Abstract
Serotonin (5-HT)-synthesizing neurons of the medullary raphe are putative central chemoreceptors, proposed to be one of potentially multiple brain stem chemosensitive cell types and loci interacting to produce the respiratory chemoreflex. Hypocretin-synthesizing neurons of the lateral hypothalamus are important contributors to arousal state, thermoregulation, and feeding behavior and are also reportedly involved in the hypercapnic ventilatory response. Recently, a functional interaction was found between the hypocretin system and 5-HT neurons of the dorsal raphe. The validity and potential significance of hypocretin modulation of medullary raphe 5-HT neurons, however, is unknown. As such, the purpose of this study was to explore functional interactions between the hypocretin system and 5-HT system of the medullary raphe on baseline respiratory output and central chemosensitivity. To explore such interactions, we used the neonatal in vitro medullary slice preparation derived from wild-type (WT) mice (normal 5-HT function) and a knockout strain lacking all central 5-HT neurons (Lmx1bf/f/p mice). We examined effects of acidosis, hypocretin-1, a hypocretin receptor antagonist (SB-408124), and the effect of the antagonist on the response to acidosis. We confirmed the critical role of 5-HT neurons in central chemosensitivity given that the increased hypoglossal burst frequency with acidosis, characteristic of WT mice, was absent in preparations derived from Lmx1bf/f/p mice. We also found that hypocretin facilitated baseline neural ventilatory output in part through 5-HT neurons. Although the impact of hypocretin on 5-HT neuronal sensitivity to acidosis is still unclear, hypocretins did appear to mediate the burst duration response to acidosis via serotonergic mechanisms.
Keywords: hypocretin, serotonin, ventilation, chemosensitivity
the brain's responsiveness to pH/CO2 (central chemosensitivity) has been proposed to be a distributed network function involving multiple chemoreceptive sites in the brain stem (Nattie and Li 2009). Growing evidence identifies a distinct subset of 5-HT neurons of the medullary raphe as critical for chemosensitivity (Brust et al. 2014; Corcoran et al. 2009, 2013; Iceman et al. 2013; Richerson 2004). Much effort has been directed at understanding how multiple chemoreceptive cells and loci interact and modulate each other and/or the respiratory rhythm itself (Dias et al. 2008, 2009; Iceman and Harris 2013; Iceman et al. 2014; Li et al. 2006; Mulkey et al. 2007).
Recently, neurons containing hypocretin neuropeptides (hypocretin-1/orexin A and hypocretin-2/orexin B) were proposed to influence CO2 sensitivity (Dias et al. 2009; Nakamura et al. 2007). The hypocretin system is anatomically linked with central chemosensory and respiratory nuclei (Fung et al. 2001; Hagan et al. 1999; Nakamura et al. 2007; Volgin et al. 2002; Young et al. 2005). The functional consequences of such interactions and the specific effects on ventilation remain unclear (Corcoran et al. 2010; Nakamura et al. 2007; Young et al. 2005; Zhang et al. 2005).
To date, most of the physiological evidence linking the hypocretin and 5-HT systems has focused on the dorsal raphe and the regulation of sleep and wakefulness (Brown et al. 2001; Takahashi et al. 2005). However, because medullary raphe neurons both express hypocretin receptors 1 and 2 (hcrt-r1 and hcrt-r2) and receive hypocretin projections, it is possible that these systems interact with the medullary raphe to promote a ventilatory response to hypercapnia (Marcus et al. 2001; Nambu et al. 1999; Zheng et al. 2005).
The present study aimed to explore potential interactions between the hypocretin system and 5-HT neurons of the medullary raphe. We tested the hypothesis that 5-HT neurons contribute to the response to acidosis of the hypoglossal motor output of an in vitro rhythmic medullary slice preparation. We compared responses to acidosis in slice preparations derived from WT (containing 5-HT neurons) and Lmx1bf/f/p (lacking central 5-HT neurons) mouse strains. Next, we tested the hypothesis that hypocretins contribute to respiratory motor output and promote the response to acidosis through 5-HT neurons. We identified whether hypoglossal nerve activity changes generated by either hypocretin-1 or a hypocretin receptor antagonist depended on the presence of 5-HT neurons. We also identified the effects of hypocretin receptor antagonism on hypoglossal nerve responses to acidosis and whether or not this was dependent on the presence of 5-HT neurons.
METHODS
All procedures and protocols were approved by the Institutional Animal Care and Use Committee of Yale University, where all of the experiments were conducted.
5-HT neuron-deficient mouse model.
Procedures using Cre-Lox site-specific recombination to produce mice lacking 5-HT neurons (Lmx1bf/f/p) have been described (Zhao et al. 2006). The breeding strategy involved mating females homozygous for the floxed Lmx1b sequence (Lmx1bf/f) with males homozygous for floxed Lmx1b and hemizygous for a Cre-recombinase sequence linked to that of the enhancer region of Pet1 (ePet1-Cre; Lmx1bflox/flox,ePet-Cre/+, also referred to as Lmx1bf/f/p). This strategy results in a 1:1 offspring ratio of Lmx1bf/f mice (WT; containing central 5-HT neurons) and Lmx1bf/f/p mice (lacking >99% of central 5-HT neurons; see Corcoran et al. 2014 for histology). Genotyping was determined by using tail tissue samples obtained from each mouse and procedures described by Zhao et al. (2006).
In vitro medullary slice preparations.
Experiments were performed on brain stem slices prepared from WT and Lmx1bf/f/p littermates (postnatal days 1–5) with the investigator blind to the genotype. Mice were decapitated and the brain stem and spinal cord removed under a flow of chilled artificial cerebrospinal fluid (aCSF) composed of (in mM) 124 NaCl, 25 NaHCO3, 3 KCl, 1.5 CaCl2, 1.0 MgSO4, 0.5 NaH2PO4, and 30 d-glucose and equilibrated with 95% O2 and 5% CO2 (pH ∼7.4). The brain stem was pinned to a wax block, and transverse slices of the medulla (550–600 μm thick) were prepared using a vibratome (see Smith et al. 1991 for details). Slices containing the pre-Bötzinger complex, the rostral portion of the medullary raphe, the hypoglossal motor nuclei, and the most rostral hypoglossal nerve rootlets were placed in a recording chamber (0.7 ml) and superfused with aCSF at a rate of 0.7 ml/min via a syringe pump (Harvard Apparatus). The aCSF was warmed to 29°C using a temperature controller (model TC-324B; Warner Instruments). Potassium concentrations were elevated to 9 mM to ensure rhythmic activity in the slice, as per standard procedures (Smith et al. 1991).
Motor discharge of the hypoglossal rootlets was recorded continuously by glass suction electrodes. Signals were amplified and filtered (×10,000; 0.3–1 kHz) using a Grass LP511 AC amplifier, a Dagan differential amplifier (EX4-400), and a Quest 60-Hz noise eliminator (HumBug; Quest Scientific). Data were digitized at 100 Hz and analyzed off-line using MATLAB software (The MathWorks). Analysis of hypoglossal root discharge included an initial integration, full-wave rectification, and moving average over 50-ms periods.
Pharmacological agents.
All pharmacological agents were obtained from Tocris, stored in stock solution at −20°C, and thawed the day of an experiment. The following agents were added to the aCSF to produce the following final concentrations: hypocretin-1 (orexin A; 300 nM) and SB-408124 (3 μM). The hypocretin receptor antagonist (SB-408124) was dissolved in DMSO before being added to the aCSF, with the final concentration <0.005% DMSO. After 30 min of baseline recording, the superfusate was switched to one containing either hypocretin-1 or SB-408124 and was applied continuously to the slice for 10 min. To administer an acidotic challenge, the superfusate (pH 7.4) was switched to pH 6.9 for 10 min. Low pH was achieved before experiments by adding HCl to the gas-equilibrated aCSF (95% O2 and 5% CO2) and continuously monitoring changes in pH with a pH electrode until the value was stable at 6.9.
Data analysis.
Integrated hypoglossal nerve discharge was analyzed to determine burst frequency, peak burst amplitude, burst duration, duration of the period between bursts (interburst interval), and the coefficient of variation of the interburst interval (CV IBI; calculated as the standard deviation divided by the mean, an indication of timing variability; Hodges et al. 2009). Values were averaged from the last 2 min of baseline immediately preceding treatment and the last 2 min of treatment (either pH change or pharmacological agent) in an effort to determine the effect of treatment relative to baseline and to establish a “steady-state” response. All data are presented as means ± SE or expressed as a proportion of the baseline value. Statistical significance was evaluated using one-way or two-way ANOVA with repeated measures, followed by Dunn's or Bonferroni's post hoc analyses for multiple comparisons, where appropriate, to compare effects of pH, genotype, or drug, as well as their interactions. Additionally, differences between genotype group means of baseline burst parameters were compared using a Student's t-test or nonparametric Mann-Whitney rank sum test. The criterion level for determination of statistical significance was set at P < 0.05 for all data analyses.
RESULTS
Genotype-dependent differences in hypoglossal burst parameters.
Under baseline conditions, hypoglossal burst patterns differed between WT and Lmx1bf/f/p preparations (Table 1, Fig. 1A). These data were combined from baseline periods of all experimental series (n = 21 WT and 19 Lmx1bf/f/p) and were consistent with our previous data sets (Corcoran et al. 2014).
Table 1.
Baseline hypoglossal burst parameters from WT and Lmx1bf/f/p rhythmic medullary slices
| Genotype | n | Frequency, bursts/min | Duration, s | CV IBI |
|---|---|---|---|---|
| WT | 19 | 9.2 ± 0.9 | 0.66 ± 0.4 | 0.28 ± 0.024 |
| Lmx1bf/f/p | 21 | 6.8 ± 0.5 | 0.68 ± 0.2 | 0.44 ± 0.026* |
Data are means ± SE for values in wild-type (WT) and Lmx1bf/f/p (lacking 5-HT neurons) mouse strains. A t-test (
P < 0.001) or Mann-Whitney rank sum test was used, as appropriate, to determine a statistically significant difference compared with WT.
CV IBI, coefficient of variation of the interburst interval.
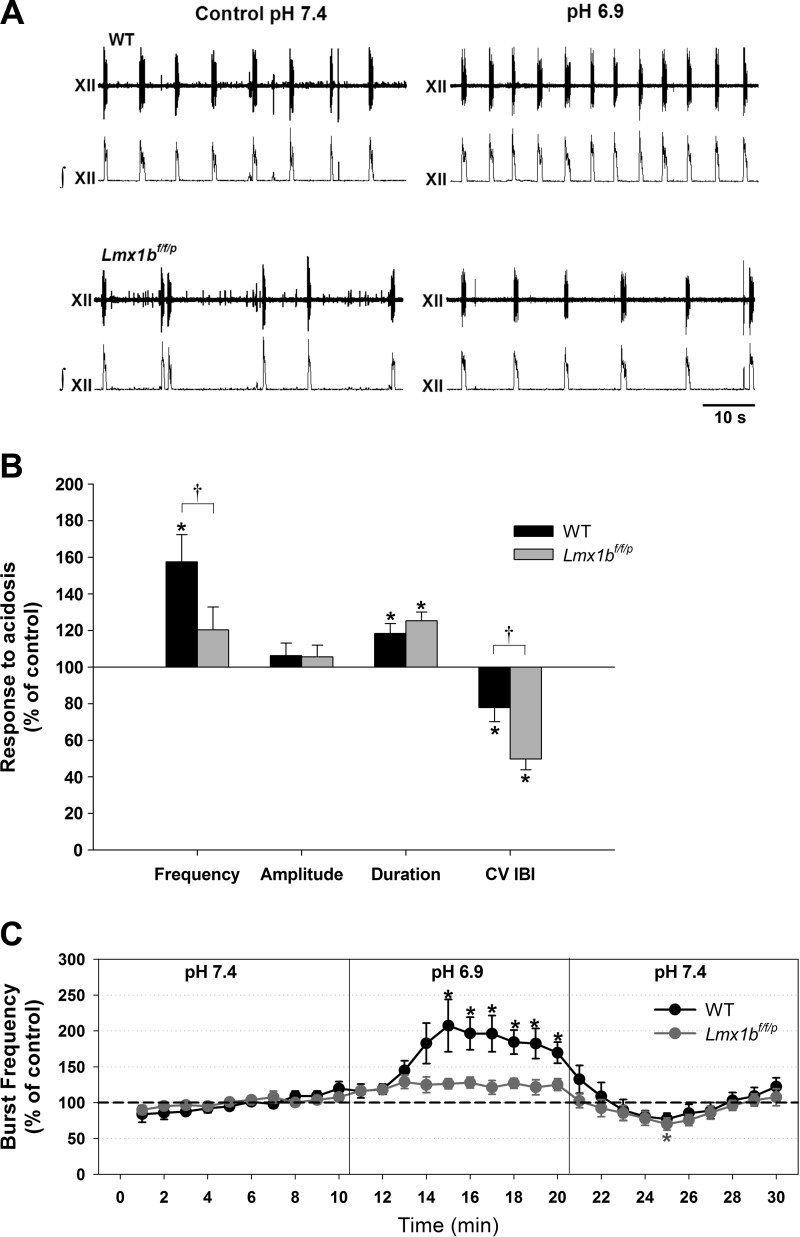
Fig. 1.
Acidosis differentially affects hypoglossal burst parameters in rhythmic slices from neonatal wild-type (WT) and Lmx1bf/f/p mice (lacking all central 5-HT neurons). A: raw and integrated traces from both genotypes at normal pH (7.4) and during acidosis (pH 6.9). Lmx1bf/f/p bursting is more irregular and slower compared with WT during control conditions. B: steady-state values (relative to baseline) of burst frequency, amplitude, duration, and coefficient of variation of the interburst interval (CV IBI) in response to acidosis. Data are means ± SE expressed as a percentage of control values. Acidosis increased burst frequency in WT preparations only. C: time course of burst frequency (means ± SE) in both genotypes during control conditions (pH 7.4), acidosis (pH 6.9), and washout (i.e., return to normal aCSF). *P < 0.05 indicates a statistically significant difference from baseline levels; †P < 0.05 indicates a statistically significant difference between genotypes (n = 12 WT and 13 Lmx1bf/f/p); determined by 2-way (B) or 1-way (C) repeated-measures (RM) ANOVA.
pH differentially affects hypoglossal burst parameters in WT and Lmx1bf/f/p preparations.
Following baseline observations at pH 7.4, preparations were exposed for 10 min to acidic aCSF (pH 6.9; Fig. 1). In WT slices, acidosis increased hypoglossal burst frequency (57 ± 15% increase from baseline; n = 12; Fig. 1B) and burst duration (18 ± 5% increase from baseline) and reduced the CV IBI (22 ± 8% from baseline). Burst amplitude was unchanged by acidosis.
Responses to acidosis in Lmx1bf/f/p preparations differed from those of WT preparations. Most notably, acidosis did not increase burst frequency in Lmx1bf/f/p preparations; however, this treatment did greatly increase burst regularity in Lmx1bf/f/p preparations: the CV IBI was reduced (50 ± 6% from baseline; n = 13) to a greater degree than that occurring in WT preparations (Fig. 1B). As with WT preparations, acidosis increased burst duration in Lmx1bf/f/p preparations (25 ± 5% increase from baseline) and did not alter burst amplitude.
In both WT and Lmx1bf/f/p preparations, effects of pH changes were reversible and burst parameters returned to baseline values within 10–15 min of reperfusion with normal aCSF (Fig. 1C).
Exogenous hypocretin affects hypoglossal burst parameters.
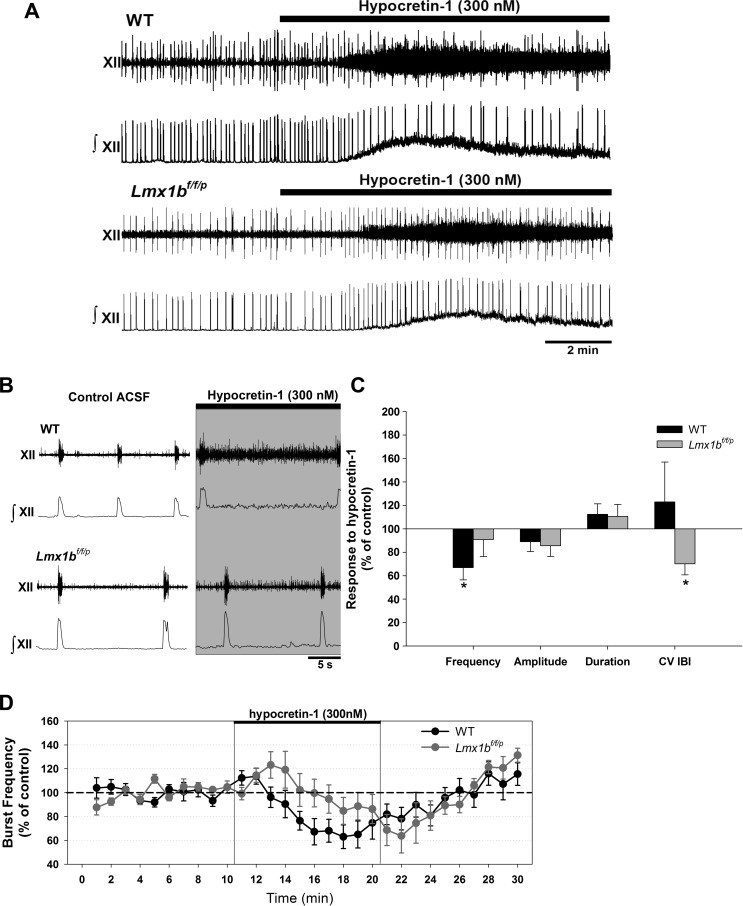
The most consistent observation in response to exogenous hypocretin-1 (300 nM), in both WT and Lmx1bf/f/p genotypes, was an increase in tonic activity in the hypoglossal nerve (Fig. 2, A and B). Exogenous hypocretin-1 had little effect on baseline burst parameters (Fig. 2C). The only significant effect in WT preparations (n = 7) was a decrease in hypoglossal burst frequency (by 33 ± 11% from baseline). This response did not occur in Lmx1bf/f/p preparations (n = 8; Fig. 2C). Exogenous hypocretin-1 did increase regularity of the hypoglossal burst pattern (illustrated by a 30 ± 9% reduction of the CV IBI from baseline) in Lmx1bf/f/p preparations. Effects of hypocretin-1 were reversible with washout, and burst parameters returned to baseline values within 10–15 min.
Fig. 2.
Effects of application of exogenous hypocretin-1 (300 nM) in WT and Lmx1bf/f/p rhythmic slices. A: raw and integrated traces from both genotypes perfused with normal artificial cerebrospinal fluid (aCSF) and during exposure to hypocretin. An increase in tonic activity was commonly observed in the presence of exogenous hypocretin. B: expanded timescale of traces shown in A. C: steady-state values of burst frequency, amplitude, duration, and CV IBI in response to hypocretin. Data are means ± SE expressed as a percentage of control values. Exogenous hypocretin reduced instability in Lmx1bf/f/p preparations. D: time course of burst frequency in both genotypes during control conditions, application of hypocretin, and washout. *P < 0.05 indicates a statistically significant difference from baseline levels (i.e., no hypocretin; n = 7 WT and 8 Lmx1bf/f/p; 2-way RM ANOVA).
Blockade of endogenous hypocretin affects hypoglossal burst parameters.
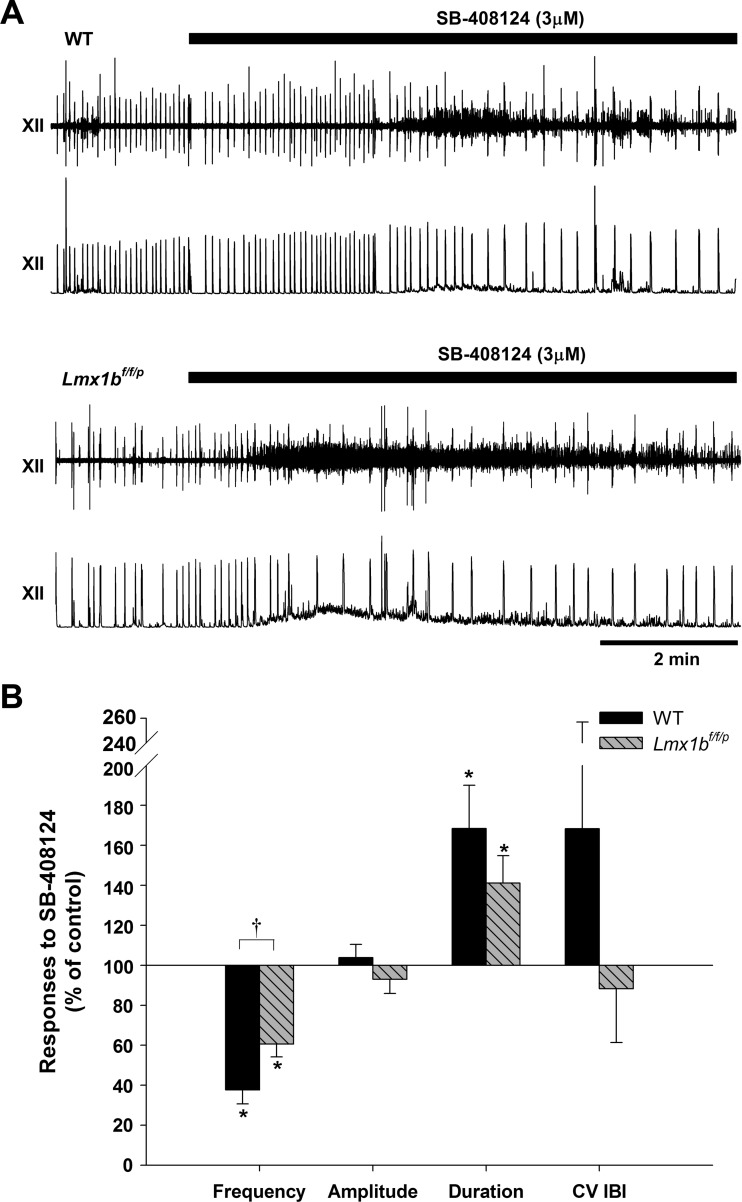
We examined the influence of endogenous hypocretin receptor activation by determining the effect of an hcrt-r1 antagonist (SB-408124) on hypoglossal nerve output (Fig. 3). In both WT and Lmx1bf/f/p preparations, SB-408124 decreased hypoglossal burst frequency. This effect was more pronounced in WT preparations (62 ± 7% reduction from baseline) compared with a more modest reduction (by 39 ± 6% from baseline) in Lmx1bf/f/p preparations (n = 6 for each genotype). The treatment did not alter burst amplitude, although burst duration was increased in preparations from both genotypes (68 ± 22% above baseline in WT, 41 ± 14% above baseline in Lmx1bf/f/p). In addition, SB-408124 did not significantly alter the regularity of the hypoglossal burst patter.
Fig. 3.
Hypocretin receptor 1 (hcrt-r1) antagonist effects on hypoglossal burst activity. A: raw and integrated traces from both genotypes perfused with normal aCSF and during exposure to 3 μM SB-408124. B: hypoglossal burst frequency, amplitude, duration, and CV IBI responses to 3 μM SB-408124 (n = 6 for each genotype) in WT and Lmx1bf/f/p preparations. Data are means ± SE expressed as a percentage of control. *P < 0.05 indicates a statistically significant difference from control; †P < 0.05 indicates a significantly different response between genotypes (2-way RM ANOVA).
Effect of hypocretin receptor antagonists on the response to acidosis.
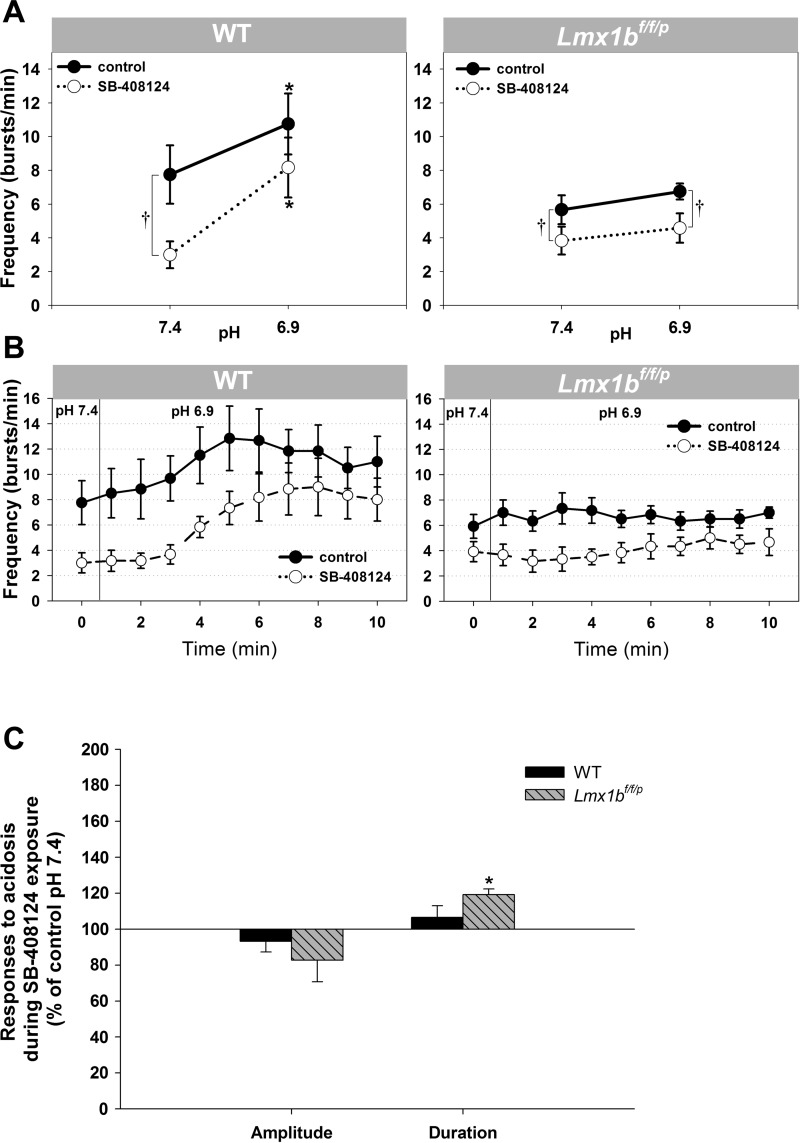
We examined the influence of endogenous hypocretins on pH sensitivity by exposing slices to solutions with pH 7.4 and 6.9 both without and with the hypocretin receptor antagonist. Figure 4, A and B, illustrates changes in hypoglossal burst frequency in response to acidosis observed under control conditions (normal aCSF) and blockade of hcrt-r1 (when aCSF contained SB-408124) in both WT and Lmx1bf/f/p preparations. Despite blockade of hypocretin receptors, WT preparations exhibited a frequency response to acidosis (increase from 3.0 ± 0.8 to 8.2 ± 1.8 bursts/min in the presence of SB-408124; n = 6). Switching to pH 6.9 restored the frequency, dampened by the antagonist to a level equivalent to baseline control values. In Lmx1bf/f/p preparations, no significant frequency response to a change in pH from 7.4 to 6.9 was observed in the presence of the hypocretin antagonist. This paralleled the responses in the absence of antagonists (n = 6). The hypocretin antagonist blocked the change in burst duration otherwise observed with acidosis in WT preparations (Fig. 4C). In contrast, burst duration increased with a switch to pH 6.9 (Fig. 4C) in both the presence and absence of the hypocretin receptor antagonist in Lmx1bf/f/p preparations. Similar to responses to acidosis alone, burst amplitude did not change in response to acidosis in either genotype while hcrt-r1 was blocked.
Fig. 4.
Hypoglossal nerve response to pH 6.9 and the impact of an hcrt-r1 antagonist on this response in WT and Lmx1bf/f/p preparations. A: application of 3 μM SB-408124 (○) compared with control (●). Data are means ± SE. *P < 0.05 indicates a statistically significant difference from values at pH 7.4. †P < 0.05 indicates a significant difference between control and SB-408124 (2-way RM ANOVA) from values at pH 7.4. B: time course of burst frequency in each genotype, both with (○) and without (●) SB-408124 in the perfusate, during control pH conditions (pH 7.4) and during acidosis (pH 6.9). C: hypoglossal nerve burst duration and amplitude responses to acidosis in WT and Lmx1bf/f/p preparations during exposure to SB-408124. Data are means ± SE expressed as a percentage of control pH 7.4 values. A paired t-test was used to determine statistical significance of values at pH 6.9 (in the presence of SB-4081240) compared with control pH 7.4 (also in the presence of SB-408124). *P = 0.001.
DISCUSSION
Lmx1bf/f/p preparations do not respond to acidosis.
One of the major findings of the current study was that, unlike the response normally exhibited in WT preparations, Lmx1bf/f/p preparations failed to increase hypoglossal burst frequency in response to acidosis. The Lmx1bf/f/p mouse, a conditional knockout, is a useful model for studying the role of 5-HT neurons in ventilatory control, including the contribution of 5-HT neurons to central chemosensitivity. Recent work has shown that breathing is relatively stable in adult Lmx1bf/f/p mice; however, the hypercapnic ventilatory response is decreased by ∼50% compared with WT mice (Hodges et al. 2008). In contrast, neonates display severely disordered breathing, which can be restored with addition of agonists for 5-HT2A in slices and in vivo and for neurokinin-1 (NK-1) receptors in slices (Corcoran et al. 2014; Hodges et al. 2009). Chemosensitivity has not yet been described in Lmx1bf/f/p mice at young ages. Our data demonstrated that whereas WT rhythmic medullary slices produced a very robust response to a pH challenge (from 7.4 to 6.9), the frequency of hypoglossal bursts in Lmx1bf/f/p preparations was not altered by the same stimulus. Because WT and Lmx1bf/f/p mice differ by an almost complete absence of 5-HT neurons in the knockout, our data illustrate that 5-HT neurons are necessary for hypoglossal chemoresponsiveness in the slice and provide further evidence that 5-HT neurons are critical in the ventilatory response to hypercapnia.
On return to baseline conditions, there was an apparent undershoot of burst frequency statistically different from baseline at one time point in Lmx1bf/f/p slices. Because both genotypes exhibited this phenomenon, it is likely independent of 5-HT neurons. One possibility is that it is due to previously characterized GABAergic mechanisms (Corcoran et al. 2008, Iceman et al. 2014; Richerson 1995; Wang et al. 1998, 2002), although this was not examined directly.
In addition, acidosis significantly decreased variability in the rate of hypoglossal bursts as determined by a decrease in the CV IBI. Barrett et al. (2012) and Fiamma et al. (2007) both reported a reduction in breathing variability in response to hypercapnia, which is in agreement with our current reported findings.
Hypocretin-1 effects on baseline bursting.
Exogenous hypocretin-1 increased tonic hypoglossal nerve activity in both WT and Lmx1bf/f/p preparations. Although our conclusions in regards to tonic activity are based on qualitative assessment alone, the observation is consistent with findings recently reported by Sugita et al. (2014). In that study, tonic discharge from a C4 recording obtained in a brain stem-spinal cord preparation was induced by the application of hypocretin-2. It was established that this tonic activity originated in the spinal cord, because it was not evident in recordings of respiratory neurons or in C4 recordings when exposure to hypocretin-2 was restricted to the medulla. G protein-coupled hypocretin receptors have been localized to a number of motor neuron pools (Fung et al. 2001; Young et al. 2005), including the hypoglossal motor nucleus, and our result may be due to general motor neuron activation.
Beyond the general tonic activation observed, the influence of exogenous hypocretin-1 was limited to its effect on hypoglossal burst frequency in WT preparations, which it significantly reduced. The physiological relevance of this effect is unclear. Previous observations suggest that exogenous hypocretin does not consistently alter phrenic burst discharge in the juvenile arterially perfused rat preparation or the hypoglossal nerve output in a rhythmic rat medullary slice (Corcoran et al. 2010).
Exogenous hypocretin-1 enhanced the regularity of the hypoglossal burst pattern in Lmx1bf/f/p preparations. Because this tendency was only observed in the Lmx1bf/f/p slices, preparations that lacked central 5-HT neurons and were more irregular at baseline compared with WT, it suggests that 5-HT is necessary for stability of the respiratory-related rhythm (also supported by evidence from Hodges et al. 2009; Pena and Ramirez 2002; Richter et al. 2003). In addition, this instability in Lmx1bf/f/p slice preparations can be rescued by exogenous hypocretin, demonstrating that 5-HT neuron contribution to the stability of the respiratory rhythm can be bypassed by a hypocretin mechanism, similar to that of substance P and elevated potassium (Hodges et al. 2009).
Hypocretin antagonism has a greater effect on hypoglossal activity in WT slices.
Addition of the hypocretin receptor antagonist to aCSF changed hypoglossal burst output in both WT and Lmx1bf/f/p preparations. We would expect this antagonist to have an influence only if hypocretin receptor activation is present; thus one interpretation is that endogenous hypocretin may still be present in the slices.
Hypocretin neuropeptides are synthesized exclusively in the lateral hypothalamus (Sakurai et al. 1998), an area that is absent in the in vitro rhythmic medullary slice preparation. However, these hypothalamic neurons project widely throughout the brain stem (Peyron et al. 1998), and it is feasible that a quantity of endogenous hypocretin is stored at synaptic terminals. We speculate that the elevated potassium concentrations used in the perfusate may induce spontaneous synaptic release of hypocretin despite the isolation of hypocretin nerve terminals, present in the slice, from their hypothalamic cell bodies. An alternative explanation, however, is that the antagonist may have a previously uncharacterized nonspecific influence on receptors other than those for hypocretin, that hypocretin receptors have a form of constitutive activation in the absence of endogenous ligand, or that the antagonist exerts partial antagonism of hypocretin receptors. The perplexing observation that the antagonist evokes the same response as the endogenous ligand suggests that the antagonist drug has partial agonist effects that we are unable to identify.
The effects of hypocretin antagonists were more profound in WT than Lmx1bf/f/p preparations, suggesting that influences on the respiratory rhythm by hypocretin were mediated, in part, through activation of 5-HT neurons. Low-density expression of hcrt-r1 occurs in the raphe magnus and raphe obscurus (Marcus et al. 2001). Also, hypocretin axon terminals are found in the raphe magnus and pallidus (Nambu et al. 1999). A much denser innervation of hypocretin-1 fibers is observed in the raphe pallidus (Zheng et al. 2005). If the effects of hypocretin antagonists are dependent on hypocretin receptors expressed on raphe 5-HT neurons, then the lack of such neurons in Lmx1bf/f/p slices would explain the limited impact of hypocretin in this genotype.
Hypocretin affects the pH response.
We also sought to determine whether hypocretins modulate the ventilatory response to acidosis, and if so, whether this is through excitation of 5-HT neurons. To do this we compared hypoglossal burst responses to low pH in rhythmic medullary slices derived from WT and Lmx1bf/f/p mice before and during exposure to a hcrt-r1 antagonist. Whereas baseline (pH 7.4) frequency was markedly lower in preparations where hcrt-r1 were blocked, burst frequency increased in response to pH 6.9 in a similar fashion to the control slices (which lacked the hypocretin receptor antagonists). This suggests that hypocretin did not enhance or promote the response to acidosis. Such a result is in contrast to our data from arterially perfused in situ rats, where systemic administration of SB-408124 inhibited the ventilatory hypercapnic response (Corcoran et al. 2010), and to other reports that hypocretins modulate chemosensitivity (Deng et al. 2007; Dias et al. 2009; Nakamura et al. 2007). Elements responsible for the observation that hypocretin receptor antagonist-mediated attenuation of chemosensitivity likely occur outside regions contained within the rhythmic slice and/or may not be active during the neonatal period used for these preparations.
Because acidosis did not induce a change in hypoglossal burst frequency in Lmx1bf/f/p preparations, the lack of response in the presence of hypocretin receptor antagonists is not unexpected and does not give us any information as to the importance of hypocretin in such a response.
The only evidence alluding to a hypocretin facilitation of the hypercapnic response due to an interaction with the 5-HT system is based on changes in hypoglossal burst duration. In WT preparations, bursts were significantly longer during exposure to pH 6.9 than during exposure to pH 7.4. Blockade of hypocretin receptors eliminated this response. In Lmx1bf/f/p preparations, a similar lengthening in hypoglossal bursts was observed at pH 6.9 compared with pH 7.4; however, hypocretin receptor antagonism did not inhibit this response as it did in WT. How and whether this increase in duration is mediated by 5-HT neurons remains unknown.
Mechanisms of chemotransduction.
Our results implicate 5-HT neurons, modulated by hypocretin/orexin A, in processes contributing to pH/CO2 chemosensitivity. Recently, a specific subset of 5-HT neurons was identified as intrinsically sensitive to changes in pH/CO2, both in vitro and in the intact nervous system (Burst et al. 2014; Iceman et al. 2013). It is likely that these are one of a number of cell types, in various nuclei, that directly influence chemosensory-related neural networks. These experiments do not identify specific mechanisms of sensory transduction, yet a mechanistic understanding is critical. Although transduction mechanisms remain undefined, a number of potential mechanisms may be excluded from consideration as contributors to the current results. For example, although pH/CO2 sensitivity in the regions such as the retrotrapezoid nucleus is contributed to by astrocytes (Moreira et al. 2015), glia are believed to play no role in raphe 5-HT neuron chemosensory transduction (Sobrinho et al. 2014; Wu et al. 2013). Similarly, despite the implication of acid-sensitive TASK channels in this process (Bayliss et al. 2015; Putnam et al. 2004), chemosensitivity of raphe 5-HT neurons does not appear to be dependent on TASK channels (Massey et al. 2015; Wu et al. 2008). Our observations suggest that input from hypocretin synthesizing neurons somehow modulates chemotransduction on 5-HT-synthesizing neurons. The role of these cells may be complex, resulting from the direct influences of hypocretin (Marcus et al. 2001; Volgin et al. 2002; Young et al. 2005) or the activity-dependent release or corelease of other transmitters such as glutamate from hypocretin neurons (Schone et al. 2012, 2014). Although the projections from the lateral hypothalamus appear to influence CO2 sensitivity in a number of nuclei (Deng et al. 2007; Dias et al. 2009; Nakamura et al. 2007) and may themselves be chemosensitive (Williams et al. 2007), their absence from our experimental preparation precludes chemotransduction at these cells from contributing to the present results.
Conclusions.
In summary, we have shown that 5-HT neurons are required for the hypoglossal nerve frequency response to severe acidosis in the in vitro rhythmic medullary slice preparations, furthering evidence that 5-HT neurons play an important role in central chemoreception. Exogenous application of hypocretin-1, as well as a hcrt-r1 antagonist, differentially altered respiratory motor output in WT and Lmx1bf/f/p preparations, suggesting that 5-HT neurons contribute to the role of hypocretin in fictive ventilation. Although we do not have evidence that hypocretin contributes to the frequency response to acidosis, this neuropeptide may facilitate the acidosis-induced increase in hypoglossal burst duration via modulation of 5-HT neurons.
GRANTS
This work was supported in part by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke. We also are grateful for support from National Institutes of Health Grants 2U54NS041069, P01HD36379, and R01HD052772 and the Iowa City Veterans Affairs Medical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.E.C. and M.B.H. conception and design of research; A.E.C. performed experiments; A.E.C. analyzed data; A.E.C., G.B.R., and M.B.H. interpreted results of experiments; A.E.C. prepared figures; A.E.C. drafted manuscript; A.E.C., G.B.R., and M.B.H. edited and revised manuscript; A.E.C., G.B.R., and M.B.H. approved final version of manuscript.
REFERENCES
- Barrett KT, Kinney HC, Li A, Daubenspeck JA, Leiter JC, Nattie EE. Subtle alterations in breathing and heart rate control in the 5-HT1A receptor knockout mouse in early postnatal development. J Appl Physiol 113: 1585–1593, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Barhanin J, Gastreau C, Guyenet PG. The role of pH-TASK channels in central respiratory chemoreception. Pflügers Arch 467: 917–929, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology 40: 457–459, 2001. [DOI] [PubMed] [Google Scholar]
- Brust RD, Corcoran AE, Richerson GB, Nattie E, Dymecki SM. Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell Rep 9: 2152–2165, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Commons KG, Wu Y, Smith JC, Harris MB, Richerson GB. Dual effects of 5-HT1a receptor activation on breathing in neonatal mice. J Neurosci 34: 51–59, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu W, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol 168: 29–58, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Richerson GB, Harris MB. Both serotonergic and GABAergic neurons contribute to central chemosensitivity in a perfused rat brainstem. Soc Neurosci Abstr 34: 383.14, 2008. [Google Scholar]
- Corcoran AE, Richerson GB, Harris MB. Modulation of respiratory activity by hypocretin-1 (orexin A) in situ and in vitro. In: New Frontiers in Respiratory Control. Advances in Experimental Medicine and Biology. New York: Springer, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Richerson GB, Harris MB. Serotonergic mechanisms are necessary for central respiratory chemoresponsiveness in situ. Respir Physiol Neurobiol 186: 214–220, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol 103: 1772–1779, 2007. [DOI] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J Appl Physiol 105: 83–90, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol 587: 2059–2067, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiamma MN, Straus C, Thibault S, Wysocki M, Baconnier P, Similowski T. Effects of hypercapnia and hypocapnia on ventilatory variability and the chaotic dynamics of ventilatory flow in humans. Am J Physiol Regul Integr Comp Physiol 292: R1985–R1993, 2007. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretin (orexin) input to trigeminal and hypoglossal motoneurons in the cat: a double-labeling immunohistochemical study. Brain Res 903: 257–262, 2001. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA 96: 10911–10916, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28: 2495–2505, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci 29: 10341–10349, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iceman KE, Corcoran AE, Taylor BE, Harris MB. CO2-inhibited neurons in the medullary raphe are GABAergic. Respir Physiol Neurobiol 203: 28–34, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iceman KE, Harris MB. A group of non-serotonergic cells is CO2-stimulated in the medullary raphé. Neuroscience 259: 203–213, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iceman KE, Richerson GB, Harris MB. Medullary serotonin neurons are CO2-sensitive in situ. J Neurophysiol 110: 2536–2544, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Zhou S, Nattie EE. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol 577: 307–318, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435: 6–25, 2001. [DOI] [PubMed] [Google Scholar]
- Massey CA, Iceman KE, Johansen SL, Wu Y, Harris MB, Richerson GB. Isoflurane abolishes spontaneous firing of serotonin neurons and masks their pH/CO2 chemosensitivity. J Neurophysiol 113: 2879–2888, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira TS, Wenker IC, Sobrinho CR, Barna BF, Takakura AC, Mulkey DK. Independent purinergic mechanisms of central and peripheral chemoreception in the rostral ventrolateral medulla. J Physiol 593: 1067–1074, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci 27: 14128–14138, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol 102: 241–248, 2007. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res 827: 243–260, 1999. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemoreception is a complex system function that involves multiple brainstem sites. J Appl Physiol 106: 1464–1466, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci 22: 11055–11064, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol 73: 933–944, 1995. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004. [DOI] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med 9: 542–548, 2003. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Lie WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G-protein-coupled receptors that regulate feeding behavior. Cell 92: 573–585, 1998. [DOI] [PubMed] [Google Scholar]
- Schöne C, Apergis-Schoute J, Sakurai T, Adamantidis A, Burdakov D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep 8: 697–704, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöne C, Cao ZF, Apergis-Schoute J, Adamantidis A, Sakurai T, Burdakov D. Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. J Neurosci 32: 12437–12443, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman L. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrinho CR, Wenker IC, Poss EM, Takakura AC, Moreira TS, Mulkey DK. Purinergic signalling contributes to chemoreception in the retrotrapezoid nucleus but not the nucleus of the solitary tract or medullary raphe. J Physiol 592: 1309–1323, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita T, Sakuraba S, Kaku Y, Yoshida K, Arisaka H, Kuwana S. Orexin induces excitation of respiratory neuronal network in isolated brainstem spinal cord of neonatal rat. Respir Physiol Neurobiol 200: 105–109, 2014. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Wang QP, Guan JL, Kayama Y, Shioda S, Koyama Y. State-dependent effects of orexins on the serotonergic dorsal raphe neurons in the rat. Regul Pept 126: 43–47, 2005. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Saghir M, Kubin L. Developmental changes in the orexin 2 receptor mRNA in hypoglossal motoneurons. Neuroreport 13: 433–436, 2002. [DOI] [PubMed] [Google Scholar]
- Wang W, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphe neurones to independent changes in pHo and Pco2. J Physiol 540: 951–970, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol 511: 433–450, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphe neurons. Neuroscience 90: 1001–1011, 1999. [DOI] [PubMed] [Google Scholar]
- Williams RT, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA 104: 10685–10690, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Zaykin A, Deneris ES, Wylie CJ, Richerson GB. Chemosensitivity of adult 5-HT neurons is not dependent on TASK channels. International Symposium on Respiratory Control Abstracts, 2008. [Google Scholar]
- Wu Y, Zaykin Wang W, Hodges MR, Wylie CJA, Deneris ES, Richerson GB. Medullary raphe serotonin neuron chemosensitivity is not dependent on ATP release or neuroglia. Soc Neurosci Abstr 39: 656.03, 2013. [Google Scholar]
- Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol 98: 1387–1395, 2005. [DOI] [PubMed] [Google Scholar]
- Zhang W, Fukuda Y, Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neurosci Lett 385: 131–136, 2005. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RW, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci 26: 12781–1278, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol 485: 127–142, 2005. [DOI] [PubMed] [Google Scholar]