Abstract
Impaired facial emotion recognition abilities in HIV+ patients are well documented, but little is known about the neural etiology of these difficulties. We examined the relation of facial emotion recognition abilities to regional brain volumes in 44 HIV-positive (HIV+) and 44 HIV-negative control (HC) adults. Volumes of structures implicated in HIV− associated neuropathology and emotion recognition were measured on MRI using an automated segmentation tool. Relative to HC, HIV+ patients demonstrated emotion recognition impairments for fearful expressions, reduced anterior cingulate cortex (ACC) volumes, and increased amygdala volumes. In the HIV+ group, fear recognition impairments correlated significantly with ACC, but not amygdala volumes. ACC reductions were also associated with lower nadir CD4 levels (i.e., greater HIV-disease severity). These findings extend our understanding of the neurobiological substrates underlying an essential social function, facial emotion recognition, in HIV+ individuals and implicate HIV-related ACC atrophy in the impairment of these abilities.
Keywords: HIV, Emotion Perception, Structural Neuroimaging, Anterior Cingulate Cortex, Amygdala, Psychosocial Functions
1. INTRODUCTION
Several studies have reported reduced facial emotion recognition abilities in disorders that, like HIV infection (Clark & Cohen, 2010), are associated with frontostriatal dysfunction. Impairments have been noted in individuals with Parkinson’s disease (Clark, Neargarder, & Cronin-Golomb, 2008; Devlin et al., 2010; Dujardin et al., 2004; Lawrence, Goerendt, & Brooks, 2007; Sprengelmeyer et al., 2003), Huntington’s disease (Johnson et al., 2007; Sprengelmeyer et al., 1996), obsessive compulsive disorder (Sprengelmeyer et al., 1997), and HIV (Clark, Cohen, Westbrook, Devlin, & Tashima, 2010). Facial emotion recognition is a core component of the human experience (Darwin, 1872/1965). Accordingly, deficits in facial emotion recognition have been tied to increased interpersonal problems in a number of patient groups (Clark et al., 2008; Kornreich et al., 2002), including HIV (Clark et al., 2010).
Despite a clear link between facial emotion recognition abilities and quality of life metrics in HIV+ individuals (Clark et al., 2010), except for a handful of studies (Baldonero et al., 2013; Clark et al., 2010; Heilman, Harden, Weber, Cohen, & Porges, 2013; Lane, Moore, Batchelor, Brew, & Cysique, 2012), facial emotion recognition impairments in HIV+ cohorts have received little investigation. Results from these studies have been somewhat varied, with most reports indicating that HIV+ patients demonstrate impairments in recognizing negative emotions (Lane et al., 2012), particularly fear (Baldonero et al., 2013; Clark et al., 2010), and one indicating that facial emotion recognition impairments are non-specific (Heilman et al., 2013).
With only a few studies on the topic to date (Schulte, Müller-Oehring, Sullivan, & Pfefferbaum, 2012), there is a dearth of information regarding the neural etiologies of emotion processing abnormalities in HIV+ patients. Improved understanding of the neural correlates of these functions in HIV+ individuals would contribute both to our general understanding of HIV-associated neuropathology in relation to neuropsychiatric functions, and to our ability to properly conceptualize and treat these impairments. An important question relating to neuropsychiatric symptoms in HIV+ patients, which has yet to be answered fully, is whether affective difficulties have a purely neurologic or more psychogenic origin. Resolution of this issue would have strong treatment-related implications for HIV+ patients with psychiatric complaints. Thus, there is a clear need to investigate the neurobiological substrates of emotion processing impairments, including facial emotion recognition difficulties, in HIV+ patients in order to better elucidate these issues.
The current study tested the hypothesis that facial emotion recognition impairments are associated with brain volume abnormalities in HIV+ patients. Emotion processing involves a large network of brain regions (Adolphs, 2002b; Fusar-Poli et al., 2009), the primary core of which are also implicated in HIV-related neuropathology, including the amygdala (Ances, Ortega, Vaida, Heaps, & Paul, 2012; Clark et al., 2012), frontal cortices (Kallianpur et al., 2012; Kuper et al., 2011; Pfefferbaum et al., 2012), and basal ganglia structures (Ances et al., 2012; Becker et al., 2011). Using structural magnetic resonance imaging (MRI), we assessed the volumes of five brain regions affected by HIV infection that are also known to contribute heavily to facial emotion recognition processes. These key regions included the amygdala (Adolphs, 2002b; Adolphs, Tranel, Damasio, & Damasio, 1994; Ances et al., 2012; Clark et al., 2012), anterior cingulate cortex (Kallianpur et al., 2012; Kuper et al., 2011; Murphy, Nimmo-Smith, & Lawrence, 2003), orbitofrontal cortex (Adolphs, 2002a; Kallianpur et al., 2012; Tsuchida & Fellows, 2012), caudate (Ances et al., 2006; Becker et al., 2011; Fusar-Poli et al., 2009), and putamen (Becker et al., 2011; Fusar-Poli et al., 2009). Based on prior data indicating that HIV+ patients perform poorly on the recognition of negative emotions (Baldonero et al., 2013; Clark et al., 2010; Lane et al., 2012), and given the strong contribution of the anterior cingulate and amygdala to these functions in particular (Adolphs et al., 1994; Fusar-Poli et al., 2009; Phan, Wager, Taylor, & Liberzon, 2002), we hypothesized that HIV-related volumetric abnormalities in these two regions would correlate with emotion recognition impairments in our HIV+ sample.
2. METHOD
2.1 Participants
Participants included 44 HIV-seropositive (HIV+) and 44 demographically comparable HIV-negative control (HC) adults whose facial emotion recognition impairments have been characterized previously (Clark et al., 2010). The current group of 88 participants represents the sub-set of individuals from the original cohort of 100 participants (Clark et al., 2010) for whom MRI data were available. As noted in the original report, HIV+ participants were recruited from The Miriam Hospital Immunology Center. HC participants were acquaintances of HIV+ participants or were recruited from the community. All participants obtained greater than 23 points on the Mini-Mental Status Exam (MMSE) (Folstein, Folstein, & McHugh, 1975) and all were native English speakers, both of which were inclusion criteria for the study. Scores on the MMSE ranged from 25 to 30 in the HIV+ group, and from 26 to 30 in the HC group. Participants were excluded on the basis of reported history of uncorrected abnormal vision; developmental or learning disability; major psychiatric illness (e.g., schizophrenia, bipolar disorder); neurological illness affecting the central nervous system; and traumatic head injury with loss of consciousness >10 minutes. Substance use exclusion criteria included current alcoholism; use of heroin/opiates or any intravenous drug within the past 12 months; and use of cocaine within the past 3 months. The Miriam Hospital’s Institutional Review Board approved this research. All participants gave their informed consent and were financially compensated for their time. See Table 1 for details of participant characteristics.
Table 1.
Demographic Characteristics of the Participant Groups
| HIV+ Group (M/F = 29/15) |
HC Group (M/F = 28/16) |
|||
|---|---|---|---|---|
|
|
||||
| Variable | Mean | SD | Mean | SD |
| Age (years) | 46.4 | 8.0 | 44.3 | 12.1 |
| Range | 23-65 | 24-74 | ||
| Education (years) | 12.4 | 2.4 | 13.6 | 3.3 |
| % Right-handed | 91 | 89 | ||
| Ethnic composition (% Caucasian) | 48* | 73* | ||
| % African American | 32 | 16 | ||
| % Hispanic American | 2 | 2 | ||
| % Asian American | 2 | 2 | ||
| % Biracial or “Other” | 16 | 7 | ||
| Mini-Mental Status Exam (/30) | 28.3 | 1.1 | 28.5 | 1.1 |
| Length of infection (years) | 13.3 | 7.7 | n/a | |
| Nadir CD4 (cells/|μl) | 157 | 123 | n/a | |
| Current CD4 (cells/|μl) | 437.7 | 261.2 | n/a | |
| Viral load, Number of patients | ||||
| Undetectable (<75 copies/mL) | 35 | n/a | ||
| Detectable (≥75 copies/mL) | 8 | n/a | ||
| % on ARV medications | 86.4 | n/a | ||
| CESD (/60) | 20.2* | 15.3 | 13.0* | 11.4 |
| KMSK – Alcohol (/13) | 9.4 | 4.1 | 9.4 | 3.7 |
| KMSK – Cocaine (/16) | 10.2** | 6.1 | 4.6** | 6.1 |
| KMSK – Opiate (/13) | 3.1* | 4.7 | 1.3* | 3.2 |
| Benton Test of Facial Recognition (/27) | 21.9 | 2.6 | 22.5 | 2.3 |
Note: HC = HIV-negative Control; M/F = Male-Female; ARV = antiretroviral; CESD = Center for Epidemiologic Studies Depression Scale; KMSK = Kreek-McHugh-Schluger- Kellogg scale. Asterisks indicate that the groups’ means are significantly different at the level.
p≤.05
p≤.01
Disease duration in the HIV+ group was estimated using self-report; this information was verified against the medical record. Average length of HIV infection was 13.3 years. History of cART use, current CD4 levels, and nadir CD4 levels (i.e., the lowest ever CD4 count on record) were obtained from the medical record. Eighty-six percent (38/44) of HIV+ participants were taking antiretroviral (ARV) medications, with nearly all of these individuals (37/38) on two or more ARV medications. Current CD4 levels were available for all but one participant and ranged from 114 to 1320 cells/μl. Nadir CD4 levels ranged from 0 to 488 cells/μl.
Current depression symptoms were estimated using the Center for Epidemiologic Studies Depression Scale (CESD) (Radloff, 1977). CESD scores were available for all HC, and all but one HIV+ participant. Clinically significant levels of depression (CESD >15) were reported by 57% of the HIV+ participants and 27% of the HC participants. The Kreek-McHugh-Schluger-Kellogg scale (KMSK), which characterizes lifetime exposure to alcohol, cocaine, and opiates (Kellogg et al., 2003), estimated history of alcohol and drug use. In the HIV+ and HC groups, 57% and 55% respectively scored above the cutoff for lifetime dependence on alcohol (KMSK-Alcohol >10), meaning that about half of the participants likely met criteria for alcohol dependence at some point in their lifetime. In terms of cocaine use, 59% and 25% of the HIV+ and HC participants respectively met criteria for lifetime dependence (KMSK-Cocaine >10). Twenty-one percent and 7% of the HIV+ and HC participants respectively met criteria for lifetime opiate dependence (KMSK-Opiates >8).
2.2 Materials
Emotion Recognition
Facial emotion recognition was assessed by presenting participants with 84 Ekman and Friesen photographs (Ekman & Friesen, 1976) on a 17” laptop computer. Twelve (6 male, 6 female) images were displayed from each of the following six prototypic emotion categories: Anger, Disgust, Fear, Happy, Sad, and Surprise, plus Neutral. E-Prime software (http://www.pstnet.com) displayed the stimuli in a random order. Participants were asked to select the emotion label that best represented the emotion expressed in the image; responses were chosen from seven emotion labels displayed below each facial image. Participants responded orally, without a time limit. Responses were entered into the computer by the experimenter. Percent correct recognition was calculated for each facial emotion category.
Landscape Categorization Control
A non-emotional image categorization control task, known to be a suitable control for the emotion recognition task (Clark et al., 2008), was administered. Participants viewed 84 black-and-white photographs of landscape images, which are matched to the difficulty level of images in the emotion recognition task (Clark et al., 2008). Twelve images were presented from each of the following categories: Canyon, City, Forest, Mountain, Shore, Town and Tropical. Administration procedures were identical to those of the emotion recognition task. Participants selected the landscape category label that best described the image from the list of seven possible categories presented on the screen below each image. Data from two HC participants were lost as a result of technical errors.
Word-Definition Matching
A word-definition matching task was administered prior to the Emotion Recognition and Landscape Categorization tasks to ensure that all participants were able to adequately conceptualize the meaning of the terms used as responses in these tasks. Participants matched the seven emotion labels (Anger, Disgust, Fear, Happy, Sad, Surprise, Neutral) and seven landscape labels (Canyon, City, Forest, Mountain, Shore, Town, Tropical) to their definitions. All participants correctly matched these terms with their definitions.
Facial Recognition
General face perception abilities were assessed using the 13-item version of the Benton Test of Facial Recognition (A. L. Benton, Sivan, Hamsher, Varney, & Spreen, 1994). During this test participants viewed target photographs of unfamiliar faces and identified images of the target from a selection of six facial photographs below. Data from this measure were not available for 2 HIV+ and 3 HC participants.
2.3 Magnetic Resonance Imaging
MRI scans were conducted within two weeks of the completion of the emotion recognition tasks described above. Structural images of the brain were obtained using a 3-Tesla scanner (Siemens TIM Trio; Siemens, New York, NY). High-resolution T1-weighted MPRAGE images were acquired in the sagittal plane (resolution=0.86mm × 0.86mm × 0.86mm; TR=2250ms; TE=3.06ms; TI=900ms; flip angle=9°; FOV=220mm).
Brain volumes were estimated using the Individual Brain Atlases using Statistical Parametric Mapping Software (IBASPM) (Alemán-Gómez, Melie-García, & Valdés-Hernández, 2006) toolbox version 1.0 for Statistical Parametric Mapping 5 (SPM5; Wellcome Department of Imaging Science; www.fil.ion.ucl.ac.uk/spm/). The automated process involves segmenting individual brain volumes into gray matter, white matter, and cerebrospinal fluid, which were then normalized using nonlinear registration to the MNI152 template. This process permitted the calculation of volume estimates for each parcellation and for total intracranial volume. Brain segmentations were visually inspected for accuracy (e.g., correct alignment); none were discarded. Grey matter voxels were then aligned to a map of 116 anatomically labeled structures (Tzourio-Mazoyer et al., 2002), and inverse-transformed into their native spaces, yielding volume measures for individual brain structures in original space based on an MNI anatomical atlas. Volumetric data were examined for five regions of interest (ROI) bilaterally: amygdala, anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), caudate, and putamen (Figure 1). ROI volumes were corrected for total intracranial volume by dividing each by the total intracranial volume.
Figure 1.
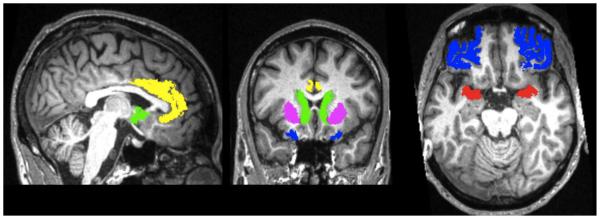
Note: Bilateral regions of interest (ROI) included the amygdala (red), anterior cingulate cortex (ACC; yellow), orbitofrontal cortex (OFC; extending into Brodmann areas 10, 11, and 47; blue), caudate (green), and putamen (violet). The images depict ROIs as defined for a typical participant in original space; sagittal (left), coronal (middle), and axial (right) views are shown in neurological orientation (right is right).
(Editorial note: Figure 1 is intended for color reproduction in print.)
2.4 Data Analysis
Independent-sample t-tests and chi-square tests assessed differences in demographic variables between the HIV+ and HC groups. A mixed design repeated measures ANOVA with factors of group (HIV+, HC) and emotion category (Anger, Disgust, Fear, Happy, Neutral, Sad, Surprise) examined performance on the facial emotion recognition task. Similarly, performance on the landscapes categorization task was examined using a mixed design repeated measures ANOVA with factors of group (HIV+, HC) and landscape category (Canyon, City, Forest, Mountain, Shore, Town, Tropical). This approach was also used to compare the HIV+ and HC groups’ ROI volumes, using factors of group (HIV+, HC) and ROI volume (amygdala, ACC, OFC, caudate, putamen). In these analyses, demographic variables that differed significantly according to group status were entered as covariates. Planned comparisons t-tests were conducted when a significant main effect of group or interaction effect was observed. For those ROIs demonstrating significant group differences, Pearson correlations were computed to explore the relation between HIV-disease factors (current CD4 levels, nadir CD4, length of HIV infection) and ROI volumes.
Linear regression was implemented to examine the association between ROI volumes and emotion recognition for those emotions in which significant group differences were observed. We restricted this analysis to the HIV+ group, as our goal was to understand the relation between brain volumes and facial emotion recognition deficits in HIV+ patients specifically. ROI volumes were entered into the model as independent variables, and performance on the emotion recognition task was entered as the dependent variable. Demographic variables that differed between the HIV+ and HC groups, and were also found to correlate with the HIV+ group’s performance on the emotion recognition task were included in the linear regression model in order to control for their potential contribution to emotion recognition performance.
3. RESULTS
3.1 Participant Characteristics
The HIV+ and HC groups did not differ significantly in age (t[74.6]=.98, p=.33), current cognitive status (MMSE score: t[86]=.99, p=.32), or gender (χ2=.00, p=1.00). There was a higher proportion of Caucasian to non-Caucasian participants in the HC group compared to the HIV+ group (χ2=4.74, p=.03). HIV+ participants showed a trend toward lower education levels (t[78.2]=1.91, p=.06), and reported significantly higher rates of depression (t[77.6]=2.50, p=.02). Significant group differences were not observed on the KMSK-Alcohol scale (KMSK-A, t[86]=.00, p=1.00); however, prior rates of cocaine (KMSK-C) and opiate (KMSK-O) use were significantly higher in the HIV+ compared to the HC group (t[86]=4.23, p<.001 and t[75.3]=2.14, p=.04, respectively). Facial perception skills did not differ significantly between HIV+ and HC (Benton Test: t[81]=1.22, p=.23). Table 1 shows mean raw scores for the HIV+ and HC groups on these measures.
3.2 Emotion Recognition and Landscape Categorization Measures
Consistent with findings from the larger cohort (Clark et al., 2010), HIV+ patients in this sub-sample exhibit a significant impairment in fear recognition. Table 2 shows group performances on the emotion recognition and landscapes categorization tasks. Analyses of the emotion recognition task revealed a significant main effect of group (F[1,86]=4.59, p=.03), a significant main effect of emotion category (F[6,516]=77.70, p<.001), and a non-significant group by emotion interaction (F[6,516]=1.68, p=.63). After correcting the model for group differences in education, the main effect of group was just above trend-level (F[1,85]=2.40, p=.06, one-tailed). Ethnicity (Caucasian/non-Caucasian), depression levels (CESD), and history of prior drug use (KMSK-C, KMSM-O) did not contribute significantly to the model when entered as covariates (p’s >.05) and were thus removed from the analyses. To examine the main effect of group, we conducted planned comparison t-tests, which indicated that the HIV+ group demonstrated significantly poorer recognition of fearful expressions compared to HC (t[86]=2.80, p=.006). The groups did not differ significantly in recognition scores for any other emotion (all p’s >.26). This group difference in fear recognition remained even after controlling for group differences in education levels, ethnicity, depression symptoms, prior cocaine use, and prior opiate use (F[1,80]=5.28, p=.02).
Table 2.
Performance on the Emotion Recognition and Matched Landscape Categorization Tasks
| HIV+ | HC | HIV+ | HC | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Emotion | Mean | SD | Mean | SD | Landscape | Mean | SD | Mean | SD |
| Anger | 75.7 | 18.3 | 79.9 | 18.0 | Canyon | 78.0 | 20.6 | 78.8 | 17.0 |
| Disgust | 79.0 | 19.5 | 83.3 | 16.6 | City | 80.3 | 14.9 | 83.9 | 11.6 |
| Fear | 51.7** | 21.0 | 64.0** | 20.1 | Tropical | 77.1 | 19.1 | 68.3 | 23.7 |
| Happy | 98.6 | 3.2 | 98.7 | 3.1 | Forest | 95.6 | 9.2 | 97.4 | 5.0 |
| Neutral | 89.1 | 15.3 | 91.5 | 10.9 | Mountain | 84.5 | 12.9 | 87.7 | 11.8 |
| Sad | 70.7 | 19.8 | 72.2 | 20.2 | Town | 73.7 | 17.5 | 71.4 | 20.5 |
| Surprise | 89.5 | 13.5 | 92.2 | 9.2 | Shore | 80.3 | 12.7 | 81.7 | 13.8 |
|
| |||||||||
| All Faces | 79.2 | 9.0 | 83.1 | 8.1 | All Landscapes | 81.4 | 7.9 | 81.3 | 5.4 |
Note: Mean percentage of facial expression and landscape images that were correctly identified by each group. HC = HIV-negative Control. Asterisks indicate that the groups’ means are significantly different at the level.
p≤.01
As in the original larger sample (Clark et al., 2010), HIV+ and HC groups in this sub-sample did not differ significantly in their performances on the non-emotion landscape categorization control task, where the main effect of group (F[1,84]=.001, p=.98), and group by landscape interaction (F[6,504]=1.70, p=.12) were non-significant. The addition of education level, ethnicity (Caucasian/non-Caucasian), CESD, and KMSK scores (KMSK-C, KMSK-O) as covariates did not significantly modify these results. Furthermore, independent t-tests did not reveal significant differences between the HIV+ and HC group’s abilities to categorize specific types of landscape stimuli (all p’s>.05). Trend-level differences were noted however for tropical images, on which the HIV+ group outperformed HC (t[84]=1.90, p=.06). This difference was reduced when controlling for the demographic variables on which the groups differed (F[1,78]=2.09, p=.15). Altogether, these results confirm the specificity of the HIV+ group’s deficits in fear recognition.
3.3 MRI Volumetrics
Mean ROI volumes for each group are shown in Table 3. Intracranial volumes did not differ significantly between groups (t[86]=1.10; p=.28), permitting valid group comparisons using intracranial-corrected ROI volumes. Results from the repeated measures ANOVA with factors of group (HIV+, HC) and ROI volume, covarying for differences in education, depression, ethnicity, and prior drug use (KMSK-C, KMSK-O), revealed a significant group by ROI interaction (F[4,320]=2.34, p=.05), a non-significant main effect of group (F[1,80]=1.66, p=.20), and a significant effect of ROI (F[4,320]=31.23 p<.001). These effects were investigated further using planned comparisons t-tests, which revealed that the HIV+ group demonstrated greater amygdala volumes (t[86]=2.02, p=.05), and reduced ACC volumes (t[86]=2.13, p=.04) compared to HC. Group differences in amygdala and ACC volumes were associated with medium effect sizes (r=.21 and .22, respectively), and were observed even after controlling for demographic differences in education, depression, ethnicity, and prior drug use (amygdala: F[1,80]=4.28, p=.04; ACC: F[1,80]=2.81, p=.10). No additional group differences in ROI volumes were significant (p’s>.05).
Table 3.
Mean ROI Volumes (cm3) for Each Group
| HIV+ Group | HC Group | |||
|---|---|---|---|---|
|
|
||||
| Brain Region (bilateral) | Mean | SD | Mean | SD |
| Orbitofrontal Cortex | 16.26 | 3.02 | 16.51 | 2.71 |
| Anterior Cingulate Cortex | 7.90* | 1.59 | 8.78* | 1.57 |
| Amygdala | 3.12* | 0.39 | 3.04* | 0.45 |
| Caudate | 8.32 | 1.56 | 8.33 | 1.24 |
| Putamen | 7.95 | 1.38 | 7.99 | 1.30 |
Note: ROI = region of interest; HC = HIV-negative Control. Asterisks indicate that the groups’ ROI means, when corrected for intracranial volume, are significantly different at the level.
p≤.05
3.4 Relation of Amygdala and ACC Volumes to HIV-Disease Characteristics
We computed Pearson correlations to examine the relation of HIV-disease factors (current CD4 levels, nadir CD4, length of HIV infection) to ACC and amygdala volumes in the HIV+ group. For the ACC, trend-level effects were seen for nadir CD4 levels (r[44]= .27, p=.08), indicating that those patients with a history of more severe immunological suppression tended to demonstrate smaller ACC volumes. A similar trend was observed for length of infection (r[44]= −.27, p=.08); however, this relation was rendered non-significant (r[41]= −.16, p=.31) after controlling for the effect of age. Correlations between amygdala volumes and HIV-disease factors were non-significant (all p’s >.05).
3.5 Relation of Amygdala and ACC Volumes to Fear Recognition in HIV+
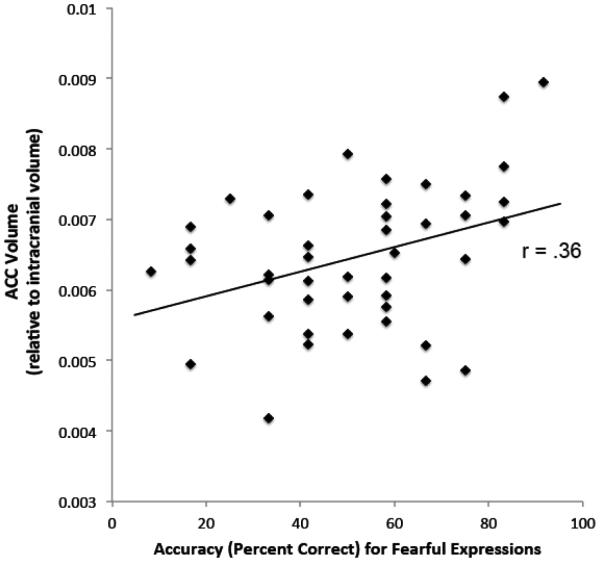
Our primary aim was to examine whether there was a relation between HIV patients’ emotion recognition impairments and ROI volumetric abnormalities. We thus conducted a linear regression analysis (R2=.13; F[2,41]=3.08, p=.06) to examine the relation of amygdala and ACC volumes to fear recognition abilities in the HIV+ group. Results revealed that fear recognition impairments were significantly related to reductions in the ACC (B=7,167.49, 95% Confidence Interval [CI]=905.83 – 13,429.16, β=.35 [t=2.31, p=.03]) (Figure 2), whereas the relation with amygdala volumes was non-significant (B=5,645.87, CI= −34,457.79 – 45,749.53, β=.04, [t=0.28, p=.78]). In this analysis, ACC volumes accounted for 11% of the variance in fear recognition. The relation between ACC volume reductions and fear recognition impairments was significant (B=6,315.87, CI=170.82 – 12,460.92, β=.31, [t=2.08, p=.04]) even after controlling for cocaine use history (B= −.69, CI= −1.73 – .35, β= −.20 [t= −1.35, p=.19]), which was the only demographic variable with between-group differences found to have a significant or trend-level correlation with fear recognition (r[44]= −.28, p=.07) in the HIV+ group. We further assessed whether the relation between ACC volumes and fear recognition was driven by their shared associations with nadir CD4 levels and, potentially, other markers of HIV-disease history and severity. We found that ACC volumes remained significantly related to fear recognition abilities (B=8,656.24, CI=2,473 – 14,838.53, β=.44, [t=2.83, p<.01]) even after controlling for HIV-disease factors (current CD4 levels, nadir CD4, length of HIV infection).
Figure 2.
Note: Relation between ACC volumes and fear recognition abilities in the HIV+ group. ACC = anterior cingulate cortex.
Amygdala and ACC volumes were not statistically related to accuracy in identifying any other emotion in the HIV+ group (all p’s >.05). Amygdala and ACC volumes did not correlate significantly with performance on any of the categories of the Landscape Categorization task (all r’s < |.20|, p's > .21).
3.6 Relation of ACC and Amygdala Volumes to Emotion Recognition in HC
As an exploratory measure in the HC group, Pearson correlations were computed to examine the relation of ACC and amygdala volumes to emotion recognition accuracy scores. A significant correlation was observed between amygdala volumes and accuracy at identifying neutral expressions (r[44]=.31, p=.04). No additional correlations were significant in the HC group.
3.7 Relation of Age to Emotion Recognition Abilities and ROI Volumes
Neuroimaging studies provide evidence of accelerated aging processes in HIV+ adults (Chang et al., 2014; Holt, Kraft-Terry, & Chang, 2012; Thomas, Brier, Snyder, Vaida, & Ances, 2013), with some indication that aging may increase HIV-related neural change (Clark & Cohen, 2010; Cysique et al., 2013). In addition, studies of facial emotion recognition in non-HIV samples indicate that older adults are less accurate at recognizing fearful expressions than younger adults (Calder et al., 2003; Circelli, Clark, & Cronin-Golomb, 2013). Accordingly, we assessed whether age was associated with performance on the Emotion Recognition task or with ROI volumes in HIV+ and HC groups using Pearson correlations. These results should be considered preliminary in light of our sample sizes and the fact that the study was not designed to examine age effects in our groups. Correlations between age and fear recognition were non-significant in the HIV+ and HC groups (r[44]= −.17, p= .26 and r[44]=.03, p=.87, respectively). In contrast, age correlated positively with sad recognition in the HIV+ group (r[44]=.35, p=.02) and disgust recognition in the HC group (r[44]=.33, p=.03), the latter of which is consistent with prior reports (Calder et al., 2003; Circelli et al., 2013; Suzuki, Hoshino, Shigemasu, & Kawamura, 2007). Correlations between age and performance on the Landscape Categorization task were non-significant in the HIV+ and HC groups (all p’s >.05), which parallels previous findings (Circelli et al., 2013).
Analyses of ROI volumes revealed that, in the HIV+ group, age correlated negatively with ACC and putamen volumes (r[44]= −.31, p=.04 and r[44]= −.36, p=.02, respectively). In the HC group, age correlated negatively with putamen and caudate volumes (r[44]= −.41, p<.01 and r[44]= −.35, p=.02, respectively). Because increasing age was associated with smaller ACC volumes in HIV+, we assessed whether age accounted for the observed relation between ACC volumes and fear recognition impairments in the HIV+ group using linear regression. This analysis indicated that ACC volumes remained significantly related to fear recognition in the HIV+ group, even after controlling for age (B=6,961.94, CI=595.49 – 13,328.39, β=.34, [t=2.21, p=.03]).
4. DISCUSSION
With the advent of cART, HIV has been transformed into a chronic illness with the possibility for long-term survival. In this context, greater emphasis is placed on reducing the burden of psychiatric difficulties in order to improve health-related quality of life for individuals with HIV (Briongos-Figuero, Bachiller-Luque, Palacios-Martin, De Luis-Roman, & Eiros-Bouza, 2011; Sherbourne et al., 2000). HIV+ adults suffer disproportionately higher rates of psychiatric symptoms (T. D. Benton, 2008; Bing et al., 2001; Ciesla & Roberts, 2001; Do et al., 2014; Morrison et al., 2002; Paul et al., 2005; Tate et al., 2003), as well as broader emotion processing impairments, including deficits in facial emotion recognition (Baldonero et al., 2013; Clark et al., 2010; Heilman et al., 2013; Lane et al., 2012). Facial emotion recognition difficulties in HIV+ adults are associated with increased interpersonal problems (Clark et al., 2010), which can exacerbate existing psychiatric symptoms (Fleishman et al., 2000; Richardson et al., 2001). Yet, the etiology of these difficulties remains largely unknown. Studies that seek to identify the neural correlates of facial emotion recognition difficulties in HIV+ adults are thus crucial, given their ability to elucidate the contribution of HIV-associated neuropathology to emotion processing impairments, and the potential for this knowledge to translate into improved conceptualizations of, and treatment approaches for HIV− related affective difficulties.
The current study examined the relation of facial emotion recognition impairments in HIV+ individuals to HIV-associated abnormalities in grey matter volume. Our MRI volumetric analyses focused on five critical brain regions known to be affected by HIV infection and also involved in facial emotion recognition processes, including the amygdala, anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), caudate, and putamen. A specific impairment in fear recognition was observed in the HIV+ sample – a finding that was initially documented in our previous study (Clark et al., 2010), from which a sub-sample of participants was drawn for the present investigation. New findings emerging from the current study include the observation that HIV+ patients demonstrate smaller ACC volumes and larger amygdala volumes compared to HIV-negative control (HC) participants. Moreover, we observed that ACC volume reductions were associated with fear recognition impairments in HIV+ individuals. We also report that individuals with a history of more severe immunosuppression (i.e., lower nadir CD4 levels) had smaller ACC volumes. This finding suggests that ACC reductions in the current sample are related to HIV infection and its neuropathological effects (Gonzalez-Scarano & Martin-Garcia, 2005), and further supports the link between low nadir CD4 levels and neural atrophy in HIV (Cohen et al., 2010; Jernigan et al., 2011). Taken as a whole, our results provide strong evidence that HIV-related atrophy in the ACC contributes to fear recognition impairments in HIV+ individuals.
A number of recent studies have reported facial emotion recognition impairments in individuals with HIV (Baldonero et al., 2013; Clark et al., 2010; Lane et al., 2012). Such findings are not unexpected given the well-characterized emotion recognition deficits in other neurological conditions with similar frontostriatal pathology, such as Parkinson’s disease, obsessive compulsive disorder, and Huntington’s disease (Clark et al., 2008; Clark, Oscar-Berman, Shagrin, & Pencina, 2007; Devlin et al., 2010; Dujardin et al., 2004; Johnson et al., 2007; Lawrence et al., 2007; Sprengelmeyer et al., 1996; Sprengelmeyer et al., 2003; Sprengelmeyer et al., 1997). Although neuropsychological evidence has been reported that suggests facial emotion recognition impairments in HIV+ adults are related to frontostriatal abnormalities (Baldonero et al., 2013; Lane et al., 2012), to our knowledge, the current study is the first to investigate the neural correlates of these impairments in HIV+ individuals using structural MRI.
Our observation that a selective deficit in fear recognition was associated with volume reductions in the ACC suggests that abnormalities in this region may underlie some of the facial emotion processing difficulties observed in HIV+ patients. These findings are in line with prior studies that have reported a relation between ACC abnormalities and facial emotion recognition impairments in other patient groups with frontostriatal dysfunction, including Parkinson’s disease (Baggio et al., 2012) and Huntington’s disease (Hobbs et al., 2011). Moreover, there is strong support for the involvement of the ACC, particularly the rostral ACC, in the processing of emotional stimuli such as faces. A meta-analysis of neuroimaging studies examining neural contributions to various emotional processing tasks found that paradigms requiring a cognitive component, such as emotional appraisal or rating of emotional stimuli, specifically engaged the ACC (Phan et al., 2002). These findings are supported by more recent functional MRI studies indicating that the ACC is reliably activated during facial emotion recognition tasks (Fusar-Poli et al., 2009; Murphy et al., 2003). The ACC has also been linked to social interactions and theory of mind (Beckmann, Johansen-Berg, & Rushworth, 2009). Taken together, our results are consistent with a larger literature implicating the ACC in psychosocial functions, particularly facial emotion recognition.
While our data indicate a strong relation between ACC volumes and fear recognition deficits in HIV+ patients, the specific contribution of the ACC to fear recognition remains unclear. The ACC shares extensive connections with the amygdala and OFC (Beckmann et al., 2009; Carmichael & Price, 1995; Vogt & Pandya, 1987). These two regions play a critical role in facial expressions recognition (Adolphs, 2002b), particularly for fearful expressions (Adolphs et al., 2005; Adolphs et al., 1999). In processing emotional stimuli, the amygdalae aid in determining the salience of a stimulus, and the OFC utilizes this information to guide decisions (Adolphs, 2010). Within this network, the ACC appears to be specifically recruited during affective tasks that possess a high cognitive-demand (e.g., rating emotional stimuli); its involvement is thought to support the integration of emotional and cognitive processes (Phan et al., 2002). In this way, the ACC is believed to help inhibit the processing of task-irrelevant information, particularly when processing ambiguous emotional stimuli (Bishop, Duncan, Brett, & Lawrence, 2004; Egner, Etkin, Gale, & Hirsch, 2008; Etkin, Egner, Peraza, Kandel, & Hirsch, 2006). Fearful expressions are unique in that they are the most difficult for healthy adults to categorize accurately within a forced-choice emotion labeling paradigm (Biehl et al., 1997; Rapcsak et al., 2000). As a result, fearful expressions are thought to be more ambiguous than other expressions in this context (Adolphs, 2002b). It is possible that these characteristics could lead to processing impairments, specifically for fearful expressions, in the context of ACC dysfunction. Interestingly, the role of the ACC in processing ambiguous stimuli is reported to be preferential for emotional (versus non-emotional) stimuli (Egner et al., 2008). In the current study, we observed a significant correlation between HIV+ patients’ ACC volumes and recognition performance for fearful expressions, whereas ACC volumes were unrelated to their performance on the equally challenging non-emotional task (i.e., Landscape Categorization). These results are consistent with, and provide support for, the notion of ACC specificity for resolving ambiguous affective stimuli (Egner et al., 2008). Accordingly, we posit that ACC atrophy might disrupt processes involved in resolving ambiguous affective stimuli, leading to the observed impairment in fear recognition in the HIV+ group. Further examination of the ACC and its network, using additional MRI techniques (e.g., fMRI, DTI), is needed to provide greater clarity regarding the ways in which ACC abnormalities might contribute to facial emotion recognition impairments in HIV+ patients.
Our results revealed relative increases in amygdala volumes in the HIV+ group when compared to HC, yet notably these enlargements were statistically unrelated to fear recognition impairments. Although amygdala enlargements have been associated with cognitive impairments in HIV+ patients (Clark et al., 2012), studies examining affective functions in non-HIV samples have indicted that larger amygdala volumes are associated with improved social functions in otherwise healthy adults (Bickart, Wright, Dautoff, Dickerson, & Barrett, 2011; Kanai, Bahrami, Roylance, & Rees, 2012; Von Der Heide, Vyas, & Olson, 2014). It is well documented that the amygdalae support social interactions and are critical to emotional information processing (Adolphs, 2010; Baxter & Croxson, 2012; Fossati, 2012). In this context, our data provide strong evidence that HIV-related increases in amygdala volumes do not underlie the observed impairments in facial emotion recognition. While our data provide the first indication that HIV-related amygdala enlargements are not associated with facial emotion recognition impairments in HIV+ patients, further investigation is required to determine whether increases in amygdala volumes may be associated with other affective and/or psychological manifestations, such as increased anxiety rates (Schienle, Ebner, & Schafer, 2011), in HIV+ patients.
In summary, the current findings extend our understanding of the neural substrates of facial emotion recognition deficits in individuals with HIV. This study provides the first evidence for a structural basis of HIV-related deficits in facial emotion recognition, and specifically implicates ACC volume reductions in these processes. We suggest that HIV-related ACC atrophy might compromise functions within the extended neural network that supports facial emotion recognition, and could thereby contribute to difficulties in processing facial expressions of fear. The current findings extend prior work (e.g., Paul et al., 2005) indicating that psychological difficulties, which occur disproportionately in HIV+ patients (T. D. Benton, 2008; Bing et al., 2001; Ciesla & Roberts, 2001; Clark et al., 2010; Do et al., 2014; Morrison et al., 2002; Paul et al., 2005; Tate et al., 2003) and significantly impact quality of life (Clark et al., 2010), might arise from HIV-associated neurological changes, as opposed to being purely psychogenic. Additional studies that utilize a variety of neuroimaging techniques (e.g., fMRI, MRS, DTI, etc.) are needed to provide a fuller understanding of the neuropathological etiology of facial emotion recognition impairments in HIV+ adults. It is likely that alterations in affective functions do not only result from regional neuroapoptosis and morphometric abnormalities, but may also evolve through additional mechanisms implicated in the development of HIV-associated neurological change, including regional alterations in neuronal excitability and changes in specific neurotransmitter systems (Berger & Arendt, 2000; Gelman et al., 2012; Hammoud et al., 2010; Keblesh, Hu, & Xiong, 2009). Future studies that illuminate these processes will increase our understanding of the neural etiologies of HIV-related psychosocial impairments, which may foster the development of novel therapeutics and interventions aimed at combating these impairments.
Highlights.
Neural correlates of facial emotion recognition (FER) deficits in HIV are unknown
FER was tested in non-demented HIV+ and control adults
Brain regions implicated in HIV-related neuropathology and FER were measured on MRI
Fear recognition deficits were associated with ACC atrophy in the HIV group
Results support the neural etiology of emotion processing impairments in HIV
Acknowledgements
This work was supported by the National Institutes of Health (Grants K23 MH096628, R01 MH074368-02S1, R01 MH074368, P01 AA019072, and P30 AI042853). We thank all of the individuals who participated in this study. Timothy P. Flanigan, MD and the medical team at The Miriam Hospital Immunology Center provided valuable aid in our recruitment efforts. Preliminary data from this study were presented at the annual meeting of the International Neuropsychological Society, 2011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002a;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Recognizing emotion from facial expressions: Psychological and neurological mechanisms. Behavioral and Cognitive Neuroscience Reviews. 2002b;1:21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, Anderson A, Lee GP, Damasio AR. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59:469–477. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Roc AC, Wang J, Korczykowski M, Okawa J, Stern J, Kim J, Wolf R, Lawler K, Kolson DL, Detre JA. Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology. 2006;66:862–866. doi: 10.1212/01.wnl.0000203524.57993.e2. [DOI] [PubMed] [Google Scholar]
- Baggio HC, Segura B, Ibarretxe-Bilbao N, Valldeoriola F, Marti MJ, Compta Y, Tolosa E, Junque C. Structural correlates of facial emotion recognition deficits in Parkinson's disease patients. Neuropsychologia. 2012;50:2121–2128. doi: 10.1016/j.neuropsychologia.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Baldonero E, Ciccarelli N, Fabbiani M, Colafigli M, Improta E, D'Avino A, Mondi A, Cauda R, Di Giambenedetto S, Silveri MC. Evaluation of emotion processing in HIV-infected patients and correlation with cognitive performance. BMC Psychology. 2013;1:1–7. doi: 10.1186/2050-7283-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Croxson PL. Facing the role of the amygdala in emotional information processing. Proc Natl Acad Sci U S A. 2012;109:21180–21181. doi: 10.1073/pnas.1219167110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Cohen B, Goodkin K, Martin E, Miller EN, Sacktor N, Alger JR, Barker PB, Saharan P, Carmichael OT, Thompson PM. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav. 2011;5:77–85. doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher KS, Varney NR, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. Second Oxford University Press; New York: 1994. [Google Scholar]
- Benton TD. Depression and HIV/AIDS. Curr Psychiatry Rep. 2008;10:280–285. doi: 10.1007/s11920-008-0045-y. [DOI] [PubMed] [Google Scholar]
- Berger JR, Arendt G. HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol. 2000;14:214–221. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat Neurosci. 2011;14:163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biehl M, Matsumoto D, Ekman P, Hearn V, Heider K, Kudoh T, Ton V. Matsumoto and Ekman's Japanese and Caucasian Facial Expressions of Emotion (JACFEE): Reliability Data and Cross-National Differences. Journal of Nonverbal Behavior. 1997;21:3–21. [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of general psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Briongos-Figuero LS, Bachiller-Luque P, Palacios-Martin T, De Luis-Roman D, Eiros-Bouza JM. Depression and health related quality of life among HIV-infected people. Eur Rev Med Pharmacol Sci. 2011;15:855–862. [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manly T, Sprengelmeyer R, Scott S, Nimmo-Smith I, Young AW. Facial expression recognition across the adult life span. Neuropsychologia. 2003;41:195–202. doi: 10.1016/s0028-3932(02)00149-5. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Chang L, Jiang C, Cunningham E, Buchthal S, Douet V, Andres M, Ernst T. Effects of APOE epsilon4, age, and HIV on glial metabolites and cognitive deficits. Neurology. 2014;82:2213–2222. doi: 10.1212/WNL.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158:725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Circelli KS, Clark US, Cronin-Golomb A. Visual scanning patterns and executive function in relation to facial emotion recognition in aging. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2013;20:148–173. doi: 10.1080/13825585.2012.675427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Cohen RA. Brain dysfunction in the era of combination antiretroviral therapy: implications for the treatment of the aging population of HIV-infected individuals. Curr Opin Investig Drugs. 2010;11:884–900. [PMC free article] [PubMed] [Google Scholar]
- Clark US, Cohen RA, Sweet LH, Gongvatana A, Devlin KN, Hana GN, Westbrook ML, Mulligan RC, Jerskey BA, White TL, Navia B, Tashima KT. Effects of HIV and early life stress on amygdala morphometry and neurocognitive function. J Int Neuropsychol Soc. 2012;18:657–668. doi: 10.1017/S1355617712000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Cohen RA, Westbrook ML, Devlin KN, Tashima KT. Facial emotion recognition impairments in individuals with HIV. J Int Neuropsychol Soc. 2010;16:1127–1137. doi: 10.1017/S1355617710001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Neargarder S, Cronin-Golomb A. Specific impairments in the recognition of emotional facial expressions in Parkinson’s disease. Neuropsychologia. 2008;46:2300–2309. doi: 10.1016/j.neuropsychologia.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Oscar-Berman M, Shagrin B, Pencina M. Alcoholism and judgments of affective stimuli. Neuropsychology. 2007;21:346–362. doi: 10.1037/0894-4105.21.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol. 2010;16:25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Moffat K, Moore DM, Lane TA, Davies NW, Carr A, Brew BJ, Rae C. HIV, vascular and aging injuries in the brain of clinically stable HIV-infected adults: a (1)H MRS study. PLoS One. 2013;8:e61738. doi: 10.1371/journal.pone.0061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The Expression of the Emotions in Man and Animals. University of Chicago Press; Chicago: 1872/1965. [Google Scholar]
- Devlin KN, Gongvatana A, Clark US, Westbrook ML, Tashima KT, Cohen RA. Center for AIDS Research Joint Symposium on HIV Research in Women. Chicago, IL: 2010. Gender Differences in HIV-Associated Cognitive Impairment. [Google Scholar]
- Do AN, Rosenberg ES, Sullivan PS, Beer L, Strine TW, Schulden JD, Fagan JL, Freedman MS, Skarbinski J. Excess burden of depression among HIV-infected persons receiving medical care in the united states: data from the medical monitoring project and the behavioral risk factor surveillance system. PLoS One. 2014;9:e92842. doi: 10.1371/journal.pone.0092842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin K, Blairy S, Defebvre L, Duhem S, Noel Y, Hess U, Destee A. Deficits in decoding emotional facial expressions in Parkinson's disease. Neuropsychologia. 2004;42:239–250. doi: 10.1016/s0028-3932(03)00154-4. [DOI] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Fleishman JA, Sherbourne CD, Crystal S, Collins RL, Marshall GN, Kelly M, Bozzette SA, Shapiro MF, Hays RD. Coping, conflictual social interactions, social support, and mood among HIV-infected persons. HCSUS Consortium. Am J Community Psychol. 2000;28:421–453. doi: 10.1023/a:1005132430171. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fossati P. Neural correlates of emotion processing: from emotional to social brain. Eur Neuropsychopharmacol. 2012;22(Suppl 3):S487–491. doi: 10.1016/j.euroneuro.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH, Jr., Soukup VM. Prefrontal dopaminergic and enkephalinergic synaptic accommodation in HIV-associated neurocognitive disorders and encephalitis. J Neuroimmune Pharmacol. 2012;7:686–700. doi: 10.1007/s11481-012-9345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Hammoud DA, Endres CJ, Hammond E, Uzuner O, Brown A, Nath A, Kaplin AI, Pomper MG. Imaging serotonergic transmission with [11C]DASB-PET in depressed and non-depressed patients infected with HIV. Neuroimage. 2010;49:2588–2595. doi: 10.1016/j.neuroimage.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KJ, Harden ER, Weber KM, Cohen M, Porges SW. Atypical autonomic regulation, auditory processing, and affect recognition in women with HIV. Biol Psychol. 2013 doi: 10.1016/j.biopsycho.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs NZ, Pedrick AV, Say MJ, Frost C, Dar Santos R, Coleman A, Sturrock A, Craufurd D, Stout JC, Leavitt BR, Barnes J, Tabrizi SJ, & Scahill RI. The structural involvement of the cingulate cortex in premanifest and early Huntington's disease. Mov Disord. 2011;26:1684–1690. doi: 10.1002/mds.23747. [DOI] [PubMed] [Google Scholar]
- Holt JL, Kraft-Terry SD, Chang L. Neuroimaging studies of the aging HIV-1-infected brain. J Neurovirol. 2012;18:291–302. doi: 10.1007/s13365-012-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR, Jr., Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011 doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Stout JC, Solomon AC, Langbehn DR, Aylward EH, Cruce CB, Ross CA, Nance M, Kayson E, Julian-Baros E, Hayden MR, Kieburtz K, Guttman M, Oakes D, Shoulson I, Beglinger L, Duff K, Penziner E, Paulsen JS. Beyond disgust: impaired recognition of negative emotions prior to diagnosis in Huntington's disease. Brain. 2007;130:1732–1744. doi: 10.1093/brain/awm107. [DOI] [PubMed] [Google Scholar]
- Kallianpur KJ, Kirk GR, Sailasuta N, Valcour V, Shiramizu B, Nakamoto BK, Shikuma C. Regional cortical thinning associated with detectable levels of HIV DNA. Cereb Cortex. 2012;22:2065–2075. doi: 10.1093/cercor/bhr285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Roylance R, Rees G. Online social network size is reflected in human brain structure. Proc Biol Sci. 2012;279:1327–1334. doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keblesh J, Hu D, Xiong H. Voltage-gated potassium channels in human immunodeficiency virus type-1 (HIV-1)-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2009;4:60–70. doi: 10.1007/s11481-008-9106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Philippot P, Foisy ML, Blairy S, Raynaud E, Dan B, Hess U, Noel X, Pelc I, Verbanck P. Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol and alcoholism. 2002;37:394–400. doi: 10.1093/alcalc/37.4.394. [DOI] [PubMed] [Google Scholar]
- Kuper M, Rabe K, Esser S, Gizewski ER, Husstedt IW, Maschke M, Obermann M. Structural gray and white matter changes in patients with HIV. J Neurol. 2011;258:1066–1075. doi: 10.1007/s00415-010-5883-y. [DOI] [PubMed] [Google Scholar]
- Lane TA, Moore DM, Batchelor J, Brew BJ, Cysique LA. Facial emotional processing in HIV infection: relation to neurocognitive and neuropsychiatric status. Neuropsychology. 2012;26:713–722. doi: 10.1037/a0029964. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Goerendt IK, Brooks DJ. Impaired recognition of facial expressions of anger in Parkinson's disease patients acutely withdrawn from dopamine replacement therapy. Neuropsychologia. 2007;45:65–74. doi: 10.1016/j.neuropsychologia.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Morrison MF, Petitto JM, Ten Have T, Gettes DR, Chiappini MS, Weber AL, Brinker-Spence P, Bauer RM, Douglas SD, Evans DL. Depressive and anxiety disorders in women with HIV infection. Am J Psychiatry. 2002;159:789–796. doi: 10.1176/appi.ajp.159.5.789. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Paul RH, Brickman AM, Navia B, Hinkin C, Malloy PF, Jefferson AL, Cohen RA, Tate DF, Flanigan TP. Apathy is associated with volume of the nucleus accumbens in patients infected with HIV. J Neuropsychiatry Clin Neurosci. 2005;17:167–171. doi: 10.1176/appi.neuropsych.17.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, Sullivan EV. Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biological psychiatry. 2012;72:361–370. doi: 10.1016/j.biopsych.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rapcsak SZ, Galper SR, Comer JF, Reminger SL, Nielsen L, Kaszniak AW, Verfaellie M, Laguna JF, Labiner DM, Cohen RA. Fear recognition deficits after focal brain damage: a cautionary note. Neurology. 2000;54:575–581. doi: 10.1212/wnl.54.3.575. [DOI] [PubMed] [Google Scholar]
- Richardson J, Barkan S, Cohen M, Back S, FitzGerald G, Feldman J, Young M, Palacio H. Experience and covariates of depressive symptoms among a cohort of HIV infected women. Soc Work Health Care. 2001;32:93–111. doi: 10.1300/J010v32n04_05. [DOI] [PubMed] [Google Scholar]
- Schienle A, Ebner F, Schafer A. Localized gray matter volume abnormalities in generalized anxiety disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261:303–307. doi: 10.1007/s00406-010-0147-5. [DOI] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Sullivan EV, Pfefferbaum A. White matter fiber compromise contributes differentially to attention and emotion processing impairment in alcoholism, HIV-infection, and their comorbidity. Neuropsychologia. 2012;50:2812–2822. doi: 10.1016/j.neuropsychologia.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbourne CD, Hays RD, Fleishman JA, Vitiello B, Magruder KM, Bing EG, McCaffrey D, Burnam A, Longshore D, Eggan F, Bozzette SA, Shapiro MF. Impact of psychiatric conditions on health-related quality of life in persons with HIV infection. Am J Psychiatry. 2000;157:248–254. doi: 10.1176/appi.ajp.157.2.248. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Calder AJ, Karnat A, Lange H, Homberg V, Perrett DI, Rowland D. Loss of disgust. Perception of faces and emotions in Huntington's disease. Brain. 1996;119:1647–1665. doi: 10.1093/brain/119.5.1647. Pt 5. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Mahn K, Schroeder U, Woitalla D, Buttner T, Kuhn W, Przuntek H. Facial expression recognition in people with medicated and unmedicated Parkinson's disease. Neuropsychologia. 2003;41:1047–1057. doi: 10.1016/s0028-3932(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Pundt I, Sprengelmeyer A, Calder AJ, Berrios G, Winkel R, Vollmoeller W, Kuhn W, Sartory G, Przuntek H. Disgust implicated in obsessive-compulsive disorder. Proc Biol Sci. 1997;264:1767–1773. doi: 10.1098/rspb.1997.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Hoshino T, Shigemasu K, Kawamura M. Decline or improvement? Age-related differences in facial expression recognition. Biol Psychol. 2007;74:75–84. doi: 10.1016/j.biopsycho.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Tate D, Paul RH, Flanigan TP, Tashima K, Nash J, Adair C, Boland R, Cohen RA. The impact of apathy and depression on quality of life in patients infected with HIV. AIDS Patient Care STDS. 2003;17:115–120. doi: 10.1089/108729103763807936. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013;80:1186–1193. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Are you upset? Distinct roles for orbitofrontal and lateral prefrontal cortex in detecting and distinguishing facial expressions of emotion. Cereb Cortex. 2012;22:2904–2912. doi: 10.1093/cercor/bhr370. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Von Der Heide R, Vyas G, Olson IR. The social network-network: size is predicted by brain structure and function in the amygdala and paralimbic regions. Soc Cogn Affect Neurosci. 2014 doi: 10.1093/scan/nsu009. [DOI] [PMC free article] [PubMed] [Google Scholar]