Abstract
The purpose of this study was to determine the effect of supplementing the diet of a mouse model of type 2 diabetes with menhaden (fish) oil or daily treatment with resolvin D1 on diabetic neuropathy. The end points evaluated included motor and sensory nerve conduction velocity, thermal sensitivity, innervation of sensory nerves in the cornea and skin, and the retinal ganglion cell complex thickness. Menhaden oil is a natural source for n-3 polyunsaturated fatty acids, which have been shown to have beneficial effects in other diseases. Resolvin D1 is a metabolite of docosahexaenoic acid and is known to have anti-inflammatory and neuroprotective properties. To model type 2 diabetes, mice were fed a high-fat diet for 8 wk followed by a low dosage of streptozotocin. After 8 wk of hyperglycemia, mice in experimental groups were treated for 6 wk with menhaden oil in the diet or daily injections of 1 ng/g body wt resolvin D1. Our findings show that menhaden oil or resolvin D1 did not improve elevated blood glucose, HbA1C, or glucose utilization. Untreated diabetic mice were thermal hypoalgesic, had reduced motor and sensory nerve conduction velocities, had decreased innervation of the cornea and skin, and had thinner retinal ganglion cell complex. These end points were significantly improved with menhaden oil or resolvin D1 treatment. Exogenously, resolvin D1 stimulated neurite outgrowth from primary cultures of dorsal root ganglion neurons from normal mice. These studies suggest that n-3 polyunsaturated fatty acids derived from fish oil could be an effective treatment for diabetic neuropathy.
Keywords: diabetic neuropathy, fish oil, resolvin, epidermal nerve fibers, corneal nerve fibers, type 2 diabetes
n-3 polyunsaturated fatty acids, derived from fish oils, have been shown to affect a myriad of molecular pathways, including alteration of physical and chemical properties of cellular membranes, direct interactions with and modulation of membrane channels and proteins, regulation of gene expression via nuclear receptors and transcription factors, changes in eicosanoid profiles, and conversion of n-3 polyunsaturated fatty acids to bioactive metabolites (Shaikh et al. 2015; Mozaffarian and Wu 2011; Spector and Yorek 1985). In animal models of obesity and diabetes, treatment with n-3 polyunsaturated fatty acids has been shown to reduce inflammation and improve impaired glucose tolerance and hepatic steatosis (Gonzalez-Periz et al. 2009; Figueras et al. 2011; Jelinek et al. 2013; Lamping et al. 2013). Eicosapentaenoic acid has also been shown to have beneficial effects on glycemic indices in patients with type 2 diabetes (Sarbolouki et al. 2013). We have shown that enriching diets with menhaden oil of type 1 or type 2 diabetic rats improved diabetic neuropathy (Coppey et al. 2012, 2015).
Many of the effects of fish oil supplements have been attributed to the production of their metabolites, which include resolvins and neuroprotectin D1. Each of these metabolites have anti-oxidant, anti-inflammatory, and neuroprotective properties (Ariel and Serhan 2007; Kohli and Levy 2009). Resolvins are oxygenated metabolites of eicosapentaenoic acid (E series resolvins) and docosahexaenoic acid (D series resolvins). It has been shown that regeneration of corneal nerves damaged by refractive surgery can be increased with treatment of docosahexaenoic acid through synthesis of neuroprotectin D1 (Cortina et al. 2010; Gordon and Bazan 2013). It was also reported that neuroprotectin D1 increases neurite outgrowth by trigeminal ganglia neurons from Swiss Webster mice (Cortina et al. 2010). Robson et al. (2010) reported that n-3 fatty acids promote neurite outgrowth by dorsal root ganglia (DRG) neurons and the effect of docosahexaenoic acid was still prominent in aged tissue. To further explore the benefits of dietary n-3 polyunsaturated fatty acid enrichment and resolvins on diabetic neuropathy, we performed a preclinical study using a mouse model of type 2 diabetes and an intervention protocol treating diabetic mice with a diet enriched with menhaden oil or with a daily injection of resolvin D1.
MATERIALS AND METHODS
Materials.
Unless stated otherwise all chemicals used in these studies were obtained from Sigma-Aldrich (St. Louis, MO).
Animals.
C57Bl/6J mice were purchased from Jackson Laboratories. Mice were housed in a certified animal care facility and standard diet and water were provided ad libitum. Measures were taken to minimize pain or discomfort and all experiments were conducted in accordance with international standards on animal welfare and were compliant with all institutional and National Institutes of Health guidelines for the use of animals (Institutional Animal Care and Use Committee Approval No. 1212258). C57Bl/6J mice at 12 wk of age were divided into four groups. After 1 wk on a standard diet (3.0 kcal/g, 13% kcal fat, 7001; Harlan Teklad, Madison, WI), three of the groups were fed a high-fat diet (5.2 kcal/g, 60% kcal fat, D12492; Research Diets, New Brunswick, NJ). To create the type 2 diabetic model, after 8 wk on the high-fat diet mice were treated with 75 mg/kg streptozotocin (EMD Chemicals, San Diego, CA) followed 3 days later with a second dose of streptozotocin (50 mg/kg) as previously described (Yorek et al. 2015). Based on the literature duration of the high-fat diet, total dosage of streptozotocin and age and strain of mice are all variables in the creation of this “type 2 diabetic mouse model.” Individual laboratories need to determine the best combination based on their own experimentation. Mice with blood glucose ≥13.8 mM (250 mg/dl) were considered diabetic (Accu-Chek; Roche, Indianapolis, IN). After 8 wk of hyperglycemia, one group of mice was continued on the high-fat diet (untreated diabetic group). A second group was fed a high-fat diet with one-half of the kilocalories derived from lard in the high-fat diet replaced with menhaden oil (menhaden oil-treated group). The third group remained on the high-fat diet and was treated with resolvin D1 (Cayman Chemical, Ann Arbor, MI) at 1 ng/g body wt (0.4% ethanol vehicle, daily ip injections; resolvin-treated group). The final group served as the control group and was fed the standard diet for the duration of the study. The treatment phase lasted for 6 wk. Fatty acid composition of the standard diet (Harlan Teklad 7001), high-fat diet (Research Diets D12492), and the custom-prepared menhaden oil supplemented high-fat diet (Research Diets) is provided in Table 1. To summarize the four groups of mice used in this study: control mice received a standard diet with no additional lard and were not treated with streptozotocin; untreated diabetic mice were treated with a high-fat diet (8-wk lard) followed by streptozotocin and continued high-fat diet (total 14 wk); menhaden oil-treated diabetic mice were treated with a high-fat diet (8 wk lard) followed by streptozotocin and 8-wk high-fat diet (lard) and then for 6 wk with a high-fat diet consisting of 50% lard and 50% menhaden oil; and resolvin D1-treated diabetic mice were treated with a high-fat diet (8 wk lard) followed by streptozotocin and continued high-fat diet (14 wk) and for the final 6 wk treated with exogenous resolvin D1.
Table 1.
Fatty acid %composition of diets measured by gas-liquid chromatography
| Diet | 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 20:5 | 22:6 |
|---|---|---|---|---|---|---|---|
| Control (3) | 15 ± 2 | 2 ± 1 | 6 ± 1 | 21 ± 3 | 47 ± 4 | 1 ± 1 | < 1 |
| High fat (3) | 17 ± 2 | 2 ± 1 | 10 ± 2 | 37 ± 4 | 29 ± 4 | < 1 | < 1 |
| Menhaden oil-enriched high fat (3) | 18 ± 2 | 8 ± 1 | 6 ± 1 | 21 ± 3 | 15 ± 2 | 9 ± 1 | 8 ± 1 |
Data are presented as the means ± SE. Parentheses indicate the number of experimental determinations. 16:0, palmitic acid; 16:1, palmitoleic acid; 18:0, stearic acid; 18:1, oleic acid; 18:2, linoleic acid; 20:5, eicosapentaenoic acid; 22:6, docosahexaenoic acid.
Glucose clearance.
Intraperitoneal glucose tolerance test was performed after an overnight fast as previously described (Coppey et al. 2011).
Behavioral response.
Thermal nociceptive response in the hindpaw was measured using the Hargreaves method with instrumentation provided by IITC Life Science (San Diego, CA) as previously described (Coppey et al. 2011).
Motor and sensory nerve conduction velocity.
Mice were anesthetized with Nembutal (75 mg/kg ip; Abbott Laboratories, North Chicago, IL) and motor and sensory nerve conduction velocities were determined as previously described (Coppey et al. 2011). Core temperature was monitored using a rectal probe and temperature regulated between 36 and 37°C using a heating pad and radiant heat. This procedure maintained a normal temperature near the sciatic nerve (Coppey et al. 2015).
Retina and cornea imaging.
Spectral domain optical coherence tomography (SD-OCT) analysis was performed to measure the retinal ganglion cell complex thickness using a Spectralis SD-OCT (Heidelberg Engineering, Vista, CA) imaging system coupled with a 25D lens (Heidelberg Engineering), as we have previously described (Yorek et al. 2014). The retinal ganglion cell complex was used because it contains the retinal ganglion cell body, the axons, and the dendrites of the retinal ganglion cell. We have determined that analysis of this region increases the dynamic range of the retina to resolve subtle structural changes (Yorek et al. 2014). Subepithelial corneal nerves were imaged in vivo using the Rostock cornea module of the Heidelberg Retina Tomograph (Heidelberg Engineering) confocal microscope as previously described (Yorek et al. 2014; Davidson et al. 2012a,b). After completion of all in vivo analyses, corneas were dissected from the eyes and corneal nerves penetrating the epithelium were visualized as previously described (Yorek et al. 2014). Analysis of corneal nerve images was completed with Imaris software version 7.6.4 X64 (Bitplane, Zurich, Switzerland).
Intraepidermal nerve fiber density in the hindpaw.
Skin from the footpads was collected and intraepidermal nerve fibers were immunohistochemically stained and counted as previously described (Yorek et al. 2014).
Biological markers and diet composition analysis.
Nonfasting blood glucose was determined using a glucometer (Accu-Chek; Roche). Hemoglobin A1C levels were determined using a Glyco-tek affinity column kit (Helena Laboratories, Beaumont, TX). To examine steatosis, liver samples were frozen in OCT compound (Sakura FineTek, Torrance, CA) in liquid nitrogen. Liver sections (5 μm) were incubated with BODIPY (Molecular Probes, Carlsbad, CA), at a 1:5,000 dilution in 1% BSA for 1 h at room temperature. After being washed, liver sections were mounted using ProLong Gold antifade reagent (Molecular Probes) and covered with a glass coverslip. Images were collected using Zeiss 710 LSM confocal laser scanning microscope. Images were analyzed for percent area fraction of lipid droplets using ImageJ software. Chymotrypsin-like proteasome activity was assayed in liver extracts using 96-well format as described by Otoda et al. (2013). The reaction mixture consisted of 100 μg of liver extract protein and 100 μM of peptide substrate Suc-Leu-Leu-Val-Tyr-AMC in assay buffer containing 50 mM HEPES (pH 7.8), 10 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 250 mM sucrose, 5 mM dithiothreitol, and 2 mM ATP. The proteasome inhibitor MG132 was added at a 20-μM concentration, and purified murine 20S proteasome (Boston Biochem, Cambridge, MA) served as a positive control in each plate. Free AMC fluorescence was measured using a 355/460 nm filter set in FLUOstar Optima microplate reader (BMG Labtech, Cary, NC). The proteasome activity was expressed in units per milligrams of protein with 1 U equal to 1 nmol of AMC released per 1 min. The protein concentration in liver extracts was measured with the bicinchoninic acid protein assay (Thermo Fisher Scientific, Waltham, MA). Serum samples were collected for determination of free fatty acid, triglyceride, free cholesterol, and resolvin D1 using commercial kits from Roche Diagnostics (Mannheim, Germany), Sigma-Aldrich, BioVision (Mountain View, CA), and Cayman Chemical, respectively. Fatty acid composition of the diets was determined by gas-liquid chromatography. Lipids were extracted from diets with a 2:1 (vol/vol) mixture of chloroform and methanol followed by phase separation with a solution of 154 mM NaCl and 4 mM HCl. Fatty acid composition was measured after the lipid fraction was transesterified in 14% boron trifluoride in methanol and the fatty acid methyl esters extracted into heptane before separation by gas-liquid chromatography (Yorek et al. 1984). Individual fatty acids peaks as percentage of total fatty acids present were identified by comparison to known fatty acid standards.
Adult mouse sensory neuron culture.
Adult mouse sensory neurons were isolated from DRG and grown in culture as described in Malin et al. (2007). Twelve-week-old C57Bl/6J mice were anesthetized with Nembutal (75 mg/kg ip; Abbott Laboratories) and euthanized by cervical dislocation. All cervical through lumbar level DRG were dissected aseptically from vertebral column and placed in ice-cold Ca2+/Mg2+ free Hank's balanced salt solution (HBSS; GIBCO, Thermo Fisher Scientific). Enzymatic dissociation was performed by incubating DRG with 40 U/ml papain and 0.7 mg/ml l-cysteine in Ca2+/Mg2+ free HBSS for 20 min at 37°C, followed by incubation with 4 mg/ml collagenase A (Roche Diagnostics) and 2 mg/ml dispase (GIBCO, Thermo Fisher Scientific) in Ca2+/Mg2+ free HBSS for 20 min at 37°C. DRG were triturated in L-15 medium (GIBCO, Thermo Fisher Scientific), and neurons were separated from debris and Schwann cells by centrifugation through a column of 15% fatty acid free bovine serum albumin (Roche Diagnostics) in L-15 as described in Singh et al. (2012). After being washed, cells were gently resuspended in complete F-12 medium (GIBCO, Thermo Fisher Scientific) containing 10 mM glucose, supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. No exogenous growth factors were added at this point. Neurons were plated on poly-d-lysine/laminin Biocoat glass coverslips (Corning, Discovery Labware, Bedford, MA) and were allowed to adhere for 2 h at 37°C and 5% CO2. Coverslips were then flooded with prewarmed complete F-12 medium with or without 50 nM resolvin D1 (Cayman Chemical) and incubated for 24 h.
β-Tubulin fluorescence immunocytochemistry and quantification of neurite outgrowth.
After 24 h the coverslips were washed twice with Ca2+/Mg2+ free 100 mM phosphate-buffered saline (PBS), pH 7.4 and fixed with 4% formaldehyde (Polysciences, Warrington, PA) in 100 mM PBS for 20 min. Nonspecific binding was blocked by 1% bovine serum albumin, 1% normal goat serum, and 0.1% Triton X-100 in 10 mM PBS at room temperature for 30 min. The blocking step was followed by incubation with neuronal class III β-tubulin rabbit polyclonal antibody (1:1,000 working dilution; Covance, Dedham, MA) overnight at 4°C and then with secondary Alexa Fluor 546 conjugated goat anti-rabbit antibody (1:2,000 working dilution; Invitrogen, Eugene, OR) for 2 h at room temperature. Coverslips were mounted on glass slides with ProLong Gold antifade reagent (Life Technologies, Carlsbad, CA). Z-stack images of neuronal cells were taken in steps of 1 μm for a total range of 5–6 μm at ×200 magnification with a Zeiss LSM710 confocal microscope and analyzed with Imaris software (version 7.6.4 X64; Bitplane). Filament tracer module of Imaris package automatically detects cell bodies, tracks neurites in three dimensions, and quantifies the neurite length in micrometers. Cell bodies were excluded from our image analyses, and the total neurite length was normalized by a number of cell bodies in each image thus producing a value of neurite length per neuron. Thirty to forty neurons were analyzed per condition (control with no growth factors added and resolvin-treated cultures) for each mouse, and the average values were used to calculate the group means.
Data analysis.
Results are presented as means ± SE. Comparisons between control and treated DRG neuron cultures were conducted using Student's t-test (Prism software; GraphPad, San Diego, CA). Comparison among control, nontreated, and treated diabetic mice were conducted using one-way ANOVA and Bonferroni posttest comparison (Prism software; GraphPad). P < 0.05 was considered significant.
RESULTS
Effect of dietary treatment with menhaden oil or exogenous resolvin D1 of mice fed a high-fat diet and treated with low dosage of streptozotocin on weight, blood glucose, serum lipid, resolvin D1 levels, liver proteasome activity, and steatosis.
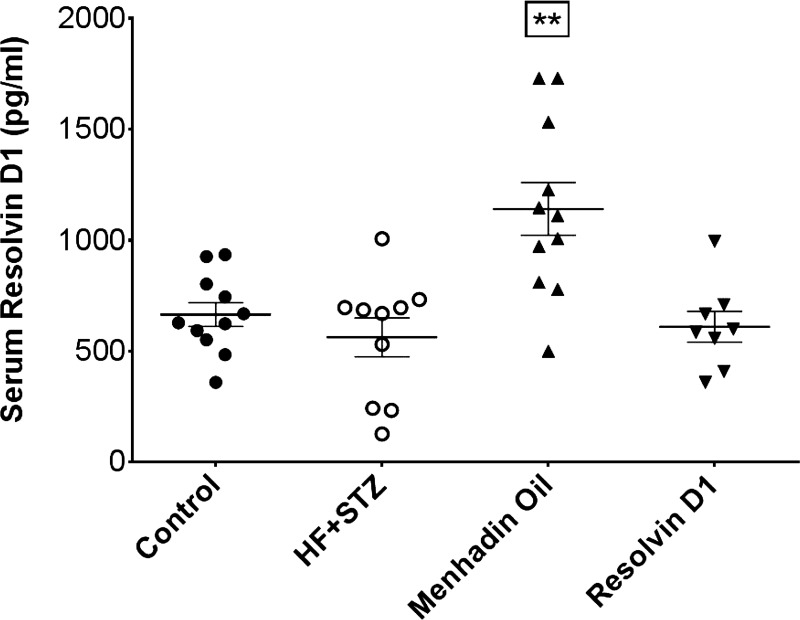
Data in Table 2 demonstrate that diabetic mice at the end of the study weighed significantly more than control mice and nonfasting blood glucose and hemoglobin A1C levels were significantly elevated. Treating diabetic mice with a dietary enrichment of menhaden oil or daily injections of resolvin D1 for the final 6 wk of the experimental period did not affect weight gain and elevated blood glucose levels or hemoglobin A1C levels. Diabetic mice were insulin resistant as shown by impaired glucose clearance, and treating diabetic mice with a dietary enrichment of menhaden oil or with exogenous resolvin D1 did not improve glucose clearance (Fig. 1). Serum triglycerides and free fatty acid levels were similar in control and diabetic mice. However, diabetic mice had a significant increase in serum free cholesterol levels compared with control mice, which was not corrected by treatments. Serum resolvin D1 levels were determined by EIA (Fig. 2). Resolvin D1 levels were significantly increased in serum from diabetic mice treated with a diet enriched with menhaden oil compared with control mice, untreated diabetic mice, and diabetic mice treated exogenously with resolvin D1. Liver proteasome activity was decreased in diabetic mice and was partially improved by dietary enrichment with menhaden oil but not resolvin D1 treatments (Table 2). Decreased liver proteasome activity is a marker for endoplasmic reticulum stress (Otoda et al. 2013). Liver steatosis (fatty liver) was significantly increased in diabetic mice (Table 2). Treating diabetic mice with a diet enriched with menhaden oil and to a greater extent with daily injections of resolvin D1 reduced liver steatosis.
Table 2.
Effect of menhaden oil dietary enrichment or daily treatment with resolvin D1 of diabetic mice on change in body weight, blood glucose, hemoglobin A1C, serum triglycerides, free fatty acids, cholesterol, liver proteasome activity, and steatosis
| Determination | Control (12) | Diabetic (12) | Diabetic + Menhaden Oil (12) | Diabetic + Resolvin D1 (12) |
|---|---|---|---|---|
| Start weight, g | 27.2 ± 0.4 | 26.4 ± 0.5 | 27.2 ± 0.4 | 27.5 ± 0.4 |
| Final weight, g | 28.0 ± 0.3 | 44.3 ± 1.5* | 44.7 ± 1.9* | 46.5 ± 2.4* |
| Blood glucose, mg/dl | 174 ± 8 | 304 ± 15* | 282 ± 16* | 312 ± 28* |
| HbA1C, % | 6.0 ± 0.3 | 8.7 ± 0.4* | 7.9 ± 0.4* | 8.3 ± 0.6* |
| Triglycerides, mg/dl | 82 ± 11 | 86 ± 10 | 100 ± 26 | 105 ± 19 |
| Free fatty acids, mmol/l | 0.37 ± 0.04 | 0.44 ± 0.05 | 0.44 ± 0.09 | 0.40 ± 0.05 |
| Cholesterol, mg/ml | 1.03 ± 0.15 | 1.81 ± 0.19* | 2.19 ± 0.22* | 2.04 ± 0.21* |
| Liver proteasome activity, U/mg protein | 0.334 ± 0.004 | 0.164 ± 0.004* | 0.222 ± 0.010*† | 0.170 ± 0.008* |
| Steatosis, %area | 7.4 ± 0.7 | 33.8 ± 3.1* | 20.5 ± 2.6*† | 10.1 ± 1.1† |
Data are presented as the means ± SE. Parentheses indicate the number of experimental animals.
P < 0.05, compared with control;
P < 0.05, compared with diabetic mice.
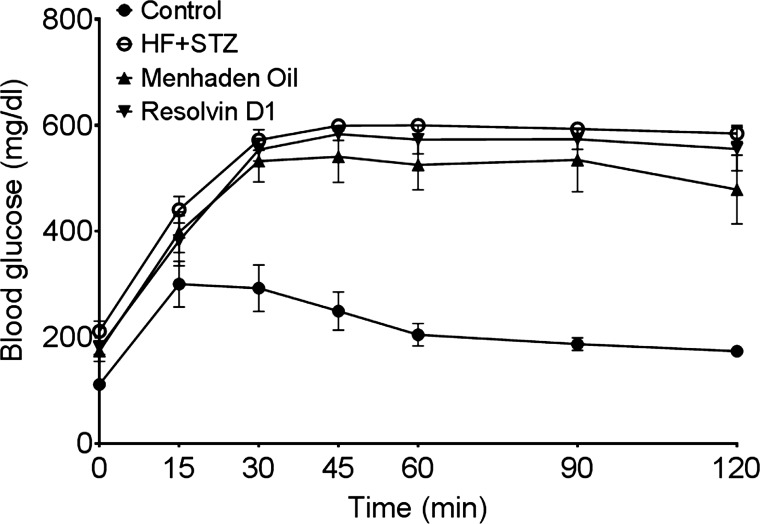
Fig. 1.
Effect of treatment of a mouse model of type 2 diabetes [high fat (HF) + streptozotocin (STZ)] with menhaden oil or resolvin D1 on glucose utilization. Fasting blood glucose at time 0 for control, diabetic mice, and diabetic mice treated with menhaden oil or resolvin D1 was 111 ± 5, 212 ± 19*, 174 ± 19*, and 180 ± 12* mg/dl, respectively (*P < 0.05, compared with control). Data are the means ± SE. The area under the curve was significantly different (P < 0.01) for untreated and treated diabetic mice vs. control. The number of mice in each group was the same as shown in Table 2.
Fig. 2.
Effect of treatment of a mouse model of type 2 diabetes (HF + STZ) with menhaden oil or resolvin D1 on serum resolvin D1 levels. Serum resolvin D1 levels was determined as described in materials and methods. Data are presented as a dot blot including the means ± SE in pg/ml. The number of mice in each group was 11, 10, 11, and 8 for control, HF + STZ, menhaden oil, and resolvin D1, respectively. **P < 0.01, compared with control mice, nontreated diabetic mice and diabetic mice treated with resolvin D1.
Effect of dietary treatment with menhaden oil or exogenous resolvin D1 of mice fed a high-fat diet and treated with low dosage of streptozotocin on nerve conduction velocity, thermal nociception, and intraepidermal nerve fiber density.
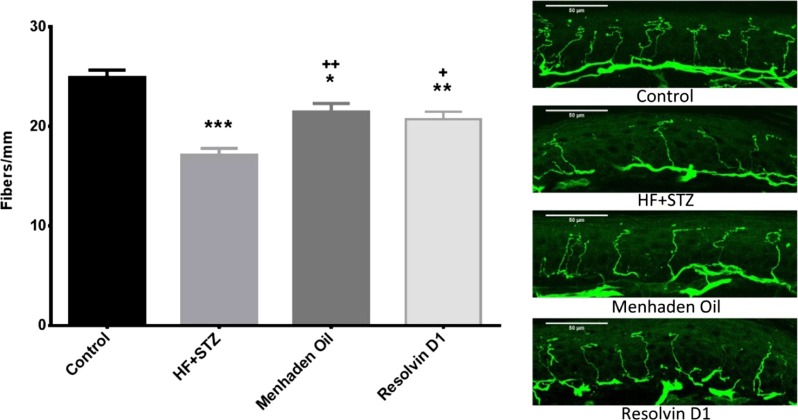
Motor and sensory nerve conduction velocities were significantly decreased in diabetic mice (Table 3). Dietary treatment of diabetic mice with menhaden oil significantly improved motor and sensory nerve conduction velocity, although motor nerve conduction velocity in diabetic mice treated with menhaden oil remained significantly slower compared with control mice. Treating diabetic mice with daily injections of resolvin D1 significantly improved both motor and sensory nerve conduction velocity and there was no statistical difference in nerve conduction velocity between control and diabetic mice treated with resolvin D1. Latency to a thermal stimulus was significantly increased in diabetic mice compared with control mice. Dietary enrichment of menhaden oil or exogenous resolvin D1 treatment reduced the increase in latency to thermal stimulus compared with untreated diabetic mice, such that there was no longer a significant difference compared with controls (Table 3). Intraepidermal nerve fiber density of the skin from the hindpaw was significantly decreased in diabetic mice (Fig. 3). Dietary enrichment with menhaden oil or daily treatments with resolvin D1 for the final 6 wk of the study period at minimum slowed the loss of these nerves, such that intraepidermal nerve fiber density in treated diabetic mice was significantly greater than in untreated diabetic mice. However, treatment for 6 wk did not fully restore intraepidermal nerve fiber density since intraepidermal nerve fiber density in treated mice remained significantly decreased compared with controls.
Table 3.
Effect of menhaden oil dietary enrichment or daily treatment with resolvin D1 of diabetic mice on motor and sensory nerve conduction velocity and thermal nociception
| Determination | Control (12) | Diabetic (12) | Diabetic + Menhaden oil (12) | Diabetic + Resolvin D1 (12) |
|---|---|---|---|---|
| MNCV, m/s | 43.9 ± 1.9 | 23.4 ± 1.0*† | 36.0 ± 1.5*† | 38.4 ± 1.4† |
| SNCV, m/s | 28.5 ± 0.7 | 22.1 ± 0.7* | 28.1 ± 1.0† | 28.9 ± 0.4† |
| Thermal nociception, s | 6.2 ± 0.2 | 9.0 ± 0.4* | 6.9 ± 0.4† | 6.8 ± 0.2† |
Data are presented as the means ± SE. Parentheses indicate the number of experimental animals. MNCV and SNCV, motor and sensory nerve conduction velocity.
P < 0.05, compared with control;
P < 0.05, compared with diabetic mice.
Fig. 3.
Effect of treatment of a mouse model of type 2 diabetes (HF + STZ) with menhaden oil or resolvin D1 on intraepidermal nerve fiber density. Intraepidermal nerve fiber density was determined as described in materials and methods. Representative images are shown for each condition. Data are presented as the means ± SE in profiles/mm. The number of mice in each group was the same as shown in Table 2. *P < 0.05, compared with control mice; **P < 0.01, compared with control mice; ***P < 0.001, compared with control mice; +P < 0.05, compared with nontreated diabetic mice; ++P < 0.01, compared with nontreated diabetic mice.
Effect of dietary treatment with menhaden oil or exogenous resolvin D1 of mice fed a high-fat diet and treated with low dosage of streptozotocin on subepithelial and epithelial corneal nerve fibers.
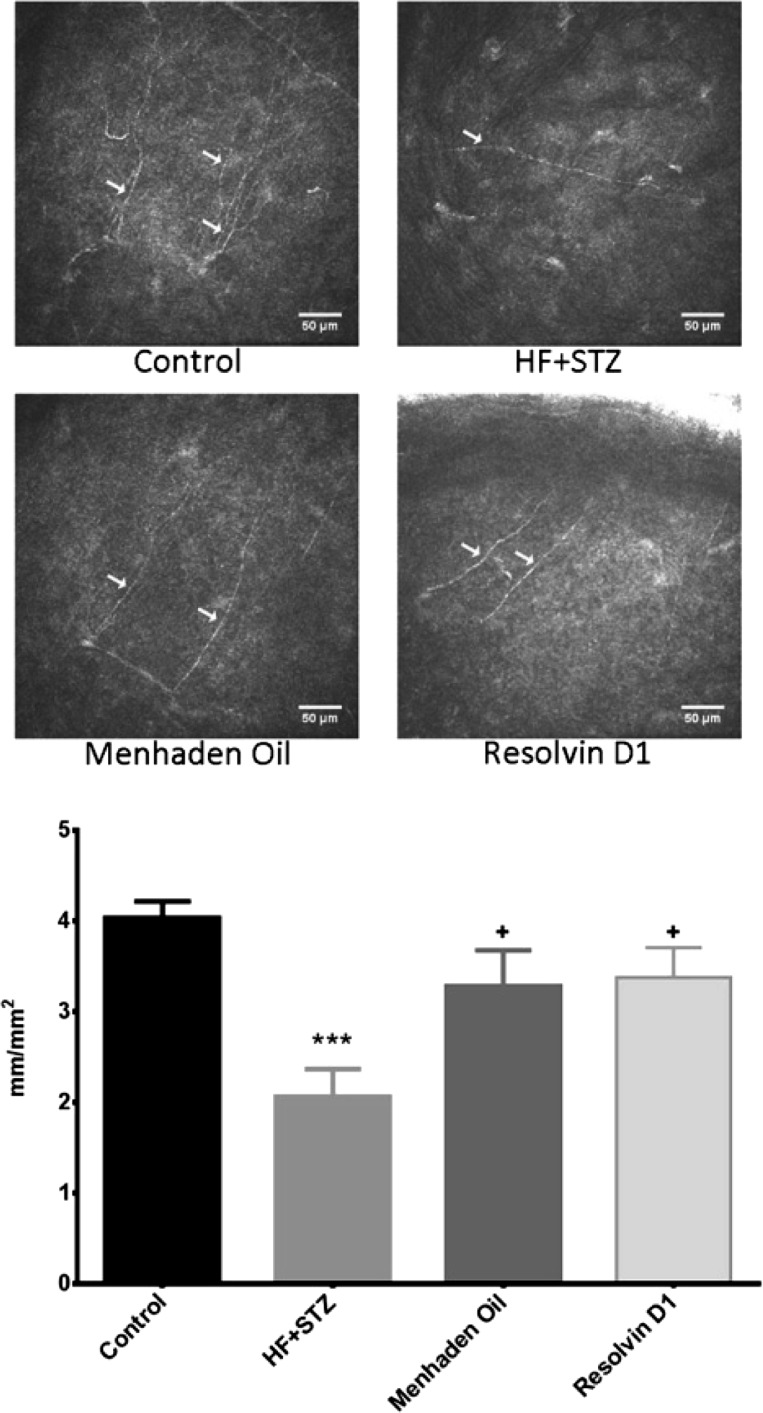
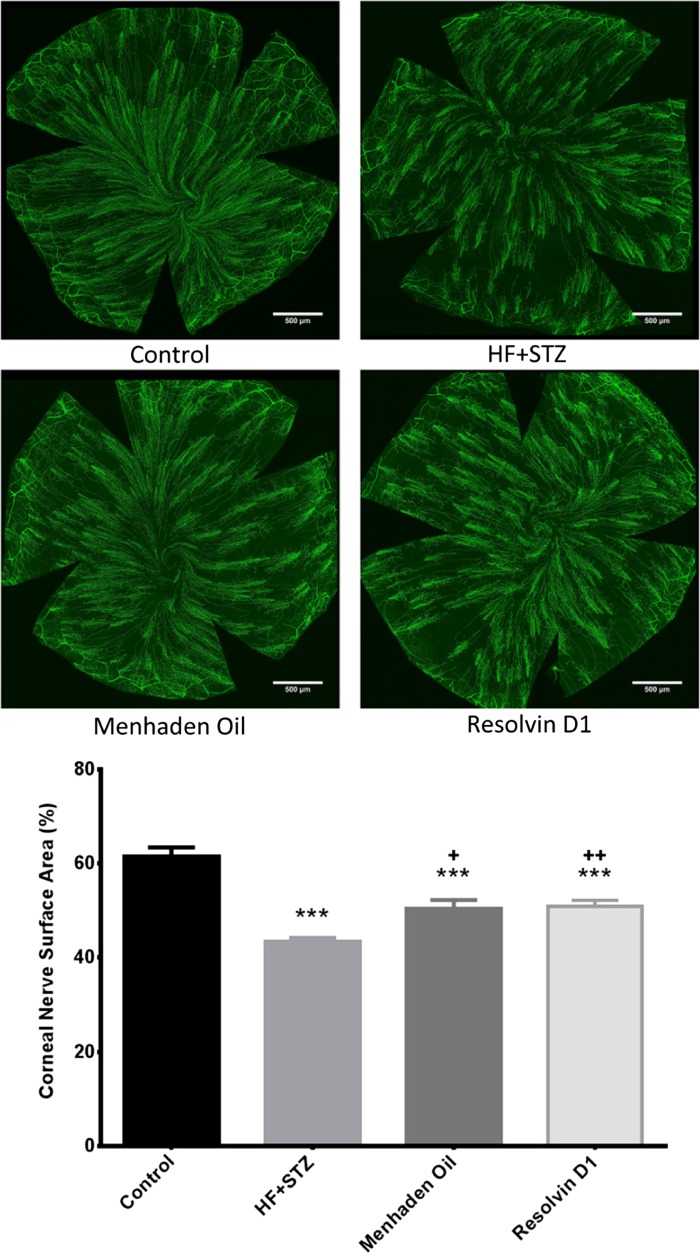
Corneal confocal microscopy, a noninvasive in vivo imaging procedure that can be performed repeatedly, provides an assessment of subbasal corneal sensory nerve structure and can detect early nerve damage in diabetic patients (Malik et al. 2003; Quattrini et al. 2007; Malik 2008). It has been proposed that imaging of diabetes-induced changes of these nerves may be a surrogate marker for early damage and repair for diabetic peripheral neuropathy (Mehra et al. 2007; Malik 2008; Tavakoli et al. 2010, 2011). We have previously reported that diabetes in rodents causes a decrease in sensory nerve density in the subepithelial layer of the cornea as well as a decrease in sensory nerves penetrating the corneal epithelium and the changes caused in the structure and density of corneal nerves by diabetes in rodents are consistent with the changes in these nerves in humans with diabetes (Coppey et al. 2015; Davidson et al. 2012a,b, 2014; Yorek et al. 2014). To further explore the potential to reduce diabetes-induced sensory nerve damage in the cornea, we examined changes in sensory nerve density in diabetic mice after 6 wk of treatment with dietary enrichment with menhaden oil or daily injections of resolvin D1. Data in Figs. 4 and 5 demonstrate that the corneal nerve fiber length of the subepithelial layer as measured by corneal confocal microscopy (Fig. 4) or corneal nerve fiber area as determined by in vitro labeling with anti-tubulin (Fig. 5) is significantly decreased in diabetic mice. Treating diabetic mice with a diet enriched with menhaden oil or with daily injections of resolvin D1 exhibited increased corneal nerve density compared with untreated diabetic mice. However, the density of corneal nerves in the subepithelial layer as determined by immunohistochemical staining with anti-tubulin remained significantly decreased in diabetic treated mice compared with control mice.
Fig. 4.
Effect of treatment of a mouse model of type 2 diabetes (HF + STZ) with menhaden oil or resolvin D1 on subepithelial cornea nerve fiber length. Subepithelial cornea nerve fiber length was determined as described in materials and methods. Representative images are shown for each condition. Data are presented as the means ± SE in mm/mm2. The number of mice in each group was the same as shown in Table 2. Scale bar = 50 μm. ***P < 0.001, compared with control mice; +P < 0.05, compared with nontreated diabetic mice.
Fig. 5.
Effect of treatment of a mouse model of type 2 diabetes (HF + STZ) with menhaden oil or resolvin D1 on innervation of subepithelial layer of the whole cornea. In vitro analysis of subepithelial corneal nerves stained with anti-tubulin. Corneas were collected and processed as described in materials and methods. Representative images are shown for each condition. Data are presented as the means ± SE in %surface area occupied by corneal nerves. The number of mice in each group was the same as shown in Table 2. Scale bar = 500 μm. ***P < 0.001, compared with control mice; +P < 0.05, compared with nontreated diabetic mice; ++P < 0.01, compared with nontreated diabetic mice.
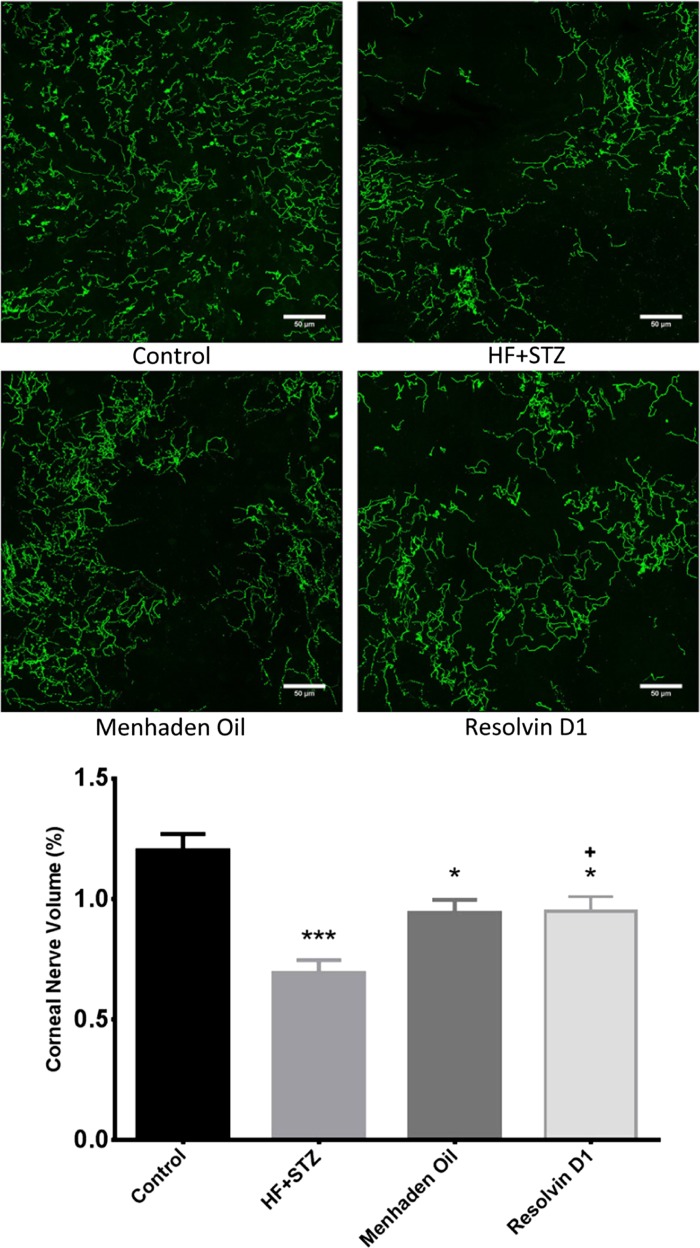
We have previously reported in a study conducted with a model of type 1 diabetic rats that diabetes-induced loss of corneal nerves can be detected earliest by examining the nerves that penetrated the corneal epithelium (Davidson et al. 2012b). This makes sense if the phenomenon of nerve axonopathy explains the loss of peripheral nerve fibers since the nerves penetrating the corneal epithelium are more distal than subbasal nerves of the cornea (Efron 2012). Data in Fig. 6 demonstrate that corneal nerves penetrating the epithelial layer of the cornea are also decreased in diabetic mice. Diabetic mice treated with a daily injection of resolvin D1 had a significantly improved innervation of the corneal epithelium compared with untreated diabetic mice. Treating diabetic mice with a diet enriched with menhaden oil also tended to reduce the loss of corneal nerves penetrating the epithelium, but these results were not significantly different from untreated diabetic mice. In both menhaden oil and resolvin D1-treated diabetic mice loss of corneal nerves penetrating the epithelium remained significantly decreased compared with control mice.
Fig. 6.
Effect of treatment of a mouse model of type 2 diabetes (HF + STZ) with menhaden oil or resolvin D1 on innervation of the corneal epithelium. In vitro analysis of corneal nerves penetrating the corneal epithelium stained with anti-tubulin. Corneas were collected and processed as described in materials and methods. Representative images are shown for each condition. Data are presented as the means ± SE in %volume occupied by corneal nerves. The number of mice in each group was the same as shown in Table 2. Scale bar = 50 μm. *P < 0.05, compared with control mice; ***P < 0.001, compared with control mice; +P < 0.05, compared with nontreated diabetic mice.
Effect of dietary treatment with menhaden oil or exogenous resolvin D1 of mice fed a high-fat diet and treated with low dosage of streptozotocin on retinal ganglion cell complex thickness.
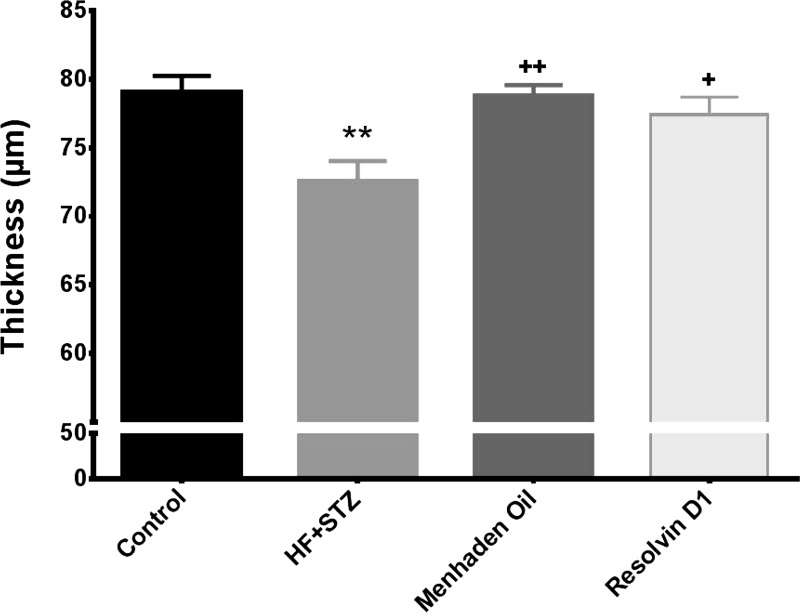
We previously reported a significant thinning of the retinal ganglion cell complex in mice models of type 1 and type 2 diabetes compared with control mice (Yorek et al. 2015). In this study we demonstrated that treating a mouse model of type 2 diabetes with a diet enriched with menhaden oil or with daily injections of resolvin D1 completely prevented the thinning of the retinal ganglion cell complex observed in untreated diabetic mice after 22 wk of a high-fat diet and 14 wk of hyperglycemia (Fig. 7).
Fig. 7.
Effect of treatment of a mouse model of type 2 diabetes (HF + STZ) with menhaden oil or resolvin D1 on retinal ganglion cell complex thickness. The retinal ganglion cell complex thickness was determined as described in materials and methods. Data are presented as the means ± SE in μm thickness. The number of mice in each group was the same as shown in Table 2. **P < 0.01, compared with control mice; +P < 0.05, compared with nontreated diabetic mice; ++P < 0.01, compared with nontreated diabetic mice.
Effect of resolvin D1 on neurite outgrowth by cultured DRG neurons.
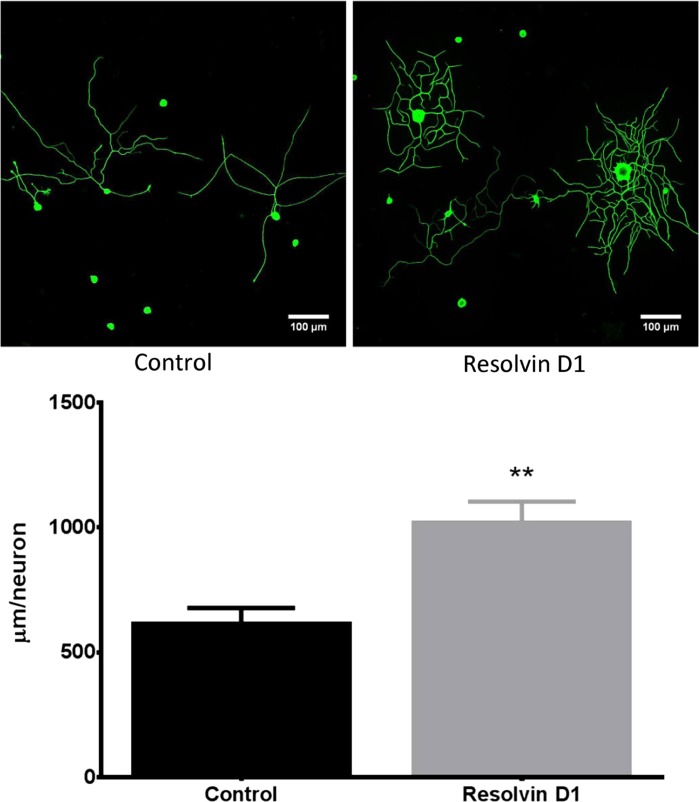
It has been previously reported that neuroprotectin D1 can increase neurite outgrowth by trigeminal ganglia neurons from Swiss Webster mice (Cortina et al. 2010). Robson, et al. (2010) also reported that n-3 fatty acids promote neurite outgrowth from DRG neurons. However, it is unknown whether resolvins can increase neurite outgrowth. Data in Fig. 8 demonstrate that a 24-h incubation with 50 nM resolvin D1 increases neurite outgrowth from DRG neurons by about twofold. In a separate study resolvin D1 increased neurite outgrowth by primary cultures of trigeminal ganglia from adult mice by ∼2.5-fold (data not shown).
Fig. 8.
Effect of resolvin D1 on neurite outgrowth of primary cultures of dorsal root ganglion neurons isolated from normal mice. Dorsal root ganglion neurons were collected and cultured as described in materials and methods. Afterwards the effect of 50 nM resolvin D1 on neurite outgrowth was determined. Data are presented as the means ± SE in μm/neuron. The number of mice in each group was 8. **P < 0.01, compared with untreated neurons.
DISCUSSION
Menhaden oil is derived from the Menhaden, a forage fish of the genera Brevoortia Ethmidium that resides in Atlantic, Pacific, and Gulf waters. Oil prepared from these fish is commonly used as a source for omega-3 (n-3) polyunsaturated fatty acids, enriched in eicosapentaenoic (20:5) and docosahexaenoic (22:6) acids for human consumption as a dietary supplement. n-3 Polyunsaturated fatty acids derived from fish oils have been demonstrated to lower plasma triglycerides, resting heart rate, blood pressure, and inflammation and improve vascular function (Mozaffarian and Wu 2011). Metabolites of eicosapentaenoic acid and docosahexaenoic acid, referred to as resolvins (resolution-phase interaction products) and neuroprotectin D1, have anti-oxidant, anti-inflammatory, and neuroprotection properties (Ariel and Serhan 2007; Kohli and Levy 2009). In nonvascular tissue 15-lipoxygenase-1 is responsible for the generation of resolvins and neuroprotectin D1 and eicosapentaenoic acid and docosahexaenoic acid are good substrates for 15-lipoxygenase-1. Resolvin formation can be increased by consuming increased amounts of eicosapentaenoic acid or docosahexaenoic acid (Ariel and Serhan 2007; Kohli and Levy 2009). In addition to being a substrate for formation of bioactive metabolites and having anti-inflammatory properties, n-3 fatty acids have been shown to affect a range of molecular pathways including alteration of physical properties of cellular membranes, modulation of membrane channels and proteins, and regulation of gene expression via nuclear receptors and transcription factors (Mozaffarian and Wu 2011). Membrane alteration with n-3 fatty acids has been shown to affect Akt signaling, impacting neuronal survival (Akbar et al. 2005).
Supplementing a high-fat diet with fish oil has been shown to improve metabolic features associated with type 2 diabetes (Jelinek et al. 2013). We have previously reported that treating rodents modeling type 1 or type 2 diabetes with menhaden oil improved many deficits associated with diabetic peripheral neuropathy (Coppey et al. 2012, 2015). In this study we focused on the effect of resolvin D1, a metabolite of docosahexaenoic acid, on diabetic neuropathy end points in a mouse model of type 2 diabetes. The hypothesis examined was if resolvin production from n-3 polyunsaturated fatty acids is responsible for the beneficial effects of menhaden oil supplementation on diabetic neuropathy end points, then direct treatment with resolvin D1 should mimic the effects of menhaden oil.
Anti-inflammatory therapies represent a potential approach for treatment of diabetes complications including neuropathy (Agrawal and Kant 2014; Sandireddy et al. 2014; Zhou and Zhou 2014). The resolvins, which are potent anti-inflammatory and pro-resolving mediators endogenously generated from n-3 polyunsaturated fatty acids, may be considered a good candidate for the treatment of diabetic neuropathy (Titos and Claria 2013). Studies in both animals and humans have demonstrated that circulating levels of the resolvins can be increased by dietary supplementation with n-3 polyunsaturated fatty acids derived from fish oil and resolvin levels can be further enhanced by aspirin (Serhan et al. 2002; Mas et al. 2012; Chen et al. 2014; Katakura et al. 2014). In this study resolvin D1 levels were increased by nearly twofold in serum derived from diabetic mice treated with menhaden oil-enriched diet compared with serum collected from control mice, untreated diabetic mice, or diabetic mice treated with daily injection of resolvin D1. A deficit in the production of resolvins has been demonstrated in obese adipose tissue, and restoration of their levels by either exogenous administration or by feeding n-3 polyunsaturated-enriched diets has been shown to improve inflammatory status, insulin sensitivity and ameliorate metabolic dysfunction (Figueras et al. 2011; Hellmann et al. 2011; Claria et al. 2012; Spite et al. 2014; Titos and Claria 2013). Resolvins are also considered to be potential treatment for other inflammatory conditions such as rheumatoid arthritis, inflammatory bowel disease, and allergic responses (Arita et al. 2005; Bento et al. 2011; Rogerio et al. 2012; Lee 2012; Norling and Perretti 2013). In this study the primary finding was that delivery of exogenous resolvin D1 through a daily intraperitoneal injection reversed deficits of some diabetic neuropathy end points and hepatic steatosis and at the very least slowed progression of loss of sensory nerves in the skin and cornea. Furthermore, the benefits observed with exogenous resolvin administration were similar or greater than the effects obtained by dietary supplementation of diabetic mice with menhaden oil. It is important to stress that the study design used was an intervention. The mice had been on a high-fat diet for 16 wk with 8 wk of hyperglycemia before the 6 wk treatment phase. Resolvin D1 also promoted neurite growth by cultured DRG neurons suggesting a direct effect by resolvins on neuron generation.
In our studies hepatic steatosis was improved to a greater extent by exogenous resolvin D1 treatment than dietary enrichment with menhaden oil. This could be due to exogenous treatment with resolvin D1 being more efficacious than resolvins produced endogenously from menhaden oil. A daily injection of resolvin D1 could have a more robust effect on signaling pathways in the liver and resolving inflammatory conditions contributing to hepatic steatosis. Our assay for resolvin D1 in serum indicated that daily treatment with resolvin D1 did not cause an increase in resolvin D1 levels compared with control or untreated diabetic mice. This is not surprising since collection of serum occurred 24 h after the final injection of resolvin D1. It is also important to stress that induction of diabetes does not appear to decrease resolvin D1 production. This suggests that the expression and activity of enzymes responsible for production of resolvins such as 15-lipoxygenase-1 are not compromised by diabetes. Improvement in hepatic steatosis occurred even though serum cholesterol and liver proteasome activity, a marker for endoplasmic reticulum stress, were not restored to control level following dietary treatment with menhaden oil or daily treatment with resolvin D1. This contrasts with a study by Jelinek et al. (2013) that showed that mice fed a high-fat diet supplemented with fish oil improved metabolic features as well as glucose tolerance and hepatic steatosis. However, the former study used a prevention design whereas we performed an intervention protocol. Hellmann et al. (2011) using db/db mice, a model of type 2 diabetes, demonstrated that 10 days of daily treatment with resolvin D1 significantly improved insulin sensitivity and glucose tolerance. However, the dose of resolvin D1 used was twofold higher than the dose used in this study (Hellmann et al. 2011). It was also unclear how long the db/db mice were diabetic or had an impaired glucose tolerance before treatment was initiated. Hepatic insulin resistance is considered to be a central player in the development of metabolic syndrome and nonalcoholic fatty liver disease (Asrih and Jornayvaz 2015). Current therapies focus on lifestyle changes and weight loss but the outcome has been disappointing (Asrih and Jornayvaz 2015). In our studies treatment with resolvin D1and to a lesser extent menhaden oil reduced liver steatosis without improving whole body insulin resistance. It is possible that a longer treatment phase or larger dose of menhaden oil or resolvin D1 in our studies would have also improved glucose utilization.
Nerve conduction velocity is decreased after 4–8 wk of hyperglycemia in diabetic rodents (Coppey et al. 2015). In the current study treatment with menhaden oil-enriched diet or daily injections of resolvin D1 was initiated 8 wk after the onset of hyperglycemia and motor nerve conduction velocity was significantly improved and sensory nerve conduction velocity was completely corrected after 6 wk of treatment. This indicates that dietary treatment with menhaden oil or exogenous treatment with resolvin D1 was able to reverse the diabetes-induced deficits in nerve conduction velocity. However, it was observed that treatment with menhaden oil or resolvin D1 was more effective in improving sensory nerve conduction velocity than motor nerve conduction velocity. This may be due to different mechanisms contributing to the slowing of motor and sensory nerve conduction velocities. It has been our experience that feeding rodents a high-fat diet reduces sensory nerve conduction velocity but not motor nerve conduction velocity (Coppey et al. 2011; Davidson et al. 2014; Yorek et al. 2015). However, with the onset of hyperglycemia that occurs after treating high-fat fed rodents with a low dose of streptozotocin there is a decrease in motor nerve conduction velocity. This suggests to us that pathological mechanisms associated with hyperglycemia are responsible for slowing of motor nerve conduction velocity and that menhaden oil or resolvin D1 is not entirely protective against these mechanisms. Treatment of diabetic mice also resulted in inconsistencies between treated and untreated diabetic mice in thermal nociception, intraepidermal nerve fiber density, corneal nerve fiber length, and density in the subbasal layer of the cornea and corneal epithelium and retinal ganglion cell complex thickness. Treatment of diabetic mice with menhaden oil or resolvin D1 appeared to be more effective in protecting thermal nociception than skin intraepidermal nerve fiber density with the latter remaining significantly decreased compared with control mice after 6 wk of treatment. This may be due to the sensitivity of mice to thermal nociception. It may not be necessary to repair or regenerate fully sensory nerves in the skin to have normal thermal nociception. However, these deficits take longer to develop and reach significance compared with slowing of nerve conduction velocities. Given the study design used we can only conclude that following 16 wk of a high-fat diet combined with 8 wk of untreated hyperglycemia dietary enrichment with menhaden oil or a daily injection of resolvin D1 for 6 wk slows progression of changes in the density and sensitivity of sensory nerves in the skin, cornea and retinal ganglion cell complex. Future studies will need to examine the effect of intervention after 20 wk of a high-fat diet and 12 wk of untreated hyperglycemia, after significant changes in nerve density of the skin, cornea, and retina have occurred, to determine whether treatment with menhaden oil or resolvin D1 can stimulate nerve regeneration and repair (Yorek et al. 2015). However, our studies did demonstrate that exogenous resolvin D1 stimulates neurite outgrowth from DRG neurons isolated from normal C57Bl/J mice. These results are similar to studies demonstrating regeneration of corneal nerves damaged by refractive surgery can be increased with treatment of docosahexaenoic acid through synthesis of neuroprotectin D1 (Cortina et al. 2010; Gordon and Bazan 2013). This group also reported that neuroprotectin D1 increases neurite outgrowth from trigeminal ganglia neurons isolated from Swiss Webster mice (Cortina et al. 2010). Furthermore, Robson et al. (2010) reported that n-3 fatty acids promote neurite outgrowth by DRG neurons and the effect of DHA was still prominent in aged tissue. Overall, these studies demonstrate that n-3 polyunsaturated fatty acids and their metabolites can promote neurite growth and nerve regeneration.
This study has several limitations. First, although we measured serum levels of resolvin D1, this study did not determine the levels of resolvins or other n-3 polyunsaturated fatty acid metabolites in target tissue. It would have been interesting to know whether diabetes and treatment with menhaden oil changes the levels of these mediators of inflammatory resolution in the cornea, sciatic nerve, and liver. Second, our study design did not account for the possible effect reducing the lard content in the diet may have on diabetic neuropathic end points when 50% of the lard was replaced with menhaden oil in the menhaden oil-treated diabetic mice. Lastly, there are always concerns regarding applicability of results from animal studies to human conditions and interpretation of results always needs to be done with awareness of this issue.
In summary, these studies demonstrate that treating a mouse model of type 2 diabetes after the onset of hyperglycemia and the development of diabetic complications associated with neuropathy with a dietary supplement of menhaden oil or daily injections of resolvin D1 reverses neuropathic deficits such as slowing of nerve conduction velocity and slows progression of loss of sensitivity and/or density of nerves in the skin, cornea, and retina. The benefits of menhaden oil and resolvin D1 on the diabetic neuropathy end points were very similar suggesting a similar mode of action and that the effect of dietary menhaden oil may be due to the endogenous production of resolvins and neuroprotectin (Bazan 2009). Resolvin D1 was also shown to stimulate neurite outgrowth suggesting that metabolites of eicosapentaenoic and docosahexaenoic acids derived from fish oils may be an effective treatment for nerve regeneration. In the study of signalolipidomics it has been suggested that docosahexaenoic acid, and perhaps eicosapentaenoic acid as well, offer emerging targets for pharmaceutical intervention for such disorders as Alzheimer's disease, macular degeneration, Parkinson's disease, and brain ischemia-reperfusion injury (Bazan et al. 2011; Palacios-Pelaez et al. 2010). These studies provide evidence that docosahexaenoic and eicosapentaenoic acids and their metabolites may also be an effective treatment for diabetic neuropathy.
GRANTS
This material is based on work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (BX001680-01), Rehabilitation Research and Development (RX000889-01), Iowa City Veterans Affairs Center of Excellence for the Prevention and Treatment of Visual Loss (C9251-C), and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-081147.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
DISCLAIMER
The content of this manuscript is new and solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies.
AUTHOR CONTRIBUTIONS
Author contributions: H.S., M.S.Y., L.J.C., A.H., and M.A.Y. performed experiments; H.S., M.S.Y., L.J.C., A.H., M.M.H., and M.A.Y. analyzed data; H.S., M.S.Y., and A.H. prepared figures; H.S., M.S.Y., L.J.C., A.H., M.M.H., R.H.K., and M.A.Y. approved final version of manuscript; M.S.Y. and M.A.Y. interpreted results of experiments; M.M.H., R.H.K., and M.A.Y. conception and design of research; M.M.H. and M.A.Y. drafted manuscript; M.A.Y. edited and revised manuscript.
REFERENCES
- Agrawal NK, Kant S. Targeting inflammation in diabetes: newer therapeutic options. World J Diabetes 5: 697–710, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA 102: 10858–10863, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol 28: 176–183, 2007. [DOI] [PubMed] [Google Scholar]
- Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA 102: 7671–7676, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrih M, Jornayvaz FR. Metabolic syndrome and nonalcoholic fatty liver disease: is insulin resistance the link? Mol Cell Endocrinol. First published February 24, 2015; doi: 10.1016/j.mce.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer's disease. J Lipid Res 50: S400–S405, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer's and other neurodegenerative diseases. Annu Rev Nutr 31: 321–351, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento AF, Claudino RF, Dutra RC, Marcon R, Calixto JB. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol 187: 1957–1969, 2011. [DOI] [PubMed] [Google Scholar]
- Chen J, Shetty S, Zhang P, Gao R, Hu Y, Wang S, Li Z, Fu J. Aspirin-triggered resolvin D1 down-regulates inflammatory responses and protects against endotoxin-induced acute kidney injury. Toxicol Appl Pharmacol 277: 118–123, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claria J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol 189: 2597–2605, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppey L, Davidson E, Lu B, Gerard C, Yorek M. Vasopeptidase inhibitor ilepatril (AVE7688) prevents obesity- and diabetes-induced neuropathy in C57Bl/6J mice. Neuropharmacology 60: 259–266, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppey LJ, Davidson EP, Obrosov A, Yorek MA. Enriching the diet with menhaden oil improves peripheral neuropathy in streptozotocin-induced type 1 diabetic rats. J Neurophysiol 113: 701–708, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppey LJ, Holmes A, Davidson EP, Yorek MA. Partial replacement with menhaden oil improves peripheral neuropathy in high-fat-fed low-dose streptozotocin type 2 diabetic rat. J Nutr Metab 2012: 950517, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina MS, He J, Li N, Bazan NG, Bazan HE. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci 51: 804–810, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E, Coppey L, Holmes A, Yorek M. Changes in corneal innervation and sensitivity and acetylcholine-mediated vascular relaxation of the posterior ciliary artery in a type 2 diabetic rat. Invest Ophthalmol Vis Sci 53: 1182–1187, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EP, Coppey LJ, Kardon RH, Yorek MA. Differences and similarities in development of corneal nerve damage and peripheral neuropathy and in diet-induced obesity and type 2 diabetic rats. Invest Ophthalmol Vis Sci 55: 1222–1230, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EP, Coppey LJ, Yorek MA. Early loss of innervation of cornea epithelium in streptozotocin-induced type 1 diabetic rats: improvement with ilepatril treatment. Invest Ophthalmol Vis Sci 53: 8067–8074, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron N. Assessing diabetic neuropathy using corneal confocal microscopy. Invest Ophthalmol Vis Sci 53: 8075, 2012. [DOI] [PubMed] [Google Scholar]
- Figueras M, Olivan M, Busquets S, Lopez-Soriano FJ, Argiles JM. Effects of eicosapentaenoic acid (EPA) treatment on insulin sensitivity in an animal model of diabetes: improvement of the inflammatory status. Obesity (Silver Spring) 19: 362–369, 2011. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martinez-Clemente M, Lopez-Parra M, Arroyo V, Claria J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by ω-3 fatty acids: a role for resolvins and protectins. FASEB J 23: 1946–1957, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon WC, Bazan NG. Mediator lipidomics in ophthalmology: targets for modulation in inflammation, neuroprotection and nerve regeneration. Curr Eye Res 38: 995–1005, 2013. [DOI] [PubMed] [Google Scholar]
- Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J 25: 2399–2407, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek D, Castillo JJ, Arora SL, Richardson LM, Garver WS. A high-fat diet supplemented with fish oil improves metabolic features associated with type 2 diabetes. Nutrition 29: 1159–1165, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakura M, Hashimoto M, Inoue T, Al Mamun A, Tanabe Y, Iwamoto R, Tsuchikura S, Shido O. Omega-3 fatty acids protect renal functions by increasing docosahexaenoic acid-derived metabolite levels in SHR. Cg-Leprcp/NDmcr rats, a metabolic syndrome model. Molecules 19: 3247–3263, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol 158: 960–971, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamping KG, Nuno DW, Coppey LJ, Holmes AJ, Hu S, Oltman CL, Norris AW, Yorek MA. Modification of high saturated fat diet with n-3 polyunsaturated fat improves glucose intolerance and vascular dysfunction. Diabetes Obes Metab 15: 144–152, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH. Resolvins as new fascinating drug candidates for inflammatory diseases. Arch Pharm Res 35: 3–7, 2012. [DOI] [PubMed] [Google Scholar]
- Malik RA. Early detection of nerve damage and repair in diabetic neuropathy. Nat Clin Pract Neurol 4: 646–647, 2008. [DOI] [PubMed] [Google Scholar]
- Malik RA, Kallinikos P, Abbott CA, van Schie CH, Morgan P, Efron N, Boulton AJ. Corneal confocal microscopy: a noninvasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 46: 683–688, 2003. [DOI] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc 2: 152–160, 2007. [DOI] [PubMed] [Google Scholar]
- Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem 58: 1476–1484, 2012. [DOI] [PubMed] [Google Scholar]
- Mehra S, Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Augustine T, Malik RA. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care 30: 2608–2612, 2007. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58: 2047–2067, 2011. [DOI] [PubMed] [Google Scholar]
- Norling LV, Perretti M. The role of omega-3 derived resolvins in arthritis. Curr Opin Pharmacol 13: 476–481, 2013. [DOI] [PubMed] [Google Scholar]
- Otoda T, Takamura T, Misu H, Ota T, Murata S, Hayashi H, Takayama H, Kikuchi A, Kanamori T, Shima KR, Lan F, Takeda T, Kurita S, Ishikura K, Kita Y, Iwayama K, Kato K, Uno M, Takeshita Y, Yamamoto M, Tokuyama K, Iseki S, Tanaka K, Kaneko S. Proteasome dysfunction mediates obesity-induced endoplasmic reticulum stress and insulin resistance in liver. Diabetes 62: 811–824, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Pelaez R, Lukiw WJ, Bazan NG. Omega-3 essential fatty acids modulate initiation and progression of neurodegenerative disease. Mol Neurobiol 41: 367–374, 2010. [DOI] [PubMed] [Google Scholar]
- Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, Marshall A, Boulton AJ, Efron N, Malik RA. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 56: 2148–2154, 2007. [DOI] [PubMed] [Google Scholar]
- Robson LG, Dyall S, Sidloff D, Michael-Titus AT. Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurons throughout development and in aged animals. Neurobiol Aging 31: 678–687, 2010. [DOI] [PubMed] [Google Scholar]
- Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol 189: 1983–1991, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int J Endocrinol 2014: 674987, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbolouki S, Javanbakht MH, Derakhshanian H, Hosseinzadeh P, Zareei M, Hashemi SB, Dorosty AR, Eshraghian MR, Djalali M. Eicosapentaenoic acid improves insulin sensitivity and blood sugar in overweight type 2 diabetes mellitus patients: a double-blind randomized clinical trial. Singapore Med J 54: 387–390, 2013. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 196: 1025–1037, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh SR, Kinnun JJ, Leng X, Williams JA, Wassall SR. How polyunsaturated fatty acids modify molecular organizations in membranes: insight from NMR studies of model systems. Biochim Biophys Acta 1848: 211–219, 2015. [DOI] [PubMed] [Google Scholar]
- Singh B, Xu Y, McLaughlin T, Singh V, Martinez JA, Krishnan A, Zochodne DW. Resistance to trophic neurite outgrowth of sensory neurons exposed to insulin. J Neurochem 121: 263–276, 2012. [DOI] [PubMed] [Google Scholar]
- Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab 19: 21–36, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA, Yorek MA. Membrane lipid composition and cellular function. J Lipid Res 26: 1015–1035, 1985. [PubMed] [Google Scholar]
- Tavakoli M, Kallinikos P, Iqbal A, Herbert A, Fadavi H, Efron N, Boulton AJ, Malik RA. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med 28: 1261–1267, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli M, Quattrini C, Abbott C, Kallinikos P, Marshall A, Finnigan J, Morgan P, Efron N, Boulton AJ, Malik RA. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 33: 1792–1797, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titos E, Claria J. Omega-3-derived mediators counteract obesity-induced adipose tissue inflammation. Prostaglandins Other Lipid Mediat 107: 77–84, 2013. [DOI] [PubMed] [Google Scholar]
- Yorek MS, Obrosov A, Shevalye H, Lupachyk S, Harper MM, Kardon RH, Yorek MA. Effect of glycemic control on corneal nerves and peripheral neuropathy in streptozotocin-induced diabetic C57Bl/6J mice. J Peripher Nerv Syst 19: 205–217, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorek MA, Bohnker RR, Dudley DT, Spector AA. Comparative utilization of n-3 polyunsaturated fatty acids by cultured human Y-79 retinoblastoma cells. Biochim Biophys Acta 795: 277–285, 1984. [DOI] [PubMed] [Google Scholar]
- Yorek MS, Obrosov A, Shevalye H, Holmes A, Harper MM, Kardon RH, Yorek MA. Effect of diet induced obesity or type 1 or type 2 diabetes on corneal nerves and peripheral neuropathy in C57Bl/6J mice. J Peripher Nerv Syst. First published April 10, 2015; doi: 10.1111/jns.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhou S. Inflammation: therapeutic targets for diabetic neuropathy. Mol Neurobiol 49: 536–546, 2014. [DOI] [PubMed] [Google Scholar]