Abstract
Neuronal microcircuits, small, localized signaling motifs involving two or more neurons, underlie signal processing and computation in the brain. Compartmentalized signaling within a neuron may enable it to participate in multiple, independent microcircuits. Each A17 amacrine cell in the mammalian retina contains within its dendrites hundreds of synaptic feedback microcircuits that operate independently to modulate feedforward signaling in the inner retina. Each of these microcircuits comprises a small (<1 μm) synaptic varicosity that typically receives one excitatory synapse from a presynaptic rod bipolar cell (RBC) and returns two reciprocal inhibitory synapses back onto the same RBC terminal. Feedback inhibition from the A17 sculpts the feedforward signal from the RBC to the AII, a critical component of the circuitry mediating night vision. Here, we show that the two inhibitory synapses from the A17 to the RBC express kinetically distinct populations of GABA receptors: rapidly activating GABAARs are enriched at one synapse while more slowly activating GABACRs are enriched at the other. Anatomical and electrophysiological data suggest that macromolecular complexes of voltage-gated (Cav) channels and Ca2+-activated K+ channels help to regulate GABA release from A17 varicosities and limit GABACR activation under certain conditions. Finally, we find that selective elimination of A17-mediated feedback inhibition reduces the signal to noise ratio of responses to dim flashes recorded in the feedforward pathway (i.e., the AII amacrine cell). We conclude that A17-mediated feedback inhibition improves the signal to noise ratio of RBC-AII transmission near visual threshold, thereby improving visual sensitivity at night.
Keywords: amacrine, feedback, GABA, retina, microcircuit
integration of excitatory and inhibitory synaptic input underlies many forms of neural computation. Inhibitory components of neural circuits can operate on spatial scales ranging from microns (local inhibition) to millimeters (i.e., global/lateral inhibition; for review see Isaacson and Scanziani 2011). Amacrine cells, a diverse class of inhibitory interneuron in the retina, exemplify this broad range (Baccus et al. 2008; Briggman et al. 2011; Euler et al. 2002; Grimes et al. 2009). A17 amacrine cells feature inhibitory feedback circuits at the very small end of the spectrum: each A17 has within its dendritic arbor hundreds of micron-sized varicosities, each of which receives synaptic input from an individual rod bipolar cell (RBC) and provides local, reciprocal inhibitory feedback to the same RBC (see Fig. 1, A–D) (Ellias and Stevens 1980; Grimes et al. 2010; Nelson and Kolb 1985; Zhang et al. 2002). In vivo and in vitro studies indicate that A17 feedback inhibition regulates the time course of light-evoked signaling in RBCs (Dong and Hare 2003) and the dynamic range of feedforward signaling from the RBC to its other postsynaptic target, the AII amacrine cell (Grimes et al. 2009).
Fig. 1.

Each A17 amacrine cell contains hundreds of tiny feedback microcircuits. A: A17 amacrine cells have many long thin (<150 nm diameter) (Grimes et al. 2010) neurites that are studded with small synaptic varicosities (∼1 μm diameter). B: each A17 amacrine cell (green) contacts hundreds of rod bipolar cell axon terminals (red), forming small appositions between each varicosity and terminal pair. C: schematic diagram of reciprocal inhibitory synapse. D: single electron miscropic (EM) section showing both proximal (yellow arrow) and distal (red arrow) feedback synapses. Rod bipolar cell (RBC) ribbon is indicated by blue arrow. E: 3-dimensional (3D) serial electron micrograph reconstructions reveal the underlying synaptic morphology of the typical feedback circuit: 1 excitatory input and 2 reciprocal output synapses per varicosity. F: of those reconstructed varicosities with the 1:2 configuration all of the feedback synapses were located within 1 μm of excitatory input synapse. G: relative distances (from the input synapse) of the feedback synapses plotted as a function of radial distance from the center of each presynaptic ribbon, indicating a bimodal distribution, i.e., each feedback circuit exhibits both a proximal and distal output synapse. Open triangles indicate mean, and filled triangles indicate median.
Three-dimensional (3D) electron microscopy (EM) reconstructions indicate that each A17 varicosity typically receives one excitatory synaptic input and returns two inhibitory synapses back onto the same RBC terminal (Grimes et al. 2010), but it remains unknown whether the two synapses exhibit distinct molecular, physiological, and functional characteristics. GABA release from A17s can be triggered by Ca2+ influx through either Ca2+-permeable AMPA receptors (AMPARs) (Chavez et al. 2006) or Cav channels, although Cav channel activation is limited by Ca2+-activated K+ (BK) channels (Grimes et al. 2009), suggesting that the two feedback synapses might employ distinct release mechanisms. Moreover, GABAA and GABAC receptors (GABAARs and GABACRs) exhibit distinct localization in RBC terminals (Fletcher et al. 1998; Frazao et al. 2007) and distinct requirements for activation (Chavez et al. 2006), suggesting that they might be at least partially segregated between the two feedback synapses.
Here, we have combined 3D EM reconstructions with immunogold EM and electrophysiology to examine the location, receptor constituents, physiological characteristics, and functional roles of feedback synapses made by A17s. Our results suggest that the feedback synapses express distinct presynaptic components and postsynaptic GABAR populations. Specifically, GABA release from the synapse closest to the RBC ribbon appears to be triggered primarily by Ca2+ influx through Ca2+-permeable AMPARs and activates mostly GABAARs in the RBC membrane. GABA released from the second synapse, by contrast, would appear to activate mostly GABACRs and be regulated by Cav-BK channel complexes. We also find that A17-mediated feedback inhibition is highly engaged in low light conditions, even in absolute darkness, and enhances visual signaling near visual threshold.
METHODS
All experiments were performed in accordance with protocols approved by Institutional Animal Care and Use Committees at the National Institute of Neurological Disorders and Stroke (NINDS) Intramural Research Program and/or the University of Washington. Cx36−/− mice were generously provided by David Paul (Harvard Medical School).
Electrophysiology.
Electrophysiology experiments (see Figs. 3, B–D, and 4) were conducted at room temperature (RT; 22–25°C) using light-adapted retinal slices (210-μm thick) taken from Sprague-Dawley Rats [postnatal days (P)17–21], as previously described (Chavez et al. 2006; Singer and Diamond 2003). Rat retinas were isolated in artificial cerebrospinal fluid (ACSF) containing the following (in mM): 119 NaCl, 26 NaHCO3, 1.25 Na2HPO4, 2.5 KCl, 2.5 CaCl2, 1.5 MgSO4, 10 glucose, 2 Na-pyruvate, 4 Na-lactate, and equilibrated with 95% O2-5% CO2. For disynaptic experiments using rat retinas (see Fig. 3), ACSF was supplemented with the group III mGluR agonist L-AP4 (10 μM) to block RBC excitatory input, strychnine (1 μM) to block glycine receptors, and tetrodotoxin (TTX; 1 μM) to block voltage-gated sodium channels. For clearer observation of voltage-step-activated BK currents (see Fig. 4), the ACSF was supplemented with 4-AP (4 mM), TTX (1 μM), and NBQX (10 μM) to block A-type potassium channels, voltage-gated sodium channels, and GluARs, respectively.
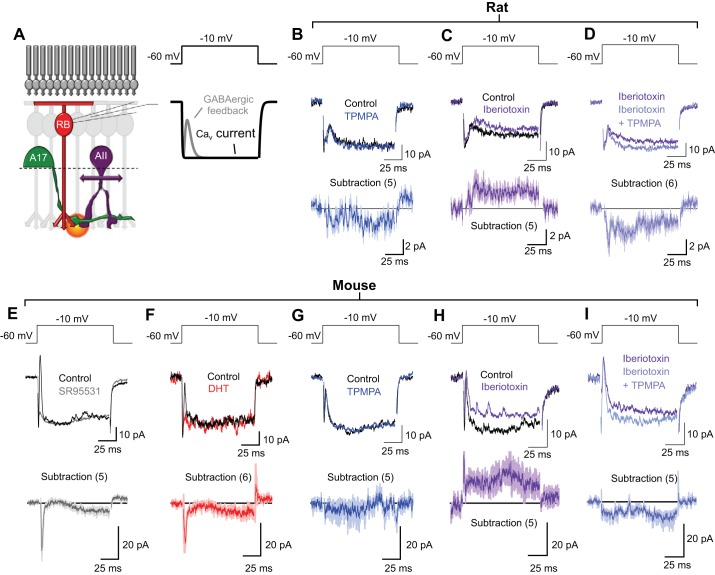
Fig. 3.
Direct recordings of feedback inhibition to RBCs indicate that Ca2+-activated K+ (BK) channels limit activation of GABACR-enriched feedback synapses onto RBC axon terminals. A: schematic diagram of a transverse retinal slice with a patch electrode recording from a RBC. B–I: voltage-clamp recordings from RBCs in rat (room temperature; B–D) and mouse (∼34°C; E–I) were used to elicit and record feedback inhibition from RBC (100-ms step from −60 to −10 mV). Top: voltage protocol for step-evoked feedback experiments. Middle: example traces from individual cells. Bottom: average subtracted current (drug minus control) across cells; thick lines represent the mean, shaded regions correspond to the standard error mean. B and G: under control conditions TPMPA had only small effects on the voltage step-evoked inhibitory postsynaptic current (vIPSC). C and H: bath application of iberiotoxin enhanced both the amplitude and prolonged the time course of vIPSCs. D and I: with BK channels blocked (i.e., the presence of iberiotoxin), TPMPA reduced the amplitude and sustained component of vIPSCs. E and F: bath application of SR95531 (E) or 5,7-dihydroxytryptamine (DHT; F) confirmed that, with BK channel activity intact, feedback inhibition in mouse is mediated primarily by GABAARs and activated by GABA release from A17s.
Fig. 4.
BK-Cav1.3 complexes are present in the retina, and functional BK-Cav1 complexes are expressed in A17 amacrine cells. A: postembedding immunogold labeling of BK channels (small arrowhead) at RBC-A17 reciprocal synapses. RBC ribbon indicated by large arrowhead. B: histogram showing distance of BK immunogold from the synaptic ribbon. C and D: coimmunoprecipitation experiments (see methods) indicated that BK channels form complexes with Cav1.3 (C) but not Cav1.2 (D) in rat retina. E–G: whole cell voltage-clamp recordings from A17 amacrine cells were used to directly probe BK-Cav interactions. A voltage step from −90 to −30 mV was used to elicit rapidly inactivating BK currents that were blocked completely by iberiotoxin. The amplitude of the iberiotoxin-sensitive (BK-mediated) current depended strongly on which exogenous chelator (EGTA or BAPTA) was included in the patch solution (recordings made ∼20 min after break-in). Recorded amplitudes were largest in those cells dialyzed with EGTA (E), a slower calcium chelator, whereas smaller BK currents were recorded from cells dialyzed with BAPTA (F). G: summary of BAPTA/EGTA experiments. *P < 0.05.
Electrophysiology experiments (see Figs. 3, E–I, 5, and 6) were conducted at near physiological temperature (∼34°C) using dark-adapted retinal slices (200-μm thick) taken from C57/BL6 mice (3–9 wk old), as previously described (Dunn et al. 2006; Dunn and Rieke 2008). Briefly, mice were dark adapted overnight before isolating retinas under infrared illumination (>950 nm). Retinas from were isolated and stored at 32°C in bicarbonate-based Ames solution equilibrated with 5% CO2-95% O2. While in the recording chamber, dark-adapted retinal mouse slices were continually perfused with Ames at a rate of 6–8 ml/min.
Whole cell voltage-clamp recordings were made from RBCs using pipettes (∼7–9 MΩ) containing the following (in mM): 100 Cs methanesulfonate, 20 TEA-Cl, 10 HEPES, 1.5 BAPTA, 10 Na phosphocreatine, 4 Mg-ATP, 0.4 Na-GTP, 10 l-glutamic acid, and 0.02 Alexa-488 or Alexa-750 hydrazide (pH 7.4). RBC access resistance was 25–50 MΩ and was not compensated. Whole cell voltage-clamp recordings were made from A17s using pipettes (∼5–6 MΩ) containing the following (in mM): 100 Cs methanesulfonate, 20 TEA-Cl, 10 HEPES, 10 EGTA, 10 Na phosphocreatine, 4 Mg-ATP, 0.4 Na-GTP, and 0.04 Alexa-594 hydrazide (pH 7.4). A17 access resistance was ≤30 MΩ and was not compensated. Whole cell voltage-clamp recordings were made from AIIs using pipettes (∼5–6 MΩ) containing the following (in mM): 105 Cs methanesulfonate, 10 TEA-Cl, 20 HEPES, 10 EGTA, 2 QX-314, 5 Mg-ATP, 0.5 Tris-GTP, and 0.1 Alexa-750 hydrazide (pH 7.2). AII access resistance was ≤25 MΩ and was ∼50% compensated.
The step-evoked feedback experiments (see Fig. 3) were limited to two conditions (e.g., control/TPMPA or iberiotoxin/iberiotoxin + TPMPA) to ensure that all data were collected while reciprocal feedback was stable (∼10–15 min). Recordings were only kept when feedback responses stabilized within the first 30–60 s after break-in.
Drugs were purchased from Sigma or Tocris (St. Louis, MO) with the exception of TTX (Alamone Labs, Jerusalem, Israel). All fluorescent dyes were purchased from Molecular Probes (Eugene, OR). Recordings were made using either an Axopatch 1D amplifier (rat) or Multiclamp 700B (mouse; Axon Instruments, Foster City, CA), which was interfaced with an Instrutech ITC-18 analog-to-digital board and controlled by custom software written for Igor Pro (Wavemetrics). Current responses (see Figs. 3 and 4) were collected at 12-, 20-, or 25-s intervals, low-pass filtered at 5 kHz, and digitized at 10 kHz.
Data analysis.
Electrophysiology data was analyzed using Igor Pro, Matlab, and/or Excel (Microsoft). Voltage steps (see Figs. 3 and 4) were leak subtracted using the p/4 subtraction protocol. The transient peak of the step-evoked feedback (see Fig. 3) was measured using the fitted baseline method described in Chavez et al. (2006). In short, the last 50 ms of the step response was fit with a straight line, and this fit was extrapolated to the first 50 ms of the step to provide a baseline for which to measure the peak response. The kinetic changes related to pharmacological manipulations were assessed for each cell by subtracting the mean response in control conditions from the mean response in the presence of pharmacological agents. Subtracted responses (see Fig. 3) represent the average subtraction across cells. Paired, two-tailed Student's t-tests were used to compare data sets, unless otherwise stated, and significance was determined as P < 0.05 or P < 0.01. Unless otherwise indicated, data are presented as means ± SD, and illustrated traces are averages of 5–10 responses.
The empirical measurements of the signal to noise ratio of dim flash responses were made using the average response to a particular flash (0.003, 0.007, 0.013, or 0.026 Rh*/rod/flash) as a template (Chichilnisky and Rieke 2005). We estimated signal and noise by first determining the correlation of the template with each flash response and with an equivalent period of recording in darkness for each flash strength. The signal was then determined as the difference between the mean correlation of the flash and dark trials. Noise was determined from the standard deviation of the dark correlations.
Temporal structure in the tonic excitatory input to AII amacrine cell was assessed using the autocorrelation function
where I(t) is the offset-corrected current recording, t is time, τ is a time lag, and σ2 is the variance of the recording.
The number of vesicles released from RBC synapses onto an AII during a single photon response (SPR; mSPR) was estimated by first calculating the charge transfer of the average response in an AII to a dim (0.0033 R*/rod) flash (2.0 ± 1.0 pC; n = 5; see Fig. 5) and dividing it by the average miniature excitatory postsynaptic currents (mEPSCs) charge transfer in mouse AIIs under similar experimental conditions (31.8 fC, adjusted to reflect different Vm; Jarsky et al. 2011). This quotient (63 ± 32) underestimates mSPR because many flashes elicit no response in the AII: One AII receives input from ∼500 rods (Tsukamoto et al. 2001), but the synapses between rods and RBCs exclude roughly 75% of the single photon signals (Field and Rieke 2002), so that each flash elicits an average of 0.41 (0.0033 × 500 × 0.25) single photon signals in the AII. The average value for mSPR, therefore, is 63 ÷ 0.41 = 152 vesicles.
Fig. 5.
Feedback circuits improve the sensitivity and fidelity of single photon transmission in the inner retina. A dim flash discrimination task used to study signal transmission near visual threshold (Chichilnisky and Rieke 2005). A: dim flashes of various strengths were delivered on subsequent trials to dark-adapted mouse retinal slices while recording EPSCs in AII amacrine cells. The signal to noise ratio (SNR) was derived by cross correlating the dim flash template (from the average flash response, blue, bottom trace) with the stimulus trials or the associated noise trials and then comparing the cross-correlations (>80 trials per flash strength; see methods). Bi and Bii: example responses recorded from an AII in a Cx36−/− retina. Very dim flashes (∼1 photon per AII collecting area per flash) gave rise to a significant number of failures and a relatively low SNR (<1). Ci and Cii: increasing the flash strength by a factor of 4 increased the mean amplitude of the response, reduced the number of failures and improved the SNR. D: average SNR measurements for 4 different flash strengths taken from wild-type (WT; n = 6) and Cx36−/− (n = 6) retinas (means ± SE). Electrical coupling in the WT retinas increases the apparent collecting area of AII amacrine cells, thereby enhancing sensitivity and SNR for dim flashes. E: eliminating A17 function with DHT (Chavez et al. 2006) reduced the SNR of responses to the dimmest flashes (flash strengths indicated by red box in D). Inset: typical average single photon responses from 1 cell in control solution and in the presence of DHT. F and G: effects of rundown during the control period (left) and subsequent application of DHT (right) on the standard deviation (σ) of noise and stimulus trials and the mean amplitude (μ) of dim flash responses in WT (F; n = 5) and Cx36−/− (G; n = 4) mice. *P < 0.05.
If each flash elicits an average of 0.41 single photon signals, Poisson statistics predicts that multiple photon signals would reach the AII on <7% of the trials.
Light stimulation.
Light from a blue LED (λpeak = 470 nm) was delivered to the dark-adapted retina slices through a custom multiport substage condenser. The light stimulus uniformly illuminated the tissue sample over a circular area with a diameter of 560 μm that was centered on the voltage-clamped AII amacrine cell. Photon flux at the retina was converted to photoisomerizations per rod (Rh*/rod) using the LED emission spectra, absorption spectra of rhodopsin, and an assumed rod collecting area of 0.5 μm2.
Serial EM reconstruction protocol.
Isolated retinas were fixed in 4% glutaraldehyde in 0.1 M cacodylate buffer at 4°C overnight and were then postfixed with 1% OsO4 for 1 h at 4°C. After being rinsed and dehydrated, tissues were embedded in Epon resin at 50°C for 1 day and then 60°C for 2 additional days. ∼70-nm serial sections were collected and counterstained with 5% uranyl acetate and 0.3% lead citrate and viewed on a JEOL 1200EX II EM. Reconstruct was used for 3D reconstruction of varicosities (details in Grimes et al. 2010).
Postembedding-immunogold EM protocol.
Immunogold labeling was performed on tissue taken from our previous study (Zhang and Diamond 2006). Briefly, ∼70-nm-thick ultrasections were collected on Formvar-Carbon coated nickel-slot grids. Grids were washed with distilled H2O followed by a Tris-buffered saline wash (TBS: 0.05 M Tris buffer, 0.7% NaCl, pH 7.6), incubated in 5% BSA in TBS for 30 min, and then incubated overnight at RT in one of several antibody solutions: 1) mouse anti-GluA3 [Millipore; monoclonal, raised against residues 245–451 (NH2 terminus); dilution 1:50]; 2) a cocktail of rabbit anti-GABACR [a generous gift from Dr. Heinz Wässle; polyclonal, raised against residues 16–171 (NH2 terminus) of the ρ-subunit; 1:100] and goat anti-GABAAR subunit (Santa Cruz Biotechnology; polyclonals, raised against the cytoplasmic domains of α1 and α3, 1:100; β2 and β3, 1:100; γ3, 1:50); 3) rabbit anti-BK α subunit [Sigma; polyclonal, raised against residues 1,184-1,200 (NH2-terminus); 1:100]. Antibody solutions included TBS-Triton (0.01% Triton X-100 in TBS, pH 7.6) with 2% BSA and 0.02 M NaN3. After being rinsed, grids were then incubated in a mixture of donkey anti-rabbit IgG (1:20) coupled to 6-nm gold particles and donkey anti-goat IgG (1:20) coupled to 10-nm gold particles (Electron Microscopy Sciences) or in donkey anti-rabbit IgG (1:20) coupled to 10-nm gold particles in TBS-Triton, pH 8.2, with 2% BSA and 0.02 M NaN3. Grids were counterstained with 5% uranyl acetate and 0.3% lead citrate and were viewed on a JEOL1200 EM and images were digitalized. A total of 21 and 23 rod bipolar cell dyads were analyzed for GABARs and BK channels, respectively, when they contained at least two gold particles at the synaptic sites.
Immunohistochemistry.
A17 amacrine cells from dissected mouse (C57/B6, 3–6 wk old) retinas were injected with 2% LY before fixation with 4% paraformaldehyde in 0.1 M phosphate buffer (PBS) for 20 min at RT. The retinas were rinsed with PBS and incubated for 3 days with rabbit polyclonal Lucifer yellow (1:500; Invitrogen) and mouse monoclonal PKC (1:500; Sigma) antibodies in PBS with 0.5% Triton and 5% goat serum. Secondary antibody incubation [anti-rabbit Alexa Fluor conjugate (Invitrogen) and anti-mouse DyLight conjugate (Jackson ImmunoResearch)] was carried out in PBS overnight, and the retinas were subsequently mounted in Vectashield (Vector Labs). Images were acquired with a Olympus FV1000 microscope and analyzed with MetaMorph (Universal Imaging) and Amira (Mercury Computer Systems).
Coimmunoprecipitation.
Rat (Sprague-Dawley; P19∼25) retinas were homogenized in cold buffer containing the following (in mM): 320 sucrose, 4 HEPES, 2 EDTA, 5 EGTA, 1 PMSF, and 1× protease inhibitors cocktail (Complete, Roche, IN), incubated with 1% Triton X-100 (vol/vol) 1 h at 4°C with rotation then centrifuged (5,000g, 20 min) at 4°C. Supernatant (SN-I) was saved. Pellet was solubilized in lysis buffer again that containing: 1% Triton X-100, 20 Tris·HCl, 2 EDTA, 5 EGTA, 1 PMSF, and 1× protease inhibitors cocktail for additional 30 min at 4°C with rotation. Unsolubilized proteins were removed by centrifuged (1,000 g, 5 min). Supernatant (SN-II) was saved and two supernatants (I and II) were pooled. Protein concentration was determined by BCA protein assay (Pierce Chemical, Rockford, IL).
Rabbit anti-Cav1.2 [Alomone Labs; polyclonal; raised against residues 848–865 (COOH terminus)], anti-Cav 1.3 [Alomone Labs; polyclonal; raised against residues 859–875 (COOH terminus)], anti-BK α-subunit [Sigma; polyclonal, raised against residues 1,184-1,200 (NH2 terminus)], or rabbit immunoglobulin G (IgG; Sigma) were mixed with lysate pooled protein (1∼2 ug/500 ug protein) and incubated 2 h to overnight with rotation at 4°C. The antibody-antigen complex was immobilized by add 30 μl of protein G plus beads (Pierce Chemical) and incubated for additional 1∼2 h with rotation at 4°C. The protein-beads mixtures were washed four times with 0.1% Triton X-100 (vol/vol) in lysis buffer. The beads were resuspended in reducing SDS sample buffer (Invitrogen, Carlsbad, CA) with 50 mM DTT and heated at 95°C for 10 min.
For immunoblot analysis, the immunoprecipitation proteins were separated by running 3∼8% NuPage Novex Tris-Acetate SDS gel (Invitrogen) electrophoresis. The separated proteins were transferred to nitrocellulose membranes and stored overnight at 4°C in transfer buffer (Invitrogen). Membranes were cut horizontally around 150 kDa. All membranes were blocked for 1 h at RT in TBST containing the following (in mM): 10 Tris·HCl pH 8.0 and 150 NaCl, with 0.1% Tween 20 plus 10% nonfat milk powder and then incubated with primary antibodies in TBST containing 2% nonfat milk powder for 2 h at RT or overnight at 4°C. Top membranes were incubated with αCav1.2 (1:400; Alomone Labs) or αCav 1.3 (1:300; Alomone Labs), and bottom membranes were incubated with αBK (1:4000; Sigma). After being rinsed, all membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:3,000; Chemicon) in TBST containing 2% nonfat milk powder and resolved by chemiluminescence (ECL; Pierce Chemical).
RESULTS
Structural and molecular assessment of the microcircuit.
Most RBC-A17 feedback microcircuits comprise one excitatory input and two inhibitory reciprocal output synapses (11 of 17 3D EM reconstructions; see Grimes et al. 2010 for details), all separated by <1 μm (Fig. 1D). In reconstructed varicosities containing two feedback synapses (n = 11), we observed that the inhibitory synapses were always located on the same side of the excitatory input (i.e., the presynaptic ribbon) and tended to align along a single axis (Fig. 1, E and F). In each varicosity, we refer to the inhibitory synapse closest to the ribbon as “proximal” (Fig. 1, D–G, yellow) and the more distant synapse as “distal” (Fig. 1, D–G, red). In 3D space, proximal synapses were located, on average, 220 ± 130 nm (mean ± SD, median = 165 nm) from the excitatory input synapse, whereas the distal synapse was located, on average, more than twice that distance (510 ± 150 nm; median = 490 nm; Fig. 1G).
Previous experiments showed that Ca2+ influx through postsynaptic AMPARs can trigger vesicular GABA release from A17s (Chavez et al. 2006). At most synapses, vesicle release machinery and Ca2+ channels are tightly colocalized within the presynaptic active zone (Issa and Hudspeth 1994; Jarsky et al. 2010). To determine where AMPARs are located relative to the feedback synapses, we examined GluAR localization with postembedding immunogold EM (see methods; Zhang and Diamond 2009). Antibodies to AMPAR subunits GluA1 and GluA2 exhibited no immunoreactivity (data not shown), consistent with previous work (Li et al. 2002). GluA3 immunoreactivity was localized to A17 varicosities (Fig. 2A) but, surprisingly, typically not in the postsynaptic membrane immediately apposed to the RBC synaptic ribbon; instead, most GluA3 immunogold was observed in the perisynaptic region, 212 ± 149 nm (median = 197 nm; n = 157 gold particles in 27 sections; Fig. 2B) from the region immediately opposite the ribbon, a distribution that overlapped substantially with that of the proximal feedback synapses (Fig. 1D). GluA4 immunoreactivity was inconsistent and was not studied further. These results suggest that GluA3-containing AMPARs are positioned to permit tight coupling between Ca2+ influx and vesicle release at the proximal feedback synapse.
Fig. 2.

Receptor localization within feedback microcircuits. A: postembedding immunogold labeling of GluA3 AMPA receptors (AMPARs) in A17 membranes. B: distribution of GluA3 immunogold relative to the synaptic ribbon. C: double immunogold labeling (see methods) was used to determine the types and distributions of inhibitory GABA receptors (GABARs) expressed at feedback synapses. D: immunogold particles, labeling GABAA (blue) or GABAC (red) receptors, were identified in single section micrographs and their locations are plotted relative to the synaptic ribbon. Approximate positions of feedback synapses, determined by vesicle clusters in the A17, are indicated by gray lines. Each row shows the location of the synapses GABAAR gold and GABACR gold in a single section. Multiple feedback synapses were distinguished in 15 of 21 sections. Bottom: histograms showing location, relative of the RBC ribbon of GABAAR and GABACR gold in all 21 sections.
Both GABAA- and GABACRs on RBC synaptic terminals can be activated by reciprocal feedback inhibition under various conditions (Chavez et al. 2010; Eggers and Lukasiewicz 2006a,b; Hartveit 1999), but the subsynaptic localization of the two receptor types relative to each other remains unclear. EM indicates that both receptor subtypes are localized to RBC-A17 synapses (Fletcher and Wassle 1999), but double-label immunofluorescence data suggest that two subtypes are typically not colocalized at the same synapses (Fletcher et al. 1998; Frazao et al. 2007).
We examined the subsynaptic localizations of GABAAR and/or GABACR using postembedding immunogold EM (Fig. 2, C and D). GABAAR antibody-tagged gold particles were typically found at feedback synapses and were located 258 ± 126 nm from the RBC ribbon (n = 49 gold particles in 21 sections; Fig. 2, C and D). GABACR antibody-tagged gold particles, by contrast, were located 362 ± 114 nm from the ribbon (n = 73 gold particles in 19 sections; Fig. 2, C and D), significantly further from the RBC ribbon than were GABAARs (GABAAR vs. GABACR: P = 7×10−6, Wilcoxon rank test). Both proximal and distal synapses were distinguished in 16 of 21 sections (Fig. 2D). Within this subset, 66% of GABAAR immunogold was located at proximal synapses, 31% was located at distal synapses, and 3% were nonsynaptic. By contrast, only 22% of GABACR immunogold were localized to proximal synapses, with 62% at distal synapses (16% nonsynaptic). These data indicate that GABAARs and GABACRs are specifically enriched at proximal and distal feedback synapses, respectively, but that segregation of the receptors is imperfect. Because GABACRs activate and deactivate much more slowly than GABAARs (Chang and Weiss 1999), even partial segregation of GABAR subtypes would suggest that the relative amount of GABA release from proximal and distal synapses may influence the dynamics of inhibitory feedback.
BK channels limit GABA release.
Previous work has shown that, when reciprocal feedback is elicited by depolarizing a single RBC under whole cell voltage clamp, the feedback inhibitory postsynaptic current (IPSC) is mediated primarily by GABAARs (Chavez et al. 2010; Singer and Diamond 2003). When synaptic activation of A17s is enhanced, either by blocking postsynaptic receptor desensitization or by augmenting release from RBCs, a GABACR-mediated component emerges in the feedback IPSC (Chavez et al. 2010; Hartveit 1999; Singer and Diamond 2003). The anatomical data presented in Fig. 1 and previous physiology results suggest two nonexclusive scenarios to explain the differential activation of GABAR subtypes:
1) Given the localization of the receptors (Fig. 2, A and B), AMPAR-mediated Ca2+ influx seems most likely to trigger GABA release preferentially at the closest (“proximal”) inhibitory synapse that contains primarily GABAARs. Calcium influx through AMPARs is likely restricted in space due to endogenous buffers, thereby limiting cross talk between the presynaptic release sites. Enhanced or desynchronized AMPAR-mediated Ca2+ signals may, however, recruit release from the “distal” synapse that is enriched with GABACRs.
2) Stronger stimulation of A17s has been shown to activate Cav channels, which also can trigger GABA release (Grimes et al. 2009). BK channels limit recruitment of Cav channels in A17s by opposing postsynaptic depolarization and may thereby regulate specifically Cav-mediated activation of distal, GABACR-containing synapses. Additionally, because GABACRs exhibit relatively slow binding and unbinding rates relative to GABAARs, they may be preferentially activated by desynchronized GABA release. By limiting prolonged depolarization of the A17 varicosity, BK channels may help to synchronize GABA release and thereby limit GABACR activation.
To test these possibilities, we examined the effects of blocking BK channels on feedback GABAergic IPSCs elicited by RBC depolarization (Fig. 3). The highly selective BK channel antagonist iberiotoxin (Galvez et al. 1990) has been shown to eliminate BK activity in A17s with no direct effects on RBCs or postsynaptic A17 AMPARs (Grimes et al. 2009). An iberiotoxin effect on the kinetics of GABAergic IPSCs recorded from RBCs would, therefore, support the second scenario proposed above. In acute rat retinal slices, RBC depolarization (a 100-ms step from −60 to −10 mV) elicited an inward, sustained Cav channel-mediated current and a transient outward feedback IPSC (Fig. 3, A and B). As shown previously (Chavez et al. 2006), the IPSC in control conditions was largely insensitive to the GABACR antagonist TPMPA (50 μM; transient peak reduced by 5 ± 7%; n = 5; P = 0.22; Fig. 3B; see methods) (Chavez et al. 2006). Blocking BK channels with iberiotoxin (100 nM) increased (transient peak increased by 60 ± 27%; n = 5; P = 0.003) and prolonged feedback inhibition (Fig. 3C); the slow component revealed by iberiotoxin was subsequently blocked by TPMPA (50 μM; Fig. 3D), suggesting that BK channels limit the activation of GABACRs. These results indicate that BK channels influence the synaptic activation of kinetically distinct GABARs, thereby regulating the dynamics of reciprocal feedback inhibition.
To facilitate comparison of these results with light-evoked responses recorded in mouse AII amacrine cells (presented below, see Figs. 5 and 6), we repeated the iberiotoxin experiments in C57/BL6 mouse retinal slices at ∼34°C. First, however, we confirmed that feedback IPSCs elicited by step depolarization of a voltage-clamped mouse RBC were, as in rat (Chavez et al. 2006), blocked by the GABAAR antagonist SR95531 (10 μM; transient peak reduced by 76 ± 17%; n = 5; P = 0.011; Fig. 3E). We also checked that reciprocal feedback inhibition in mouse RBCs was eliminated by 5,7-dihydroxytryptamine (DHT; 50 μM; transient peak reduced by 65 ± 7%; n = 6; P = 0.0007; Fig. 3F), a toxic serotonin analog that acutely eliminates A17 function (Chavez et al. 2006; Dong and Hare 2003), confirming previous results in rat (Chavez et al. 2006). Feedback IPSCs in mouse RBCs were slightly increased (transient peak increased by 9 ± 4%; n = 5; P = 0.015) and greatly prolonged by iberiotoxin (Fig. 3H): IPSCs were unaffected by the GABACR antagonist TPMPA in control conditions (transient peak reduced by 6 ± 9%; n = 5; P = 0.21; Fig. 3G), but iberiotoxin revealed a slower component in the IPSC that was sensitive to TPMPA (Fig. 3, H and I). These experiments indicate that BK channels regulate the time course of GABAergic feedback by limiting activation of GABACRs. They do not, however, exclude the possibility that BK channels may influence the synchrony of GABA release in a way that reduces GABACR activation or that GABA spillover may also contribute to GABACR activation.
Fig. 6.
Feedback inhibition from A17s shapes dark noise. A: dark noise recorded from an AII amacrine cell in control solution (i) and in the presence of DHT (ii). B: autocorrelations (30 s recording epochs from AII amacrine cells; see methods) indicated that DHT affects the temporal structure of the dark noise on multiple time scales. Insets: large synaptic noise events typically comprised numerous quantal-like synaptic events (arrows) that were more tightly clustered in control conditions (i) than in the presence of DHT (ii). C: full width at half maximum (FWHM) of the autocorrelations was broadened by DHT (i) but not by iberiotoxin (ii). D: temporal correlations on longer time scales (40–100 ms) were enhanced by DHT (i) and reduced by iberiotoxin (ii). Data in presented in C and D are presented as the means (thick line) and SE (shaded region) across cells. E: summarized effects of DHT and a cocktail of GABAR antagonists (INH: SR95531 + TPMPA), iberiotoxin (IBTX), and IBTX applied following application of DHT (means ± SD; no. of cells in parentheses). *P < 0.05 or **P < 0.01.
BK-Cav channel complexes are localized to distal synapses.
The results presented above indicate that BK channel activity in A17s limits GABA release and the activation of slower GABACRs. BK channels require high concentrations of intracellular Ca2+ to shift their voltage dependence of activation into the neuron's physiological range (Fakler and Adelman 2008); such large calcium signals (>10 μM) (Brenner et al. 2000) are likely restricted to the immediate vicinity of the Ca2+ influx through channels in the plasma membrane. In some neurons, BK channels form multimolecular complexes with Cav channels (Berkefeld et al. 2006), thereby ensuring access to Ca2+. The significant Ca2+ influx through calcium-permeable AMPARs in A17 synaptic varicosities (Chavez et al. 2006; Grimes et al. 2009), however, may be sufficient to activate BK channels and limit activation of CaV channels, provided that the BK channels are located close to the calcium-permeable AMPARs in the postsynaptic density of the excitatory ribbon synapse. To test this possibility, we examined the membrane localization of BK channels using postembedding immunogold directed toward the BK channel α subunit.
BK immunoreactivity was present near feedback synapses in A17 varicosities, consistent with previous immunofluorescence data (Grimes et al. 2009), but gold particles were localized 408 ± 144 nm from the ribbon synapse (mean ± SD; n = 78 particles in 23 sections, Fig. 4, A and B). The substantial distance between BK channel-associated gold particles and the synaptic ribbon suggests that BK channels are located primarily at distal feedback synapses. These results suggest that most BK channels in A17 varicosities are unlikely to be activated by Ca2+ influx through postsynaptic calcium-permeable AMPARs. It seems more likely, therefore, that BK channels are typically activated by Ca2+ influx through L-type Cav (Cav1) channels, which are also located in A17 feedback varicosities and mediate BK channel activation induced by membrane depolarization (Grimes et al. 2009).
To test whether BK channels form multimolecular complexes with Cav1 channels, we performed coimmunoprecipitation experiments with homogenized tissue from rat retina using antibodies to Cav1.2, Cav1.3, and the BK α-subunit (see methods). BK channels coimmunoprecipitated with Cav1.3 channels but not Cav1.2 channels (Fig. 4, C and D), even though Cav1.2 is also expressed in retina and has been shown to form complexes with BK channels in other brain areas (Berkefeld et al. 2006).
The coimmunoprecipitation experiments provide direct evidence that BK-Cav complexes are expressed in mammalian retina but, because the experiments were conducted on tissue from whole retina, they do not indicate specifically whether BK-Cav complexes are present in A17s. To test this, we made whole cell recordings from rat A17s and evoked transient, outward, iberiotoxin-sensitive currents with voltage steps from −90 to −30 mV (Fig. 4, E and F). Intracellular Ca2+ was buffered by either a slow (EGTA, 10 mM) or fast (BAPTA, 10 mM) Ca2+ chelator included in the patch pipette (>20-min dialysis period; see methods). If BK and Cav channels shared a highly localized (∼10 nm) signaling domain, BAPTA would disrupt BK activation by Cav-mediated Ca2+ influx more effectively than would EGTA (Fakler and Adelman 2008). If, however, BK and Cav channels were separated by a greater distance, BAPTA and EGTA would affect BK activation similarly. Larger BK currents were elicited with EGTA in the patch pipette compared with BAPTA (P = 0.015, unpaired t-test; n = 6; Fig. 4G), suggesting that BK and Cav channels are tightly colocalized in A17s.
These experiments indicate that most BK channels in A17s are exposed to cytoplasmic Ca2+ nanodomains surrounding open Cav channels. This, together with the localization of BK channels near distal feedback synapses (Fig. 4, A and B), suggests that BK channels may be activated by, and subsequently regulate, the same Cav channels that trigger GABA release at the distal feedback synapse.
A17 feedback enhances sensitivity at visual threshold.
A17-mediated feedback inhibition has been shown to shape rod pathway-mediated visual responses in vivo (Dong and Hare 2003), but it is not known how these feedback microcircuits influence retinal signaling near visual threshold, a condition for which the rod pathway is optimized (Field et al. 2005). To answer this question, we recorded light-evoked responses from mouse AII amacrine cells, which provide the feedforward signaling pathway that conveys RBC synaptic output to downstream targets (Fig. 5A). Synapses between RBCs and AII amacrine cells have a high gain in darkness; this, coupled with convergence from multiple RBCs (∼3–4 RBCs make direct contact, while gap junctions with other AIIs increases the collection to ∼20 RBCs), enables AIIs to collect input (indirectly) from ∼500 rods (Sterling et al. 1988; Tsukamoto et al. 2001). Consequently, AII amacrine cells are very sensitive to dim flashes but also highly susceptible to upstream noise sources (Dunn et al. 2006; Grimes et al. 2014).
To test the impact of feedback microcircuits on signaling at visual threshold, we recorded EPSCs from voltage-clamped AII amacrine cells while delivering dim flashes to dark-adapted mouse retinal slices (Fig. 5, B and C). Synaptic input to AII amacrine cells, both in the dark and following a dim flash, was highly variable. To quantify the sensitivity of the AII responses, we measured the signal to noise ratio (SNR) for a given flash strength. The dimmest of the four flash strengths tested (0.0033 photoisomerizations or Rh* per rod) produced SPRs or failures in individual RBCs (Poisson statistics predict that, at this intensity, <3% of the flashes would produce multiple photon absorptions in the ∼20 rods providing input to an RBC; see methods).
Examining the role of A17-mediated reciprocal feedback inhibition in signaling evoked by single photons is complicated by the fact that GABARs also mediate inhibitory inputs onto RBCs from other amacrine cells (Chavez et al. 2010; Eggers and Lukasiewicz 2006a) as well as inhibition between amacrine cells (Eggers and Lukasiewicz 2010). The effect of blocking either GABAARs or GABACRs would, therefore, be ambiguous under physiological conditions. Instead, we tested the effects of DHT, the A17-specific toxin (Fig. 3F; see methods) (Chavez et al. 2006, 2010; Dong and Hare 2003). Flashes were chosen to be just detectable (SNR ∼0.5) while minimizing contamination from coincident SPRs traversing individual RBCs (Fig. 5D, red box). Elimination of reciprocal feedback with 50 μM DHT reduced the SNR of the response (P = 0.005; n = 9; Fig. 5E) but did not affect the response time course (Fig. 5E, inset).
This data set included recordings from five retinas taken from wild-type (WT) mice and four retinas taken from transgenic mice lacking the gene (Gjd2) that encodes connexin 36 (Cx36) proteins (see methods; Fig. 5D); recordings from mice lacking Cx36-containing gap junctions (between neighboring AII amacrine cells and between AII amacrine cells and ON cone bipolar cells) ensured that dim flash responses were mediated entirely by direct synaptic input from RBC synapses. As expected from a reduction in the effective collecting area of the AII amacrine cell, the SNR of responses recorded in Cx36−/− retinas was lower than those measured in WT retinas (Fig. 5D) (Dunn et al. 2006). Elimination of reciprocal feedback with 50 μM DHT reduced the SNR of dim flash responses in Cx36−/− mice (P = 0.048; n = 4; Fig. 5E).
The observed reduction in SNR by DHT could reflect changes in signal, noise, or both. To control for the possible effects of rundown, we compared the mean response size (μ) and standard deviation (σ) of the background noise during the first and last 50 trials of the control component of an experiment (Fig. 5, F and G, left; the number of control trials ranged from 190 to 390). Recordings in WT retinas showed no significant changes in the signal and noise distributions over this time frame (Fig. 5F, left; n = 5). Cx36−/− mice did show time-dependent rundown, but all parameters were affected similarly (Fig. 5G, left; n = 4). Subsequent application of DHT affected both signal and noise, but the largest effect was on the signal (WT: 51 ± 7% of control, P = 0.024, n = 5; Cx36−/−: 42 ± 24% of control, P = 0.041, n = 4; Fig. 5, F and G). The increase in the coefficient of variation (CV = σ/μ) of responses to the criterion flash is consistent with a reduction in presynaptic release and not primarily a reduction of postsynaptic sensitivity, as the latter should affect signal and noise equally (Faber and Korn 1991). This mechanistic interpretation is consistent with evidence that presynaptic depression mediated through stimulus-dependent reductions in the readily releasable pool (RRP) of synaptic vesicles figures prominently in the operation of this synapse (Dunn and Rieke 2008; Oesch and Diamond 2011; Singer and Diamond 2006). These results collectively indicate that reciprocal feedback inhibition enhances visual sensitivity in darkness by reducing presynaptic depression, possibly by minimizing spontaneous vesicle release in the dark and hence maximizing the RRP.
Feedback microcircuits are highly engaged in darkness.
The RBC-A17 synaptic microcircuit allows feedback inhibition to influence presynaptic release both rapidly and locally, suggesting that reciprocal inhibition may influence the amplitude and time course of release at individual synapses. Surprisingly, we found that the amplitude of the average dim flash response decreased when feedback was eliminated, whereas the influence on response kinetics was highly variable. To investigate the impact of A17 feedback on the kinetics of release, we recorded tonic excitatory input to AII amacrine cells in WT and Cx36−/− mouse retinas in complete darkness (Fig. 6). These recordings revealed that tens to hundreds of large-amplitude spontaneous excitatory synaptic events were transmitted to each AII amacrine cell per second in the absence of any visual stimulus (i.e., synaptic noise; Grimes et al. 2014; Fig. 6Ai). As indicated in Fig. 5, B and C (a Cx36−/− example), noise events were similar in amplitude to the dim flash responses but had much faster kinetics. The high frequency and apparently compound nature of these noise events (Fig. 6Bi) precluded standard deconvolution analysis based on quantal events. Instead, we examined autocorrelations of the raw traces to extract the dominant temporal structure of the fluctuating synaptic input (see methods; Fig. 6B). The full width at half maximum (FWHM) of the autocorrelation function reflected the time course of the compound noise events in darkness (25 ± 7 ms, n = 28; Fig. 6B), which was broader than expected for independent AMPAR-mediated mEPSCs (Singer et al. 2004). We also observed correlations on time scales longer that the apparent duration of individual noise events (Δtlag = 40–100 ms; average AC40-100ms: 0.11 ± 0.04, n = 28), which might reflect occasional desynchronized neurotransmitter release from RBCs or single-photon-like (low frequency) noise events generated in rods.
Bath application of DHT (50 μM) altered the temporal structure of the noise events (Fig. 6, A and B) (Freed et al. 2003), indicating that reciprocal inhibition from A17s to RBCs shapes inner-retinal synaptic noise under dark-adapted conditions. In particular, DHT extended the time course of the brief noise events, as evinced by the increase in the FWHM of the autocorrelation function (Fig. 6Ci). We interpret these effects to indicate that feedback inhibition elicited by the initial EPSCs within a compound event inhibits subsequent release from the RBC, thereby sharpening the temporal characteristics of the compound event (Freed et al. 2003); the time course of individual quantal events within the compound event appeared to be unaffected. DHT also altered the autocorrelation function on longer time scales, particularly in the 40- to 100-ms time lag window (Fig. 6Di). These results suggest that A17 feedback microcircuits are active in darkness and help to synchronize spontaneous transmitter release from RBCs in the absence of visual stimuli. Similar results were obtained using a full inhibitory receptor antagonist cocktail (20 μM SR95531, 50 μM TPMPA, and 1 μM strychnine), suggesting that A17-mediated feedback inhibition dominates inhibitory control of rod bipolar output in darkness (Fig. 6E).
As mentioned above, the multifarious nature of synaptic inhibition in the inner retina complicates direct interpretation of GABAAR and/or GABACR antagonists applied in isolation. However, the results presented in Fig. 3 suggest that BK channels limit the slower, GABACR-mediated component of reciprocal feedback inhibition, suggesting that GABACR-mediated feedback should suppress correlations in the 40- to 100-ms time lag window of the autocorrelation function in darkness. To examine the regulation of feedback inhibition by BK channels in complete darkness, we bath applied the BK channel antagonist iberiotoxin (100 nM) while recording from AII amacrine cells. As indicated by the autocorrelation function, iberiotoxin reduced temporal correlations in synaptic dark noise specifically on longer time scales (Fig. 6Dii). Importantly, iberiotoxin's effects were occluded by prior application of DHT (Fig. 6E), indicating that its actions were due specifically to blockade of BK channels on A17s.
Together these results indicate that reciprocal feedback from A17 shapes the time course of synaptic release from RBCs in the dark; this process is mediated primarily by GABAARs as GABACRs are largely held in check by the BK complexes present in A17 feedback varicosities. We conclude that feedback synapses are highly engaged in complete darkness and speed the time course of synaptic dark noise. By keeping noise events brief, feedback microcircuits minimize the inevitable reduction in presynaptic occupancy while ongoing microcircuit activity maintains high sensitivity to presynaptic voltage fluctuations.
DISCUSSION
Signaling fidelity, specificity, and speed often rely on tight colocalization of interacting proteins. In A17-RBC feedback synapses, receptors, channels, and release sites are arranged to create distinct signaling compartments that give rise to two inhibitory feedback synapses with distinct GABA release and response characteristics. Our experiments reveal that even very small circuit motifs can exhibit complex morphology and multimodal synaptic function. Feedback synapses with different GABA release mechanisms (i.e., Ca2+ influx through Ca2+-permeable AMPARs vs. Cavs) in the A17 and different receptor populations (GABAARs vs. GABACRs) in the RBC appear to exert distinct effects on feedforward signaling in the retina. Furthermore, we show that the relative activation of these distinct feedback synapses is regulated by macromolecular BK-complexes in a way that influences signaling near visual threshold.
Reciprocal feedback shapes signaling and enhances sensitivity at visual threshold.
Due to convergence and divergence in the mouse rod pathway, a SPR is passed to an AII amacrine cell via 10–50 ribbon synapses (Tsukamoto and Omi 2013). SPRs observed here in AIIs exhibit a synaptic quantal content of ∼150 (see methods), suggesting that each participating ribbon synapse releases, on average, at least three synaptic vesicles, which is a significant fraction of its maximal RRP (∼7–8 vesicles) (Singer and Diamond 2006). Accordingly, SPRs induce synaptic depression, probably by partially depleting the RRP (Dunn and Rieke 2008; Singer and Diamond 2006). Our results indicate that A17-mediated feedback inhibition increases the quantal content of the SPR measured in AII amacrine (Fig. 5). By limiting background release from RBC terminals, feedback inhibition may allow more vesicles to occupy the RRP in darkness (Oesch and Diamond 2011) so that more vesicles are available to contribute to the SPR. In this scenario, blocking A17 feedback would be expected to increase dark synaptic activity recoded in AIIs. In our AII recordings, however, the holding current in darkness, and DHT's effect on it, was highly variable across cells (data not shown), preventing us from confirming this prediction with statistical confidence. Regardless of the specific mechanism underlying enhancement of the SPR, our results indicate that both feedforward and feedback signaling pathways are highly engaged in darkness and suggest that the correct balance of excitation and inhibition optimizes the sensitivity and gain of the rod pathway under very dim lighting conditions.
These interpretations were formed under the assumption that DHT acts specifically on A17 amacrine cells, based on previous work showing that DHT does not affect photoreceptor light responses, RBC Cav currents or sensitivity to GABA, postsynaptic responsivity of AII amacrine cells, or glycinergic and other GABAergic feedback inhibition onto RBC terminals (Chavez and Diamond 2008; Chavez et al. 2006, 2010; Dong and Hare 2003; Grimes et al. 2009; Nakatsuka and Hamasaki 1985). In rabbit retina, however, three different amacrine cell types accumulate indoleamines (Sandell and Masland 1988), and all appear susceptible to DHT (Dong and Hare 2003), which must be taken up and metabolized inside the cell by monoamine oxidase to become toxic (Baumgarten et al. 1978; Chavez et al. 2006). Two types, S1 and S2, have been studied most extensively (Sandell and Masland 1988; Vaney 1986; Zhang et al. 2002); A17s in rat retina morphologically correspond to the S2 in rabbit (Grimes et al. 2010; Menger and Wassle 2000). Rodent correlates to the other indoleamine-accumulating amacrine cells have not been reported, but we cannot definitively exclude the possible existence of other DHT-sensitive cells in the retinal circuitry.
Reciprocal feedback inhibition sharpens the temporal components of synaptic dark noise (at the level of RBC output), shifting the power of background synaptic signals in AIIs to higher frequencies (Fig. 6) while enhancing the gain of SPRs, which comprise lower frequency components (Fig. 5). These transformations may have important implications for the next step in the rod pathway, wherein AIIs transmit the light-evoked signal through gap junctions to ON cone bipolar cells (Kolb 1979). These electrical synapses constitute a static low-pass filter with a cutoff frequency of ∼100 Hz (Veruki and Hartveit 2002). By speeding feedforward transmission (in darkness) on this time scale (5–20 ms; Fig. 6), feedback inhibition may improve the selective suppression of dark noise by these gap junctions, clearing the way for single-photon transmission to the ON cone bipolar cell terminal from the rod pathway. Further experiments are required to test these predictions.
Dual-component GABAergic inhibition.
Reciprocal feedback connections between RBCs and A17 amacrine cells employ specialized morphological and biophysical features to shape feedforward signaling in the rod pathway (Chavez et al. 2006; Grimes et al. 2009; Grimes et al. 2010). Here, we show that feedback inhibition within these microcircuits is dispensed through adjacent, molecularly distinct GABAergic synapses. Ca2+ influx through postsynaptic Ca2+-permeable AMPARs triggers GABA release at a nearby feedback synapse apposed primarily to GABAARs in the RBC terminal membrane (Chavez et al. 2006). Release from a second, more distant feedback synapse appears to be regulated by BK channels, which form tight signaling complexes with Cav1.3 channels, limiting Cav channel activation and their consequent contribution to GABA release under some conditions. When this BK-mediated suppression is overcome, GABA release from the distal synapse recruits the activation of GABACRs in the RBC membrane. Accordingly, in the rabbit retina GABACR-mediated feedback from A17s shapes visual responses to bright flashes from darkness in vivo (Dong and Hare 2003). Similar experiments in mice showed that genetic elimination of BK channels also shapes the visual response to brighter flashes on a dark background (Tanimoto et al. 2012).
Our study provides insight into the role of A17-mediated feedback inhibition at visual threshold, conditions for which visual signals are often very sparse in space and time. Here, we show that reciprocal feedback enhances the SNR of SPRs recorded in AII amacrine cells; in the absence of light stimuli, BK channels regulate slower correlations in “dark noise,” suggesting that BK channels limit GABACR-mediated feedback under dark-adapted conditions. Taken together, our results suggest that GABAAR-mediated feedback is highly engaged in darkness and plays an important role in shaping tonic network activity while maximizing visual sensitivity. These results demonstrate a remarkable level of complexity within a small (∼1 μm) microcircuit and show, for the first time, that A17-mediated feedback inhibition influences retinal signaling at visual threshold.
GABACRs are expressed in many different retinal neurons, including horizontal cells (Blanco et al. 1996; Dong and Werblin 1996; Enz et al. 1996; Qian and Dowling 1993), cone photoreceptors (Pattnaik et al. 2000), a subset of ganglion cells (Rotolo and Dacheux 2003), and, most notably, bipolar cells (Feigenspan et al. 1993; Lukasiewicz and Werblin 1994; Matthews et al. 1994; Pan and Lipton 1995). GABACRs do not appear to be expressed by amacrine cells and do not mediate inhibition within amacrine cell networks (Lukasiewicz and Shields 1998; Zhou and Fain 1995). Although GABACRs may mediate feedforward inhibition onto some ganglion cells (Rotolo and Dacheux 2003), they appear primarily involved, together with GABAARs, in feedback inhibition onto bipolar cell synaptic terminals (Lukasiewicz et al. 2004; Lukasiewicz and Shields 1998; Sagdullaev et al. 2006). Feedback inhibition extends the dynamic range of photopic (day vision) responses in ganglion cells (Sagdullaev et al. 2006), but specific roles for GABAAR- and GABACR-mediated components have not been determined.
Our results indicate that, in the rod pathway, features of the A17-RBC feedback microcircuit allow for distinct activation of GABAARs and GABACRs. First, the two receptor types are largely segregated at adjacent synapses (Fig. 2), a morphological arrangement that may resolve the paradoxical observations that both receptors are expressed at A17-RBC synapses but are typically not colocalized (Fletcher et al. 1998; Frazao et al. 2007). This segregation also may explain why feedback miniature IPSCs in RBCs are mediated only by GABAARs (Palmer 2006), although it is also possible that GABACRs, with a relatively slow GABA binding rate (Chang and Weiss 1999), are less readily activated by a very brief GABA transient arising from the release of a single vesicle (Chavez et al. 2010).
Second, BK channels in A17 feedback varicosities limit postsynaptic depolarization, Cav channel activation, and the consequent Cav-triggered GABA release (Grimes et al. 2009). Our results suggest that BK channels preferentially limit GABA release at distal feedback synapses that are enriched with GABACRs (Fig. 3). These results suggest that GABA release at proximal, GABAAR-enriched synapses is triggered by Ca2+ influx through calcium-permeable AMPARs (Chavez et al. 2006), whereas release at distal, GABACR-enriched synapses is triggered by Cav channels that are regulated by BK channels. One result countering this idea is that feedback elicited by exogenous glutamate application onto A17s can activate GABACRs on RBCs even when Cavs are blocked (Chavez et al. 2006). This result may, however, reflect GABA spillover between adjacent synapses (but see Chavez et al. 2010) or the exogenous activation of extrasynaptic AMPARs on A17 neurites. Additionally, Ca2+ released from ryanodine receptor-operated intracellular stores enhances GABA release from A17s (Chavez et al. 2006) and may also mediate interactions between the two synapses.
GRANTS
This work was supported by the NINDS Intramural Research Program (to J. S. Diamond) and the Howard Hughes Medical Institute (to F. Rieke). M. Hoon was supported by National Eye Institute Grant EY-10699 (to R. Wong).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.N.G., F.R., and J.S.D. conception and design of research; W.N.G., J.Z., H.T., C.W.G., and M.H. performed experiments; W.N.G., J.Z., H.T., C.W.G., M.H., and F.R. analyzed data; W.N.G., H.T., C.W.G., F.R., and J.S.D. interpreted results of experiments; W.N.G., J.Z., H.T., M.H., and J.S.D. prepared figures; W.N.G. drafted manuscript; W.N.G., F.R., and J.S.D. edited and revised manuscript; W.N.G., J.Z., H.T., C.W.G., M.H., F.R., and J.S.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Paul Newman, Mike Ahlquist, Mark Cafaro, and the NINDS EM facility for their technical assistance.
REFERENCES
- Baccus SA, Olveczky BP, Manu M, Meister M. A retinal circuit that computes object motion. J Neurosci 28: 6807–6817, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten HG, Klemm HP, Lachenmayer L, Bjorklund A, Lovenberg W, Schlossberger HG. Mode and mechanism of action of neurotoxic indoleamines: a review and a progress report. Ann NY Acad Sci 305: 3–24, 1978. [DOI] [PubMed] [Google Scholar]
- Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science 314: 615–620, 2006. [DOI] [PubMed] [Google Scholar]
- Blanco R, Vaquero CF, de la Villa P. The effects of GABA and glycine on horizontal cells of the rabbit retina. Vision Res 36: 3987–3995, 1996. [DOI] [PubMed] [Google Scholar]
- Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000. [DOI] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature 471: 183–188, 2011. [DOI] [PubMed] [Google Scholar]
- Chang Y, Weiss DS. Channel opening locks agonist onto the GABAC receptor. Nat Neurosci 2: 219–225, 1999. [DOI] [PubMed] [Google Scholar]
- Chavez AE, Diamond JS. Diverse mechanisms underlie glycinergic feedback transmission onto rod bipolar cells in rat retina. J Neurosci 28: 7919–7928, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci 30: 2330–2339, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature 443: 705–708, 2006. [DOI] [PubMed] [Google Scholar]
- Chichilnisky EJ, Rieke F. Detection sensitivity and temporal resolution of visual signals near absolute threshold in the salamander retina. J Neurosci 25: 318–330, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CJ, Hare WA. Temporal modulation of scotopic visual signals by A17 amacrine cells in mammalian retina in vivo. J Neurophysiol 89: 2159–2166, 2003. [DOI] [PubMed] [Google Scholar]
- Dong CJ, Werblin FS. Use-dependent and use-independent blocking actions of picrotoxin and zinc at the GABAC receptor in retinal horizontal cells. Vision Res 36: 3997–4005, 1996. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the Mammalian retina. J Neurosci 26: 3959–3970, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Rieke F. Single-photon absorptions evoke synaptic depression in the retina to extend the operational range of rod vision. Neuron 57: 894–904, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABA(A), GABA(C) and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol 572: 215–225, 2006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. J Neurophysiol 103: 25–37, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci 26: 9413–9425, 2006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellias SA, Stevens JK. The dendritic varicosity: a mechanism for electrically isolating the dendrites of cat retinal amacrine cells? Brain Res 196: 365–372, 1980. [DOI] [PubMed] [Google Scholar]
- Enz R, Brandstatter JH, Wassle H, Bormann J. Immunocytochemical localization of the GABAc receptor rho subunits in the mammalian retina. J Neurosci 16: 4479–4490, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 418: 845–852, 2002. [DOI] [PubMed] [Google Scholar]
- Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J 60: 1288–1294, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron 59: 873–881, 2008. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Wassle H, Bormann J. Pharmacology of GABA receptor Cl- channels in rat retinal bipolar cells. Nature 361: 159–162, 1993. [DOI] [PubMed] [Google Scholar]
- Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron 34: 773–785, 2002. [DOI] [PubMed] [Google Scholar]
- Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: from behavior to mechanism. Annu Rev Physiol 67: 491–514, 2005. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Koulen P, Wassle H. GABAA and GABAC receptors on mammalian rod bipolar cells. J Comp Neurol 396: 351–365, 1998. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Wassle H. Indoleamine-accumulating amacrine cells are presynaptic to rod bipolar cells through GABA(C) receptors. J Comp Neurol 413: 155–167, 1999. [DOI] [PubMed] [Google Scholar]
- Frazao R, Nogueira MI, Wassle H. Colocalization of synaptic GABA(C)-receptors with GABA (A)-receptors and glycine-receptors in the rodent central nervous system. Cell Tissue Res 330: 1–15, 2007. [DOI] [PubMed] [Google Scholar]
- Freed MA, Smith RG, Sterling P. Timing of quantal release from the retinal bipolar terminal is regulated by a feedback circuit. Neuron 38: 89–101, 2003. [DOI] [PubMed] [Google Scholar]
- Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem 265: 11083–11090, 1990. [PubMed] [Google Scholar]
- Grimes WN, Hoon M, Briggman KL, Wong RO, Rieke F. Cross-synaptic synchrony and transmission of signal and noise across the mouse retina. Elife 3: e03892, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Li W, Chavez AE, Diamond JS. BK channels modulate pre- and postsynaptic signaling at reciprocal synapses in retina. Nat Neurosci 12: 585–592, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Zhang J, Graydon CW, Kachar B, Diamond JS. Retinal parallel processors: more than 100 independent microcircuits operate within a single interneuron. Neuron 65: 873–885, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol 81: 2923–2936, 1999. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron 72: 231–243, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa NP, Hudspeth AJ. Clustering of Ca2+ channels and Ca2+-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc Natl Acad Sci USA 91: 7578–7582, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Cembrowski M, Logan SM, Kath WL, Riecke H, Demb JB, Singer JH. A synaptic mechanism for retinal adaptation to luminance and contrast. J Neurosci 31: 11003–11015, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Tian M, Singer JH. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J Neurosci 30: 11885–11895, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol 8: 295–329, 1979. [DOI] [PubMed] [Google Scholar]
- Li W, Trexler EB, Massey SC. Glutamate receptors at rod bipolar ribbon synapses in the rabbit retina. J Comp Neurol 448: 230–248, 2002. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Eggers ED, Sagdullaev BT, McCall MA. GABAC receptor-mediated inhibition in the retina. Vision Res 44: 3289–3296, 2004. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Shields CR. A diversity of GABA receptors in the retina. Semin Cell Dev Biol 9: 293–299, 1998. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Werblin FS. A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. J Neurosci 14: 1213–1223, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G, Ayoub GS, Heidelberger R. Presynaptic inhibition by GABA is mediated via two distinct GABA receptors with novel pharmacology. J Neurosci 14: 1079–1090, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger N, Wassle H. Morphological and physiological properties of the A17 amacrine cell of the rat retina. Vis Neurosci 17: 769–780, 2000. [DOI] [PubMed] [Google Scholar]
- Nakatsuka K, Hamasaki DI. Destruction of the indoleamine-accumulating amacrine cells alters the ERG of rabbits. Invest Ophthalmol Vis Sci 26: 1109–1116, 1985. [PubMed] [Google Scholar]
- Nelson R, Kolb H. A17: a broad-field amacrine cell in the rod system of the cat retina. J Neurophysiol 54: 592–614, 1985. [DOI] [PubMed] [Google Scholar]
- Oesch NW, Diamond JS. Ribbon synapses compute temporal contrast and encode luminance in retinal rod bipolar cells. Nat Neurosci 14: 1555–1561, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MJ. Functional segregation of synaptic GABAA and GABAC receptors in goldfish bipolar cell terminals. J Physiol 577: 45–53, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZH, Lipton SA. Multiple GABA receptor subtypes mediate inhibition of calcium influx at rat retinal bipolar cell terminals. J Neurosci 15: 2668–2679, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik B, Jellali A, Sahel J, Dreyfus H, Picaud S. GABAC receptors are localized with microtubule-associated protein 1B in mammalian cone photoreceptors. J Neurosci 20: 6789–6796, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Dowling JE. Novel GABA responses from rod-driven retinal horizontal cells. Nature 361: 162–164, 1993. [DOI] [PubMed] [Google Scholar]
- Rotolo TC, Dacheux RF. Two neuropharmacological types of rabbit ON-alpha ganglion cells express GABAC receptors. Vis Neurosci 20: 373–384, 2003. [DOI] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron 50: 923–935, 2006. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Masland RH. Photoconversion of some fluorescent markers to a diaminobenzidine product. J Histochem Cytochem 36: 555–559, 1988. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci 23: 10923–10933, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol 95: 3191–3198, 2006. [DOI] [PubMed] [Google Scholar]
- Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci 7: 826–833, 2004. [DOI] [PubMed] [Google Scholar]
- Sterling P, Freed MA, Smith RG. Architecture of rod and cone circuits to the on-beta ganglion cell. J Neurosci 8: 623–642, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto N, Sothilingam V, Euler T, Ruth P, Seeliger MW, Schubert T. BK channels mediate pathway-specific modulation of visual signals in the in vivo mouse retina. J Neurosci 32: 4861–4866, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci 21: 8616–8623, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Omi N. Functional allocation of synaptic contacts in microcircuits from rods via rod bipolar to AII amacrine cells in the mouse retina. J Comp Neurol 521: 3541–3555, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI. Morphological identification of serotonin-accumulating neurons in the living retina. Science 233: 444–446, 1986. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Hartveit E. Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. J Neurosci 22: 10558–10566, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Diamond JS. Distinct perisynaptic and synaptic localization of NMDA and AMPA receptors on ganglion cells in rat retina. J Comp Neurol 498: 810–820, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Diamond JS. Subunit- and pathway-specific localization of NMDA receptors and scaffolding proteins at ganglion cell synapses in rat retina. J Neurosci 29: 4274–4286, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li W, Trexler EB, Massey SC. Confocal analysis of reciprocal feedback at rod bipolar terminals in the rabbit retina. J Neurosci 22: 10871–10882, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZJ, Fain GL. Neurotransmitter receptors of starburst amacrine cells in rabbit retinal slices. J Neurosci 15: 5334–5345, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]