Abstract
Gamma oscillations are generated in networks of inhibitory fast-spiking (FS) parvalbumin-positive (PV) interneurons and pyramidal cells. In animals, gamma frequency is modulated by the velocity of visual motion; the effect of velocity has not been evaluated in humans. In this work, we have studied velocity-related modulations of gamma frequency in children using MEG/EEG. We also investigated whether such modulations predict the prominence of the “spatial suppression” effect (Tadin D, Lappin JS, Gilroy LA, Blake R. Nature 424: 312-315, 2003) that is thought to depend on cortical center-surround inhibitory mechanisms. MEG/EEG was recorded in 27 normal boys aged 8–15 yr while they watched high-contrast black-and-white annular gratings drifting with velocities of 1.2, 3.6, and 6.0°/s and performed a simple detection task. The spatial suppression effect was assessed in a separate psychophysical experiment. MEG gamma oscillation frequency increased while power decreased with increasing velocity of visual motion. In EEG, the effects were less reliable. The frequencies of the velocity-specific gamma peaks were 64.9, 74.8, and 87.1 Hz for the slow, medium, and fast motions, respectively. The frequency of the gamma response elicited during slow and medium velocity of visual motion decreased with subject age, whereas the range of gamma frequency modulation by velocity increased with age. The frequency modulation range predicted spatial suppression even after controlling for the effect of age. We suggest that the modulation of the MEG gamma frequency by velocity of visual motion reflects excitability of cortical inhibitory circuits and can be used to investigate their normal and pathological development in the human brain.
Keywords: magnetoencephalography, visual cortex, frequency of gamma oscillations, electroencephalography, spatial suppression
neural gamma oscillations are generated in excitatory-inhibitory neural circuits and mutually connected inhibitory interneuron networks. These oscillations depend crucially on inhibitory GABA pathways, with fast-spiking (FS) parvalbumin-expressing (PV) interneurons playing a central role (Cardin et al. 2009; Carlen et al. 2012; Gulyas et al. 2010; Sohal et al. 2009; Volman et al. 2011). High-frequency gamma oscillations seem to be of particular interest for understanding various brain dysfunctions in neuropsychiatric disorders (Uhlhaas et al. 2011).
Gamma oscillations are reliably induced in the visual cortex of human subjects by drifting gratings (Hoogenboom et al. 2006; Muthukumaraswamy and Singh 2013; Rice et al. 2013). The peak frequency of magnetoencephalography (MEG)-recorded visually-induced gamma oscillations is an individually stable (Muthukumaraswamy et al. 2010) and highly genetically determined (van Pelt et al. 2012) trait. However, the neurophysiological mechanisms that control the frequency of gamma oscillations in human subjects have been intensely debated (Brunet et al. 2014; Cousijn et al. 2014; Schwarzkopf et al. 2012; Swettenham et al. 2009).
Some insight into the neural basis of gamma frequency control is coming from recent animal studies. It has been shown that gamma frequency is affected by genetic and pharmacological manipulations of the excitability of FS PV interneuron (Anver et al. 2011; Ferando and Mody 2015; Mann and Mody 2010). In particular, Mann and Mody (2010) found that the frequency of hippocampal gamma oscillations can be modulated through altering the balance between tonic excitation of inhibitory interneurons through the specific NR2D subunit-containing N-methyl-d-aspartate receptors (NMDARs) and tonic inhibition of these interneurons through δ-GABAa receptors.
Changes to the properties of sensory stimulation also may affect gamma frequency. Gray and Di Prisco (1997) observed a substantial increase in visual gamma oscillation frequency with increasing velocity of motion of high-contrast gratings in cats. These velocity-related modulations of gamma frequency might reflect the intensity of the excitatory drive to FS PV interneurons and provide valuable information on FS PV dysfunction in brain disorders. However, the effect of motion velocity on gamma frequency has not been tested in human subjects to date.
In the present study, we aimed to investigate the frequency modulation of gamma oscillations in typically developing children using visual gratings that move with a range of velocities close to those previously used in animal studies (1.2–6.0°/s). We expected to find an increase in gamma frequency with increasing stimulus velocity similar to that found for the local field potential (LFP) in animals (Gray and DiPrisco 1997). If present in humans, the velocity-related changes in gamma frequency might be less affected by morphological differences between individuals and more sensitive to functional aspects of inhibitory circuitry than the gamma frequency measured in response to a single type of stimulation.
Apart from controlled experimental manipulations, the frequency of visual gamma oscillations in pediatric populations may be affected by development of GABAergic inhibitory circuitry that continues up to early adulthood (Le Magueresse and Monyer 2013). Indeed, synaptic elimination proceeds beyond adolescence into the third decade of life and occurs predominantly in supragranular cortical layers that are thought to be the main source of gamma oscillations (reviewed in Cho et al. 2015; also see Oke et al. 2010). Investigations of gamma oscillations in young participants may cast light on the typical development of inhibitory cortical circuits and their pathology in neurodevelopmental disorder. To assess maturational change in gamma frequency, we included children and adolescents aged 8 to 15 yr in this study.
The other aim of this study was to investigate whether interindividual variations in frequency characteristics of visual gamma oscillations have consequences for visual perception, particularly for the spatial suppression effect. It has been shown that it is normally more difficult to detect direction of motion of large (i.e., filling ≥5° of the visual field) rather than small (∼1° of the visual field) high-contrast visual stimuli (Foss-Feig et al. 2013; Tadin et al. 2003; Tadin et al. 2011). This difficulty with detecting of motion direction (the so-called spatial suppression effect) is thought to result from stronger surround inhibition with large high-contrast stimuli (Tadin et al. 2003; Tadin et al. 2011). It is likely that the interindividual differences in neuronal inhibition (and in gamma oscillation frequency) may affect the magnitude of this spatial suppression effect. Therefore, we have also tested for a putative link between gamma frequency parameters and the spatial suppression effect assessed in a separate psychophysical experiment as a part of this study.
METHODS
Subjects.
Twenty-seven boys (8–15 yr; mean age = 136.1 mo, SD = 23 mo) were recruited from local schools via advertisements. The criteria for inclusion were the absence of neurological abnormalities in the subject's medical history and normal or corrected to normal vision.
This study was approved by the local ethics committee of the Moscow University of Psychology and Education and was conducted following the ethical principles regarding human experimentation (Helsinki Declaration). Written informed consent was obtained from a parent/guardian of each child.
Experimental task.
Subjects sat in a magnetically shielded room (AK3b; Vacuumschmelze, Hanau, Germany), with the back of the head resting against the helmet-shaped surface of the helium dewar. The stimulus comprised a black and white annular sine wave grating with a spatial frequency of 1.66 circles per degree and a diameter of 18° of visual angle (Fig. 1A). The stimulus was generated using Presentation software (Neurobehavioral Systems) and projected to a screen located 1.1 m in front of the participants with a Panasonic PT-D7700E-K DLP projector. The moving image was presented with 1,280 × 1,024 screen resolution and a 60-Hz refresh rate. The grating appeared in the center of the screen over a black background and “contracted” to the central point at one of three velocities, 1.2, 3.6, or 6°/s, referred to below as “low,” “medium,” and “fast,” respectively. The frequency of black to white transitions at a given position in the visual field produced by these velocities was 4, 12, and 20 Hz, respectively. In the intervals between the stimuli, a small white fixation cross was presented on the black background in the center of the screen. The participants were instructed to constantly maintain their gaze at the fixation cross between stimuli.
Fig. 1.
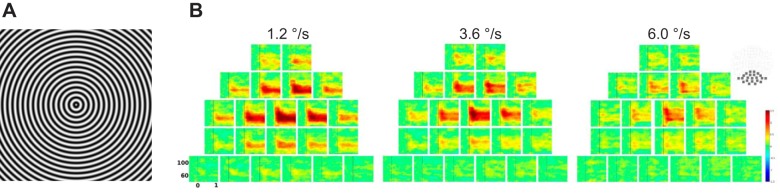
A: stimulus used in the study. B: grand average time-frequency plots of the baseline-normalized gamma power (in dB) in response to gratings moving with 3 velocities. A selection of posterior channels is shown at far right. Vertical lines mark stimulus onset. The most prominent gamma increase is observed at the middle occipital gradiometer sensor pair (MEG2112-MEG2113).
Each trial began with the presentation of the fixation cross for 1,200 ms, followed by the moving grating. The moving grating appeared for an interval that randomly varied between 1,200 and 3,000 ms. The stimulus then stopped moving and remained on the screen for an additional individually adjusted time (“response period:” ∼900 ms) during which the participant was instructed to detect the termination of the motion and respond by pressing a button as quickly as possible. If no response occurred within the response period, the grating was replaced with a discouraging message “too late!” that remained on the screen for 2,000 ms. The next trail began immediately after the button press or the display of the discouraging message. To minimize visual and mental fatigue, short (3–6 s) animated cartoon characters were presented during the rest period between every 2 and 5 grating stimuli.
The length of the response period was adjusted individually for each participant during a short training session that directly preceded the main experiment. During this training session, 18 stimuli (6 of each type) were presented and the mean reaction time and its standard deviation calculated. The individual response time for the main experiment was then set at the mean reaction time (RT) plus three standard deviations.
Individual RT and commission/omission errors were measured for all but one participant. The experiment included three blocks of trials; stimuli of different velocities were presented in all three blocks in a pseudorandom order. The participants responded with either the right or left hand in a sequence that was counterbalanced between blocks and participants. Each stimulus velocity was presented 30 times within each experimental block, resulting in 90 trials per stimulus velocity.
MEG and electroencephalography recording.
Although MEG is more sensitive to visual gamma oscillations than electroencephalography (EEG) (Muthukumaraswamy and Singh 2013), its application is limited in some groups of subjects (e.g., young children and individuals with low IQ). In the present study, we compared the effectiveness of MEG and EEG for detection of the effects of stimulus velocity on sustained visual gamma oscillations induced by moving gratings.
Neuromagnetic activity was recorded with a helmet-shaped 306-channel detector array (Vectorview; Neuromag, Helsinki, Finland) that comprised 102 identical triple sensor elements. Each sensor element consisted of three superconducting quantum interference devices, two with orthogonal planar gradiometer pickup coils and one with a magnetometer pickup coil configuration. In this study, the recordings from the 204 planar gradiometers were used for analyses. We also recorded EEG (in all but 1 subject) at the Fz, Pz, Oz, O1, and O2 positions referenced to the left earlobe. EEG impedance was kept below 15 kΩ. The Oz electrode (that demonstrated the highest power of gamma response on the group level) was the only one used for the EEG analysis.
Prior to the MEG session, the positions of a set of four HPI coils were digitized together with fiducial points using a Polhemus Fastrak 3D digitizer (Fastrak; Polhemus, Colchester, VT); the HPI coil positions were further used to track each subject's head position inside the helmet during recordings. The spatiotemporal signal space separation method method (Taulu et al. 2004) implemented by MaxFilter (Elekta Neuromag software) was used to suppress interference signals generated outside of the helmet/sensor array volume. Head movement compensation was used as well. The data were converted to standard head position (x = 0, y = 0, and z = 45 mm) across all experimental blocks.
Four electrooculogram (EOG) electrodes were used to record horizontal and vertical eye movements. EOG electrodes were placed at the outer canti of the eyes and above and below the left eye. EEG and EOG signals were recorded with a high-pass filter of 0.1 Hz. MEG, EEG, and EOG signals were recorded with a band-pass filter of 0.03–330 Hz, digitized at 1,000 Hz, and stored for off-line analysis.
MEG and EEG data preprocessing.
The correction of vertical eye movement artifacts was performed for the continuous MEG data with Brainstorm (http://neuroimage.usc.edu/brainstorm) (Tadel et al. 2011), using the SSP algorithm (Tesche et al. 1995; Uusitalo and Ilmoniemi 1997). The subsequent analyses were done using the SPM12 toolbox (http://www.fil.ion.ucl.ac.uk/spm) (Litvak et al. 2011). The pipeline was optimized for analysis of visual gamma oscillations through defining a “spatio-spectral” region of interest over the occipital cortex.
Data were epoched from −0.5 to 1.2 s relative to the stimulus onsets. The MEG and EEG epochs containing strong muscle artifacts were excluded by thresholding the mean absolute values of high-frequency signals. The threshold was set at five standard deviations from the amplitude of the 70-Hz high-passed signal averaged across channels. The remaining epochs were further visually inspected in SPM12, and the epochs that displayed longer than 100-ms episodes of MEG average absolute amplitude exceeding the baseline value approximately three times or more were also excluded from the analysis. The average number of artifact-free epochs per condition was 83 (range: 56–90).
For efficient spectral estimation from a relatively small number of trials, we used multitaper spectral analysis (Thomson 1982). This method is based on premultiplying the data with a series of tapers optimized for producing uncorrelated estimates of the spectrum in a given frequency band. This approach sacrifices some of the frequency resolution for an increase in signal-to-noise ratio. It does so by effectively multiplying the number of trials by the number of tapers used. We estimated the spectra in overlapping windows of 400 ms (shifted by 50 ms). The frequency resolution was set to the inverse of the time window (2.5 Hz) for ≤25 Hz, 0.1 times the frequency for 25–50 Hz. A constant 5-Hz resolution was used for 50–100 Hz; 7.5-Hz resolution was used for 102.5–120 Hz. These settings resulted in a single taper being used for 2.5–30 Hz, two tapers for 32.5–42.5 Hz, three tapers for 45–100 Hz, and four tapers for 102.5–120 Hz. The resulting time-frequency images had no discontinuities thanks to the continuous frequency resolution function.
The time-frequency data were averaged across epochs using a robust averaging procedure (Holland and Welsch 1977; Litvak et al. 2011). To reduce intersubject variability and to normalize power changes across different frequency bands, the averaged power was log transformed and baseline corrected using a −500- to −100-ms interval before stimulus onset as the baseline (LogR option in SPM); this resulted in power changes in dB relative to baseline. Planar MEG gradiometer channels were then combined by adding time-frequency data for pairs of channels corresponding to orthogonal sensors at the same location. Based on previous neuroimaging studies (Hoogenboom et al. 2006; Muthukumaraswamy and Singh 2013), we expected the visual sustained gamma response to be restricted to posterior MEG sensors overlaying the occipital lobe. Therefore, we used a posterior selection of planar gradiometers (Fig. 1B) for subsequent analysis.
Assessment of gamma response.
We expected that the faster motion of the annual grating would result in higher frequency of gamma response. To reveal velocity-specific power changes at each of the three stimulus velocities, we performed group level statistical analysis of the stimulus-related changes in spectral power. The analysis proceeded in the following steps. First, we calculated average log-transformed baseline-normalized power (in dB) in 400- to 1,000-ms poststimulus intervals for each frequency bin within 50–120 Hz. This time window contains a strong sustained gamma response to visual motion and is not contaminated by stimulus onset response (Hoogenboom et al. 2006; Muthukumaraswamy and Singh 2013). Second, we selected a gradiometer pair that displayed the highest gamma power increase in response to motion in this time-frequency range. For all three velocities, this was the gradiometer pair channel nos. 2,112 and 2,113 that correspond to the sensor element located approximately at the middle occipital position. Third, we searched the data from this gradiometer pair for velocity-specific frequencies that demonstrate baseline-normalized spectral power for a given stimulus velocity exceeding that of the two other velocities (while properly correcting for family-wise error using the permutation approach). To correct for multiple comparisons, the baseline-normalized spectral power values at each frequency bin within the 50- to 120-Hz range were randomly permuted 1,000 times between the two data sets (e.g., low- and medium-velocity conditions). The maximum t-value from each permuted data set was taken across all frequency values when forming the empirical null distribution and comparing it with original statistics, with P < 0.05 as the significance threshold. The velocity-specific frequencies were computed as an overlap between the results of the two unidirectional pairwise comparisons (e.g., “low > medium” and “low > fast” for the slow velocity; see the blue bar under the top right graph in Fig. 4).
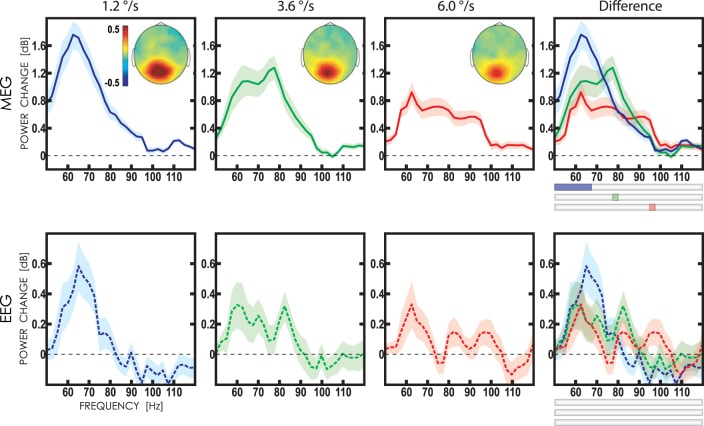
Fig. 4.
Gamma responses to moving gratings measured with MEG (sensors MEG2112-MEG2113; top) and EEG (Oz derivation; bottom). Grand average spectra of normalized gamma power are shown for the three stimulus velocities: ”slow“ (1.2°/s; blue line), ”medium“ (3.6°/s; green line), and ”fast“ (6°/s; red line). The shadows mark 95% confidence intervals. The ”velocity-specific“ gamma frequencies (P < 0.05, corrected for multiple comparisons) are marked at top far right by the corresponding color on the horizontal bars. Insets show spatial distribution of the mean MEG gamma power (in dB) in the 50- to 120-Hz range.
In each subject and for each stimulus velocity, we also calculated individual velocity-specific peak frequencies (VSPFs). Considering possible individual variations in head position and cortical morphology, the individual VSPF was always defined at the gradiometer pair, where the maximal gamma response was observed in this particular subject for a particular velocity. To ensure that the individual values comply with the group gamma response, the search for the gamma VSPF was limited to a certain frequency window. This window was centered at the frequency of the group average gamma peak that was closest to the velocity-specific frequencies. This peak overlapped with “velocity specific” frequencies (see Fig. 4) for the slow and medium velocities (62.5 Hz for the slow and 80 Hz for the medium velocities) and was close to the velocity-specific frequencies for the fast velocity (where it constituted 92.5 Hz). The width of each velocity-specific window was set at the full width at half-maximum. This resulted in velocity-specific frequency windows of 52.5–77.5 Hz for the slow velocity, 55.0–87.5 Hz for the medium velocity, and 55.0–97.5 Hz for the high velocity. The individual spectra were smoothed with a three-point moving average filter. After the smoothing, the algorithm searched for the peaks of the baseline-normalized power within the velocity-specific windows. Given that a specified frequency window may cover several local maxima, the peak of the highest frequency was always taken from all of the peaks that exceeded the baseline value by at least two standard deviations. This approach makes explicit the assumption that there may be several spectral peaks in gamma response (see, e.g., Gray and DiPrisco 1997) and is less susceptible to arbitrary detection of a local maximum. As we discuss later, the applied approach to the gamma peak frequency definition reduces the possible contribution from the photic driving effect at 60 Hz.
The average baseline-normalized MEG gamma power in the 50- to 120-Hz band was always calculated for the same gradiometer pair that was used to define VSPF. A similar strategy was used to define EEG VSPF and the average gamma power in the Oz derivation.
Only those participants for which the sustained gamma response met the criterion for a peak for all three stimuli velocities (19 of 27 participants) were included in the VSPF analysis.
Psychophysical task.
Detection of motion direction is hampered for large, as opposed to small, high-contrast gratings (Betts et al. 2009; Betts et al. 2005; Tadin et al. 2007; Tadin et al. 2003; Tadin et al. 2011). This decrease in motion sensitivity or spatial suppression was suggested to reflect surround inhibition of motion-direction-selective neurons. We tested this spatial suppression effect on a separate day, within 2 mo, after the main MEG experiment. Visual stimuli were presented with PsychoToolbox (Brainard 1997), a free Matlab application. To assess spatial suppression, we used the experimental approach described by Foss-Feig et al. (2013). The psychophysical data were available from 22 participants, 16 of which were also included in VSPF analysis.
The stimuli in this task consisted of drifting vertical sine wave gratings (1 cycle/degree, 4°/s), covered by a two-dimensional Gaussian envelope whose width defined the stimulus size. There were four types of stimuli, low (1%) and high contrast (100%) stimuli of either small (2° of visual field/Gaussian width) or large (12°) size. The direction of motion (left or right) was determined randomly for each trial.
Participants sat 60 cm from a monitor (Benq XL2420T, 24”W LED, 1,920 × 1,080 resolution, 120 Hz). An assistant seated next to the participant controlled for the correct distance from the monitor, vertical head position, and adequate task performance. Participants were asked to make an unspeeded, two-alternative, forced-choice response indicating the perceived direction of motion by pressing the left or right arrows on the keyboard. Each block consisted of either high- or low-contrast trials, with the block order counterbalanced across participants. The intertrial interval was 400 ms. In the beginning of each trial, a flicking central dot appeared for 100 ms, followed by the stimulus. The initial stimulus duration was set to 150 ms. The duration was further adjusted depending on the participant's response using two (1 for small and 1 for large stimuli) interleaved one-up two-down staircases (8.3-ms step) that converged on 71% correct performance. The block continued until both staircases completed nine reversals typically lasting ∼4 min. The first two reversals of each staircase were excluded from the analysis; the threshold was computed by taking the mean over the remaining reversals. Most participants (and 14 of those 16 that had the VSPFs defined for all stimuli types) performed two runs of low- and high-contrast blocks. The final thresholds were averaged over the two runs, when available. A training block was presented at the beginning of the session to acquaint subjects with the task and stimulus types.
For the purpose of the present study, we calculated the spatial suppression (SS) index as the difference between thresholds for the large and small high-contrast stimuli normalized by their sum. The greater SS index values correspond to a stronger SS effect.
RESULTS
MEG experiment: behavioral performance.
The behavioral data were available from 26 of 27 participants. The number of omission and commission errors was low (omission: mean = 2, range 0–19 per condition; commission: mean = 0.6, range 0–5 per condition) and did not differ between conditions. The reaction time significantly decreased with increasing stimulus velocity [ANOVA: F(2, 48)= 18.2, P < 1e-05], being on average 435.0 ms for the slow, 413.1 ms for the medium, and 406.3 for the fast velocities.
Gamma response to visual motion.
In line with the previous studies in adults, we observed a strong increase in gamma power in response to visual motion in children. All three types of stimuli (”slow,“ ”medium,“ and ”fast“) caused maximal response over the occipital midline (Fig. 1B). The gamma response to moving gratings was observed also in EEG, but its signal-to-noise ratio was much lower than in MEG channels (Fig. 2).
Fig. 2.
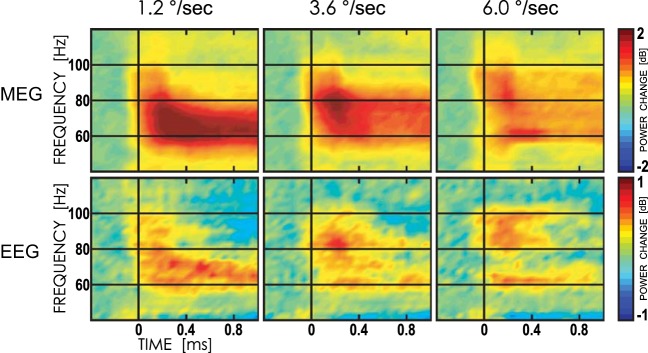
Grand average time-frequency plots of gamma power changes (in dB) elicited by gratings drifting at 1.2, 3.6, and 6.0°/s. Top: magnetoencephalographic (MEG) sensors with maximal gamma response (MEG2112 and MEG2113). Bottom: electroencephalographic (EEG) channel Oz. Note different scales for MEG and EEG.
Effect of stimulus velocity on the magnitude of gamma response.
To test for the effect of velocity on gamma power, we calculated mean baseline-normalized power (in dB) in the 50- to 120-Hz/400- to 1,000-ms range. Repeated-measures ANOVA revealed strong reduction of the power of the gamma response with increasing stimulus velocity [F(2, 52) = 44.8, P < 10e-5; Fig. 3]. A smaller but still significant effect of velocity was found also for the EEG gamma power [in Oz: F(2, 50) = 5.0, P < 0.05].
Fig. 3.
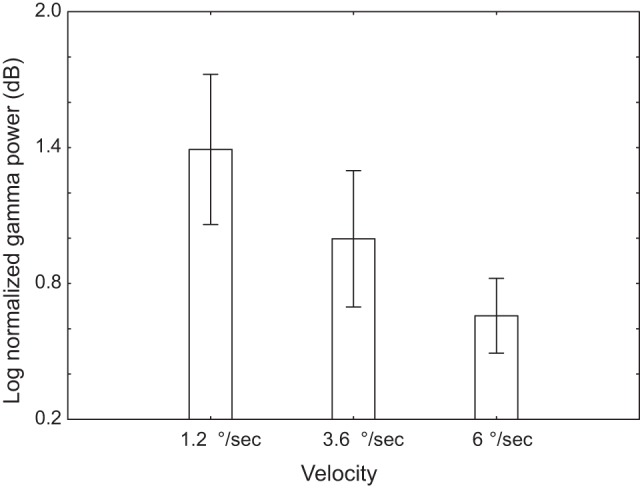
Effect of stimulus velocity on the magnitude of the visual gamma response. Vertical bars denote 0.95 confidence intervals.
Velocity-specific increases in gamma power.
To check whether the visual gamma response differentiated reliably between the three types of stimuli at some ”velocity-specific“ frequencies, we used permutation analysis (see methods). The significant velocity-specific increase of MEG gamma power was observed at 50–67.5 Hz for the slow motion velocity, at 77.5–82.5 Hz for the medium velocity, and at 95–97.5 Hz for the fast velocity (Fig. 4). ANOVA with factors ”velocity“ and velocity-specific frequency range (”range“) revealed the presence of the strong velocity × range interaction [F(4, 104) = 27.9, ε = 0.64, P < 1e-5].
Velocity-specific gamma responses in EEG (channel Oz) occurred at the same frequencies as in MEG, but they were less prominent, and no velocity-specific frequencies were detected after correction for multiple comparisons. Considering that no significant differences were found with EEG, we further limited analysis to the MEG data.
Possible confounding effects of photic driving.
The visual stimuli were projected at the 60-Hz refresh rate. It is possible that oscillations synchronized to the refresh frequency could account for some percentage of gamma synchronization during stimuli presentation (photic driving effect). To assess the contribution of the 60-Hz refresh rate in the motion-related gamma response, we computed evoked activity phase-locked to the start of the visual stimulation separately for the three stimulus velocities. We then estimated the reliability of the 60-Hz evoked response using the same ”maximal threshold“ permutation approach that was used to estimate the reliability of VSPFs on the group level (see methods). The effect of the 60-Hz refresh rate was significant for all three velocities (slow, P < 0.003; medium, P < 0.0004; fast, P < 10e-5). To assess possible differences in contribution of the evoked activity at different velocities, we further performed repeated-measures ANOVA with factor velocity. The effect of velocity was significant [F(2, 52) = 20.8, P < 10e-5]. Planned comparisons revealed an increase in evoked 60-Hz power from the medium to the high velocity [F(1, 26) = 25.3, P < 0.0001], whereas no difference between the slow and the medium velocities was found (P = 0.2). The significantly higher 60-Hz evoked response to the fast velocity condition could be explained by contribution of the third harmonic of its black-to-white transition frequency (∼20 Hz for the fast velocity).
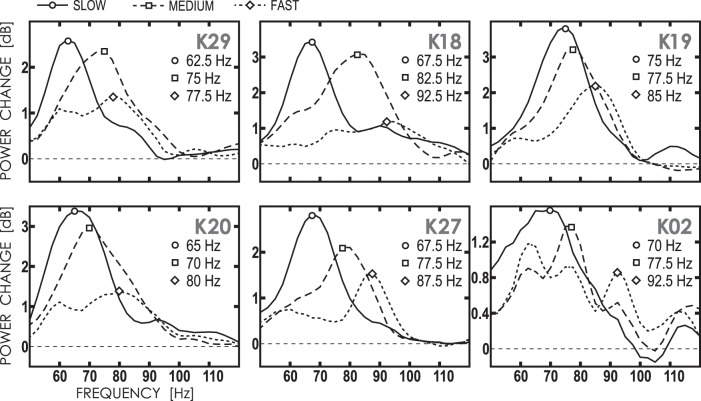
Worthy of note is the fact that our approach of defining the gamma VSPF minimized the contribution of the 60-Hz evoked activity because it was based on the choice of the ”right-most“ peak (see methods). Indeed, inspection of the individual VSPFs in Fig. 6A shows that only three subjects displayed VSPFs at 60 Hz (2 for the slow and 1 for the medium velocities).
Fig. 6.
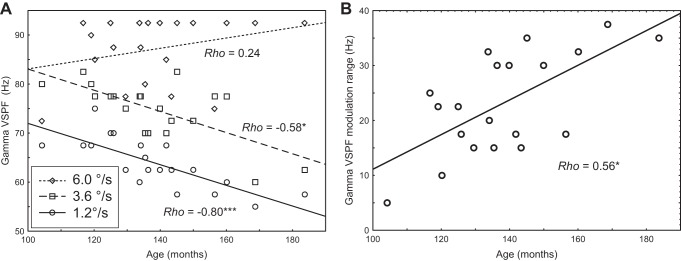
Age-related changes in VSPF of gamma response elicited by visual stimuli moving with different velocities (A) and in gamma VSPF modulation range (B). *P < 0.01, ***P < 0.0001.
Gamma VSPFs.
Inspection of individual spectra revealed a velocity-related shift of gamma frequency in virtually all participants who displayed reliable gamma peaks. Figure 5 shows normalized power spectra for six representative individuals. The VSPFs constituted on average 64.9 Hz (SD = 5.2) for the slow, 74.8 Hz (SD = 6.0) for the medium, and 87.1 Hz (SD = 6.9) for the fast velocity, thus suggesting a 22-Hz increase in frequency with increasing velocity of visual motion from 1.2 to 6.0°/s (later addressed as VSPF modulation range).
Fig. 5.
Baseline-normalized power spectra of gamma oscillations induced by the 3 types of stimuli (slow, medium, and fast) in 6 representative participants. Individual velocity-specific peak frequenies (VSPFs) are marked as follows: ○, slow; □, medium; ◇, fast velocities. Note the shift of frequency maximum with increasing stimulus velocity.
Swettenham et al. (2009) have observed previously that the individuals with a higher peak frequency in their gamma response to stationary gratings also displayed a smaller frequency increase in response to motion. These authors suggested the presence of a saturation effect that limited the frequency of gamma oscillations. To test this hypothesis, we checked whether the VSPF modulation range could be predicted from the VSPF to the slow velocity. We also included age as an independent factor. The regression with factors ”age“ and ”slow-velocity VSPF“ was significant [F(2, 17) = 8.3, adjusted to r2 = 0.43, P < 0.01]. However, the inspection of partial correlations has shown that the slow velocity VSPF did not predict the VSPF modulation range independently from age (P = 0.13).
Regarding the response to the fast velocity stimuli, 50% of participants had VSPF of 92.5 Hz, and none of the participants displayed VSPF that exceeded this value (Fig. 6A). This finding cannot be explained by the ”border effect“ because the upper border of the velocity-specific gamma frequency range for the fast velocity was 97.5 Hz. On the other hand, this 92.5-Hz frequency corresponds to the frequency of the ”highest-frequency gamma peak“ on the group spectrum (Fig. 4C). This observation confirms the presence of a saturation effect for the highest motion velocity.
Effect of age.
No correlations with age were found for the power of the gamma response within the 50- to 120-Hz range at any stimulus velocity (Pearson r, all P > 0.28). The correlations between gamma power measured at the VSPFs and subjects' age were also not significant (slow: Rho = −0.12, P = 0.6; medium: Rho = −0.12, P = 0.6; fast: Rho = −0.36, P = 0.13). The gamma VSPF reliably decreased with age for the slow (Rho = −0.8, P < 0.0002) and medium (Rho = −0.6, P < 0.005) velocities but did not change with age for the fast velocity (Rho = 0.26, P = 0.26) (Fig. 6A). The VSPF modulation range reliably increased with age (Rho = 0.56, P < 0.01, Fig. 6B).
Gamma frequency and spatial suppression.
Spatial suppression results were available from 22 participants. In 16 of these participants, VSPF has been detected for all three stimulus velocities. The mean time required for correct detection (TCD) of motion direction was 92.2 ms (SD = 45.0) for the large stimuli and 40.8 ms (SD = 15.6) for the small stimuli. The increase of the detection time with increasing stimulus size was highly significant [F(1, 21) = 54.9, P < 10e-5], indicating the presence of a strong spatial suppression effect. We further calculated an SS index as a difference between TCD for the high-contrast stimuli of large and small size normalized to the subject's mean TCD. The correlation between this SS index and age was nearly significant (n = 22, Pearson r = 0.41, P = 0.061), suggesting a tendency toward a developmental increase in spatial suppression.
To test whether the visual gamma peak frequency predicts this SS index, we performed an exploratory regression analysis with one of the gamma parameters (VSPF for the slow, medium, or fast velocity or the VSPF range) and age as independent variables. Results of these analyses are summarized in Table 1. The analysis revealed that the broader VSPF range predicts greater SS index independently of age. There was also a tendency for a greater SS index in children with higher VSPF values to the fast velocity (P = 0.08). No link between SS index and frequency of the VSPF to the slow and medium velocities was found.
Table 1.
Prediction of the spatial suppression from the gamma frequency characteristics and age: results of the multiple regression analysis
| VSPF Slow, Age | VSPF Medium, Age | VSPF Fast, Age | VSPF Range, Age | |
|---|---|---|---|---|
| SS index (n=16) | r2adj = 0.04 | r2adj = 0.10 | r2adj = 0.21# | r2adj = 0.29* |
| βage = 0.07 | βage = 0.60# | βage = 0.14 | βage = −0.17 | |
| βVSPF slow = −0.36 | βVSPF medium = 0.40 | βVSPF fast = 0.48# | βVSPF range = 0.73* |
SS, spatial suppression; VSPF, velocity-specific peak frequency; r2adj, modified version of r2 that has been adjusted for the no. of predictors in the model.
P < 0.1;
P < 0.05, VSPF range = (VSPF fast − VSPF slow).
DISCUSSION
In the present study, we recorded MEG and EEG in children during visual presentation of annular gratings contracting at three different velocities and analyzed gamma oscillations elicited by this stimulation. Our results can be summarized as follows. First, the frequency of gamma oscillations increased with increasing velocity of the visual motion. Second, the older children demonstrated a wider range of velocity-related modulation of their gamma peak frequency. The frequency of gamma induced by the slow (1.2°/s) and medium (3.6°/s) velocities decreased in children from 8 to 15 yr of age, whereas no age-related changes were observed for the fast (6.0°/s) motion. Third, the broader range of gamma frequency modulation predicted stronger spatial suppression measured in children in a separate psychophysical experiment.
Modulation of gamma oscillations by velocity.
The increase of peak frequency of gamma oscillations with increasing velocity of visual motion has been reported previously in LFP recordings in animals (Gray and DiPrisco 1997). The present study is the first to demonstrate this effect in human subjects.
The effect of velocity on gamma frequency was visible in both MEG and EEG (Figs. 2 and 4). However, the estimation of individual gamma peaks in EEG was problematic due to the low signal-to-noise ratio. Therefore, we limit our further discussions to the analysis of gamma oscillations as recorded by MEG. The substantially higher sensitivity of MEG than EEG to gamma oscillations has been reported previously in adults (Muthukumaraswamy and Singh 2013). The advantage of MEG over EEG might be even higher in children because they are more likely to move during the experiment, and EEG was suggested to be more sensitive to muscular artifacts than MEG (Muthukumaraswamy and Singh 2013). However, because we used a single Oz EEG electrode in our study, it is possible that future multichannel EEG recordings could provide a better signal-to-noise ratio than what we report herein.
We note that although the 60-Hz photic driving effect was present in our data and its magnitude depended on stimulus velocity, this effect could only reduce the observed velocity-related modulation of gamma frequency. Indeed, the 60-Hz photic driving effect was highest for the fast velocity. As such, photic driving cannot be used to explain the observed shift to higher velocity-specific peak frequencies (74.8 Hz for medium and 87.1 Hz for fast velocities) or velocity-specific frequency ranges (77.5–80 Hz for medium and 95–97.5 Hz for fast velocities) with increasing velocity of visual motion (Fig. 4).
Unlike numerous findings regarding gamma power (e.g., see Hermes et al. 2014; Koelewijn et al. 2013; Uhlhaas et al. 2009), the reports about changes in gamma frequency produced by manipulating features of visual stimuli in humans are rare. For example, the effect of stimulus size previously reported in intracranial studies in animals (Ray and Maunsell 2010) was shown to be either small (van Pelt and Fries 2013) or absent (Perry et al. 2013) in MEG recordings of human subjects. A slightly (on average 5 Hz) lower gamma peak frequency was observed in MEG recordings on adult subjects in response to stimuli presented in the periphery (6° of visual angle) compared with stimuli presented in the center of the visual field (van Pelt and Fries 2013). The studies linked most closely to ours have investigated how the transition from stationary to moving gratings changed MEG-recorded gamma activity in the visual cortex of healthy adults (Muthukumaraswamy and Singh 2013; Naue et al. 2011; Swettenham et al. 2009). There are notable distinctions between results of the present study and these previous studies. First, we observed a large increase in VSPF (22 Hz on average) when the stimulus velocity increased from 1.2 to 6.0°/s. The previous studies, on the other hand, reported smaller increases in frequency. Swettenham et al. (2009) found an average 10-Hz difference in gamma peak frequency when they compared static gratings with those moving at 1.33°/s (that is close to the slow velocity presented herein). The same group later reported an even more moderate increase of 5 Hz for a larger group of participants using the same paradigm (Muthukumaraswamy and Singh 2013). Second, we observed that increasing the velocity from 1.2 to 6°/s was associated with a drop in gamma power (Fig. 3) in addition to the increase in gamma frequency. Although the transition from the stationary to the slowly moving grating led to an increase in gamma frequency, it either did not affect (for 1.75°/s, as in Naue et al. 2011) or increased gamma power (for 1.33°/s, as in Muthukumaraswamy and Singh 2013). Such uncorrelated changes in gamma frequency and power have been found previously in animal studies (Jia et al. 2013) and can be explained by different control mechanisms of these properties of gamma oscillations.
What neural mechanism mediates changes in gamma frequency? The finding of a positive correlation between an individual's peak frequency of MEG-recorded visual gamma oscillations and concentration of GABA in the visual cortex gave rise to the hypothesis that higher gamma frequency reflects stronger GABAergic inhibition (Edden et al. 2009). However, the recent studies in humans have shown that facilitation of GABAergic transmission either did not affect (Muthukumaraswamy et al. 2013; Saxena et al. 2013) or decreased (Campbell et al. 2014; Lozano-Soldevilla et al. 2014) frequency of visual gamma oscillations as measured with MEG. Importantly, nonspecific pharmacological GABA modulations may affect GABA transmission on both inhibitory FS PV interneurons and principle cells (Edden et al. 2009; Muthukumaraswamy et al. 2013; Saxena et al. 2013; Campbell et al. 2014; Lozano-Soldevilla et al. 2014). On the other hand, experimental studies in animals and brain tissues in vitro provide evidence that the frequency of gamma oscillations is determined mainly by the net excitation of the inhibitory circuitry. Indeed, manipulations that affect excitability of FS PV interneurons effectively modulate gamma frequency, whereas those selectively influencing principle cells do not produce noticeable frequency changes (Mann and Mody 2010). A large increase in gamma frequency may be elicited either by excitation of FS PV cells through FS-specific NMDARs or by a reduction in their inhibition through abolishment of FS-specific δ-unit-containing GABA receptors (Ferando and Mody 2015; Mann and Mody 2010). In contrast, the application of NMDA antagonists decreases the frequency of high-frequency gamma oscillations (Anver et al. 2011; Oke et al. 2010). Furthermore, alcohol, a drug that both increases GABAergic inhibition at GABAA receptors and decreases glutamatergic excitation at NMDARs, reliably reduces the frequency of visual MEG gamma oscillations in humans (Lozano-Soldevilla et al. 2014). Considering the results of animal studies, it is likely that alcohol might operate through either potentiation of inhibition or reduction of excitation of the FS PV cells. Modeling studies also support the role of inhibitory neurons in the regulating frequency of gamma oscillations (Kirli et al. 2014). Considering these previous animal and computational studies, it is likely that our observation of a robust increase in gamma frequency with an increase in the velocity of visual motion can be explained by augmented excitatory drive to the FS PV interneurons.
It is also likely that modulations of gamma frequency by other properties of visual stimuli (e.g., contrast, eccentricity, size) are mediated through the stimulus capacity to activate the inhibitory circuitry in visual cortex. Indeed, the higher frequency of LFP gamma oscillations in response to gratings of relatively higher contrasts in monkey V1 (Jia et al. 2013; Ray and Maunsell 2010) and in human MEG (Hadjipapas et al. 2015) can be explained by a greater capacity of higher-contrast stimuli to activate neurons' inhibitory surround (Paffen et al. 2005). In addition, the decrease in gamma peak frequency with decreasing visual eccentricity observed in MEG on humans (van Pelt and Fries 2013) and in LFP of monkeys (Lima et al. 2010) is consistent with the eccentricity-dependent scaling of intensity of surround inhibition in the areas V1 and V2 (Zuiderbaan et al. 2012). The modulation of LFP gamma frequency by size of a visual stimulus also depends on stimulation of inhibitory interneurons (Gieselmann and Thiele 2008). Gieselmann and Thiele (2008) demonstrated that the gratings centered at the receptive field of the recorded neurons induced gamma oscillations in LFP only if they also stimulated a neuron's inhibitory surround. They also observed that the frequency of oscillations depended on the area of the stimulated inhibitory surround.
It has been suggested that apart from the strength of the inhibition, the frequency of gamma oscillations might depend on some constitutional factors, such as the size of the visual cortical areas (Schwarzkopf et al. 2012) and conduction delays (Gieselmann and Thiele 2008). Relative changes in gamma frequency caused by changes in motion velocity are likely to be less sensitive to these factors than the absolute values, and therefore, they may more closely reflect functioning of FS PV neurons, one of the major classes of inhibitory neurons in the mammalian cortex. In particular, experimental modulations of gamma frequency by motion velocity may appear useful to probe the function of inhibitory networks in disorders characterized by abnormalities of FS PV neurons, e.g, schizophrenia (Gonzalez-Burgos and Lewis 2012; Lewis 2014) and autism (Lawrence et al. 2010).
Although a shift in FS PV neurons' excitability is the most likely cause of the velocity-related increase in gamma peak frequency observed in our study, other factors might also contribute. In particular, stimulation with different velocities may result in different involvement of cortical regions. For example, neurons in the area MT are more sensitive to higher velocities (Yang et al. 2009), whereas the motion-sensitive neurons in the posteriomedial visual area (PM) are ”tuned“ to slower drift rates compared with those in V1 (Roth et al. 2012). Therefore, MT and PM may differently modulate the frequency of gamma oscillations in V1, depending on the speed of visual motion. It has been shown, however, that feedback projections to V1 from extrastriate areas target mostly pyramidal cells, whereas terminals on inhibitory interneurons are rare (see discussion in Gieselmann and Thiele 2008). Therefore, considering the role of inhibitory cells in regulating gamma frequency, the contribution of feedback projections into gamma frequency modulation is unclear. Future source localization and connectivity studies may help to investigate the contribution of different cortical sources in the modulation of gamma frequency by the velocity of visual motion.
In our study, the velocity-related increase in gamma frequency was associated with a remarkable reduction of power in the gamma oscillations (Fig. 3). Differing contributions of a number of factors may explain the nonlinear dynamics of gamma amplitude as well as the generally inconsistent relationship between gamma amplitude and frequency modulation (Jia et al. 2013). Similar to the present study, some studies in animals also reported a reduction in amplitude of visual gamma oscillations with increasing gamma frequency (Gieselmann and Thiele 2008; Lima et al. 2010). For example, Gieselmann and Thiele (2008) have found that an increase in stimulus size led to augmentation of the LFP gamma response with a corresponding drop in frequency of gamma oscillations. These authors suggested that the greater strength of gamma LFP resulted from a bigger size of the stimulated area, whereas the concomitant slowing down of gamma frequency was explained by longer conduction times required for synchronization of activity of relatively remote neural populations. The changes in gamma frequency described by Lima et al. (2010), on the other hand, are difficult to explain by changes in conduction times. These authors observed that, when compared with a single moving grating, superimposition of two moving gratings (depth-order plaids) increased the gamma peak frequency while reducing the power of gamma oscillations. They hypothesized that this finding reflects a competition and increased mutual inhibition between subsets of neurons activated by the two component gratings. In this case, the increase in frequency of the gamma oscillations could be explained by a stronger excitation of the inhibitory network in response to the plaids, as opposed to the gratings.
Jia et al. (2013) reported that a transition from low to medium (50%) contrast in visual gratings is accompanied by an increase in both gamma frequency and power, whereas further contrast increases, while inducing an increased gamma frequency, led to a reduction of the peak gamma power (Fig. 4) (Jia et al. 2013). The effect of the velocity of visual stimuli on gamma characteristics resembles that of the contrast. Indeed, gamma frequency and power displayed the concordant changes during transition from stationary to moving gratings in the study by Muthukumaraswamy and Singh (2013) but changed in opposite directions with an increase in stimulus motion velocity in our experiment. Most probably, the concomitant growth in gamma power and frequency resulted from a strengthening of excitatory drive to both principal and inhibitory cells. Modulation of gamma frequency by feedback projections (Jia et al. 2013; Kang et al. 2010) could also contribute. Further strengthening of the input (and of the excitatory drive to the inhibitory FS PV cells) could reduce the amplitude of gamma oscillations via affecting the balance between excitation and inhibition (Atallah and Scanziani 2009). Another possible explanation for the drop in gamma power is a reduced neuronal synchronization at high gamma frequencies. It has been suggested that the gamma cycle creates a ”window of opportunity“ for synchronization between spatially distant neuronal assembles (Fries 2005). Conceivably, the shorter window of opportunity at high gamma frequency reduces the number of neuronal assemblies involved in synchronous membrane potential fluctuations, which may in turn lead to a reduction in gamma power.
Swettenham et al. (2009) have observed previously that the higher oscillation frequency for the static visual stimuli was related to a smaller frequency increase in response to motion in adult subjects. These authors suggested the presence of an ”upper oscillation frequency“ for the motion-driven gamma responses and proposed that some individuals are naturally close to this limit. Our study in children indicates that the frequency of gamma oscillations in response to the slowly moving gratings does not predict a frequency increase in response to the fast motion when age is accounted for. Moreover, the strong velocity-related increase of gamma frequency suggests that if the ”limit of upper gamma frequency“ does exist, it is clearly not reached in children in response to the stimuli moving with velocities of 1.2 to 3.6°/s. On the other hand, the observations that none of the participants displayed gamma VSPF of >92.5 Hz and that 50% of the children had 92.5 Hz VSPF for the fast velocity suggest that this frequency may be close to a physiological limit for this type of stimulation.
Development of gamma oscillations.
Our results are in line with the previous studies that reported a developmental decrease in gamma frequency in response to stationary visual grating between 8 and 45 yr (Gaetz et al. 2012) and 20 and 45 yr (Muthukumaraswamy et al. 2010). We extended this finding by showing that similar developmental dynamics characterize the response to the gratings moving with slow and medium velocities (1.2 and 3.6°/s) in boys aged 8–15 yr. No such age-related decline in frequency was observed in case of the fast motion (6.0°/s). Similarly to Gaetz et al. (2012), we did not find significant developmental changes in the power of sustained visual gamma oscillations.
Considering that increased myelination was suggested to produce faster EEG frequencies (Nunez 2000), the observed maturational decrease in gamma frequency seems counterintuitive. Indeed, developmental studies typically report stable frequency growth of slow oscillations during the course of maturation (Hudspeth and Pribram 1992; Orekhova et al. 2006; Stroganova et al. 1999). Presumably, the different developmental courses of gamma and slow oscillations can be explained by differing mechanisms in their generation. Slow oscillations arise in broadly synchronized cortico-subcortical loops (Hughes and Crunelli 2007; Miller 1991). Myelination of these loops undergoes a long developmental course, and their conductivity increases with age (Paus et al. 2001). In contrast, visual gamma oscillations depend to a greater extent on horizontal intrinsic connections within visual cortical areas (Ray and Maunsell 2010) and may depend predominantly on the properties of local inhibitory circuits.
Gaetz et al. (2012) tested whether the age-related changes in morphology of visual cortical areas (i.e., cortical volume or thickness) could account for reduction in gamma frequency, but they found no significant links. Hence, it is unlikely that the developmental decline in gamma frequency observed in case of slow and medium velocities in our study can be explained by changes in brain macroanatomy occurring during childhood and adolescence. More likely, this decline reflects functional maturation of visual cortical areas. Maturation of the FS PV interneurons might be of particular relevance for the developmental changes in gamma frequency. Unlike other interneurons, the FS cells undergo protracted development that proceeds well into adolescence and involves both inhibitory and excitatory transmission in FS cells (Pinto et al. 2010; Wang and Gao 2009).
Concentration of the α1- and δ-subunits of δ-GABAA receptors (δ-GABAAR) on inhibitory neurons increases with age (Le Magueresse and Monyer 2013; Pinto et al. 2010). Concentration of the GABA-synthesizing enzyme GAD65 also increases from early in life (<4 yr) to the teenage and adult years, leading to increase in ”on-demand“ GABA release (Pinto et al. 2010). Together, these factors may contribute to an increase in δ-GABAAR-mediated tonic inhibition of FS PV interneurons.
Wang and Gao (2009) reported that expression of NMDARs in PV interneurons is higher during development than in adulthood. In the frontal cortex, the number of FS cells demonstrating NMDAR-mediated currents progressively drops from ∼75% in young to 25% in adult rats (Wang and Gao 2009). The similar developmental decline of NMDA-mediated neurotransmission may also take place in the visual cortex (Carmignoto and Vicini 1992). The developmental reduction in the number of NMDARs may contribute to a reduction in FS PV cells' excitability with age (Anver et al. 2011; Mann and Mody 2010).
As a result of the combination of the factors listed above (and possibly also others), the excitability of PV neurons decreases with age, and a greater level of input is needed to recruit PV cells in the mature compared with the immature brain (Le Magueresse and Monyer 2013). Considering that higher excitability of FS PV neurons is associated with a higher gamma frequency (Anver et al. 2011; Ferando and Mody 2015; Mann and Mody 2010), the reduction in gamma oscillation frequency in older children is well in line with the developmental decrease in FS PV interneurons' excitability.
Despite the age-related slowing of gamma oscillations to low/medium-velocity stimuli (1.2 and 3.6°/s), the frequency of gamma response to the fast moving gratings was the same in the younger and the older children. As a result, the range of frequency modulation by velocity becomes higher with age. Although the causes of such an increase are unknown, maturational changes in kinetics of the FS PV inhibitory neurons may contribute. It has been found that duration of miniature inhibitory postsynaptic potentials is shorter in postpubertal compared with prepubertal monkeys (Hashimoto et al. 2009). This shortening may appear to be a universal phenomenon in all cortical neurons (Hashimoto et al. 2009; Le Magueresse and Monyer 2013). The decay time of NMDAR-mediated synaptic currents also decreases during development (Carmignoto and Vicini 1992; Paoletti et al. 2013), probably as a result of a developmental switch in NMDAR subunit composition (Paoletti et al. 2013). The decrease in the ratio of slow NMDA to fast AMPA glutamate receptors in FS neurons during the adolescent period (Gonzalez-Burgos and Alejandre-Gomez 2005; Wang and Gao 2010) may further speed up the kinetics of inhibitory interneurons. The faster kinetics of FS PV cells may then refine their temporal integration properties and allow greater gamma frequency modulation under an increasing strength of visual input.
It has been suggested that developmental changes in inhibitory neurotransmission may have essential consequences for information processing (Ferando and Mody 2015; Pinto et al. 2010). Considering the high concentration of GABA in the visual cortex (Zilles et al. 2002), the developmental refinement of inhibitory processes may be needed for optimal visual perceptual functions such as orientation discrimination (Edden et al. 2009), contrast sensitivity (Benedek et al. 2010), contour integration (Braunitzer et al. 2011; Kiorpes and Bassin 2003), and perception of moving stimuli (Lewis 2014; Tadin et al. 2003). Indeed, studies in children and monkeys show that these ”low-order“ visual functions undergo long developmental trajectories that proceed into the teen years (Braunitzer et al. 2011; Kiorpes and Bassin 2003; Lewis 2014; Palomares et al. 2011). A wider gamma frequency modulation range may be associated with greater maturity and/or efficiency of the visual perceptual functions. In particular, we hypothesize that the broader range of gamma frequency modulations might relate to a more efficient neural inhibition as assessed through measuring the effect of spatial suppression in the visual cortex (Tadin et al. 2003; Tadin et al. 2011).
Gamma frequency and spatial suppression.
It has been noted previously that detection of motion direction is hampered for large, compared with small, high-contrast gratings (Tadin et al. 2003). This decrease in motion sensitivity or spatial suppression was suggested to reflect surround inhibition of direction-selective neurons. The decline in neural inhibition related to aging (Betts et al. 2009; Betts et al. 2005) or disease (Golomb et al. 2009; Tadin et al. 2007) leads to a reduction of the spatial suppression effect. Just the opposite, maturational increase in GABAergic inhibition has been proposed to lead to greater spatial suppression in adults compared with children (Lewis 2014). The tendency for a correlation between spatial suppression magnitude and a child's age observed in our study is in line with this previous finding.
Notably, we have found that the greater magnitude of the spatial suppression effect correlated with the range of gamma VSPF modulation and that this correlation was not explained by age-related changes in the VSPF modulation range (Table 1). There was also a tendency for correlation between spatial suppression and peak gamma frequency in response to the fast motion of the gratings (Table 1). This pattern of results suggests that the more prominent spatial suppression might be linked to faster kinetics of FS PV cells and greater diapason of their modulation by excitation.
Although the intact surround inhibition in MT/V5 seems to be critical for spatial suppression (Tadin et al. 2011), its exact neural mechanisms are yet unknown. Our data suggest that either the properties of FS PV neuron circuitry in V1 or some external influences modulating their activity are among the factors affecting the magnitude of spatial suppression in children.
In conclusion, children in our study exhibited robust frequency modulation of MEG-recorded gamma oscillations by velocity of visual motion. We propose that modulation of gamma frequency by motion velocity may help to investigate typical development of inhibitory networks in the visual cortex and its disturbance in neurodevelopmental and psychiatric disorders.
GRANTS
The study has been supported by Russian Science Foundation Grant no. 14-35-00060 and the Charity Foundation for Autism “Way Out.” The MEG Centre is supported by core funding from the Russian Ministry of Education and Science (RFMEFI61914X0006).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.V.O., O.V.S., and T.A.S. conception and design of research; E.V.O., A.V.B., and T.A.S. analyzed data; E.V.O. and T.A.S. interpreted results of experiments; E.V.O., A.V.B., and A.O.P. prepared figures; E.V.O. drafted manuscript; E.V.O., A.V.B., O.V.S., A.O.P., A.Y.N., and T.A.S. approved final version of manuscript; O.V.S., A.O.P., and A.Y.N. performed experiments; O.V.S. and T.A.S. edited and revised manuscript.
ACKNOWLEDGMENTS
We heartily thank the children and their families for their participation in this study.
REFERENCES
- Anver H, Ward PD, Magony A, Vreugdenhil M. NMDA receptor hypofunction phase couples independent gamma-oscillations in the rat visual cortex. Neuropsychopharmacology 36: 519–528, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah BV, Scanziani M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron 62: 566–577, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek K, Janaky M, Braunitzer G, Rokszin A, Keri S, Benedek G. Parallel development of contour integration and visual contrast sensitivity at low spatial frequencies. Neurosci Lett 472: 175–178, 2010. [DOI] [PubMed] [Google Scholar]
- Betts LR, Sekuler AB, Bennett PJ. Spatial characteristics of center-surround antagonism in younger and older adults. J Vis 9: 25.1–25.15, 2009. [DOI] [PubMed] [Google Scholar]
- Betts LR, Taylor CP, Sekuler AB, Bennett PJ. Aging reduces center-surround antagonism in visual motion processing. Neuron 45: 361–366, 2005. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Braunitzer G, Rokszin A, Kobor J, Nagy A, Sztriha L, Benedek G. Development of visual contour integration in children with migraine without aura. Cephalalgia 31: 1048–1056, 2011. [DOI] [PubMed] [Google Scholar]
- Brunet N, Vinck M, Bosman CA, Singer W, Fries P. Gamma or no gamma, that is the question. Trends Cogn Sci 18: 507–509, 2014. [DOI] [PubMed] [Google Scholar]
- Campbell AE, Sumner P, Singh KD, Muthukumaraswamy SD. Acute effects of alcohol on stimulus-induced gamma oscillations in human primary visual and motor cortices. Neuropsychopharmacol 39: 2104–2113, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663–667, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai LH. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry 17: 537–548, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science 258: 1007–1011, 1992. [DOI] [PubMed] [Google Scholar]
- Cho RY, Walker CP, Polizzotto NR, Wozny TA, Fissell C, Chen CM, Lewis DA. Development of sensory gamma oscillations and cross-frequency coupling from childhood to early adulthood. Cereb Cortex 25: 1509–1518, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn H, Haegens S, Wallis G, Near J, Stokes MG, Harrison PJ, Nobre AC. Resting GABA and glutamate concentrations do not predict visual gamma frequency or amplitude. Proc Natl Acad Sci USA 111: 9301–9306, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci 29: 15721–15726, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferando I, Mody I. In vitro gamma oscillations following partial and complete ablation of delta subunit-containing GABAA receptors from parvalbumin interneurons. Neuropharmacology 88: 91–98, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig JH, Tadin D, Schauder KB, Cascio CJ. A substantial and unexpected enhancement of motion perception in autism. J Neurosci 33: 8243–8249, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 9: 474–480, 2005. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Roberts TP, Singh KD, Muthukumaraswamy SD. Functional and structural correlates of the aging brain: relating visual cortex (V1) gamma band responses to age-related structural change. Hum Brain Mapp 33: 2035–2046, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieselmann MA, Thiele A. Comparison of spatial integration and surround suppression characteristics in spiking activity and the local field potential in macaque V1. Eur J Neurosci 28: 447–459, 2008. [DOI] [PubMed] [Google Scholar]
- Golomb JD, McDavitt JR, Ruf BM, Chen JI, Saricicek A, Maloney KH, Hu J, Chun MM, Bhagwagar Z. Enhanced visual motion perception in major depressive disorder. J Neurosci 29: 9072–9077, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophrenia Bull 38: 950–957, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Burgos I, Alejandre-Gómez M. Cerebellar granule cell and Bergmann glial cell maturation in the rat is disrupted by pre- and postnatal exposure to moderate levels of ethanol. Int J Dev Neurosci 23: 383–388, 2005. [DOI] [PubMed] [Google Scholar]
- Gray C, DiPrisco G. Stimulus-dependent neuronal oscillations and local synchronization in striate cortex of the alert cat. J Neurosci 17: 3239–3253, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Szabo GG, Ulbert I, Holderith N, Monyer H, Erdelyi F, Szabo G, Freund TF, Hajos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci 30: 15134–15145, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjipapas A, Lowet E, Roberts M, Peter A, De Weerd P. Parametric variation of gamma frequency and power with luminance contrast: A comparative study of human MEG and monkey LFP and spike responses. Neuroimage 112: 327–340, 2015. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, Gonzalez-Burgos G, Lewis DA. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry 65: 1015–1023, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes D, Miller KJ, Wandell BA, Winawer J. Stimulus dependence of gamma oscillations in human visual cortex. Cereb Cortex. First published May 22, 2014; doi: 10.1093/cercor/bhu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PW, Welsch RE. Robust regression using iteratively re-weighted leastsquares. Commun Stat Theory Methods 6: 813–827, 1977. [Google Scholar]
- Hoogenboom N, Schoffelen JM, Oostenveld R, Parkes LM, Fries P. Localizing human visual gamma-band activity in frequency, time and space. Neuroimage 29: 764–773, 2006. [DOI] [PubMed] [Google Scholar]
- Hudspeth WJ, Pribram KH. Psychophysiological indices of cerebral maturation. Int J Psychophysiol 12: 19–29, 1992. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Crunelli V. Just a phase they're going through: the complex interaction of intrinsic high-threshold bursting and gap junctions in the generation of thalamic alpha and theta rhythms. Int J Psychophysiol 64: 3–17, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia XX, Xing DJ, Kohn A. No consistent relationship between gamma power and peak frequency in macaque primary visual cortex. J Neurosci 33: 17–25, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Shelley M, Henrie JA, Shapley R. LFP spectral peaks in V1 cortex: network resonance and cortico-cortical feedback. J Comput Neurosci 29: 495–507, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, Bassin SA. Development of contour integration in macaque monkeys. Vis Neurosci 20: 567–575, 2003. [DOI] [PubMed] [Google Scholar]
- Kirli KK, Ermentrout GB, Cho RY. Computational study of NMDA conductance and cortical oscillations in schizophrenia. Front Comput Neurosci 8: 133, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelewijn L, Rich AN, Muthukumaraswamy SD, Singh KD. Spatial attention increases high-frequency gamma synchronisation in human medial visual cortex. Neuroimage 79: 295–303, 2013. [DOI] [PubMed] [Google Scholar]
- Lawrence YA, Kemper TL, Bauman ML, Blatt GJ. Parvalbumin-, calbindin-, and calretinin-immunoreactive hippocampal interneuron density in autism. Acta Neurol Scand 121: 99–108, 2010. [DOI] [PubMed] [Google Scholar]
- Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron 77: 388–405, 2013. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol 26: 22–26, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima B, Singer W, Chen NH, Neuenschwander S. Synchronization dynamics in response to plaid stimuli in monkey V1. Cereb Cortex 20: 1556–1573, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Mattout J, Kiebel S, Phillips C, Henson R, Kilner J, Barnes G, Oostenveld R, Daunizeau J, Flandin G, Penny W, Friston K. EEG and MEG data analysis in SPM8. Comput Intell Neurosci 2011: 852961, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Soldevilla D, ter Huurne N, Cools R, Jensen O. GABAergic modulation of visual gamma and alpha oscillations and its consequences for working memory performance. Curr Biol 24: 2878–2887, 2014. [DOI] [PubMed] [Google Scholar]
- Mann EO, Mody I. Control of hippocampal gamma oscillation frequency by tonic inhibition and excitation of interneurons. Nat Neurosci 13: 205–212, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. Cortico-Hippocampal Interplay and the Representation of Contexts in the Brain. Berlin and New York: Springer-Verlag, 1991. [Google Scholar]
- Muthukumaraswamy SD, Myers JF, Wilson SJ, Nutt DJ, Hamandi K, Lingford-Hughes A, Singh KD. Elevating endogenous GABA levels with GAT-1 blockade modulates evoked but not induced responses in human visual cortex. Neuropsychopharmacol 38: 1105–1112, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Singh KD. Visual gamma oscillations: the effects of stimulus type, visual field coverage and stimulus motion on MEG and EEG recordings. Neuroimage 69: 223–230, 2013. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Singh KD, Swettenham JB, Jones DK. Visual gamma oscillations and evoked responses: variability, repeatability and structural MRI correlates. Neuroimage 49: 3349–3357, 2010. [DOI] [PubMed] [Google Scholar]
- Naue N, Strüber D, Fründ I, Schadow J, Lenz D, Rach S, Körner U, Herrmann CS. Gamma in motion: pattern reversal elicits stronger gamma-band responses than motion. Neuroimage 55: 808–817, 2011. [DOI] [PubMed] [Google Scholar]
- Nunez PL. Toward a quantitative description of large-scale neocortical dynamic function and EEG. Behav Brain Sci 23: 371–398; discussion 399–437, 2000. [DOI] [PubMed] [Google Scholar]
- Oke OO, Magony A, Anver H, Ward PD, Jiruska P, Jefferys JG, Vreugdenhil M. High-frequency gamma oscillations coexist with low-frequency gamma oscillations in the rat visual cortex in vitro. Eur J Neurosci 31: 1435–1445, 2010. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN, Elam M. EEG theta rhythm in infants and preschool children. Clin Neurophysiol 117: 1047–1062, 2006. [DOI] [PubMed] [Google Scholar]
- Paffen CL, van der Smagt MJ, te Pas SF, Verstraten FA. Center-surround inhibition and facilitation as a function of size and contrast at multiple levels of visual motion processing. J Vis 5: 571–578, 2005. [DOI] [PubMed] [Google Scholar]
- Palomares M, Englund JA, Ahlers S. Patterns and trajectories in Williams Syndrome: the case of visual orientation discrimination. Res Dev Disabil 32: 1021–1029, 2011. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14: 383–400, 2013. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull 54: 255–266, 2001. [DOI] [PubMed] [Google Scholar]
- Perry G, Hamandi K, Brindley LM, Muthukumaraswamy SD, Singh KD. The properties of induced gamma oscillations in human visual cortex show individual variability in their dependence on stimulus size. Neuroimage 68: 83–92, 2013. [DOI] [PubMed] [Google Scholar]
- Pinto JG, Hornby KR, Jones DG, Murphy KM. Developmental changes in GABAergic mechanisms in human visual cortex across the lifespan. Front Cell Neurosci 4: 16, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron 67: 885–896, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JK, Rorden C, Little JS, Parra LC. Subject position affects EEG magnitudes. Neuroimage 64: 476–484, 2013. [DOI] [PubMed] [Google Scholar]
- Roth MM, Helmchen F, Kampa BM. Distinct functional properties of primary and posteromedial visual area of mouse neocortex. J Neurosci 32: 9716–9726, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena N, Muthukumaraswamy SD, Diukova A, Singh K, Hall J, Wise R. Enhanced stimulus-induced gamma activity in humans during propofol-induced sedation. PLoS One 8: e57685, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf DS, Robertson DJ, Song C, Barnes GR, Rees G. The frequency of visually induced gamma-band oscillations depends on the size of early human visual cortex. J Neurosci 32: 1507–1512, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698–702, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroganova TA, Orekhova EV, Posikera IN. EEG alpha rhythm in infants. Clin Neurophysiol 110: 997–1012, 1999. [DOI] [PubMed] [Google Scholar]
- Swettenham JB, Muthukumaraswamy SD, Singh KD. Spectral properties of induced and evoked gamma oscillations in human early visual cortex to moving and stationary stimuli. J Neurophysiol 102: 1241–1253, 2009. [DOI] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011: 879716, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadin D, Kim J, Park S. Weakened center-surround interactions in visual motion processing in schizophrenia. Schizophrenia Bull 33: 545–545, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadin D, Lappin JS, Gilroy LA, Blake R. Perceptual consequences of centre-surround antagonism in visual motion processing. Nature 424: 312–315, 2003. [DOI] [PubMed] [Google Scholar]
- Tadin D, Silvanto J, Pascual-Leone A, Battelli L. Improved motion perception and impaired spatial suppression following disruption of cortical area MT/V5. J Neurosci 31: 1279–1283, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Kajola M, Simola J. Suppression of interference and artifacts by the Signal Space Separation Method. Brain Topogr 16: 269–275, 2004. [DOI] [PubMed] [Google Scholar]
- Tesche CD, Uusitalo MA, Ilmoniemi RJ, Huotilainen M, Kajola M, Salonen O. Signal-space projections of MEG data characterize both distributed and well-localized neuronal sources. Electroencephalogr Clin Neurophysiol 95: 189–200, 1995. [DOI] [PubMed] [Google Scholar]
- Thomson DJ. Spectrum estimation and harmonic analysis. Proc IEEE 70: 1055–1096, 1982. [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolic D, Singer W. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci 3: 17, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Neuenschwander S, Wibral M, Singer W. A new look at gamma? High- (>60 Hz) γ-band activity in cortical networks: function, mechanisms and impairment. Prog Biophys Mol Biol 105: 14–28, 2011. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput 35: 135–140, 1997. [DOI] [PubMed] [Google Scholar]
- van Pelt S, Boomsma DI, Fries P. Magnetoencephalography in twins reveals a strong genetic determination of the peak frequency of visually induced γ-band synchronization. J Neurosci 32: 3388–3392, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pelt S, Fries P. Visual stimulus eccentricity affects human gamma peak frequency. Neuroimage 78: 439–447, 2013. [DOI] [PubMed] [Google Scholar]
- Volman V, Behrens MM, Sejnowski TJ. Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci 31: 18137–18148, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacol 34: 2028–2040, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex. J Physiol 588: 2823–2838, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang J, Liang Z, Li GX, Wang YC, Ma YY, Zhou YF, Leventhal AG. Aging affects the neural representation of speed in Macaque area MT. Cereb Cortex 19: 1957–1967, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N, Grefkes C, Scheperjans F, Boy C, Amunts K, Schleicher A. Architectonics of the human cerebral cortex and transmitter receptor fingerprints: reconciling functional neuroanatomy and neurochemistry. Eur Neuropsychopharmacol 12: 587–599, 2002. [DOI] [PubMed] [Google Scholar]
- Zuiderbaan W, Harvey BM, Dumoulin SO. Modeling center-surround configurations in population receptive fields using fMRI. J Vis 12: 10, 2012. [DOI] [PubMed] [Google Scholar]