Abstract
Resting state functional magnetic resonance imaging (rsfMRI) results have indicated that network mapping can contribute to understanding behavior and disease, but it has been difficult to translate the maps created with rsfMRI to neuroelectrical states in the brain. Recently, dynamic analyses have revealed multiple patterns in the rsfMRI signal that are strongly associated with particular bands of neural activity. To further investigate these findings, simultaneously recorded invasive electrophysiology and rsfMRI from rats were used to examine two types of electrical activity (directly measured low-frequency/infraslow activity and band-limited power of higher frequencies) and two types of dynamic rsfMRI (quasi-periodic patterns or QPP, and sliding window correlation or SWC). The relationship between neural activity and dynamic rsfMRI was tested under three anesthetic states in rats: dexmedetomidine and high and low doses of isoflurane. Under dexmedetomidine, the lightest anesthetic, infraslow electrophysiology correlated with QPP but not SWC, whereas band-limited power in higher frequencies correlated with SWC but not QPP. Results were similar under isoflurane; however, the QPP was also correlated to band-limited power, possibly due to the burst-suppression state induced by the anesthetic agent. The results provide additional support for the hypothesis that the two types of dynamic rsfMRI are linked to different frequencies of neural activity, but isoflurane anesthesia may make this relationship more complicated. Understanding which neural frequency bands appear as particular dynamic patterns in rsfMRI may ultimately help isolate components of the rsfMRI signal that are of interest to disorders such as schizophrenia and attention deficit disorder.
Keywords: resting state fMRI, dynamic analysis, quasi-periodic patterns, sliding window correlation, simultaneous electrophysiology and MRI
resting state functional magnetic resonance imaging (rsfMRI) has become an important tool for psychologists and neuroscientists. It works by examining correlations (or other similarity measures) in the blood oxygenation level dependent (BOLD) signal over time between different brain regions and organizing them into “networks” of highly correlated regions (Biswal et al. 1995). Unlike traditional functional magnetic resonance imaging, which examines the response to a task or stimulus, rsfMRI can map networks during undirected cognition, which provides incredibly flexible experimental design and analysis (Raichle 2011; Raichle et al. 2001). rsfMRI has been used to identify alterations in network connectivity due to neurological and psychiatric disorders (Greicius et al. 2004; Rombouts et al. 2005; van den Heuvel and Hulshoff Pol 2010). Network connectivity has also been linked to behavior and cognitive performance in healthy subjects (examples covering a broad range of tasks include Weissman et al. 2006, Tambini et al. 2010 and Magnuson et al. 2015).
The majority of these studies used entire 6 min or longer rsfMRI runs to calculate their correlations. However, the time scale for activity related to cognition is much shorter, from milliseconds for a single action potential to seconds for a cohesive thought. To address this difference in temporal resolution, the investigation of dynamic changes in rsfMRI was proposed (Chang and Glover 2010; Kiviniemi et al. 2011; Majeed et al. 2009, 2011). As the resting state has (by definition) no stimulus for comparison, it is not trivial to extract meaningful dynamic information from it. Techniques proposed have included finding characteristic spatiotemporal dynamics (Majeed et al. 2009), wavelet analysis (Chang and Glover 2010), sliding-window correlation (Hutchison et al. 2013; Keilholz et al. 2013), independent component analysis (Kiviniemi et al. 2011), and averaging the rsfMRI signal near known events (Liu and Duyn 2013; Magri et al. 2012; Petridou et al. 2013). While much work remains, evidence is emerging that such dynamics do indeed reflect the underlying neural activity (Keilholz 2014; Thompson et al. 2013b, 2013c).
The rsfMRI signal is not a simple reporter of neural activity, however, instead being a complex combination of blood oxygenation, blood volume and blood flow (Logothetis 2008) and potentially relating to both synaptic activity and action potentials from neurons (Hyder and Rothman 2012). Therefore, it is possible that these different ways to characterize variations in rsfMRI over time are reporting different aspects of the underlying neural signaling and physiology. Electrophysiological signals are often separated into frequency bands, with the hypothesis that different frequencies represent different underlying neuronal or physiological processes (Magri et al. 2012). A broad range of frequencies has been linked to spontaneous BOLD signal fluctuations (Lu et al. 2007; Pan et al. 2011; Scholvinck et al. 2010; Shmuel and Leopold 2008). Therefore, this study attempts to determine whether different types of dynamic rsfMRI spatial and temporal patterns relate to different frequency bands of neural electrophysiology.
Our group has previously studied the relationship between two different types of dynamic rsfMRI and neural activity. The first study examined sliding window correlation (SWC) between the rsfMRI signal from left and right primary somatosensory and found that it was linked to changes in correlation in gamma, beta and theta power (Thompson et al. 2013b). The second study generated a characteristic pattern that occurs multiple times in the rsfMRI signal in a periodic, although not constant, manner, referred to as a quasi-periodic pattern (QPP) (Majeed et al. 2011) and found that this pattern was linked to infraslow (<1 Hz) electrical activity (Thompson et al. 2013c). However, the first of these studies was limited to comparing SWC to band-limited power (BLP) in frequencies above 0.1 Hz due to the recording hardware. The second of these studies, conversely, did not examine the relationship between QPP and higher frequencies, as these frequencies did not exhibit rsfMRI correlates similar to QPP in a prior study (Pan et al. 2013).
Hypothetical models for the origin of the rsfMRI signal (Raichle 2011) and results from noninvasive electroencephalography (EEG) recording (Hiltunen et al. 2014; Monto et al. 2008) have suggested that low-frequency rsfMRI measurements may be directly linked to low-frequency neural activity that follows changes in the power of high-frequency neural activity. As a preliminary attempt to determine whether the high and low frequencies represented separable processes, we examined phase amplitude coupling between high- and low-frequency electrical activity and compared static rsfMRI to both of these frequency bands (Thompson et al. 2014). We discovered that the different frequencies of electrical activity appeared to contribute to two separate patterns of BOLD signal fluctuations that were then combined into the measured rsfMRI signal. However, this study did not investigate dynamic activity, leaving the connection to dynamic rsfMRI hypothetical at the time.
The present study expands on our previous work, generating both types of dynamic rsfMRI patterns (SWC and QPP) and comparing them to the two frequency bands in the electrical signal that correlated most strongly with them in previous studies. Previously collected data (Pan et al. 2013; Thompson et al. 2013c) were examined from a rat model where electrical frequencies as low as 0 Hz were recorded as local field potentials (LFPs) simultaneously with rsfMRI. BLP of high-frequency electrical activity and amplitudes of infraslow LFP were compared with both QPP and SWC.
Our results show that SWC correlated with only the high-frequency BLP, whereas QPP strength consistently correlated only with the infraslow LFP. Under isoflurane anesthesia, where the rsfMRI signal becomes much more widespread (Liu et al. 2012), QPP strength also correlated with high-frequency BLP, but this did not occur under dexmedetomidine anesthesia, which is a lighter anesthesia and closer to an awake state (See Appendix A of Thompson et al. 2013c). Our results suggest that different types of dynamic rsfMRI do indeed reflect different types of underlying neural activity, and that anesthesia influences this relationship. Our results may help researchers better isolate parts of the rsfMRI signal that are important to the types of neural activity of interest for a given study.
MATERIALS AND METHODS
Data used.
This study used data which has previously been reported in either Pan et al. (2013) (low-dose isoflurane), Thompson et al. (2013c) (high-dose isoflurane) or both of those papers overlapping (dexmedetomidine). All experimental procedures were done in compliance with the Emory University Institutional Animal Care and Use Committee. A video of the experimental procedure can be found in the Journal of Visual Experiments (Pan et al. 2010). To summarize, glass electrodes with silver-silver chloride leads (allowing recording of very low frequencies) were implanted in the left and right primary somatosensory cortices of the lower forelimb region (S1FL) of 10 healthy, adult, male Sprague-Dawley rats while they were anesthetized with 2% or higher isoflurane (greater if required to have no response to a toe pinch). Rats were transferred inside a 9.4-Tesla MRI scanner, and, during recording, isoflurane anesthesia was either reduced to 1.3–2.0%, or the rat was transferred to dexmedetomidine anesthesia. Dexmedetomidine dose was a 0.025 mg/kg bolus followed by 0.05 mg·kg−1·h−1 infusion, which was increased to 0.15 mg·kg−1·h−1 after 1.5 h (Pan et al. 2013). All dexmedetomidine data used here were acquired after a minimum of 2 h to produce stable physiology (Magnuson et al. 2014b). Echo-planar, gradient-echo, BOLD functional magnetic resonance imaging (rsfMRI) was recorded with repetition time 500 ms (2 Hz), echo time 15 ms, one 2-mm slice, field of view 1.92 × 1.92 cm, 64 × 64 voxels and 1,000 images (∼8 min). Simultaneously, while rsfMRI data were recorded, LFP data were recorded from the implanted electrodes at 12 kHz with a low-pass filter of 100 Hz and no high-pass filter. Artifacts were removed from the LFP signal using a previously described algorithm (Pan et al. 2010, 2011), and the use of glass electrodes limited the artifacts in the BOLD images to a minimum.
Data were separated into three groups based on anesthesia. Low-dose isoflurane was defined as isoflurane at concentrations of 1.3–1.5% of which there were 14 scans (3 each from rats 1 and 2, 2 each from rats 3, 4, 5 and 6). High-dose isoflurane was defined as isoflurane at concentrations of 1.7–2.0% of which there were 17 scans (4 from rat 1, 8 from rat 2, 2 from rat 3, 3 from rat 4). Dexmedetomidine anesthesia was the third group of which there were 46 scans (5 from rat 4, 7 from rat 5, 3 from rat 6, 5 from rat 7, 5 from rat 8, 14 from rat 9, 7 from rat 10).
Processing of electrical signals.
To generate the infraslow LFP signals, the LFP time courses were resampled to 4 Hz, then filtered using a finite impulse response (FIR) filter with a hamming window. A pass-band of 0.038–0.184 Hz was used for isoflurane (both low and high dose) data and 0.045–0.304 Hz for dexmedetomidine data. These pass-bands matched the filter used on BOLD data (see next section). A discussion of how differing pass-bands per anesthesia affect the results is given in the original paper (Thompson et al. 2013c). The signals were then normalized to zero mean, unit variance.
To generate the high-frequency BLP signals, the LFP time courses were resampled to 200 Hz, then filtered using a FIR filter with a hamming window. A pass-band of 25–40 Hz or “high beta” was used for both dexmedetomidine and isoflurane because, of all frequency bands tested, this band's SWC was most strongly correlated with BOLD-generated SWC (Thompson et al. 2013b). Data were normalized to zero mean, unit variance. The time courses were converted to BLP by taking the magnitude of the Hilbert transformation of the data. This effectively produced the envelope of power changes in the high-beta-band over time.
For comparison to the resting state SWC, SWC time courses were generated for both the infraslow LFP signals and the high-frequency BLP signals. A window length of 50 s was used to match the rsfMRI window length (see next section), and this window was advanced across the time course, beginning at from 0 s to 50 s and ending at 450 s to 500 s, moving one sample at a time and at each window returning the correlation coefficient between the left and right S1FL electrodes' signals as the SWC signal at that time point.
Generation of dynamic rsfMRI.
Prior to generating dynamic rsfMRI, regions of interest (ROIs) were manually drawn in the left and right S1FL of the BOLD image using a rat brain atlas (Paxinos and Watson 2005). The mean signal across the slice was regressed from each voxel, and each voxel was linearly detrended. The BOLD signals in each voxel were resampled to 4 Hz, then filtered using a FIR filter with a hamming window. A pass-band of 0.038–0.184 Hz was used for isoflurane (both low and high dose) data and 0.045–0.304 Hz for dexmedetomidine data. These pass-bands come from the empirical filter that was generated in Thompson et al. (2013c). A discussion of how differing pass-bands per anesthesia affects the results is given in that paper. The signals were then normalized to zero mean, unit variance.
SWC was generated using windows of 50 s in length. This length was chosen to be consistent with our group's previous studies (Keilholz et al. 2013) and also gives a good compromise of high-correlation scores vs. low error (Thompson et al. 2013b). The mean signal over time across all voxels was calculated in both the left and right S1FL ROIs. A 50-s window was advanced across these mean signals, beginning at from 0 s to 50 s and ending at 450 s to 500 s, moving one sample at a time, and at each window returning the correlation coefficient between the left and right S1FL ROI mean BOLD signals as the SWC signal at that time point. SWC correlation coefficients were converted to Z-scores using a normalizing fisher transformation (equation 1 from Thompson et al. 2013b), then normalized to zero mean, unit variance per individual run.
QPPs were generated using the algorithm described in Majeed et al. (2011). To summarize, this algorithm takes an epoch of a spatiotemporal signal, compares it to the rest of the same signal and then averages together likely instances of the same pattern being repeated. This is iterated many times until a high-confidence template of a characteristic spatiotemporal pattern in the signal is returned. The algorithm returns both the template as well as a time course of the strength of the pattern over time. Template lengths of 11.25 s for isoflurane (low and high dose) and 6.5 s for dexmedetomidine were used as these were the inverse of the frequency centers of the empirical filters generated in our previous work (Thompson et al. 2013c). The template start point was arbitrarily set to the signals' centers (250 s). The ROI used to calculate the QPPs was the combination of the left and right S1FL ROIs. All other parameters were as described in Appendix B of Thompson et al. (2013c). As the start positions of the QPP templates were arbitrary, following their generation, QPP templates were aligned to all others within the same anesthetic state (thus separately for dexmedetomidine, low-dose isoflurane and high-dose isoflurane runs) based on the first rat's first run, using spatial correlation as per Thompson et al. (2013c), and these alignments were then applied to the QPP strengths over time. QPP strengths over time were normalized to zero mean, unit variance per individual run.
While numerous methods of quantifying dynamic rsfMRI patterns exist (Chang and Glover 2010; Grigg and Grady 2010; Hutchison et al. 2013; Keilholz et al. 2013; Kiviniemi et al. 2011; Liu and Duyn 2013; Magri et al. 2012; Majeed et al. 2009; Petridou et al. 2013), QPP and SWC were chosen for this study as they are at opposite ends of the spectrum of methods, with SWC (as generated here) providing spatially-localized and temporally-windowed information, whereas QPP identifies spatially-extended and quasi-periodic to periodic processes.
Data exclusion due to SWC abnormalities.
In the time series of SWC generated from high-frequency BLP, large, instantaneous baseline shifts were observed. Sometimes these occurred on the edge of the signal, but more commonly occurred as a plateau in the center of the signal. Inspection of the underlying electrophysiology time courses revealed that this occurred when a spike occurred and the sliding window was overlapping the spike. These spikes appeared unrelated to the rsfMRI artifacts that were removed and were in many cases difficult to observe without using the SWC to find them. While the source was unknown, possibilities include slight movements, unexpected acoustic noise, or transient alterations in the blood-brain barrier (Voipio et al. 2003), caused by anesthesia.
To avoid possible errors caused by this, the high-frequency BLP SWC time courses, which showed a change of 0.01 in uncorrected correlation coefficient between two adjacent windows, were removed. These removals included 2 scans for low-dose isoflurane (both scans from rat 4), no scans for high-dose isoflurane and 11 scans for dexmedetomidine (2 from rat 4, all 3 scans from rat 6, 1 scan from rat 7, 1 scan from rat 8, 2 scans from rat 9, 2 scans from rat 10).
Comparison between dynamic rsfMRI and electrical signals.
Four comparisons were performed between dynamic rsfMRI and the electrical signals, reflecting the two types of dynamic patterns (SWC and QPP) and the two frequency bands (infraslow LFP and high-frequency BLP). The comparisons were as follows: 1) SWC generated from high-frequency BLP vs. SWC generated from BOLD; 2) SWC generated from infraslow LFP vs. SWC generated from BOLD; 3) the high-frequency BLP signal itself vs. QPP strength over time; and 4) the infraslow LFP signal itself vs. QPP strength over time. These comparisons were done both directly, using Pearson correlation and also using partial correlation. In partial correlation, the correlation coefficients are calculated accounting for the influence of a third signal, ideally returning a correlation coefficient that removes correlations between the original two signals that are due to the third signal. The third signal, or “controlling variable,” here was set to the other type of dynamic rsfMRI. Thus for 1 and 2, the controlling variable was QPP strength (i.e., how closely it resembled the template) over time and for 3 and 4 it was SWC generated from BOLD. Note that for 3 and 4 each electrode's correlation with QPP strength over time was calculated separately, whereas for 1 and 2 using SWC only gave a single signal, as it was based on correlation between both electrodes. The high-frequency BLP and SWC generated from it were resampled to 4 Hz prior to correlation to match the equivalent dynamic rsfMRI's sampling rate. If signals were of uneven length for Pearson correlation, they were padded with trailing zeros.
As the time shift of QPPs is arbitrary (Majeed et al. 2011) and as it is an open question where electrical activity would align with BOLD averaged over long windows (Thompson et al. 2013b), correlation was calculated at many time shifts between the electrical signals and the dynamic rsfMRI. Time shifts were done from the BOLD-derived signals shifted forwards in time up to 10 s relative to the LFP-derived signals (−10 s on Figs. 2–4) to the BOLD-derived signals shifted backwards in time up to 10 s relative to the LFP-derived signals (10 s on Figs. 2–4). Including no shift (0 s on Figs. 2–4), this is a total of 81 time shifts at 4 Hz.
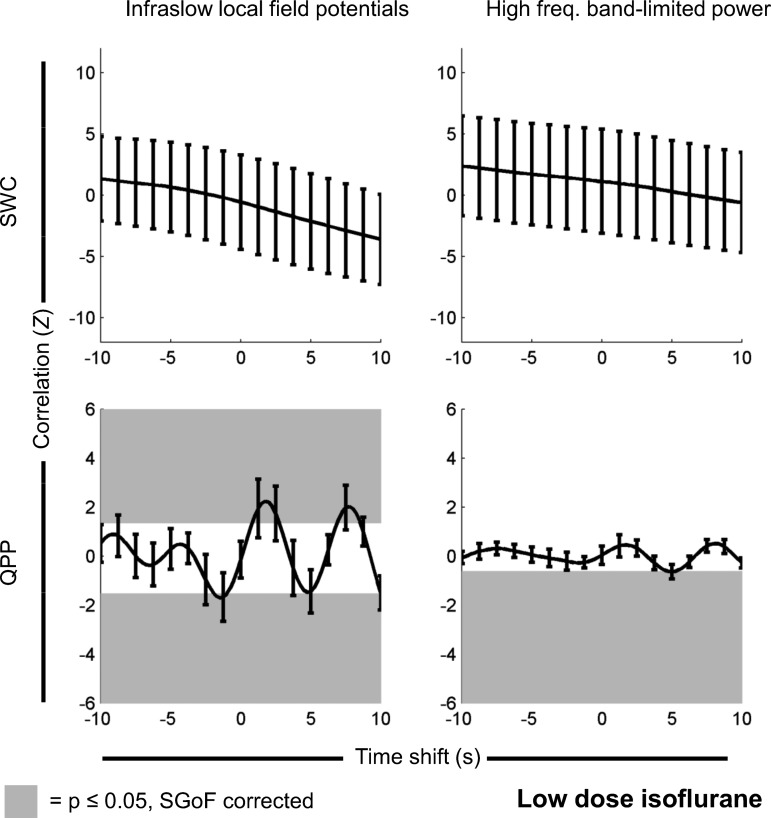
Fig. 2.
Correlation between dynamic rsfMRI and electrophysiology-derived signals recorded under a low dose of isoflurane (N = 12 scans). The top row is correlation between the SWC calculated from rsfMRI and SWC either calculated from the infraslow (low-frequency) LFP directly (left) or the high-frequency BLP signal (right). The bottom row is correlation between the strength of the QPP over time (generated from rsfMRI) and either the infraslow (low-frequency) LFP directly (left) or the high-frequency BLP signal (right). The y-axis on each plot is normalized Z value, corresponding to number of standard deviations from the null hypothesis of no correlation. The x-axis on each plot is the time shift between electrophysiology and rsfMRI-derived measures, with positive indicating that electrophysiology events precede rsfMRI events and negative indicating that rsfMRI events precede electrophysiology events. Significant Z values have a gray background. If no gray background is present, then nothing passed multiple comparison corrections [using sequential goodness of fit (SGoF)]. Error bars are 1 SE of the mean. Under a low dose of isoflurane, there is significant correlation between the change in QPP strength over time and the infraslow LFP signal, as well as a significant correlation between the change in QPP strength over time and the high-frequency BLP signal (note that, as only one point is significant, the line of correlation vs. time shift is only tangent to the significance zone at that point). Note that, for QPP, time shifts are arbitrary, and positive and negative correlations are expected (see Significance of QPP correlation vs. time shift in materials and methods). Partial correlation results (with SWC acting as a controlling variable for QPP strength vs. electrophysiology correlations and QPP strength acting as a controlling variable for SWC vs. electrophysiology correlations) had no statistically significant differences and appeared identical when plotted.
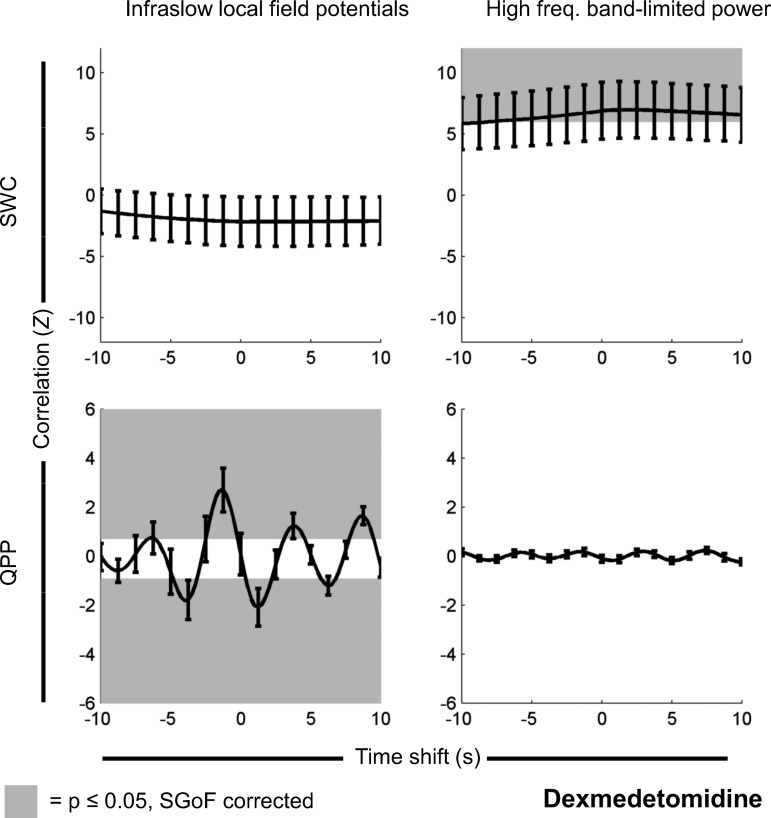
Fig. 4.
Same as Fig. 2, but for signals recorded under dexmedetomidine (n = 35 scans). There is significant correlation between SWC derived from rsfMRI and SWC derived from high-frequency BLP. There is also a significant correlation between the change in QPP strength over time and the infraslow LFP. However, the reverse pairings are not significant. Note that, for QPP, time shifts are arbitrary, and positive and negative correlations are expected (see Significance of QPP correlation vs. time shift in materials and methods). Partial correlation results had no statistically significant differences and appeared identical when plotted. As dexmedetomidine is the lightest anesthetic and close to an awake state, this suggests that different types of dynamic rsfMRI may reflect different frequency bands in the underlying electrophysiology, but deeper anesthesia (Figs. 2 and 3) obscures this.
Finally, following the procedure from our previous studies, (Pan et al. 2013; Thompson et al. 2013c), the sign of correlation coefficients resulting from correlation with infraslow LFP (itself only, not SWC of it) was reversed. This was done as the direction of bursting in the layer from which data were recorded is downward. This reversal was not necessary in the other cases, as BLP calculated from LFP and SWC calculated from any signal will not be sensitive to the positive/negative direction of the original LFP signal, only the fluctuations within it.
Significance testing.
To test if correlations between dynamic rsfMRI and the electrical signals were significant, a surrogate data set was generated using every possible mismatch of one rat's BOLD data with another rat's LFP data (without exclusion, 182 mismatched scans for low-dose isoflurane, 272 mismatched scans for high-dose isoflurane, 2,070 mismatched scans for dexmedetomidine; with exclusion, 132, 272, and 1,190 mismatched scans for each anesthetic state, respectively). The surrogate data were otherwise generated identically to the real data, as described above.
For regular correlation, two-tailed, equal variance t-tests were performed between the set of actual Z values and the respective surrogate Z values for a given anesthesia (low-dose isoflurane, high-dose isoflurane, dexmedetomidine), rsfMRI metric (SWC, QPP), frequency band (infraslow LFP, high-frequency BLP) and time shift (−10 s to 10 s). Resulting P values for all time shifts were then corrected for multiple comparisons using sequential goodness of fit (SGoF) (Carvajal-Rodriguez et al. 2009), a binomial method that looks for significantly large clusters of small P values. Thus there were a total of 12 statistical families (three anesthesia, two frequency bands and two types of dynamic rsfMRI). Effect sizes in results will be reported as the mean of significant Z values from actual data minus the mean of corresponding Z values from mismatched data. This effect size was taken over significant time shifts, with positive and negative Z values separated.
Instead of being tested vs. surrogate data, each set of partial correlation Z values (for a given type of dynamic rsfMRI, frequency band and time shift) were tested vs. the equivalent set of regular correlation Z values. This was done because a significant difference between partial and regular correlation here would indicate an influence of one dynamic on the other, suggesting they may be measuring the same thing. Resulting P values were also corrected using SGoF within the 12 families, as described in the previous paragraph.
Significance of QPP correlation vs. time shift.
The plots of correlation vs. time shift between periodic signals of the same frequency will not be a single peak as is commonly seen when correlating neural activity and rsfMRI (e.g., Shmuel and Leopold 2008). Instead, they will appear as an autocorrelation plot of a periodic process. As Pearson correlation normalizes signals to zero mean, this means that such a plot always has both significant positive and negative peaks, given sufficient signal-to-noise ratio (SNR). These peaks can be used to estimate the frequency of the periodic process, as shown in Equation 1 in Thompson et al. (2013c). In the present study, we do not have as much SNR as we had in Thompson et al. due to data exclusion due to SWC abnormalities (see above) and also due to use of a standard filter rather than a filter empirically created from the data. However, as QPPs are periodic (by definition) and their correlates in electrophysiology would hypothetically have the same period, we still expect to see positive and negative peaks in the plots of correlation vs. time shifts for them, even if it does not appear as a clean autocorrelation plot.
All QPP templates were aligned to the first rat's first run (per anesthesia) so they would match each other in terms of phase. However, the algorithm given in Majeed et al. (2011) does not give “start” or “end” points for QPPs. Rather the start point will be arbitrary, as the resulting template can be seen as a single (assuming the window length is chosen as the period) or multiple (if it is chosen longer than the period) period of a periodic process, chosen at an arbitrary starting phase. As QPP templates differ between anesthetic states (Magnuson et al. 2014b; Majeed et al. 2009, 2011; Thompson et al. 2013c), alignments from one anesthetic state will not be compared with the others. Therefore, as the first rat's first run's QPP template is at an arbitrary phase, and also as comparisons between anesthetic states are not possible, the specific time shift where QPP strength has highest correlation with electrophysiology will also be arbitrary. [This is assuming that enough time shifts are tested to determine if significant peak(s) exist; slightly less than the length of the QPP template in each direction should be sufficient.] The peaks' time shifts will only reflect where the particular template for the first rat's first run (per anesthesia) was aligned with the electrophysiology, which is arbitrary, likely to be unique to that run (Majeed et al. 2011) and also likely to be different for each anesthetic state.
However, what can be tested from the time shifts between QPP strength and electrophysiology is the distance between peaks on an individual plot. Unlike our previous study (Thompson et al. 2013c), we lack sufficient SNR and thus sufficiently autocorrelation-like plots to characterize the period of the correlated patterns exactly. However, we can still examine the change in time shift between the highest significant positive peak (hypothetically where the autocorrelation would be centered) and the lowest significant negative peak next to it (hypothetically one of the side peaks). This difference should be greater for slower patterns and lesser for faster patterns.
RESULTS
Two frequency bands of neural electrophysiology were generated as signals: the infraslow LFP signal and the high-frequency BLP signal. Two patterns of dynamic rsfMRI were generated as well: the SWC between left and right S1FL and the strength of the QPP over time. SWC was also generated from the electrophysiological signals for comparison to the rsfMRI-generated SWC. The electrophysiological signals were then compared with the dynamic rsfMRI with regular and partial correlation at time shifts of up to 10 s in each direction. Figure 1 shows examples of each type of dynamic rsfMRI, as well as electrophysiological SWC for comparison.
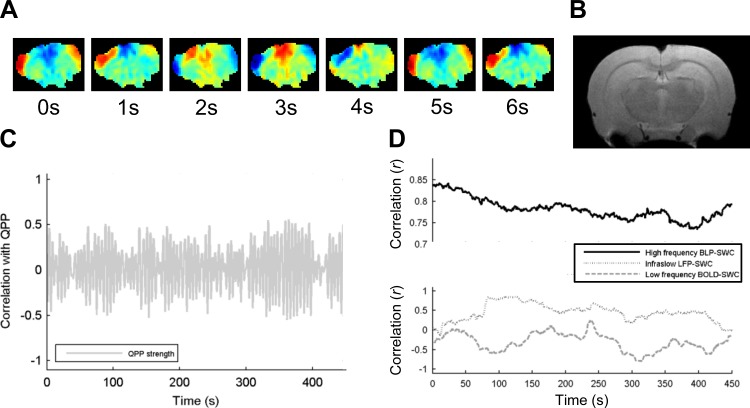
Fig. 1.
Examples of the dynamics generated in this study. A: quasi-periodic patterns (QPP) template, generated with the algorithm from Majeed et al. (2011). Every 4th frame is shown; range is approximately from −1 for dark blue to +1 for dark red. Notice that an alternating positive/negative wave moves from the ventral-lateral to dorsal-medial cortex. B: anatomical MRI rat brain image in the same plane as A, for comparison. C: the strength of the QPP, over time. The higher points are where the resting state functional magnetic resonance imaging (rsfMRI) signal is spatiotemporally similar to the pattern in A, and the low points are where it is not. Notice that there are periods of stronger and weaker periodicity. D: examples of sliding window correlation (SWC) calculated from the high-frequency band-limited power (BLP; solid line, changes are smaller than others in terms of absolute r values), the infraslow (low-frequency) local field potential (LFP; thin dashed line) and the infraslow blood oxygenation level dependent (BOLD) signal (thick dashed line). Notice that correlation changes dynamically over time, with some similarities between the three time courses.
Regular correlation results.
Under a low dose of isoflurane, QPP strength over time was significantly correlated with infraslow LFP (P ≤ 0.0217, SGoF corrected); there was significant negative correlation at time shifts of −1.75 s to −1 s (mean actual minus mean mismatched Z = −1.82) and significant positive correlation at time shifts of 1 s to 2.75 s and 7.5 s to 8.25 s (mean actual minus mean mismatched Z = 2.03). QPP strength over time was significantly correlated with high-frequency BLP (P ≤ 0.0121, SGoF corrected); there was significant negative correlation at the time shifts of 5 s (mean actual minus mean mismatched Z = −0.640; note that, as only one point is significant, the line of correlation vs. time shift is only tangent to the significance zone at that point) but no significant positive correlation. There were no significant correlations between any SWC time courses. These results are shown in Fig. 2.
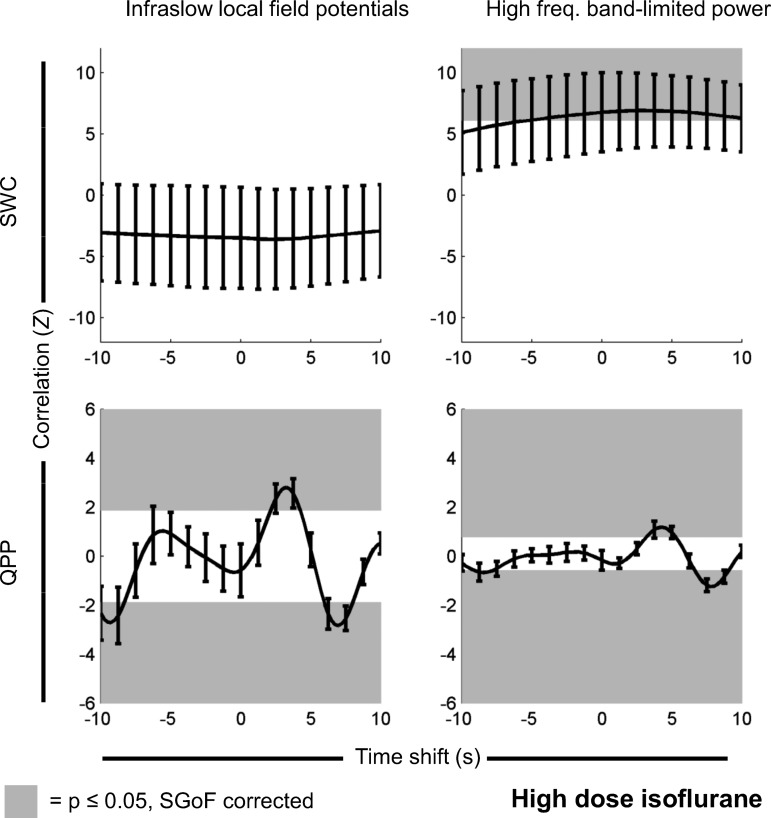
Under a high dose of isoflurane, QPP strength over time was significantly correlated with infraslow LFP (P ≤ 0.00143, SGoF corrected); there was significant negative correlation at time shifts of −10 s to −8.5 s and 6.25 s to 8 s (mean actual minus mean mismatched Z = −2.43) and significant positive correlation at time shifts of 2.25 s to 4.25 s (mean actual minus mean mismatched Z = 2.47). QPP strength over time was significantly correlated with high-frequency BLP (P ≤ 0.00307, SGoF corrected); there was significant positive correlation at time shifts of 3.25 s to 5.25 s (mean actual minus mean mismatched Z = 1.04) and significant negative correlation at time shifts of −9 s to −7.75 s and 6.75 s to 8.75 s (mean actual minus mean mismatched Z = −0.872). SWC generated from BOLD was significantly correlated with SWC generated from high-frequency BLP (P ≤ 0.0434, SGoF corrected); there was significant positive correlation at time shifts of −5.25 s to 9.75 s (mean actual minus mean mismatched Z = 6.95), but no significant negative correlation. There was no significant correlation between SWC generated from infraslow LFP and SWC generated from BOLD. These results are shown in Fig. 3.
Fig. 3.
Same as Fig. 2, but for signals recorded under a high dose of isoflurane (n = 17 scans). There is a significant positive correlation relationship between SWC derived from rsfMRI and SWC derived from high-frequency BLP. There are also significant positive and negative correlations between the change in QPP strength over time and both the infraslow LFP and the high-frequency BLP. Note that, for QPP, time shifts are arbitrary, and positive and negative correlations are expected (see Significance of QPP correlation vs. time shift in materials and methods). Partial correlation results had no statistically significant differences and appeared identical when plotted.
Under dexmedetomidine, QPP strength over time was significantly correlated with infraslow LFP (P ≤ 0.0167, SGoF corrected), there was significant negative correlation at time shifts of −4.75 s to −3.25 s, 0.5 s to 2 s, and 5.75 s to 6.75 s (mean actual minus mean mismatched Z = −1.49) and significant positive correlation at time shifts of −6.25 s, −2.25 s to −0.25 s, 3.25 s to 4.25 s and 7.75 s to 9.25 s (mean actual minus mean mismatched Z = 1.53). SWC generated from BOLD significantly correlated with SWC generated from high-frequency BLP (P ≤ 0.0165); there was significant positive correlation at time shifts of −8 s to 10 s (mean actual minus mean mismatched Z = 6.51) but no significant negative correlation. There was no significant correlation between QPP strength over time and high-frequency BLP, nor between SWC generated from infraslow LFP and SWC generated from BOLD. These results are shown in Fig. 4.
These results suggest that, under dexmedetomidine, QPP relate primarily to infraslow frequencies in electrical signals, while SWC relate primarily to power changes in high frequencies. This effect is modulated by anesthesia, however, as under isoflurane QPP relate to power changes in high frequencies as well.
Seven of twelve statistical families show significant results, indicating a chance of only 1.6 × 10−6 % that the presented results are a false positive (based on a binomial distribution of 12 trials, 5% chance of false positive per trial). This strong significance confirms previous observations of the relationship between dynamic rsfMRI and neural electrophysiology (Keilholz 2014).
Partial correlation results.
No t-tests between regular and partial correlation Z values produced P values lower than 0.988. Partial correlation Z values were nearly identical to regular correlation Z values, differing on average only by Z = 0.0043 and at most by Z = 0.0828 across all equivalent partial vs. regular correlation values. This result suggests that there is no linear influence from one of the types of dynamic rsfMRI upon the other, when the other is correlated to its analog in the neural signal.
Peak time shifts.
Table 1 shows the range of time shifts where high-frequency BLP-derived SWC was significantly correlated with rsfMRI-derived SWC, as well as peak correlation time locations. Nonsignificant comparisons are not shown. While the range is very broad (significance covers >10 s of shifts), it is biased toward positive time shifts and has a peak at a slightly longer time shift under isoflurane (2.75 s) vs. dexmedetomidine (2.25 s).
Table 1.
Time shift ranges for significance for sliding window correlation
| Anesthesia | Frequency Band | Range Start | Range End | Peak Location |
|---|---|---|---|---|
| High-dose isoflurane | High frequency | −5.25 | 10 | 2.75 |
| Dexmedeotomidine | High frequency | −8 | 10 | 2.25 |
Columns indicate the anesthetic state, frequency band for electrophysiology, start and end time shifts for significance, and the time shift where the peak Z value occurred. Rows are individual comparisons (i.e., plots in Figs. 2–4); only significant rows are shown. The range is very large for both significant cases, and biased toward positive time shifts, indicating that events in the electrical signal come before equivalent events in the resting state functional magnetic resonance imaging (rsfMRI) signal. The peak time shift under isoflurane is slightly greater, agreeing with previous studies of static rsfMRI and electrophysiology (Pan et al. 2013).
Table 2 shows the time shift of the largest significant positive correlation between QPP and infraslow LFP, the time shift of the nearest significant negative peak correlation, as well as the difference between the two. Nonsignificant comparisons are not shown. The distance between peaks increases as anesthetic depth increases, with 2.25 s for dexmedetomidine, 3.25 s for low-dose isoflurane and 3.5–3.75 s for high-dose isoflurane.
Table 2.
Time shifts between peaks for significance for QPP strength
| Anesthesia | Frequency Band | Positive Peak | Nearest Negative Peak | Difference |
|---|---|---|---|---|
| Low-dose isoflurane | High frequency | N/A | 5 | N/A |
| Low-dose isoflurane | Infraslow | 1.75 | −1.5 | 3.25 |
| High-dose isoflurane | High frequency | 4.25 | 7.75 | 3.5 |
| High-dose isoflurane | Infraslow | 3.25 | 7 | 3.75 |
| Dexmedetomidine | Infraslow | −1.5 | −4 | 2.5 |
Columns indicate the anesthetic state, frequency band for electrophysiology, maximum significant positive peak, nearest significant negative peak to it, and the difference in time shifts between them. Rows are individual comparisons (i.e., plots in Figs. 2–4); only significant rows are shown. As anesthetic depth increases from dexmedetomidine to low-dose isoflurane to high-dose isoflurane, the difference in time shift between the peaks increases. This suggests that quasi-periodic pattern (QPP) rate decreases as anesthetic depth increases, agreeing with previous studies (Thompson et al. 2013c), but adds low-dose isoflurane as a middle point between high-dose isoflurane and dexmedetomidine. N/A, not applicable.
Results without data exclusion.
To ensure that the exclusion of scans (due to rapid signal changes observed in SWC generated from high-frequency BLP) did not affect results, all tests were run without these exclusions. All statistical families that showed significance for the regular study also were significant without the data exclusion, except that, under a low dose of isoflurane, QPP did not significantly correlate with high-frequency BLP (not passing SGoF as minimum P = 0.0125 and only 6 of 81 P values are <0.05; with exclusion the same test passed SGoF with minimum P = 0.0121 and 9 of 81 P values <0.05). As low-dose isoflurane had the fewest scans of any anesthetic agent (14 before exclusion, vs. 17 or 46 for the other anesthetic agents), it had the least statistical power and thus was most susceptible to lowered data quality. However, all other results being the same suggest that these exclusions did not influence our conclusions.
DISCUSSION
Summary of results.
Using simultaneously recorded rsfMRI and neural electrophysiology data, we compared two types of dynamic rsfMRI patterns and two different frequency bands of electrical activity. This study presents a more direct comparison between the electrophysiological correlates of dynamic rsfMRI patterns than has been previously possible due to limitations of previous analyses (Thompson et al. 2013b, 2013c). Our results showed that SWC significantly correlated only to the high-frequency BLP, except under a low dose of isoflurane, whereas QPP strength over time significantly correlated to the infraslow LFP in all cases. The QPP strength over time also related to the high-frequency BLPs, but only under isoflurane anesthesia. In addition, partial correlation results suggested that there was no influence from SWC on QPP correlations, or vice versa.
These findings suggest that the two different types of dynamic rsfMRI patterns reflect different underlying neurophysiological sources. SWC appears more closely linked to high-frequency neural activity, whereas QPPs appear more closely linked to the very-low-frequency neural activity. As SWC in this study was generated only from two small brain regions, whereas QPPs were generated from the entire slice, this result supports the general idea in neuroscience that high frequencies tend to be more local, while low frequencies tend to be more global (Buzsaáki 2006). This idea is also supported by mathematical models of the LFP signal, which indicate that cutoff distance decreases as frequency increases (Leski et al. 2013).
Our group has previously shown these relationships individually, comparing SWC to BLP of high-frequency neural signals (Thompson et al. 2013b) and QPPs to the infraslow neural signal (Thompson et al. 2013c). However, these previous studies did not investigate SWC vs. low-frequency electrophysiology nor QPP vs. high-frequency electrophysiology. For the former, the fact that LFP data under 0.1 Hz was unavailable precluded comparison between SWC and infraslow neural activity. For the latter, the nonobservation of QPPs in correlates of the high-frequency BLP in a prior study (Pan et al. 2013) led us to focus solely on the QPP-infraslow relationship. The present study fills those gaps and helps explain why QPPs were not observed in correlates of high-frequency BLP in Pan et al. (2013). The observation that different ways of measuring dynamic rsfMRI may reflect different underlying processes is an important consideration for dynamic rsfMRI studies moving forward.
Correlation strengths and time shifts.
Correlation between rsfMRI-derived SWC and high-frequency BLP-derived SWC had the highest mean Z values observed in this study, and these Z values were comparable between both of the anesthetic agents where significance was observed (compare Figs. 3 and 4, top right). It only included positive correlations, indicating that increases in correlation in the rsfMRI signal correspond to increases in correlation in the electrical activity, as has been observed previously by our group (Thompson et al. 2013b). The time shifts where this correlation was significant trended positive, indicating that, as with direct correlation of static signals, the changes in correlation seen in rsfMRI come after the equivalent changes in correlation seen in the high-frequency BLP (Magri et al. 2012; Pan et al. 2011; Shmuel and Leopold 2008). However, compared with those studies, the range of significant positive correlation observed here was very broad. This was likely because the window used for correlation in the present study was much smaller (50 s vs. 300 s or longer), making estimation of the time lag difficult. This suggests that SWC, while useful for dynamic analysis, may not be effective at estimating rsfMRI vs. electrophysiology time lags. The peak time shift was slightly greater under isoflurane (Table 1), indicating that, also similar to results seen using static correlation, the electrophysiology-to-functional magnetic resonance imaging time lag under dexmedetomidine is lower than under isoflurane (Pan et al. 2013).
Correlation between QPP strength and infraslow LFP had lower mean Z values than SWC correlations, but was still statistically significant (Figs. 2–4, bottom left). The plot of correlation vs. time shift resembled an autocorrelation plot in the sense that there were both significant positive and negative correlations. This suggests that the QPP strength over time is periodic and correlates with an electrical signal that has the same period, as has been observed previously by our group (Thompson et al. 2013c). Correlation between QPP strength and high-frequency BLP was only significant under isoflurane and had lower mean Z values than vs. infraslow LFP, but otherwise followed a similar pattern vs. time shift (Figs. 2 and 3, bottom right). The particular time shifts of significance in these plots differ because, first, all QPP templates were aligned to the first rat, first run (per anesthesia) which was at an arbitrary phase (Majeed et al. 2011), and, second, each anesthetic state was aligned separately, as anesthetic state will influence QPP templates (Magnuson et al. 2014b; Majeed et al. 2009, 2011; Thompson et al. 2013c). However, the distance between peak time shifts was informative. As anesthetic depth increases, so does the difference between the largest significant positive peak and the closest significant negative peak to it (Table 2). As these peaks likely approximate an autocorrelation plot (which we lack the SNR to fully resolve), the decreased distance indicates higher frequency, and thus as anesthetic depth increases the frequency of QPP decrease. This is an identical result to our previous result comparing only high-dose isoflurane to dexmedetomidine (Thompson et al. 2013c) with the addition of low-dose isoflurane as a middle point between dexmedetomidine and high-dose isoflurane.
While not done here, future work could attempt to determine the “start point” of QPP templates in relation to neural activity. Similar patterns, referred to as “coactivation patterns” (CAPs), are produced by an algorithm which uses signal peaks (Liu and Duyn 2013). As it is possible that the CAP and QPP algorithms are returning some of the same patterns, a combination of these algorithms could hypothetically be used to find such a start point. Doing this would be beyond the scope of the present study.
Dynamic rsfMRI modulated by anesthetic agent.
Anesthesia modulates the relationship between dynamic rsfMRI and electrophysiology, as under isoflurane QPPs become correlated with the high-frequency BLP. There are several possible explanations for this. First, high doses of isoflurane induce a burst state, which means that all neural activity alternates between a “low” state where there is little activity and a “high,” activated state (see Appendix A of Thompson et al. 2013c). In our previous work, we used the burst state to explain observing correlation between high and low neural frequencies as they related to rsfMRI in only a single case under isoflurane (Thompson et al. 2014). This is because during a low state most activity is suspended simultaneously and thus would correlate whether or not it would have correlated had there been no bursting. However, in the present study, our data includes low-dose isoflurane where bursting was not frequently observed. Therefore, the artificial correlations created due to bursting may not tell the entire story.
A second possible explanation is that isoflurane is a very different anesthetic from dexmedetomidine. Isoflurane is a powerful vasodilator and very strong anesthetic, whereas dexmedetomidine has more of a sedative effect and is a vasoconstrictor (see Appendix A of Thompson et al. 2013c). Such changes in vascular tone alter the frequency of vasomotor waves which likely alters QPP power (Kiviniemi et al. 2005; Masamoto and Kanno 2012). Hypothetically related to the vasodilation it causes, isoflurane has been shown to increase the correlations seen in rsfMRI in a dose-dependent manner (Liu et al. 2012). As the rsfMRI signal is a surrogate measure that does not measure the underlying electrical potentials, large-scale vasodilation could periodically obscure and reveal all activity and thus create correlations between any two factors that might be measured. This is especially likely to obscure results on the infraslow time scale (Mayhew et al. 1996).
A less likely possibility is that QPPs are indeed correlated with high-frequency activity under dexmedetomidine, but that our study was not able to detect the link. This could be due to insufficient statistical power (which is unlikely, as the most scans were from dexmedetomidine, 35 scans after exclusion) or because the wrong frequency band was used for either QPP or LFP [also unlikely, as the frequency bands used here were chosen empirically based on our group's previous work (Thompson et al. 2013c)].
Additionally, correlation was not seen between SWC and high-frequency electrical activity under a low dose of isoflurane, unlike the other two anesthetic conditions. This is unusual, as the low dose of isoflurane otherwise appears as intermediate between the strongly anesthetized high-dose isoflurane and the lightly anesthetized dexmedetomidine. This may potentially be due to lack of statistical power, as the fewest scans were performed under the low dose of isoflurane (only 12 scans after exclusion).
Limitations.
While partial correlation was used in this study to control for the effects of one type of dynamic rsfMRI upon the other, it was not a direct comparison as SWC was used to control against QPP's relationship with the electrical signal directly, and QPP strength directly was used to control against the rsfMRI signal's SWC vs. the electrical signal's SWC. This limits us to being able to claim that the SWC signal does not affect the relationship between QPP and original (not SWC) electrical signals, and the QPP does not affect the relationship between SWC generated from electrical signals and SWC generated from BOLD. While our results indicate no direct effect between SWC and QPP, it is probable that there is a more sophisticated relationship between the two, at least because QPP tend to be bilateral (Majeed et al. 2009), and here we calculated SWC from bilateral brain regions. In a study by Ko et al. (2011), electrocorticography from humans undergoing surgery for epilepsy showed quasi-periodic fluctuations at infraslow frequencies (∼0.017 Hz) in BLP calculated from high-frequency gamma (70–120 Hz) in the default mode network. The results of Ko et al. indicate that a link between the QPP and SWC observed here may be possible. Thus, further work is needed to better separate SWC and QPP and determine which parts of the rsfMRI signal come from one or both of them.
The use of anesthesia in this study was a necessary limitation due to the combination of rsfMRI and LFP, along with fragile electrodes which needed to be implanted acutely. However, SWC has been often observed in awake humans (Allen et al. 2014; Chang and Glover 2010; Hutchison et al. 2013; Sakoglu et al. 2010; Thompson et al. 2013a) and has been correlated with BLP in the alpha-, beta- and gamma-bands from EEG (Chang et al. 2013; Tagliazucchi et al. 2012). QPPs have also been observed in awake humans (Majeed et al. 2011), and similar patterns including CAPs have been observed in awake humans (Liu and Duyn 2013) and awake rats (Liang et al. 2015). In particular, the patterns seen in the study by Liang et al. are very similar to those found here. Preliminary research indicates that a low-frequency electrical basis of QPPs may be observable in humans as well (Korhonen et al. 2014). The ubiquity of QPPs, CAPs and SWC in awake and anesthetized humans and rodents suggests the broader applicability of these results.
It is important to note that the results presented here are by no means exhaustive. Methods of generating dynamic activity, such as using wavelets (Chang and Glover 2010), partial least squares (Grigg and Grady 2010) and event-based (Magri et al. 2012) or amplitude-based [including CAPs (Liu and Duyn 2013; Petridou et al. 2013)] averaging methods were not used here and may have different neural correlates. QPP themselves also can be compared with neural activity in many ways other than strength over time; some of these include examining template properties (Magnuson et al. 2014a; Thompson et al. 2013c), occurrence vs. nonoccurrence (Magnuson et al. 2014b) and phase or power envelope strength rather than raw strength values. Also, to limit our scope, only frequency bands that had shown positive results in our previous studies were used for LFP analysis. For a discussion of how these bands were generated and how per-anesthesia differences may have affected results, see the original papers (Thompson et al. 2013b, 2013c). With a larger dataset, measurement of more physiological factors to reduce noise, and EEG data from human subjects, a much more comprehensive analysis could be performed in the future. Furthermore, interventions can be performed to begin to determine the causal relationships governing neural activity, neurophysiology and dynamic rsfMRI patterns. For example, the intrahemispheric patterns of the QPPs are preserved after the corpus callosum is severed, but the interhemispheric coordination of these patterns is lost (Magnuson et al. 2014a).
Dynamic rsfMRI, behavior and disease.
As rsfMRI has been shown to be altered in many neuropsychological diseases (van den Heuvel and Hulshoff Pol 2010), it is possible that the link between dynamic rsfMRI and the underlying electrical potentials could be exploited to better diagnose and treat diseases. While this is currently very speculative, some evidence indicates that this link may be possible. For example, EEG at gamma frequencies is altered in schizophrenia (Cho et al. 2006), gamma frequencies correlate with SWC in invasive recordings in rats (Thompson et al. 2013b) and SWC is more diagnostic of schizophrenia than correlation calculated using entire rsfMRI runs (Sakoglu et al. 2010). Conversely, infraslow EEG potentials have been linked to attention deficit disorder (Helps et al. 2008, 2010), QPPs in humans linked to the default mode network (Majeed et al. 2011) and default mode network regions linked to attention deficit disorder as well (Tian et al. 2006).
If it is the case that certain types of dynamic rsfMRI are more diagnostic to certain diseases than others, then it would be advantageous to minimize the contribution from the nondiagnostic dynamic patterns. QPPs can be removed from a signal using regression, and leaving the rest of the functional connectivity intact (Majeed 2010). The algorithm which generates QPP templates removes much of the local SWC through repeated averaging (Majeed et al. 2011), and other possibilities exist to focus on local rsfMRI. For example, separate maps of long-range connections and short-range connections can be generated (Tomasi and Volkow 2010), and these could be hypothetically used to bias data toward long- or short-range dynamics.
Conclusion.
There are many different ways to measure dynamic spatial and temporal changes in rsfMRI, and this study shows that at least two of them relate to different frequency bands of the electrical signal. The relationship is altered by anesthesia, but some correlations are preserved across the very different anesthetic states that were examined. While our data are limited by being recorded under anesthesia, the observation of similar dynamics in awake rats and humans (Chang and Glover 2010; Liang et al. 2015; Majeed et al. 2011) suggest these results may have broader application. Understanding which type of dynamic pattern present in rsfMRI relates to a given type of underlying neural activity could have broad application in the diagnosis and treatment of disease. Researchers could potentially remove unwanted contributions to focus their analysis on the dynamics that most relate to the disease or dysfunction of interest.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grants 1R21-NS-072810-01A1, 1R21-NS-057718-01A2, and 1R01-NS-078095-01A1, and the Scholarly Inquiry and Research at Emory (SIRE) Fellowship Program at Emory University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.J.T. and S.D.K. conception and design of research; G.J.T. analyzed data; G.J.T. and S.D.K. interpreted results of experiments; G.J.T. prepared figures; G.J.T. drafted manuscript; G.J.T., W.-J.P., and S.D.K. edited and revised manuscript; G.J.T., W.-J.P., and S.D.K. approved final version of manuscript; W.-J.P. performed experiments.
ACKNOWLEDGMENTS
G. J. Thompson is now working at Dr. Fahmeed Hyder's laboratory in the Department of Diagnostic Radiology at Yale University; however, this work was done at his former affiliation, shown above. The authors thank Jacob Billings, Joshua Grooms and Sadia Shakil for input into the laboratory's projects, Steven Marzec for necessary IT help, and Wendy Hu for proofreading. The authors also thank Dieter Jaeger, Jeremy Edgerton, and Collin Lobb for help in initial setup of electrophysiology experiments.
REFERENCES
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24: 663–676, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34: 537–541, 1995. [DOI] [PubMed] [Google Scholar]
- Buzsaáki G. Rhythms of the Brain. Oxford, UK: Oxford University Press, 2006, p. xiv, 448. [Google Scholar]
- Carvajal-Rodriguez A, de Una-Alvarez J, Rolan-Alvarez E. A new multitest correction (SGoF) that increases its statistical power when increasing the number of tests. BMC Bioinformatics 10: 209, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 50: 81–98, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Liu Z, Chen MC, Liu X, Duyn JH. EEG correlates of time-varying BOLD functional connectivity. Neuroimage 72: 227–236, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A 103: 19878–19883, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 101: 4637–4642, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg O, Grady CL. Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS One 5: e13311, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helps S, James C, Debener S, Karl A, Sonuga-Barke EJ. Very low frequency EEG oscillations and the resting brain in young adults: a preliminary study of localisation, stability and association with symptoms of inattention. J Neural Transm 115: 279–285, 2008. [DOI] [PubMed] [Google Scholar]
- Helps SK, Broyd SJ, James CJ, Karl A, Chen W, Sonuga-Barke EJ. Altered spontaneous low frequency brain activity in attention deficit/hyperactivity disorder. Brain Res 1322: 134–143, 2010. [DOI] [PubMed] [Google Scholar]
- Hiltunen T, Kantola J, Abou Elseoud A, Lepola P, Suominen K, Starck T, Nikkinen J, Remes J, Tervonen O, Palva S, Kiviniemi V, Palva JM. Infra-slow EEG fluctuations are correlated with resting-state network dynamics in fMRI. J Neurosci 34: 356–362, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp 34: 2154–2177, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Rothman DL. Quantitative fMRI and oxidative neuroenergetics. Neuroimage 62: 985–994, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz S, Magnuson ME, Pan WJ, Willis M, Thompson G. Dynamic properties of functional connectivity in the rodent. Brain Connect 3: 31–40, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz SD. The neural basis of time-varying resting state functional connectivity. Brain Connect 4: 769–779, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V, Vire T, Remes J, Elseoud AA, Starck T, Tervonen O, Nikkinen J. A sliding time-window ICA reveals spatial variability of the default mode network in time. Brain Connect 1: 339–347, 2011. [DOI] [PubMed] [Google Scholar]
- Kiviniemi VJ, Haanpaa H, Kantola JH, Jauhiainen J, Vainionpaa V, Alahuhta S, Tervonen O. Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Magn Reson Imaging 23: 531–537, 2005. [DOI] [PubMed] [Google Scholar]
- Ko AL, Darvas F, Poliakov A, Ojemann J, Sorensen LB. Quasi-periodic fluctuations in default mode network electrophysiology. J Neurosci 31: 11728–11732, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen V, Hiltunen T, Myllyla T, Wang X, Kantola J, Nikkinen J, Zang YF, LeVan P, Kiviniemi V. Synchronous multiscale neuroimaging environment for critically sampled physiological analysis of brain function: hepta-scan concept. Brain Connect 4: 677–689, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leski S, Linden H, Tetzlaff T, Pettersen KH, Einevoll GT. Frequency dependence of signal power and spatial reach of the local field potential. PLoS Comput Biol 9: e1003137, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Liu X, Zhang N. Dynamic resting state functional connectivity in awake and anesthetized rodents. Neuroimage 104: 89–99, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Duyn JH. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc Natl Acad Sci U S A 110: 4392–4397, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu XH, Zhang Y, Chen W. The change of functional connectivity specificity in rats under various anesthesia levels and its neural origin. Brain Topogr 26: 363–377, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature 453: 869–878, 2008. [DOI] [PubMed] [Google Scholar]
- Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci U S A 104: 18265–18269, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson ME, Thompson GJ, Pan WJ, Keilholz SD. Effects of severing the corpus callosum on electrical and BOLD functional connectivity and spontaneous dynamic activity in the rat brain. Brain Connect 4: 15–29, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson ME, Thompson GJ, Pan WJ, Keilholz SD. Time-dependent effects of dexmedetomidine on functional connectivity and frequency and spatial distribution of spontaneous BOLD fluctuations. NMR Biomed 27: 291–303, 2014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson ME, Thompson GJ, Schwarb H, Pan WJ, McKinley A, Schumacher EH, Keilholz SD. Errors on interrupter tasks presented during spatial and verbal working memory performance are linearly linked to large-scale functional network connectivity in high temporal resolution resting state fMRI. Brain Imaging Behav. In press, 2015. [DOI] [PubMed] [Google Scholar]
- Magri C, Schridde U, Murayama Y, Panzeri S, Logothetis NK. The amplitude and timing of the BOLD signal reflects the relationship between local field potential power at different frequencies. J Neurosci 32: 1395–1407, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed W. Spatiotemporal dynamics of low frequency fluctuations in BOLD fMRI. In: Bioengineering. Atlanta, GA: Georgia Institute of Technology, 2010, p. 106. [Google Scholar]
- Majeed W, Magnuson M, Hasenkamp W, Schwarb H, Schumacher EH, Barsalou L, Keilholz SD. Spatiotemporal dynamics of low frequency BOLD fluctuations in rats and humans. Neuroimage 54: 1140–1150, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed W, Magnuson M, Keilholz SD. Spatiotemporal dynamics of low frequency fluctuations in BOLD fMRI of the rat. J Magn Reson Imaging 30: 384–393, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Kanno I. Anesthesia and the quantitative evaluation of neurovascular coupling. J Cereb Blood Flow Metab 32: 1233–1247, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew JE, Askew S, Zheng Y, Porrill J, Westby GW, Redgrave P, Rector DM, Harper RM. Cerebral vasomotion: a 0.1-Hz oscillation in reflected light imaging of neural activity. Neuroimage 4: 183–193, 1996. [DOI] [PubMed] [Google Scholar]
- Monto S, Palva S, Voipio J, Palva JM. Very slow EEG fluctuations predict the dynamics of stimulus detection and oscillation amplitudes in humans. J Neurosci 28: 8268–8272, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Thompson G, Magnuson M, Majeed W, Jaeger D, Keilholz S. Broadband local field potentials correlate with spontaneous fluctuations in functional magnetic resonance imaging signals in the rat somatosensory cortex under isoflurane anesthesia. Brain Connect 1: 119–131, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Thompson G, Magnuson M, Majeed W, Jaeger D, Keilholz S. Simultaneous FMRI and electrophysiology in the rodent brain. J Vis Exp 42: e1901, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Thompson GJ, Magnuson ME, Jaeger D, Keilholz S. Infraslow LFP correlates to resting-state fMRI BOLD signals. Neuroimage 74C: 288–297, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam: Elsevier, 2005. [Google Scholar]
- Petridou N, Gaudes CC, Dryden IL, Francis ST, Gowland PA. Periods of rest in fMRI contain individual spontaneous events which are related to slowly fluctuating spontaneous activity. Hum Brain Mapp 34: 1319–1329, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The restless brain. Brain Connect 1: 3–12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A 98: 676–682, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp 26: 231–239, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoglu U, Pearlson GD, Kiehl KA, Wang YM, Michael AM, Calhoun VD. A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. MAGMA 23: 351–366, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A 107: 10238–10243, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest. Hum Brain Mapp 29: 751–761, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, von Wegner F, Morzelewski A, Brodbeck V, Laufs H. Dynamic BOLD functional connectivity in humans and its electrophysiological correlates. Front Hum Neurosci 6: 339, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron 65: 280–290, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Magnuson ME, Merritt MD, Schwarb H, Pan WJ, McKinley A, Tripp LD, Schumacher EH, Keilholz SD. Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually. Hum Brain Mapp 34: 3280–3298, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Merritt MD, Pan WJ, Magnuson ME, Grooms JK, Jaeger D, Keilholz SD. Neural correlates of time-varying functional connectivity in the rat. Neuroimage 83C: 826–836, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Pan WJ, Billings JC, Grooms JK, Shakil S, Jaeger D, Keilholz SD. Phase-amplitude coupling and infraslow (<1 Hz) frequencies in the rat brain: relationship to resting state fMRI. Front Integr Neurosci 8: 41, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Pan WJ, Magnuson ME, Jaeger D, Keilholz SD. Quasi-periodic patterns (QPP): Large-scale dynamics in resting state fMRI that correlate with local infraslow electrical activity. Neuroimage 84C: 1018–1031, 2013c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, Sui M, Cao Q, Hu S, Peng M, Zhuo Y. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett 400: 39–43, 2006. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional connectivity density mapping. Proc Natl Acad Sci U S A 107: 9885–9890, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. European Neuropsychopharmacol 20: 519–534, 2010. [DOI] [PubMed] [Google Scholar]
- Voipio J, Tallgren P, Heinonen E, Vanhatalo S, Kaila K. Millivolt-scale DC shifts in the human scalp EEG: evidence for a nonneuronal generator. J Neurophysiol 89: 2208–2214, 2003. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci 9: 971–978, 2006. [DOI] [PubMed] [Google Scholar]