Abstract
In individuals with motor-complete spinal cord injury, epidural stimulation of the lumbosacral spinal cord at 2 Hz evokes unmodulated reflexes in the lower limbs, while stimulation at 22–60 Hz can generate rhythmic burstlike activity. Here we elaborated on an output pattern emerging at transitional stimulation frequencies with consecutively elicited reflexes alternating between large and small. We analyzed responses concomitantly elicited in thigh and leg muscle groups bilaterally by epidural stimulation in eight motor-complete spinal cord-injured individuals. Periodic amplitude modulation of at least 20 successive responses occurred in 31.4% of all available data sets with stimulation frequency set at 5–26 Hz, with highest prevalence at 16 Hz. It could be evoked in a single muscle group only but was more strongly expressed and consistent when occurring in pairs of antagonists or in the same muscle group bilaterally. Latencies and waveforms of the modulated reflexes corresponded to those of the unmodulated, monosynaptic responses to 2-Hz stimulation. We suggest that the cyclical changes of reflex excitability resulted from the interaction of facilitatory and inhibitory mechanisms emerging after specific delays and with distinct durations, including postactivation depression, recurrent inhibition and facilitation, as well as reafferent feedback activation. The emergence of large responses within the patterns at a rate of 5.5/s or 8/s may further suggest the entrainment of spinal mechanisms as involved in clonus. The study demonstrates that the human lumbosacral spinal cord can organize a simple form of rhythmicity through the repetitive activation of spinal reflex circuits.
Keywords: human, repetitive nerve stimulation, rhythm generation, spinal cord stimulation, spinal reflexes
the soleus hoffmann reflex (H reflex) is widely used in human neurophysiological studies that explore the organization of spinal reflexes and the sensorimotor integration in neural control of movement (Capaday and Stein 1986; Knikou 2008; Pierrot-Deseilligny and Burke 2012; Pierrot-Deseilligny and Mazevet 2000; Schiepatti 1987). It is evoked by electrical stimulation of large-diameter Ia afferents from muscle spindles in the posterior tibial nerve with subsequent recruitment of motoneurons largely through monosynaptic connections in the spinal cord (Magladery and McDougal 1950; Pierrot-Deseilligny and Burke 2012). Changes in the H-reflex amplitude caused by conditioning inputs indicate modulation of the excitability of the reflex pathway by peripheral influence and central circuitry (Knikou 2008; Pierrot-Deseilligny and Burke 2012).
Each sensory input volley to the spinal cord elicited by stimulation of the tibial nerve not only evokes the H reflex but also initiates central processes that modify the responsiveness to subsequent stimulus-induced input (Crone and Nielsen 1989; Magladery and McDougal 1950). When the tibial nerve is repetitively stimulated, there is a progressive fall in the amplitudes of the H reflexes (Ishikawa et al. 1966; Schindler-Ivens and Shields 2000). Conditioning inputs through stimulation of other peripheral nerves activate spinal circuits that are otherwise not engaged and lead to different patterns of H-reflex modulation (Mao et al. 1984; Meunier et al. 1993; Pierrot-Deseilligny and Burke 2012). Such conditioning test paradigms have described various time-dependent facilitatory or inhibitory processes, based on the widespread spinal interconnections between the reflex circuits of different motoneuron pools (Delwaide et al. 1976).
Applying sensory inputs to several lumbar and upper sacral spinal cord segments simultaneously became possible with the application of epidural spinal cord stimulation (SCS) for the treatment of upper motor neuron disorders (Cook and Weinstein 1973) and the specific electrode placement required for the control of lower-limb spasticity (Pinter et al. 2000). When repetitively applied, such simultaneous, bilateral stimulation of multiple posterior roots produces motor outputs in the lower limbs of individuals with motor-complete spinal cord injury (SCI) that cannot be evoked by stimulation of a single peripheral nerve alone (Danner et al. 2015; Dimitrijevic et al. 1998; Gerasimenko et al. 2002; Jilge et al. 2004; Shapkova and Schomburg 2001). The SCS-elicited motor outputs are comprised of series of stimulus pulse-related posterior root-muscle (PRM) reflexes, i.e., large-afferent-induced spinal reflexes initiated in the posterior roots and recorded by surface electromyography (EMG) from many lower-limb muscles (Minassian et al. 2004). Stimulation at 2 Hz elicits unmodulated, monosynaptic PRM reflexes (Murg et al. 2000), while 22–60 Hz can lead to rhythmic burstlike motor activity with waxing and waning of the PRM reflexes, modulated by concomitantly activated spinal interneuronal mechanisms (Danner et al. 2015; Dimitrijevic et al. 1998; Minassian et al. 2004; cf. Wenger et al. 2014).
In the present work, we elaborate on an output phenomenon encountered when applying intermediate SCS frequencies of 5–26 Hz. Instead of leading to a progressive reflex depression or random variability in reflex size as would be suggested by H-reflex studies, the repetitive stimulation often produced a pattern with the amplitudes of the successive responses alternating between large and small. Here we provide a detailed description of this modulation pattern, including its prevalence, dependence on the applied stimulation parameters, and observations hinting at the coupling of reflexes evoked in pairs of muscle groups. The cycle frequency of this periodic reflex modulation is well above the range of burst frequencies adequate for locomotion (Danner et al. 2015) and hence renders involvement of the spinal locomotor rhythm generator unlikely. This study therefore adds to the understanding of how the human lumbar spinal reflex circuits can process repetitive inputs to regulate and sustain a simple form of oscillating motor behavior.
MATERIALS AND METHODS
Subjects.
Data derived from eight individuals with traumatic, clinically motor-complete SCI in the chronic stage of recovery were analyzed (Table 1). The subjects' neurological status was evaluated according to the International Standards for Neurological Classification of Spinal Cord Injury (Kirshblum et al. 2011). They had epidural SCS systems implanted for the control of spinal spasticity affecting the lower extremities (Pinter et al. 2000). Informed written consent was obtained from the subjects prior to their participation. All procedures were approved by the Ethics Committee of the City of Vienna and conformed to the Declaration of Helsinki.
Table 1.
Subject demographic data
| Subject | Sex | Age, yr | AIS | Neurological Injury Level | Years After Injury |
|---|---|---|---|---|---|
| 1 | M | 29 | B | C6 | 3 |
| 2 | M | 22 | A | C6 | 5 |
| 3 | M | 29 | A | T4 | 8 |
| 4 | M | 18 | A | C5 | 3 |
| 5 | M | 25 | B | C7 | 1 |
| 6 | F | 31 | A | T5 | 1 |
| 7 | F | 25 | A | T6 | 4 |
| 8 | M | 33 | A | T5 | 13 |
AIS, American Spinal Injury Association Impairment Scale.
Epidural spinal cord stimulation.
Epidural SCS was applied via a cylindrical electrode lead (Pisces-Quad electrode, model 3487A, Medtronic, Minneapolis, MN) that was connected to a programmable pulse generator (Itrel 3, model 7425, Medtronic) placed subcutaneously in the abdominal wall. The lead carried four electrodes, referred to as 0 to 3 from rostral to caudal direction, each with a diameter of 1.3 mm, a length of 3 mm, and an interelectrode distance of 6 mm. The electrodes were positioned longitudinally in the dorsal epidural space, targeting the lumbosacral spinal cord. Vertebral levels ranged from the lower half of T11 to the lower third of L1 across the eight subjects. Each electrode could be independently set to +, −, or “off,” allowing for various bipolar electrode combinations. Monopolar stimulation was carried out by selecting one of the epidural electrodes as cathode and the active area of the implanted pulse generator case (“c”) as anode. The pulse generator delivered capacitively decoupled monophasic rectangular constant-voltage pulses of 210-μs width, each followed by an exponentially decaying phase to adjust charge balance for each stimulus and avoid delivery of direct current (Rattay et al. 2000). Programmable stimulation frequencies and intensities were 2–130 Hz and 0–10.5 V.
The rostrocaudal position (Pos) of the selected cathode relative to the spinal cord segments was physiologically estimated based on the thresholds of the PRM reflexes elicited at 2 Hz in the L2–L4 innervated quadriceps (Q) and the L5–S2 innervated triceps surae (TS), respectively (Minassian et al. 2007a; Murg et al. 2000): Pos 1, Q but not TS recruited with maximum applied stimulation intensity, active cathode position estimated to be rostral, yet close, to the L2 spinal cord segment; Pos 2, lower thresholds for Q than TS responses, cathode position over L2–L4 segments; Pos 3, same thresholds for Q and TS, cathode position over L5–S2 segments; Pos 4, lower thresholds for TS than Q, cathode position caudal to the S2 segment.
Surface polyelectromyography.
EMG activity was recorded from Q, hamstrings (Ham), tibialis anterior (TA), and TS bilaterally with pairs of silver-silver chloride surface electrodes (Intec Medizintechnik, Klagenfurt, Austria) with an interelectrode distance of 3 cm (Sherwood et al. 1996). Additional EMG recordings were taken from the lumbar paraspinal trunk muscles to monitor stimulation artifacts. Abrasive paste (Nuprep, Weaver, Aurora, CO) was used for skin preparation to reduce contact resistance. EMG signals were amplified with a Grass 12D-16-OS Neurodata Acquisition System (Grass Instruments, Quincy, MA) adjusted to a gain of 2,000 over a 3-dB bandwidth of 30–700 Hz. Data were digitized at 2,002 samples per second and channel with a Codas ADC system (Dataq Instruments, Akron, OH).
Stimulation protocol.
For a given selection of active electrodes, stimulation was initially applied at 2 Hz and intensity gradually increased in 1-V increments until PRM reflexes were elicited. At this intensity, the stimulation frequency was increased stepwise, including 5, 11, 16, 22, and 26 Hz. The frequency variation was then repeated for intensities up to 10 V. Stimulation intensity and frequency variation were subsequently repeated for different epidural electrode combinations. All recordings were conducted with the subjects relaxed, lying comfortably supine.
Data analysis and statistics.
Data were analyzed off-line with MATLAB R2012a (The MathWorks, Natick, MA). Statistical analyses were performed with SPSS 22 for Mac OS X (10.8.5), and α-errors of P < 0.05 were considered significant. For Student's t-tests, the assumptions of normality and equality of variances were verified.
Recordings obtained during SCS at 2, 5, 11, 16, 22, and 26 Hz were selected for analysis. The stimulation elicited PRM reflexes in Q, Ham, TA, and TS that were electromyographically recorded as stimulus pulse-related reflex-compound muscle action potentials (Minassian et al. 2004). The number of PRM reflexes collected with unchanged parameter settings ranged from 20 to ∼1,600 per muscle group studied, also depending on the applied stimulation frequency. The EMG of individual PRM reflexes was analyzed for 50 ms after stimulus. Peak-to-peak amplitudes of the PRM reflexes were calculated.
For the detection of patterns characterized by an amplitude alternation of successively elicited reflexes between large and small, henceforth referred to as periodic reflex modulation, EMG sections under unchanged stimulation conditions were sequenced into shorter segments. The first segment contained the responses to the first 20 stimuli, and the respective next segments were each shifted by one stimulus (i.e., segment 2, responses to stimuli 2–21; segment 3, responses to stimuli 3–22, etc.). For each muscle group and recording segment, the first-order differences of the peak-to-peak amplitudes of pairs of consecutive responses were calculated (response2 − response1, response3 − response2, . . . , response20 − response19) to determine the signs of the 19 resulting differences. A steady pattern of periodic modulation was identified for a given 20-response segment if the complete series of 19 signs was alternating, i.e., either +,−,+,−, etc. or −,+,−,+, etc. The upper limit of the α-error considering the largest sample size of 1,600 responses per muscle is 0.003. To compare the prevalence of periodic reflex modulation between muscle groups, a Pearson's χ2-test was used. We introduced the consistency of modulation to describe the prevalence of the periodic modulation within each recording section with given SCS parameters per muscle. It was calculated as the number of 20-response segments with a steady pattern relative to the total number of segments into which the recording section had been sequenced. A consistency of, e.g., 50% would indicate that half of the 20-response segments of a recording section had steady patterns. To calculate the attenuation, the ratios of the mean peak-to-peak amplitudes of the 10 smaller and the 10 larger PRM reflexes within each patterned segment were calculated and averaged for each muscle group and recording section. Following the hypothesis that the attenuation correlated with the consistency, a Spearman's rank order correlation test was performed for each muscle group and stimulation frequency.
The phase relation of periodic reflex modulation concomitantly occurring in 2 or 4 muscle groups unilaterally or in pairs of muscle groups bilaterally was determined for each patterned 20-response segment by comparing the signs of the respective 19 first-order differences calculated. Synchronous modulation was identified if the series started with the same sign, and reciprocal modulation otherwise. Fluctuations were detected if at least one 20-response segment each with synchronized as well as reciprocal modulation occurred within a recording section. Consistency and attenuation of periodic modulation occurring in one muscle group only were compared to the respective values when these patterns occurred concomitantly with modulation in its antagonist or in the same group on the contralateral side with a Student's t-test.
For the responses of each muscle group to 2-Hz SCS, the occurrence of postactivation depression was tested by comparing the amplitude of the PRM reflex elicited by the 1st stimulus to the mean amplitudes of the responses to the 5th to 14th stimulus pulses with a paired Student's t-test.
Onset latencies were determined for PRM reflexes elicited by 2-Hz SCS as well as for those constituting patterns with periodic reflex modulation as the times between the stimulus artifact and the first deflection from baseline exceeding 5% of the respective peak-to-peak amplitude. For each muscle group, mean onset latencies were calculated from all responses within a recording section with unchanged stimulation conditions. For each subject, these mean values were then averaged per muscle group, separately for the responses to 2-Hz SCS and for the periodically modulating responses. Group results were then obtained by averaging the corresponding mean values of the eight subjects. Mean latencies of responses to 2-Hz SCS and of the periodically modulating responses were compared with Student's t-tests. Furthermore, for each muscle group and epidural electrode combination, threshold intensities were defined as the minimum intensity required to consistently elicit responses at 2 Hz with amplitudes > 50 μV.
RESULTS
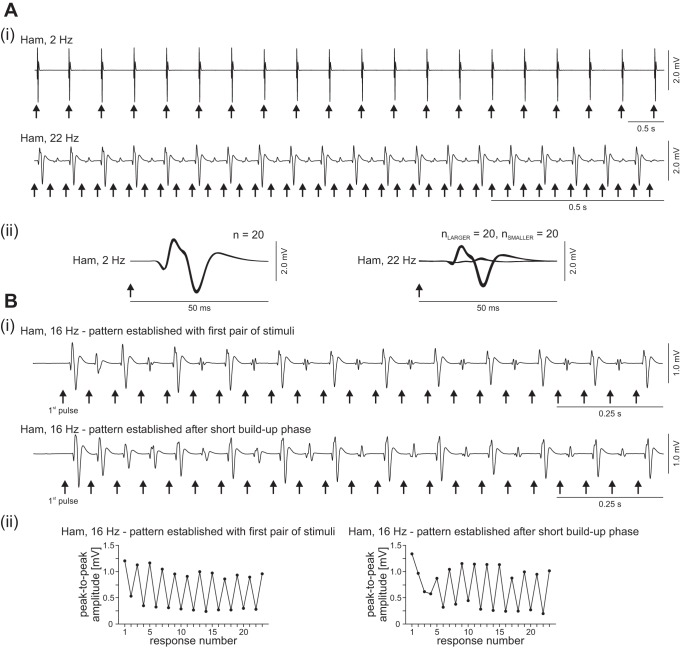
Epidural SCS at 2–26 Hz evoked series of stimulus-locked PRM reflexes in the lower-limb muscle groups of the eight subjects studied. Stimulation at 2 Hz elicited highly repeatable responses (Fig. 1A) with no significant change in reflex size within the series of PRM reflexes (paired Student's t-test; Q, difference: 31.02 ± 201.10 μV, t101 = 1.550, P = 0.124, r = 0.15; Ham, difference: 6.40 ± 301.17 μV, t101 = 0.215, P = 0.830, r = 0.02; TA, difference: −1.986 ± 104.73 μV, t72 = 0.162, P = 0.871, r = 0.02; TS, difference: −30.890 ± 349.84 μV, t85 = 0.819, P = 0.415, r = 0.09). When SCS was applied at 5–26 Hz, patterns emerged in which PRM reflex amplitudes alternated between large and small (Fig. 1A, 22 Hz). This periodic reflex modulation could occur either in a single or in several uni- or bilateral muscle groups. Of the 1,374 recording sections available (each with unchanged stimulation parameters) at 5–26 Hz, 431 (31.4%) included segments with periodic amplitude modulation. Characteristically, these patterns had short buildup phases (Fig. 1B), with 26.2% already established with the first pair of stimuli and an additional 32.5% starting within the first 10 stimuli applied. Another 16.9% of the patterns were established within the 11th to 30th stimulus pulses. The remaining 24.4% cases appeared after the 30th stimulus pulse. Across all patterned EMG segments of the various muscle groups and subjects, even the larger of the alternating responses (0.84 ± 0.85 mV) were significantly smaller than the unconditioned initial responses to the first stimulus of the train of stimuli applied during a given recording section (1.44 ± 1.12 mV; Student's t-test; difference: −0.6 ± 0.69 mV, t399 = −17.314, P < 0.00001, r = 0.65); muscle-specific ratios were 0.55 ± 0.36 (Q), 0.75 ± 0.35 (Ham), 0.54 ± 0.31 (TA), and 0.46 ± 0.30 (TS).
Fig. 1.
Series of posterior root-muscle (PRM) reflexes without and with periodic amplitude modulation elicited by epidural stimulation of the lumbosacral spinal cord. Ai, top: responses of hamstrings (Ham) to 2-Hz stimulation with unmodulated amplitudes. Bottom: periodic reflex modulation elicited at 22 Hz in the same muscle group (note different time scaling). ii: Same PRM reflexes as shown in i but presented stimulus triggered in enlarged timescale. n, Numbers of superimposed responses. Bi: examples illustrating the characteristic short buildup phase of periodic modulation patterns, here elicited at 16 Hz, established either with the first pair of stimuli applied (top) or after a few stimulus pulses (bottom). ii: Peak-to-peak amplitudes (black circles) of the consecutively elicited PRM reflexes shown in i. Arrows mark times of stimulus application. Data derived from subject 2, epidural electrode combination 0− 3+, 6 V in Ai, both traces, and Bi, bottom trace; and 0+ 3−, 3 V in Bi, top trace.
Stimulation parameter dependence of periodic reflex modulation.
Periodic reflex modulation was most likely to be elicited at 16 Hz across all muscle groups studied, and its prevalence rapidly decreased when lower or higher stimulation frequencies were applied (Fig. 2A). Stimulation frequencies producing the patterns ranged from 5 to 26 Hz for Q and Ham and from 11 to 22 Hz for TA and TS, respectively. Across muscle groups, the prevalence of recording sections with invariant stimulation parameters containing at least one patterned segment decreased with increasing stimulation intensities (Fig. 2B). There was no specific relationship between the site of stimulation and the occurrence of the patterns, except for stimulation from Pos 4 that was least effective (Fig. 2C). Overall, the prevalence of the periodic reflex modulation (Fig. 3A) was higher in the proximal muscles (Q: 27.7% of the available data sets; Ham: 29.1%) than in the distal muscles [TA: 18.2%; TS: 21.0%; χ2(3) = 13.617, P = 0.0035, odds ratio = 1.44].
Fig. 2.
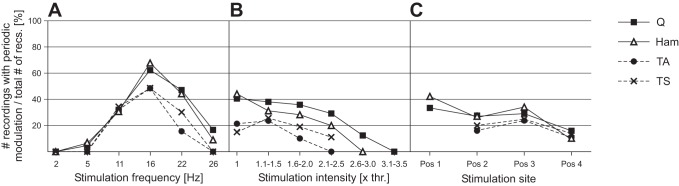
Relationship between the prevalence of periodic reflex modulation and stimulation parameters: no. of recording sections (each with constant stimulation parameters) with periodic reflex modulation of quadriceps (Q), hamstrings (Ham), tibialis anterior (TA), and triceps surae (TS) relative to the total number of available recording sections across subjects at a given stimulation frequency (A), intensity [in multiples of the respective reflex thresholds (thr.), B], and site (C).
Fig. 3.
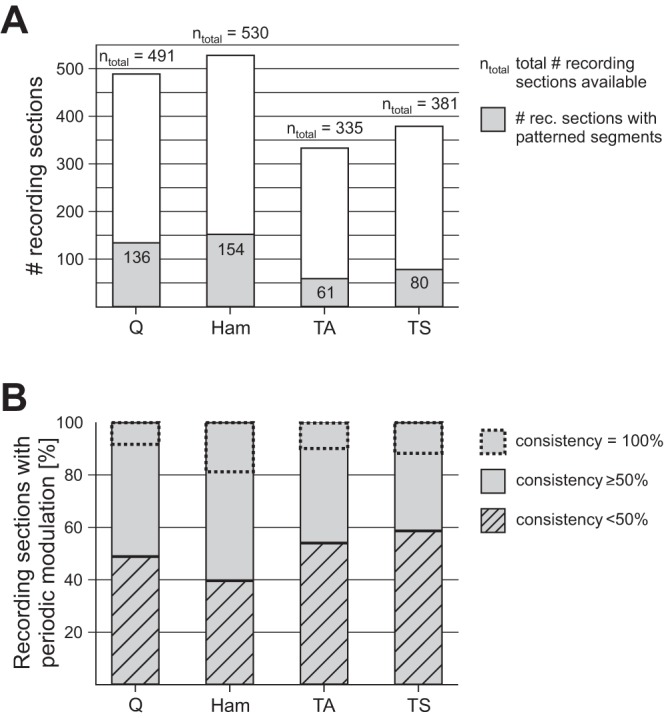
Prevalence and consistency of periodic reflex modulation per muscle group. A: number of recording sections with segments featuring periodic reflex modulation (gray areas within bars) in relation to total number ntotal of recording sections (each with a given set of stimulation parameters) from quadriceps, hamstrings, tibialis anterior, and triceps surae across subjects. B: % of recording sections with periodic reflex modulation per muscle with a consistency below 50% or above 50%. The percentage of modulation patterns occurring with a consistency of 100% (i.e., throughout a recording section) is marked by dashed rectangles within bars.
Also, the consistency of the periodic reflex modulation was higher in the thigh than in the leg muscles (Fig. 3B). In >50% of all recording sections of Q or Ham with periodic reflex modulation, more than half of the successively elicited PRM reflexes alternated between large and small, while this was not the case in the distal muscles. A consistency of even 100%, i.e., modulation occurring throughout a recording section, was found in all muscle groups studied and occurred most commonly in Ham.
The mean consistency was stimulation frequency dependent and peaked at 16 Hz for Q, Ham, and TS, and at 11-Hz SCS for TA (Fig. 4A). The attenuation of the small-amplitude PRM reflexes relative to the larger ones ranged from 0.42 to 0.86 for the different stimulation frequencies, with the strongest attenuation found at 16-Hz SCS across muscles (Fig. 4B). Furthermore, in Q and Ham, the attenuation correlated with the consistency of the patterns (Q, ρ = −0.677, P = 0.00061; Ham, ρ = −0.436, P = 0.039). In other words, the stronger the relative attenuation of the smaller responses, the higher was the prevalence of patterned segments to occur within a given recording section. No such statistical relationship was found for the leg muscle groups (TA, ρ = −0.381, P = 0.171; TS, ρ = −0.364, P = 0.133).
Fig. 4.
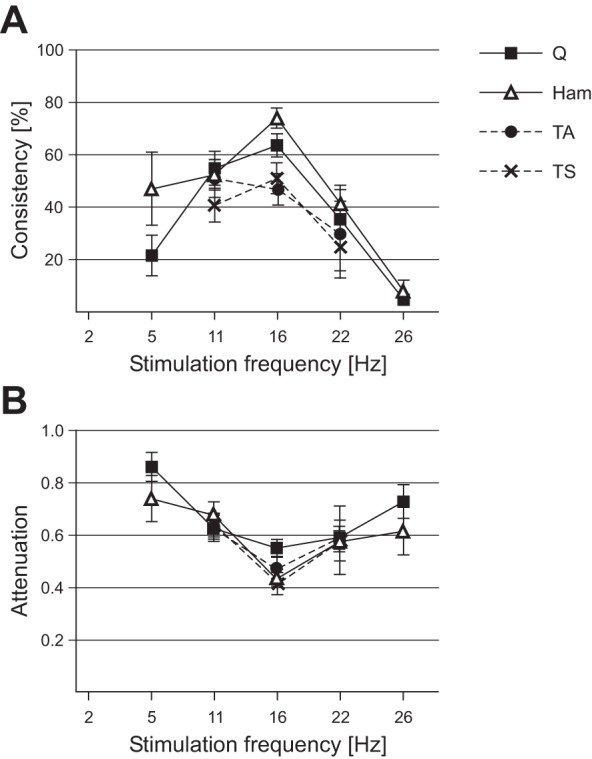
Stimulation frequency dependence of periodic reflex modulation. A: average consistency of periodic reflex modulation (describing its prevalence per recording section with unchanged stimulation parameters) within each muscle. B: attenuation of the smaller relative to the larger responses as a function of the stimulation frequency. Error bars are SE.
Coupling of reflex modulation in pairs of related muscle groups.
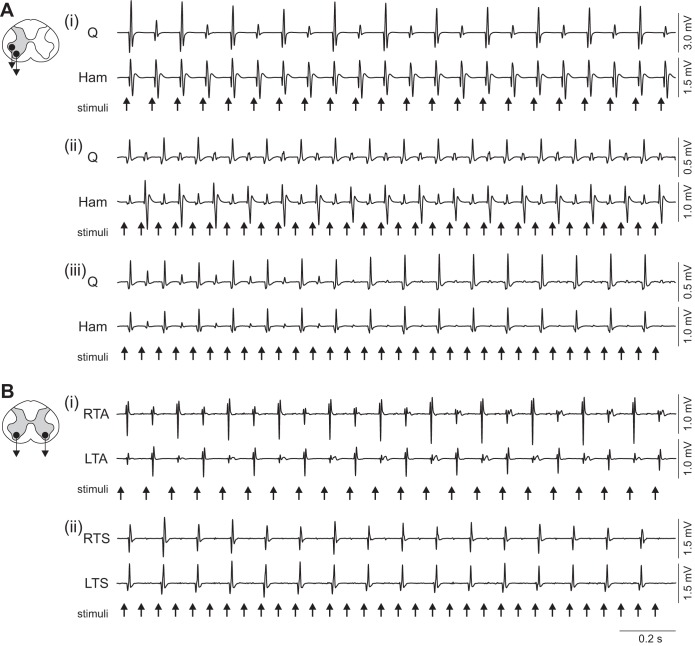
Periodic reflex modulation could be generated in one muscle group only (Fig. 5Ai) but had a 2.0 times higher prevalence in a given muscle when the amplitude modulation concomitantly occurred in its antagonist (cf. Table 2). The periodic reflex modulation in antagonistic muscle groups occurred with reciprocal (Fig. 5Aii) and synchronized (Fig. 5Aiii) relationships. Both output relationships were produced with similar prevalence in Q and Ham, while in TA and TS synchronized modulation was almost exclusively elicited (Table 2). Fluctuations between reciprocal and synchronized modulation were exceptions.
Fig. 5.
Coordination of periodic reflex modulation in pairs of related muscles. A: relationship of motor outputs in antagonistic muscle groups with periodic modulation occurring in one of the antagonists only (i), reciprocal modulation (ii), and synchronous modulation (iii). B: periodic modulation patterns occurring in the same muscle group bilaterally with reciprocal (i) and synchronous (ii) relationships. Recordings from unilateral quadriceps and hamstrings as well as right (R) and left (L) tibialis anterior and triceps surae, subject 2. Stimulation parameters: Ai: 0+ 2−, 11 Hz, 5 V; ii: 0− 3+, 16 Hz, 5 V; iii: 0+ 2−, 16 Hz, 4 V; Bi: 0− 3+, 11 Hz, 7 V; ii: 0− 3+, 16 Hz, 7 V.
Table 2.
Relationship of periodic reflex modulation in pairs of antagonistic muscle groups
| nmod_total | nsingle | % | nreciprocal | % | nsynchron | % | nfluctuating | % | |
|---|---|---|---|---|---|---|---|---|---|
| Q | 136 | 36 | 26.5 | 52 | 38.2 | 45 | 33.1 | 3 | 2.2 |
| Ham | 154 | 54 | 35.1 | 52 | 33.8 | 45 | 29.2 | 3 | 1.9 |
| TA | 61 | 17 | 27.9 | 2 | 3.3 | 41 | 67.2 | 1 | 1.6 |
| TS | 80 | 36 | 45.0 | 2 | 2.5 | 41 | 51.3 | 1 | 1.3 |
Q, quadriceps; Ham, hamstrings; TA, tibialis anterior; TS, triceps surae; nmod_total, total no. of recording sections containing segments with periodic reflex modulation per muscle group; nsingle, periodic alternation in 1 of the antagonistic muscles only; nreciprocal, reciprocal modulation; nsynchron., synchronous modulation in pairs of antagonistic muscle groups; nfluctuating, fluctuation between reciprocal and synchronous modulation.
Similarly, periodic reflex modulation in a given muscle occurred with a 1.7 times higher prevalence when such pattern was concomitantly produced in the same muscle group on the contralateral side (Fig. 5B). These patterns were most commonly interrelated by synchronous modulation in Ham, TA, and TS, whereas in Q reciprocal and synchronous modulation had similar prevalence (Table 3). The occurrence of the different types of patterns in pairs of related muscle groups is described in Table 4 for each subject.
Table 3.
Relationship of periodic reflex modulation in a given muscle group bilaterally
| nbilateral | % | nreciprocal | % | nsynchron | % | nfluctuating | % | |
|---|---|---|---|---|---|---|---|---|
| Q bilateral | 82 | 60.3 | 40 | 48.8 | 36 | 43.9 | 6 | 7.3 |
| Ham bilateral | 106 | 68.8 | 16 | 15.1 | 84 | 79.2 | 6 | 5.7 |
| TA bilateral | 36 | 59.0 | 8 | 22.2 | 24 | 66.7 | 4 | 11.1 |
| TS bilateral | 46 | 57.5 | 6 | 13.0 | 36 | 78.3 | 4 | 8.7 |
nbilateral, no. of recording sections with periodic modulation patterns occurring bilaterally in a given muscle group, of which nreciprocal are modulating reciprocally and nsynchron synchronously; nfluctuating, fluctuation between reciprocal and synchronous modulation.
Table 4.
Observed patterns of periodic reflex modulation across subjects
| Modulation in Pairs of Antagonists |
Modulation Occurring in a Muscle Bilaterally |
|||||||
|---|---|---|---|---|---|---|---|---|
| Subject | Single | Recip. | Synch. | Fluct. | Recip. | Synch. | Fluct. | Modulation in All 4 Unilateral Muscles |
| 1 | x | x | x | x | x | x | ||
| 2 | x | x | x | x | x | x | x | |
| 3 | x | x | ||||||
| 4 | x | x | x | x | x | |||
| 5 | x | x | x | x | x | x | x | x |
| 6 | x | x | x | x | ||||
| 7 | x | x | x | x | x | |||
| 8 | x | x | x | x | x | x | x | x |
Single, periodic reflex modulation in 1 of the antagonistic muscle groups only; Recip. or Synch., periodic reflex modulation occurring concomitantly in pairs of related muscle groups with reciprocal (Recip.) or synchronous (Synch.) relationship; Fluct., fluctuation between reciprocal and synchronous modulation; x, pattern present.
The consistency of the periodic reflex modulation within a recording section of a given muscle as well as the relative attenuation of the smaller responses were significantly higher when elicited in pairs of either antagonistic (Fig. 6A) or bilateral (Fig. 6B) muscle groups compared with those patterns occurring in one muscle of the pair only (all P < 0.00001).
Fig. 6.
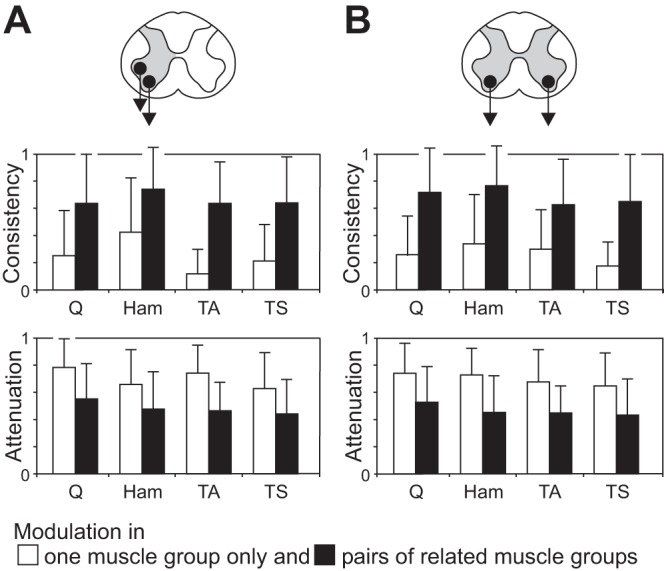
Consistency and attenuation of periodic reflex modulation patterns: patterns found in 1 or both muscle groups of a pair with antagonistic relation (A) and bilaterally (B). The consistency of a pattern, measured within each muscle, is significantly larger, and the attenuation of the smaller responses relative to the larger ones significantly stronger when the periodic reflex modulation is concomitantly occurring in the related muscle (bars are mean values across all available data sets, error bars are SE; all P < 0.00001).
Phase relation of reflex modulation across all four unilateral muscle groups.
Periodic reflex modulation across all four unilateral muscle groups studied was detected in three of the eight subjects (Table 4) and 16.7% of the 431 recording sections with patterned EMG segments. The most frequent output relationships were those of reciprocal modulation of the Q responses with respect to Ham, TA, and TS (7 examples, 38.9%; Fig. 7A) and of reciprocal reflex modulation of Ham with respect to Q, TA, and TS activity (6 examples, 33.3%; Fig. 7B). Synchronous modulation of the PRM reflexes in the proximal muscles occurring reciprocally to the synchronized modulation of the responses in the distal muscles was found in another three examples (16.7%). One example (5.6%) was found with synchronized modulation of Q and TA responses occurring reciprocally to the synchronized modulation of Ham and TS responses and one other example (5.6%) with synchronized response modulation across all four muscle groups. No other output relationship was detected.
Fig. 7.
The 2 most frequently detected output relationships of periodic reflex modulation across muscles of the same side. Data derived from subject 2, 0− 3+, 16 Hz, 6 V (A) and subject 5, c+ 1−, 16 Hz, 6 V (B). Arrows mark times of stimulus application.
EMG waveforms and latencies of modulated PRM reflexes.
The vast majority of the amplitude-modulated PRM reflexes elicited at 5–26 Hz closely resembled the unmodulated responses at 2 Hz. The responses to 2-Hz SCS had muscle-specific EMG waveforms and short onset latencies amounting to 9.5 ± 0.9 ms (Q), 10.0 ± 0.6 ms (Ham), 18.2 ± 0.9 ms (TA), and 17.9 ± 1.0 ms (TS) across subjects (Fig. 8A). The PRM reflexes within the patterns with periodic modulation had the same waveforms in Q in 99.3% of all examples, in Ham in 85.1%, in TA in 63.9%, and in TS in 91.3% (Fig. 8B). The onset latencies of these PRM reflexes were 10.2 ± 0.7 ms (Q), 11.1 ± 1.2 ms (Ham), 18.5 ± 1.1 ms (TA), and 18.7 ± 1.9 ms (TS) and did not differ from those of the responses to 2-Hz SCS (Student's t-test; Q, t7 = 1.928, P = 0.095, r = 0.59; Ham, t7 = 2.318, P = 0.054, r = 0.66; TA, t6 = 0.533, P = 0.613, r = 0.21; TS, t6 = 0.800, P = 0.454, r = 0.31).
Fig. 8.
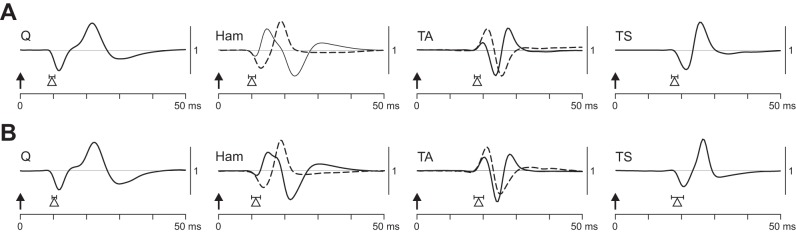
Characteristic EMG waveforms and onset latencies of PRM reflexes. A: responses to 2-Hz stimulation. B: responses from patterns of periodic amplitude modulation. Arrows indicate times of stimulus delivery; EMG waveforms are exemplary and were characteristic across subjects. Triangles represent onset latencies averaged over all available data sets and subjects; horizontal lines show SE. Two characteristic waveforms were found in Ham and TA. A: data derived from Q, subject 4, 0+ 3−, 8 V; Ham, subject 8, 0+ 3−, 4 V (solid line) and subject 2, 0+ 3−, 5 V (dashed line); TA, subject 8, 2+ 3−, 5 V (solid line) and subject 2, 0+ 2−, 6 V (dashed line); and TS, subject 4, 1+ 3−, 9 V. B: data derived from Q, subject 4, 0+ 3−, 11 Hz, 5 V; Ham, subject 2, 0+ 3−, 16 Hz, 4 V (solid line) and subject 8, 1+ 3−, 16 Hz, 4 V (dashed line); TA, subject 2, 0+ 2−, 11 Hz, 5 V (solid line) and subject 8, c+ 3−, 11 Hz, 4 V (dashed line); and TS, subject 2, 0+ 2−, 11 Hz, 5 V. Waveforms are presented amplitude matched.
DISCUSSION
Epidural stimulation of the lumbosacral spinal cord at 2 Hz evoked stimulus pulse-locked PRM reflexes in the lower-limb muscle groups that did not demonstrate postactivation depression or periodic modulation. When the stimulation frequency was increased to 5–26 Hz, patterns emerged after short buildup phases within the series of PRM reflexes with an alternation between larger and smaller response amplitudes. The prevalence of the periodic reflex modulation, its consistency, and the relative attenuation of the smaller-amplitude responses depended on the applied stimulation frequency, maximizing at 16 Hz. While the amplitude modulation could be elicited in one muscle group only, its consistency and the relative attenuation were significantly higher when generated in pairs of related muscle groups. Patterns occurring in pairs of related muscle groups were coupled by synchronous modulation of the responses in both rather than reciprocating.
Nature of periodically modulated PRM reflexes.
The neural structures electrically stimulated by the longitudinal, cylindrical electrode type as employed here, placed at close distance to the posterior aspect of the lumbosacral spinal cord, are sensory fibers, while the immediate stimulation of motor fibers within the anterior roots can be ruled out even with the maximum available stimulation intensity (Minassian et al. 2004, 2007a; Rattay et al. 2000). Here the vast majority of the periodically modulating responses had the same EMG waveforms and short onset latencies as the PRM reflexes evoked by 2-Hz SCS (cf. Minassian et al. 2004; Murg et al. 2000). These responses were previously suggested to be initiated in large-diameter afferents within the posterior roots/rootlets (Rattay et al. 2000) and transmitted via monosynaptic reflex arcs (Minassian et al. 2004, 2007a; cf. Capogrosso et al. 2013). They also closely resemble the lower-limb responses evoked by transcutaneous posterior root stimulation (Hofstoetter et al. 2008, 2014; Ladenbauer et al. 2010; Minassian et al. 2007b, 2011). Physiologically, PRM reflexes elicited by single stimuli share similarities with the H reflex (Maertens de Noordhout et al. 1988), such as attenuation by sustained tendon vibration (Minassian et al. 2007b, 2011) and motor task-specific amplitude modulation (Courtine et al. 2007; Hofstoetter et al. 2008; Minassian et al. 2007b). Also, when pairs of stimuli are delivered with an interstimulus interval of 50 ms, both the H reflex (Paillard 1959; Schiepatti 1987) and the PRM reflexes (Minassian et al. 2004, 2007b; Roy et al. 2014) evoked by the second pulse are attenuated or completely suppressed. Differences between these two types of reflexes become obvious when evoked by trains of stimuli. The H-reflex size progressively decreases with increasing stimulation frequencies from 0.1 to 10 Hz (Calancie et al. 1993; Schindler-Ivens and Shields 2000), and its variability at these depressed levels appears to be random (Ishikawa et al. 1966). The different behavior of the repetitively elicited PRM reflexes may arise from the synchronous stimulation of afferents of several spinal cord segments bilaterally, the resulting mixed homo- and heteronymous inputs and the recruitment of a wider range of interneurons, as well as the concomitant elicitation of reflex responses in several lower-limb muscle groups.
Entrainment of spinal reflex circuits by repetitive inputs and interaction with peripheral events.
Synaptic summation of inhibitory and excitatory actions with different time courses may have been underlying the periodic PRM reflex modulation. Such summation processes would require at least one component to not have fully decayed by the time the next activation arrives at the motoneuron pool, or secondary actions that affect the PRM reflex transmission only after a certain delay. Candidate mechanisms include postactivation depression, presynaptic inhibition, recurrent inhibition and facilitation, as well as reafferent feedback activation following the muscle twitches.
Recurrent inhibition is mediated by Renshaw cells that respond to an activation with a burst of spikes, lasting for 40–100 ms (Eccles et al. 1954; Hultborn et al. 1971a; Katz and Pierrot-Deseilligny 1999). Axon collaterals of alpha motoneurons synapse on the Renshaw cells, which in turn project back to populations of homonymous and heteronymous motoneurons, including those that excited them (Pierrot-Desseilligny and Burke 2012). Renshaw cells would discharge after the motoneuronal activity of a PRM reflex evoked by a given stimulus pulse. An input volley elicited by a succeeding stimulus pulse that arrives to the motoneuron pool before full decay of the ongoing Renshaw cell activity, as could be the case at, e.g., 16-Hz stimulation, would recruit a smaller number of motoneurons. In turn, the smaller reflex discharge would activate fewer Renshaw cells, and the degree of recurrent inhibition upon the motoneuron pool would be reduced when the next stimulus pulse is applied. This process may eventually lead to a temporarily stable state of alternating degrees of recurrent inhibition exerted on the motoneuron pools and hence to the observed PRM reflex modulation.
Another mechanism contributing to the PRM reflex modulation could be a periodically emerging excitation superimposed upon a longer-lasting reduced reflex transmission. Such excitation could be caused by reafferent feedback activation induced by the substantial stimulation-evoked muscle twitch contractions and the subsequent relaxation. Reafferent feedback activation has been suggested to contribute to the facilitation of H reflexes elicited by double stimuli at interstimulus intervals of 100–400 ms (Kagamihara et al. 1998; Paillard 1959; Schiepatti 1987). Here postactivation depression and presynaptic inhibition presumably led to a longer-lasting reduction in reflex transmission when SCS was applied at 5–26 Hz, since even the larger responses within the periodic modulation patterns were smaller than the unconditioned initial response to the first stimulus of the respective train. Thus for a train of epidural stimulation delivered at a frequency around 16 Hz, the first pulse would set inhibitory mechanisms into action, leading to a smaller response to the second stimulus pulse. The afferent discharge following the muscle twitch contractions induced by the first pulse would cause rebound volleys to the spinal cord that would arrive in time to facilitate the response to the third stimulus (after 2 × 62.5-ms interstimulus interval = 125 ms). The weaker contractions evoked by the second stimulus pulse would lead to less facilitatory impact on the PRM reflex elicited by the fourth stimulus pulse, and, eventually, an alternation between smaller and larger PRM reflexes may develop.
Periodic reflex modulation occurred in about 30% of the recording sections of the proximal and 20% of the distal muscle groups. This uneven distribution may in part result from differences in efficacy of recurrent inhibition to motoneurons innervating proximal and distal muscles. Contraction and relaxation times of the large thigh muscles together with their shorter reflex arcs may result in different timing and patterns of rebound facilitation compared with the leg muscles. Differences in the secondary activation of muscle spindle afferents may also arise from muscle receptor sensitivity and the range of twitch-related movements. Furthermore, different interactions between proximal and distal stretch reflexes elicited in succession could either facilitate or impede the generation of the periodic reflex modulation. Delwaide and colleagues (1976) demonstrated that the reflex activation of the soleus muscle resulted in two distinct phases of facilitation of the quadriceps stretch reflex, whereas the stretch reflex of quadriceps led to a long-lasting inhibition of the soleus reflex.
The occurrence of the periodic reflex modulation could be partially linked to the reorganization of the spinal circuitry following SCI (Edgerton et al. 2001). These changes together with an elevated central state of excitability alter the input processing by the neural circuitry, which may increase the probability of the periodic reflex modulation to occur. Furthermore, some descending long-tract fibers or propriospinal connections may survive even severe SCI clinically classified as motor complete (Kakulas 2004; McKay et al. 2004; Sherwood et al. 1992) and may influence the modulation patterns produced across subjects.
The lack of attenuation of the PRM reflexes elicited by 2-Hz SCS may have resulted from the multiroot input provided by SCS together with alterations in the spinal circuits' excitability after SCI (Grey et al. 2008; Ishikawa et al. 1966). At such stimulation frequency, the net results of inhibition and excitation may have evened each other out. Also, time-dependent mechanisms related to recurrent inhibition and rebound discharges would have ceased before the next response was elicited.
Coupling of mechanisms underlying reflex modulation in pairs of muscle groups.
Periodic PRM reflex modulation generated in a single muscle group showed that the mechanisms modulating the excitability of the respective motoneuron pool did not require the occurrence of such a pattern in other muscles (cf. Clair et al. 2011). However, prevalence, consistency, and attenuation were significantly higher when the periodic patterns occurred in pairs of related muscle groups. This observation hints at interactions and mutual drive of oscillating or time-dependent mechanisms acting on separate motoneuron pools. Coupling effects are also put forward by the observation that not all possible phase relations of reflex modulation between muscle groups occurred with the same odds. Influences affecting different motoneuron pools may arise from interconnections of myotatic reflex arcs (Cheng et al. 1995; Delwaide et al. 1976; Meunier et al. 1990) as well as crossed spinal connections (Koceja and Kamen 1992; Mezzarane and Kohn 2002). For instance, recurrent inhibition of Ia inhibitory interneurons would periodically release antagonistic motoneurons from their tonic inhibitory activity, a phenomenon known as recurrent facilitation (Hultborn et al. 1971b; Pierrot-Desseilligny and Burke 2012). Independently, the preferential generation of synchronous reflex modulation in pairs of muscle groups may be explained by the same initial conditions across muscles with the first stimulus pulse leading to large, unconditioned PRM reflexes.
Involvement of spinal oscillating networks.
An alternative mechanism underlying the periodic reflex modulation could be the activation of a spinal network with inherent oscillating capability that cyclically modified the excitability of the central components of the PRM reflex arcs. One network capable of producing rhythmic motor output in response to tonic SCS is the spinal locomotor rhythm and pattern generator (Danner et al. 2015). However, the higher stimulation frequencies of 22–60 Hz required for its activation as well as the lower cycle frequency of the produced rhythmic outputs of 0.27–1.84 Hz (Danner et al. 2015; Dimitrijevic et al. 1998; Minassian et al. 2004) render its involvement in the patterns described here highly unlikely.
Another spinal oscillating system that may underlie the periodic reflex modulation is indicated by the occurrence of the larger-amplitude PRM reflexes within these patterns at 5.5–8 Hz that are the usual rates of sustained clonus (Dimitrijevic et al. 1980). One hypothesis for the generation of clonus is that peripheral events such as stretch interact with a spinal generator that produces alternating excitatory-refractory cycles that sustain and regulate the repetitive clonic bursts (Beres-Jones et al. 2003; Dimitrijevic et al. 1980). Dimitrijevic and colleagues (1980) also found that Achilles tendon reflexes evoked in clonic muscles attained large EMG amplitudes when tapping at rates similar to that of clonus. When tapping rates were further increased, reflex attenuation occurred, affecting each response, or—with tapping rates close to twice the rate of clonus—a large response to alternate taps was evoked. The periodic reflex modulation described here is reminiscent of the latter finding. Hence, the SCS inputs may have initiated and entrained centrally organized cyclical changes of excitability similar to those underlying clonus, which in turn affected the excitability cycles of the PRM reflexes.
Role of stimulation frequency in central and muscular effects.
Clinical applications of epidural SCS in individuals with severe SCI are motivated by the finding that sustained stimulation at 5–15 Hz can generate bilateral extension (Jilge et al. 2004), sufficient to induce standing (Harkema et al. 2011), while 22–60 Hz can produce alternating flexion-extension movements (Dimitrijevic et al. 1998). It was suggested that the different frequencies recruited different elements of spinal pattern generating networks (Danner et al. 2015; Jilge et al. 2004; Minassian et al. 2007a). Since the SCS-induced motor outputs are composed of series of stimulus-triggered PRM reflexes (Jilge et al. 2004; Minassian et al. 2004), the stimulation frequency entrains the motoneuron firing rate and thus critically influences the muscle force produced. This relationship between the stimulation frequency and muscle force was also used in a recent study to achieve control of hindlimb kinematics during SCS-induced locomotion in paralyzed rats (Wenger et al. 2014). The phenomenon described in the present report shows that, under specific conditions, the effective output frequencies (given by the larger-amplitude responses within the periodic modulation) may be lower than the stimulation frequency applied. For inducing standing, increasing the stimulation frequency slightly above those optimal to centrally organize an extension function may be required to avoid PRM reflex alternation (cf. Fig. 2A) and to produce adequate levels of extensor muscle force.
Conclusions.
The repetitive elicitation of monosynaptic spinal reflexes at distinct rates led to a simple form of oscillating motor output with unanticipated prevalence in individuals after chronic loss of supraspinal input. Apparently, the bilateral, multisegmental activation as provided by SCS was essential to reveal this type of input processing and output generation by the human lumbosacral spinal circuitry. The recent renaissance of epidural lumbar SCS in SCI patients (Angeli et al. 2014; Danner et al. 2015; Harkema et al. 2011) as well as novel neurophysiological studies employing transcutaneous SCS (Dy et al. 2010; Hofstoetter et al. 2013, 2014; Knikou 2014; Roy et al. 2014) may provide means to further advance the current understanding of human spinal sensorimotor integration.
GRANTS
This work was supported by the Vienna Science and Technology Fund (WWTF; Grant LS11-057), the Wings for Life Spinal Cord Research Foundation (Grant WFL-AT-007/11), and the Austrian Science Fund (FWF; Grant L512-N13).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: U.S.H., F.R., and K.M. conception and design of research; U.S.H., S.M.D., and K.M. analyzed data; U.S.H., H.B., and K.M. interpreted results of experiments; U.S.H. and K.M. prepared figures; U.S.H., S.M.D., and K.M. drafted manuscript; U.S.H., S.M.D., B.F., H.B., W.M., F.R., and K.M. edited and revised manuscript; U.S.H., S.M.D., B.F., H.B., W.M., F.R., and K.M. approved final version of manuscript; B.F. performed experiments.
ACKNOWLEDGMENTS
Special thanks are due to Milan R. Dimitrijevic, Dept. of Physical Medicine and Rehabilitation, Baylor College of Medicine, Houston, TX, and Foundation for Movement Recovery, Oslo, Norway, and W. Barry McKay, Hulse Spinal Cord Injury Lab, Crawford Research Institute, Shepherd Center, Atlanta, GA, for their insightful comments on the present manuscript. We acknowledge the continuous support of Maria Auer, Renate Preinfalk, and Christa Schneider, all affiliated with the Neurological Centre, Maria Theresien Schloessel, Otto Wagner Hospital, Vienna, Austria.
REFERENCES
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137: 1394–1409, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beres-Jones JA, Johnson TD, Harkema SJ. Clonus after human spinal cord injury cannot be attributed solely to recurrent muscle-tendon stretch. Exp Brain Res 149: 222–236, 2003. [DOI] [PubMed] [Google Scholar]
- Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr Clin Neurophysiol 89: 177–186, 1993. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci 6: 1308–1313, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Bassi Luciani L, Courtine G, Micera S. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci 33: 19326–19340, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Brooke JD, Staines WR, Misiaszek JE, Hoare J. Long-lasting conditioning of the human soleus H reflex following quadriceps tendon tap. Brain Res 681: 197–200, 1995. [DOI] [PubMed] [Google Scholar]
- Clair JM, Anderson-Reid JM, Graham CM, Collins DF. Postactivation depression and recovery of reflex transmission during repetitive electrical stimulation of the human tibial nerve. J Neurophysiol 106: 184–192, 2011. [DOI] [PubMed] [Google Scholar]
- Cook AW, Weinstein SP. Chronic dorsal column stimulation in multiple sclerosis. Preliminary report. NY State J Med 73: 2868–2872, 1973. [PubMed] [Google Scholar]
- Courtine G, Harkema SJ, Dy CJ, Gerasimenko YP, Dyhre-Poulsen P. Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J Physiol 582: 1125–1139, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Methodological implications of the post-activation depression of the soleus H-reflex in man. Exp Brain Res 78: 28–32, 1989. [DOI] [PubMed] [Google Scholar]
- Danner SM, Hofstoetter US, Freundl B, Binder H, Mayr W, Rattay F, Minassian K. Human spinal locomotor control is based on flexibly organized burst generators. Brain 138: 577–588, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide PJ, Cordonnier M, Charlier M. Functional relationships between myotatic reflex arcs of the lower limb in man: investigation by excitability curves. J Neurol Neurosurg Psychiatry 39: 545–554, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann NY Acad Sci 860: 360–376, 1998. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Nathan PW, Sherwood AM. Clonus: the role of central mechanisms. J Neurol Neurosurg Psychiatry 43: 321–332, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dy CJ, Gerasimenko YP, Edgerton VR, Dyhre-Poulsen P, Courtine G, Harkema SJ. Phase-dependent modulation of percutaneously elicited multisegmental muscle responses after spinal cord injury. J Neurophysiol 103: 2808–2820, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol 126: 524–562, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J Physiol 533: 15–22, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko YP, Makarovskii AN, Nikitin OA. Control of locomotor activity in humans and animals in the absence of supraspinal influences. Neurosci Behav Physiol 32: 417–423, 2002. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Klinge K, Crone C, Lorentzen J, Biering-Sørensen F, Ravnborg M, Nielsen JB. Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Exp Brain Res 185: 189–197, 2008. [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377: 1938–1947, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstoetter US, Hofer C, Kern H, Danner SM, Mayr W, Dimitrijevic MR, Minassian K. Effects of transcutaneous spinal cord stimulation on voluntary locomotor activity in an incomplete spinal cord injured individual. Biomed Tech (Berl) (September 7, 2013). doi: 10.1515/bmt-2013-4014. [DOI] [PubMed] [Google Scholar]
- Hofstoetter US, McKay WB, Tansey KE, Mayr W, Kern H, Minassian K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J Spinal Cord Med 37: 202–211, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstoetter US, Minassian K, Hofer C, Mayr W, Rattay F, Dimitrijevic MR. Modification of reflex responses to lumbar posterior root stimulation by motor tasks in healthy subjects. Artif Organs 32: 644–648, 2008. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Jankowska E, Lindström S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol 215: 591–612, 1971a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Jankowska E, Lindström S, Roberts W. Neuronal pathway of the recurrent facilitation of motoneurons. J Physiol 218: 495–514, 1971b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Ott K, Porter RW, Stuart D. Low frequency depression of the H wave in normal and spinal man. Exp Neurol 15: 140–156, 1966. [DOI] [PubMed] [Google Scholar]
- Jilge B, Minassian K, Rattay F, Pinter MM, Gerstenbrand F, Binder H, Dimitrijevic MR. Initiating extension of the lower limbs in subjects with complete spinal cord injury by epidural lumbar cord stimulation. Exp Brain Res 154: 308–326, 2004. [DOI] [PubMed] [Google Scholar]
- Kagamihara Y, Hayashi A, Okuma Y, Nagaoka M, Nakajima Y, Tanaka R. Reassessment of H-reflex recovery curve using the double stimulation procedure. Muscle Nerve 21: 352–360, 1998. [DOI] [PubMed] [Google Scholar]
- Kakulas BA. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord 42: 549–563, 2004. [DOI] [PubMed] [Google Scholar]
- Katz R, Pierrot-Deseilligny E. Recurrent inhibition in humans. Prog Neurobiol 57: 325–355, 1999. [DOI] [PubMed] [Google Scholar]
- Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Mulcahey MJ, Schmidt-Read M, Waring W. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 34: 535–546, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knikou M. The H reflex as a probe: pathway and pitfalls. J Neurosci Methods 171: 1–12, 2008. [DOI] [PubMed] [Google Scholar]
- Knikou M. Transpinal and transcortical stimulation alter corticospinal excitability and increase spinal output. PLoS One 9: e102313, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koceja DM, Kamen G. Contralateral influences on triceps surae motoneuron excitability. Electroencephalogr Clin Neurophysiol 85: 177–182, 1992. [DOI] [PubMed] [Google Scholar]
- Ladenbauer J, Minassian K, Hofstoetter US, Dimitrijevic MR, Rattay F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans Neural Syst Rehabil Eng 18: 637–645, 2010. [DOI] [PubMed] [Google Scholar]
- Maertens de Noordhout A, Rothwell JC, Thompson PD, Day BL, Marsden CD. Percutaneous electrical stimulation of lumbosacral roots in man. J Neurol Neurosurg Psychiatry 51: 174–181, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magladery JW, McDougal DB Jr. Electrophysiological studies of nerve and reflex activity in normal man. I. Identification of certain reflexes in the electromyogram and the conduction velocity of peripheral nerve fibers. Bull Johns Hopkins Hosp 86: 265–290, 1950. [PubMed] [Google Scholar]
- Mao CC, Ashby P, Wang M, McCrea D. Synaptic connections from large muscle afferents to the motoneurons of various leg muscles in man. Exp Brain Res 56: 341–350, 1984. [DOI] [PubMed] [Google Scholar]
- McKay WB, Lim HK, Priebe MM, Stokic DS, Sherwood AM. Clinical neurophysiological assessment of residual motor control in post-spinal cord injury paralysis. Neurorehabil Neural Repair 18: 144–153, 2004. [DOI] [PubMed] [Google Scholar]
- Meunier S, Penicaud A, Pierrot-Deseilligny E, Rossi A. Monosynaptic Ia excitation and recurrent inhibition from quadriceps to ankle flexors and extensors in man. J Physiol 423: 661–675, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of monosynaptic heteronymous Ia connections in the human lower limb. Exp Brain Res 96: 534–544, 1993. [DOI] [PubMed] [Google Scholar]
- Mezzarane RA, Kohn AF. Bilateral soleus H-reflexes in humans elicited by simultaneous trains of stimuli: symmetry, variability, and covariance. J Neurophysiol 87: 2074–2083, 2002. [DOI] [PubMed] [Google Scholar]
- Minassian K, Hofstoetter U, Rattay F. Transcutaneous lumbar posterior root stimulation for motor control studies and modification of motor activity after spinal cord injury. In: Restorative Neurology of Spinal Cord Injury, edited by Dimitrijevic MR, Kakulas BA, Vrbova G, McKay WB. New York: Oxford Univ. Press, 2011, p. 226–255. [Google Scholar]
- Minassian K, Jilge B, Rattay F, Pinter MM, Binder H, Gerstenbrand F, Dimitrijevic MR. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord 42: 401–416, 2004. [DOI] [PubMed] [Google Scholar]
- Minassian K, Persy I, Rattay F, Pinter MM, Kern H, Dimitrijevic MR. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum Mov Sci 26: 275–295, 2007a. [DOI] [PubMed] [Google Scholar]
- Minassian K, Persy I, Rattay F, Dimitrijevic MR, Hofer C, Kern H. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 35: 327–336, 2007b. [DOI] [PubMed] [Google Scholar]
- Murg M, Binder H, Dimitrijevic MR. Epidural electric stimulation of posterior structures of the human lumbar spinal cord. 1. Muscle twitches—a functional method to define the site of stimulation. Spinal Cord 38: 394–402, 2000. [DOI] [PubMed] [Google Scholar]
- Paillard J. [Characteristics of the cumulative depression of the Hoffmann reflex to a slow rate of stimulation.] J Physiol (Paris) 51: 545–546, 1959. [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord: Spinal and Corticospinal Mechanisms of Movement. New York: Cambridge Univ. Press, 2012. [Google Scholar]
- Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiol Clin 30: 67–80, 2000. [DOI] [PubMed] [Google Scholar]
- Pinter MM, Gerstenbrand F, Dimitrijevic MR. Epidural electrical stimulation of posterior structures of the human lumbosacral cord. 3. Control of spasticity. Spinal Cord 38: 524–531, 2000. [DOI] [PubMed] [Google Scholar]
- Rattay F, Minassian K, Dimitrijevic MR. Epidural electrical stimulation of posterior structures of the human lumbosacral cord. 2. Quantitative analysis by computer modeling. Spinal Cord 38: 473–489, 2000. [DOI] [PubMed] [Google Scholar]
- Roy FD, Bosgra D, Stein RB. Interaction of transcutaneous spinal stimulation and transcranial magnetic stimulation in human leg muscles. Exp Brain Res 232: 1717–1728, 2014. [DOI] [PubMed] [Google Scholar]
- Schiepatti M. The Hoffmann reflex: a means of assessing spinal excitability and its descending control in man. Prog Neurobiol 28: 345–376, 1987. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S, Shields RK. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp Brain Res 133: 233–241, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapkova EY, Schomburg ED. Two types of motor modulation underlying human stepping evoked by spinal cord electrical stimulation (SCES). Acta Physiol Pharmacol Bulg 26: 155–157, 2001. [PubMed] [Google Scholar]
- Sherwood AM, Dimitrijevic MR, McKay WB. Evidence of subclinical brain influence in clinically complete spinal cord injury: discomplete SCI. J Neurol Sci 110: 90–98, 1992. [DOI] [PubMed] [Google Scholar]
- Sherwood AM, McKay WB, Dimitrijevic MR. Motor control after spinal cord injury: assessment using surface EMG. Muscle Nerve 19: 966–979, 1996. [DOI] [PubMed] [Google Scholar]
- Wenger N, Moraud EM, Raspopovic S, Bonizzato M, DiGiovanna J, Musienko P, Morari M, Micera S, Courtine G. Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci Transl Med 6: 255ra133, 2014. [DOI] [PubMed] [Google Scholar]