Abstract
High affinity antibodies result from interactions between B cells and T follicular helper (Tfh) cells in germinal centers (GCs). Recent studies have identified an effector subset of T regulatory cells termed T follicular regulatory (Tfr) cells that specifically controls GC responses by suppressing Tfh and B cells. The discovery of Tfr cells has shed new light on pathways regulating humoral immunity that enable potent and specific responses to pathogens while restricting autoimmunity. Here, we review the current understanding of the cellular and molecular mechanisms underlying the differentiation and function of Tfr cells. In this context we discuss recent insights into the role of Tfh cells in disease, how this knowledge may be translated therapeutically, and important areas of further research.
Keywords: Humoral Immunity, Vaccination, regulatory T cells, Tfr, Tfh, Germinal Center
New Insights into Regulation of B Cell Responses
Production of high-affinity class-switched antibodies and memory B cells is essential for clearance of pathogens and immunity elicited by vaccination. These antibodies, as well as memory B cells, are produced during a multistep process called the germinal center (GC) reaction. During the GC reaction, B cells interact with T follicular helper (Tfh) cells, which specialize in providing B cell help. These interactions result in somatic hypermutation, affinity maturation and class switch recombination. The antibodies produced can clear invading pathogens through neutralization, opsonization, and/or antibody dependent cell cytotoxicity (ADCC)[1, 2].
Elegant studies of B cell and Tfh cell dynamics in the germinal center have elucidated many key steps in the GC response [3-5]. The GC reaction requires regulation so as to ensure appropriate levels of antibody production, while limiting inflammation and autoimmunity. Central tolerance is one mechanism that prevents autoimmunity because self-reactive T and B cells are largely deleted. Some self-reactive cells, however, escape into the periphery[6]. In the periphery, the requirement for innate receptor-mediated “stranger/danger” signals for antibody production is another mechanism that prevents autoimmunity [7, 8]. These mechanisms only partially control B cell responses. It has been hypothesized that more direct regulation is necessary to control the GC. Therefore, a central question has been how are GC B and Tfh cells regulated after the start of the germinal center reaction?
Recent work has identified a subset of CD4+ T regulatory cells that potently and specifically inhibit B cells responses [9-11]. Here we review the current understanding of the phenotype and functions of these cells, termed T follicular regulatory (Tfr) cells. We discuss how Tfr cells exert their suppressive functions, the roles of Tfr cells in health and disease, and important areas for future inquiry.
Discovery of Tfr cells
Studies of FoxP3+ T regulatory (Treg) cells suggested that Treg cells may control B cell responses. The absence of Treg cells results in increased antibody production. Scurfy and FoxP3 knockout mice (which lack Treg cells due to absence of functional FoxP3 expression) have sharp increases in serum IgG1 and IgE levels[12, 13] basally, and in the context of allergic responses[14]. Similarly, patients with IPEX syndrome (in which Treg cells are lacking) have a broad spectrum of autoantibodies in their sera [15, 16]. Treg-specific deletion (using a FoxP3-driven Cre strain) of molecules important in Treg effector function, such as IRF4 or CTLA-4, also results in heightened levels of serum IgG and IgE antibodies [17, 18].
Complementary studies linked Treg populations with B cell responses. Human CD57-CD69-CD25+CD4+ cells (which are enriched for Treg populations) can inhibit IgA production and AID expression when cultured with B cells[19]. Murine CD25+CD4+ cells can kill B cells through cytolysis [20]. FoxP3+ cells can express CXCR5 and be found in GCs of human tonsils or immunized mice [19, 21, 22]. However, it was not clear whether the CXCR5-expressing Treg cells represented a specialized cell subset or represented Tregs that entered GCs stochastically. Also unclear was whether CXCR5+ Treg cells could specifically suppress B cell responses in vivo. These ambiguities reflected the lack of functional and definitive experiments and the need for strategies to rigorously purify these cells.
In 2011, three separate papers described a specialized population of Treg cells that express CXCR5, Bcl6, PD-1 and ICOS, and therefore phenotypically resembled Tfh cells (Table 1) [9-11]. By demonstrating that Bcl6, SAP or B cell deficient mice lack CXCR5+FoxP3+cells (but not Treg cells), and that CXCR5+FoxP3+ cells have a distinct transcriptional signature compared to other Treg cells, these studies conclusively showed that CXCR5+FoxP3+ cells are a distinct effector subset of Treg cells, T Follicular regulatory (Tfr) cells[9-11]. Demonstrating specialized in vivo function was essential for proving that Tfr cells were a distinct lineage/subset. Three initial in vivo experiments showed that Tfr cells specifically inhibit B cell responses. Chung et al. performed an adoptive transfer experiment in which CD4 T cells were transferred with WT or Bcl6−/− CD25hi (i.e. Treg enriched) CD4 T cells into Tcrb−/− mice that were immunized with NP-KLH [10]. Recipients of Bcl6−/− CD25hi cells (which cannot form Tfr cells) exhibited higher antigen-specific antibody production. Linterman et al. generated chimeric mice (in which Rag2−/− mice were reconstituted with FoxP3-DTR and either WT or SAP deficient cells) that were immunized with sheep red blood cells (SRBCs) and given tamoxifen to delete FoxP3 Tregs [9]. The SAP deficient chimeras (which cannot generate Tfr cells) had elevated germinal center B cells. Wollenberg et al. used an adoptive transfer approach in which OTII+ CD4 T cells were transferred along with WT or Cxcr5−/− FoxP3+ Tregs to Tcra−/− recipients which were immunized with OVA. The Cxcr5−/− Treg group had substantially increased antigen-specific antibody levels[11]. Together, these initial Tfr studies not only elucidated the precise phenotype of Tfr cells, but also demonstrated their specialized function in suppressing B cell responses in vivo.
Table 1.
Molecular phenotype of Tfr cells as compared to naïve CD4+ T cells, Tfh cells and Treg cells
| Tfr | Treg | Tfh | Tnaive | Reference | |
|---|---|---|---|---|---|
| CD4 | ++ | ++ | ++ | ++ | [9-11, 24] |
| CXCR5 | ++ | − | +++ | − | [9-11, 24, 25] |
| FoxP3 | ++ | ++ | − | − | [9-11, 24] |
| ICOS | +++ | + | ++ | + | [9, 24, 25] |
| PD1 | ++ | −,+ | ++ | − | [9-11, 24, 87] |
| Bcl6 | + | − | ++ | − | [9, 10] |
| Blimp1 | + | + | − | − | [9, 24, 48] |
| CTLA-4 | +++ | ++ | + | − | [9, 40, 41] |
| CD25 | ++ | +++ | − | − | [9, 24] |
| GITR | +++ | ++ | − | − | [9-11, 24] |
| Ki67 | ++ | −,+ | +++ | −,+ | [11, 24] |
| CD44 | ++ | − | ++ | − | [10, 24] |
| IL-21 | − | − | + | − | [9] |
Currently, Tfr cells are defined as an effector subset of Tregs that express CXCR5, which directs them by gradients of CXCL13 to migrate to GCs and suppress B cell responses. Tfr cells phenotypically resemble Tfh cells: both Tfr and Tfh cells express CXCR5, PD-1, ICOS and Bcl6 (Table 1) (Box 1). Although phenotypically similar, Tfr cells originate from natural Treg precursors, whereas Tfh cells originate from FoxP3-naïve CD4 T cells[9, 10, 23]. Tfr cells can be distinguished from Tfh cells by expression of FoxP3, CD25 and/or GITR[9-11, 24].
Box 1. Tfr Cell Gating and Analysis.
Gating Tfr cells by flow cytometry can be difficult, due to poor detection reagents for CXCR5 expression. Currently, Tfr cells are defined by a number of different methods that vary with experimental model and anatomical location. Each method requires CXCR5 and FoxP3 staining, as well as staining for a costimulatory receptor or Bcl6. For CXCR5 expression, most laboratories use a biotin-conjugated anti-CXCR5 followed by a streptavidin secondary reagent to boost signal. CXCR5 staining should be confirmed with knockout mice (such as CXCR5−/− or mice that lack Tfr cells such as ICOS−/−, CD28−/−, Bcl6−/− mice) or suitable controls because autofluorescence and/or spectral compensation may give false CXCR5 positivity. Recently, protocols for staining have been published [72, 73]. Additionally, bona fide Tfr cells should be validated through functional assays.
When quantifying Tfr cells four parameters are informative: total cell numbers, percent of total CD4 T cells, percent of FoxP3+ CD4 Treg cells and percent of CD4+CXCR5+ (follicular T) cells. Total numbers indicate relative differentiation/expansion among experimental groups. Percent of total CD4 T cells provides an indication of relative Tfr cell development compared to total CD4 T cells. Percent of FoxP3+ CD4 Treg indicates Tfr cell development versus expansion of precursor Treg cells. Percent of CD4+CXCR5+ cells indicates the Tfr/Tfh ratio, which serves as an important way to gauge the GC reaction.
It is recommended that a number of anatomical locations and time points be examined to determine if results are unique to one particular organ. Tfr cells have been found in LN, spleen, blood and Peyer’s patches (PP). Tfr cells from all of these organs express CXCR5 and other typical Tfr cell surface receptors. However, there are subtle differences. CTLA-4 is consistently highly expressed in dLN, blood and PP Tfr cells. CXCR5 is slightly lower on circulating Tfr cells than dLN, spleen or PP Tfr cells. ICOS seems to be the most divergent. ICOS is most highly expressed on dLN Tfr cells, but is expressed on efferent lymph and circulating Tfr cells at much lower levels, and has intermediate expression on PP Tfr cells[25].
Figure 1.
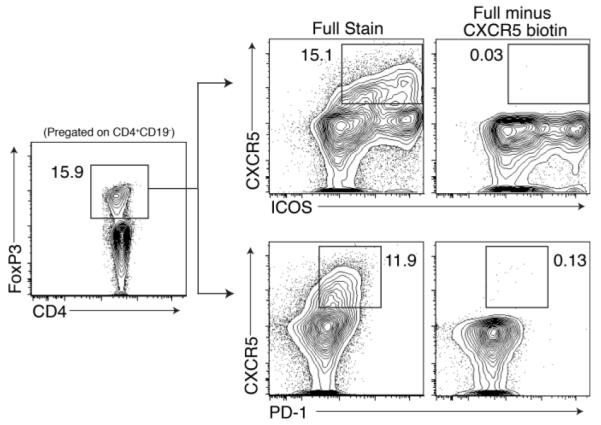
Representative gating protocols for Tfr cells based on CXCR5 and ICOS or on CXCR5 and PD-1 expression. Both strategies pregate on CD4+CD19− FoxP3+cells. “Full minus CXCR5 biotin” indicates staining control in which the CXCR5 biotin is omitted.
Signals for Tfr Cell Differentiation
Types of APC needed for Tfr cell generation
The cues responsible for Tfr cell differentiation are currently being elucidated. Tfr cells differentiate in response to a wide variety of stimuli including SRBCs, foreign antigens such as ovalbumin (OVA) or keyhole limpet hemocyanin (KLH) in adjuvant, self-antigens such as myelin oligodendrocyte glycoprotein (MOG), and viruses including LCMV and influenza (Box2)[9, 10, 24, 25]. Tfr cells in skin draining lymph nodes (dLN) require DCs for optimal differentiation after subcutaneous immunization with NP-OVA. When mice that express diphtheria toxin receptor (DTR) on DCs were immunized and given diphtheria toxin to deplete DCs [25], there was a marked reduction in the percentage of Tfr cells. The DC subsets most directly responsible for stimulating Tfr cell differentiation remain unclear. A recent study suggested that Tfh cell development requires contributions by both non-migratory and migratory DCs for complete differentiation[26]. Therefore, it is plausible that Tfr cells may require multiple lineages of DCs for optimal differentiation. Tfr cells have been found in LNs, spleen, blood, lymph and Peyer’s patches. Since Tfr cells are present in a number of tissues and differentiate in response to a number of different stimuli, it is likely that many types of DCs/APCs may promote Tfr cell generation, and the most important DC subset may be depend on the tissue and stimulus.
Box 2. Tfr cells following antigenic challenge and in autoimmune disease.
Tfr cells have been studied in a limited number of settings during both antigenic stimulation or in the context of autoimmunity in both mice and humans. Although much of the data on Tfr cells are an indirect result of studies focusing on Tfh cell biology, a number of important aspects of Tfr cell differentiation and/or function have been elucidated (see Table I below).
Autoantibodies can elicit immunopathologies either directly or indirectly in a number of disease settings including Systemic Lupus Erythematosus (SLE), myasthenia gravis, Sjogren’s syndrome and rheumatoid arthritis (RA). Patients with these autoimmune diseases have increased Tfh cells [4, 31, 74, 75]. Since Tfr cells are thought to be specialized in controlling antibody production by directly suppressing GC B and Tfh cells, it is likely that altered Tfr cell function may contribute to the progression of humoral autoimmunity. Although in depth studies are lacking, diminished Tfr cells have been correlated with extent of autoantibody production in several settings. Altered Tfr:Tfh cell ratios have been implicated in autoimmunity in induced [51, 53] and spontaneous lupus-like disease settings [43, 44], in murine arthritis models [76, 77], in human patients with ankylosing spondylitis [78] and immunodysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX)-like disease[79].
Table I.
Summary of studies examining Tfr cell responses to antigenic challenge and in autoimmune settings
| Species | Organ | Immunizing Agent |
Disease | Reference |
|---|---|---|---|---|
| Mouse | S | SRBC, KLH Ribi | [9] | |
| Mouse, Human |
LN | KLH in CFA | [10] | |
| Mouse | LN | OVA/alum | [11] | |
| Mouse | LN, blood | MOG/NPOVA | [24] | |
| Mouse | S | SRBC | [45] | |
| Mouse | S | [80] | ||
| Mouse | S, mLN | KLH/Alum | Nephritis | [53] |
| Mouse | PP | [81] | ||
| Mouse | PP, SILP | Gut | [82] | |
| Mouse | S | BXD2 arthritis | [76] | |
| Mouse | LN | KLH/CFA | dsDNA | [57] |
| Human | LN | [28] | ||
| Mouse | mLN | [35] | ||
| Mouse | LN, blood, lymph | OVA/Flu/LCMV | [25] | |
| Mouse | Ln, PP | OVA/CFA | [40] | |
| Mouse | S, LN, PP | OVA/CFA | [41] | |
| Mouse | S | OVA/CFA | ANA | [51] |
| Mouse | S | ANA, Nephritis | [43] | |
| Mouse | S | ANA, Nephritis | [44] | |
| Human | blood | IPEX | [79] | |
| Human | blood | AS | [78] | |
| Mouse | LN | [54] | ||
| Mouse | Spleen | BXD2 arthritis | [77] | |
| Mouse | Spleen | [39] |
Abbreviations: LN (lymph node), S (spleen), mLN (mesenteric lymph node), SILP (small intestine lamina propria), lymph (efferent lymph), SRBC (sheep red bolld cell), CFA (complete Freund’s adjuvant), Alum (aluminum hydroxide), IPEX (immunodysregulation polyendocrinopathy enteropathy X-linked), AS (ankylosing spondylitis). Blank columns indicate unimmunized (in Imm Agent column) or not associated with a specific disease (Disease column).
Similar to Tfh cells, Tfr cells in the LNs and spleen require B cells for optimal differentiation and/or expansion [9, 25, 27]. However, one study reported that human patients treated with rituximab (anti-CD20) and tacrolimus after renal transplant have reduced naïve and GC B cell numbers, but roughly similar Tfr cell numbers compared to non-rituximab treated patients[28]. These findings may be unique to transplantation settings or due to immunosuppression. In murine systems, Tfh cells require prolonged interaction with GC B cells to fully develop an effector phenotype. Further work is needed to understand if similar interactions with GC B cells influence Tfr cell generation and maintenance [29].
Circulating Tfr cells are memory-like cells that persist for long periods of time, similarly to circulating Tfh cells in both mice and humans [25, 30-32]. Interestingly, neither circulating Tfr nor Tfh cells require B cells for differentiation [25, 31]. Circulating Tfr (and Tfh) cells do need DCs for differentiation, similar to their LN counterparts[25]. Efferent lymph and circulating Tfr cells express lower levels of ICOS compared to LN Tfr cells. Since circulating Tfr cells are thought to bypass the B cell zone and exit the lymph node, it is likely that B cells are responsible for stimulating maximum expression of ICOS on Tfr cells. These studies suggest that the APC requirements for differentiation of lymph node and circulating Tfr cells are distinct. The LN Tfr cell “effector” phenotype is probably initiated during contact with DCs in the T cell zone, strengthened in the interfollicular region during contact with B cells, and optimized in the GC after prolonged contact with cognate GC B cells (Figure 1). Circulating Tfr cells likely divert away from the B cell zone and migrate to the efferent lymph before the full effector program occurs. The transcriptional programs of circulating memory-like Tfr cells and dLN “effector” Tfr cells probably differ to some extent, considering changes in expression of molecules such as ICOS on circulating and dLN Tfr cells.
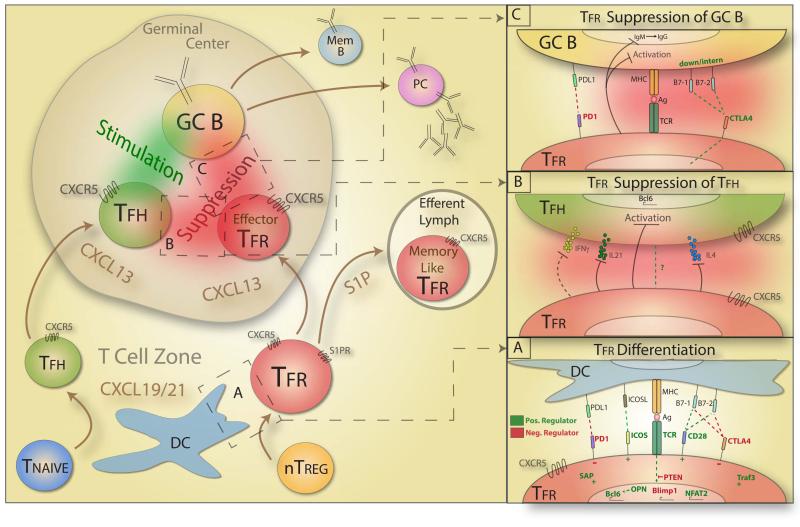
Figure 1. Model of Tfr cell Differentiation and Suppression.
Exposure to invading pathogens results in DC activation and antigen presentation in the T cell zone. A population of naïve T cells (Tnaive) that interact with DCs differentiate into T follicular helper (Tfh) cells. Tfh cells express CXCR5, which senses gradients of CXCL13 within the GC and directs Tfh cells into the GC. Simultaneously, a population of natural Tregs (nTreg) interacts with DCs and differentiates into T follicular regulatory (Tfr) cells. Box A shows the cues that modulate Tfr cell differentiation with positive regulators of differentiation denoted in green and negative regulators denoted in red font (OPN= osteopontin). Tfr cells in the T cell zone then diverge. A subset of Tfr cells with lower CXCR5 (and lower CD69) expression follows S1P gradients and exits the LN via the efferent lymph, and are destined to become memory-like cells. A second subset of Tfr cells with high CXCR5 (and high CD69) expression follows CXCL13 gradients to the GC. In the GC, Tfh cells interact with GC B cells, stimulating them to undergo activation, class switch recombination, affinity maturation, and differentiation into Memory B cells (Mem B) and plasma cells (PC). In the GC, Tfr cells interact with Tfh and GC B cells, leading to suppression of both Tfh and GC B cells. Box B shows a schematic of Tfr suppression of Tfh cells. Tfr cells suppress Tfh cell production of cytokines such as IL-21, IL-4 and IFNγ that stimulate B cells. Box C shows a schematic of Tfr suppression of GC B cells where positive mediators of Tfr suppression are in green and negative regulators of Tfr suppression are in red.
Costimulatory and coinhibitory signals regulate Tfr cell generation
Tfr cells require T cell costimulation for differentiation. Cd28−/− mice have a 90% reduction in Tfr cells in LN, spleen and blood after immunization [9, 24]. CD28 induces optimal FoxP3 expression and optimal proliferation of Treg cell subsets [33, 34]. Therefore, CD28 signaling likely maintains FoxP3 expression and promotes proliferation and maintenance of developing Tfr cells. Consistent with this, mice in which CD28 was specifically deleted after T cell priming (through use of an Ox40 driven Cre) had a ~80% reduction in Tfr cells in the mediastinal LN [35]. CD28 also is critical for Tfh cell differentiation. Similarly, ICOS is crucial for Tfr and Tfh cell development. Mice lacking ICOS have severe defects in Tfr cells, similar in magnitude to Cd28−/− mice [24]. ICOS mediates expression of Bcl6 and c-Maf in Tfh cells, as well as Tfh cell movement into GCs [36-38]. Moreover, mice lacking miR-146a (which represses ICOS) have enhanced Tfh and Tfr cells[39].
While Tfr cell differentiation is promoted by T cell costimulatory signals, Tfr cell differentiation is restrained by coinhibitory signals. Tfr cells express high levels of PD-1. Pdcd1−/− mice have large increases in Tfr cell percentages in LNs[24] following immunization with antigen in CFA. PD-1 most likely interacts with PD-L1 on APCs to inhibit Tfr cell development, because PD-L1 (but not PD-L2) and PD-1 deficient mice have similar increases in Tfr cell percentages. PD-1 deficient mice also have increased circulating Tfr cells[24].
The inhibitory receptor CTLA-4 also inhibits Tfr cell differentiation. CTLA-4 conditional knockout mice in which CTLA-4 is inducibly deleted in all cells or only in Tregs have profound increases in Tfr cells in LN, blood and Peyer’s patches following immunization with NP-OVA [40]. Mice that constitutively lack CTLA-4 in Treg cells have a similar increase in Tfr cells, even without immunization [41]. CTLA-4 deficiency results in substantially increased ICOS expression on Tfr cells, suggesting that CTLA-4 not only inhibits the differentiation of Tfr cells, but also modulates expression of key immunoregulatory molecules on Tfr cells [40].
TCR Signals and Tfr cell Generation
Tfr cell generation appears be modulated by TCR signal strength, similar to Tfh cells. Strong TCR signaling favors Tfh cell differentiation/expansion[42]. Treg-specific deletion of phosphatase and tensin homolog (PTEN; which negatively regulates phosphatidylinositol-3-OH kinase (PI(3)K)) leads to increased Tfr cells [43, 44], suggesting that more potent downstream TCR signaling enhances Tfr cell generation/maintenance. Consistent with this concept, mice lacking TRAF3 specifically in Tregs have minor defects in Tfr cell development, presumably due to diminished ERK signaling (TCR signals promote ERK signaling)[45]. Although strong TCR signals may benefit Tfr cell differentiation, too much stimulation may hinder downstream effector responses. It is difficult to test this experimentally because Treg cell subsets and conventional T cells have distinct TCR affinities and TCR transgenic mice often do not have antigen-specific Treg cells[46, 47].
Transcription factors controlling Tfr cell differentiation
Bcl6 is thought to be the master transcription factor for Tfh cells that controls the “Tfh program”. However, it is unclear how Bcl6 mediates this transcriptional program [48-50]. Although Bcl6 is expressed at lower levels in mouse Tfr cells compared to mouse Tfh cells, Tfr cells also need Bcl6 for differentiation. Bcl6−/− mice have an almost complete loss of Tfr cells [9]. In Tfr cells, Bcl6 may be shielded from ubiquination by osteopontin which helps to maintain Bcl6 signaling [51]. Bcl6 also antagonizes the transcription factor Blimp1/Prdm1[48]. Deletion of Blimp1 results in heightened Tfr cell differentiation, further suggesting that Bcl6 function is essential for proper Tfr cell development [9]. Paradoxically, Blimp1 is needed for the differentiation and/or homeostasis of other Treg subsets[52]. The interplay between Bcl6 and Blimp1 in Tfr cell differentiation and function needs to be studied in more detail to understand the reason for the distinct functions of Blimp1 in Tfr and non-Tfr Treg cells.
Other transcription factors also control Tfr cells. NFAT2 seems to enhance Tfr cells, as deletion of NFAT2 specifically in Treg cells results in slightly diminished numbers of Tfr cells in the GC [53]. In contrast, FOXO1 seems to potently limit Tfr cell differentiation, possibly through inhibiting ICOS expression [54].
Mechanisms of Tfr Suppression
The suppressive function of Tfr cells was initially demonstrated indirectly by adoptive transfer studies using total Treg populations from mice that cannot generate Tfr cells (described above). More direct in vivo evidence for Tfr cell suppression of B cell responses comes from adoptive transfer experiments using differentiated Tfr cells. For these studies, mice were immunized with NP-OVA and 7 days later differentiated LN Tfh and Tfr or CXCR5- Treg cells were adoptively transferred into Tcrα−/− recipients that were immunized with NP-OVA. Tfr cells potently suppressed antigen-specific antibodies (as indicated by reduced serum NP-specific antibody) but CXCR5- Tregs could not exert these suppressive effects. These studies reinforce the potent capacity of Tfr cells to suppress B cell responses in vivo, as well as the specialized function of Tfr cells [24]. Additional work has corroborated these findings [51]. Lymphopenic recipients were used in initial adoptive transfer studies, but this approach may lead to alterations in Tfh and Tfr functions due to homeostatic proliferation. The use of Cd28−/− mice as recipients is a useful alternative approach, since these mice lack Tfh and Tfr cells, but have otherwise wild type percentages of naïve T cells [25, 40].
Although in vivo assays of Tfr cell suppressive function are important because they are physiological, in vitro suppression assays also have helped elucidate how Tfr cells suppress B and Tfh cells. The first in vitro Tfr cell suppression assay system cultured Tfh and B cells sorted from dLN of NP-OVA immunized mice with (or without) Tfr cells in the presence of anti-CD3/IgM [24]. IgG in the supernatant was substantially diminished when Tfr cells were present in these cultures. Suppression assays also have used specific antigen instead of polyclonal activation to study interactions among sorted Tfh, Tfr and B cells in vitro [25].
Beyond confirmation of Tfr cell suppression of antibody production by B cells, in vitro assays have helped elucidate how Tfr cells alter Tfh and B cells. Tfh cells did not lose CXCR5 nor Bcl6 expression in B cell cultures with Tfr cells, suggesting that Tfr cells may not alter the transcriptional “Tfh program” in Tfh cells [25]. However, Tfh cell activation, assessed by Ki67+ staining, and production of key Tfh cell cytokines, such as IL-4 and IL-21, was potently suppressed by Tfr cells. Tfr cells suppressed most cytokines tested (including IFNγ and IL-10), prevented GL7 and B7-1 expression on B cells, and limited class switch recombination in B cells [25]. Similar, but more limited, experiments have been performed with human cells, and demonstrated that human Tfr cells can suppress antibody production in vitro[28]. In these studies, memory B cells (CD27+IgD-CD19+) were cultured with Tfh cells (CXCR5+CD57+CD25-CD4+) with or without Tfr (CXCR5+CD57+CD25+CD127-CD4+) cells from iliac LNs in the presence of anti-CD3/CD28 beads. Cultures with Tfr cells had a ~70% reduction in IgA production in supernatants, demonstrating that Tfr cells can suppress IgA production in vitro.
Although it is clear that Tfr cells can suppress Tfh and B cells, how they exert these suppressive effects remains uncertain. Altered Tfr:Tfh ratios control B cell responses, but it is difficult to distinguish alterations in Tfr cell differentiation from Tfr cell function without direct suppression assays. One clearly defined mechanism of Tfr suppression is through CTLA-4. Two recent studies identified CTLA-4 as a key mediator of Tfr cell suppressive function. Selective deletion of CTLA-4 on Treg cells resulted in heightened GC responses [40, 41]. When CTLA-4 was deleted on Tfr cells after their differentiation (to distinguish effects of CTLA-4 on Tfr cell differentiation and suppressive function), these CTLA-4 deficient Tfr cells were less suppressive than control Tfr cells in vitro and in vivo [40]. Further work is needed to understand how CTLA-4 controls Tfr cells. CTLA-4 may mediate signaling important for the Tfr cell suppressive program. Although there is evidence that CTLA-4 can cause downregulation/transcytosis of B7-2 on B cells, this probably happens at the T-B border and not in the GC [40, 41]. CTLA-4 also might exert suppressive effects by facilitating tighter contact between Tfr cells and B cells through a high affinity interaction of CTLA-4 with B7-1 or B7-2 expressed on GC B cells, allowing soluble mediators or receptors that mediate suppression to be more effective.
Production of inhibitory cytokines such as IL-10 or TGFβ may be another means by which Tfr cells inhibit B cell responses (Figure 2). IL-10 can modulate antibody production in vivo and B7-1 levels[55, 56]. However, the effect of IL-10 on the GC reaction may be an indirect effect of non-Tfr Treg subsets, because when Tfr cells were added to Tfh/B cell cultures, IL-10 was reduced in culture supernatants[25]. TGFβ made by Tfr cells may inhibit Tfh cells since Tfh cells are suppressed by TGFβ[57]. Tfr cells also might suppress the GC reaction by directly killing GC B and/or Tfh cells, as CD4+CD25+ Tregs are capable of directly killing B lymphocytes in vitro in a perforin dependent manner[20]. Although Tfr cells have lower levels of granzyme B compared to other Tregs[9], it possible that direct killing of GC B and/or Tfh cells by Tfr cells serves as a means to control the GC reaction.
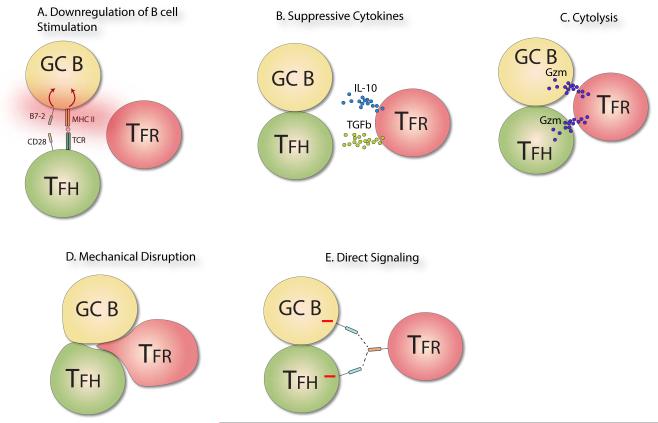
Figure 2. Proposed mechanisms of suppression of B cell Responses by Tfr cells.
A) Model in which Tfr cells limit B cell stimulation by causing downregulation of MHC and B7-1 or B7-2 on the GC B cell. Tfr expressed CTLA-4, for example, may downregulate B7-1/B7-2. However, downregulation also may result from soluble factors (e.g., cytokine) or other receptor based mechanisms. B) Model in which Tfr cells produce suppressive cytokines, such as IL-10 and TGF-β which may inhibit activation of GC B and/or Tfh cells. C) Model in which Tfr cells suppress B cell responses by directly killing activated GC B and/or Tfh cells. Cytolysis could be mediated by granzymes produced by the Tfr cell and directed towards the GC B and/or Tfh cell. D) Model in which Tfr cells mechanically disrupt interactions between Tfh and B cells. Tfr cells bind to GC B cells and/or Tfh cells and push the cells apart, resulting in the separation of Tfh and GC B contacts. This separation reduces both Tfh and GC B activation since costimulation and antigen presentation are disrupted. E) Model in which a ligand on Tfr cells engages a receptor on GC B cells and/or Tfh cells, resulting in negative signaling into the GC B and/or Tfh cell and dampening of BCR and TCR signals.
We hypothesize that another mechanism by which Tfr cells might suppress GC B and Tfh cells is through mechanical disruption of Tfh and B cell contacts; Tfr cells may physically interact with GC B cells and dislodge the close contact between the GC B and Tfh cells. Since GC B cells require prolonged costimulation through Tfh cells, and Tfh cells require antigenic stimulation through GC B cells, this mechanism would suppress both Tfh and GC B cells. Another potential means by which Tfr cells might exert their suppressive effects is by expression of a ligand that can engage a receptor that signals into GC B and Tfh cells. For instance, if an inhibitory receptor were expressed on both GC B and Tfh cells, ligation of that receptor by a ligand on Tfr cells could reduce the activation state and functionality of both cells. A receptor fitting these criteria has yet to be described.
Concluding Remarks
The discovery of Tfr cells is leading to a revised view of how antibody responses are regulated. The identification and characterization of this specialized Treg subset is providing insights into regulation of GC B and Tfh cells during the GC reaction. However, there are many unanswered questions about Tfr cell biology as well as the role of Tfr cells in specific disease settings. The answers to these questions should provide a mechanistic understanding of how humoral immunity is regulated in health and disease, and suggest new strategies for enhancing beneficial or limiting pathogenic antibody responses.
Several key aspects of Tfr cell biology are not understood. More in depth study of the Tfr cell transcriptional signature is needed to determine how Tfr cell differentiation and function are controlled. Delineating mechanisms of Tfr suppression is essential for creating new therapeutics that target Tfr cells. Also unclear is how Tfr cells in different organs functionally relate. Tfr cells in the circulation have memory-like properties - they can recirculate throughout lymphoid organs for long time periods and upon reactivation, can home to GCs and suppress B cell responses[25]. Further work is needed to understand why circulating memory Tfr cells are somewhat less suppressive than dLN Tfr cells and the functional consequences of these differences. In addition, it is not known if plasticity exists for Tfr cells, and whether some non-Tfr Tregs could be ex-Tfr cells. Also unclear is whether these putative ex-Tfr cells might regain their phenotype with activation or become Tfh-like cells that contribute to inflammation and disease. Better lineage markers and tools are needed in order to address these questions. Another key issue relates to how vaccines affect Tfr cell generation and memory. Understanding how different vaccine adjuvants affect Tfr cell differentiation and memory may reveal how to attenuate Tfr cells to enhance B cell memory in healthy people and people with inadequate B cell responses, such as the elderly or HIV-infected individuals.
The roles of Tfr cells in diseases also need to be elucidated. Tfr cells have been studied the most in autoimmune diseases (Box 2), but many questions remain. Further work is needed to determine whether alterations in Tfr cell generation, function, maintenance or memory contribute to initiation and progression of humoral autoimmunity. Ectopic lymphoid structures (ELS) are seen in tissues from many autoimmune diseases including RA, SLE and Sjogren’s syndrome[58], but it is not clear if Tfr cells regulate Tfh and B cell responses within ELS or control ELS formation.
Tfr cells likely have roles in other types of diseases. In the transplant setting chronic rejection of human allografts is associated with a broad range of autoantibody responses, and Tfh cells may promote the production of these antibodies[59, 60]. Moreover, chronic graft-versus-host disease (GVHD) has a strong antibody component[61] and transfer of Tregs can benefit patients with graft rejection or GVHD[62, 63]. An important area for future investigation is whether Tfr cell generation, function or maintenance is altered in chronic graft rejection or GVHD. Augmenting Tfr cells may be a way to treat chronic graft rejection and/or GVHD.
Another understudied area in which Tfr cells may play an important role is allergies. Many allergic responses rely on IgE antibodies, and IgE class switched B cells can be found in GCs. Since Treg cells have been associated with controlling allergies, Tfr cells may regulate allergic responses by controlling IgE production [13, 64-66]. If correct, then enhancing Tfr function may help to attenuate IgE production and therefore ameliorate allergies.
Tfr cells have not been studied in cancer. Anti-tumor antibodies elicited by vaccines can correlate with tumor clearance in melanoma patients [67], and class switched antibodies produced in patients with melanoma can potently kill melanoma cells in vitro, suggesting that humoral immune responses may contribute to anti-tumor immunity [68]. Whether checkpoint blockade elicits antibodies that promote tumor clearance or elicit adverse events is unclear. ELSs can form near tumors and ELSs containing Tfh cells have been correlated with tumor clearance [69]. These ELSs contain class switched B cells and plasma cells that are thought to actively produce antibody [58, 70, 71]. It will be important to understand beneficial and pathogenic antibody responses in cancer and the functions of Tfr cells in regulating these responses.
In summary, given the recent discovery and analyses of Tfr cells, we are only beginning to understand the roles that Tfr cell play in regulating humoral immune responses in health and disease. The limited number of studies of Tfr cells published to date have pointed to a central role for these cells in controlling the GC reaction and downstream B cell responses. Further studies of Tfr cell should provide insights into the pathogenesis of diseases in which antibodies can be protective (infections, cancer) or pathogenic (autoimmune diseases, chronic transplant rejection, allergies), and may suggest new strategies for modulating humoral immune responses.
Box 3. Tfr cells in relation to Tfh and non-Tfr Treg cells.
Tfr cells resemble Tfh cells in a number of ways, including expression of CXCR5, PD-1, ICOS and Bcl6. However, there are subtle differences. LN Tfr cells have slightly reduced expression of CXCR5 and Bcl6, slightly elevated (or similar) PD-1 expression, and much higher ICOS expression compared to LN Tfh cells [9, 24, 25]. Tfr cells also express much higher levels of CTLA-4, CD25 and GITR than Tfh cells, which may be controlled by FoxP3 expression [9, 40]. Although Tfr cells differentiate from different precursors than Tfh cells, it is likely that Tfr cells partially coopt the Tfh transcriptional program, including Bcl6 expression, to aid in suppression of Tfh cells. Other effector Treg subsets that suppress specific effector T cells, such as adipose tissue Treg, similarly share transcriptional programs with the cells they suppress, suggesting that local environmental cues may be involved [17, 83]. Although upregulation of FoxP3 may be possible in limited circumstances in human Tfh cells, it is not generally thought that Tfh cells can become Tfr cells and vice versa[84].
Tfr cells seem to be in a more heightened activation state than conventional Tregs, due to their high GITR, CTLA-4 and Ki67 staining [9, 25, 85]. Whether this activation state is due to heightened antigenic stimulation or cytokine milieu, or both, is not known. The role of conventional (non-Tfr) Tregs in controlling B cells remains unclear. Without CXCR5 expression, conventional nTregs probably cannot access GCs, but may still influence antibody production at the T-B border. There are conflicting data about the role of CXCR5- Tregs in controlling antibody production. Some studies do not support a strong role for CXCR5- Tregs in inhibiting B cell responses. Adoptive transfer of CXCR5- Tregs along with Tfh cells resulted in enhanced, not suppressed B cell responses in recipient mice [24, 40]. Studies in influenza models also demonstrated that CXCR5- Tregs promote B cell responses, possibly by limiting IL-2 levels and enhancing Tfh cell differentiation[86]. Until an experimental approach is developed to delete genes selectively on Tfr cells or non-Tfr Treg cells, the role of non-Tfr Tregs in modulating B cell responses is likely to remain unclear.
Highlights.
Tfh cells promote the germinal center reaction and antibody production
Tfr cells control the germinal reaction by suppressing Tfh cells and B cells
Tfr cells are found in many tissues and can respond to both foreign and self antigens
Altered Tfr:Tfh cell ratios correlate with autoimmune disease
Acknowledgements
This work is supported through NIH grants T32HL007627, P01AI56299 and R37 AI38310.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vidarsson G, et al. IgG subclasses and allotypes: from structure to effector functions. Frontiers in immunology. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joller N, et al. Antibody-Fc receptor interactions in protection against intracellular pathogens. Eur J Immunol. 2011;41:889–897. doi: 10.1002/eji.201041340. [DOI] [PubMed] [Google Scholar]
- 3.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 4.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson RW, et al. T cell receptor cross-reactivity between similar foreign and self peptides influences naive cell population size and autoimmunity. Immunity. 2015;42:95–107. doi: 10.1016/j.immuni.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawlings DJ, et al. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat Rev Immunol. 2012;12:282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12:479–491. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- 9.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wollenberg I, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 12.Godfrey VL, et al. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol. 1991;138:1379–1387. [PMC free article] [PubMed] [Google Scholar]
- 13.Lin W, et al. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. The Journal of allergy and clinical immunology. 2005;116:1106–1115. doi: 10.1016/j.jaci.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 14.Curotto de Lafaille MA, et al. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Tsuda M, et al. The spectrum of autoantibodies in IPEX syndrome is broad and includes anti-mitochondrial autoantibodies. Journal of autoimmunity. 2010;35:265–268. doi: 10.1016/j.jaut.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Lampasona V, et al. Autoantibodies to harmonin and villin are diagnostic markers in children with IPEX syndrome. PLoS One. 2013;8:e78664. doi: 10.1371/journal.pone.0078664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 19.Lim HW, et al. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 20.Zhao DM, et al. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim HW, et al. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004;114:1640–1649. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander CM, et al. T regulatory cells participate in the control of germinal centre reactions. Immunology. 2011;133:452–468. doi: 10.1111/j.1365-2567.2011.03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 24.Sage PT, et al. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nature immunology. 2013;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sage PT, et al. Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest. 2014;124:5191–5204. doi: 10.1172/JCI76861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerner MY, et al. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity. 2015;42:172–185. doi: 10.1016/j.immuni.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Kerfoot SM, et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34:947–960. doi: 10.1016/j.immuni.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallin EF, et al. Human T-follicular helper and T-follicular regulatory cell maintenance is independent of germinal centers. Blood. 2014;124:2666–2674. doi: 10.1182/blood-2014-07-585976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumjohann D, et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Locci M, et al. Human circulating PD-(+)1CXCR3(−)CXCR5(+) memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He J, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Hale JS, et al. Distinct Memory CD4 T Cells with Commitment to T Follicular Helper- and T Helper 1-Cell Lineages Are Generated after Acute Viral Infection. Immunity. 2013 doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Q, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 34.Tai X, et al. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nature immunology. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 35.Linterman MA, et al. CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection. eLife. 2014;3 doi: 10.7554/eLife.03180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi YS, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauquet AT, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature immunology. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu H, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- 39.Pratama A, et al. MicroRNA-146a regulates ICOS-ICOSL signalling to limit accumulation of T follicular helper cells and germinal centres. Nature communications. 2015;6:6436. doi: 10.1038/ncomms7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sage PT, et al. The coinhibitory receptor ctla-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wing JB, et al. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41:1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Fazilleau N, et al. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nature immunology. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huynh A, et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nature immunology. 2015;16:188–196. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shrestha S, et al. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nature immunology. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang JH, et al. TRAF3 regulates the effector function of regulatory T cells and humoral immune responses. J Exp Med. 2014;211:137–151. doi: 10.1084/jem.20131019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee HM, et al. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity. 2012;37:475–486. doi: 10.1016/j.immuni.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Josefowicz SZ, et al. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu D, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Leavenworth JW, et al. A p85alpha-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nature immunology. 2015;16:96–106. doi: 10.1038/ni.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cretney E, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nature immunology. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 53.Vaeth M, et al. Follicular regulatory T cells control humoral autoimmunity via NFAT2-regulated CXCR5 expression. J Exp Med. 2014;211:545–561. doi: 10.1084/jem.20130604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stone EL, et al. ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity. 2015;42:239–251. doi: 10.1016/j.immuni.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai G, et al. A regulatory role for IL-10 receptor signaling in development and B cell help of T follicular helper cells in mice. J Immunol. 2012;189:1294–1302. doi: 10.4049/jimmunol.1102948. [DOI] [PubMed] [Google Scholar]
- 56.Ding L, et al. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 57.McCarron MJ, Marie JC. TGF-beta prevents T follicular helper cell accumulation and B cell autoreactivity. J Clin Invest. 2014;124:4375–4386. doi: 10.1172/JCI76179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pitzalis C, et al. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14:447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- 59.Porcheray F, et al. Chronic humoral rejection of human kidney allografts associates with broad autoantibody responses. Transplantation. 2010;89:1239–1246. doi: 10.1097/TP.0b013e3181d72091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conlon TM, et al. Germinal center alloantibody responses are mediated exclusively by indirect-pathway CD4 T follicular helper cells. J Immunol. 2012;188:2643–2652. doi: 10.4049/jimmunol.1102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Srinivasan M, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119:1570–1580. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brunstein CG, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wood KJ, et al. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12:417–430. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 64.He JS, et al. The distinctive germinal center phase of IgE+ B lymphocytes limits their contribution to the classical memory response. J Exp Med. 2013;210:2755–2771. doi: 10.1084/jem.20131539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agua-Doce A, Graca L. Regulatory T cells and the control of the allergic response. Journal of allergy. 2012;2012:948901. doi: 10.1155/2012/948901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu LC, Zarrin AA. The production and regulation of IgE by the immune system. Nat Rev Immunol. 2014;14:247–259. doi: 10.1038/nri3632. [DOI] [PubMed] [Google Scholar]
- 67.Miller K, et al. Improved survival of patients with melanoma with an antibody response to immunization to a polyvalent melanoma vaccine. Cancer. 1995;75:495–502. doi: 10.1002/1097-0142(19950115)75:2<495::aid-cncr2820750212>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 68.Gilbert AE, et al. Monitoring the systemic human memory B cell compartment of melanoma patients for anti-tumor IgG antibodies. PLoS One. 2011;6:e19330. doi: 10.1371/journal.pone.0019330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu-Trantien C, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123:2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cipponi A, et al. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 71.Dieu-Nosjean MC, et al. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35:571–580. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Maceiras AR, Graca L. Identification of foxp3(+) T follicular regulatory (tfr) cells by flow cytometry. Methods Mol Biol. 2015;1291:143–150. doi: 10.1007/978-1-4939-2498-1_12. [DOI] [PubMed] [Google Scholar]
- 73.Sage PT, Sharpe AH. In Vitro Assay to Sensitively Measure TFR Suppressive Capacity and TFH Stimulation of B Cell Responses. Methods Mol Biol. 2015;1291:151–160. doi: 10.1007/978-1-4939-2498-1_13. [DOI] [PubMed] [Google Scholar]
- 74.Simpson N, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis and rheumatism. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 75.Szabo K, et al. Follicular helper T cells may play an important role in the severity of primary Sjogren’s syndrome. Clinical immunology. 2013;147:95–104. doi: 10.1016/j.clim.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 76.Ding Y, et al. Interleukin-21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis & rheumatology. 2014;66:2601–2612. doi: 10.1002/art.38735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim YU, et al. Regulation of Autoimmune Germinal Center Reactions in Lupus-Prone BXD2 Mice by Follicular Helper T Cells. PLoS One. 2015;10:e0120294. doi: 10.1371/journal.pone.0120294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shan Y, et al. Higher frequency of peripheral blood follicular regulatory T cells in patients with new onset ankylosing spondylitis. Clinical and experimental pharmacology & physiology. 2015;42:154–161. doi: 10.1111/1440-1681.12330. [DOI] [PubMed] [Google Scholar]
- 79.Charbonnier LM, et al. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. The Journal of allergy and clinical immunology. 2015;135:217–227. doi: 10.1016/j.jaci.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goenka R, et al. Local BLyS production by T follicular cells mediates retention of high affinity B cells during affinity maturation. J Exp Med. 2014;211:45–56. doi: 10.1084/jem.20130505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moriyama S, et al. Sphingosine-1-phosphate receptor 2 is critical for follicular helper T cell retention in germinal centers. J Exp Med. 2014;211:1297–1305. doi: 10.1084/jem.20131666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kawamoto S, et al. Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity. 2014;41:152–165. doi: 10.1016/j.immuni.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 83.Cipolletta D, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brady MT, et al. Mesenchymal stromal cells support the viability and differentiation of follicular lymphoma-infiltrating follicular helper T-cells. PLoS One. 2014;9:e97597. doi: 10.1371/journal.pone.0097597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huehn J, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leon B, et al. FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability. Nature communications. 2014;5:3495. doi: 10.1038/ncomms4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Francisco LM, et al. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2009;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]