Abstract
Hypercapnia-induced arousal from sleep is an important protective mechanism pertinent to a number of diseases. Most notably among these are the sudden infant death syndrome, obstructive sleep apnea and sudden unexpected death in epilepsy. Serotonin (5-HT) plays a significant role in hypercapnia-induced arousal. The mechanism of 5-HT's role in this protective response is unknown. Here we sought to identify the specific 5-HT receptor subtype(s) involved in this response. Wild-type mice were pretreated with antagonists against 5-HT receptor subtypes, as well as antagonists against adrenergic, cholinergic, histaminergic, dopaminergic, and orexinergic receptors before challenge with inspired CO2 or hypoxia. Antagonists of 5-HT2A receptors dose-dependently blocked CO2-induced arousal. The 5-HT2C receptor antagonist, RS-102221, and the 5-HT1A receptor agonist, 8-OH-DPAT, attenuated but did not completely block CO2-induced arousal. Blockade of non-5-HT receptors did not affect CO2-induced arousal. None of these drugs had any effect on hypoxia-induced arousal. 5-HT2 receptor agonists were given to mice in which 5-HT neurons had been genetically eliminated during embryonic life (Lmx1bf/f/p) and which are known to lack CO2-induced arousal. Application of agonists to 5-HT2A, but not 5-HT2C, receptors, dose-dependently restored CO2-induced arousal in these mice. These data identify the 5-HT2A receptor as an important mediator of CO2-induced arousal and suggest that, while 5-HT neurons can be independently activated to drive CO2-induced arousal, in the absence of 5-HT neurons and endogenous 5-HT, 5-HT receptor activation can act in a permissive fashion to facilitate CO2-induced arousal via another as yet unidentified chemosensor system.
Keywords: serotonin, hypercapnia, CO2, arousal, sleep, Lmx1b, mouse
sleep is a vital homeostatic function conserved across a wide range of species from nematodes and fruit flies to mice and men (Campbell and Tobler 1984; Zimmerman et al. 2008). While the reason that sleep is required remains elusive (Brown et al. 2012), without it rats die (Rechtschaffen et al. 1983), and humans have profound neuropsychological, physiological, and behavioral consequences (Killgore 2010). Organisms must strike a delicate balance between achieving adequate amounts of sleep to optimally function during the waking hours and maintaining safety during the sleeping hours by retaining the ability to respond (e.g., with arousal) to a variety of stimuli. One such arousal stimulus is hypercapnia (Berthon-Jones and Sullivan 1984; Phillipson et al. 1977). Hypercapnia encountered during sleep likely signifies a respiratory obstruction of some manner, such as is seen with obstructive sleep apnea (OSA) or from sleeping prone with the face in a pillow. Although hypercapnia has long been recognized as an arousal stimulus, the mechanisms by which it induces arousal are not well understood.
Serotonin (5-HT) neurons located in the rostral brain stem comprise a component of the ascending arousal system that is responsible for regulating sleep-wake transitions (Brown et al. 2012; Ito et al. 2013). 5-HT neurons, including those in this region, are chemosensitive (Richerson 2004; Severson et al. 2003). Mice with a genetic lesion of 5-HT neurons (Lmx1bf/f/p; Zhao et al. 2006) do not arouse from sleep in response to hypercapnia, but do arouse to other stimuli such as hypoxia, or auditory or tactile stimulation (Buchanan and Richerson 2010). Hypercapnia-induced arousal is thought to be an important factor in diseases such as OSA (Berthon-Jones and Sullivan 1984), panic disorder (Klein 1993), and sudden infant death syndrome (SIDS) (Kinney et al. 2009) and may also be important in sudden unexpected death in epilepsy (SUDEP) (Massey et al. 2014; Richerson and Buchanan 2011; Sowers et al. 2013). For instance, babies who die of SIDS have abnormalities in their brain stem 5-HT system (Kinney et al. 2009). A leading theory is that these abnormalities result in an inability to sense the CO2 that accumulates when a sleeping infant is in the prone position and, consequently, a lack of arousal (Kinney and Thach 2009). Understanding the specific serotonergic mechanisms involved in CO2-induced arousal will be important for treating and preventing these disorders (Richerson 2013). Here we used a system our laboratory has previously employed for assessing CO2-induced arousal in mice (Buchanan and Richerson 2010) to identify specific 5-HT receptor subtypes underlying CO2 arousal. We also probed for a role of involvement of other ascending arousal system components. Finally, we examined whether 5-HT receptor activation in Lmx1bf/f/p mice would be sufficient to recover the arousal response to CO2, which is known to otherwise be absent in these animals (Buchanan and Richerson 2010).
METHODS
Experimental animals.
All procedures and protocols were approved by the Institutional Animal Care and Use Committee at Yale University. Adult male wild-type (WT) (28–40 g) and Lmx1bf/f/p (24–35 g) littermate pairs and male C57BL/6J mice (26–36 g; Jackson Laboratories, Bar Harbor, ME) were housed in standard cages in a 12:12-h light-dark regimen with food and water available ad libitum. Generation, breeding, and genotyping (Hodges et al. 2009; Zhao et al. 2006) of Lmx1bf/f/p mice have been previously described. Briefly, females homozygous for floxed Lmx1b (Lmx1bf/f; phenotypically normal “WT”) were mated with males homozygous for floxed Lmx1b and hemizygous for ePet1-Cre (Lmx1bf/f;e-Pet1-Cre/+ or Lmx1bf/f/p) to produce progeny of these two genotypes in a slightly less than 1:1 ratio (Hodges et al. 2009). At the conclusion of all experimental trials, the animals were euthanized with an overdose of pentobarbital sodium (150 mg/kg ip).
EEG/EMG headmount and activity/temperature telemeter implants.
EEG/EMG headmounts (8201; Pinnacle Technology; Lawrence, KS) and temperature/activity telemeters (G2 E-Mitter; Mini-Mitter, Bend, OR) were implanted as previously described (Buchanan and Richerson 2010). Briefly, under ketamine (100 mg/kg ip) and xylazine (10 mg/kg ip) anesthesia, the skull was exposed, and the headmount was attached to the skull with two 0.1-in. (anterior) and two 0.125-in. (posterior) stainless steel machine screws (3/64 in. diameter; Pinnacle Technologies, Lawrence, KS). EMG leads emanating from the posterior portion of the headmount were sutured into nuchal muscles bilaterally 3 mm from midline. The base of the headmount, screw heads and EMG leads were anchored with dental acrylic (Jet Acrylic; Lang Dental, Wheeling, IL), and the skin sutured closed leaving only the headmount socket exposed. Telemeters (G2 E-mitter; Mini-Mitter) were implanted intraperitoneally as previously described (Buchanan and Richerson 2010). Animals received pre- and postoperative analgesia with meloxicam (0.3 mg/kg ip pre-op; 0.05 mg·kg−1·d−1 post-op in drinking water for 7 days) and were allowed to recover for at least 10 days before being studied.
EEG/EMG data acquisition.
Animals were fit with a preamplifier (8202-SL; Pinnacle Technology) attached directly to the implanted headmount, introduced to the recording chamber, and allowed to acclimate as described below. EEG and EMG leads were then passed through a tether and commutator (8204; Pinnacle Technology) and into an analog conditioning amplifier (model 440 Instrumentation Amplifier; Brownlee Precision, San Jose, CA). Data were digitized with an analog-to-digital converter (PCI-6221 or NI-USB-6009; National Instruments, Austin, TX) in a Dell desktop computer and acquired using software custom written in MATLAB (Mathworks, Natick, MA). EEG signals were amplified by 50,000 and band-pass filtered from 0.3 to 200 Hz. EMG signals were amplified by 50,000 and band-pass filtered from 10 to 1000 Hz. All signals were digitized at 1,000 samples per second. Body temperature and activity were captured by a telemetry receiver (ER-4000, Energizer Receiver; Mini-Mitter) situated directly beneath the recording chamber, transmitted to the computer via a serial port, and sampled every 10 s with the same custom software.
EEG/EMG analysis and sleep state determination.
Data were manually scored in 10-s epochs as waking (W), non-rapid eye movement sleep (NREM) or rapid eye movement sleep (REM) using software custom written in MATLAB. Vigilance state was assigned using a standard approach (Franken et al. 1998) based on the EEG/EMG frequency characteristics as follows: W, low-amplitude, high-frequency (7–13 Hz) EEG with high EMG power; NREM, high-amplitude, low-frequency (0.5–4 Hz) EEG with moderate to low EMG power and lack of voluntary motor activity; REM, moderate-amplitude, moderate-frequency (4.5–8 Hz) EEG with minimal EMG power except for brief bursts and minimal activity correlating with EMG bursts. Fast Fourier transform power spectra were created with MATLAB for each 10-s epoch of data and used along with EEG and EMG characteristics to verify scoring.
Plethysmography.
For quantification of ventilation, the recording chamber was fit with an ultra-low-pressure/high-sensitivity pressure transducer (DC002NDR5; Honeywell International, Minneapolis, MN). The analog output from the pressure transducer was digitized by an A/D converter (PCI-6221; National Instruments), displayed on a computer monitor in real time using an acquisition program custom written in MATLAB and saved on a computer hard drive. The signal was calibrated by delivering metered breaths (300 μl; 150 breaths/min) via a mechanical ventilator (Mini-Vent; Harvard Apparatus) to the recording chamber. Breathing parameters including respiratory rate (RR), tidal volume and minute ventilation (V̇e) were assessed with software custom written in MATLAB using previously described methods (Hodges et al. 2008).
Hypercapnic/hypoxic challenges.
Animals were placed into a 350-cm3 cast acrylic recording chamber equipped with bedding, food, and water and allowed to acclimate for at least 1 h on 3 consecutive days prior to the actual experimental trial. The gas within the chamber at baseline was room air (RA; 21% O2, 79% N2) and was changed to hypercapnia (7% CO2 with 21% O2 and 72% N2; 10 min) or hypoxia (5% O2, 95% N2; 2 min or 10% O2, 90% N2; 10 min), as described previously (Buchanan and Richerson 2010). After a baseline recording period in RA for at least 60 min, animals received an injection (intraperitoneal or subcutaneous) of test substance or vehicle. At least 30 min after the injection, the gas in the chamber was changed to either hypercapnia or hypoxia. At least 20 min after the end of the first gas challenge, the animals were challenged with the other test gas. One-half of the animals were randomly exposed to hypercapnia first, and one-half were exposed to hypoxia first. The gas was changed back to RA between test gas exposures. Each test gas exposure was initiated after mice were noted to be asleep, as determined by real-time observation of the EEG/EMG recording, eye closure, and absence of motor activity using frequency and amplitude parameters, as described above. It should be noted that systemic application of many 5-HT agonists and antagonists acutely alter sleep architecture; however, animals are able to fall back asleep after drug treatment (Monti et al. 1990), and thus we were able to conduct our studies with the animals asleep. Flow rates were maintained at 710–760 ml/min with a flow meter (WU-32446-33; Cole-Parmer, Hoffman Estates, IL). All compressed gas containers were obtained from Airgas East (Cheshire, CT). To determine if the responses could be due to auditory, olfactory or tactile (pressure) cues that might be associated with a gas change, control experiments were performed by changing the gas line from one delivering RA to another delivering RA.
Drugs.
Atropine (Hentschke et al. 2007), DOI hydrochloride (Monti et al. 1990), ketanserin tartrate (Kirov and Moyanova 1998), Ro4368554 (Monti and Jantos 2011), and SB-269970 hydrobromide (Westrich et al. 2013) were obtained from Sigma-Aldrich (St. Louis, MO). Cimetidine (Monti et al. 1986), diphenhydramine hydrochloride (Monti et al. 1986), GR-125487 (Galeotti et al. 1998), MDL 11,939 (Dudley et al. 1988), methysergide maleate (Ishida et al. 2007), MK-212 hydrochloride (Hemrick-Luecke and Fuller 1996), nicergoline (Ogawa et al. 1993), ondansentron hydrochloride (Ye et al. 2001), propranolol hydrochloride (Zarrindast et al. 2004), RS-102221 hydrochloride (Bonhaus et al. 1998), SB-699551 (Thomas et al. 2006), SKF83566 hydrobromide (Meyer et al. 1993), (S)-(-)-sulpiride (Dimpfel 2008), TCB-2 (Fox et al. 2010), TCS-1102 (Winrow et al. 2010), thioperamide (Monti et al. 1991), and 8-OH-DPAT hydrobromide (Yoshitake and Kehr 2004) were obtained from Tocris Bioscience (Ellisville, MO). Cimetidine (10 mg/kg ip), diphenhydamine (10 mg/kg ip), DOI (0.3–3 mg/kg ip), GR-125487 (1–30 mg/kg ip), methysergide (0.3–10 mg/kg ip), MK-212 (3–30 mg/kg ip), ondansetron (0.3–10 mg/kg ip), propranolol (10 mg/kg ip), SB-269970 (0.3–10 mg/kg ip), SKF83566 (1 mg/kg ip), TCB-2 (0.3–10 mg/kg ip), and 8-OH-DPAT (0.3–3 mg/kg ip) were dissolved in double-distilled H2O; atropine (10 mg/kg sc), ketanserin (0.1–3 mg/kg ip), MDL 11,939 (0.3–10 mg/kg ip), sulpiride (10 mg/kg ip), thioperamide (4 mg/kg sc), and TCS-1102 (40 mg/kg sc) were dissolved in ethanol; nicergoline (1 mg/kg ip), Ro4368554 (0.3–10 mg/kg ip), RS-102221 (0.3–10 mg/kg ip), and SB-699551 (1–30 mg/kg ip) were dissolved in dimethyl sulfoxide. Drugs were diluted to the appropriate concentration with saline (0.9% NaCl) to ensure all injection volumes were 100 μl. Vehicle treatments consisted of 100-μl injections of saline, 10% ethanol in saline, or 1% dimethyl sulfoxide in saline as appropriate. All drugs tested have been previously shown to affect the central nervous system (CNS) when applied systemically via intraperitoneal or subcutaneous injections in rodents (see cited references listed for each drug above).
Statistical analysis.
Interactions between genotype, gas composition, drug, and vigilance state were analyzed for all physiological variables using two-way ANOVA, paired t-test, or two-tailed t-test assuming unequal variance as appropriate. The significance threshold was P < 0.05 for all conditions. Analyses were accomplished using Microsoft Excel (Redmond, WA), OriginPro 9.0 (OriginLab, Northampton, MA), and Systat 11.0.
RESULTS
Antagonists of 5-HT2 receptor subtypes blocked CO2-induced arousal in WT animals.
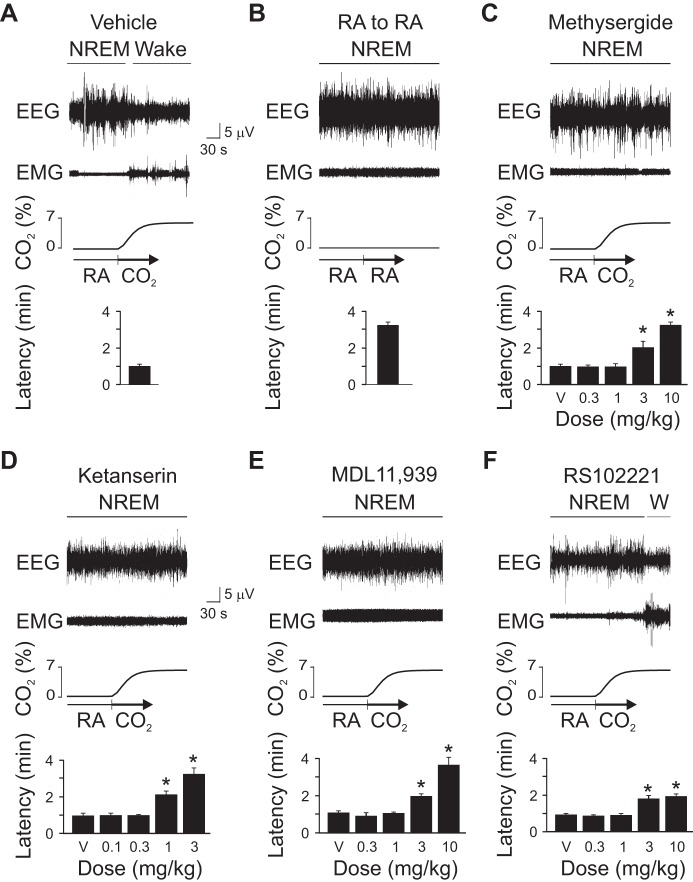
Consistent with what we have shown previously (Buchanan and Richerson 2010), 7% CO2 by itself (data not shown) or 30 min after vehicle injection rapidly induced arousal from sleep (Fig. 1A). Note that mice normally cycle rapidly between sleep states, so a latency of only 3–5 min from sleep onset to arousal is expected in a control mouse (Buchanan and Richerson 2010). Changing gas flow from one RA tank to another had no effect on sleep state (Fig. 1B). The nonspecific 5-HT receptor antagonist, methysergide; the nonselective 5-HT2 receptor antagonist, ketanserin; and the 5-HT2A selective antagonist, MDL 11,939 all dose-dependently blocked CO2-induced arousal (Fig. 1, C–E). At higher doses, the 5-HT2C selective antagonist, RS-102221, attenuated but did not completely block CO2-induced arousal (Fig. 1F). Note that the representative traces in each panel of these figures are at the highest dose.
Fig. 1.
Serotonin (5-HT) 2A receptor blockade prevented CO2-induced arousal in wild-type (WT) mice. Five-minute EEG, EMG and chamber CO2 concentration ([CO2]) data traces depict arousal, or lack of arousal, following a 7% CO2 (A) or room air (RA; B) challenge preceded by vehicle (V) injection, or CO2 challenge preceded by V or several different doses of methysergide (C), ketanserin (D), MDL 11,939 (E), or RS-102221 (F). In each panel, the top three traces are EEG, EMG, and chamber [CO2] traces, respectively, followed by a bar graph depicting latency to arousal. Values are means ± SE; n = 6 for all conditions. *P < 0.05 compared with V. W, wake; NREM, non-rapid eye movement sleep.
Blockade of 5-HT3, 5-HT4, 5-HT5, 5-HT6 or 5-HT7 receptors had no effect on CO2-induced arousal.
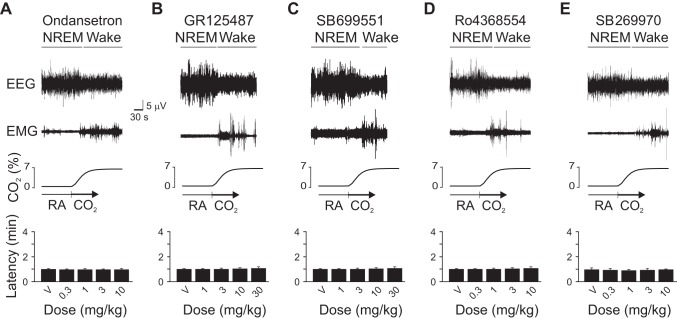
Given that other 5-HT receptors are also present in the thalamus and cortex and have been implicated in sleep-wake regulation (Monti 2011; Volk et al. 2010), we tested whether blockade of any of these receptor subtypes could impair CO2-induced arousal. The 5-HT3 receptor antagonist, ondansetron; the 5-HT4 receptor antagonist, GR-125487; the 5-HT5 receptor antagonist, SB-699551; the 5-HT6 receptor antagonist, Ro4368554; and the 5-HT7 receptor antagonist SB-269970 had no effect on CO2-induced arousal (Fig. 2). Doses for each drug were within or above the dose range used in the literature to have effects on a variety of behavioral and physiological measures when applied systemically (see references in methods).
Fig. 2.
5-HT3, 5-HT4, 5-HT5, 5-HT6 or 5-HT7 receptor blockade did not affect CO2-induced arousal latency. Data are presented as in Fig. 1. Latency to arousal following CO2 challenge preceded by V or several doses of ondansetron (A), GR-125487 (B), SB-699551 (C), Ro4368554 (D) or SB-269970 (E) are shown. Values are means ± SE; n = 6 for all conditions.
Antagonists of 5-HT receptors have no effect on hypoxia-induced arousal.
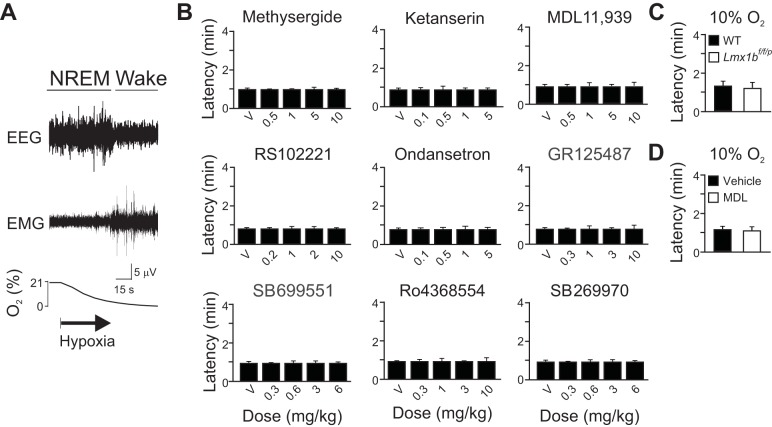
To determine whether the effects of 5-HT receptor blockade were specific for hypercapnia-induced arousal, we tested all of the drugs against hypoxia-induced arousal, which we previously showed to be unaffected by genetic deletion of 5-HT neurons in Lmx1bf/f/p mice (Buchanan and Richerson 2010). Blockade of 5-HT receptors with the same pharmacological agents tested above did not affect hypoxia-induced arousal at any of the doses tested (Fig. 3, A and B).
Fig. 3.
5-HT receptor blockade had no effect on hypoxia-induced arousal. A: 90-s EEG, EMG, and chamber [O2] traces depicting arousal from sleep after [O2] within the chamber has reached 8–9% O2. B: bar graphs depicting lack of effect of any concentration of any of the drugs (as labeled on graphs) on latency to hypoxia-induced arousal. Values are means ± SE; n = 6 for each condition. C: latency to arousal following 10% O2 challenge in WT (solid bar) and Lmx1bf/f/p (open bar) mice. Values are means ± SE; n = 6 for each genotype. D: bar graph depicting lack of effect of V (solid bar) or MDL 11,939 (10 mg/kg ip; open bar) on latency to arousal following 10% O2 challenge in WT mice. Values are means ± SE; n = 5 for each condition.
To determine whether 5-HT might be involved in arousal to less severe hypoxia, the arousal response to 10% O2 was tested in a separate set of WT and Lmx1bf/f/p mice. Both genotypes aroused equally well to the less severe hypoxic stimulus (Fig. 3C). Similarly, to determine whether 5-HT2A receptor blockade would be sufficient to prevent arousal to the less severe hypoxic stimulus, five WT mice were challenged with 10% O2 following treatment with MDL 11,939 (10 mg/kg ip). Blockade of 5-HT2A receptors in this manner had no effect on the latency to arousal to 10% O2 (Fig. 3D).
Activation of 5-HT1A receptors attenuates arousal to CO2, but not to hypoxia.
The 5-HT1A receptor is an inhibitory somato-dendritic autoreceptor on 5-HT neurons as well as on postsynaptic neurons. When activated, these receptors hyperpolarize and thereby inhibit 5-HT neuron firing (Verge et al. 1985). Activation of 5-HT1A receptors with 8-OH-DPAT attenuated but did not completely block CO2-induced arousal (Fig. 4). At the doses tested, 8-OH-DPAT had no effect on hypoxia-induced arousal (Fig. 4C).
Fig. 4.
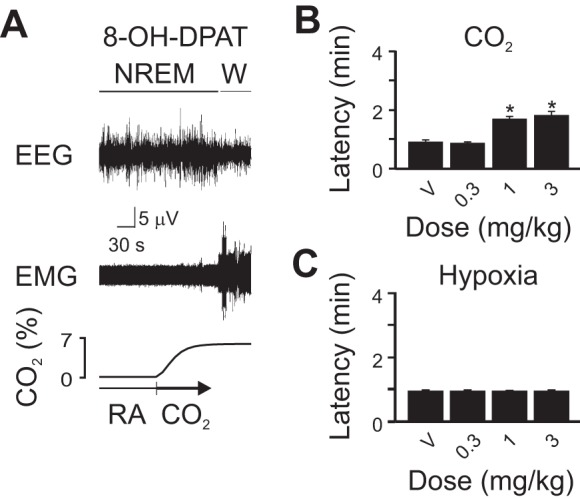
5-HT1A receptor activation attenuated, but did not completely block, CO2-induced arousal and had no effect on hypoxia-induced arousal. A: 5-min EEG, EMG, and chamber [CO2] traces depicting arousal from sleep after [CO2] within the chamber has reached a plateau. B and C: bar graphs depicting latency to arousal to CO2 (B) and hypoxia (C) following V or several doses of 8-OH-DPAT. Values are means ± SE; n = 6 for each condition. *P < 0.05 compared with V.
The pharmacological profile for CO2-induced arousal is the same in C57BL/6J and phenotypically WT Lmx1bf/f mice.
The phenotypically WT Lmx1bf/f mice tested above are littermates of the genetically 5-HT neuron-deficient mice and therefore carry two copies of the loxP-flanked Lmx1b gene (Zhao et al. 2006). To control for possible effects of these transgenes, C57BL/6J mice were tested for their ability to arouse from sleep in response to hypercapnia and hypoxia. C57BL/6J mice were aroused from sleep in response to both hypercapnia and hypoxia with similar latencies (0.99 ± 0.37 min for hypercapnia; 0.90 ± 0.28 min for hypoxia; n = 6 per condition). Vehicle pretreatment had no significant effect on arousal latencies (1.01 ± 0.41 min for hypoxia; 0.92 ± 0.35 min for hypercapnia; n = 6 per condition). MDL 11,939 (1 and 10 mg/kg) blocked CO2-induced arousal (latency 3.86 ± 0.39 min with 1 mg/kg; 4.03 ± 0.46 min with 10 mg/kg; n = 6 per dose). RS-102221 (10 mg/kg) and 8-OH-DPAT (2 mg/kg) attenuated, but did not completely block CO2-induced arousal (latency 2.25 ± 0.29 min with RS-102221; 2.01 ± 0.30 min with 8-OH-DAT; n = 6 per drug). Ondansetron (5 mg/kg) had no effect on CO2-induced arousal latency (0.94 ± 0.42 min; n = 6). None of these drugs had any effect on hypoxia-induced arousal at the doses tested (MDL 11,939, 10 mg/kg: 0.88 ± 0.25 min; RS-102221: 0.84 ± 0.34 min; 8-OH-DPAT: 0.95 ± 0.32 min; ondansetron: 0.91 ± 0.43 min; n = 6 per drug).
Antagonists of other neurotransmitters of the arousal system had no effect on CO2- or hypoxia-induced arousal.
Given that there are a number of other neurotransmitters that have been implicated in sleep-wake regulation (Saper et al. 2010) and neurons that contain and release some of these neurotransmitters have been reported to be chemosensitive (Pineda and Aghajanian 1997; Williams et al. 2007), we examined whether systemic blockade of receptors for any of these neurotransmitters could prevent CO2- or hypoxia-induced arousal. Antagonists of receptors for norepinephrine (nicergoline, propranolol), muscarine (atropine), histamine (diphenhydramine, cimetidine, thioperamide), dopamine (SKF83566, sulpiride), or orexin (TCS-1102) had no effect on CO2- or hypoxia-induced arousal (Fig. 5). α2-Receptors were not specifically targeted; however, the 5-HT7 antagonist SB-269970 also has α2 activity (Westrich et al. 2013) and had no effect on CO2- or hypoxia-induced arousal, also arguing against a major role for α2 involvement.
Fig. 5.
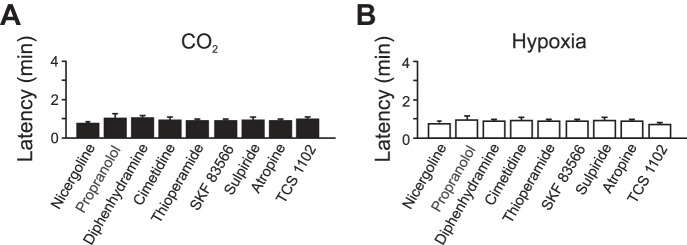
Blockade of non-5-HT receptors had no effect on CO2- or hypoxia-induced arousal. Bar graphs depict latency to arousal to CO2 (A) or hypoxia (B) following systemic application of (in mg/kg) 1 nicergoline, 10 propranolol, 10 diphenhydramine, 10 cimetidine, 4 thioperamide, 1 SKF83566, 10 sulpiride, 10 atropine, and 40 TCS-1102. Values are means ± SE; n = 6 for each condition.
5-HT2A receptor activation restores CO2-induced arousal in Lmx1bf/f/p mice.
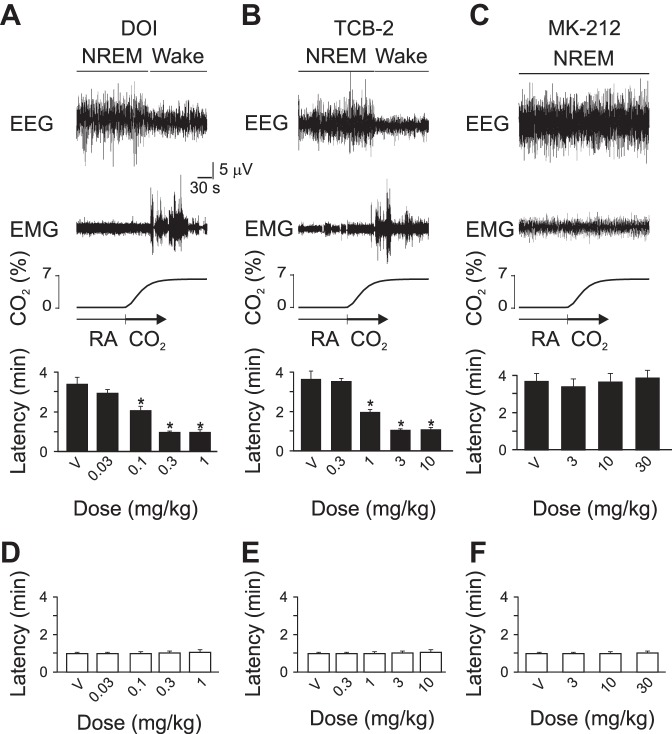
Lmx1bf/f/p mice have an impaired hypercapnic ventilatory response (HCVR) (Hodges et al. 2008) and lack the ability to arouse from sleep in response to CO2 (Buchanan and Richerson 2010). Direct activation of 5-HT receptors by intracerebroventricular application of 5-HT can restore the HCVR in Lmx1bf/f/p mice (Hodges et al. 2008). To test whether 5-HT agonists can recover hypercapnia-induced arousal, we pretreated Lmx1bf/f/p mice with the 5-HT2A/2C selective agonist DOI, the 5-HT2A selective agonist TCB-2, or the 5-HT2C agonist MK-212 prior to exposure to 7% CO2 or hypoxia. DOI and TCB-2 recovered CO2-induced arousal in a dose-dependent fashion. MK-212 had no effect on CO2-induced arousal (Fig. 6, A–C). None of these drugs had any effect on the arousal response to hypoxia (Fig. 6, D–F). Application of DOI (1 mg/kg ip) or TCB-2 (10 mg/kg ip) to WT mice had no effect on the latency for CO2-induced arousal (0.95 ± 0.27 min and 0.90 ± 0.38 min, respectively; n = 6) compared with vehicle (0.89 ± 0.33 min and 0.96 ± 0.40 min, respectively; n = 6). There was no significant change in the latency to arousal following the change from one RA tank to another after treatment with DOI (1 mg/kg ip; 3.21 ± 0.29 min; n = 6) or TCB-2 (10 mg/kg ip; 3.37 ± 0.35 min, respectively; n = 6) compared with vehicle (3.23 ± 0.46 min and 3.48 ± 0.43 min, respectively; n = 6).
Fig. 6.
Systemic application of 5-HT2A receptor agonists recovered CO2-induced arousal in 5-HT neuron-deficient mice. Five-minute EEG and EMG data traces are shown depicting arousal, or lack of arousal, following 7% CO2 challenge preceded by injection of V or of several different doses of DOI (A), TCB-2 (B) or MK-212 (C). Panel layout and abbreviations are as in Fig. 1. Values are means ± SE; n = 6 for all conditions. *P < 0.05 compared with V. D–F: bars graphs depicting lack of effect of any concentration of DOI (D), TCB-2 (E) or MK-212 (F) on latency to hypoxia-induced arousal.
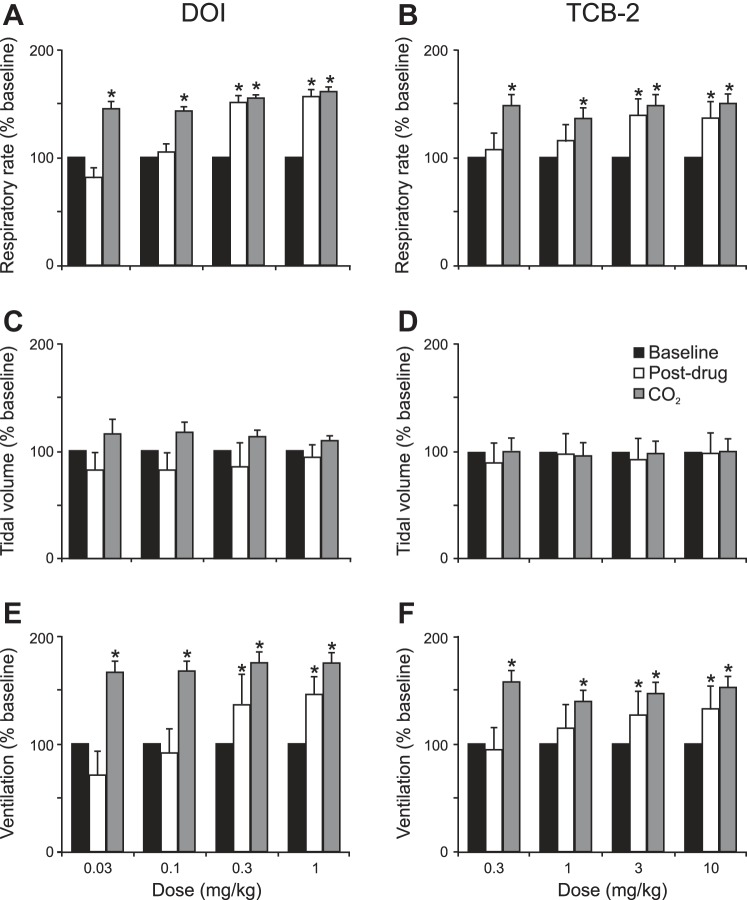
DOI and TCB-2 increased RR and V̇e in Lmx1bf/f/p mice, but had little effect on tidal volume (Fig. 7). Similar effects were seen in WT mice (data not shown). There was an increase in RR and V̇e following all CO2 challenges, whether or not there was arousal from the CO2 stimulation (Fig. 7). In Lmx1bf/f/p mice that did not arouse to CO2, there was still an increase in ventilation, similar to that which has been previously described (Hodges et al. 2008).
Fig. 7.
5-HT2A receptor agonists increase respiratory rate and minute ventilation without much effect on tidal volume in Lmx1bf/f/p mice. Bar graphs depict effect of DOI (left) and TCB-2 (right) on breathing frequency (A and B), tidal volume (C and D), and minute ventilation (E and F). Values are means ± SE; n = 6 for each drug and dose. All data are normalized to baseline. *P < 0.05 with respect to baseline.
DISCUSSION
Impairment of CO2-induced arousal contributes significantly to morbidity and mortality associated with OSA and SIDS and may be involved in SUDEP (Kinney et al. 2009; Sowers et al. 2013). The specific mechanisms underlying CO2-induced arousal are uncertain. The data presented here demonstrate that, under normal circumstances, CO2-induced arousal is mediated via the 5-HT2A receptor subtype. Blockade of other 5-HT receptors, or a number of nonserotonergic neurotransmitter receptors, had no effect on CO2-induced arousal at the doses tested. Activation of 5-HT2A receptors in the 5-HT neuron deficient Lmx1bf/f/p mice, which are known to lack CO2-induced arousal (Buchanan and Richerson 2010), was sufficient to recover the arousal response to CO2 in these animals, suggesting that CO2-induced arousal can be mediated via an alternative chemoreceptive system.
Receptor mechanisms in CO2-induced arousal.
Identification of a key role for 5-HT2A receptors in CO2-induced arousal is not surprising. 5-HT2A receptors are found in cortex, thalamus, and locus coeruleus among other sites (Pazos et al. 1985; Pazos and Palacios 1985) and are thought to play a prominent role in sleep-wake regulation with activation increasing wake probability and inactivation increasing slow-wave sleep (Kirov and Moyanova 1998; Monti and Jantos 2006). The thalamus and cortex are final common pathways for arousal; therefore, stimulation of 5-HT2A receptors in these sites could lead to arousal. Similarly, activation of 5-HT2A receptors on neurons in other sites known to contribute to sleep-wake regulation, such as the locus coeruleus, could cause subsequent activation of cortical and thalamic sites, leading to arousal. We screened for involvement of nonserotonergic transmitter systems by testing a single high dose of a number of non-5-HT receptor antagonists. We believe that, based on the literature, we tested sufficiently high doses that these drugs should have at least attenuated CO2-induced arousal if these receptors were prominently involved in the response. This does not eliminate the possibility that other systems may have a minor contribution that becomes more apparent under certain circumstances, such as in the absence of an intact 5-HT system. We did not specifically test this possibility in this study.
Administration of a 5-HT2C antagonist, RS-102221, attenuated but did not completely block CO2-induced arousal. 5-HT2C receptors are not as well distributed in cortical and thalamic sites as are 5-HT2A receptors; however, 5-HT2C receptors are abundantly expressed in limbic structures (Palacios et al. 1991). Thus the modest effect of RS-102221 may represent a limbic component of CO2-induced arousal. It may also reflect a small contribution of the 5-HT2C receptors that are found in cortical sites. Finally, RS-102221 is a weaker antagonist of 5-HT2A receptors as well (Barnes and Sharp 1999); therefore, the effect on CO2-induced arousal may simply be explained by the off-target effect on 5-HT2A receptors.
Along with 5-HT2A receptors, 5-HT3, 5-HT6, and 5-HT7 are found in thalamic and cortical loci and have been implicated in sleep-wake regulation (Monti 2011). However, blockade of these receptors, and of 5-HT4 and 5-HT5 receptors, had no effect on the arousal responses to hypercapnia or hypoxia. The doses tested in these experiments have been shown to have CNS effects in mice when applied systemically. It is intriguing that there is such a clear effect when 5-HT2A receptors are blocked, but not any effect upon blockade of all the other tested receptors (except maybe 5-HT2C). Not all neurons within the raphe are chemosensitive. It may be that the subset of raphe 5-HT neurons that are activated by acidosis (Richerson 2004) project to target areas with high density of 5-HT2A receptor expression, or activation of the other receptors is not sufficient to induce arousal from sleep.
Raphe subnuclei in CO2-induced arousal.
There are two major populations of 5-HT neurons in the CNS: a midbrain group (dorsal and median raphe) that projects primarily rostrally, and a medullary group (raphe magnus, pallidus, and obscuris) that projects primarily caudally. While subsets of 5-HT neurons in both the rostral (Severson et al. 2003; Veasey et al. 1997) and caudal (Bernard et al. 1996; Bradley et al. 2002; Wang et al. 2001) sites can sense changes in serum pH and modulate their firing frequency in response to these changes, it is primarily the rostral 5-HT neuronal groups that are associated with sleep-wake regulation (Saper et al. 2010). Therefore, we postulate that, under normal circumstances, stimulation of these groups is most likely to be responsible for causing arousal in response to inspired CO2. In this study, drugs were applied systemically and thus could also activate the caudal 5-HT neurons. A subset of these neurons are thought to be primarily responsible for modulating breathing in response to hypercapnia and not necessarily involved in CO2-gated sleep-wake modulation (Richerson 2004). Disruption of certain populations of medullary 5-HT neurons in piglets, for example in the raphe paragigantocellularis, alters sleep architecture and may play a role in hypoxia-induced arousal (Darnall et al. 2005).
Activation of medullary raphe 5-HT neurons rapidly causes an increase in ventilation (Depuy et al. 2011). This suggests a primary role of these neurons in regulating breathing and not simply a modulatory one, as might otherwise be expected for a monoaminergic mechanism. Activation of midbrain 5-HT neurons would be expected to similarly rapidly cause arousal.
Profound respiratory stimuli, such as those causing augmented breaths, can activate respiratory mechanoreceptors which can cause arousal (Gleeson et al. 1990). 5-HT2A receptors are present throughout respiratory networks, and 5-HT2A agonists increase breathing frequency and ventilation (Cayetanot et al. 2002). Hypercapnia is thought to cause arousal independent of its effect on breathing (Buchanan and Richerson 2010). In our study, increases in breathing in response to CO2 were observed even in animals that did not arouse to CO2. This suggests that, if the respiratory component contributes to arousal, the degree of respiratory enhancement caused by 7% CO2 is not sufficient to cause arousal. Our studies do not address whether arousal can occur without the breathing change, because the state change from NREM to wake is associated with increased breathing, making it difficult to separate these two intertwined responses. Mechanoreceptor activation may play a larger role in hypoxia-induced arousal, as hypoxia leads to a greater occurrence of augmented breaths and a greater degree of mechanoreceptor activation (Bell and Haouzi 2010).
Beyond 5-HT in CO2-induced arousal.
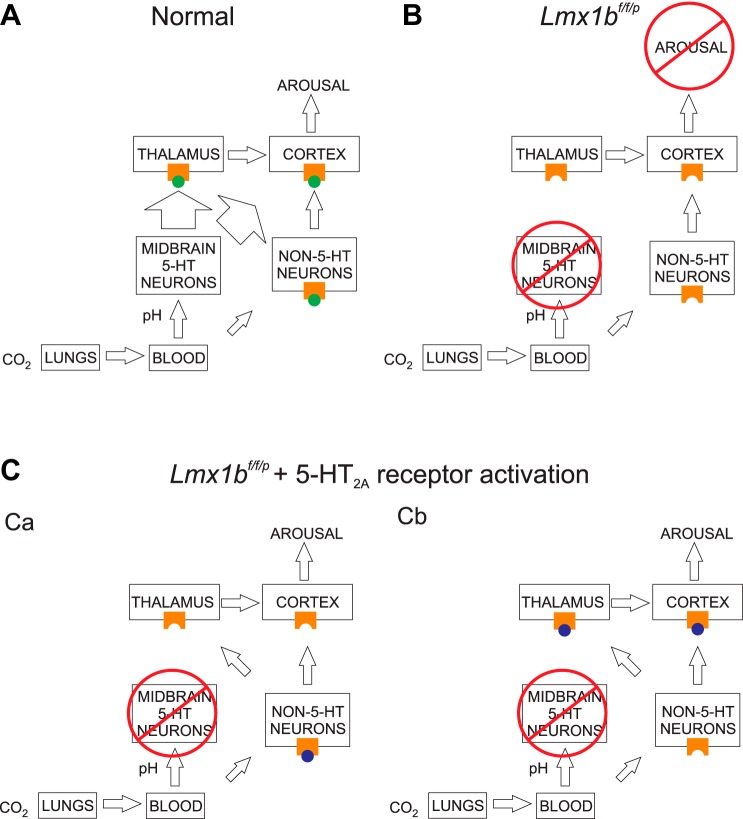
CO2-induced arousal could be recovered in Lmx1bf/f/p mice that do not have 5-HT neurons in the CNS by pretreatment with agonists of the 5-HT2A receptor (DOI and TCB-2), but not the 5-HT2C receptor (MK-212). A similar phenomenon was seen in these mice for the HCVR (Hodges et al. 2008). This adds a layer of complexity to our previous finding that CO2-induced arousal is lost in Lmx1bf/f/p mice. One plausible explanation is that activation of 5-HT2A receptors on some other nonserotonergic neurons enhances their CO2 sensitivity. At baseline (i.e., in the presence of normal 5-HT neurons and endogenous 5-HT), although the alternative CO2-sensing system is present and available to be activated, the effect of activation of 5-HT neurons by CO2 might be sufficiently robust that the effect of activation of the other chemosensors by CO2 is less important. In the absence of 5-HT neurons (and endogenous 5-HT), these neurons do not sense CO2 sufficiently to induce arousal from sleep (Buchanan and Richerson 2010); however, with 5-HT2A receptor activation such as by DOI or TCB-2, the mechanism for sensing CO2 is positively modulated, allowing those neurons to cause arousal from sleep (Fig. 8). A similar mechanism was proposed for the HCVR (Hodges et al. 2008). Such enhancement of chemosensitivity could occur via activation of 5-HT2A receptors directly on these alternate chemosensitive neurons, or on neurons within downstream target structures. DOI is known to alter sleep architecture (Monti and Jantos 2006). It is possible that the perceived recovery of CO2-induced arousal with DOI and TCB-2 in the Lmx1bf/f/p mice could simply have been a reflection of an increased wake probability caused by the 5-HT2A agonists. However, if this were the case, we would have expected to see a reduced arousal latency during the RA-to-RA control experiments. A final possibility is that the “alternate” chemosensor discussed here is the primary sensor but requires 5-HT and more specifically 5-HT2A receptor activation to mediate arousal to CO2.
Fig. 8.
Proposed model of CO2-induced arousal. A: under normal conditions, elevations in the concentration of CO2 inspired into the lungs are transmitted as increases in H+ ion concentration (decreases in pH) via the bloodstream to midbrain 5-HT neurons, thereby activating these neurons and causing release of 5-HT (green circles) onto arousal centers (presumably thalamic and cortical neurons), where it activates 5-HT2A receptors on these neurons to induce arousal from sleep. There is also lesser stimulation of non-5-HT chemosensitive neurons. B: in the absence of 5-HT neurons, there is no 5-HT release following decrease in pH, and no subsequent 5-HT2A receptor activation, no arousal center stimulation, and no arousal from sleep. Stimulation of non-5-HT neurons fails to induce arousal in the absence of 5-HT2A receptor activation. C: in the absence of 5-HT neurons, there is still activation of non-5-HT chemosensitive neurons. Ca: activation of 5-HT2A receptors on non-5HT neurons by exogenously applied 5-HT2A receptor agonists (e.g., DOI, TCB-2; purple circles) could enhance the chemosensitivity of these neurons and lead to arousal center stimulation. Cb: alternatively, 5-HT2A receptors could be activated directly at the arousal centers, and receptor binding permits stimulation by the non-5-HT neurons, leading to arousal.
There are a number of possibilities for which nonserotonergic neurons could have their chemosensitivity enhanced, either directly or indirectly, by 5-HT2A receptor activation. Other neuronal populations involved in sleep-wake regulation have chemoreceptive capabilities. These include noradrenergic neurons of the locus coeruleus (Pineda and Aghajanian 1997), histaminergic neurons of the tuberomammillary nucleus (Johnson et al. 2005), and orexinergic neurons in the lateral hypothalamus (Johnson et al. 2010, 2012; Williams et al. 2007). Another chemosensitive region, the retrotrapezoid nucleus, may be involved in sleep-wake regulation (Abbott et al. 2013; Guyenet and Abbott 2013). Glutamatergic neurons of the parabrachial nucleus have also been implicated in CO2-induced arousal (Kaur et al. 2013), although these neurons are not known to be intrinsically chemosensitive.
In this study, we tested a wide variety of antagonists against receptors for neurotransmitters that are released from the aforementioned putative chemoreceptive nuclei. None of these drugs had any effect on CO2-induced arousal at the doses tested. This may suggest that we did not target the appropriate transmitter systems. For instance, we used nicergoline, which has catecholaminergic activity beyond α1-adrenergic activity, and we did not test glutamatergic or GABAergic receptor antagonists, largely because systemic application of these agents would have widespread consequences. Given the possible role for glutamatergic inputs from regions such as retrotrapezoid nucleus and parabrachial nucleus, attempting to target contributions of these regions with a targeted approach will likely prove informative in the future. We relied on the literature to guide the doses we tested. Therefore, it is possible that we did not test high enough doses; however, we believe we should have at least seen some attenuation of the arousal response if these other neurotransmitter system played a prominent role.
Another possibility is that 5-HT2A receptor activation leads to increased excitability of nonserotonergic neurons. Finally, 5-HT2A receptor activation could cause upregulation of some other chemosensors, such as the ubiquitously expressed acid sensing ion channels. Although, under normal conditions acid sensing ion channels 1 and 2 are not involved in CO2-induced arousal or the HCVR (Price et al. 2014).
The ability to arouse from sleep in response to hypercapnia is an important protective reflex. This reflex may be lost in diseases such as SIDS (Kinney et al. 2009) and SUDEP (Richerson and Buchanan 2011) and may be important in OSA apnea (Berthon-Jones and Sullivan 1984; Kaur et al. 2013). In all of these diseases, hypoxia is encountered along with hypercapnia. The relative contributions of each of these aberrations to the morbidity and mortality associated with these diseases in unclear. 5-HT appears to be more prominently involved in CO2-induced arousal with little, if any, role in hypoxia-induced arousal in our hands. Understanding the specific mechanisms underlying CO2- and hypoxia-induced arousal may lead to pharmacotherapy that could be employed in susceptible individuals to reduce morbidity and mortality from these diseases.
GRANTS
This work was supported by National Institutes of Health Grants K08-NS-069667 (G. F. Buchanan), R01-HD-052772 (G. B. Richerson), P20-NS-076916 (G. B. Richerson); the Veterans Affairs Medical Center (G. B. Richerson); a Veterans Affairs Special Fellowship in Neuroscience (G. F. Buchanan); and the Beth & Nathan Tross Epilepsy Research Fund (G. F. Buchanan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.F.B. and G.B.R. conception and design of research; G.F.B., H.R.S., and A.M. performed experiments; G.F.B. and H.R.S. analyzed data; G.F.B. and G.B.R. interpreted results of experiments; G.F.B. prepared figures; G.F.B. drafted manuscript; G.F.B., H.R.S., and G.B.R. edited and revised manuscript; G.F.B. and G.B.R. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of H. R. Smith: Neuroscience Graduate Program, Albert Einstein College of Medicine, Bronx, NY 10461.
REFERENCES
- Abbott SB, Coates MB, Stornetta RL, Guyenet PG. Optogenetic stimulation of c1 and retrotrapezoid nucleus neurons causes sleep state-dependent cardiorespiratory stimulation and arousal in rats. Hypertension 61: 835–841, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083–1152, 1999. [DOI] [PubMed] [Google Scholar]
- Bell HJ, Haouzi P. The hypoxia-induced facilitation of augmented breaths is suppressed by the common effect of carbonic anhydrase inhibition. Respir Physiol Neurobiol 171: 201–211, 2010. [DOI] [PubMed] [Google Scholar]
- Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphe. J Appl Physiol 80: 108–115, 1996. [DOI] [PubMed] [Google Scholar]
- Berthon-Jones M, Sullivan CE. Ventilation and arousal responses to hypercapnia in normal sleeping humans. J Appl Physiol 57: 59–67, 1984. [DOI] [PubMed] [Google Scholar]
- Bonhaus D, Rocha C, Dawson M, Eglen R. Absorption and brain penetration of a high affinity, highly selective 5-HT2C receptor antagonist, RS-102221. Ann NY Acad Sci 861: 269, 1998. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci 5: 401–402, 2002. [DOI] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev 92: 1087–1187, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A 107: 16354–16359, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev 8: 269–300, 1984. [DOI] [PubMed] [Google Scholar]
- Cayetanot F, Gros F, Larnicol N. Postnatal changes in the respiratory response of the conscious rat to serotonin 2A/2C receptor activation are reflected in the developmental pattern of fos expression in the brainstem. Brain Res 942: 51–57, 2002. [DOI] [PubMed] [Google Scholar]
- Darnall RA, Harris MB, Gill WH, Hoffman JM, Brown JW, Niblock MM. Inhibition of serotonergic neurons in the nucleus paragigantocellularis lateralis fragments sleep and decreases rapid eye movement sleep in the piglet: implications for sudden infant death syndrome. J Neurosci 25: 8322–8332, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci 31: 1981–1990, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimpfel W. Pharmacological modulation of dopaminergic brain activity and its reflection in spectral frequencies of the rat electropharmacogram. Neuropsychobiology 58: 178–186, 2008. [DOI] [PubMed] [Google Scholar]
- Dudley M, Wiech N, Miller F, Carr A, Cheng H, Roebel L, Doherty N, Yamamura H, Ursillo R, Palfreyman M. Pharmacological effects of MDL 11,939: a selective, centrally acting antagonist of 5-HT2 receptors. Drug Dev Res 13: 29–43, 1988. [Google Scholar]
- Fox MA, French HT, LaPorte JL, Blackler AR, Murphy DL. The serotonin 5-HT(2A) receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology (Berl) 212: 13–23, 2010. [DOI] [PubMed] [Google Scholar]
- Franken P, Malafosse A, Tafti M. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol Regul Integr Comp Physiol 275: R1127–R1137, 1998. [DOI] [PubMed] [Google Scholar]
- Galeotti N, Ghelardini C, Bartolini A. Role of 5-HT4 receptors in the mouse passive avoidance test. J Pharmacol Exp Ther 286: 1115–1121, 1998. [PubMed] [Google Scholar]
- Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis 142: 295–300, 1990. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Abbott SB. Chemoreception and asphyxia-induced arousal. Respir Physiol Neurobiol 188: 333–343, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemrick-Luecke SK, Fuller RW. Involvement of 5-HT2A receptors in the elevation of rat serum corticosterone concentrations by quipazine and MK-212. Eur J Pharmacol 311: 207–211, 1996. [DOI] [PubMed] [Google Scholar]
- Hentschke H, Perkins MG, Pearce RA, Banks MI. Muscarinic blockade weakens interaction of gamma with theta rhythms in mouse hippocampus. Eur J Neurosci 26: 1642–1656, 2007. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28: 2495–2505, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci 29: 10341–10349, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Sato T, Irifune M, Tanaka K, Nakamura N, Nishikawa T. Effect of acetaminophen, a cyclooxygenase inhibitor, on Morris water maze task performance in mice. J Psychopharmacol 21: 757–767, 2007. [DOI] [PubMed] [Google Scholar]
- Ito H, Yanase M, Yamashita A, Kitabatake C, Hamada A, Suhara Y, Narita M, Ikegami D, Sakai H, Yamazaki M, Narita M. Analysis of sleep disorders under pain using an optogenetic tool: possible involvement of the activation of dorsal raphe nucleus-serotonergic neurons. Mol Brain 6: 59, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Moratalla R, Lightman SL, Lowry CA. Are tuberomammillary histaminergic neurons involved in CO2-mediated arousal? Exp Neurol 193: 228–233, 2005. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Lightman SL, Lowry CA, Shekhar A. Activation of the orexin 1 receptor is a critical component of CO2-mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacology 37: 1911–1922, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A. A key role for orexin in panic anxiety. Nat Med 16: 111–115, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, Saper CB. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J Neurosci 33: 7627–7640, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res 185: 105–129, 2010. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol 4: 517–550, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med 361: 795–805, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov R, Moyanova S. Age-dependent effect of ketanserin on the sleep-waking phases in rats. Int J Neurosci 93: 257–264, 1998. [DOI] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions: an integrative hypothesis. Arch Gen Psychiatry 50: 306–317, 1993. [DOI] [PubMed] [Google Scholar]
- Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol 10: 271–282, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer ME, Cottrell GA, Van HC, Potter TJ. Effects of dopamine D1 antagonists SCH23390 and SK&F83566 on locomotor activities in rats. Pharmacol Biochem Behav 44: 429–432, 1993. [DOI] [PubMed] [Google Scholar]
- Monti JM. Serotonin control of sleep-wake behavior. Sleep Med Rev 15: 269–281, 2011. [DOI] [PubMed] [Google Scholar]
- Monti JM, Jantos H. Effects of the serotonin 5-HT2A/2C receptor agonist DOI and of the selective 5-HT2A or 5-HT2C receptor antagonists EMD 281014 and SB-243213, respectively, on sleep and waking in the rat. Eur J Pharmacol 553: 163–170, 2006. [DOI] [PubMed] [Google Scholar]
- Monti JM, Jantos H. Effects of the 5-HT(6) receptor antagonists SB-399885 and RO-4368554 and of the 5-HT(2A) receptor antagonist EMD 281014 on sleep and wakefulness in the rat during both phases of the light-dark cycle. Behav Brain Res 216: 381–388, 2011. [DOI] [PubMed] [Google Scholar]
- Monti JM, Jantos H, Boussard M, Altier H, Orellana C, Olivera S. Effects of selective activation or blockade of the histamine H3 receptor on sleep and wakefulness. Eur J Pharmacol 205: 283–287, 1991. [DOI] [PubMed] [Google Scholar]
- Monti JM, Pellejero T, Jantos H. Effects of H1- and H2-histamine receptor agonists and antagonists on sleep and wakefulness in the rat. J Neural Transm 66: 1–11, 1986. [DOI] [PubMed] [Google Scholar]
- Monti JM, Pineyro G, Orellana C, Boussard M, Jantos H, Labraga P, Olivera S, Alvarino F. 5-HT receptor agonists 1-(2,5-dimethoxy-4-iodophenyl)−2-aminopropane (DOI) and 8-OH-DPAT increase wakefulness in the rat. Biogen Amine 7: 145–151, 1990. [Google Scholar]
- Ogawa N, Asanuma M, Hirata H, Kondo Y, Kawada Y, Mori A. Cholinergic deficits in aged rat brain are corrected with nicergoline. Arch Gerontol Geriatr 16: 103–110, 1993. [DOI] [PubMed] [Google Scholar]
- Palacios J, Waeber C, Mengod G, Hoyer D. Autoradiography of 5-HT receptors: a critical appraisal. Neurochem Int 18: 17–25, 1991. [DOI] [PubMed] [Google Scholar]
- Pazos A, Cortes R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res 346: 231–249, 1985. [DOI] [PubMed] [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res 346: 205–230, 1985. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Kozar LF, Rebuck AS, Murphy E. Ventilatory and waking responses to CO2 in sleeping dogs. Am Rev Respir Dis 115: 251–259, 1977. [DOI] [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience 77: 723–743, 1997. [DOI] [PubMed] [Google Scholar]
- Price MP, Gong H, Parsons MG, Kundert JR, Reznikov LR, Bernardinelli L, Chaloner K, Buchanan GF, Wemmie JA, Richerson GB, Cassell MD, Welsh MJ. Localization and behaviors in null mice suggest that ASIC1 and ASIC2 modulate responses to aversive stimuli. Genes Brain Behav 13: 179–194, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep deprivation in rats. Science 221: 182–184, 1983. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Buchanan GF. The serotonin axis: shared mechanisms in seizures, depression, and SUDEP. Epilepsia 52, Suppl 1: 28–38, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson G. Serotonin: the anti-suddendeathamine. Epilepsy Curr 13: 241–244, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron 68: 1023–1042, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci 6: 1139–1140, 2003. [DOI] [PubMed] [Google Scholar]
- Sowers LP, Massey CA, Gehlbach BK, Granner MA, Richerson GB. Sudden unexpected death in epilepsy: fatal post-ictal respiratory and arousal mechanisms. Respir Physiol Neurobiol 189: 315–323, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR, Soffin EM, Roberts C, Kew JN, de la Flor RM, Dawson LA, Fry VA, Coggon SA, Faedo S, Hayes PD, Corbett DF, Davies CH, Hagan JJ. SB-699551-A (3-cyclopentyl-N-[2-(dimethylamino)ethyl]-N-[(4′-{[(2-phenylethyl)amino]methyl}-4-biphenylyl)methyl]propanamide dihydrochloride), a novel 5-ht5A receptor-selective antagonist, enhances 5-HT neuronal function: evidence for an autoreceptor role for the 5-ht5A receptor in guinea pig brain. Neuropharmacology 51: 566–577, 2006. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neuroscience 79: 161–169, 1997. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Patey A, Gozlan H, el MS, Hamon M. Presynaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites but not terminals are of the 5-HT1A subtype. Eur J Pharmacol 113: 463–464, 1985. [DOI] [PubMed] [Google Scholar]
- Volk B, Nagy BJ, Vas S, Kostyalik D, Simig G, Bagdy G. Medicinal chemistry of 5-HT5A receptor ligands: a receptor subtype with unique therapeutical potential. Curr Top Med Chem 10: 554–578, 2010. [DOI] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol 85: 2224–2235, 2001. [DOI] [PubMed] [Google Scholar]
- Westrich L, Sprouse J, Sanchez C. The effects of combining serotonin reuptake inhibition and 5-HT7 receptor blockade on circadian rhythm regulation in rodents. Physiol Behav 110–111: 42–50, 2013. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A 104: 10685–10690, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winrow CJ, Tanis KQ, Reiss DR, Rigby AM, Uslaner JM, Uebele VN, Doran SM, Fox SV, Garson SL, Gotter AL, Levine DM, Roecker AJ, Coleman PJ, Koblan KS, Renger JJ. Orexin receptor antagonism prevents transcriptional and behavioral plasticity resulting from stimulant exposure. Neuropharmacology 58: 185–194, 2010. [DOI] [PubMed] [Google Scholar]
- Ye JH, Ponnudurai R, Schaefer R. Ondansetron: a selective 5-HT(3) receptor antagonist and its applications in CNS-related disorders. CNS Drug Rev 7: 199–213, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitake T, Kehr J. Differential effects of (R)-, (R, S)- and (S)-8-hydroxy-2-(di-n-propylamino)tetralin on hippocampal serotonin release and induction of hypothermia in awake rats. Life Sci 74: 2865–2875, 2004. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Haidari H, Jafari MR, Djahanguiri B. Influence of beta-adrenoceptor agonists and antagonists on baclofen-induced memory impairment in mice. Behav Pharmacol 15: 293–297, 2004. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RW, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci 26: 12781–12788, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JE, Naidoo N, Raizen DM, Pack AI. Conservation of sleep: insights from non-mammalian model systems. Trends Neurosci 31: 371–376, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]