Abstract
Visuomotor learning results in changes in both motor and sensory systems (Cressman EK, Henriques DY. J Neurophysiol 102: 3505–3518, 2009), such that reaches are adapted and sense of felt hand position recalibrated after reaching with altered visual feedback of the hand. Moreover, visuomotor learning has been shown to generalize such that reach adaptation achieved at a trained target location can influence reaches to novel target directions (Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. J Neurosci 20: 8916–8924, 2000). We looked to determine whether proprioceptive recalibration also generalizes to novel locations. Moreover, we looked to establish the relationship between reach adaptation and changes in sense of felt hand position by determining whether proprioceptive recalibration generalizes to novel targets in a similar manner as reach adaptation. On training trials, subjects reached to a single target with aligned or misaligned cursor-hand feedback, in which the cursor was either rotated or scaled in extent relative to hand movement. After reach training, subjects reached to the training target and novel targets (including targets from a second start position) without visual feedback to assess generalization of reach adaptation. Subjects then performed a proprioceptive estimation task, in which they indicated the position of their hand relative to visual reference markers placed at similar locations as the trained and novel reach targets. Results indicated that shifts in hand position generalized across novel locations, independent of reach adaptation. Thus these distinct sensory and motor generalization patterns suggest that reach adaptation and proprioceptive recalibration arise from independent error signals and that changes in one system cannot guide adjustments in the other.
Keywords: altered visual feedback, generalization, proprioception, reach adaptation, recalibration
movements must be continually adapted in order to compensate for changes within the environment and one's body. A key feature of motor adaptation is the ability to generalize what one learns in one context and apply it to other, similar contexts. Previous research examining visuomotor learning, by having subjects reach in a virtual reality environment with distorted visual feedback of their hand, has demonstrated that subjects quickly adjust their movements so that the “seen” hand moves to the target (Flanagan and Rao 1995; Ghahramani et al. 1996; Krakauer 2009; Simani et al. 2007; Vetter et al. 1999; Wolpert et al. 1995). Moreover, learning has been shown to generalize. However, the magnitude to which learning generalizes or transfers to novel untrained movements has been shown to depend on the type of visuomotor distortion introduced. Specifically, adapting to a visuomotor rotation tends to lead to narrow generalization patterns (mainly limited to trained target directions), whereas adaptation to a cursor-gain distortion has been shown to lead to fairly broad generalization, showing extensive transfer of learning to untrained locations across the workspace (Ghahramani et al. 1996; Krakauer et al. 2000; Vetter et al. 1999; Wang and Sainburg 2005).
In addition to changes in reaches (or reach adaptation), visuomotor learning has been shown to lead to sensory recalibration. In particular, proprioceptive information has been suggested to be recalibrated after reaches made while wearing laterally displacing prism goggles (Harris 1963; Hay and Pick 1966; Redding et al. 2005) and in a virtual reality environment (Cressman and Henriques 2009, 2011; Henriques and Cressman 2012), such that subjects shift the position they feel their hand in toward the direction of the visual feedback provided. Ostry et al. (2010) have also shown shifts in the direction of perceived hand motion after reach training in a velocity-dependent force-field perturbation. As well, predictions regarding the visual consequences of one's actions have been shown to be updated after reaching with altered visual feedback of the hand (Block and Bastian 2012; Izawa et al. 2012; Synofzik et al. 2006, 2008).
While changes in reaches and shifts in felt hand position are seen after visuomotor learning, the relationship between these changes remains unclear. We have suggested that these changes in movement and shifts in felt hand position arise independently. In support of this proposal, we have shown that reach adaptation transfers from the trained to the untrained limb (intermanual transfer) in the absence of changes in felt hand position transferring to the untrained hand (Mostafa et al. 2014). Moreover, we have consistently found that sensory and motor adaptation are not significantly correlated (Cressman et al. 2010; Cressman and Henriques 2009; Salomonczyk et al. 2011, 2012). Likewise, changes in the predicted sensory consequences of one's movements have been shown to occur both in the absence and in the presence of reach adaptation in cerebellar patients (Block and Bastian 2012; Izawa et al. 2012; Synofzik et al. 2006, 2008). In contrast to our proposal that changes in the motor and sensory systems arise independently, it has been suggested that these changes arise in parallel. Specifically, it has been put forth that the processes giving rise to motor learning drive sensory changes, as motor adaptation appears to be a prerequisite for somatosensory recalibration (Mattar et al. 2013; Ostry et al. 2010; Wong et al. 2011).
One difficulty in establishing the relationship between motor and sensory changes to date is that the two changes typically occur in parallel under experimental conditions (i.e., motor changes are accompanied by changes in the sensory system or vice versa). In this study, we looked to establish whether proprioceptive recalibration is dependent on changes in the motor system and/or its potential contribution to reach adaptation. To establish the relationship between proprioceptive recalibration and reach adaption we compared the generalization pattern for proprioceptive recalibration at novel targets to that of reach adaptation after subjects trained to reach to a single target with altered visual feedback of the hand.
Subjects trained to reach to one target in a virtual reality environment when a visuomotor distortion was introduced. Similar to Krakauer and colleagues (2000), we had subjects reach to the training target when a visuomotor rotation or cursor gain distortion was introduced. After subjects reached with altered visual feedback of the hand, we then examined generalization of 1) reach adaptation, by having subjects reach to novel visual targets without any visual feedback in locations different from the trained target or in a direction similar to the trained target but from a new starting position, and 2) proprioceptive recalibration, by having subjects judge the position of their unseen hand with respect to visual reference markers at similar novel locations. Thus we looked to establish whether proprioceptive recalibration generalized across the workspace. Furthermore, by having a second start position, we were able to determine whether generalization patterns for reach adaptation and proprioceptive recalibration were dependent on initial hand position. By comparing the patterns of motor and sensory generalization after training with the two cursor distortions (visuomotor rotation and a cursor gain), we looked to determine whether it is possible for proprioceptive recalibration to arise in the absence of reach adaptation (or vice versa). Similar to previous results, we hypothesized that reach adaptation would be more limited after reaches with a visuomotor rotation compared with after reaches with a cursor gain. Of primary concern was how these generalization patterns of reach adaptation would compare to changes in felt hand position across the workspace. We hypothesized that changes in felt hand position would generalize broadly across the workspace after both visuomotor distortions and hence we would find proprioceptive recalibration at locations in the workspace where we did (e.g., after training with a cursor gain) and did not (e.g., after training with a visuomotor rotation) find any reach adaptation. These results would suggest that the processes underlying reach adaptation and proprioceptive recalibration are independent.
METHODS
Overview
Subjects.
Thirty-four young right-handed adults (mean age = 23.5 yr, SD = 6.1 yr; 25 women, 9 men) were recruited from York University and volunteered to participate in the tasks described below. Subjects were prescreened and verbally reported being right-handed and having no history of visual, neurological, and/or motor dysfunction. After prescreening, 24 subjects completed the Rotation Generalization protocol (14 in a Left Rotation Generalization group and 10 in a Right Rotation Generalization group; the difference in the number of subjects between the 2 groups is due to 3 subjects in the Right Rotation Generalization group failing to complete all testing sessions). Ten additional subjects completed the Gain Generalization protocol. All subjects provided informed consent in accordance with ethical guidelines set by and protocols reviewed and approved by the York University Human Participants Review Subcommittee.
General experimental setup.
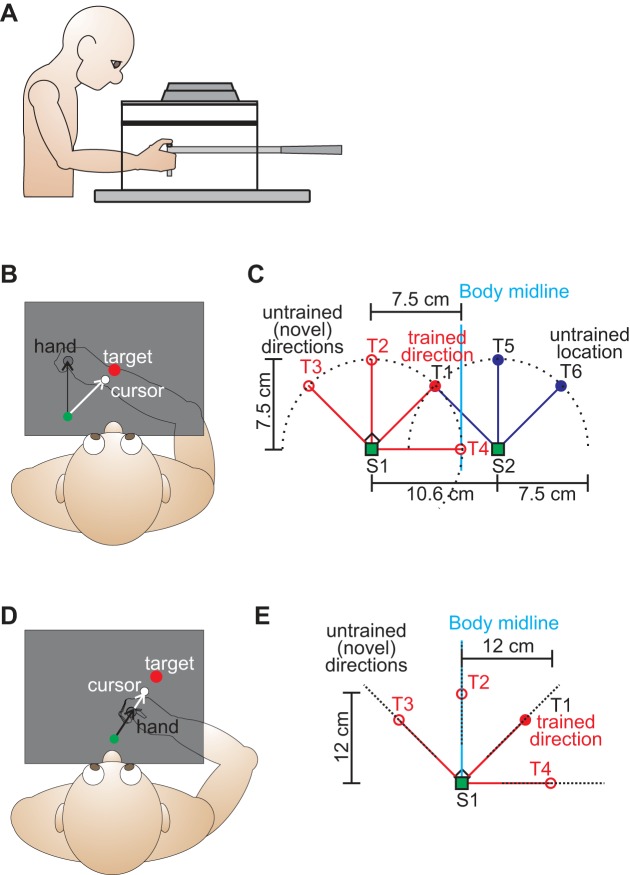
A side view of the setup is illustrated in Fig. 1A and is similar to that used in Cressman and Henriques (2009, 2010). Subjects were seated at a table such that the distance of the chair from the table and the height of the chair were adjusted in order to ensure that subjects could comfortably see and reach to all target positions. Once the chair was adjusted, it remained in the same position for the entire experiment, across the different testing sessions. Subjects were instructed to grasp the vertical handle of a two-joint robot manipulandum (Interactive Motion Technologies) with their right hand such that their thumb was positioned on a top marker (1.4 cm in diameter). Visual stimuli were projected from a monitor (model: Samsung 510N; refresh rate: 72 Hz) installed 17 cm above the robot and viewed by subjects as a reflected image. The display subtended a visual angle of 67°. The reflective surface was opaque and positioned such that the displayed images appeared to lie in the same horizontal plane as the robot handle. The room lights were dimmed, and subjects' view of their right hand was blocked by the reflective surface and a black cloth draped between the experimental setup and subjects' right shoulder.
Fig. 1.
Experimental setup and design. A: side view of the experimental setup. B and C: top view of the experimental surface visible to subjects in the Rotation Generalization protocol. B: visuomotor distortion introduced in the rotated Reach Training task. The white cursor (representing the hand) was rotated 45° clockwise with respect to the actual hand location (gray disk) during the rotated reach training block. The reach training target (red filled circle) was located 7.5 cm from the home position (S1: green circle) at an angle of 45° with respect to the home position. C: locations of the 7 no-cursor reach targets (trained and novel locations) and reference markers at which generalization of reach adaptation and shifts in felt hand position were assessed. All targets/markers were located 7.5 cm from 1 of the 2 home positions (S1: 4 targets/reference markers; S2: 3 targets/reference markers). With respect to S1, targets/markers were located at the same position as the reach training target, 45° left or right of the reach training target, and 90° left of the reach training target. With respect to S2, targets/markers were located in the same direction as the reach training target (45° right of S2), as well as 45° and 90° left of the reach training direction. The blue vertical line indicates body midline. D and E: top view of the experimental surface visible to subjects in the Gain Generalization protocol. D: visuomotor distortion introduced in the cursor gain Reach Training task. In this case, a cursor increase was introduced such that the white cursor (representing the hand) moved 1.5 times more than the hand (gray disk) during the misaligned reach training block. The reach training target (red filled circle) was located 12 cm from the home position (S1: green circle) at an angle of 45° with respect to the home position. E: locations of the 4 no-cursor reach targets (trained and novel) and reference markers at which generalization of motor adaptation and proprioceptive recalibration were assessed. All targets/markers were located 12 cm from the home position (S1), at the same position as the reach training target, 45° left or right of the reach training target, and 90° left of the reach training target.
Rotation Generalization Protocol
Stimulus display.
At the start of each reach training trial described below, the robot manipulandum was positioned below a home (or start) position, ∼28 cm in front of subjects. This position was indicated visually by a green filled circle, 1 cm in diameter. As depicted in Fig. 1, B and C, this home position (S1) was located 7.5 cm left of the subject's midline. An additional home position (S2) was located 10.6 cm to the right of S1 (i.e., 3.1 cm right of the subject's midline) as seen in Fig. 1C. Visual stimuli (yellow circles, 1 cm in diameter) were displayed 7.5 cm from the two home positions as illustrated in Fig. 1C. Specifically, from S1 visual stimuli (red circles in Fig. 1C) were displayed directly above the home position at center (0°; T2), 45° left (counterclockwise, CCW; T3) and right (clockwise, CW; T1) of center, and 90° right of center (T4). From S2, visual stimuli (blue circles in Fig. 1C) were displayed directly above the second home position (0°; T5) and 45° left (CCW; T1) and right (CW; T6) of center.
Procedure.
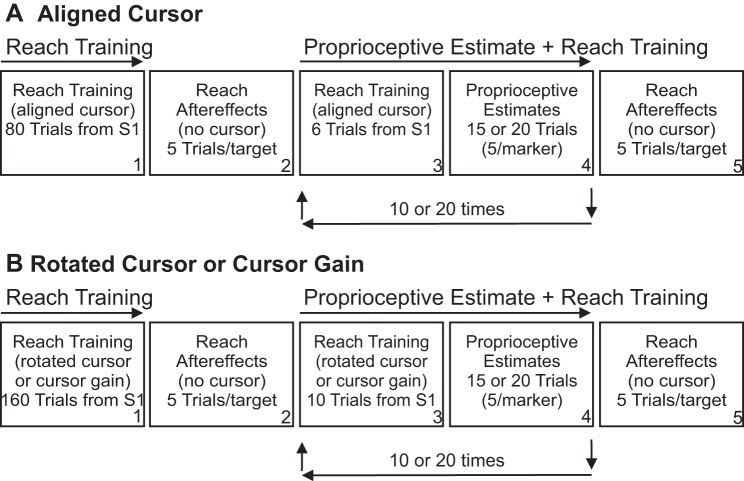
Subjects completed two testing sessions on different days such that there was a minimum of 2 days to a maximum of 3 wk between sessions. Within each testing session, subjects completed the tasks outlined below and illustrated in Fig. 2. The first testing session (Fig. 2A) provided baseline measures. In particular, we assessed reaching errors in the No-cursor reaching task described below and proprioceptive estimates of hand position after subjects reached to the trained target with a cursor that was aligned with their hand during the reach training task. In the second testing session (Fig. 2B), we assessed no-cursor reaching errors (i.e., visuomotor adaptation) and felt hand position at the trained and novel reference marker locations after subjects reached with altered visual feedback of their hand during the reach training task.
Fig. 2.
Breakdown of testing sessions within the experiment. In the first testing session (A), subjects reached with an aligned cursor that accurately represented the position of their hands to the 45° reach training target. In the second testing session (B), subjects reached with a misaligned cursor that was either rotated 45° clockwise with respect to the actual hand location during the reach training trials or moved to a greater (1.5 times: Cursor Increase) or lesser (0.67 times: Cursor Decrease) amount than the hand. After completing the reach training trials with an aligned (box A1) or misaligned (box B1) cursor, subjects reached to each no-cursor reach target 5 times without a cursor in order to assess generalization of reach adaptation (reach aftereffect trials, boxes A2 and B2). Subjects then completed 6 or 10 more reach training trials to the reach training target with the cursor present (boxes A3 and B3) before completing the first set of 15 or 20 proprioceptive estimate trials (boxes A4 and B4). This cycle of visually guided reaches to the trained target (boxes A3 and B3) followed by proprioceptive estimates (boxes A4 and B4) was completed a total of 10 or 20 times. Subsequently, subjects reached to all no-cursor reach targets 5 times without a cursor in order to assess maintenance of reach aftereffects (boxes A5 and B5).
We begin by describing the tasks used in the first part of the testing session (Fig. 2A). The tasks are described in the order in which they were completed.
Reach Training Task
In this first task (Fig. 2A, box A1), subjects began each trial with the robot, and hence their hand, positioned directly below the visual home position (S1). After the hand was maintained at the home position for 300 ms, the reach training target (T1) located 45° right of center would appear (yellow circle, 1 cm in diameter; depicted as a red filled circle in Fig. 1C). Subjects were instructed to move as quickly and accurately as possible to the target while gripping the handle of the free-moving robot manipulandum. Once subjects started moving, the home position was turned off. The position of the unseen hand was represented by a cursor (1-cm green disk; depicted as a white filled circle in Fig. 1B). The cursor appeared after the robot handle had moved 4 cm outward from the home position and remained visible until the reach was complete (when the center of the cursor had moved to within 0.5 cm of the target's center). At that point, both the target and cursor were removed and the robot was locked to a constrained, grooved path. This constrained path guided subjects back to the home position by a direct linear route in the absence of visual feedback. If subjects attempted to move outside of the established path, a resistance force [proportional to the depth of penetration with a stiffness of 2 N/mm and a viscous damping of 5 N(s/mm)] was generated perpendicular to the constrained path (Cressman and Henriques 2009; Henriques and Soechting 2003; Jones et al. 2010). Subjects completed 80 trials.
No-Cursor Reaching Task: To Assess Generalization of Reach Errors (and Aftereffects)
This task (Fig. 2A, box A2) was performed immediately after the Reach Training task. A trial would start with the robot handle positioned at one of the two visible home positions. A target would then appear (yellow circle, 1 cm in diameter; depicted as red and blue circles in Fig. 1C). This was the cue for subjects to reach out using the robot handle, without the cursor or any visual feedback of the hand, to the still-visible target. Similar to the Reach Training task, the start position would disappear once a subject started moving. The reach was considered complete once a final position was held for 250 ms. At this point, the target would disappear and subjects would move back to the home position along a constrained linear path in order to complete the subsequent trial.
Subjects completed five reaches to the reach training target and each of the six novel targets. Specifically, subjects reached to visual target T1 from S1 to assess reach adaptation at the trained target. To assess motor generalization, subjects reached to three novel visual targets (T2, T3, and T4) from S1 and three novel visual targets (T1, T5, and T6) from S2 as shown in Fig. 1C. Reaches to T1 from S2 are referred to as novel given the new start position, even though T1 is in the same target location at the trained target. This target arrangement thus allowed us to examine motor generalization with respect to movement direction and start position.
Subjects completed all reaches from a given home position before moving to the second home position. Subjects in the Left Rotation Generalization group completed all 20 reaches (4 targets × 5 reaches/target) from S1, before moving to S2 to complete 15 more no-cursor reaches. In contrast, subjects in the Right Rotation Generalization group completed all 15 reaches (3 targets × 5 reaches/target) from S2, before moving to S1 to complete 20 more no-cursor reaches.
Proprioceptive Estimate and Reach Training Task: To Assess Generalization of Sense of Felt Hand Position
In this task, proprioceptive estimates and reach training trials (boxes A3 and A4 in Fig. 2A) were systematically interleaved. Subjects began by completing an additional six reach training trials with a visual cursor to the reach training target located 45° right of center from S1. These reaches were then immediately followed by sets of 20 (or 15) proprioceptive estimate trials. Subjects completed the interleaving sets of reach training trials and proprioceptive estimates 20 times. Similar to the no-cursor reaches, half of the subjects completed the 10 sets of 20 proprioceptive estimates (5 to each of the 4 reference markers) from S1 before moving to S2 to complete the 10 additional sets of 15 proprioceptive estimate trials (5 to each of the 3 reference markers). The other subjects completed all 10 sets of proprioceptive estimates from S2 before moving to S1 to complete the last 10 sets of proprioceptive estimate trials from S1. Between each set of proprioceptive estimates, subjects completed six reaches to the reach training target from S1 when a visual cursor was displayed. Thus subjects completed 120 reach training trials and 350 proprioceptive estimates in total in the Proprioceptive Estimate and Reach Training task.
In the proprioceptive estimation trials, subjects grasped the handle of the robot manipulandum at one of the visible home positions (S1 or S2) for 500 ms. After 500 ms, the home position was removed, and subjects were to actively push the robot handle out along a constrained linear path. The path guided the hand to a location somewhere along the black dotted lines shown in Fig. 1C, which are provided as reference. Once the hand arrived at its final position a reference marker appeared (yellow circle, 1 cm in diameter), and subjects made a 2-AFC judgment about the position of their hand (left or right) relative to the reference marker. There was no time constraint during the task, and subjects were encouraged to take as long as they needed before pressing a left or right arrow key to indicate that they felt their hand was to the left or to the right of the reference marker, respectively. After entering the response, subjects' hands were guided back to the home position (either S1 or S2) by a constrained linear path.
The reference marker locations were the same as the no-cursor reach targets (red and blue circles in Fig. 1C). The position of the hand with respect to each reference marker was adjusted over trials with an adaptive staircase algorithm (Kesten 1958; Treutwein 1995) as outlined by Cressman and Henriques (2009). For each reference marker, there were two staircases, one staircase starting 20° to the left (CCW) of the reference marker and one starting 20° to the right (CW). Thus when subjects first pushed their hand out along the constrained path they ended up 20° left (or right) of the reference marker along the black dotted lines in Fig. 1C. For each reference marker the two staircases were adjusted independently and randomly interleaved. Specifically, the position of the hand was adjusted over trials depending on a subject's pattern of responses, such that the differences between hand locations on subsequent trials (step size) decreased each time subjects reversed their response pattern from left (right) to right (left) within a particular staircase. This ensured that subjects were tested more frequently at positions closer to their sensitivity threshold. If subjects responded consistently, the two staircases for each reference marker converged toward a certain position at which subjects had an equal probability of reporting left or right. This position represented the location at which subjects perceived that their hand was aligned with the reference marker.
No-Cursor Reaching Task: To Assess Maintenance of Reach Errors (and Aftereffects)
Immediately after completing the Proprioceptive Estimate and Reach Training task, subjects completed the No-cursor reaching task for a second time. These reaches were carried out in the same manner as described above. Again, subjects reached to all visual targets five times.
This ended the first testing session. The tasks in the second testing session (Fig. 2B) were similar to those outlined above, except that the Reach Training task was replaced with the Reach Training with altered visual feedback task described below. Furthermore, the same cursor distortion introduced in the Reach Training with altered visual feedback task was present during all reach training trials with the cursor in the Proprioceptive Estimate and Reach Training task. In the second testing session, subjects completed 10 interleaved reach training trials in the Proprioceptive Estimate and Reach Training task, as opposed to the 6 reaches completed in the first testing session (Fig. 2, box A3 vs. box B3). These additional reaches were completed because this was a novel visuomotor environment and we wanted to ensure that adaptation of reaching movements was maintained.
Reach Training with Altered Visual Feedback Task
The second part of the testing session began with the Reach Training with altered visual feedback task (Fig. 2B, box B1). This task was very similar to the Reach Training task described above. However, instead of having a cursor accurately represent the position of subjects' hands while they reached to the reach target located 45° right of center, the cursor was gradually rotated 45° CW with respect to the robot over the first 60 trials in increments of 0.75°. Subjects completed 160 trials.
Gain Generalization Protocol
In outlining this experiment, we focus on describing differences between the tasks completed in the Gain Generalization protocol compared with the Rotation Generalization protocol discussed above. The order that the tasks were completed in was the same between the two experiments.
Stimulus display.
In the Gain Generalization protocol, there was only one home position (S1) located directly in front of the subject's midline as indicated in Fig. 1, D and E. Visual stimuli (yellow circles, 1 cm in diameter depicted as red circles in Fig. 1E) were displayed 12 cm from the home position at center (0°; T2), 45° left (CCW; T3) and right (CW; T1) of center, and 90° right of center (T4).
Procedure.
Subjects completed three testing sessions, a minimum of 2 h apart to a maximum of 3 wk apart. Three sessions were included in the experiment, as session 1 had subjects complete the reach training trials with a cursor that was aligned with the hand and sessions 2 and 3 included reach training with altered visual feedback such that the cursor moved a greater (Cursor Increase) or smaller (Cursor Decrease) amount than the hand. The order of these two sessions was counterbalanced across subjects.
Reach Training Task
This task was the same as in the Rotation Generalization protocol. Subjects reached 80 times to the reach training target located 45° right of center from S1 (red filled circle in Fig. 1E).
No-Cursor Reaching Task: To Assess Generalization of Reach Errors (and Aftereffects)
Subjects completed 20 no-cursor reaches, 5 to the reach training target and 5 to each of the three novel visual targets (T2–T4) from S1 (as shown in Fig. 1E). This task was completed immediately after the reach training trials and again after the Proprioceptive Estimate and Reach Training task discussed below.
Proprioceptive Estimate and Reach Training Task: To Assess Generalization of Sense of Felt Hand Position
The reference marker locations were the same as the no-cursor reach targets (red circles in Fig. 1E). Specifically, proprioceptive estimates of felt hand location were determined at a reference marker (yellow circle, 1 cm in diameter) located at the reach training target location and three novel locations (T2–T4) from S1 (as shown in Fig. 1E). Subjects indicated whether their hand was farther (i.e., above) or closer (i.e., below) than the reference marker relative to the hand's starting position (S1) by pressing an up or down arrow key, respectively.
Reach Training with Altered Visual Feedback Task
Subjects completed this testing session twice. In one session the cursor gain was set at 1.5, meaning that the distance moved by the cursor on the screen was 1.5 times the distance moved by the hand (by the end of the first 60 trials; Cursor Increase). In another session the cursor gain was set at 0.67, meaning that the distance moved by the cursor on the screen was 0.67 times the distance moved by the hand (by the end of the first 60 trials; Cursor Decrease). Subjects completed 160 trials.
Data Analyses
No-cursor reach errors.
We examined the no-cursor reaches to determine whether (and at which of the trained and/or novel targets) subjects adapted their reaches in response to altered visual feedback. Specifically, we examined position data of the hand related to angular error or path length at movement end point and peak velocity. As indicated below, performance assessed at movement end point showed patterns of results similar to those when performance was assessed at peak velocity. Thus we focus our discussion on the results achieved at movement end point, given that we wanted to compare where subjects reached to with where subjects felt their hands were located at similar positions/distances in the proprioceptive estimation trials. For each no-cursor reach trial in the Rotation Generalization protocol, we calculated the reaching error by determining the angular difference between a reference vector joining the center home position and the target and the vector joining the center home position and the position of the hand at reach end-point location or peak velocity. To determine whether subjects adapted their reaches after reaching with the rotated cursor at any of the targets in the Rotation Generalization protocol, we analyzed mean reaching errors in the no-cursor reaches in a 2 Visual Feedback during the Reach Training task (i.e., visual training completed with an Aligned vs. Rotated cursor) × 2 Time (trials completed before vs. after the proprioceptive estimate and reach trials) × 7 Target repeated-measures analyses of variance (RM-ANOVA). Given that we were interested in examining generalization, we primarily discuss the Visual Training × Target interactions in results and compare performance at trained and novel target locations. In the Gain Generalization protocol, we calculated the distance that subjects moved in the no-cursor reach trials. Specifically, the path length of a given trial was calculated by determining the magnitude of the vector between the initial and final (x, y) positions of the hand or initial position of the hand and position at peak velocity. In the Gain Generalization protocol we also calculated peak velocity achieved. Mean reaching distance and peak velocity in the no-cursor reaches were then analyzed in a 3 Visual Training (Aligned vs. Cursor Increase vs. Cursor Decrease) × 2 Time × 4 Target RM-ANOVA.
Proprioceptive estimates of hand position.
To determine the locations at which subjects felt their hands were aligned with the reference markers, we fitted binary logistic regression functions in SPSS to the proprioceptive estimates for each reference marker for each subject in each testing session. The binary logistic regression function in SPSS is of the form P(Y) = 1/{1 + e^[−(βo + β1X)]}, where P(Y) represents the probability of Y occurring, e is the base of the natural logarithms, X is the predictor variable, and the coefficients βo and β1 refer to the intercept and a coefficient (weight) attached to the predictor variable, respectively, to form a linear regression equation. This procedure fits a generalized linear model with a binomial distribution and logit link function to each subject's responses. Based on each logistic function, we then calculated each subjects' bias (the point of 50% probability) and uncertainty range (the difference between the values at which the response probability was 25% and 75%) for each reference marker (Cressman and Henriques 2009; Henriques and Soechting 2003; Wong and Henriques 2009). Bias is a measure of the accuracy of hand-reference marker alignment, and the magnitude of the uncertainty range defines its precision. Bias and uncertainty related to a particular reference marker were excluded if the associated uncertainty was greater than the mean uncertainty across all reference markers within either the Rotated or Gain Generalization protocol + 2 SDs, as a large uncertainty range implies that subjects did not consistently report where their hand was in space (i.e., the staircases for a particular reference marker did not converge). On the basis of this analysis, 7% of all hand-reference marker estimates were excluded.
To determine whether (and at which reference marker locations) proprioception was recalibrated after reaching with a rotated cursor in the Rotation Generalization protocol, we compared the biases and the uncertainty ranges after Reach Training with an aligned cursor vs. a rotated cursor, using a RM-ANOVA. The RM-ANOVA included Visual Training (aligned vs. rotated cursor) and Reference Marker location (7 locations) as factors. In the Gain Generalization protocol, we analyzed biases and uncertainty ranges in a 3 Visual Training (Aligned vs. Cursor Increase vs. Cursor Decrease) × 4 Reference Marker location RM-ANOVA.
Differences with a probability of <0.05 were considered to be significant, and multiple comparisons with Bonferroni correction were used to determine the locus of these differences.
RESULTS
The goals of this study were to determine whether reaching with altered visual feedback in a localized area of the workspace leads to global changes in felt hand position and to establish the relationship between proprioceptive recalibration and reach adaptation by comparing generalization patterns.
Reach Adaptation: Rotation Generalization Protocol
To establish reach adaptation and generalization, we first determined whether subjects adapted their reaches and the magnitude of visuomotor adaptation (or aftereffects) at each of the no-cursor reach targets. Table 1 indicates subjects' angular errors at movement end point and peak velocity after reaching with an aligned cursor and a rotated cursor. As the same pattern of results is reflected for both measures, we focus on discussing errors at movement end point. Specifically, Fig. 3 displays mean changes in reaching errors at movement end point for reach trials completed without a cursor to each no-cursor reach target as a percentage of the rotation introduced in the reach trials with altered visual feedback. Given that we were interested in examining generalization at the novel no-cursor reach targets relative to reaching performance at the reach training target, target positions are referenced relative to the reach training target direction (set at 0°). At the reach training target (open black circle in Fig. 3A), subjects reached such that their hand was significantly more to the left of the target (13.8°) after completing the reach training trials with a cursor that was rotated rightward with respect to the hand compared with an aligned cursor [F(1,23) = 63.419, P < 0.001]. These results indicate that subjects adapted their reaches to the trained target, and we next looked to establish whether reach adaptation generalized to novel target locations. Similar to Krakauer and colleagues (2000), we found that the magnitude of reach adaptation was less when reaching in novel directions compared with the trained direction. Specifically, as seen by the generalization curve in Fig. 3A (black line joining black circles), subjects reached such that their hand was less left of the targets that were 45° left or right of the trained target position compared with the reach training target (45° left target = 10.4°; 45° right target = 8.9°). The difference in reaching errors after reaching with a rotated vs. aligned cursor was even smaller for the target that was 90° left of the trained target (90° left target = 2.8°). Taken together, these results indicate that reaches were not adapted to a similar magnitude for all target positions [F(6,138) = 7.365, P < 0.001]. The reach errors with respect to S2 (Fig. 3A, gray line joining gray circles) followed a similar pattern of generalization. In particular, reach adaptation was the greatest for the novel target located in the same direction as the trained target and decreased the further a target was from the trained reaching direction, indicating that reach adaptation had a very narrow generalization pattern. In accordance with these observations, multiple comparisons with Bonferroni correction revealed that subjects showed significant and similar aftereffects at the trained target (13.8°) and at the novel target located in the same 45° rightward direction from S2 (i.e., the target located at 45° right of center from S2 in the same direction as the trained target = 12.6° leftward reaching error). Significant reach adaptation was also observed at all novel targets that were within 45° of the trained target direction, such that reaches were adapted at the center (0°) target and the 90° rightward target with respect to S1 and the center target (9.9°) with respect to S2. Errors (and hence aftereffects) were not significantly different after reaching with an aligned vs. rotated cursor at the novel targets located 90° left/CCW of the trained target direction (i.e., 45° left of center) from either of the two starting positions, even though this target position from S2 corresponded to the reach training target (90° left target from S2 = 2.8°; open gray circle in Fig. 3A). Thus the greatest reach adaptation was observed for the trained target and a target located at a similar direction relative to a second starting position (S2). Subjects reached with similar errors across the testing session, such that reaching with an aligned or rotated cursor had a similar influence on performance regardless of whether the reaches were completed before or after the Proprioceptive Estimate and Reach Training task [F(1,23) < 1].
Table 1.
Reaching errors at movement end point and peak velocity in Rotation Generalization Protocol
| Targets [start position-target relative to trained target direction (°)] |
|||||||
|---|---|---|---|---|---|---|---|
| Reach Training | S1-0 | S1-45 CW | S1-45 CCW | S1-90 CCW | S2-0 | S2-45 CCW | S2-90 CCW |
| End-point angular errors, ° | |||||||
| Aligned cursor | −4.7 (1.8) | −6.7 (2.7) | 0.0 (2.1) | 6.0 (3.0) | −2.2 (2.1) | 4.1 (1.8) | 5.7 (2.0) |
| Rotated cursor | −18.4 (2.2) | −15.7 (2.5) | −10.5 (2.1) | 3.2 (2.2) | −14.8 (1.9) | −5.7 (1.6) | 2.9 (1.9) |
| Errors at peak velocity, ° | |||||||
| Aligned cursor | −5.5 (2.6) | −2.6 (3.0) | −5.9 (3.6) | 2.0 (5.0) | −1.3 (5.5) | 0.7 (3.3) | 10.1 (4.5) |
| Rotated cursor | −21.4 (3.4) | −14.3 (3.5) | −14.9 (3.8) | 0.8 (3.7) | −14.3 (3.1) | 10.6 (3.1) | 8.8 (2.6) |
Values are mean (SE) reaching errors at movement end point and peak velocity after reach training with an aligned cursor or misaligned visual feedback of the hand. Angular errors in the Rotation Generalization Protocol are reported with respect to target position [positive value = error to the right (CW) of the target and negative value = error to the left (CCW) of the target]. Values are reported for each target, after subjects trained with the visual cursor (i.e., performance is shown in the first no-cursor reaching task completed).
Fig. 3.
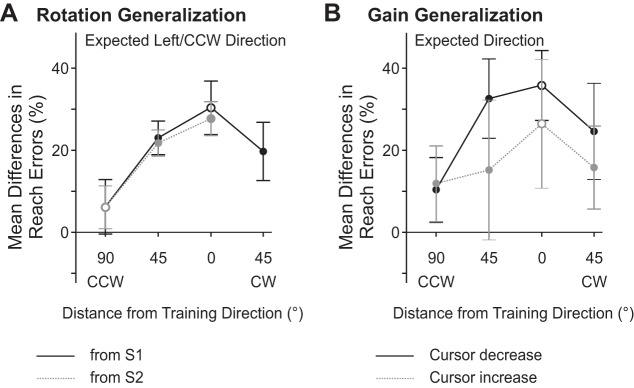
Aftereffects after reach training with misaligned visual feedback of the hand in which feedback of the hand was rotated (A) or a cursor gain was introduced (B). A positive value indicates that subjects adapted their reaches in the expected direction. In A, angular reach end-point errors in the no-cursor reach trials are shown after training with a rotated cursor relative to reach errors achieved after reaching with an aligned cursor as % of the distortion introduced. Errors are shown for each no-cursor reach target relative to the trained target direction (0° distance) from both S1 (solid black line) and S2 (dashed gray line). Open symbols represent errors made at the reach training target from S1 (open black circle) or S2 (open gray circle). In B, distance errors in the no-cursor reach trials are shown after training with a cursor gain. Errors are shown for each no-cursor reach target relative to the trained target direction (0° distance) after training with a Cursor Decrease (solid black line) and after training with a Cursor Increase (dashed gray line) as % of the distortion introduced. Open symbols represent errors made at the reach training target. Error bars reflect SE.
Reach Adaptation: Gain Generalization Protocol
Table 2 provides insight into the distance subjects had reached at movement end point and peak velocity after reaching with an aligned cursor and a cursor gain. Moreover, the peak velocity achieved across the different targets after training with an aligned cursor and a cursor gain is reported in Table 2. Similar to the Rotation Generalization protocol, the same patterns of results are reflected across measures at movement end point and peak velocity. Thus we focus on discussing errors at movement end point. In Fig. 3B, we plot the differences in path length after trials in which subjects had trained with a Cursor Decrease (black symbols) or Cursor Increase (gray symbols) compared with trials in which the cursor was aligned with the hand as a percentage of the distortion introduced during the reach training trials with altered visual feedback. Averaged across the reach training and novel targets, path length was 12.7 cm after training with an aligned cursor, 14.2 cm after training with a Cursor Decrease, and 12.0 cm after training with a Cursor Increase. As illustrated by the black curve in Fig. 3B, subjects significantly adapted their reaches, thus reaching to a greater extent overall after training with a Cursor Decrease compared with reaches made after training with an aligned cursor [1.5 cm; F(2,18) = 22.860, P < 0.001]. In contrast, as indicated by post hoc analyses, reaches were not significantly shorter after training with a Cursor Increase (gray line in Fig. 3B) compared with after training with an aligned cursor (0.7 cm; P > 0.05); however, there was a trend indicating reach adaptation in that the gray symbols in Fig. 3B fall above 0 in the expected direction. Similar to the results discussed above in the Rotation Generalization protocol, the influence of reach training on aftereffects was similar regardless of whether subjects completed the no-cursor reaches before or after the Proprioceptive Estimate and Reach task [F(2,18) < 1].
Table 2.
Reaching errors and performance at movement end point and peak velocity in Gain Generalization Protocol
| Targets [start position-target relative to trained target direction (°)] |
||||
|---|---|---|---|---|
| Reach Training | S1-0 | S1-45 CW | S1-45 CCW | S1-90 CCW |
| End-point distance errors, cm | ||||
| Aligned cursor | 1.02 (0.56) | −0.09 (0.40) | 0.76 (0.54) | 1.05 (0.32) |
| Cursor decrease | 3.16 (0.51) | 1.38 (0.57) | 2.71 (0.48) | 1.67 (0.52) |
| Cursor increase | −0.04 (0.49) | −0.70 (0.52) | 0.15 (0.46) | 0.58 (0.43) |
| Path length at peak velocity, cm | ||||
| Aligned cursor | 6.00 (0.22) | 6.11 (0.43) | 5.78 (0.11) | 5.44 (0.24) |
| Cursor decrease | 7.54 (0.29) | 7.50 (0.47) | 6.82 (0.26) | 6.04 (0.30) |
| Cursor increase | 5.57 (0.26) | 5.96 (0.35) | 5.90 (0.26) | 5.72 (0.18) |
| Peak velocity, cm/s | ||||
| Aligned cursor | 23.97 (2.08) | 22.22 (2.18) | 23.16 (1.80) | 24.87 (2.15) |
| Cursor decrease | 29.38 (2.49) | 27.71 (2.02) | 28.74 (2.74) | 30.74 (2.92) |
| Cursor increase | 22.94 (2.22) | 22.93 (2.41) | 24.50 (2.20) | 26.40 (2.36) |
Values are mean (SE) reaching errors and performance at movement end point and peak velocity after reach training with an aligned cursor or misaligned visual feedback of the hand. Movement distance errors in the Gain Generalization Protocol are reported with respect to the target (positive value = overshot the target and negative value = undershot the target). As well, path length achieved at peak velocity and peak velocity are reported. Values are reported for each target, after subjects trained with the visual cursor (i.e., performance is shown in the first no-cursor reaching task completed).
The effect of reach training with a cursor gain on movement distance varied as a function of target position [F(6,54) = 4.994, P < 0.001]. Specifically, aftereffects after training with a Cursor Decrease (black symbols in Fig. 3B) were localized, such that the greatest reach adaptation was at the trained target (2.2 cm; Cursor Decrease = 15.2 cm vs. Aligned Cursor = 13.0 cm; open black circle in Fig. 3B). Subjects also exhibited significant aftereffects at the novel center target located 45° left of the trained target (2.0 cm; Cursor Decrease = 14.7 cm vs. Aligned Cursor = 12.8 cm; P < 0.05) but not at the other two novel target locations (45° right of the trained target = 1.5 cm; Cursor Decrease = 13.4 cm vs. Aligned Cursor = 11.9 cm and 90° left of the trained target = 0.6 cm; Cursor Decrease = 13.7 cm vs. Aligned Cursor = 13.0 cm).
Proprioceptive Recalibration: Rotation Generalization Protocol
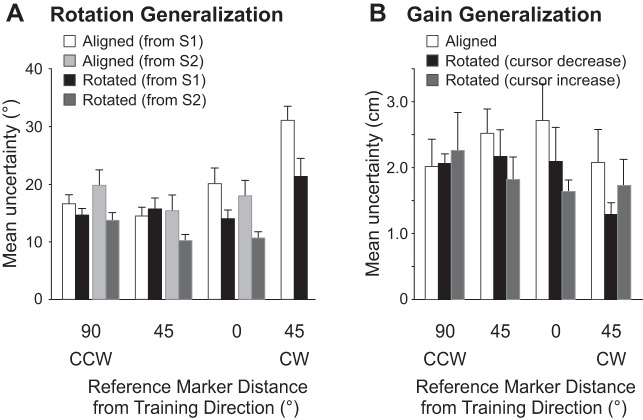
To determine whether subjects recalibrated their sense of felt hand position and whether these changes generalized across reference markers, we examined subjects' proprioceptive biases at each reference marker. Figure 4A shows changes in biases after subjects reached with a rotated cursor compared with an aligned cursor for reference markers that corresponded to both S1 (black symbols) and S2 (gray symbols). In general, after training with a rotated cursor, subjects had a greater leftward bias when estimating the position of their hand with respect to all reference markers compared with after training with an aligned cursor [rotated cursor = 18.6° vs. aligned cursor = 13.5°, F(1,6) = 10.914, P = 0.003]. More specifically, for the reference marker at the same location as the reach training target (open black circle in Fig. 4A), subjects estimated their hands to be at the reference marker when it was shifted 5.8° more leftward after training with a rotated cursor compared with an aligned cursor. Subjects had a similar shift in felt hand position at the reference marker located 45° CW with respect to S2 (shift in bias of 4.8°). Moreover, in contrast to the reaching results reported above, biases in felt hand position were shifted a similar amount at the other reference markers located 45° (7.1°) as well as 90° (3.7°) away from the reach training target, as indicated by the relatively flat curves (black and gray) in Fig. 4A. In other words, the change in felt hand position did not vary as a function of reference marker location [F(6,138) = 1.201, P = 0.310], suggesting that generalization of proprioceptive recalibration was broader than reach adaptation.
Fig. 4.
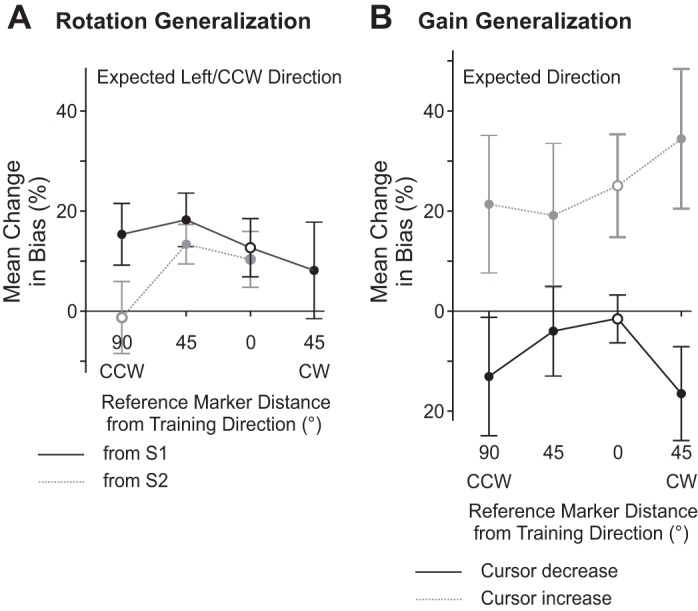
Changes in proprioceptive biases after training with misaligned visual feedback of the hand compared with aligned visual feedback of the hand as % of the distortion introduced. A positive value indicates that subjects recalibrated proprioception in the expected direction. In A, changes in biases after training with a rotated cursor are shown for each reference marker relative to the trained target direction (0° distance) from both S1 (solid black line) and S2 (dashed gray line). Open symbols represent errors made at a similar location to the reach training target from S1 (open black circle) or S2 (open gray circle). In B, changes in biases after training with a Cursor Decrease (solid black line) and after training with a Cursor Increase (dashed gray line) are shown at each reference marker relative to the trained target direction (0° distance). Error bars reflect SE.
While this significant effect of visual training on proprioceptive recalibration did not vary across reference marker locations, looking at Fig. 4A it appears that there was no proprioceptive recalibration at the reference marker 90° CCW with respect to the reach training direction (or 45° CCW; open gray circle in Fig. 4A) relative to the S2 start position. This was indeed the case, as confirmed by post hoc comparison (P > 0.05). The lack of proprioceptive recalibration at this particular reference marker from S2 may have been due to the fact that the range of angular hand positions used to measure biases in felt hand position with respect to this reference marker (rightward dotted arc crossing T1 in Fig. 1C) was oriented differently to that from the trained location from S1 (leftward dotted arc in Fig. 1C). Specifically, the angular hand positions were not vertically consistent (but were laterally consistent). While these differing axes for T1 from S2 vs. S1 were necessary to maintain consistency with the measures of angular aftereffects after visuomotor rotation of the cursor, this vertical discrepancy between the ranges of measured hand positions may have been enough to allow participants to overcome any recalibration along the consistent lateral direction.
Uncertainty levels were on average 16.8° across subjects, reference markers, and training sessions. As seen in Fig. 5A, uncertainty ranges significantly decreased as a function of training [F(1,23) = 12.823, P = 0.002], such that subjects were slightly more precise (14.3°) after training to reach with a rotated cursor (black and dark gray bars) compared with an aligned cursor (19.3°) (white and light gray bars). This effect of reach training did not vary across reference marker locations [F(6,138) = 1.766, P = 0.110], even for locations with overall (and significantly) larger ranges of uncertainty, specifically the reference marker located 45° CW from the trained target location (rightmost bars in Fig. 5A; T4 in Fig. 1C).
Fig. 5.
Magnitude of the uncertainty ranges in the proprioceptive estimate trials are shown after training with aligned (white or light gray bars) and misaligned (black and dark gray bars) visual feedback of the hand at each reference marker. A: Rotation Generalization protocol: magnitude of the uncertainty ranges at each reference marker with respect to the trained target direction (0°) made from S1 (after aligned reach training, white bars; after reach training with a rotated cursor, black bars) and S2 (after aligned reach training, light gray bars; after reach training with a rotated cursor, dark gray bars). B: Gain Generalization protocol: magnitude of the uncertainty ranges at each reference marker with respect to the trained target direction (0°) after reach training with an aligned cursor (white bars), a Cursor Decrease (black bars), and a Cursor Increase (gray bars). Error bars reflect SE.
Proprioceptive Recalibration: Gain Generalization Protocol
Subjects were very accurate when judging whether their hand was above or below a reference marker. In fact, average estimate errors after training with an aligned cursor were only 0.3 cm above the reference markers when averaged across reference markers and subjects (which is ∼2.5% of the entire distance to the marker). In Fig. 4B, we show changes in estimates of hand position after reaches made with a Cursor Decrease (black symbols) and after training with a Cursor Increase (gray symbols) relative to estimates made after training with an aligned cursor. These changes in proprioceptive bias depended on the type of visual feedback experienced during the reach training trials [F(2,18) = 4.166, P = 0.033]. Specifically, subjects did not shift their felt hand position at any of the reference markers, including the reference marker at the reach training target location (open black circle in Fig. 4B) after reaching with a Cursor Decrease (i.e., the line joining the black symbols in Fig. 4B is approximately at 0). However, after training with a Cursor Increase, subjects significantly shifted their felt hand position such that they felt that their hand was at the reference markers when it was on average 0.7 cm below the reference markers (i.e., closer to the home position). This was the case for the reference marker in the reach training direction, as well as the novel directions [F(3,27) < 1], indicating a broad generalization of proprioceptive changes.
With respect to precision, as illustrated in Fig. 5B, subjects were fairly consistent when estimating the position of their hand across the three Visual Training conditions, such that the average uncertainty range was 2 cm. These uncertainty ranges did not vary with visual training [F(2,18) = 1.896, P = 0.179] or reference marker location [F(3,27) < 1] or as a function of visual training × reference marker location [F(6,54) = 1.016, P = 0.425].
Relationship Between Reach Adaptation and Proprioceptive Recalibration
The generalization patterns outlined above for reach adaptation and changes in felt hand position differed when both cursor rotation and cursor gain distortion were introduced. In particular, results indicated that for both cursor distortions reach adaptation did not generalize that broadly to novel target directions, with the greatest change in reaches occurring at the trained target. In contrast, changes in felt hand position generalized more broadly across all reference marker locations in the expected direction by a similar magnitude. Given these differences in patterns, it is not that surprising that we found no significant correlation between reach adaptation and changes in felt hand position at either the trained target location or across any of the novel target locations (all P > 0.05), such that the magnitude of proprioceptive recalibration was similar regardless of the level of reach adaptation achieved.
DISCUSSION
In the present study, we looked to establish whether proprioceptive recalibration generalizes to novel locations across the workspace and/or directions. This generalization pattern was then compared to generalization of reach adaptation at similar locations in order to determine the relationship between changes in the motor and sensory systems. Subjects trained to reach to a single target while a cursor representing the hand was rotated 45° CW with respect to the hand, or a cursor gain distortion was introduced, such that the cursor moved 1.5 or 0.67 times the distance moved by the hand. We found that subjects only adapted their movements to some targets after reaching with a distorted cursor. In contrast, subjects recalibrated proprioception across a greater area of the workspace.
Motor Adaptation
With respect to reach adaptation we found a generalization pattern similar to that seen by Krakauer and colleagues (2000) after subjects trained with a rotated cursor, in that the greatest amount of reach adaption was observed at the training target and adaptation fell off the farther a novel target was from the training target. In fact, there was no reach adaptation observed at a novel target located 90° CCW of the training target. We found a similar pattern of reach adaptation even when subjects initiated their movements from a novel start position. Specifically, the greatest adaptation was observed in the same direction as the training target, and reach adaptation was not observed at a target located 90° CCW of this trained target direction, even though this 90° CCW target was at the same spatial location as the trained target. This same generalization pattern was observed when we analyzed angular errors at movement end point or peak velocity. Thus, similar to Wang and Sainburg (2005), results indicated that subjects remapped their trajectories (i.e., movement vectors) as opposed to final end-point positions.
We also found that subjects adapted their reaches after training with a cursor gain. Subjects adapted their reaches more after training with a Cursor Decrease (i.e., cursor that moved to a lesser extent than their hand) than a Cursor Increase (i.e., cursor that moved to a greater extent than their hand), as shown by Pearson et al. (2010). In contrast to our hypothesis, we found that generalization of reach adaptation was dependent on direction (Ghahramani et al. 1996; Krakauer et al. 2000; Pearson et al. 2010; Pine et al. 1996), such that the greatest reach adaptation was again seen in the trained target direction. Thus, after training with both distortions, subjects adapted their reaches in particular directions (i.e., not across all targets).
While subjects adapted their reaches in the present study, the magnitude of reach adaptation observed initially appears to be less than that in previous reports after reach training with a rotated cursor or cursor gain. The differences between the reach aftereffects we report and previous findings can be partially explained by such factors as how many trials were included in the average that made up the reach aftereffects reported and how frequently no-cursor trials occurred relative to reach training trials. The reach aftereffects reported in the present study reflect the average of the five no-cursor trials completed per target, and all no-cursor reaches were completed in one block of trials without any interleaved reach training trials with visual feedback (35 trials in the Rotation Generalization paradigm and 20 trials in the Gain Generalization paradigm). Thus some decay likely occurred. This method of assessing reach adaptation differs from that done previously (Izawa et al. 2012; Krakauer et al. 2000), in which no-cursor reaching trials were intermixed with reach training trials (in which visual feedback was provided). Intermixing reach training trials may potentially lead to less decay of reach adaptation and hence greater aftereffects. In addition, we did not impose a time constraint on subjects' reaching speed, another factor that may have reduced the aftereffects seen in the present study. Nonetheless, despite these differences in paradigms and analyses, which may reduce the size of our reach aftereffects, we find the same localized pattern of reach generalization as shown previously (Izawa et al. 2012; Krakauer et al. 2000; Wang and Sainburg 2005).
The Gain Generalization results reported in the present experiment are not as consistent with previous reports and were not as broad as we had hypothesized. Vindras and Vivani (2002) demonstrated broader generalization, as they found that subjects adapted their movements to slightly less than 60% of the cursor gain introduced during training in a paradigm in which reach training trials were interleaved with no-cursor aftereffect trials. In contrast, we found that reach adaptation varied between 10% and 40% of the cursor gain introduced depending on target position relative to the trained target. Again, the reduction in reach aftereffects we observed in the present study compared with previous reports could be partially due to the fact that we had subjects perform a number of no-cursor reaches in a row (with no interleaved reach training trials with visual feedback) and then averaged errors at reach end point across trials. In attempt to better replicate previous results, we ran multiple versions of the Gain Generalization protocol; however, on all versions of our protocol we found reach aftereffects that were similar in magnitude to the results reported.
Sensory Recalibration
In contrast to the narrow generalization patterns for reach adaptation, subjects recalibrated their sense of felt hand position more broadly when both a cursor rotation and gain were introduced. In particular, subjects recalibrated their sense of felt hand position across a greater area of the workspace after training with a cursor rotation, such that changes in felt hand position were observed even at the reference markers located 90° CCW from the trained target direction. Moreover, the magnitude of recalibration was consistent across reference markers and did not peak at the reach training target location. Finally, results demonstrated that changes in felt hand position were independent of the starting position of the hand and path taken by the hand, as changes in felt hand position were observed even when subjects' hands were moved outward from a novel home position. Subjects did not recalibrate their sense of felt hand position after training with a Cursor Decrease despite adapting their movements. In contrast, subjects recalibrated their sense of felt hand position across all reference markers after training with a Cursor Increase even though reach adaptation was not significant. Taken together, the results indicate that when proprioceptive recalibration occurs it is independent of movement direction, such that it extends across the workspace to novel locations.
We discuss these changes in felt hand position as proprioceptive recalibration, as opposed to a combination of proprioceptive and visual recalibration and/or motor adaptation. We think that it is unlikely that vision is being recalibrated based on our experimental manipulation and results across several previous studies. First, unlike prism adaptation studies, the present study manipulated visual feedback of the hand only, not the entire workspace, avoiding (or limiting) visual recalibration. As well, we have previously shown similar changes in felt hand position when subjects estimated the position of their hand relative to a proprioceptive reference marker (i.e., body midline) without visual cues as we find when subjects indicated the position of their hand relative to a visual reference marker (Clayton et al. 2014; Cressman and Henriques 2009; Mostafa et al. 2014). Moreover, we found that reaches to the unseen adapted hand with the visible left hand also lead to a similar shift in localization of the target-hand (Clayton et al. 2014). Finally, we have recently demonstrated that changes in felt hand position do not transfer from the trained hand to the untrained after reach adaptation, which we would expect if vision was also recalibrated (Mostafa et al. 2014). We also do not expect motor adaptation to be contaminating our proprioceptive estimates. In our initial work looking at proprioceptive recalibration, we either had subjects voluntarily move their limb into position or had the robot move their limb for them during the proprioceptive estimation trials (Cressman and Henriques 2009). We found no difference in estimates between subjects who moved their hand into position and those who had their hand passively placed into position by the robot, suggesting that movement of the hand in the proprioceptive estimation task does not contaminate the results. The lack of influence of the movement on proprioceptive estimates is likely due to the fact that it is not a voluntary movement in the traditional sense. Specifically, in our task there is no target that subjects are reaching to (the reference marker turns on after subjects have stopped moving) and subjects do not have control of planning the direction or extent of their movement.
Motor Adaptation vs. Sensory Recalibration
The different generalization patterns for reach adaptation and proprioceptive recalibration found in the present study and recent work by Mostafa and colleagues (2015) suggest that proprioceptive recalibration may arise independently of motor changes. Specifically, we found evidence of proprioceptive recalibration in the absence of reach adaptation and vice versa. In support of our claim of independence, Block and Bastian (2012) have shown that cerebellar patients are able to recalibrate proprioception such that proprioceptive estimates are shifted to match visual estimates of target positions in the absence (or lack) of motor adaptation. In this task, participants were required to reach to visual and proprioceptive targets when visual and proprioceptive information were gradually misaligned. Results indicated that after reach training patients realigned proprioceptive end points to the same amount as control subjects when end-point visual feedback was not available. However, when visual feedback was available patients recalibrated proprioception less than control subjects.
In addition to evidence of sensory recalibration in the absence of motor adaptation, updating predictions regarding the sensory consequences of one's movements (i.e., the forward model) have also been shown to be independent from reach adaptation (Izawa et al. 2012; Synofzik et al. 2008). For example, Synofzik and colleagues (2008) showed that while cerebellar patients and control subjects were both able to adapt their reaches to a visual distortion, perceived pointing direction, as indicated with a mouse-guided cursor by the left untrained hand, was recalibrated to a lesser amount in patients than in control subjects. Moreover, Izawa and colleagues (2012) recently showed that cerebellar patients were unable to learn to predict the visual sensory consequences of their motor commands like control subjects, despite showing similar levels of adaptation in their reaching movements. Taken together, results looking at proprioceptive recalibration and predictions regarding the sensory consequences of one's movements suggest that sensory changes may arise independently from motor changes.
We have previously suggested that two independent error signals, 1) the discrepancy between the desired and actual movement, known as the sensorimotor error signal (Wong and Shelhamer 2011), and 2) the discrepancy between visual and proprioceptive estimates of hand position, which we refer to as the cross-sensory error signal, may be primarily responsible for changes in movements and felt hand position, respectively (Cressman and Henriques 2010). Furthermore, changes in felt hand position may contribute to reach adaptation when only a cross-sensory error signal is present (Cressman and Henriques 2010; Salomonczyk et al. 2013). The results of the present study further reveal that proprioceptive recalibration does not always contribute to reach adaptation. In other words, movements do not always take into account shifted perceptual boundaries of hand-target alignment. In particular for movements to novel target directions, but also for the trained target after subjects reached with a cursor gain, the motor system did not appear to take into account a recalibrated visual-proprioceptive mapping and plan a corresponding movement vector based on changes in felt hand position. Instead, the sensory and motor systems use different estimates to determine when the hand is at the target.
Our suggestion of independent error signals giving rise to reach adaptation and proprioceptive recalibration is in line with Berniker and Kording's (2008) source-estimation model. Specifically, differences in sensory and motor end-point positions may arise because of how the nervous system estimates the sources of the motor errors experienced, for example, whether the errors are attributed to estimated changes in limb properties vs. changes in the environment. The cross-sensory discrepancy that leads to changes in felt hand position may be due to an adapted representation or internal model specific to the limb, while changes in movement may arise because of adaptation to the internal representation or model of the world. Moreover, these different error signals may also arise from the engagement of different brain areas and thus reflect specialized functions of brain areas such as the parietal cortex and cerebellum. For example, proprioceptive recalibration could be occurring within the parietal cortex (along with the somatosensory cortex and premotor cortical areas), while changes in motor commands likely involve modifications within the cerebellum. In a recent review, Shadmehr and Krakauer (2008) proposed that a possible function of the parietal cortex is to update and integrate actual and predicted sensory feedback of the limb for state estimation while the function of the cerebellum involves forming the internal models necessary for predicting the sensory outcome of motor commands and correcting these motor commands through internal feedback. Block and Bastian (2012) have also proposed that sensory realignment depends on regions of the posterior parietal cortex after demonstrating that individuals with cerebellar damage recalibrated proprioception despite impaired motor adaptation. Furthermore, Clower and colleagues (1996) interpreted their neuroimaging results to directly implicate the posterior parietal cortex in sensory recalibration. In addition to the parietal cortex, Vahdat and colleagues (2011) have recently reported the engagement of a cortical network, involving the second somatosensory cortex, ventral premotor cortex, and supplementary motor cortex, responsible for sensory plasticity after reach training in a velocity-dependent force field. This activity was distinct from activation in a second network, involving the cerebellum, primary motor cortex, and dorsal premotor cortex, that was implicated in reach adaption, suggesting that the sensory and motor changes may be distinct even after force-field learning.
The suggestion that proprioceptive recalibration and reach adaptation are independent processes, perhaps arising because of processing of different error signals by different areas of the brain, is consistent with previous literature demonstrating visuomotor adaptation in the absence and/or degradation of proprioceptive input. For example, deafferented individuals have been shown to adapt their reaches in response to altered visual feedback of the hand (Bernier et al. 2006; Ingram et al. 2000; Miall and Cole 2007). As well, it has recently been demonstrated that healthy subjects adapt their reaches in response to a visuomotor distortion even when proprioceptive feedback is degraded by agonist-antagonist muscle vibration (Bock and Thomas 2011; Pipereit et al. 2006). In fact, Bernier and colleagues (2009) showed that when proprioceptive input is intact, healthy subjects attenuate this input (as measured by median nerve somatosensory evoked potentials) in the primary somatosensory cortex upon exposure to misaligned visual feedback of the hand. Together, these findings imply that sensory and motor adaptation are independent processes, such that motor adaptation can arise independently of sensory recalibration and that part of the sensory adaptive process may be to reduce conflicting proprioceptive input before it is recalibrated.
Conclusions
In summary, we have demonstrated that reaching with altered visual feedback of the hand gives rise to different generalization patterns with respect to proprioceptive recalibration and reach adaptation. These results imply that movement trajectories do not necessarily follow changes in sensory boundaries and that motor changes do not give rise to proprioceptive recalibration. Instead, we propose that sensory and motor plasticity arise because of two different error signals processed in different cortical networks.
GRANTS
This research was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council to E. K. Cressman and D. Y. P. Henriques.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.K.C. and D.Y.P.H. conception and design of research; E.K.C. performed experiments; E.K.C. analyzed data; E.K.C. and D.Y.P.H. interpreted results of experiments; E.K.C. and D.Y.P.H. prepared figures; E.K.C. and D.Y.P.H. drafted manuscript; E.K.C. and D.Y.P.H. edited and revised manuscript; E.K.C. and D.Y.P.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Holly Clayton and Yulia Metersky for their assistance with data collection.
REFERENCES
- Bernier PM, Burle B, Vidal F, Hasbroucq T, Blouin J. Direct evidence for cortical suppression of somatosensory afferents during visuomotor adaptation. Cereb Cortex 19: 2106–2113, 2009. [DOI] [PubMed] [Google Scholar]
- Bernier PM, Chua R, Bard C, Franks IM. Updating of an internal model without proprioception: a deafferentation study. Neuroreport 17: 1421–1425, 2006. [DOI] [PubMed] [Google Scholar]
- Berniker M, Kording K. Estimating the sources of motor errors for adaptation and generalization. Nat Neurosci 11: 1454–1461, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block HJ, Bastian AJ. Cerebellar involvement in motor but not sensory adaptation. Neuropsychologia 50: 1766–1775, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock O, Thomas M. Proprioception plays a different role for sensorimotor adaptation to different distortions. Hum Mov Sci 30: 415–423, 2011. [DOI] [PubMed] [Google Scholar]
- Clayton HA, Cressman EK, Henriques DY. The effect of visuomotor adaptation on proprioceptive localization: the contributions of perceptual and motor changes. Exp Brain Res 232: 2073–2086, 2014. [DOI] [PubMed] [Google Scholar]
- Clower DM, Hoffman JM, Votaw JR, Faber TL, Woods RP, Alexander GE. Role of posterior parietal cortex in the recalibration of visually guided reaching. Nature 383: 618–621, 1996. [DOI] [PubMed] [Google Scholar]
- Cressman EK, Henriques DY. Sensory recalibration of hand position following visuomotor adaptation. J Neurophysiol 102: 3505–3518, 2009. [DOI] [PubMed] [Google Scholar]
- Cressman EK, Henriques DY. Reach adaptation and proprioceptive recalibration following exposure to misaligned sensory input. J Neurophysiol 103: 1888–1895, 2010. [DOI] [PubMed] [Google Scholar]
- Cressman EK, Henriques DY. Motor adaptation and proprioceptive recalibration. Prog Brain Res 191: 91–99, 2011. [DOI] [PubMed] [Google Scholar]
- Cressman EK, Salomonczyk D, Henriques DY. Visuomotor adaptation and proprioceptive recalibration in older adults. Exp Brain Res 205: 533–544, 2010. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Rao AK. Trajectory adaptation to a nonlinear visuomotor transformation: evidence of motion planning in visually perceived space. J Neurophysiol 74: 2174–2178, 1995. [DOI] [PubMed] [Google Scholar]
- Ghahramani Z, Wolpert DM, Jordan MI. Generalization to local remappings of the visuomotor coordinate transformation. J Neurosci 16: 7085–7096, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CS. Adaptation to displaced vision: visual, motor, or proprioceptive change? Science 140: 812–813, 1963. [DOI] [PubMed] [Google Scholar]
- Hay JC, Pick HL Jr. Visual and proprioceptive adaptation to optical displacement of the visual stimulus. J Exp Psychol 71: 150–158, 1966. [DOI] [PubMed] [Google Scholar]
- Henriques DY, Cressman EK. Visuomotor adaptation and proprioceptive recalibration. J Mot Behav 44: 435–444, 2012. [DOI] [PubMed] [Google Scholar]
- Henriques DY, Soechting JF. Bias and sensitivity in the haptic perception of geometry. Exp Brain Res 150: 95–108, 2003. [DOI] [PubMed] [Google Scholar]
- Ingram HA, van Donkelaar P, Cole J, Vercher JL, Gauthier GM, Miall RC. The role of proprioception and attention in a visuomotor adaptation task. Exp Brain Res 132: 114–126, 2000. [DOI] [PubMed] [Google Scholar]
- Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci 32: 4230–4239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Cressman EK, Henriques DY. Proprioceptive localization of the left and right hands. Exp Brain Res 204: 373–383, 2010. [DOI] [PubMed] [Google Scholar]
- Kesten H. Accelerated stochastic-approximation. Ann Math Stat 29: 41–59, 1958. [Google Scholar]
- Krakauer JW. Motor learning and consolidation: the case of visuomotor rotation. Adv Exp Med Biol 629: 405–421, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20: 8916–8924, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar AA, Darainy M, Ostry DJ. Motor learning and its sensory effects: time course of perceptual change and its presence with gradual introduction of load. J Neurophysiol 109: 782–791, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Cole J. Evidence for stronger visuo-motor than visuo-proprioceptive conflict during mirror drawing performed by a deafferented subject and control subjects. Exp Brain Res 176: 432–439, 2007. [DOI] [PubMed] [Google Scholar]
- Mostafa AA, Kamran-Disfani R, Bahari-Kashani G, Cressman EK, Henriques DY. Generalization of reach adaptation and proprioceptive recalibration at different distances in the workspace. Exp Brain Res 233: 817–827, 2015. [DOI] [PubMed] [Google Scholar]
- Mostafa AA, Salomonczyk D, Cressman EK, Henriques DY. Intermanual transfer and proprioceptive recalibration following training with translated visual feedback of the hand. Exp Brain Res 232: 1639–1651, 2014. [DOI] [PubMed] [Google Scholar]
- Ostry DJ, Darainy M, Mattar AA, Wong J, Gribble PL. Somatosensory plasticity and motor learning. J Neurosci 30: 5384–5393, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TS, Krakauer JW, Mazzoni P. Learning not to generalize: modular adaptation of visuomotor gain. J Neurophysiol 103: 2938–2952, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine ZM, Krakauer JW, Gordon J, Ghez C. Learning of scaling factors and reference axes for reaching movements. Neuroreport 7: 2357–2361, 1996. [DOI] [PubMed] [Google Scholar]
- Pipereit K, Bock O, Vercher JL. The contribution of proprioceptive feedback to sensorimotor adaptation. Exp Brain Res 174: 45–52, 2006. [DOI] [PubMed] [Google Scholar]
- Redding GM, Rossetti Y, Wallace B. Applications of prism adaptation: a tutorial in theory and method. Neurosci Biobehav Rev 29: 431–444, 2005. [DOI] [PubMed] [Google Scholar]
- Salomonczyk D, Cressman EK, Henriques DY. Proprioceptive recalibration following prolonged training and increasing distortions in visuomotor adaptation. Neuropsychologia 49: 3053–3062, 2011. [DOI] [PubMed] [Google Scholar]
- Salomonczyk D, Cressman EK, Henriques DY. The role of the cross-sensory error signal in visuomotor adaptation. Exp Brain Res 228: 313–325, 2013. [DOI] [PubMed] [Google Scholar]
- Salomonczyk D, Henriques DY, Cressman EK. Proprioceptive recalibration in the right and left hands following abrupt visuomotor adaptation. Exp Brain Res 217: 187–196, 2012. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res 185: 359–381, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simani MC, McGuire LM, Sabes PN. Visual-shift adaptation is composed of separable sensory and task-dependent effects. J Neurophysiol 98: 2827–2841, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synofzik M, Lindner A, Thier P. The cerebellum updates predictions about the visual consequences of one's behavior. Curr Biol 18: 814–818, 2008. [DOI] [PubMed] [Google Scholar]
- Synofzik M, Thier P, Lindner A. Internalizing agency of self-action: perception of one's own hand movements depends on an adaptable prediction about the sensory action outcome. J Neurophysiol 96: 1592–1601, 2006. [DOI] [PubMed] [Google Scholar]
- Treutwein B. Adaptive psychophysical procedures. Vision Res 35: 2503–2522, 1995. [PubMed] [Google Scholar]
- Vahdat S, Darainy M, Milner TE, Ostry DJ. Functionally specific changes in resting-state sensorimotor networks after motor learning. J Neurosci 31: 16907–16915, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter P, Goodbody SJ, Wolpert DM. Evidence for an eye-centered spherical representation of the visuomotor map. J Neurophysiol 81: 935–939, 1999. [DOI] [PubMed] [Google Scholar]
- Vindras P, Viviani P. Altering the visuomotor gain. Evidence that motor plans deal with vector quantities. Exp Brain Res 147: 280–295, 2002. [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Adaptation to visuomotor rotations remaps movement vectors, not final positions. J Neurosci 25: 4024–4030, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science 269: 1880–1882, 1995. [DOI] [PubMed] [Google Scholar]
- Wong AL, Shelhamer M. Sensorimotor adaptation error signals are derived from realistic predictions of movement outcomes. J Neurophysiol 105: 1130–1140, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JD, Wilson ET, Gribble PL. Spatially selective enhancement of proprioceptive acuity following motor learning. J Neurophysiol 105: 2512–2521, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T, Henriques DY. Visuomotor adaptation does not recalibrate kinesthetic sense of felt hand path. J Neurophysiol 101: 614–623, 2009. [DOI] [PubMed] [Google Scholar]