Abstract
Previous research on reach planning in humans has implicated a frontoparietal network, including the precuneus (PCu), a putative human homolog of the monkey parietal reach region (PRR), and the dorsal premotor cortex (PMd). Using a pro-/anti-reach task, electrophysiological studies in monkeys have demonstrated that the movement goal rather than the location of the visual cue is encoded in PRR and PMd. However, if only the effector but not the movement goal is specified (underspecified condition), the PRR and PMd have been shown to represent all potential movement goals. In this functional magnetic resonance imaging study, we investigated whether the human PCu and PMd likewise encode the movement goal, and whether these reach-related areas also engage in situations with underspecified compared with specified movement goals. By using a pro-/anti-reach task, we spatially dissociated the location of the visual cue from the location of the movement goal. In the specified conditions, pro- and anti-reaches activated similar parietal and premotor areas. In the PCu contralateral to the moving arm, we found directionally selective activation fixed to the movement goal. In the underspecified conditions, we observed activation in reach-related areas of the posterior parietal cortex, including PCu. However, the activation was substantially weaker in parietal areas and lacking in PMd. Our results suggest that human PCu encodes the movement goal rather than the location of the visual cue if the movement goal is specified and even engages in situations when only the visual cue but not the movement goal is defined.
Keywords: precuneus, premotor cortex, reach planning, visuomotor integration, human functional magnetic resonance imaging
previous research in monkeys has identified two regions being crucially involved in reach planning: the parietal reach region (PRR) (Snyder et al. 1997; Batista and Andersen 2001; Gail and Andersen 2006; for a review see Andersen and Buneo 2002) and the dorsal premotor cortex (PMd) (Crammond and Kalaska 1996; Cisek and Kalaska 2002, 2005). The PRR is located at the medial bank of the intraparietal sulcus (Snyder et al. 1997) and receives direct input from extrastriate visual areas and projects to the PMd (Johnson et al. 1996; Tanné-Gariépy et al. 2002; for a review see Wise et al. 1997). It is thus an important interface between sensory and motor cortices (Mountcastle et al. 1975). A subset of PRR neurons codes both the movement goal for an action and the effector to perform the action, whereas other subpopulations fire in the absence of spatial movement goal information if only the effector is specified and vice versa (Calton et al. 2002). The PMd has likewise been shown to integrate positional information of the movement goal and the effector (Hoshi and Tanji 2006). Using target-selection tasks, it has been demonstrated that PMd neurons simultaneously encode multiple movement goals if more than one potential reach goal is present (Cisek and Kalaska 2002, 2005). As soon as the correct movement goal is specified the corresponding directional signal is enhanced while the signals of the nonchosen movement goals are suppressed. Thus, both areas, the PRR and the PMd, which are reciprocally connected (Johnson et al. 1996), contribute to sensorimotor integration.
A recent inactivation study in PRR found lesion effects specific to contralateral limb movements but independent of the spatial location of the reach goal (Yttri et al. 2014). Based on this result, the authors suggested limb-specific movement planning in area PRR and therefore characterized the PRR as a motor area situated early in the visuomotor pathway. However, other inactivation studies observed stronger lesion effects in a region slightly anterior to PRR for reach goals presented contralateral to the injection site arguing for target-selectivity (Battaglia-Mayer et al. 2013; Hwang et al. 2012).
In contrast to target-selection tasks, rule-selection tasks have been applied to answer the question whether PMd and PRR neurons represent the location of the visual cue or the movement goal. To disentangle the location of the visual cue from the location of the movement goal, context rules are applied to the visual cue that either instruct a reach toward the visual cue (rule pro) or toward its mirrored location (rule anti) (Gail and Andersen 2006; Gail et al. 2009; Westendorff et al. 2010; Klaes et al. 2011). Delay-related directional tuning signals in PRR neurons revealed selective coding of the movement goal rather than the memorized position of the visual cue irrespective of whether it was directly cued by the physical visual cue (pro-reach) or inferred from the rule applied to the visual cue (anti-reach) (Gail and Anderson 2006). This suggests that PRR translates current sensory information into reach plans rather than storing the visual cue location in visual memory. Similar results have been revealed for PMd neurons (Gail et al. 2009), indicating an important role of PMd and PRR in space-context integration to encode the desired movement goal. By introducing different precueing conditions, it has been shown that movement goal representations in PMd and PRR neurons are modulated by contextual information, i.e., by the information given before the movement planning period. For example, PRR neurons were stronger engaged in planning of pro-reaches while PMd neurons showed stronger overall activity during planning of anti-reaches (Gail et al. 2009). Moreover, motor-related latencies were shorter for PMd than PRR neurons for inferred movement goals, i.e., during anti-reach planning, suggesting that PMd initiates movement goal remapping in PRR (Westendorff et al. 2010). In these experiments, the point in time when the context rule was given varied between trials; the context rule was either presented before or after a variable instructed delay period. This also allowed for differentiating movement planning based on specified movement goals (visual cue and context rule given before the delay) from movement planning based on underspecified movement goals (only the visual cue given before the delay). In the underspecified condition, monkeys were uninformed whether they should perform a reach toward the visual cue (pro-reach) or toward its mirrored position (anti-reach) until an additional rule cue was given after the delay specifying the movement goal. The underspecified movement goal condition yielded spatial tuning preferences for the inferred anti-movement goal in both PRR and PMd (Westendorff et al. 2010). Likewise, a preference for the encoding of the inferred movement goal in underspecified conditions was also observed in free-choice trials where the monkeys were free to choose the pro- or anti-reach goal (Klaes et al. 2011). However, if the free-choice behavior was controlled for by a bias-minimizing reward schedule, the delay-period activity indicated that PMd and PRR simultaneously encoded the two alternative movement plans when only the visual cue was given.
In humans, a broad frontoparietal network, likewise including strongly connected areas of the posterior parietal cortex (PPC) and dorsal premotor cortex (Tomassini et al. 2007), is involved in the preparation of goal-directed reaching movements (Prado et al. 2005; Beurze et al. 2007; Busan et al. 2009; Lindner et al. 2010; Parkinson et al. 2010; for a review see Culham et al. 2006). Consistent with electrophysiological findings in monkeys, the human PPC and PMd have been demonstrated to represent both the spatial location of the movement goal and the effector selected for that action, e.g., left vs. right arm (Beurze et al. 2007). Using a sequential cueing task, Beurze et al. (2007) also found activation in the PPC and the PMd even if only information of the movement goal or the effector was available, which is in line with previous findings in monkey PRR (Calton et al. 2002). However, activity in PPC and PMd was more pronounced when both the movement goal and the effector were cued by showing stronger effector- than target-selectivity (Beurze et al. 2007). The double coding of movement goal and effector signals together with stronger activation when both the movement goal and the effector were specified let the authors argue for a role of the human PPC and PMd in sensorimotor integration. Vice versa, the weaker activation in PPC and PMd when only the movement goal or the effector was specified argues for an incomplete stage of sensorimotor integration. One goal of the present study was to examine whether and how PPC and PMd engage in underspecified conditions in a pro-/anti-reach rule-selection task.
Within the human PPC, a dorsomedial area of the SPL seems to be crucially involved in the planning of hand and arm movements and has been discussed as a putative human homolog of monkey area PRR (Astafiev et al. 2003; Connolly et al. 2003; Pellijeff et al. 2006; Fernandez-Ruiz et al. 2007; Hagler et al. 2007; Vesia and Crawford 2012). Consistent with previous reports on monkey PRR (e.g., Snyder et al. 1997), the “human PRR” elicits higher activation for the planning of goal-directed pointing movements compared with the planning of goal-directed saccades (Connolly et al. 2003). In the following, we will label this region as PCu, since it gives an anatomical reference.
To examine the spatial code maintained in the PCu during movement planning, Fernandez-Ruiz et al. (2007) used left/right reversing prisms. By doing so, they were able to dissociate the visually perceived direction of a pointing movement toward a spatially corresponding visual cue from the actual (physical) pointing direction, e.g., an actual rightward movement to a right visual cue was seen as a leftward movement to a left visual cue. During movement planning, they found higher activation to contralateral visual cues in conditions without the prism and a reversed activation pattern with higher activation to ipsilateral visual cues (which are now visually perceived in contralateral space) in conditions with the prism. This effect was only significant for the left PCu contralateral to the moving hand. The authors concluded that the PCu encodes the location of the visual movement goal rather than the direction of the actual limb movement, i.e., the physical movement goal. However, this task design does not spatially dissociate the visual cue presented before the delay from the visual movement goal representation since participants always reached to the visual cue, i.e., performed pro-reaches in visual coordinates. Thus, it remains unclear whether human PCu maintains the visual cue in visuospatial memory or represents the visual movement goal. Pro-/anti-reach tasks are suitable to answer this question. Therefore, a second goal of this study was to examine whether the human PCu and the PMd encode the location of the visual cue or the (inferred) visual movement goal by applying a pro-/anti-reach rule-selection task.
We used functional magnetic resonance imaging (fMRI) to investigate whether human reach-related areas, in particular PCu and PMd, represent the visual cue or the visual movement goal, and how strongly these areas are engaged during reach planning when the movement goal is not specified. We applied an adapted version of the pro-/anti-reach rule-selection task from an electrophysiological study in monkeys (Westendorff et al. 2010). This task allows us to 1) dissociate the location of the previously presented visual cue from the location of the (inferred) visual movement goal and 2) compare movement planning activity in situations with specified or underspecified movement goals. First, we hypothesize that visual movement goals are encoded in the human PCu and PMd as it has previously been shown in monkey electrophysiological research (Gail et al. 2009, Westendorff et al. 2011). If the human PCu and PMd represent the visual movement goal, we expect higher activation for contralateral visual cues in pro-trials and a reversed activation pattern for anti-trials, i.e., higher activation for ipsilateral than contralateral visual cues. Second, we hypothesize that PCu and PMd also engage in reach movement planning in underspecified conditions (cf., Westendorff et al. 2010; Klaes et al. 2011). To further specify the characteristics of this engagement we examined the activation strength and lateralization effects in underspecified conditions.
MATERIALS AND METHODS
Participants
Twenty-five subjects participated in this fMRI experiment. We discarded 3 subjects due to motion artifacts and another 3 subjects due to poor performance in the behavioral task (<70% correct trials), leaving 19 participants (age range 20–29 yr; 11 females). All participants were right-handed as assessed with the Edinburgh Handedness Inventory (Oldfied 1971), had normal vision, and had no history of neurological or psychiatric disorders or chronic diseases. They were financially compensated or received course credit for their participation. All subjects gave informed written consent according to the Declaration of Helsinki (2008) before the experiment in accordance with the study procedure approved by the local ethics committee.
Experimental Design and Conditions
To investigate brain areas involved in movement planning in specified and underspecified conditions, we adapted a delayed reach task with different cueing conditions from an electrophysiological study in monkeys (Westendorff et al. 2010; Fig. 1). This task allowed us to separate the position of the visual cue (visuospatial memory) from the position of the movement goal (movement goal encoding) by introducing a context rule (pro- vs. anti-reach) that had to be applied to the visual cue. By applying the context rule either before (specified condition) or after (underspecified condition) the delay, we were able to manipulate the amount of information available during the delay period resulting in conditions with specified or underspecified movement goals.
Fig. 1.
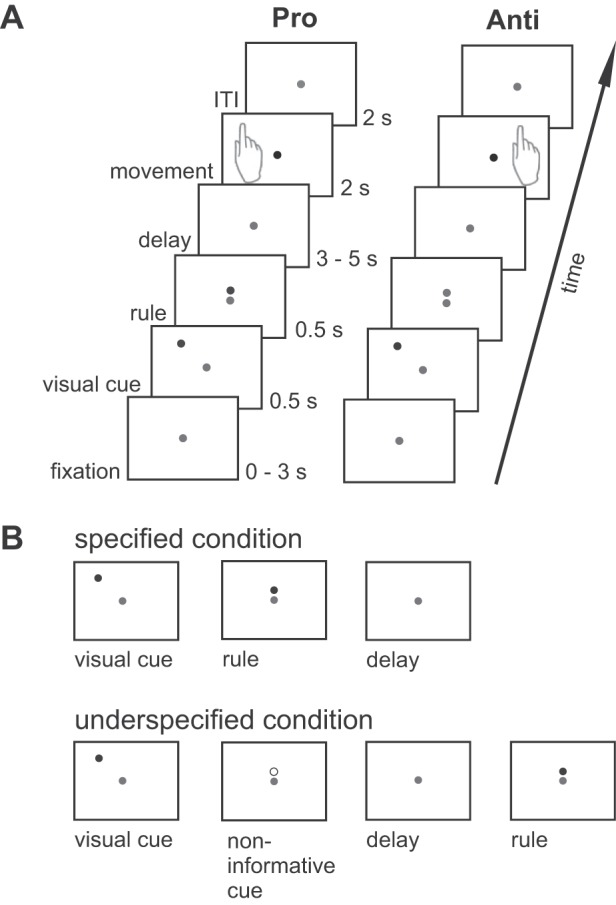
Delayed pro-/anti-reach task with different precueing conditions. A: different context rules had to be applied to visual cues at four possible locations to infer the movement goal. The example shows a single-reach trial with only one visual cue indicated by a green light-emitting diode (LED) (here depicted as a dark gray circle). In double-reach trials, two visual cues were presented consecutively indicating the order of the reach sequence. A red fixation LED (here in light gray) was visible at the center of the screen throughout the whole trial, and a change of its brightness served as a go-cue (black circle). In condition Pro, the context rule was indicated by a green LED (here dark gray) above the fixation LED, and memory-guided reaches were performed toward the position of the previously presented visual cue after a variable memory delay. In condition Anti, the context rule was indicated by a red LED (depicted in light gray) above the fixation LED, and reaches were performed to the mirror-imaged position of the visual cue. B: different precueing conditions were introduced to vary the information available during the memory delay. In specified conditions Pro and Anti, both the visual cues and the context rule were available during the delay. In the underspecified condition, only the visual cues were available during the memory delay, whereas the context rule was given immediately after the delay prompting participants to start the respective reaching movement. An additional yellow cue (depicted as an open circle) was presented before the delay to keep visual input constant but contained no information about the task.
Light-emitting diodes (LEDs) served as spatial cues, rule cues, and fixation point. Subjects were instructed to maintain fixation on a red central fixation LED throughout the trial. Green LEDs served as spatial cues that were presented at one of four possible locations left or right from the fixation point. We varied the number of reaches by presenting one or two visual cues, resulting in single-reach or double-reach trials, respectively. In double-reach trials, the two visual cues were always presented sequentially. Before the experiment, subjects were instructed to reach from the start location to the first visual cue location and from there directly to the second visual cue location following the order of the visual cue presentation. Both reach goals always fell into the same visual hemifield so that all reaches were either performed within the left or right visual field. With this division, we were able to investigate lateralization effects of the visual cue or the movement goal. We varied the location of the visual cue and the number of reaches (single vs. double) to keep participants attentive and to reduce the predictability of the upcoming movement and thus the preplanning of the movement.
In the specified condition, visual cue and rule cue were presented consecutively. The color of the LED located right above the fixation point specified the context rule that subjects had to apply to the visual cue to infer the movement goal. A green LED indicated that participants had to perform a reach toward the remembered position of the visual cue (pro-reach), whereas a red LED required moving toward the position mirrored to the centrally located fixation point (anti-reach). In this condition, all information required for building up a movement plan was available during the following delay period. Participants started right arm reaches as soon as the central fixation LED was dimmed after the delay (go-cue). In the underspecified condition the visual cue and an additional noninformative cue were presented before the delay. Importantly, the rule cue was presented after the delay. Thus, during the delay period, participants knew the location of the visual cue but were unaware about the movement goal (pro- vs. anti-reach). We introduced an additional noninformative cue in this condition to keep visual information constant and to inform subjects about the underspecified condition with the delay preceding the rule cue. Subjects performed reaches after the presentation of the rule cue. Other than that, specified and underspecified conditions were identical, and trials were presented interleaved in random order. Subjects did not receive feedback about the correct reach goal location.
Apparatus and Stimuli
To enable a direct view of the visual stimuli, subjects were positioned in the scanner with their head tilted with wedges (∼20–30°) inside the head coil. A custom-made MR-compatible plastic table, adjustable in distance and height, was mounted over the subjects' hips and fixed to the scanner bed. At the front end of the table a vertical plastic board with six holes with a diameter of 1 mm was attached. Inside each hole one fiber optic cable ended, which was connected with an LED placed in the control room. One red LED was positioned centrally and served as the fixation point and go-cue. Four green LEDs served as visual cues with one LED each positioned at the upper left and upper right workspace (5 cm horizontal and 5 cm vertical deviation from the fixation LED) and at the lower left and lower right workspace (8 cm horizontal and 1 cm vertical deviation from the fixation LED). An additional bicolor (green/red) LED right above the central LED indicated the context rule. Directly in front of the plastic board an MR-compatible 10.4-in. touchscreen panel (Magic Touch; Keytec, Garland, TX) was attached at an eye-to-screen distance of about 50 cm to record reaching endpoints with a resolution of 1,024 × 768 pixels. To reduce effects of eye movements on brain activation we recorded the eyes using an infrared camera (MRC Systems, Heidelberg, Germany) attached to the head coil and visually inspected the data offline for constant fixation throughout the trials. Due to the tilted head position we could not conduct eye tracking. The use of a camera allowed for a general control of constant fixation but not for a quantitative analysis of single eye movements. However, we instructed and trained our subjects thoroughly to maintain fixation, especially during the delay period. In addition, the subtle change in brightness of the fixation LED serving as go-cue along with the variable delay interval encouraged subjects to fixate until they started the movement. For all remaining subjects (see Participants) this rough criterion of constant fixation was fulfilled.
The right upper arm was strapped to the bed to minimize movement artifacts during reaching. Yet, it was ensured that participants could freely move their right forearm and reach toward all locations of the touchscreen without moving the upper arm or shoulder. Before and after movement execution, subjects continuously pressed a button of an MR-compatible button box placed on their abdomen with their right index finger. To assess individual reach endpoint errors, we subtracted the observed reach endpoints from the physical location of the visual cue. To this end, all visual cue LEDs were turned on subsequently after the end of the experiment, and subjects touched each location accordingly. Based on the individual reach endpoint errors, reaches were classified offline in correct and incorrect movements (see Behavioral Analyses in results).
All LEDs and response devices were controlled by Presentation software (Neurobehavioral Systems, Albany, CA).
Trial Timing
We used a rapid event-related design to study the neural correlates of movement planning. Each trial started with a fixation period of random duration varying from 0 to 3 s with 15-ms steps (repetition time/no. of trials = 3 s/192). Next, the visual cue was presented for 500 ms. In the specified condition, the visual cue was succeeded by the rule cue (500 ms) and a random delay of 3, 3.6, 4.3, or 5 s (see Fig. 1A). After the delay, the go-cue was presented initiating the movement interval (2 s). We varied the duration of the delay to minimize the predictability of the movement onset that might reduce activation associated with movement preparation. In the underspecified condition, the visual cue was followed by a noninformative cue (500 ms; see Fig. 1B) and then the delay period (3, 3.6, 4.3, or 5 s). Afterward the rule cue (500 ms) and the go-cue were presented successively followed by the movement interval (2 s). In the specified conditions and the underspecified condition, a new trial started after an intertrial interval of 2 s with the next excitation pulse.
One trial lasted on average 10.75 s (8–13.5 s). Each condition (specified conditions Pro and Anti and underspecified condition) consisted of 64 trials, resulting in 192 trials in total and a duration of about 35 min. The experiment in the scanner lasted about 1.5 h, including the set-up time, the functional scan, and the anatomical scan. Participants practiced the task on a computer outside the scanner before the experiment.
Behavioral Analyses
Behavioral data refer to the movement execution phase. At the time of the go-cue, participants were informed about the movement goal and performed either pro- or anti-reaches depending on the contextual cue given before (specified condition) or after (underspecified condition) the delay period. We thus analyzed pro- and anti-reaches separately in the specified and underspecified conditions.
In a first step, we analyzed the rate of correct responses. To do so, we separated the area of the touchscreen into individual quadrants (mean size, width × height: 10.06 × 7.9 cm) based on the vertical and horizontal centerlines between the coordinates of the calibration touches to the visible spatial cues acquired after the experiment. Responses were classified as correct if touches fell into the correct individual quadrant. Three subjects performed poorly in all conditions with a rate of correct responses of <70% and were discarded from further analyses. For the remaining subjects (n = 19) the amount of correct responses was compared across conditions using a one-way repeated-measures (RM) ANOVA with the factor condition (specified conditions Pro and Anti, underspecified conditions Pro and Anti) and an α-level of 0.05. When the assumption of sphericity was violated according to Mauchly's test for sphericity, F-statistics were corrected according to the procedure of Greenhouse-Geisser. Two-tailed post hoc t-tests were Bonferroni-Holm corrected for multiple comparisons, if necessary.
Second, we analyzed the response time for all participants, defined as the time elapsed after the onset of the go-cue and until the first touch. We used a one-way RM ANOVA with the factor condition (specified conditions Pro and Anti, underspecified conditions Pro and Anti) and an α-level of 0.05. Corrections for multiple comparisons or for violations of sphericity were carried out as described above.
Imaging Parameters
Functional and anatomical MRI data were acquired at the Bender Institute of Neuroimaging (Giessen, Germany) using a 1.5-Tesla Siemens Symphony whole body MRI system with a quantum gradient system (Siemens, Erlangen, Germany) and a standard one-channel head coil. A gradient-echo field map was measured before the functional run to receive information about inhomogeneities in the static magnetic field. For functional imaging, a total of 794 volumes were registered on average, varying from 786 to 802 volumes due to the combined duration of the randomized trial ordering with jittered fixation intervals and delay periods. A T2*-weighted gradient-echo-planar imaging (EPI) sequence was used with 30 axial slices covering the whole brain [slice thickness: 4 mm; 1 mm gap; descending slice order; echo time (TE): 59 ms; repetition time (TR): 3 s; flip angle: 85°; field of view: 192 mm; matrix size: 64 × 64 mm; voxel size: 3.0 × 3.0 × 4.0 mm]. The orientation of the slices was selected to cover superior parietal areas and was tilted to parallel the inferior border of the orbitofrontal cortex to reduce signal losses due to susceptibility artifacts. Structural images consisting of 160 sagittal slices were acquired using a T1-weighted magnetization-prepared, rapid-acquisition gradient echo sequence (matrix size: 256 × 180 mm; field of view: 250 mm; TE: 4.18 ms; TR: 1,990 ms; voxel size: 1.4 × 1.0 × 1.0 mm).
Preprocessing
Imaging data were preprocessed and analyzed using the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL; version 5.0.2; http://www.fmrib.ox.ac.uk/fsl). The first four volumes (12 s) were discarded due to an incomplete steady state of the magnetic field. We manually screened the motion parameters (rotations, translation) along the x-, y-, and z-axes of each participant. After realignment and motion correction using FSL's motion correction tool MCFLIRT (Jenkinson et al. 2002) we used a custom-made FSL tool to detect EPI outlier volumes by calculating the mean squared difference in brightness values to the respective adjacent volumes. These deviation scores were thresholded according to an outlier detection method for skewed data (Hubert and van der Veeken 2008) globally for the whole dataset. Three participants were discarded from further analyses due to large motion artifacts defined by >10% outlier volumes (Hubert and van der Veeken 2008).
Nonbrain tissue was removed from all images using the FSL's brain extraction tool (Smith 2002). Further preprocessing included the following steps: 1) B0 unwarping using field maps, 2) spatial normalization to the Montreal Neurological Institute (MNI) space, 3) slice timing correction, 4) spatial smoothing using a Gaussian kernel of 5 mm full-width half-maximum, and 5) temporal high-pass filtering with a cutoff of 144 s to remove low-frequency drift.
Data Analyses
Data analyses were performed using the general linear model (GLM) implemented in FSL's FMRI Expert Analysis Tool version 6.00 (Smith et al. 2004). We defined the delay phase as the period of interest for putative movement planning. We modeled one separate delay predictor for each experimental condition (specified conditions Pro and Anti, underspecified condition) and position of the visual cue (left or right visual field), resulting in six predictors of interest: Pro Left, Pro Right, Anti Left, Anti Right, Underspecified Left, Underspecified Right. In addition to these delay regressors, we defined the fixation interval (Fix), the presentation of the visual cue, the presentation of the rule cue, and the movement period as predictors of no interest. Each predictor was defined as a boxcar function with the value one for the duration of the respective event. Regressors were convolved with a double-γ hemodynamic response function to model the late undershoot. We also included the temporal derivative to our model to achieve a better fit to the data (Friston et al. 1998).
We conducted three different types of analyses. To identify brain areas active during the delay period in the specified and underspecified conditions, we performed whole brain voxelwise analyses. Additionally, we performed a conjunction analysis across the specified and underspecified conditions to extract activation in common brain regions. Finally, we conducted region of interest (ROI) analyses based on our prior hypotheses about cortical areas involved in movement planning.
Voxelwise Analyses
Whole brain voxelwise analyses were conducted using standard multiple-regression procedures. We calculated one baseline contrast for each experimental condition to test our hypothesis that areas of the reaching network are involved in specified and underspecified conditions. The two-delay regressors of each condition were combined and compared with the fixation interval: (Pro Left + Pro Right) > Fix, (Anti Left + Anti Right) > Fix, and (Underspecified Left + Underspecified Right) > Fix. Additionally, we identified differences in activation strength between conditions by calculating the differential contrasts: (Anti Left + Anti Right) > (Pro Left + Pro Right) and vice versa, (Pro Left + Pro Right) > (Underspecified Left + Underspecified Right), and (Anti Left + Anti Right) > (Underspecified Left + Underspecified Right).
For individual analyses, Z-statistic images were thresholded at P < 0.05, corrected for multiple comparisons using Gaussian random field theory (GRF; Worsley et al. 1996). For group-level analyses, parameter estimates were assessed with a mixed-effects model, with the random effects component of variance estimated using FSL's FLAME stage 1 procedure (Beckmann et al. 2003; Woolrich et al. 2004). Before thresholding, the statistical images were masked by a maximum probability gray matter mask based on the Harvard-Oxford cortical structural atlas provided by the Harvard Center for Morphometric Analysis (http://www.cma.mgh.harvard.edu/fsl_atlas.html) available with FSL. We did so to restrict our analyses to gray matter and thereby reduce the cluster criteria for statistical significance. Z (Gaussianized T)-statistic images were generated using a Z-statistics threshold of 2.1 and a corrected cluster probability threshold of P = 0.05 using GRF (Worsley et al. 1996).
We applied a custom-made FSL tool to locate signal peaks of clusters and label anatomical regions according to the Juelich probabilistic cytoarchitectonic atlas (Eickhoff et al. 2007).
Conjunction Analysis
To identify a general reaching network being involved in movement planning independent of the context rule, we conducted a second-level conjunction analysis on baseline contrasts from specified conditions Pro and Anti: [(Pro Left + Pro Right) − Fix] ∩ [(Anti Left + Anti Right) − Fix]. A custom-made FSL tool was used to create a minimum z-image from the second-level z-statistics images (z > 2.1, P = 0.05) of the respective contrasts and to perform a clusterwise test. Note that for easier reading the conjunction analysis will be denoted as Pro ∩ Anti.
ROI Analyses
We conducted second-level ROI analyses on three regions that were activated during movement planning in the specified conditions as revealed by previous whole brain analyses: the left PMd and left and right PCu. We defined the ROIs independently of our analyses on the basis of the study by Lindner et al. (2010), who found sustained activation in PMd and PCu during the delay period associated with reach movement planning. ROIs were created by specifying spheres with a radius of 5 mm centered at the reported coordinates (left PCu: −17.6 −64.9 60.0; right PCu: 17.6 −64.9 59.6; left PMd: −26.6 −8.6 58.1).
In a first step, we confirmed our ROI selection by testing for a main effect of condition using the following contrasts: (Pro Left + Pro Right) > Fix, (Anti Left + Anti Right) > Fix, (Underspecified Left + Underspecified Right) > Fix. Z-statistic images were thresholded at P = 0.05, corrected for multiple comparisons using GRF (Worsley et al. 1996). For further analyses, we extracted the mean percent signal change (%SC) per subject for each delay regressor from each ROI using Featquery (http://fsl.fmrib.ox.ac.uk/fsl/fsl4.0/feat5/featquery.html). We hypothesized that movement goals are encoded in PCu and PMd leading to higher %SC in response to contralateral visual cues in condition Pro, but higher %SC for ipsilateral visual cues in condition Anti (indicating a contralateral movement goal). To test this assumption, we conducted a 2 × 2 RM ANOVA with the factors condition (Pro vs. Anti) and visual field (left vs. right) with an α-level of 0.05. One-tailed post hoc t-tests were Bonferroni-Holm corrected for multiple comparisons, if necessary.
Second, we tested how activation strength changed in the underspecified condition compared with specified conditions in areas that showed a main effect for all three conditions, namely the left and right PCu. To do so, we analyzed %SC as a function of condition (three levels: Pro, Anti, underspecified) in a one-way RM ANOVA with an α-level of 0.05. When the assumption of sphericity was violated according to Mauchly's test for sphericity F-statistics were corrected according to the procedure of Greenhouse-Geisser. Two-tailed post hoc t-tests were Bonferroni-Holm corrected for multiple comparisons, if necessary. Finally, we examined if the left and right PCu show a preference for the left or right visual cue by testing for lateralization differences performing two-tailed paired-sample t-tests.
RESULTS
In the present study, we first analyzed brain activations in the specified conditions Pro and Anti to examine whether the brain encodes the reach movement goal or the physical location of the visual cue. Here, we focused on three regions of the reaching network, the left PMd and the left and right PCu. Second, we investigated how delay period activation differs if the movement goal is underspecified compared with when it is specified. In the following, we provide an overview of the behavioral results, and then report the results of the voxelwise whole brain group analyses and the ROI analyses.
Behavioral Analyses
Across all conditions subjects' reaches fell into the correct quadrant of the touchscreen in 85.8% of all trials (90.2% for the specified condition Pro, 83.7% for the specified condition Anti, 86.0% for the underspecified condition Pro, and 83.1% for the underspecified condition Anti), with a mean deviation across all trials of 2.1 ± 1.9 cm. There was no significant effect of condition on the percentage of correct responses [F(3,54) = 1.954, p = 0.146]. Response time also did not differ between the four conditions [F(3,54) = 1.115, P = 0.318]: specified Pro (M = 1,299 ms, SD = 261), specified Anti (M = 1,317 ms, SD = 295), underspecified Pro (M = 1,254 ms, SD = 483), and underspecified Anti (M = 1,369 ms, SD = 519).
Planning Pro- and Anti-Reaches in Specified Conditions
Voxelwise analyses.
To identify brain areas active during the delay period in the specified conditions, we performed baseline contrasts for the specified conditions Pro and Anti on the group data. The activation of both conditions with labels of the signal peaks according to the Juelich cytoarchitectonic atlas (Eickhoff et al. 2007), MNI coordinates, and z-values are listed in Table 1.
Table 1.
Voxelwise analyses of pro- and anti-reaches
| Condition SR Pro MNI Coordinates |
Condition SR Anti MNI Coordinates |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anatomic Region | Hemisphere | x | y | Z | z Value | x | y | z | z Value |
| SPL 7P (PCu) | R | 10 | −72 | 54 | 3.82 | 10 | −72 | 54 | 3.83 |
| L | −12 | −68 | 56 | 3.23 | −10 | −78 | 52 | 3.43 | |
| SPL7A (aPCu) | R | 30 | −62 | 64 | 3.4 | 28 | −64 | 64 | 3.44 |
| L | −12 | −64 | 66 | 3.3 | −12 | −64 | 66 | 3.28 | |
| SPL 7PC | L | −42 | −48 | 58 | 3.88 | −42 | −48 | 58 | 4.06 |
| SPL 5M | L | −6 | −50 | 56 | 2.27 | ||||
| Angular gyrus | R | 52 | −54 | 44 | 2.46 | ||||
| L | −52 | −58 | 44 | 3.08 | −52 | −56 | 42 | 3.11 | |
| Supramarginal gyrus | R | 54 | −30 | 44 | 3.07 | 54 | −30 | 44 | 3.3 |
| L | −48 | −46 | 54 | 3.47 | −48 | −46 | 54 | 3.64 | |
| aIPS (hIP1) | R | 38 | −56 | 46 | 3.18 | 38 | −56 | 46 | 3.4 |
| L | −38 | −50 | 44 | 3.56 | −36 | −52 | 40 | 3.6 | |
| aIPS (hIP2) | R | 40 | −46 | 50 | 3.27 | 40 | −48 | 50 | 3.41 |
| L | −46 | −44 | 44 | 3.03 | −40 | −46 | 48 | 3.58 | |
| aIPS (hIP3) | R | 32 | −56 | 52 | 2.63 | ||||
| L | −26 | −56 | 52 | 2.56 | |||||
| Postcentral gyrus (BA 1, S1) | R | 46 | −38 | 64 | 3.02 | 46 | −38 | 64 | 2.74 |
| L | −44 | −38 | 60 | 3.78 | −44 | −38 | 60 | 3.65 | |
| Postcentral gyrus (BA 2, S1) | L | −46 | −36 | 48 | 3.73 | −46 | −42 | 58 | 3.72 |
| Precentral gyrus (BA 4a, M1) | L | −42 | −16 | 48 | 2.67 | ||||
| Precentral gyrus (BA6, PMd) | L | −26 | 4 | 68 | 3.02 | −26 | −18 | 74 | 2.38 |
| SFG | R | 22 | 4 | 68 | 3.25 | ||||
| L | −24 | 14 | 62 | 3 | |||||
| MFG | R | 48 | 12 | 50 | 2.4 | ||||
| L | −46 | 36 | 32 | 2.8 | −50 | 28 | 30 | 2.39 | |
| Frontal Pole | R | 30 | 56 | −4 | 2.42 | 32 | 60 | −2 | 2.56 |
| L | −40 | 46 | −2 | 3.83 | −40 | 46 | −2 | 3.76 | |
Montreal Neurological Institute (MNI) coordinates of local maxima in clusters showing significantly more activation in specified conditions Pro and Anti compared with fixation (cluster corrected, z >2.1, P = 0.05). Functional labels are in parentheses. SPL, superior parietal lobule; PCu, precuneus; aPCu, anterior precuneus; aIPS, anterior intraparietal sulcus; hIP, human intraparietal area; S1, primary somatosensory cortex; M1, primary motor cortex; PMd, dorsal premotor cortex; SFG, superior frontal gyrus; MFG, middle frontal gyrus; R, right; L, left.
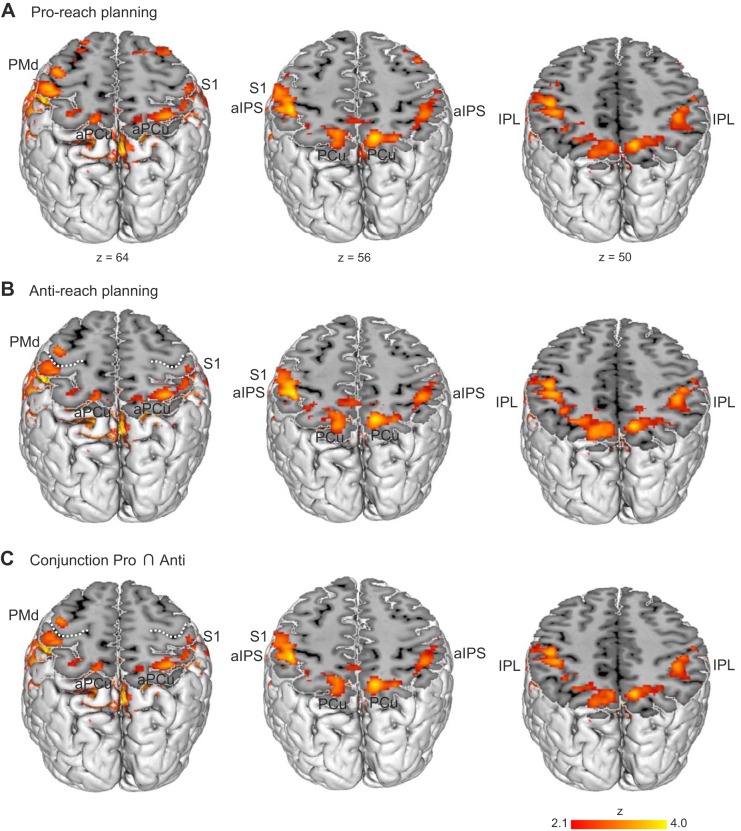
For the planning of pro-reaches, we calculated the baseline contrast (Pro Left + Pro Right) > Fix and found activation in frontoparietal areas comprising the reaching network (Fig. 2A). Specifically, the left and right SPL were activated. The cluster included the PCu comprising the medial portions of the SPL, anterior to the parietooccipital sulcus. This region has previously been suggested as a putative human homolog of monkey area PRR (Connolly et al. 2003; Fernandez-Ruiz et al. 2007; Fabbri et al. 2010). Moreover, activation occurred in the left and right inferior parietal lobules (IPL), the left and right anterior intraparietal sulcus (aIPS) and adjacent primary somatosensory cortex (S1), the left M1, and the left PMd spreading into the left superior frontal gyrus (SFG). We also found activation in the left frontal pole extending into the left middle frontal gyrus (MFG), and in the right frontal pole spreading into the right MFG and SFG.
Fig. 2.
Delay period activation for the specified conditions Pro (A) and Anti (B) obtained by calculating the respective baseline contrasts (Pro Left + Pro Right) > Fix and (Anti Left + Anti Right) > Fix. C: the overlap of activation in both specified conditions, Pro ∩ Anti, as revealed by the conjunction analysis of the two contrasts shown in A and B. White broken lines denote the central sulcus. S1, primary somatosensory cortex; aIPS, anterior intraparietal sulcus; IPL, inferior parietal lobule; PCu, precuneus; PMd, dorsal premotor cortex; aPCu, anterior precuneus.
Figure 2B shows the results for the specified condition Anti contrasted against fixation, (Anti Left + Anti Right) > Fix. The planning of anti-reaches activated a similar frontoparietal network as we found for pro-reaches which contained bilateral activation in the SPL (including the left and right PCu), the IPL, the aIPS, and S1 and a left-lateralized activation in the PMd which also covered the left M1. In addition, the specified condition Anti activated the left and right frontal pole, with the left activation spreading into the left MFG.
Descriptively, condition Pro yields activation in the left and right SFG and the right MFG, whereas we see no such effect for the specified condition Anti. To examine whether activation differences were statistically significant between planning pro- and anti-reaches, we calculated the contrasts (Anti Left + Anti Right) > (Pro Left + Pro Right) and (Pro Left + Pro Right) > (Anti Left + Anti Right). These contrasts revealed no cluster more strongly activated in planning anti-reaches compared with pro-reaches and vice versa, suggesting that the planning of pro- and anti-reaches recruits similar brain areas. To substantiate this result, we conducted a conjunction analysis on the two specified conditions, Pro ∩ Anti. Consistent with the results described above, the conjunction analysis revealed an activation overlap in a large frontoparietal network extending from the bilateral SPL (signal peaks in the left hemisphere: z = 3.88, right hemisphere: z = 3.82) to the aIPS, the IPL, and S1, as well as to the left PMd and M1 (see Fig. 2C). These clusters comprised the PCu in both the left and right hemispheres. The activation patterns further overlapped in the left frontal pole (z = 3.76) spreading into the left MFG and the right frontal pole (z = 3.34). In short, in the specified pro- and anti-reach conditions delay period activation expanded throughout posterior parietal and premotor areas and did not differ between conditions.
ROI analyses.
Based on our hypotheses on movement goal encoding within the reaching network, we conducted ROI analyses on the PCu and the PMd, using the MNI coordinates reported by Lindner et al. (2010). Because the whole brain analyses revealed that only the left PMd was activated during reach planning in specified conditions, we restricted our ROI analyses to the left PMd in addition to the left and right PCu. For both specified conditions Pro and Anti we found a main effect in the three ROIs left PCu (Pro: z = 3.09, Anti: z = 3.03), right PCu (Pro: z = 3.17, Anti: z = 3.43), and left PMd (Pro: z = 2.29, Anti: z = 2.8).
Based on previous findings in monkeys (Gail and Andersen 2006), we hypothesized that the movement goal rather than the physical location of the visual cue is encoded in the reach-related areas PCu and PMd. Therefore, we conducted a two-way RM ANOVA with the factors condition (2) × visual field (2) on the mean %SC in the respective areas across subjects (see Table 3). If the visual movement goal is represented, we expected higher signal changes in the left PCu for contralateral (right) visual cues in condition Pro. However, in condition Anti ipsilateral (left) visual cues should yield higher signal changes, since left visual cues combined with the context rule “anti” indicated movement goals in the right contralateral visual field. This should result in an interaction of condition and visual field. Indeed, we found a significant condition × visual field interaction indicating that the movement goal rather than the physical location of the visual cue is represented in the left PCu [F(1,18) = 9.68, P = 0.006]. This finding is shown in Fig. 3 showing higher %SC for the specified condition Pro right compared with Pro left, and for the specified condition Anti left compared with Anti right.
Table 3.
Mean percent signal change per ROI and condition
| Pro |
Anti |
Underspecified |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Mean | Left | Right | Mean | Left | Right | Mean | |
| PCu L | 0.21 ± 0.35 | 0.28 ± 0.35 | 0.23 ± 0.33 | 0.26 ± 0.4 | 0.20 ± 0.32 | 0.23 ± 0.35 | 0.14 ± 0.39 | 0.07 ± 0.29 | 0.10 ± 0.31 |
| PCu R | 0.20 ± 0.16 | 0.19 ± 0.17 | 0.19 ± 0.15 | 0.20 ± 0.18 | 0.20 ± 0.18 | 0.20 ± 0.16 | 0.1 ± 0.17 | 0.06 ± 0.16 | 0.07 ± 0.15 |
| PMd L | 0.04 ± 0.12 | 0.07 ± 0.15 | 0.05 ± 0.13 | 0.07 ± 0.14 | 0.06 ± 0.17 | 0.07 ± 0.14 | |||
Data are means ± SD. ROI, region of interest.
Fig. 3.
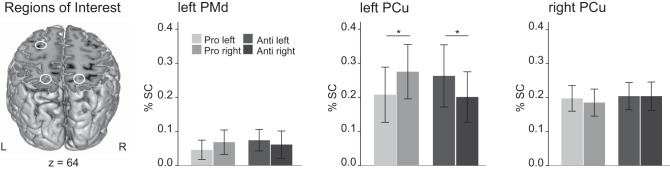
Percent signal change (%SC) for specified conditions Pro and Anti for the regions of interest (ROIs): left PMd and left and right PCu. In the left PCu, %SC for right visual cues is significantly higher than for left visual cues in condition Pro, whereas the pattern reverses in condition Anti. In both conditions, right movement goals thus lead to higher %SC, indicating movement goal encoding. No such interaction effect occurs in the right PCu and the left PMd. Error bars denote SE (*P < 0.05, Bonferroni-Holm corrected; 1-sided t-test).
To further test for movement goal encoding within the conditions Pro and Anti, we performed the respective post hoc paired t-tests using Bonferroni-Holm adjusted α-levels (P < 0.025 and 0.05). In condition Pro, contralateral right visual cues elicited a significantly higher %SC than ipsilateral left visual cues [t(18) = 2.53, P = 0.0105, 1 sided]. In condition Anti, the %SC for ipsilateral left visual cues were significantly higher than for contralateral right visual cues [t(18) = 1.74, P = 0.0495, 1 sided]. The overall response pattern speaks in favor of movement goal encoding within the left PCu.
For the right PCu and the left PMd, the %SC did not differ between conditions Pro and Anti or left and right visual cue positions.
Activation of Reach-Related Areas in Underspecified Conditions
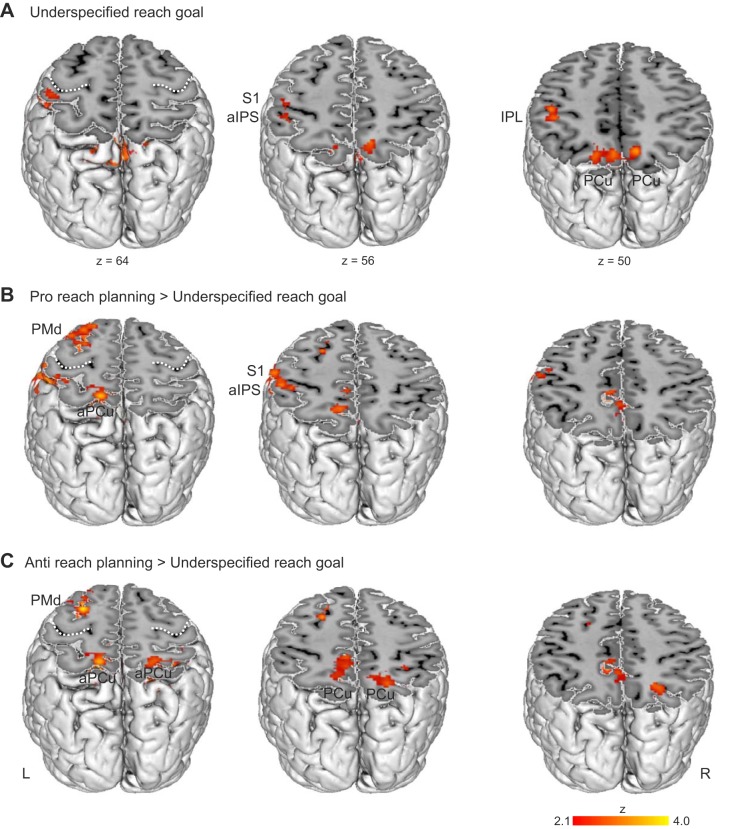
To investigate whether reach-related brain areas are active during the delay period when movement-relevant information is insufficient to prepare the final reach (underspecified situation), we first calculated a baseline contrast for the underspecified condition (Underspecified Left + Underspecified Right) > Fix. As depicted in Fig. 4A, we observed activation in the left parietal cortex, including IPL, aIPS, S1, and, importantly, the left and right PCu and anterior PCu (Table 2). Furthermore, the underspecified condition activated the left frontal pole. The results clearly show that posterior parietal areas of the reaching network were active even in situations where the movement goal was underspecified. However, in contrast to the specified conditions, we found no activation in the left PMd in the underspecified condition. Therefore, we performed the ROI analyses on the left and right PCu only.
Fig. 4.
A: delay period activation in the underspecified condition obtained by the baseline contrast (Underspecified Left + Underspecified Right) > Fix. Areas eliciting higher activation in the specified conditions compared with the underspecified condition are shown in B for the differential contrast (Pro Left + Pro Right) > (Underspecified Left + Underspecified Right) and in C for the differential contrast (Anti Left + Anti Right) > (Underspecified Left + Underspecified Right). White broken lines denote the central sulcus.
Table 2.
Voxelwise analyses of specified and underspecified movement goals
| MNI coordinates |
|||||
|---|---|---|---|---|---|
| Anatomic Region | Hemisphere | x | y | z | z Value |
| Underspecified condition | |||||
| SPL 7P (PCu) | R | 10 | −74 | 50 | 3.24 |
| L | −4 | −76 | 50 | 2.96 | |
| Supramarginal Gyrus | L | −48 | −48 | 54 | 2.34 |
| aIPS (hIP1) | L | −36 | −54 | 42 | 3.28 |
| Postcentral Gyrus (BA 2, S1) | L | −44 | −38 | 48 | 3.15 |
| Postcentral Gyrus (BA 1, S1) | L | −46 | −36 | 62 | 2.89 |
| Frontal Pole | L | −40 | 46 | −2 | 3.81 |
| Pro > underspecified | |||||
| SPL 7A (aPCu) | R | 8 | −60 | 44 | 2.83 |
| L | −14 | −64 | 64 | 3.6 | |
| Supramarginal Gyrus | L | −56 | −30 | 52 | 3.08 |
| aIPS (hIP2) | L | −46 | −44 | 56 | 2.53 |
| Postcentral Gyrus (BA 2, S1) | L | −52 | −28 | 46 | 2.15 |
| Postcentral Gyrus (BA 1, S1) | L | −50 | −24 | 52 | 2.56 |
| Precentral Gyrus (BA6, PMd) | L | −30 | −8 | 70 | 3.09 |
| Anti > underspecified | |||||
| SPL 7P (PCu) | R | 18 | −74 | 54 | 3.14 |
| L | −2 | −58 | 48 | 2.96 | |
| SPL 7A (aPCu) | R | 30 | −60 | 60 | 3.25 |
| L | −14 | −64 | 64 | 3.45 | |
| Precentral Gyrus (BA6, PMd) | L | −26 | −6 | 64 | 3.84 |
| SFG | L | −20 | 18 | 64 | 2.4 |
MNI Coordinates of local maxima in clusters showing significantly more activation in the underspecified condition compared with fixation and in the specified conditions Pro and Anti compared with the underspecified condition (all cluster corrected, z >2.1). Functional labels are in parentheses.
Next, we identified areas showing higher activation in specified compared with underspecified conditions by calculating the differential contrasts (Pro Left + Pro Right) > (Underspecified Left + Underspecified Right) and (Anti Left + Anti Right) > (Underspecified Left + Underspecified Right). Comparing the delay period activations from condition Pro with the underspecified condition revealed stronger activation in the specified condition in the left PMd (z = 3.09), and in clusters in the left parietal cortex extending throughout the SPL, the IPL, S1, along the aIPS, and to the right SPL (see Fig. 4B). Similarly, the specified condition Anti yielded higher activation in the left PMd, extending to the left SFG, and in the left and right SPL including the PCu as shown in Fig. 4C.
ROI analyses.
In addition to the whole brain frontoparietal areas showing higher activation in specified compared with underspecified conditions we examined whether the activation strength differed likewise in the left and right PCu. Moreover, we tested for putative lateralization effects between left and right visual cues that may indicate movement planning or visual memory processes within the ROIs.
Again, we first confirmed the validity of our ROIs based on the results by Lindner et al. (2010) by showing delay period activation in the left PCu (z = 1.92; P < 0.05, uncorrected) and the right PCu (z = 2.23) in the underspecified condition. Within the left PCu the %SC differed significantly between conditions [F(2,36) = 17.14; P < 0.001]. Post hoc t-tests revealed that %SC were significantly higher in condition Pro [t(18) = 4.88, P < 0.017, corrected] and Anti [t(18) = 4.75, P < 0.025, corrected] compared with the underspecified condition, respectively. However, there was no significant difference between the specified conditions [t(18) = 0.25; P = 0.81]. Similarly, %SC differed significantly between conditions in the right PCu [F(1.41,25.40) = 16.11; P < 0.001]. Again, we observed higher %SC in the specified conditions Pro [t(18) = 3.72, P < 0.025, corrected] and Anti [t(18) = 5.03, P < 0.017, corrected] compared with the underspecified condition, whereas %SC from the specified conditions did not differ [t(18) = 0.908, P = 0.376]. These results extend the findings from the whole brain contrasts showing that a specified movement goal leads to higher activation compared with underspecified movement goals in the predefined PCu regions.
In addition, we aimed to explore lateralization preferences in the PCu in the underspecified condition. Left and right visual cues did not elicit significant differences in %SC (Table 3) either in the left PCu [t(18) = 1.69, P = 0.109] or in the right PCu [t(18) = 1.42, P = 0.173].
DISCUSSION
In the current study we used a pro-/anti-reach rule-selection task to examine the nature of movement planning processes in conditions with specified and underspecified movement goals. For specified conditions, we identified a reaching network comprising the PPC bilaterally and the left PMd with a large activation overlap between planning pro- and anti-reaches. Within this network, the PCu contralateral to the moving effector elicited directionally selective activation depending on the location of the movement goal and not on the location of the visual cue. If the movement goal was not specified, areas of the reaching network within the PPC but not the PMd were recruited and showed weaker activation than in the specified conditions.
Pro- and Anti-Reach Planning in Specified Conditions
In the specified conditions that required either a pro- or an anti-reach, we identified a broad frontoparietal network involved in the planning of reaching movements. This network included the bilateral PCu and the left PMd, which has also been described in earlier fMRI studies on reach planning and execution (Prado et al. 2005; Beurze et al. 2007; Lindner et al. 2010; Parkinson et al. 2010; Fabbri et al. 2012). Area PCu, labeled human PRR by Connolly et al. (2003), plays a crucial role in planning and executing reaching and pointing movements (Connolly et al. 2003; Fernandez-Ruiz et al. 2007; Bernier et al. 2012) with and without visual feedback from the hand (Filimon et al. 2009) and even engages in imagined and observed reaching (Filimon et al. 2007). The PCu seems to functionally differ from an area in the superior parietooccipital cortex (SPOC) which is located more posterior and within the parietooccipital sulcus (Gallivan et al. 2009). SPOC has been shown to be specialized for the transport component of reach and grasp movements (Cavina-Pratesi et al. 2010), but also processes object's reachability (Gallivan et al. 2009), and hand orientation (Monaco et al. 2011) as well as grasp axes (Monaco et al. 2014) for grasping movements. Therefore, SPOC rather than PCu has been discussed as a putative human homolog of monkey area V6A (Gallivan et al. 2009), which does not only contain reach-related neurons but also neurons selective for different grip types (Fattori et al. 2010). However, the exact human homologs of monkey PRR, reach-related area 5, V6A, and medial intraparietal area remain unclear.
Our results showed that pro- and anti-reach movements activated similar brain areas that did not differ in activation strength. This is consistent with findings from Connolly et al. (2003) who also observed no activation differences between pro- and anti-reach movements during the planning period. We did not find activation in additional brain areas for anti- compared with pro-reaches, as has been reported in an earlier study by Connolly et al. (2000). However, these results were based on activation during both movement planning and execution and thus are hardly comparable to the present findings where we exclusively focused on activation during movement planning.
Movement Goal vs. Visual Cue Representation in Specified Conditions
To investigate whether PCu and PMd represent the visual movement goal or maintain the presented visual cue in visual memory during movement planning, we applied an adapted version of the pro-/anti-reach rule-selection task by Westendorff et al. (2010). Here, we focused on the left and right PCu and the left PMd. Based on previous findings from electrophysiological studies in monkeys (Westendorff et al. 2010; Klaes et al. 2011), we hypothesized that the position of the movement goal rather than the position of the visual cue is encoded in PCu and PMd.
In the left PCu, we observed a preference for contralateral visual cues in pro-reach trials where the visual cue coincided with the movement goal. Importantly, this pattern reversed for anti-reach trials showing a preference for ipsilateral visual cues where the visual cue indicated the contralateral movement goal. By showing that human PCu encodes the visual movement goal rather than the location of the visual cue preceding the movement preparation period, we substantially extend the results by Fernandez-Ruiz et al. (2007) who demonstrated a preference for visual over physical movement goals in PCu. Taken together with our findings, it is unlikely that the activation observed in PCu in the study by Fernandez-Ruiz et al. (2007) reflects the visual memorization of the visual cue rather than the visual movement goal.
The present finding is also in line with the results on reach planning in monkeys demonstrating directional selectivity of PRR neurons fixed to the motor goal rather than the visual cue in a similar pro-/anti-reach task (Westendorff et al. 2010). However, caution is needed when comparing fMRI activations in humans with single-unit recordings in monkeys. Kuang et al. (2015) combined a reversing-prism task with an anti-reach task in monkeys and found that the majority of PRR neurons encode the physical movement goal while only a small portion encodes the visual movement goal, a finding incompatible with previous fMRI work (Fernandez-Ruiz et al. 2007) and the present findings in humans. However, when they analyzed the local field potentials instead of single-neuron spiking activity from the same brain areas, they found evidence for pure visual movement goal encoding. Furthermore, they demonstrated that the observed movement goal encoding was unrelated to visual memory in line with our fMRI results in humans. This suggests similar spatial coding mechanisms for reach movement planning in monkey PRR and human PCu.
We found evidence for (visual) movement goal encoding in the left PCu, contralateral to the moving effector (right arm reaches), while no such effect occurred in the right PCu although both the left and right PCu were activated during movement planning. Similarly, Fernandez-Ruiz et al. (2007) also found PCu activation related to movement goal encoding only in the hemisphere contralateral to the moving arm. The movement goal thus seems to be reliably encoded in the contralateral PCu for right arm reaches, whereas movement goal encoding in the ipsilateral PCu seems to be less robust. One explanation for the lacking effect might be that univariate GLM analyses of fMRI data are not sensitive enough to assess movement goal encoding in the ipsilateral hemisphere. By applying fMRI adaptation, Fabbri et al. (2010) were able to find directional selectivity in the contralateral left and the ipsilateral right PCu for right arm reaches. However, for left arm reaches this effect only occurred for the contralateral right PCu and was absent for the ipsilateral left PCu. Given the fact that the PCu represents both the effector and the movement goal (Beurze et al. 2007) reach movement goals might preferably be encoded in the human PCu contralateral to the moving arm.
During the movement preparation period only the left PMd contralateral to the reaching hand was activated. Such a contralateral bias was also observed in earlier studies on reach planning (Medendorp et al. 2005; Bernier et al. 2012). The activation maximum we observed for planning reaches with specified movement goals was located near an area previously labeled PMd proper due to its movement-specific functions, in contrast to the pre-PMd, which has been associated with higher-order processes, such as response selection or motor imagery (for a review, see Picard and Strick 2001). The PMd proper has been suggested to transform visuospatial information into motor codes by double coding of movement goal and effector signals with a preference for the latter (Medendorp et al. 2005; Beurze et al. 2007). Here, we found no directionally selective activation in the left PMd. Our results thus speak in favor of a stronger effector- and weaker target-specificity in PMd compared with PCu in the human brain. Although earlier studies on reach planning indicated that PMd is primarily modulated by the effector hand (Beurze et al. 2007; Medendorp et al. 2005) more recent work using multivariate decoding approaches on fMRI data also demonstrated target-selectivity in PMd (Gallivan et al. 2011; Fabbri et al. 2014).
Activation of Reach-Related Areas in Underspecified Conditions
In conditions with underspecified movement goals, participants knew the location of the visual cue but were uninformed about whether they should perform a pro- or an anti-reach movement after the movement preparation period. We observed activation in reach-related parietal regions similar to the areas activated in the specified conditions, again comprising the bilateral PCu. Yet, the PCu as well as other parietal areas showed stronger activation in conditions with specified movement goals. Moreover, we found no activation in the PMd in the underspecified conditions, in contrast to the specified conditions. In summary, situations with underspecified movement goals recruit fewer areas that are restricted to the parietal cortex and show weaker activation than in specified conditions.
The lower activation in reach-related areas might be caused by an incomplete stage of sensorimotor integration since only the effector and the visual cue but not the visual movement goal was given. A similar conclusion has been derived from results of a sequential cueing task presenting separate cues for the movement goal and the effector (left vs. right arm) (Beurze et al. 2007). They showed that conditions in which the effector was specified but the visual movement goal was unknown (corresponding to our underspecified condition) yield activation in reach-related areas of the PPC, but activations were stronger and broader when both the effector and the movement goal were specified (after cue 2) compared with situations when only the effector or only the movement goal was given (after cue 1) (Beurze et al. 2007). In a similar study, Bernier et al. (2012) cued the effector (left vs. right arm) either before or simultaneously with the presentation of the movement goal. They tested whether frontoparietal reach areas represent the movement goal for both arms if the effector is unknown, or if the movement goal is only formed after the information about the effector is given. They found evidence for the latter case and concluded that a motor goal is specified only if both the movement goal and the effector information are given. Thus, the specification of both the effector and the movement goal seems to be necessary to reach complete sensorimotor integration in the frontoparietal reaching network. However, the tasks applied by Beurze et al. (2007) and Bernier et al. (2012) differ in various aspects from our pro-/anti-reach rule selection task, e.g., single reaching vs. sequential reaching or reaching to the visual cue vs. the inferred mirrored location, limiting a direct comparison of the findings.
An alternative explanation for the lower activation in reach-related areas could be that in underspecified conditions participants have planned all potential pro- and anti-reach movements, but mutual inhibition of competing movement plans led to lower net activation in reach-planning areas like PCu and PMd. Evidence for this second possibility comes from electrophysiological studies in monkeys demonstrating that neural activity of spatially selective neuronal populations first spans the entire angular range of potential reach directions and after movement goal specification sharpens to reflect the choice (Bastian et al. 1998, 2003). Selection of one movement plan is achieved by mutual inhibition among neuronal populations with different tuning properties (Cisek 2006) and/or through differential selection in corticostriatal circuits (Leblois et al. 2006). Simultaneous encoding of multiple movement plans has been identified in frontoparietal areas, including PMd and PRR, in conditions with underspecified movement goals and even with single cue presentation like in pro-/anti-reach tasks (Cisek and Kalaska 2002, 2005; Klaes et al. 2011). In humans, evidence for the coactivation of multiple movement plans comes from magnetoencephalographic (Tzagarakis et al. 2010) and electroencephalographic (EEG) studies (Rawle et al. 2012) showing weaker β-band suppression, and thus attenuated activation, in motor-related frontal areas when multiple potential movement goals were presented before the delay period. In accordance, EEG recorded delay period activity in (pre)motor cortex inversely scaled with the number of possible reach goals presumably caused by mutually suppressive interactions between cell populations encoding different movement directions (Praamstra et al. 2009).
With the present task design, we cannot exclude one of the two aforementioned possibilities. The lack of directional selectivity we observed in the left PCu in the underspecified condition (in contrast to the specified condition) might suggest that human PCu encodes both potential pro- and anti-reach movements in parallel. Future brain imaging studies should investigate in more detail whether reach-related areas in the human brain plan all potential movements in advance and later select the appropriate one or wait until the movement goal is specified and then plan the appropriate movement.
Conclusions
Our study aimed to clarify the nature of movement planning processes within the human reaching network in a pro-/anti-reach rule selection task. The present findings demonstrate that the reach-related area PCu encodes the visual movement goal rather than the viewed visual cue if the movement goal is specified and even engages in situations when only the visual cue but not the movement goal is available. Visual movement goal specificity was only found in the left hemisphere, suggesting preferred encoding in the PCu contralateral to the reaching hand.
GRANTS
This work was supported by the German Research Foundation (Grant No. DFG Fi 1567/4-1) assigned to K. Fiehler.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.G. and K.F. conception and design of research; H.G. performed experiments; H.G. analyzed data; H.G. and K.F. interpreted results of experiments; H.G. prepared figures; H.G. drafted manuscript; H.G. and K.F. approved final version of manuscript; K.F. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Immo Schütz for help with the experimental set up and Bertram Walter and Immo Schütz for helpful advice regarding data analyses.
REFERENCES
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci 25: 189–220, 2002. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci 23: 4689–4699, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian A, Riehle A, Erlhagen W, Schöner G. Prior information preshapes the population representation of movement direction in motor cortex. Neuroreport 9: 315–320, 1998. [DOI] [PubMed] [Google Scholar]
- Bastian A, Schoner G, Riehle A. Preshaping and continuous evolution of motor cortical representations during movement preparation. Eur J Neurosci 18: 2047–2058, 2003. [DOI] [PubMed] [Google Scholar]
- Batista AP, Andersen RA. The parietal reach region codes the next planned movement in a sequential reach task. J Neurophysiol 85: 539–544, 2001. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Ferrari-Toniolo S, Visco-Comandini F, Archambault PS, Saberi-Moghadam S, Caminiti R. Impairment of online control of hand and eye movements in a monkey model of optic ataxia. Cereb Cortex 23: 2644–2656, 2013. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage 20: 1052–1063, 2003. [DOI] [PubMed] [Google Scholar]
- Bernier PM, Cieslak M, Grafton ST. Effector selection precedes reach planning in the dorsal parietofrontal cortex. J Neurophysiol 108: 57–68, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurze SM, de Lange FP, Toni I, Medendorp WP. Integration of target and effector information in the human brain during reach planning. J Neurophysiol 97: 188–199, 2007. [DOI] [PubMed] [Google Scholar]
- Busan P, Barbera C, Semenic M, Monti F, Pizzolato G, Pelamatti G, Battaglini PP. Effect of transcranial magnetic stimulation (TMS) on parietal and premotor cortex during planning of reaching movements. PLos One 4: e4621, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton JL, Dickinson AR, Snyder LH. Non-spatial, motor-specific activation in posterior parietal cortex. Nat Neurosci 5: 580–588, 2002. [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Monaco S, Fattori P, Galletti C, McAdam TD, Quinlan DJ, Goodale MA, Culham JC. Functional magnetic resonance imaging reveals the neural substrates of arm transport and grip formation in reach-to-grasp actions in humans. J Neurosci 30: 10306–10323, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Simultaneous encoding of multiple potential reach directions in dorsal premotor cortex. J Neurophysiol 87: 1149–1154, 2002. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron 45: 801–814, 2005. [DOI] [PubMed] [Google Scholar]
- Cisek P. Integrated neural processes for defining potential actions and deciding between them: a computational model. J Neurosci 26: 9761–70, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Desouza JFX, Menon RS, Vilis T. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. J Neurophysiol 84: 1645–1655, 2000. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Andersen RA, Goodale MA. FMRI evidence for a “parietal reach region” in the human brain. Exp Brain Res 153: 140–145, 2003. [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Differential relation of discharge in primary motor cortex and premotor cortex to movements versus actively maintained postures during a reaching task. Exp Brain Res 108: 45–61, 1996. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavina-Pratesi C, Singhal A. The role of parietal cortex in visuomotor control: what have we learned from neuroimaging? Neuropsychologia 44: 2668–2684, 2006. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36: 511–521, 2007. [DOI] [PubMed] [Google Scholar]
- Fabbri S, Caramazza A, Lingnau A. Tuning curves for movement direction in the human visuomotor system. J Neurosci 30: 13488–98, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri S, Caramazza A, Lingnau A. Distributed sensitivity for movement amplitude in directionally tuned neuronal populations. J Neurophysiol 107: 1845–1856, 2012. [DOI] [PubMed] [Google Scholar]
- Fabbri S, Strnad L, Caramazza A, Lingnau A. Overlapping representations for grip type and reach direction. Neuroimage 94: 138–46, 2014. [DOI] [PubMed] [Google Scholar]
- Fattori P, Raos V, Breveglieri R, Bosco A, Marzocchi N, Galletti C. The dorsomedial pathway is not just for reaching: grasping neurons in the medial parieto-occipital cortex of the macaque monkey. J Neurosci 30: 342–349, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Goltz HC, DeSouza JFX, Vilis T, Crawford JD. Human parietal “reach region” primarily encodes intrinsic visual direction, not extrinsic movement direction, in a visual motor dissociation task. Cereb Cortex 17: 2283–2292, 2007. [DOI] [PubMed] [Google Scholar]
- Filimon F, Nelson JD, Hagler DJ, Sereno MI. Human cortical representations for reaching: mirror neurons for execution, observation, and imagery. Neuroimage 37: 1315–1328, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filimon F, Nelson JD, Huang RS, Sereno MI. Multiple parietal reach regions in humans: cortical representations for visual and proprioceptive feedback during on-line reaching. J Neurosci 29: 2961–2971, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage 7: 30–40, 1998. [DOI] [PubMed] [Google Scholar]
- Gail A, Andersen RA. Neural dynamics in monkey parietal reach region reflect context-specific sensorimotor transformations. J Neurosci 26: 9376–9384, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gail A, Klaes C, Westendorff S. Implementation of spatial transformation rules for goal-directed reaching via gain modulation in monkey parietal and premotor cortex. J Neurosci 29: 9490–9499, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, Cavina-Pratesi C, Culham JC. Is that within reach? fMRI reveals that the human superior parieto-occipital cortex encodes objects reachable by the hand. J Neurosci 29: 4381–4391, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, McLean DA, Smith FW, Culham JC. Decoding effector-dependent and effector-independent movement intentions from human parieto-frontal brain activity. J Neurosci 31: 17149–68, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Riecke L, Sereno MI. Parietal and superior frontal visuospatial maps activated by pointing and saccades. Neuroimage 35: 1562–1577, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J Neurophysiol 95: 3596–3616, 2006. [DOI] [PubMed] [Google Scholar]
- Hubert M, Van der Veeken S. Outlier detection for skewed data. J Chemom 22: 235–246, 2008. [Google Scholar]
- Hwang EJ, Hauschild M, Wilke M, Andersen RA. Inactivation of the parietal reach region causes optic ataxia, impairing reaches but not saccades. Neuron 76: 1021–1029, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841, 2002. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex 6: 102–119, 1996. [DOI] [PubMed] [Google Scholar]
- Klaes C, Westendorff S, Chakrabarti S, Gail A. Choosing goals, not rules: deciding among rule-based action plans. Neuron 70: 536–548, 2011. [DOI] [PubMed] [Google Scholar]
- Kuang S, Morel P, Gail A. Planning movements in visual and physical space in monkey posterior parietal cortex. Cereb Cortex In press. [DOI] [PubMed] [Google Scholar]
- Leblois A, Boraud T, Meissner W, Bergman H, Hansel D. Competition between feedback loops underlies normal and pathological dynamics in the basal ganglia. J Neurosci 26: 3567–3583, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner A, Iyer A, Kagan I, Andersen RA. Human posterior parietal cortex plans where to reach and what to avoid. J Neurosci 30: 11715–11725, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Crawford JD, Vilis T. Integration of target and effector information in human posterior parietal cortex for the planning of action. J Neurophysiol 93: 954–962, 2005. [DOI] [PubMed] [Google Scholar]
- Monaco S, Cavina-Pratesi C, Sedda A, Fattori P, Galletti C, Culham JC. Functional magnetic resonance adaptation reveals the involvement of the dorsomedial stream in hand orientation for grasping. J Neurophysiol 106: 2248–2263, 2011. [DOI] [PubMed] [Google Scholar]
- Monaco S, Chen Y, Medendorp WP, Crawford JD, Fiehler K, Henriques DYP. Functional magnetic resonance imaging adaptation reveals the cortical networks for processing grasp-relevant object properties. Cereb Cortex 24: 1540–1554, 2014. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol 38: 871–908, 1975. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Condon L, Jackson SR. Parietal cortex coding of limb posture: in search of the body-schema. Neuropsychologia 48: 3228–3234, 2010. [DOI] [PubMed] [Google Scholar]
- Pellijeff A, Bonilha L, Morgan PS, McKenzie K, Jackson SR. Parietal updating of limb posture: an event-related fMRI study. Neuropsychologia 44: 2685–2690, 2006. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672, 2001. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Kourtis D, Nazarpour K. Simultaneous preparation of multiple potential movements: opposing effects of spatial proximity mediated by premotor and parietal cortex. J Neurophysiol 102: 2084–95, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado J, Clavagnier S, Otzenberger H, Scheiber C, Kennedy H, Perenin MT. Two cortical systems for reaching in central and peripheral vision. Neuron 48: 849–858, 2005. [DOI] [PubMed] [Google Scholar]
- Rawle CJ, Miall RC, Praamstra P. Frontoparietal theta activity supports behavioral decisions in movement-target selection. Front Hum Neurosci 6: 1–11, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast Robust Automated Brain Extraction. Hum Brain Mapp 17: 143–155, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23: S208–S219, 2004. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature 386: 167–170, 1997. [DOI] [PubMed] [Google Scholar]
- Tanné-Gariépy J, Rouiller EM, Boussaoud D. Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res 145: 91–103, 2002. [DOI] [PubMed] [Google Scholar]
- Tomassini V, Jbabdi S, Klein JC, Behrens TEJ, Pozzilli C, Matthews PM, Rushworth MFS, Johansen-Berg H. Diffusion-weighted imaging tractography-based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomical and functional specializations. J Neurosci 27: 10259–10269, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G. Beta-band activity during motor planning reflects response uncertainty. J Neurosci 30: 11270–11277, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesia M, Crawford JD. Specialization of reach function in human posterior parietal cortex. Exp Brain Res 221: 1–18, 2012. [DOI] [PubMed] [Google Scholar]
- Westendorff S, Klaes C, Gail A. The cortical timeline for deciding on reach motor goals. J Neurosci 30: 5426–5436, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci 20: 25–42, 1997. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using bayesian inference. Neuroimage 21: 1732–1747, 2004. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73, 1996. [DOI] [PubMed] [Google Scholar]
- Yttri EA, Wang C, Liu Y, Snyder LH. The parietal reach region is limb specific and not involved in eye-hand coordination. J Neurophysiol 111: 520–532, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]