontal dashed lines indicate the response strength at the target-discrimination time. Gray ticks and black circles above the spike density functions indicate the action potentials and saccade onsets, respectively. Each row of rasters indicates 1 trial. Triangles above the raster plots indicate the mean saccadic reaction times. B: time-course of the discrimination probability at each millisecond as evaluated using receiver operating characteristic (ROC) analysis (circles) and best-fit Weibull functions (thick traces). The goodness of fit (r2) in the Bright-Difficult and Dim-Easy conditions was 0.98 and 1.0 for stimulus-aligned activity, and 0.92 and 0.99 for saccade-aligned activity, respectively. RF, receptive field.
For the primary purpose of this study, we compared the target-discrimination time (vertical dashed lines) between the two stimulus conditions. In stimulus-aligned activity, the prediscrimination interval was 31 ms shorter in the Dim-Easy condition (117 ms) compared with the Bright-Difficult condition (148 ms). Conversely, in saccade-aligned activity, the postdiscrimination interval was 36 ms longer in the Dim-Easy condition (82 ms) compared with the Bright-Difficult condition (46 ms). Figure 2B shows the time course of the discrimination probability and the best-fit Weibull functions in each condition. The discriminability increased earlier and was stronger in the Dim-Easy condition compared with the Bright-Difficult condition. Obvious differences were observed ∼100 ms after the stimulus and before the saccade onset.
In addition to the difference in the target-discrimination time, we also noticed differences in the response strength at the target-discrimination times (horizontal dashed lines in Fig. 2A). In both stimulus- and saccade-aligned activity, the instantaneous discharge rate at the target-discrimination time was ∼30% stronger in the Bright-Difficult condition (33 spikes/s) compared with the Dim-Easy condition (25–26 spikes/s) (Wilcoxon rank-sum test, P < 0.001). Therefore, the results of the example neuron suggest that the target-discrimination time and response strength at that time could differ among stimulus conditions, even if they were followed by similar reaction-time saccades.
Population Analyses of Target-discrimination Time under Different Stimulus Conditions
The response profiles observed in the example neuron shown in Fig. 2 were preserved at the population level. Figure 3A shows the normalized population responses under the Bright-Difficult and Dim-Easy conditions obtained from 44 LIP neurons, which revealed significantly discriminated activity in all four stimulus conditions (Wilcoxon rank-sum test, P < 0.01). Figure 3, B and C, shows the distributions of the target-discrimination times and the response strengths at the target-discrimination times, respectively, obtained from the individual neurons. The target-discrimination time was significantly earlier in the Dim-Easy condition compared with the Bright-Difficult condition (Wilcoxon signed-rank test, P < 0.0001), and the response strength at the target-discrimination time was significantly stronger in the Bright-Difficult condition than in the Dim-Easy condition (Wilcoxon signed-rank test, P < 0.01). Consistent with this, the strength of the target-discrimination probability increased earlier and was stronger in the Dim-Easy condition compared with the Bright-Difficult condition (Fig. 3D). A significant difference in the discrimination probability started ∼100 ms after the stimulus onset and remained continuous until the saccade-initiation time (thick lines; Wilcoxon signed-rank test, P < 0.01).
Fig. 3.
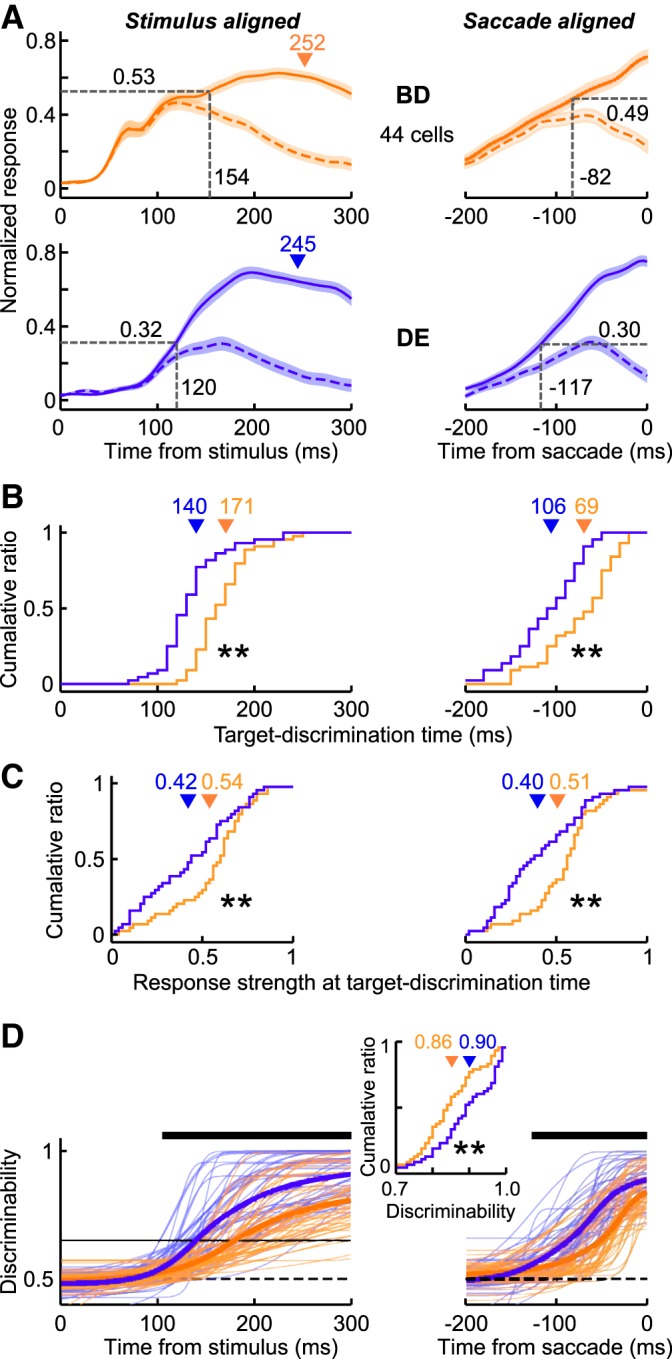
Population responses of LIP neurons in the Bright-Difficult and Dim-Easy conditions. A: normalized population responses obtained from 44 neurons. Spike density functions in the Bright-Difficult (orange traces) and Dim-Easy (blue traces) trials. Distribution of the target-discrimination times (B) and response strength at the target-discrimination time (C). Triangles indicate the mean of each distribution. **Significant differences at P < 0.01 (Wilcoxon signed ranks test). D: discrimination probability. Thin and thick traces indicate the best-fit Weibull functions for the individual neurons and their means, respectively. Horizontal bars indicate the period during which the discrimination probability difference was significant (Wilcoxon signed-rank test, P < 0.01). The goodness of fit (r2) in the Bright-Difficult and Dim-Easy conditions was 0.83 ± 0.02 (mean ± SE) and 0.93 ± 0.01 for stimulus-aligned activity, and 0.92 ± 0.07 and 0.94 ± 0.08 for saccade-aligned activity, respectively. Central inset, the distribution of the discrimination probability for the individual neurons at the saccade-initiation timing.
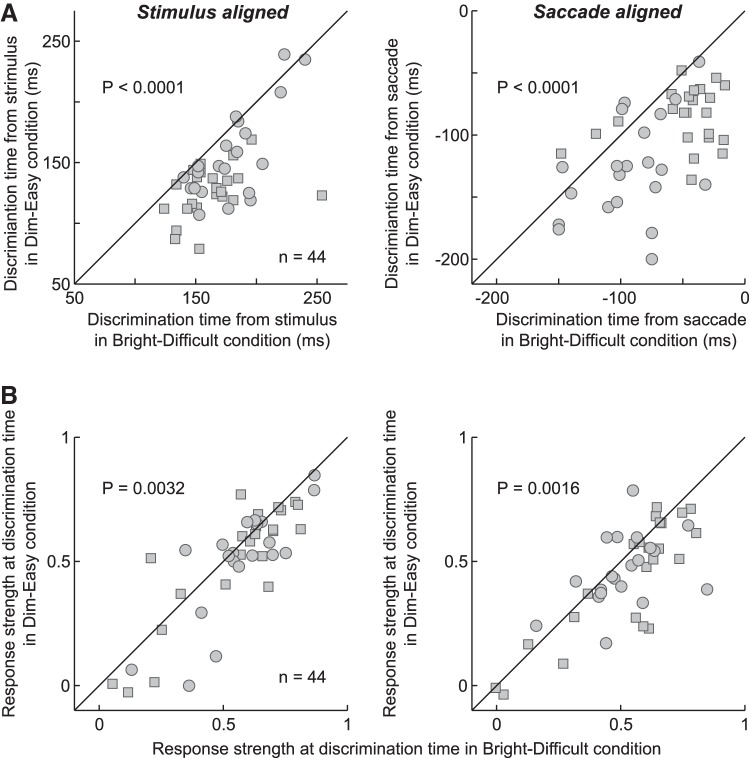
The results of Fig. 3 were generally consistent across neurons and among monkeys. Figure 4 shows the changes in either the target-discrimination time (Fig. 4A) or the response strength at that time (Fig. 4B) for individual neurons between the Dim-Easy and Bright-Difficult conditions. Squares and circles indicate the data from monkeys Y and S, respectively. The target discrimination occurred earlier in the Dim-Easy condition than in the Bright-Difficult condition for 95% (42/44) of the neurons in stimulus-aligned activity and 84% (37/44) of the neurons in saccade-aligned activity. The ratio was significantly higher than would be expected by chance in both cases (χ2-test, P < 0.0001). The strength of the response at the target-discrimination time was greater in the Bright-Difficult condition than in the Dim-Easy condition in 68% (30/44) of the neurons in stimulus-aligned activity and in 73% (32/44) of the neurons in saccade-aligned activity (χ2-test, P < 0.005).
Fig. 4.
Target-discrimination time and strength of the response for individual neurons. A: comparisons of the target-discrimination times between the Bright-Difficult and Dim-Easy conditions for individual neurons (n = 44). Analyses were made using neuronal data aligned at the stimulus onset (left) and saccade onset (right). Squares and circles indicate the data from monkeys Y and S, respectively. Data points located below the diagonal line indicate that the target-discrimination time was earlier in the Dim-Easy condition than in the Bright-Difficult condition. B: comparisons of the strength of the response at the target-discrimination time between the 2 stimulus conditions. Data points located below the diagonal line indicate that the strength of the response at the target-discrimination time was stronger in the Bright-Difficult condition than in the Dim-Easy condition.
Taken together, the population analyses reveal that target discrimination occurred earlier in the Dim-Easy condition than in the Bright-Difficult condition, whereas the strength of the response at the discrimination time was greater in the Bright-Difficult condition compared with the Dim-Easy condition. In other words, late target discrimination was associated with a strong response (Bright-Difficult), whereas early target discrimination was associated with a weak response (Dim-Easy). The observation that the postdiscrimination interval differed among stimulus conditions suggests that this interval is not devoted solely to processing spatial information for saccade preparation/initiation.
Reaction-time Matching
Although the mean reaction times were balanced between the Bright-Difficult and Dim-Easy conditions (Figs. 3, 4), the reaction-time variability was not matched (Fig. 1D). To confirm that the present results were not due to this difference, we performed reaction-time matching between the two stimulus conditions for each neuron to equalize the reaction-time distributions in either the target and distractor trials (AUC = 0.46–0.56; Kolmogorov-Smirnov test, P = 0.54–1.0). Figure 5A shows the population mean of the reaction-time distributions across neurons, which were almost perfectly matched between the Bright-Difficult and Dim-Easy conditions (Kolmogorov-Smirnov test, P = 1.00). Figure 5, B–E, shows the results when the same analyses used in Fig. 4, A–D, were applied to neuronal data after reaction-time matching. The results described in Fig. 4 remained the same; therefore, the target-discrimination time could differ under different stimulus conditions, even when the reaction times were almost perfectly matched between conditions.
Fig. 5.
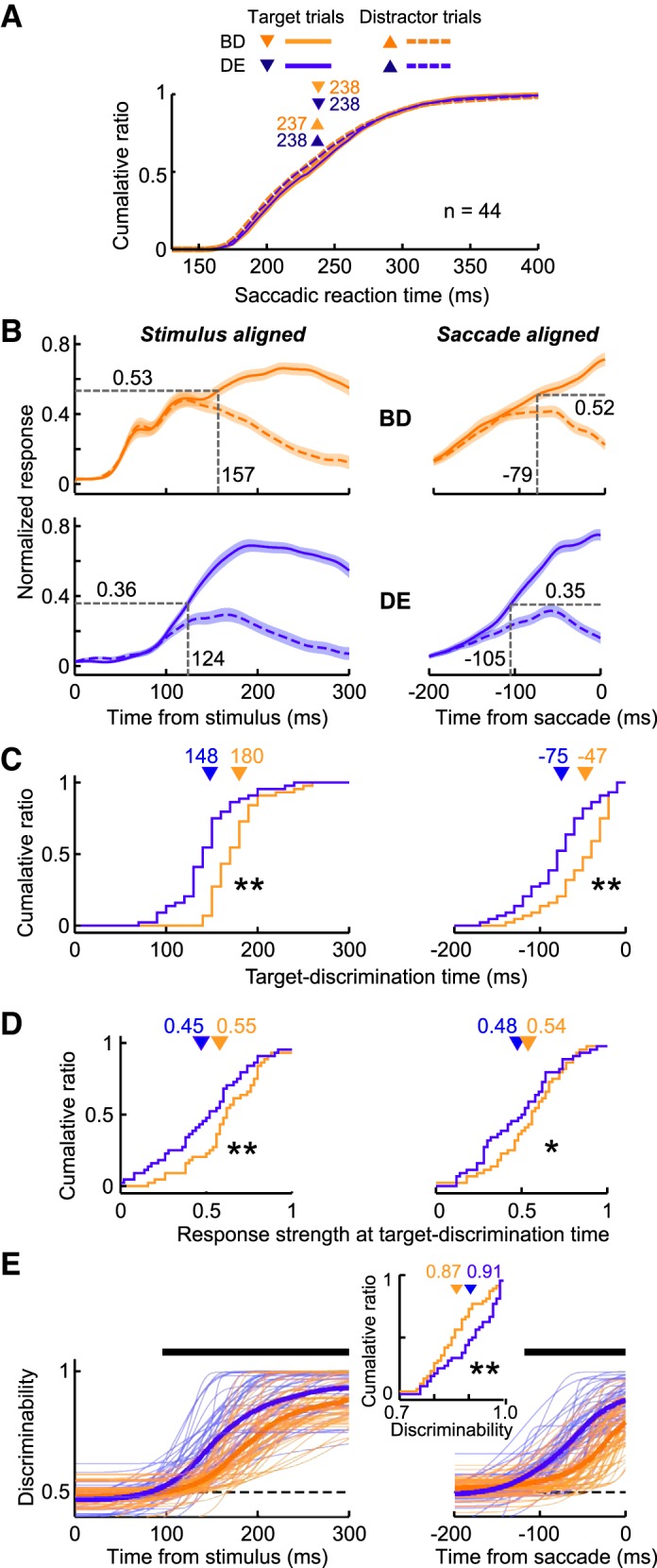
Population responses of reaction time-matched Bright-Difficult and Dim-Easy trials. A: cumulative probability of the saccadic reaction times after reaction-time matching (n = 44). Orange and blue traces indicate the data from the Bright-Difficult and Dim-Easy trials, respectively. Solid and dashed traces indicate the data from the target and distractor trials, respectively. Triangles with numbers above indicate the mean saccadic reaction times. There were no significant differences between any pair of the distributions (Wilcoxon signed-rank test, P > 0.1). B: normalized population responses calculated from the reaction-time matched trials. C, D: the distribution of the target-discrimination time and the strength of the response at the target-discrimination time, respectively. Significant differences at *P < 0.01 and **P < 0.05 (Wilcoxon signed-rank test), respectively. E: discrimination probability. The goodness of fit (r2) in the Bright-Difficult and Dim-Easy conditions was 0.74 ± 0.03 (mean ± SE) and 0.84 ± 0.03 for stimulus-aligned activity, and 0.72 ± 0.22 and 0.86 ± 0.15 for saccade-aligned activity, respectively.
Which is More Important, Stimulus Luminance or Target-distractor Similarity, for Determining the Postdiscrimination Interval?
Because both luminance and target-distractor similarity (search difficulty) differed between the Bright-Difficult and Dim-Easy conditions, it was unclear which factor was more important for producing changes in the postdiscrimination interval. To explore this further, we performed two-way ANOVA using neuronal data obtained during all four stimulus conditions (Bright-Easy, Bright-Difficult, Dim-Easy, and Dim-Difficult conditions). Figure 6A shows the target-discrimination time in stimulus-aligned activity. The interval increased with search difficulty and decreased with increasing luminance. Data revealed significant main effects of both target-distractor similarity and luminance [target-distractor similarity, F(1,172) = 158.9, P < 0.0001; luminance, F(1,172) = 40.1, P < 0.0001] but no interaction [F(1,172) = 183, P = 0.18]. In contrast, the target-discrimination time in saccade-aligned activity varied only with stimulus luminance (Fig. 6B). Two-way ANOVA showed a significant main effect of luminance [F(1,172) = 17.0, P < 0.0001], but not target-distractor similarity [F(1,172) = 1.35, P = 0.25]; there was no interaction [F(1,172) = 0.01, P = 0.92]. Therefore, the changes at the postdiscrimination interval were likely due to changes at the luminance level, but not in target-distractor similarity (search difficulty).
Fig. 6.
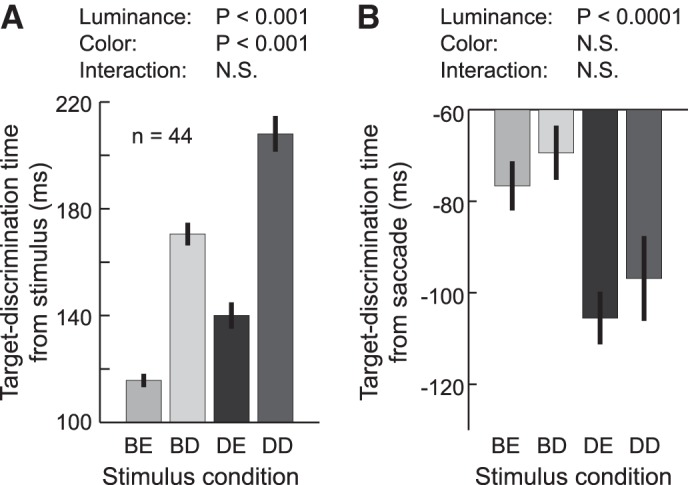
Dependence of target-discrimination time on stimulus parameters. A: mean target-discrimination time in stimulus-aligned activity (the mean prediscrimination interval) across the 44 neurons in the four stimulus conditions. Error bars indicate ± SE. The significance of the effects of luminance (Luminance), target-distractor color similarity (Color), and their interaction (Interaction) were determined by 2-way ANOVA. N.S. indicates P > 0.05. B: mean target-discrimination time in saccade-aligned activity (its absolute value represents the mean postdiscrimination interval).
Possible Influences of Different Correct Response Rates
Although the mean saccadic reaction times were balanced between the Bright-Difficult and Dim-Easy conditions (Fig. 1D), a difference in the correct response rates remained (Fig. 1E). Therefore, the reward probability/saccade response probability differed between conditions, and this may have affected neuronal activity in the visual-motor areas (e.g., Platt and Glimcher 1999; Sugrue et al. 2004; Thompson et al. 2005). If this were the case, the postdiscrimination interval would vary with search difficulty (i.e., target-distractor color similarity) because the correct response rates significantly varied between the Easy and Difficult conditions (Wilcoxon signed-rank test, P < 0.001), but it did not vary with luminance because the correct response rate did not significantly vary between the Bright and Dim conditions (Wilcoxon signed-rank test, P > 0.05). However, the present results revealed that the opposite was true (Fig. 6B), indicating that the observed difference in the postdiscrimination interval was likely not explained by a difference in the correct response rate.
Dependence on History of Search Difficulty
As shown in Fig. 1D, the correct response rates in the Easy search condition were significantly higher than were those in the Difficult search condition, suggesting that a different degree of search accuracy may be required in the Easy and Difficult search conditions. To ascertain whether the effects of this difference in the speed stress remained in the following trials, we compared the behavioral and neuronal performances in each of the Bright-Difficult and Dim-Easy conditions when the preceding trial was an Easy condition and when it was a Difficult condition. The data were analyzed only when two successive search trials were completed successfully. The results revealed that the saccadic reaction times were statistically indistinguishable (Wilcoxon signed-rank test, P = 0.23–0.84), and the strength of the activity did not differ at any millisecond time point 250 ms after stimulus onset (Wilcoxon signed-rank test, P > 0.05), irrespective of the search difficulty of the preceding trial. This result suggested that the history of search difficulty did not have a substantial influence on the behavioral and neuronal responses of the following trial.
Covariability of Target-discrimination Time and Saccade-Initiation Time within Stimulus Conditions
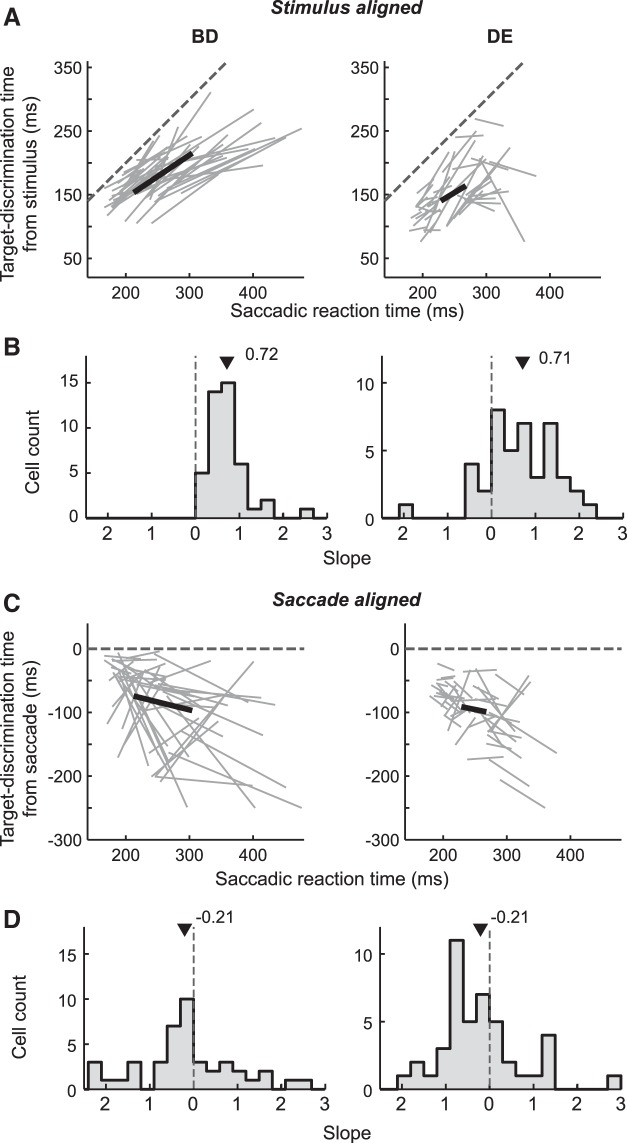
Previous studies in the LIP revealed that the variability of the saccadic reaction times could be explained by variability in the target-discrimination times (Ipata et al. 2006; Thomas and Paré 2007). To show that the current sample neurons had similar response properties, we examined the relationship between the target-discrimination time and the saccadic reaction times by dividing the trials into two equally sized (short and long reaction-time) groups for each neuron and then calculated the target-discrimination times and the mean reaction times for each group for each neuron. Figure 7A shows the relationship between the target-discrimination time in stimulus-aligned activity (prediscrimination interval) and the saccadic reaction time obtained under the Bright-Difficult (Fig. 7A, left) and Dim-Easy (Fig. 7A, right) conditions. Each line plot illustrates how a change in target-discrimination time was related to a change in the mean reaction time for each neuron. Figure 7B shows the slopes of the individual line segments (change in the prediscrimination interval/change in the saccadic reaction time). Their distributions were significantly different from both 0 (Wilcoxon signed-rank test, P < 0.001) and 1 (P < 0.01) in both conditions. The mean (± SE) slopes were 0.72 ± 0.07 and 0.71 ± 0.13 in the Bright-Difficult and Dim-Easy conditions, respectively, indicating that the variability in the prediscrimination interval partially (∼70%) accounted for the variability in the reaction times. In contrast, the target-discrimination time in saccade-aligned activity (postdiscrimination interval) covaried less with the saccadic reaction time (Fig. 7C). The slope values were significantly smaller than 1 under both conditions (Wilcoxon signed-rank test, P < 0.001), and there was no systematic difference from 0 [marginally significant under the Dim-Easy condition (P = 0.024), but this was not significant under the Bright-Difficult condition (P = 0.17)]. The mean slopes were −0.21 ± 0.18 and −0.21 ± 0.14 in the Bright-Difficult and Dim-Easy conditions, respectively (Fig. 7D).
Fig. 7.
Covariability between target-discrimination time and saccadic reaction time. A: relationship between the target-discrimination time in the stimulus-aligned activity and the saccadic reaction time of each of the 44 neurons. The target-discrimination time in the Bright-Difficult (left) and Dim-Easy (right) conditions, respectively. Each gray line indicates 1 neuron, the thick black line indicates the population mean among neurons, and the dotted line indicates unity. B: distributions of the slopes of the line segments in A. Triangles indicate the means; the slope distributions differed significantly from 0 (Wilcoxon signed-rank test, P < 0.0001) and 1 (P < 0.01) in both stimulus conditions. C, D: relationship between target-discrimination time in saccade-aligned activity and the saccadic reaction time. The slope distributions were significantly smaller than 1 in both stimulus conditions (Wilcoxon signed-rank test, P < 0.0001). The difference from 0 was not significant in the Bright-Difficult condition (P = 0.17) and marginally significant in the Dim-Easy condition (P = 0.024).
The above results reveal that a major part of the reaction-time variability could be explained by variability in the prediscrimination interval. This is roughly consistent with previous reports (Ipata et al. 2006; Thomas and Paré 2007). However, the reduced covariability between the prediscrimination interval and the saccadic reaction time observed in the current study (mean slope ∼0.7) compared with previous studies (∼1) might be due to differences in the current task requirement. Although multiple saccade responses were allowed before foveating the target in the previous studies, only a single saccade was allowed in the current study. Such a difference might affect the preservation of the postdiscrimination interval constancy (Shen and Paré 2011).
Applicability of the Leaky Integration Model
Different luminance levels evoke different magnitude visual responses in LIP neurons (Tanaka et al. 2013), and our control analysis suggested that the postdiscrimination interval decreased with increasing luminance (Fig. 6B). These findings raised the possibility that different magnitude responses were transformed into different postdiscrimination intervals. First, a possible mechanism for this effect was the accumulation-to-threshold (rise-to-threshold) mechanism (Carpenter and Williams 1995; Cisek 2006; Hanes and Schall 1996; Mazurek et al. 2003; Ratcliff et al. 2003; Reddi et al. 2003; Smith and Ratcliff 2004). This model was recently used (Heitz and Schall 2012; Purcell et al. 2010, 2012; Schall et al. 2011) to explain how the activity of visually responsive neurons in the LIP, FEF, and SC was transformed into the activity of movement neurons in the FEF, SC, and the brain stem, which reaches a fixed threshold level of activation at the time of saccade initiation (e.g., Hanes and Schall 1996; Paré and Hanes 2003; but see Everling et al. 1999, 2000; Jantz et al. 2013). Second, one might argue that another mechanism is also possible. The fact that the raw activity in the four stimulus conditions indistinguishably converged into a common activation level at around 80 ms before saccade (the mean strength was 0.49 at 79 ms before saccade, arrow in Fig. 8A) suggests that the time when the activity achieved this activation level might determine the saccadic reaction time. However, the observation that the response strength of the stimulus-aligned activity in the Bright-Difficult condition (in Fig. 3A, left) exceeded this activation level (= 0.49) at 119 ms after stimulus onset, occurring far before the target-discrimination time (154 ms), is difficult to reconcile with the second hypothesis (Schall et al. 2011). Therefore, we preferred the first hypothesis.
Fig. 8.
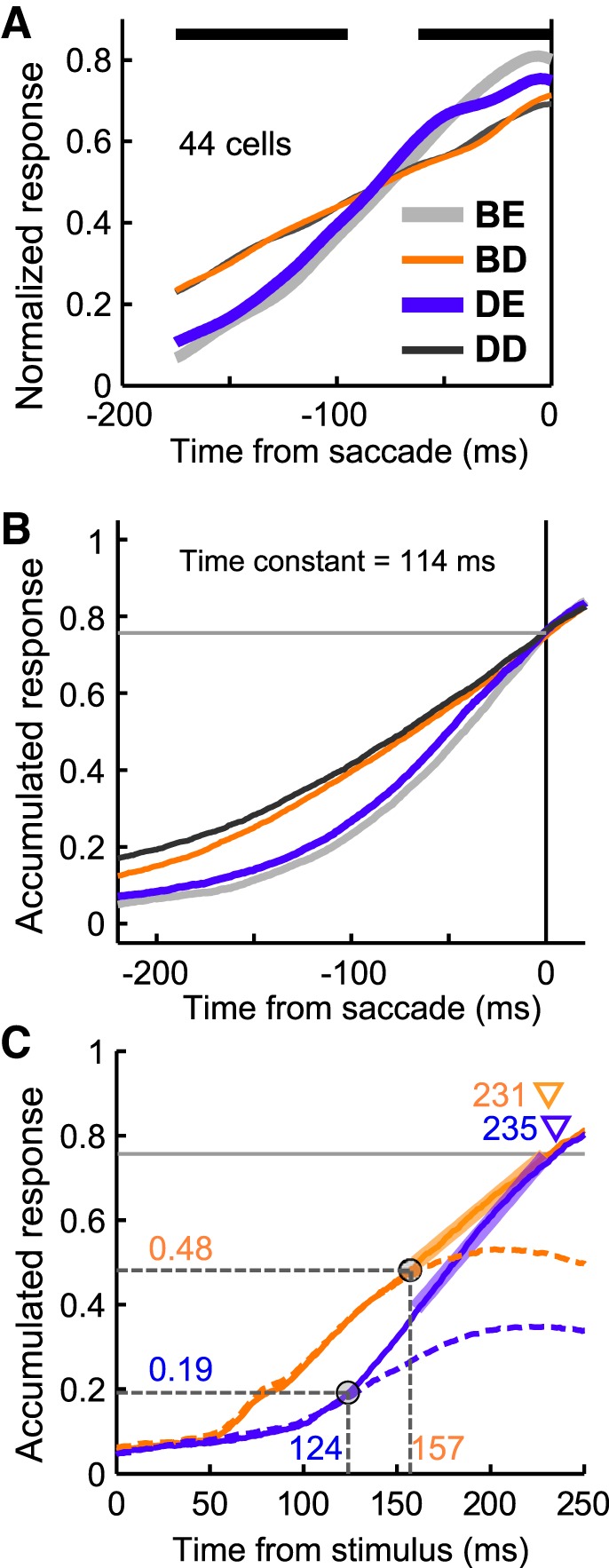
Applicability of a leaky-integrator model. A: normalized population activity aligned according to saccade onset in the 44 neurons. The 4 traces indicate the spike density functions in the 4 stimulus conditions. Horizontal thick bars indicate a significance difference in the strength of the response among the four stimulus conditions (1-way repeated ANOVA, P < 0.01). Gray, orange, blue, and black traces indicate the Bright-Easy, Bright-Difficult, Dim-Easy, and Dim-Difficult conditions, respectively. B: accumulated responses aligned according to saccade onset. The raw spike-timing histogram (1 ms resolution) aligned according to saccade onset for each neuron was convolved with a leaky integrator and averaged across neurons. An optimal time constant for leaky integration was determined (114 ms) such that the summed difference between each pair of accumulated responses was minimal at the time of saccade initiation. Horizontal gray lines indicate the threshold level (the mean strength of the accumulated responses at saccade onset). C: stimulus-aligned accumulated responses, which were derived from the reaction-time matched Bright-Difficult and Dim-Easy trials (Fig. 5). Because the reaction times were matched between conditions the 2 accumulated responses were compared from the stimulus to the saccade onset. The time constant was set as in B. Solid and dashed traces indicate the accumulated responses when the target appeared in and away from the receptive field, respectively. Target-discrimination times in the accumulated responses (circles on the traces) were the same as the target-discrimination times shown in Fig. 5B. Horizontal gray lines indicate the same threshold level shown in B. Triangles indicate the threshold-crossing times. The increasing accumulated response for each condition was evaluated by linear fitting 160–230 ms after stimulus onset (thick line segments).
To address the hypothesis that LIP activity is accumulated and used to drive the movement-related neurons in the downstream areas (such as the FEF and/or SC), we initially showed that the accumulation-to-threshold model could explain the saccade-initiation timing, irrespective of stimulus conditions by examining whether the accumulated responses of the LIP samples could reach a common threshold level at saccade onset under different stimulus conditions. Figure 8A shows the raw population activities under the four stimulus conditions, which did not converge into a common activation level at saccade onset (one-way repeated-measures ANOVA, P < 0.01). However, the accumulated responses produced by applying a leaky integration function (time constant = 114 ms) to the raw population activities reached a common threshold level at the saccade onset (one-way repeated-measures ANOVA, P > 0.05) (Fig. 8B).
Next, we show that this accumulation-to-threshold model could explain the dependence of the postdiscrimination interval on stimulus luminance. The accumulated responses calculated from the reaction-time matched trials (Fig. 5) were compared between the Bright-Difficult and Dim-Easy conditions (Fig. 8C). The time constant was set to the optimal value found in Fig. 8B (i.e., 114 ms). The advantage of using reaction-time matched data was that we were able to compare the accumulated responses throughout the trials in the stimulus-aligned format. This allowed us to confirm that the accumulated responses did not exceed the threshold level until saccade onset even when the accumulated responses were aligned to stimulus onset. The result shows that although the target-discrimination times (circles on the accumulated responses) were substantially different (33 ms, 157 vs. 124 ms) between the Bright-Difficult and Dim-Easy conditions, the difference in the threshold-crossing time (i.e., the predicted saccade-initiation timing) was small (4 ms, 231 vs. 235 ms).
Our computer simulation might explain why the postdiscrimination interval differed among conditions. Importantly, the strength of the response at the target-discrimination time differed substantially between the two conditions: the response strength was lower in the Dim-Easy condition (normalized response strength = 0.19) compared with the Bright-Difficult condition (0.48). Therefore, the remaining height, which was increased to reach the threshold, was greater in the Dim-Easy condition than in the Bright-Easy condition. This difference in the response strength meant that it took a longer time for the accumulated response to reach the threshold level after the target-discrimination time in the Dim-Easy condition compared with the Bright-Difficult condition, although the accumulated response rose faster in the Dim-Easy condition (rising rate = 5.2 units/s) than in the Bright-Hard condition (3.7 units/s). Thus, the results of the current simulation suggest that the difference in the strength of the response at the target-discrimination time is an important factor that determines the length of the postdiscrimination interval.
Target-discrimination Time is Independent of Neuron Type
Visually responsive LIP neurons can be classified into subpopulations according to the existence of saccade-burst activity or delay activity. To determine whether the target-discrimination time was different depending on the type of neuron, we calculated the target-discrimination time separately for the different types of neurons (Table 1). For 35 of the 44 neurons being studied, the datasets obtained from the memory-guided saccade task were large enough (≥5 trials) to enable this analysis. Of these, 16 and 19 were classified into visual and visual-movement cells, and 19 and 16 possessed and did not possess delay-period activity, respectively. In both cases, the discrimination times were significantly earlier in the Bright-Difficult than in the Dim-Easy condition (Wilcoxon signed-rank test, P < 0.01) and were not significantly different among neuron types (Wilcoxon rank-sum test, P > 0.05). This indicates that the present results were preserved regardless of neuron type.
Table 1.
Target-discrimination time is independent of neuron type
| Target-discrimination Time, mean ± SD |
|||
|---|---|---|---|
| Bright-Difficult | Dim-Easy | P Values across Visual Stimulus | |
| Visual (n = 16) | 170 ± 20.0 ms | 147 ± 30.6 ms | P < 0.01 |
| Visual-movement (n = 19) | 176 ± 18.8 ms | 134 ± 25.7 ms | P < 0.001 |
| P values among neuron types | N.S. | N.S. | |
| With delay activity (n = 19) | 175 ± 19.9 ms | 146 ± 29.7 ms | P < 0.01 |
| Without delay activity (n = 16) | 171 ± 18.9 ms | 132 ± 25.7 ms | P < 0.001 |
| P values among neuron types | N.S. | N.S. | |
Lateral intraparietal area neurons were classified into subpopulations based on whether they had saccade-burst or delay-period activity in the memory-guided saccade task, and the target-discrimination times were compared among neuron types (Wilcoxon signed-rank test) and stimulus conditions (Wilcoxon rank-sum test). N.S., not significant (P > 0.05).
Behavioral and Neuronal Performances in False-alarm Error Trials
To test whether the present findings were preserved in the data from the erroneous trials, we examined the behavioral and neuronal performances when the monkeys made false-alarm errors (fixation breaks) in the catch condition [the dataset from the erroneous trials in the search condition were not large enough for analysis due to the high rate of correct responses (e.g., >95% in the Dim-Easy search)]. We analyzed the data from the 33 neurons in which the monkeys made ≥7 false-alarm responses (erroneous saccades) to both the receptive field and the position opposite to the receptive field in each of the Bright and Dim catch conditions. The mean false-alarm error rate (± SD) was significantly higher in the Bright catch condition (55.9 ± 9.2%) than in the Dim catch condition (43.8 ± 11.5%) (signed-rank test, P < 0.0001), and the mean reaction time (± SD) of the false-alarm responses was significantly shorter in the Bright catch condition (292 ± 57 ms) than in the Dim catch condition (311 ± 62 ms) (signed-rank test, P < 0.0001), indicating that the erroneous saccades occurred more frequently and quickly in the Bright condition compared with the Dim condition.
Figure 9A shows the average population activity of the above 33 cells in the false-alarm error trials in the Bright and Dim catch conditions. The activity was greater when the false-alarm saccade goal was in the receptive field (solid traces) than when it was opposite to the receptive field (dashed traces). In the saccade-aligned activity (Fig. 9A, right), the postdiscrimination interval [the length from goal-discrimination time (signed-rank test, P < 0.01) to saccade onset] was longer in the Dim catch condition (110 ms) than in the Bright catch condition (86 ms), which is consistent with the results obtained from the successful search trials. Figure 9B shows the accumulated responses produced by applying a leaky integration function (time constant = 128 ms) to the activity of the 33 neurons. The time constant was determined in the same way as in Fig. 8B. As illustrated in the figure, the accumulated responses in both the false-alarm trials (thick lines) and the successful search trials (thin lines) converged into a common activation level at the time of saccade onset, irrespective of the stimulus condition (one-way repeated-measures ANOVA, P > 0.1), suggesting that the saccade-initiation timing on error trials is also explained by the same accumulation-to-threshold model.
Fig. 9.
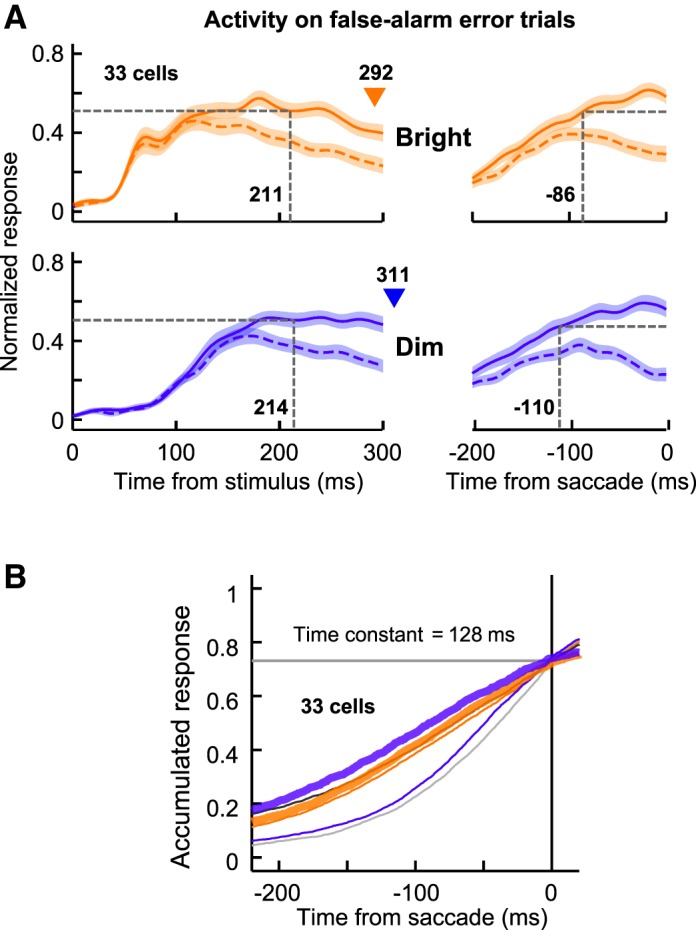
Population responses of LIP neurons in the Bright and Dim catch conditions. A: normalized population responses obtained from 33 neurons when the monkeys made false-alarm erroneous saccades (fixation breaks) in the catch condition. Orange and blue traces indicate the spike density functions in the Bright and Dim catch trials, respectively. Solid and dashed traces denote the activity when the monkeys made the erroneous saccades to the receptive field and to the location opposite to the receptive field, respectively. Vertical dashed lines indicate the time at which LIP neurons reliably predicted the saccade goal in their activity. B: accumulated responses aligned according to the onset of the false-alarm saccades. The responses are illustrated using a format similar to that of Fig. 8B, and the optimal time constant for the 33 neurons was 128 ms. Two thick traces indicate the Bright and Dim catch conditions, and 4 thin traces indicate the 4 search conditions.
DISCUSSION
The results from the present study reveal that the length of the postdiscrimination interval during a visual search was different when the stimulus condition was altered, even when the saccadic reaction times were matched between stimulus conditions in a trial-based manner (Figs. 3–5). Furthermore, a control analysis suggests that the postdiscrimination interval was altered by the stimulus luminance (Fig. 6). In addition, the simulation results using an accumulation-to-threshold model reveal that the across-stimulus variation in the postdiscrimination interval could be explained by the luminance-dependent changes in the strength of the response at the target-discrimination time (Fig. 8).
Visual Stimulus Parameters Affect Pre-/Postdiscrimination Intervals in Different Ways
As shown in Fig. 6, the two stimulus parameters (luminance and target-distractor color similarity) affected the pre- and postdiscrimination intervals in different ways. First, the prediscrimination interval was affected by both luminance and target-distractor similarity (Fig. 6A), but the neuronal substrate affected by these two variables might differ. Previous studies using a visual detection paradigm (in which only an isolated target was presented) revealed that luminance-level increases evoke an earlier response onset and stronger visual responses in the LIP (Tanaka et al. 2013) and SC (Marino et al. 2012) and shorten the behavioral reaction time (e.g., Boch et al. 1984; Jaskowski and Sobieralska 2004; Marino and Munoz 2009). In contrast, studies using a visual search paradigm (in which a target is embedded in multiple distractors) demonstrated that decreases in the target-distractor similarity did not change the visual responses but shortened the target-discrimination time and the saccadic reaction time (Bichot et al. 2001; Sato et al. 2001; Thompson et al. 2005). Therefore, magnification of the stimulus luminance might shorten the target-discrimination time by enhancing the stimulus detectability against the background, whereas reducing the target-distractor similarity could shorten the target-discrimination time by enhancing the neuronal target-distractor discriminability.
Second, the postdiscrimination interval was altered by the stimulus luminance (Fig. 6B). A similar dependence on luminance was observed in a visual detection task in which the length of time from the onset of the neuronal response to the saccade onset increased with decreasing luminance (Fig. 8 in Tanaka et al. 2013). Importantly, neurons are capable of specifying the target location at the time of response onset in a visual detection task (because only an isolated target was presented); therefore, the response-onset time in a visual detection task might correspond functionally to the target-discrimination time in a visual search task. As such, the postdetection/postdiscrimination intervals were lengthened with decreasing luminance in both the visual detection and search tasks, suggesting the existence of a common luminance-dependent process in the downstream structures.
The fact that the pre- and postdiscrimination intervals could be differentially altered under varying stimulus conditions suggests that multiple neuronal discrimination times are possible for a single behavioral reaction time. Specifically, the equivalence of behavioral reaction times does not always assume equivalent neuronal discrimination times. This suggests that, although behavioral reaction times are measured conventionally to evaluate the difficulty of a task in psychophysiological studies of perceptual discrimination, differences in behavioral reaction times do not always indicate the same differences in neuronal discrimination times in the brain under different stimulus conditions.
Relationship with Previous Studies of Saccade Target Selection in Different Visual Conditions
Although previous studies have not explicitly examined this issue, extant results indicate that the postdiscrimination interval represented in the activity of neurons in the FEF (Cohen et al. 2009) and the LIP (Balan et al. 2008) is prolonged as the number of array elements increases. Importantly, the strength of visually responsive activity decreased when the number of array elements increased, which might be caused by the effects of surround suppression (Falkner et al. 2010; Schall et al. 2004). Namely, an increase in the number of array elements would reduce the magnitude of the visual responses at the target-discrimination time. Therefore, the prolonged postdiscrimination interval observed in previous studies might be explained by the same mechanism postulated in the current study.
A previous study examined the effects of a change in luminance on SC neurons during a color-singleton search task (White and Munoz 2011). When the achromatic contrast was removed by making an array stimulus equiluminant with the background, the peak response of target activity was unchanged, whereas distractor activity was greatly attenuated. These neuronal modulations induced no change in the neuronal target-discrimination time but substantially delayed saccadic initiation to prolong the postdiscrimination interval. Therefore, prolongation of the postdiscrimination interval was unlikely to be explained by reduced response strength. This discrepancy might be due to functional differences in LIP and SC neurons or to differences in the stimulus condition; the background luminance was relatively high (20.5 cd/m2), and the array stimulus was darker than the background in the SC study. Further studies are needed to adequately address this question.
Saccade Target Selection in Different Brain Areas
Previous studies have revealed that the profiles of target-discriminating activity during visual search vary across brain areas. First, the degree of dependence on stimulus features varies among areas. When monkeys searched for a singleton target that was unique in the instructed stimulus dimension (shape or color), target discrimination for most V4 neurons (Ogawa and Komatsu 2004) and for one-third of LIP neurons (Ogawa and Komatsu 2009) occurred only when the target was defined by one particular dimension. However, half of the LIP neurons and most FEF neurons discriminated the target, irrespective of the target-defining dimension (Ogawa and Komatsu 2006, 2009). Consistently, the percentage of shape-selective neurons was significantly lower in the FEF compared with the LIP (Lehky and Sereno 2007; Peng et al. 2008). Second, saccadic reaction times covaried with the target-discrimination time for neurons in the LIP and FEF (Bichot et al. 2001; Ipata et al. 2006; Sato et al. 2001; Thomas and Paré 2007; but see Thompson et al. 1996) and for some neurons in the SC (McPeek and Keller 2002) but not for V4 neurons (Gee et al. 2010). These differences in the target-discriminating response profiles may reflect the different hierarchies in the transition from stimulus-dependent to spatially based target discrimination (Gottlieb and Snyder 2010). Taken together with the present finding that the postdiscrimination interval can differ across stimulus conditions, it is suggested that the temporal relationship between the target-discrimination time and the saccade-initiation time may vary not only across stimulus conditions but also among brain areas.
Discrete vs. Continuous Flow Models
Two conceptual models have been suggested to explain the nature of sensorimotor transformation: discrete- and continuous-flow models (Costello et al. 2013; Eriksen and Schultz 1979; Miller 1982; Smid et al. 1991; Woodman et al. 2008). A discrete-flow model assumes that different independent stages are processed in a sequential manner, which is consistent with the conception of a subtraction method (Donders 1868/1969). This model proposes that target discrimination and saccade preparation/generation are independently and discontinuously processed, and it therefore predicts that the postdiscrimination interval will be the same regardless of stimulus condition. In contrast, a continuous-flow model allows that the effects of the stimulus remain even after the target-discrimination time, and it therefore predicts that the neuronal activity during the postdiscrimination interval will differ across different stimulus conditions. Thus, the current results are more likely explained by a continuous-flow model rather than by a discrete-flow model.
Woodman et al. (2008) examined the same problem by recording the activity of FEF movement neurons during a visual search and proposed the opposite conclusion. Nevertheless, we think that their and our conclusions can be reconciled. As proposed recently (Purcell et al. 2012), if the inputs from visual neurons to movement neurons are gated until the target discriminability achieves a threshold, movement neurons do not start the activation. In such a situation, LIP visual neurons behave in a continuous-flow manner, whereas FEF movement neurons behave in a discrete-flow manner. Thus, it is possible that continuous- and discrete-flow processing occur concurrently in the activity of the different types of neuron in the brain.
Exogenous and Endogenous Influences on the Postdiscrimination Interval
The results from the present study suggest that visual stimulus (luminance) affected the determination of the postdiscrimination interval. In addition to this exogenous factor, previous studies have suggested that endogenous factors also affect the postdiscrimination interval. Although it is known that the length of time from target discrimination to saccade onset tends to be constant despite the variability of the saccadic reaction time in a given stimulus condition (Ipata et al. 2006; Sato et al. 2001; Thomas and Paré 2007), the degree of this constancy could be modified by differences in the task design of the visual search. When monkeys were required to foveate the target by a single saccade in the visual search (slow-search-speed/high-search-accuracy condition), constancy in the postdiscrimination interval was observed in approximately half of the visual movement neurons in the SC (McPeek and Keller 2002). In contrast, when monkeys were allowed to foveate the target by multiple saccades (fast-search-speed/low-search-accuracy condition), constancy in the postdiscrimination interval was observed across the entire population of SC neurons (Shen and Paré 2011). In addition, a recent study reported that when a speed-accuracy tradeoff was manipulated using a prestimulus instruction cue, the length of the postdiscrimination interval increased with search accuracy even when an identical array stimulus was presented (Heitz and Schall 2012). These results suggest that the length of the postdiscrimination interval could be altered by endogenous factors.
In the current study, the reaction-time trials were randomly interleaved with no explicit instruction, and the monkeys were unable to anticipate the upcoming stimulus condition. Furthermore, the present results demonstrate that the length of the postdiscrimination interval differed even when the saccadic reaction times were almost perfectly matched between the two different stimulus conditions, suggesting that the present results could not be explained by the above endogenous factors. Nevertheless, it is still possible that the monkeys rapidly altered their search speed/search accuracy within a trial after seeing a presented array stimulus. The monkeys might strengthen their speed-stress response after seeing an Easy stimulus compared with a Difficult stimulus, because they would have had a higher probability of getting a reward due to the increased correct response rate in the Easy compared with the Difficult condition (Fig. 1). However, this was not the case because the postdiscrimination interval was not statistically different between the Easy and Difficult conditions at each luminance level (Fig. 6B). Taken together, the present findings suggest that changes in the postdiscrimination interval were likely not due to the endogenous factors but, rather, were due to exogenous factors (the visual stimulus).
GRANTS
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) to T. Ogawa (20033010, 20500283, 23500391, and 26290004).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.T. and T.O. performed experiments; T.T. and T.O. analyzed data; T.T., S.N., and T.O. interpreted results of experiments; T.T. and T.O. prepared figures; T.T. and T.O. drafted manuscript; T.T., S.N., and T.O. approved final version of manuscript; T.O. conception and design of research; T.O. edited and revised manuscript.
ACKNOWLEDGMENTS
We acknowledge Nihon University and the National Bio-Resource Center for supplying the monkeys used in this study. We also acknowledge two anonymous reviewers for crucial comments regarding our early version of the manuscript.
REFERENCES
- Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol 296: 65–113, 1990. [DOI] [PubMed] [Google Scholar]
- Balan PF, Oristaglio J, Schneider DM, Gottlieb J. Neuronal correlates of the set-size effect in monkey lateral intraparietal area. PLoS Biol 6: e158, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. I. Temporal properties; comparison with area 7a. J Neurophysiol 66: 1095–1108, 1991a. [DOI] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol 66: 1109–1124, 1991b. [DOI] [PubMed] [Google Scholar]
- Bell AH, Everling S, Munoz DP. Influence of stimulus eccentricity and direction on characteristics of pro- and antisaccades in non-human primates. J Neurophysiol 84: 2595–2604, 2000. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Effects of similarity and history on neural mechanisms of visual selection. Nat Neurosci 2: 549–554, 1999. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD, Thompson KG. Visual feature selectivity in frontal eye fields induced by experience in mature macaques. Nature 381: 697–699, 1996. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Thompson KG, Chenchal Rao S, Schall JD. Reliability of macaque frontal eye field neurons signaling saccade targets during visual search. J Neurosci 21: 713–725, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichot NP, Rossi AF, Desimone R. Parallel and serial neural mechanisms for visual search in macaque area V4. Science 308: 529–534, 2005. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33: 1–21, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch R, Fischer B, Ramsperger E. Express-saccades of the monkey: reaction times versus intensity, size, duration, and eccentricity of their targets. Exp Brain Res 55: 223–231, 1984. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315: 1860–1862, 2007. [DOI] [PubMed] [Google Scholar]
- Carpenter RH, Williams ML. Neural computation of log likelihood in control of saccadic eye movements. Nature 377: 59–62, 1995. [DOI] [PubMed] [Google Scholar]
- Cisek P. Integrated neural processes for defining potential actions and deciding between them: a computational model. J Neurosci 26: 9761–9770, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Heitz RP, Woodman GF, Schall JD. Neural basis of the set-size effect in frontal eye field: timing of attention during visual search. J Neurophysiol 101: 1699–1704, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol 76: 2841–2852, 1996. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Neuronal responses in area 7a to multiple-stimulus displays: I. neurons encode the location of the salient stimulus. Cereb Cortex 11: 581–591, 2001. [DOI] [PubMed] [Google Scholar]
- Costello MG, Zhu D, Salinas E, Stanford TR. Perceptual modulation of motor-but not visual-responses in the frontal eye field during an urgent-decision task. J Neurosci 33: 16394–16408, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders FC. Die Schnelligkeit psychischer Processe (On the speed of mental processes), translated by Kostner WG. Acta Psychol (Amst) 30: 412–431, 1969. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, Schultz DW. Information processing in visual search: a continuous flow conception and experimental results. Percept Psychophys 25: 249–263, 1979. [DOI] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Klein RM, Munoz DP. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. J Neurosci 19: 2740–2754, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci 20: 387–400, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner AL, Krishna BS, Goldberg ME. Surround suppression sharpens the priority map in the lateral intraparietal area. J Neurosci 30: 12787–12797, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol 21: 1068–1070, 1966. [DOI] [PubMed] [Google Scholar]
- Gee AL, Ipata AE, Goldberg ME. Activity in V4 reflects the direction, but not the latency, of saccades during visual search. J Neurophysiol 104: 2187–2193, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford GW 3rd, Cohen YE. Effect of a central fixation light on auditory spatial responses in area LIP. J Neurophysiol 91: 2929–2933, 2004. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res 70: 216–220, 1988. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci 30: 535–574, 2007. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Snyder LH. Spatial and non-spatial functions of the parietal cortex. Curr Opin Neurobiol 20: 731–740, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber N, Muri RM, Mosimann UP, Bieri R, Aeschimann A, Zito GA, Urwyler P, Nyffeler T, Nef T. Effects of age and eccentricity on visual target detection. Front Aging Neurosci 5: 101, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science 274: 427–430, 1996. [DOI] [PubMed] [Google Scholar]
- Heitz RP, Schall JD. Neural mechanisms of speed-accuracy tradeoff. Neuron 76: 616–628, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol 49: 1268–1284, 1983. [DOI] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. J Neurosci 26: 3656–3661, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantz JJ, Watanabe M, Everling S, Munoz DP. Threshold mechanism for saccade initiation in frontal eye field and superior colliculus. J Neurophysiol 109: 2767–2780, 2013. [DOI] [PubMed] [Google Scholar]
- Jaskowski P, Sobieralska K. Effect of stimulus intensity on manual and saccadic reaction time. Percept Psychophys 66: 535–544, 2004. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980. [DOI] [PubMed] [Google Scholar]
- Lehky SR, Sereno AB. Comparison of shape encoding in primate dorsal and ventral visual pathways. J Neurophysiol 97: 307–319, 2007. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yttri EA, Snyder LH. Intention and attention: different functional roles for LIPd and LIPv. Nat Neurosci 13: 495–500, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimon G, Assad JA. A cognitive signal for the proactive timing of action in macaque LIP. Nat Neurosci 9: 948–955, 2006. [DOI] [PubMed] [Google Scholar]
- Marino RA, Levy R, Boehnke S, White BJ, Itti L, Munoz DP. Linking visual response properties in the superior colliculus to saccade behavior. Eur J Neurosci 35: 1738–1752, 2012. [DOI] [PubMed] [Google Scholar]
- Marino RA, Munoz DP. The effects of bottom-up target luminance and top-down spatial target predictability on saccadic reaction times. Exp Brain Res 197: 321–335, 2009. [DOI] [PubMed] [Google Scholar]
- Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. A role for neural integrators in perceptual decision making. Cereb Cortex 13: 1257–1269, 2003. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol 88: 2019–2034, 2002. [DOI] [PubMed] [Google Scholar]
- Miller J. Discrete versus continuous stage models of human information processing: in search of partial output. J Exp Psychol Hum Percept Perform 8: 273–296, 1982. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol 38: 871–908, 1975. [DOI] [PubMed] [Google Scholar]
- Nishida S, Tanaka T, Ogawa T. Separate evaluation of target facilitation and distractor suppression in the activity of macaque lateral intraparietal neurons during visual search. J Neurophysiol 110: 2773–2791, 2013. [DOI] [PubMed] [Google Scholar]
- Nishida S, Tanaka T, Shibata T, Ikeda K, Aso T, Ogawa T. Discharge-rate persistence of baseline activity during fixation reflects maintenance of memory-period activity in the macaque posterior parietal cortex. Cereb Cortex 24: 1671–1685, 2014. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Komatsu H. Target selection in area V4 during a multidimensional visual search task. J Neurosci 24: 6371–6382, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, and Komatsu H. Neuronal dynamics of bottom-up and top-down processes in area V4 of macaque monkeys performing a visual search. Exp Brain Res 173: 1–13, 2006. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Komatsu H. Condition-dependent and condition-independent target selection in the macaque posterior parietal cortex. J Neurophysiol 101: 721–736, 2009. [DOI] [PubMed] [Google Scholar]
- Pare M, Hanes DP. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J Neurosci 23: 6480–6489, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Sereno ME, Silva AK, Lehky SR, Sereno AB. Shape selectivity in primate frontal eye field. J Neurophysiol 100: 796–814, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature 400: 233–238, 1999. [DOI] [PubMed] [Google Scholar]
- Purcell BA, Heitz RP, Cohen JY, Schall JD, Logan GD, Palmeri TJ. Neurally constrained modeling of perceptual decision making. Psychol Rev 117: 1113–1143, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell BA, Schall JD, Logan GD, Palmeri TJ. From salience to saccades: multiple-alternative gated stochastic accumulator model of visual search. J Neurosci 32: 3433–3446, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Cherian A, Segraves M. A comparison of macaque behavior and superior colliculus neuronal activity to predictions from models of two-choice decisions. J Neurophysiol 90: 1392–1407, 2003. [DOI] [PubMed] [Google Scholar]
- Reddi BA, Asrress KN, Carpenter RH. Accuracy, information, and response time in a saccadic decision task. J Neurophysiol 90: 3538–3546, 2003. [DOI] [PubMed] [Google Scholar]
- Richmond BJ, Optican LM. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. II. Quantification of response waveform. J Neurophysiol 57: 147–161, 1987. [DOI] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 22: 9475–9489, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Murthy A, Thompson KG, Schall JD. Search efficiency but not response interference affects visual selection in frontal eye field. Neuron 30: 583–591, 2001. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci 15: 6905–6918, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci 22: 241–259, 1999. [DOI] [PubMed] [Google Scholar]
- Schall JD, Sato TR, Thompson KG, Vaughn AA, Juan CH. Effects of search efficiency on surround suppression during visual selection in frontal eye field. J Neurophysiol 91: 2765–2769, 2004. [DOI] [PubMed] [Google Scholar]
- Schall JD, Pare M, Woodman GF. Comment on “Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices”. Science 318: 44, 2007. [DOI] [PubMed] [Google Scholar]
- Schall JD, Purcell BA, Heitz RP, Logan GD, Palmeri TJ. Neural mechanisms of saccade target selection: gated accumulator model of the visual-motor cascade. Eur J Neurosci 33: 1991–2002, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol 86: 1916–1936, 2001. [DOI] [PubMed] [Google Scholar]
- Shen K, Valero J, Day GS, Pare M. Investigating the role of the superior colliculus in active vision with the visual search paradigm. Eur J Neurosci 33: 2003–2016, 2011. [DOI] [PubMed] [Google Scholar]
- Shive J, Francis G. Choosing colors for map display icons using models of visual search. Hum Factors 55: 373–396, 2013. [DOI] [PubMed] [Google Scholar]
- Smid HG, Lamain W, Hogeboom MM, Mulder G, Mulder LJ. Psychophysiological evidence for continuous information transmission between visual search and response processes. J Exp Psychol Hum Percept Perform 17: 696–714, 1991. [DOI] [PubMed] [Google Scholar]
- Smith PL, Ratcliff R. Psychology and neurobiology of simple decisions. Trends Neurosci 27: 161–168, 2004. [DOI] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science 304: 1782–1787, 2004. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Nishida S, Aso T, Ogawa T. Visual response of neurons in the lateral intraparietal area and saccadic reaction time during a visual detection task. Eur J Neurosci 37: 942–956, 2013. [DOI] [PubMed] [Google Scholar]
- Thomas NW, Paré M. Temporal processing of saccade targets in parietal cortex area LIP during visual search. J Neurophysiol 97: 942–947, 2007. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Sato TR. Frontal eye field activity before visual search errors reveals the integration of bottom-up and top-down salience. J Neurophysiol 93: 337–351, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol 76: 4040–4055, 1996. [DOI] [PubMed] [Google Scholar]
- Wardak C, Olivier E, Duhamel JR. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. J Neurosci 22: 9877–9884, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BJ, Munoz DP. Separate visual signals for saccade initiation during target selection in the primate superior colliculus. J Neurosci 31: 1570–1578, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, Kang MS, Thompson K, Schall JD. The effect of visual search efficiency on response preparation: neurophysiological evidence for discrete flow. Psychol Sci 19: 128–136, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]