Abstract
Background
Although magnetic resonance imaging (MRI) is a useful imaging modality for invasive cancer, its role in preoperative surgical planning for ductal carcinoma in situ (DCIS) has not been established. We sought to determine whether preoperative MRI affects surgical treatment and outcomes in women with pure DCIS.
Patients and Methods
We reviewed consecutive records of women diagnosed with pure DCIS on core biopsy between 2000 and 2007. Patient characteristics, surgical planning, and outcomes were compared between patients with and without preoperative MRI. Multivariable regression was performed to determine which covariates were independently associated with mastectomy or sentinel lymph node biopsy (SLNB).
Results
Of 149 women diagnosed with DCIS, 38 underwent preoperative MRI. On univariate analysis, patients undergoing MRI were younger (50 years vs. 59 years; P < .001) and had larger DCIS size on final pathology (1.6 cm vs. 1.0 cm; P = .007) than those without MRI. Mastectomy and SLNB rates were significantly higher in the preoperative MRI group (45% vs. 14%, P < .001; and 47% vs. 23%, P = .004, respectively). However, there were no differences in number of re-excisions, margin status, and margin size between the two groups. On multivariate analysis, preoperative MRI and age were independently associated with mastectomy (OR, 3.16, P = .018; OR, 0.95, P = .031, respectively), while multifocality, size, and family history were not significant predictors.
Conclusion
We found a strong association between preoperative MRI and mastectomy in women undergoing treatment for DCIS. Additional studies are needed to examine the increased rates of mastectomy as a possible consequence of preoperative MRI for DCIS.
Keywords: Breast imaging, DCIS, Preinvasive breast cancer, Mastectomy, MRI
Introduction
Ductal carcinoma in situ (DCIS) accounts for about 20% of all female breast cancers and is now diagnosed ten times more frequently than before the use of increased screening mammography.1–3 DCIS is thought to be a precursor to invasive cancer, and is therefore generally treated by surgical excision to prevent possible progression to invasive disease. Accurate preoperative diagnostic imaging to determine size and extent of the disease is necessary in order to optimize surgical outcome. Currently, DCIS is primarily detected by mammography, but recent studies have investigated the use of other modalities such as ultrasound and breast magnetic resonance imaging (MRI).4–12 Although MRI is thought by many to be a useful imaging modality for invasive cancer, its role and impact in preoperative surgical planning for DCIS is unclear.
Studies have shown that breast MRI is an important supplemental diagnostic tool for invasive cancer due to its high sensitivity in detection of occult disease that may otherwise go unrecognized on the basis of clinical examination, mammography, or ultrasound.13–16 Nevertheless, there are some important limitations. One significant issue associated with breast MRI is the high rate of false-positive findings, in which overestimates the extent of disease, possibly resulting in unnecessary biopsies and more extensive surgery.17,18 Another limitation is its relatively low specificity. Peters et al performed a meta-analysis of 44 studies conducted between 1985 and 2005 to investigate the diagnostic value of breast MRI for invasive cancer, which showed an overall specificity of 72% compared with a sensitivity of 90%.19 Breast MRI specificity can differ widely, ranging from 21% to 100%.19,20 Furthermore, although the ACR BIRADS MRI lexicon has now been established, there remains substantial variability for breast MRI interpretation and reporting.
These considerations may be even more problematic when considering the value of MRI in the evaluation of DCIS. MRI detection of DCIS is challenging due to the heterogeneity of DCIS histology, which produces variable enhancement patterns on magnetic resonance images. While some studies have shown that MRI has higher sensitivity than mammography for detecting DCIS, others have indicated that MRI may fail to distinguish benign proliferative changes from DCIS, leading to false-positive imaging findings that are more difficult to discern from DCIS than from invasive cancers.7–9,21 In addition, the ability of MRI to accurately predict the extent of DCIS is not well established. A few studies have shown that MRI assessment of DCIS size is well correlated with pathology size, though MRI tended to overestimate the extent of disease.21,22 Despite these challenges, breast MRI is still often used as part of the preoperative evaluation for DCIS because of its high sensitivity and its potential value in guiding surgical management.
Although several studies have investigated the impact of preoperative MRI on the management of invasive breast cancers,15,18,23–26 few have examined its effects on surgical management specifically for DCIS.27,28 In this study, we sought to determine whether the use of preoperative MRI in women diagnosed with pure DCIS on core biopsy is associated with differences in surgical treatment and outcome when compared with those women who did not undergo MRI evaluation.
Patients and Methods
The study consisted of a consecutive cohort of patients who were diagnosed with DCIS on core biopsy and subsequently underwent surgery at the University of California, San Francisco (UCSF). The research protocol was reviewed and approved by the UCSF institutional review board (H10367-32903-02). A cohort of 149 women diagnosed with DCIS without invasive cancer on stereotactic core biopsy between 2000 and 2007 were identified retrospectively through medical chart review. Women who received neoadjuvant treatment for DCIS were excluded from the study, as were 3 women identified to have bilateral DCIS were excluded from the study cohort. All women included in the study underwent all of their diagnostic evaluation and definitive surgical treatment for DCIS at UCSF.
Patient characteristics, choice of operative procedure, and surgical outcomes were compared between patients who underwent preoperative MRI and patients who did not. Patient variables and DCIS characteristics were collected for each patient, including age at diagnosis, highest nuclear grade of DCIS determined by core biopsy, size of DCIS, family history of breast cancer (first, second, or third degree), presence of multifocality, and presence of invasive carcinoma on surgical pathology. Pathology characteristics were recorded according to College of American Pathologist guidelines. DCIS nuclear grade was scored as low, intermediate, or high. DCIS pathologic size was determined as the single greatest measurement in one dimension. For women who initially underwent lumpectomy and required subsequent re-excisions, the DCIS size was measured as the aggregate size of all contiguous pathology specimens. Surgical treatment planning was assessed by whether patients underwent mastectomy as the primary extirpative procedure and whether sentinel lymph node biopsy (SLNB) was undertaken during the initial surgery. Endpoints for surgical outcome in the lumpectomy subset included the achievement of negative margins (classified as greater than or equal to 0.1 cm), the size of margins from the primary surgery, and the number of re-excisions. Re-excision was defined as an additional surgical procedure required to clear the margins in those patients who initially underwent lumpectomy. Only 2 patients who initially underwent mastectomy required re-excisions and were excluded from analysis of surgical outcomes.
Continuous variables were compared between the two groups using the Student t test, while dichotomous variables were compared between the groups using the χ2 test. The rank sum test was used to compare median age between the two groups. Multivariable logistic regression was performed to determine whether any predictor was independently associated with mastectomy or SLNB outcome. Correlation between year and proportion of patients receiving MRI was determined by Spearman’s rank correlation coefficient. For all statistical analyses, significance was determined at the P = .05 level.
Results
Of 149 women diagnosed with DCIS in this cohort, 38 underwent preoperative MRI, of whom 37 underwent diagnostic core biopsy (n = 34) or fine-needle aspiration (n = 3) before MRI. Median age, median DCIS size on pathology, DCIS grade on presurgical core biopsy, presence of invasive cancer at surgery, and family history of breast cancer were compared between the two groups (Table 1). Women who underwent preoperative MRI were significantly younger (50 years vs. 59 years; P < .001) and had larger DCIS size (1.6 cm vs. 1.0 cm; P = .007) compared with those without preoperative MRI. DCIS grade, presence of invasive cancer, and family history between the two groups did not differ significantly.
Table 1.
Patient and Ductal Carcinoma In Situ Characteristics
| No MRI | MRI | P Value | |
|---|---|---|---|
| N | 111 | 38 | |
| Median Age, Years (Range) | 59 (38–86) | 50 (24–71) | < .001a |
| DCIS Grade (%) | |||
| Low | 19 (17%) | 2 (5%) | .10b |
| Intermediate | 43 (39%) | 13 (34%) | |
| High | 49 (44%) | 23 (61%) | |
| Median DCIS Size, cm (Range) | 1.0 (0.1–15.0) | 1.6 (0.1–15.4) | .007a |
| Occult Invasive Cancer | 21 (19%) | 9 (24%) | .53b |
| Family History (1st, 2nd or 3rd Degree) | 35 (34%) | 14 (40%) | .52b |
Rank sum test.
χ2 test.
Abbreviations: MRI = magnetic resonance imaging; DCIS = ductal carcinoma in situ
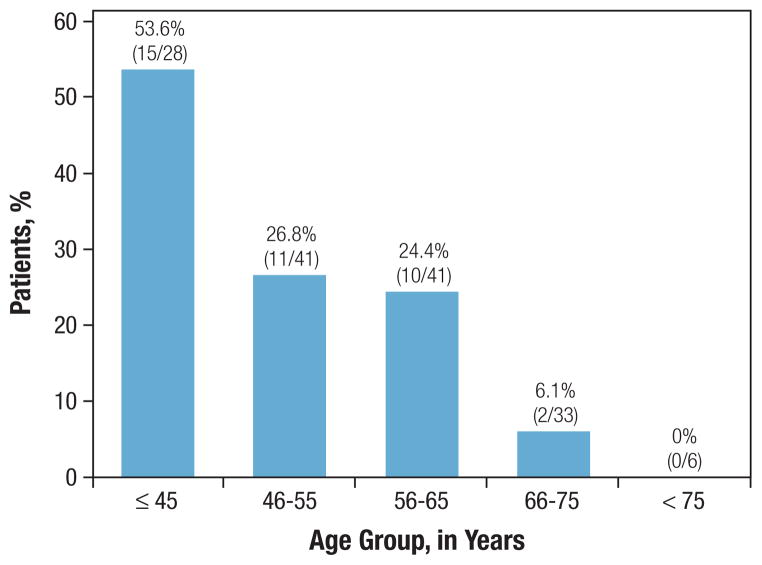
During the period of the study, the proportion of patients undergoing preoperative MRI for DCIS did not show a clear trend (P = .32; Figure 1A) and ranged from 13.3% to 33.3%. However, use of MRI appeared clearly correlated with age at diagnosis over the entire cohort (Figure 1B). Of the 28 patients under the age 45 in our cohort, over half (53.6%) underwent preoperative MRI. In contrast, of the 39 patients over the age of 65, only two women underwent preoperative MRI. Among the 38 women with preoperative MRI, 10 had incidental MRI findings that warranted an additional biopsy before surgery. In the 4/10 women who had a negative biopsy, all underwent partial mastectomy. In the 6/10 women who had a positive biopsy, all 6 had additional DCIS diagnosed in the ipsilateral breast and chose to undergo mastectomy.
Figure 1.
Figure 1A. Proportion of Patients Undergoing Preoperative MRI, by Year of Diagnosis (2000–2007)
Figure 1B. Age Distribution Among Patients Undergoing Preoperative Breast MRI for DCIS
Univariate comparison of mastectomy and SLNB rates between the two groups found that both procedures were more commonly associated with the MRI group. The mastectomy rate was 45% for women who had preoperative MRI and 14% for those who did not (P < .001). The SLNB rate was 47% for women had preoperative MRI and 23% for those who did not (P = .004) (Table 2).
Table 2.
Choice of Initial Surgical Procedure and Surgical Outcomes
| Surgical Measure | No MRI (n = 111) | MRI (n = 38) | P Value |
|---|---|---|---|
| Initial Surgical Procedure | |||
| Lumpectomy | 95 (86%) | 19 (50%) | < .001a |
| Unilateral mastectomy | 16 (14%) | 17 (45%) | < .001a |
| Bilateral mastectomy | 0 (0%) | 2 (5%) | .015a |
| Sentinel node biopsy | 25 (23%) | 18 (47%) | .004a |
| Surgical Outcomes (Overall Cohort) | |||
| Negative margins | 99 (89%) | 32 (84%) | .42a |
| Margins larger than 10 mm | 30 (27%) | 5 (13%) | .082a |
| Closest marginc (mean, cm) | 0.26 | 0.21 | .50b |
| Surgical Outcomes (Lumpectomy Subset) | |||
| Closest margin in lumpectomy subsetc (mean, cm) | 0.14 | 0.11 | .47b |
| Number reexcisions (mean) | 0.42 | 0.58 | .31b |
χ2 test.
Student t test.
At first surgery, excludes widely negative margins.
Abbreviation: MRI = magnetic resonance imaging
The number of re-excisions, margin status, and margin size were also compared between the two groups. Preoperative MRI was not significantly associated with wider surgical margins or higher proportion of patients with negative margins. Furthermore, the mean number of re-excisions was comparable in both groups (0.58 vs. 0.42; Table 2).
On multivariable analysis including MRI, age at diagnosis, multifocality, family history, and DCIS size, use of MRI was found to be an independent predictor for mastectomy; women who had preoperative MRI were more likely to choose mastectomy than women without MRI (OR, 3.2; 95% CI, 1.22–8.18). Young age was also an independent predictor of mastectomy, with older women less likely to undergo mastectomy. However, presence or absence of multifocal disease, family history, and DCIS size were not significant predictors (Table 3). Use of SLNB was independently associated with mastectomy (P < .001); SLNB was used in 28 of 35 women (80%) undergoing mastectomy, compared with 15 of 114 women (13%) undergoing lumpectomy. Presence or absence of MRI, decade of age, grade, and DCIS size were not significant predictors of SLNB.
Table 3.
Multivariable Analysis of Factors Associated With Mastectomy for DCIS
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| MRI | 3.16 | 1.22–8.18 | .018 |
| Agea | 0.95 | 0.90–0.99 | .031 |
| Multifocality | 1.98 | 0.73–5.35 | .18 |
| Family History | 1.35 | 0.53–3.41 | .53 |
| DCIS Size, cm | 1.12 | 0.99–1.27 | .080 |
Compared with youngest age category (≤ 45) as referent group.
Abbreviations: DCIS = ductal carcinoma in situ; MRI = magnetic resonance imaging
Two patients chose to undergo contralateral prophylactic mastectomy (CPM). One patient, age 50 years, had a negative MRI of the contralateral breast but elected to undergo CPM because she had a prior history of contralateral invasive cancer. The second patient was 52 years old, and her preoperative MRI showed a 0.5 mm enhancement in the contralateral breast, which had benign enhancement characteristics. The final pathology showed a micro-focus of intraductal hyperplasia without atypia and no carcinoma in the contralateral breast.
Discussion
These results indicate that the rates of mastectomy and SLNB were significantly higher among women who underwent preoperative MRI than those without preoperative MRI. In a multivariable analysis of factors associated with mastectomy, we found that women who received preoperative MRI were more likely to undergo mastectomy than their counterparts who did not receive preoperative MRI. The multivariable analysis also identified age to be an independent predictor of mastectomy. However, DCIS size, family history, and multifocality, three factors considered to be particularly clinically relevant in surgical decision making, were not found to be statistically significant predictors of mastectomy. In a multivariable analysis of factors associated with SLNB, the only independent predictor of SLNB was whether patients had a mastectomy. Thus, the increased rate of SLNB among the MRI group seems to be a reflection of the higher mastectomy rate in this group. This finding is expected since SLNB is commonly performed at the same time as mastectomy in patients with DCIS as it is generally accepted that ability to perform SLNB may be compromised after mastectomy. Thus, a SLNB at the time of the mastectomy obviates the need for an axillary node dissection if the patient were found to have invasive disease at surgical excision.29–32
Few other studies have examined the impact of preoperative MRI on surgical management specifically for DCIS. In a study of women with early-stage breast cancer, Katipamula et al also found a strong association between the use of preoperative MRI and mastectomy rate, although this study was not confined to women with DCIS.33 Another study found that MRI altered surgical management in 14 of 54 patients (26%), of whom 8 patients (15%) received more appropriate therapy. In 28 patients planning to undergo local excision, 5 patients changed from lumpectomy to mastectomy due to preoperative MRI findings. However, this study was not limited to women with pure DCIS.27
Other studies have evaluated the impact of breast MRI on the surgical management of invasive breast tumors.18,23–26,34 Overall, these studies have found that 13%–26% of women diagnosed with invasive cancer have a change in surgical management based on preoperative MRI evaluation. In these studies, the percentage of women who alter their surgical treatment from lumpectomy to mastectomy based on MRI findings ranged from 7% to 17%. In addition, 14%–26% underwent additional biopsies or wider surgical excisions due to MRI evaluation. False-positive rates in these studies ranged from 29% to 80%.15,18,24,34–36 These previous studies demonstrate that preoperative MRI can have variable effects on surgical management. Although MRI findings allowed improved assessment of disease extent in many cases, the relatively high false-positive rate of MRI has also likely led to unwarranted biopsies and more extensive surgeries.
Our study found that women who received preoperative MRI were significantly younger than those without MRI. As shown in Figure 1B, the proportion of patients receiving MRI decreased as age increased. One possible explanation for this finding is that younger women typically have more dense breast tissue. This may prompt the decision to acquire additional imaging, as MRI has been shown to provide superior imaging in this setting.37–39 Another possible explanation for the tendency of younger women to receive MRI is that physicians may be inclined to request MRI for young women with strong family history of breast cancer. Previous studies have demonstrated MRI to have approximately twice the sensitivity of mammography when screening women at hereditary risk for breast cancer.40–42
In our study, age was also a significant independent predictor of mastectomy, with younger women more likely to undergo mastectomy. To further explore the factors that contributed to the younger age in the MRI group as well as the higher mastectomy rate among younger women, we investigated BRCA status as a possible explanation. A recent study by our group showed that BRCA-positive patients with DCIS were more than 12 years younger at diagnosis than those with DCIS in the general population.43 However, of the 38 women in our current study who underwent preoperative MRI, we found that none underwent BRCA testing at the UCSF Genetic Risk Program. This does not preclude the possibility that the patients were tested elsewhere, although they would have been most likely to be referred for genetic testing at our center. Thus, BRCA mutation status could not account for the increased use of MRI in younger patients in our cohort. The low identification rate of mutation carriers can in part be attributed to the fact that few patients with pure DCIS patients are referred for genetic testing, with only recent NCCN guidelines suggesting the value of genetic counseling in women with DCIS.43 Moreover, we found that family history of breast cancer did not differ significantly between the two groups.
Of 35 patients who underwent mastectomy in our study cohort, two, both of whom had preoperative MR imaging, elected to undergo contralateral prophylactic mastectomy (CPM). Neither had occult cancer on the contralateral side on final surgical pathology. A recent study reported a CPM rate of 13.5% for DCIS patients undergoing mastectomy from 1998 to 2005.44 This previous study suggested the increased use of breast MRI as a possible rationale for the increasing rate of CPM. While there could be a relationship between preoperative MRI and the decision favoring CPM, there were too few events in our study to reach any meaningful conclusions.
To investigate the accuracy of MRI in assessing DCIS size to guide surgical treatment, we compared the margin status of those undergoing preoperative MRI to those who did not. The percentage of tumor-negative margins, the size of closest margins, and the number of reexcisions did not significantly differ between both groups. We found no difference in surgical outcomes between the two groups, which raises the concern that breast MRI does not improve preoperative assessment of disease extent over mammography alone. Future studies evaluating comprehensive radiologic-pathologic correlation are needed to address this important issue.
Although our findings are of interest, we recognize that there are inherent limitations in the retrospective study design. As an observational study, we could not adjust for referral bias and indeed, we observed a clear association between MRI use and young patient age. In addition, we could not exclude a common covariate associated with age, referral for MRI, and choice of mastectomy; an example of such a variable might be the possibility of heightened anxiety in younger patients. MRI is not routinely indicated for newly diagnosed DCIS. Consequently, we are uncertain whether the choice of MRI was based on patient preference or physician recommendation, although the cohort was treated by 4 different fellowship-trained surgeons mitigating a provider bias. In this patient cohort, MRI remained the only factor which conferred a significant 3-fold increased odds of mastectomy. However, only a prospective trial assessing those factors which most affect decision-making will determine a causal relationship.
Conclusion
In conclusion, our data demonstrate that patients with DCIS who had preoperative MRI were more likely to undergo mastectomy and SLNB than those patients without preoperative MRI. Younger patients were also more likely to undergo mastectomy. Furthermore, we did not find any benefit in surgical outcome in favor of the preoperative MRI group with respect to the proportion of negative margins, margin size, and number of re-excisions. Until the benefits of preoperative MRI for patients with DCIS are prospectively evaluated, health care providers should carefully consider the possible unintended consequences of MRI before surgery for DCIS.
Figure 2.
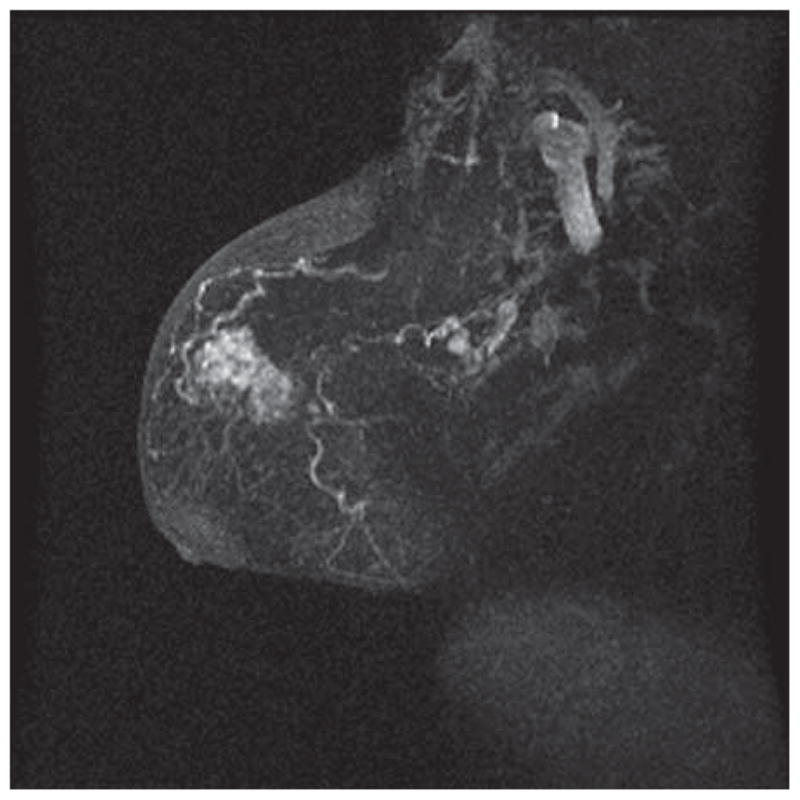
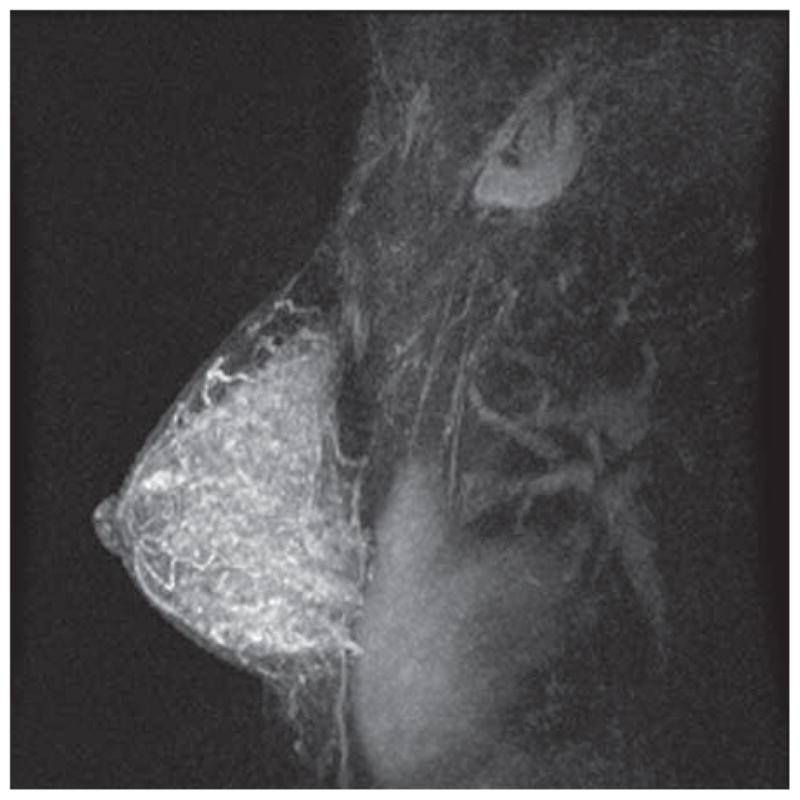
Figure 2A. MRI of a Postmenopausal Woman, With DCIS Clearly Visible as an Area of Enhancement in the Upper Breast
Figure 2B. MRI of a Premenopausal Woman with DCIS
The DCIS is visible but more difficult to identify amid extensive background enhancement. At surgical excision, the patient was found to have 1.3 cm of low-intermediate grade DCIS without invasion.
Acknowledgments
The authors thank the contributions of Alyssa Throckmorton for her thoughtful comments and edits on the manuscript, and Sachiko Suzuki for her assistance in procuring and selecting our figures. This research was supported by NIH K23 CA0977181-01A10 (ESH) and the Bay Area Breast Cancer SPORE P50 CA58207.
Footnotes
Disclosures
All authors have no conflicts of interest.
References
- 1.Burstein HJ, Polyak K, Wong JS, et al. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430–41. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- 2.Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94:1546–54. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 3.Rosner D, Bedwani RN, Vana J, et al. Noninvasive breast carcinoma: results of a national survey by the American College of Surgeons. Ann Surg. 1980;192:139–47. doi: 10.1097/00000658-198008000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izumori A, Takebe K, Sato A. Ultrasound findings and histological features of ductal carcinoma in situ detected by ultrasound examination alone. Breast Cancer. 2009 doi: 10.1007/s12282-009-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagashima T, Hashimoto H, Oshida K, et al. Ultrasound demonstration of mammographically detected microcalcifications in patients with ductal carcinoma in situ of the breast. Breast Cancer. 2005;12:216–20. doi: 10.2325/jbcs.12.216. [DOI] [PubMed] [Google Scholar]
- 6.Shin HJ, Kim HH, Kim SM, et al. Screening-detected and symptomatic ductal carcinoma in situ: differences in the sonographic and pathologic features. AJR Am J Roentgenol. 2008;190:516–25. doi: 10.2214/AJR.07.2206. [DOI] [PubMed] [Google Scholar]
- 7.Menell JH, Morris EA, Dershaw DD, et al. Determination of the presence and extent of pure ductal carcinoma in situ by mammography and magnetic resonance imaging. Breast J. 2005;11:382–90. doi: 10.1111/j.1075-122X.2005.00121.x. [DOI] [PubMed] [Google Scholar]
- 8.Schouten van der Velden AP, Boetes C, Bult P, et al. The value of magnetic resonance imaging in diagnosis and size assessment of in situ and small invasive breast carcinoma. Am J Surg. 2006;192:172–8. doi: 10.1016/j.amjsurg.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Hwang ES, Kinkel K, Esserman LJ, et al. Magnetic resonance imaging in patients diagnosed with ductal carcinoma-in-situ: value in the diagnosis of residual disease, occult invasion, and multicentricity. Ann Surg Oncol. 2003;10:381–8. doi: 10.1245/aso.2003.03.085. [DOI] [PubMed] [Google Scholar]
- 10.Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370:485–92. doi: 10.1016/S0140-6736(07)61232-X. [DOI] [PubMed] [Google Scholar]
- 11.Shiraishi A, Kurosaki Y, Maehara T, et al. Extension of ductal carcinoma in situ: histopathological association with MR imaging and mammography. Magn Reson Med Sci. 2003;2:159–63. doi: 10.2463/mrms.2.159. [DOI] [PubMed] [Google Scholar]
- 12.Hata T, Takahashi H, Watanabe K, et al. Magnetic resonance imaging for preoperative evaluation of breast cancer: a comparative study with mammography and ultrasonography. J Am Coll Surg. 2004;198:190–7. doi: 10.1016/j.jamcollsurg.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Bluemke DA, Gatsonis CA, Chen MH, et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA. 2004;292:2735–42. doi: 10.1001/jama.292.22.2735. [DOI] [PubMed] [Google Scholar]
- 14.Orel SG, Schnall MD. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology. 2001;220:13–30. doi: 10.1148/radiology.220.1.r01jl3113. [DOI] [PubMed] [Google Scholar]
- 15.Furman B, Gardner MS, Romilly P, et al. Effect of 0. 5 Tesla magnetic resonance imaging on the surgical management of breast cancer patients. Am J Surg. 2003;186:344–7. doi: 10.1016/s0002-9610(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 16.Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology. 1999;213:881–8. doi: 10.1148/radiology.213.3.r99dc01881. [DOI] [PubMed] [Google Scholar]
- 17.Van Goethem M, Schelfout K, Dijckmans L, et al. MR mammography in the preoperative staging of breast cancer in patients with dense breast tissue: comparison with mammography and ultrasound. Eur Radiol. 2004;14:809–16. doi: 10.1007/s00330-003-2146-7. [DOI] [PubMed] [Google Scholar]
- 18.Bilimoria KY, Cambic A, Hansen NM, et al. Evaluating the impact of preoperative breast magnetic resonance imaging on the surgical management of newly diagnosed breast cancers. Arch Surg. 2007;142:441–5. doi: 10.1001/archsurg.142.5.441. discussion 445–7. [DOI] [PubMed] [Google Scholar]
- 19.Peters NH, Borel Rinkes IH, Zuithoff NP, et al. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246:116–24. doi: 10.1148/radiol.2461061298. [DOI] [PubMed] [Google Scholar]
- 20.Hrung JM, Sonnad SS, Schwartz JS, Langlotz CP. Accuracy of MR imaging in the work-up of suspicious breast lesions: a diagnostic meta-analysis. Acad Radiol. 1999;6:387–97. doi: 10.1016/s1076-6332(99)80189-5. [DOI] [PubMed] [Google Scholar]
- 21.Kumar AS, Chen DF, Au A, et al. Biologic significance of false-positive magnetic resonance imaging enhancement in the setting of ductal carcinoma in situ. Am J Surg. 2006;192:520–4. doi: 10.1016/j.amjsurg.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esserman LJ, Kumar AS, Herrera AF, et al. Magnetic resonance imaging captures the biology of ductal carcinoma in situ. J Clin Oncol. 2006;24:4603–10. doi: 10.1200/JCO.2005.04.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pediconi F, Catalano C, Padula S, et al. Contrast-enhanced magnetic resonance mammography: does it affect surgical decision-making in patients with breast cancer? Breast Cancer Res Treat. 2007;106:65–74. doi: 10.1007/s10549-006-9472-9. [DOI] [PubMed] [Google Scholar]
- 24.Schelfout K, Van Goethem M, Kersschot E, et al. Contrast-enhanced MR imaging of breast lesions and effect on treatment. Eur J Surg Oncol. 2004;30:501–7. doi: 10.1016/j.ejso.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Bagley FH. The role of magnetic resonance imaging mammography in the surgical management of the index breast cancer. Arch Surg. 2004;139:380–3. doi: 10.1001/archsurg.139.4.380. discussion 383. [DOI] [PubMed] [Google Scholar]
- 26.Merchant TE, Obertop H, de Graaf PW. Advantages of magnetic resonance imaging in breast surgery treatment planning. Breast Cancer Res Treat. 1993;25:257–64. doi: 10.1007/BF00689840. [DOI] [PubMed] [Google Scholar]
- 27.Chung A, Saouaf R, Scharre K, et al. The impact of MRI on the treatment of DCIS. Am Surg. 2005;71:705–10. [PubMed] [Google Scholar]
- 28.Solin LJ, Orel SG, Hwang WT, et al. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol. 2008;26:386–91. doi: 10.1200/JCO.2006.09.5448. [DOI] [PubMed] [Google Scholar]
- 29.Dominguez FJ, Golshan M, Black DM, et al. Sentinel node biopsy is important in mastectomy for ductal carcinoma in situ. Ann Surg Oncol. 2008;15:268–73. doi: 10.1245/s10434-007-9610-6. [DOI] [PubMed] [Google Scholar]
- 30.Pendas S, Dauway E, Giuliano R, et al. Sentinel node biopsy in ductal carcinoma in situ patients. Ann Surg Oncol. 2000;7:15–20. doi: 10.1007/s10434-000-0015-z. [DOI] [PubMed] [Google Scholar]
- 31.Intra M, Veronesi P, Mazzarol G, et al. Axillary sentinel lymph node biopsy in patients with pure ductal carcinoma in situ of the breast. Arch Surg. 2003;138:309–13. doi: 10.1001/archsurg.138.3.309. [DOI] [PubMed] [Google Scholar]
- 32.Zavagno G, Carcoforo P, Marconato R, et al. Role of axillary sentinel lymph node biopsy in patients with pure ductal carcinoma in situ of the breast. BMC Cancer. 2005;5:28. doi: 10.1186/1471-2407-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: Effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tillman GF, Orel SG, Schnall MD, et al. Effect of breast magnetic resonance imaging on the clinical management of women with early-stage breast carcinoma. J Clin Oncol. 2002;20:3413–23. doi: 10.1200/JCO.2002.08.600. [DOI] [PubMed] [Google Scholar]
- 35.Bedrosian I, Mick R, Orel SG, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer. 2003;98:468–73. doi: 10.1002/cncr.11490. [DOI] [PubMed] [Google Scholar]
- 36.Del Frate C, Borghese L, Cedolini C, et al. Role of pre-surgical breast MRI in the management of invasive breast carcinoma. Breast. 2007;16:469–81. doi: 10.1016/j.breast.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Olivas-Maguregui S, Villasenor-Navarro Y, Ferrari-Carballo T, et al. Importance of the preoperative evaluation of multifocal and multicentric breast cancer with magnetic resonance imaging in women with dense parenchyma. Rev Invest Clin. 2008;60:382–9. [PubMed] [Google Scholar]
- 38.Kristoffersen Wiberg M, Aspelin P, Perbeck L, et al. Value of MR imaging in clinical evaluation of breast lesions. Acta Radiol. 2002;43:275–81. doi: 10.1080/j.1600-0455.2002.430308.x. [DOI] [PubMed] [Google Scholar]
- 39.Yoshikawa MI, Ohsumi S, Sugata S, et al. Comparison of breast cancer detection by diffusion-weighted magnetic resonance imaging and mammography. Radiat Med. 2007;25:218–23. doi: 10.1007/s11604-007-0128-4. [DOI] [PubMed] [Google Scholar]
- 40.Leach MO. Breast cancer screening in women at high risk using MRI. NMR Biomed. 2009;22:17–27. doi: 10.1002/nbm.1326. [DOI] [PubMed] [Google Scholar]
- 41.Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005;365:1769–78. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 42.Warner E, Messersmith H, Causer P, et al. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008;148:671–9. doi: 10.7326/0003-4819-148-9-200805060-00007. [DOI] [PubMed] [Google Scholar]
- 43.Hwang ES, McLennan JL, Moore DH, et al. Ductal carcinoma in situ in BRCA mutation carriers. J Clin Oncol. 2007;25:642–7. doi: 10.1200/JCO.2005.04.0345. [DOI] [PubMed] [Google Scholar]
- 44.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27:1362–7. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]