Abstract
Acute renal failure is a common complication caused by Bothrops viper envenomation. In this study, the nefrotoxicity of a main component of B. leucurus venom called L-aminoacid oxidase (LAAO-Bl) was evaluated by using tubular epithelial cell lines MDCK and HK-2 and perfused kidney from rats. LAAO-Bl exhibited cytotoxicity, inducing apoptosis and necrosis in MDCK and HK-2 cell lines in a concentration-dependent manner. MDCK apoptosis induction was accompanied by Ca2+ release from the endoplasmic reticulum, reactive oxygen species (ROS) generation and mitochondrial dysfunction with enhanced expression of Bax protein levels. LAAO-Bl induced caspase-3 and caspase-7 activation in both cell lines. LAAO-Bl (10 μg/mL) exerts significant effects on the isolated kidney perfusion increasing perfusion pressure and urinary flow and decreasing the glomerular filtration rate and sodium, potassium and chloride tubular transport. Taken together our results suggest that LAAO-Bl is responsible for the nephrotoxicity observed in the envenomation by snakebites. Moreover, the cytotoxic of LAAO-Bl to renal epithelial cells might be responsible, at least in part, for the nephrotoxicity observed in isolated kidney.
Introduction
Envenomation by snakebites, recognized as a neglected tropical disease (NDT) by the World Health Organization (WHO), constitutes an important worldwide public health problem, particularly in the rural areas of tropical countries as Africa, Asia and Latin America. In Brazil, most accidents with species notification are due to vipers of the Bothrops genus (83.8%) [1, 2].
Bothrops venoms are especially interesting as they contain a complex mixture of proteins and peptides, with a wide range of biological and pharmacological activities. More than 90% of their dry weight consists of proteins that are capable of interfering with a variety of physiological processes [3, 4].
An important protein superfamily present in all snake venoms is L-amino acid oxidase (LAAO), a flavoenzyme that catalyzes oxidative deamination of L-amino acids to synthetize the corresponding α-ketoacid, hydrogen peroxide (H2O2) and ammonia. LAAOs are present in most snake venoms, with approximately 30% of the total protein content and contributes to venom toxicity upon envenomation [5, 6].
LAAOs have been studied for the last decade and have attracted the interest of researchers due to their polyfunctional effects on different biological systems. LAAO has been recognized as a multifunctional enzyme with evident biological effects on platelet aggregation, edema formation, hemorrhage, antimicrobial, antiviral, anti-HIV action and is cytotoxic to tumor cells. Although most of LAAOs biological effects might be mediated by H2O2, the mechanism of their actions is still unclear [7].
The pit viper Bothrops leucurus (White-tailed-jararaca) is a poisonous snake whose natural habitat is the northeast of Brazil. The biological effects due to envenomation have a similar profile to those observed with other Bothrops snakes, such as coagulant, hemorrhagic and fibrinolytic activity, as well as renal failure in vivo [8, 2].
Recently, we observed that Bothrops leucurus venom induces nephrotoxicity in isolated perfused kidneys of rats, associated with cytotoxicity against renal tubular epithelial cells [2]. Here, we extended these studies by evaluating the precise role of a major component of B. leucurus venom, LAAO, to better understand the biological effects produced by envenomation. In order to clarify LAAO nephrotoxicity, we investigated not only its nephrotoxic potential, but also its cytotoxicity against renal epithelial cell lines.
Materials and Methods
Venom, chemicals and drugs
LAAO from B. leucurus venom was isolated and purified as described by Torres et al. (2010) [9]. DMEM medium, phenylhydrazone (FCCP), 3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) and catalase kit were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Tetramethylrhodamine ethyl ester (TMRE), Fura-2AM and 2,7dichlorodihydrofluorescein diacetate (DCFH-DA) were purchased from Molecular Probes (Eugene, OR, USA). AnnexinV/FITC Apoptosis Detection Kit was from BD Pharmigen (CA, USA). Z-Val-Ala-Asp (OMe)-fluoromethylketone (zVAD), catalase and BAPTA-AM were from Tocris (Bristol, UK).
Cell culture
Human kidney (HK-2) and Canine kidney (MDCK) cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich), 2 mM L-glutamine, 1% penicillin/streptomycin solution at 37°C
MTT mitochondrial dysfunction assay
Cell viability was also measured using a standard methyl thiazol tetrazolium (MTT) assay. Briefly, viable cells were plated into 96-well flat microplates (Corning, USA) in DMEM medium, treated with different concentrations of LAAO-Bl and incubated at 37°C at 12 h. It was added 10 μg/well of MTT (5 mg/mL) and incubated for 4 h, when DMSO (Merck, Germany) was added to each well to solubilize the formazan. The cell viability measurements were accessed using 570 nm on Wallac 1420 workstation.
Lactate Dehydrogenase (LDH) Release
Cytotoxicity induced by LAAO-Bl was assessed by lactate dehydrogenase (LDH) leakage into the culture medium. After 12 hours of treatment, cells supernatant was removed and reactive mixture was added for 30 min at room temperature under protection from light for determination LDH release using the Promega kit (6179A). Plates were read at 440 nm on Wallac 1420 workstation.
Annexin V-FITC and propidium iodide (PI) staining
Cells treated with different concentrations of LAAO-Bl were stained with fluorescein isothiocyanate (FITC)-conjugated to annexinV/propidium iodide (PI) according to manufacturer’s instructions. The population of annexinV-PI viable cells and annexinV+ apoptotic cells was evaluated by flow-cytometry. Data were collected in a FACS Calibur (Becton-Kickinson, Mountain View, Calif.) and analyzed using Cell Quest software (Becton-Dickinson).
Mitochondrial membrane potentiality (ΔΨm) measurements
Fluorescence Microscope
To evaluate the effects of LAAO-Bl (50 μg/mL) on ΔΨm, cells were plated on coverslips and incubated with TMRE (25 nM, 15 min at 37°C), a potentiometric and cationic indicator dye that accumulates preferentially into the energized mitochondria. Before the addition of LAAO-Bl, cells were exposed to light for at least 5 min to ensure a stable baseline. The experiments were performed in the presence of the dye to diminish the decrease in fluorescence due to photo bleaching [10]. TMRE fluorescence (548 nm excitation and 585 nm emission) was acquired using a high resolution fluorescence microscope (Nikon TE 300, Nikon, Osaka, Japan) coupled to a CCD camera (CoolSnap-Roper Scientific Inc., Princeton Instruments, Princeton, NJ, USA). Images were acquired by BioIP software (Anderson Eng., USA).
Flow cytometry
MDCK cells were treated with LAAO-Bl (50 μg/mL, 12 h). After this period, the collected cells incubated with TMRE (25 nM, 15 min, 37°C) in the dark. The cells were analyzed using FL2 by flow cytometry.
Ca2+ measurements
To evaluate the Ca2+ handling, MDCK cells were plated on coverslips after loaded with 3 μM of acetoxymethyl ester of fura-2 (Fura-2AM) in a regular buffer containing (mM): 130 NaCl, 5.36 KCl, 0.8 MgSO4, 1 Na2HPO4, 25 glucose, 20 HEPES, pH 7.3 for 30 min. Cytoplasmic Ca2+ measurements were evaluated by fluorescence microscopy (Nikon TE 300; Nikon, Osaka, Japan) coupled to a CCD camera (Quantix 512-Roper Scientific Inc., Princeton Instruments, Princeton, NJ). Images were acquired in BioIP software (Anderson Eng, Delaware, USA). Basal Ca2+ levels were considered the first 15 images, and then exposed to LAAO-Bl (50 μg/mL). Fura-2 fluorescence (emission = 510 nm) was monitored following alternate excitation at 340 and 380 nm. Percentages were expressed as ratio (340/380) values, normalized from the basal fluorescence and data were normalized by the (F − F0)/F0 × 100 formula, in which F0 represents the basal Ca2+ levels.
Reactive oxygen species (ROS) measurements
Cytosolic ROS was measured using 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA), which can readily enter cells and be cleaved by esterase to yield DCFH, a polar, nonfluorescent product. ROS in cells promotes the oxidation of DCFH to yield the fluorescent product, dichlorofluorescein. After treatment (LAAO-Bl 50 μg/mL, 12 h), cells were collected and then incubated in PBS containing the reagent DCFH-DA (5 μM, 30 min at 37°C). After that, submitted to flow cytometric analysis using a FACScan flow cytometer. Tert-Butyl hydroperoxide (t-BHP, 5 μM) was used as positive control. Data based on the FL1 channel were analyzed with the CellQuest program.
Caspase 3/7 activity measurements
Extracts collected from LAAO- Bl (50 μg/mL, 12 h)-treated cells were lysed in extraction buffer (50 mM PIPES, 50 mM KCl, 5 mM EDTA, 2 mM MgCl2, 2 mM DTT, supplemented with protease inhibitors). After 30 min on ice, cell lysates were centrifuged at 13.2000 rpm for 10 min, then the supernatants collected were quantified using the BCA protein assay. Total protein (40 μg) was mixed with 200 μL of caspase assay buffer (PBS, 10% glycerol, 0.1 mM EDTA, 2 mM DTT) containing 20 μM of Ac-DEVD-afc (Enzo Life Sciences) of the caspase-3 substrate. DVDase activity was continuously monitored following the release of fluorescent afc at 37°C with Wallac 1420 Workstation (λ exc = 400 nm; λ em = 508 nm).
Immunoblotting
Whole cell extracts were obtained by lysing cells in a buffer containing 25 mM Tris-HCl pH 7.4, 1 mM EDTA, 1 mM EGTA, 1% SDS, plus protease and phosphatase inhibitors. The protein concentration was determined by the BCA protein assay. Cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose membranes, blocked with 5% non fat milk, washed with 0.1% Tween/PBS and incubated overnight with a specific primary antibody. Membranes were washed and probed with the appropriate secondary antibody conjugated to horseradish peroxidase (Amersham Pharmacia Biotech). The antibodies against Caspase 3 (#96615) and Caspase 7(#9492) were obtained from Cell Signaling, α-tubulin antibody (#T8203) was obtained from Sigma-Aldrich and Bax (Sc 493) was obtained from Santa Cruz Biotechnology.
Kidney perfusion
Adult male Wistar rats (260–320 g) were fasted for 24 h with free access to water. The rats were anesthetized with sodium pentobarbitone (50 mg/kg, i.p) and after careful dissection of the right kidney; the right renal artery was cannulated via the mesenteric artery without interrupting the blood flow as described by Bowman (1970) [11]. The perfusate consisted of a modified Krebs–Henseleit solution (MKHS) of the following composition (in mMol/L): 114.00 NaCl, 4.96 KCl, 1.24 KH2PO4, 0.5 MgSO4.7H2O, 2.10 CaCl2 and 24.99 NaHCO3. Bovine serum albumin (BSA 6 g%; fraction V), urea (0.075 g), inulin (0.075 g) and glucose (0.15 g) were added to the solution, resulting in a final perfusate volume of 100 mL. The pH was adjusted to 7.4. In each experiment, 100 mL of MKHS were recirculated for 120 min. The pressure perfusion (PP) was measured at the tip of the stainless steel cannula in the renal artery. Samples of urine and perfused were collected at 10 min for analysis of the sodium, potassium and chloride levels by ion-selective electrodes (Rapid chen 744, Bayer Diagnostic, UK); inulin, as described by Walser et al. (1955) and modified by Fonteles et al. (1983); and osmolality, which was measured in vapor pressure osmometer (Wescor 5100C, USA). LAAO-Bl (10 μg/mL) was added to the system 30 min after the beginning of each perfusion. The perfusion pressure (PP), renal vascular resistance (RVR), urinary flow (UF), glomerular filtration rate (GFR), the percentage of sodium (% TNa+), potassium (%TK+) and chloride (%TCl) tubular transport were determined (Martinez-Maldonado and Opava-Stitzer, 1978). The results were compared to the internal control group at 30 min previously in each experiment (n = 6). This study was approved by the Ethics Committee on Animal Research (CEPA) of the Federal University of Ceara (Permit Number: 79/08). All efforts were made to minimize suffering.
Statistical Analysis
The values given are mean ± S.E.M of triplicate values for each experiment. The significance of difference between the experimental groups and controls was accessed by a one-way analysis of variance (ANOVA). Significance was defined as p<0.05.
Results
LAAO-Bl induces apoptosis and necrosis in both renal epithelial cell lines
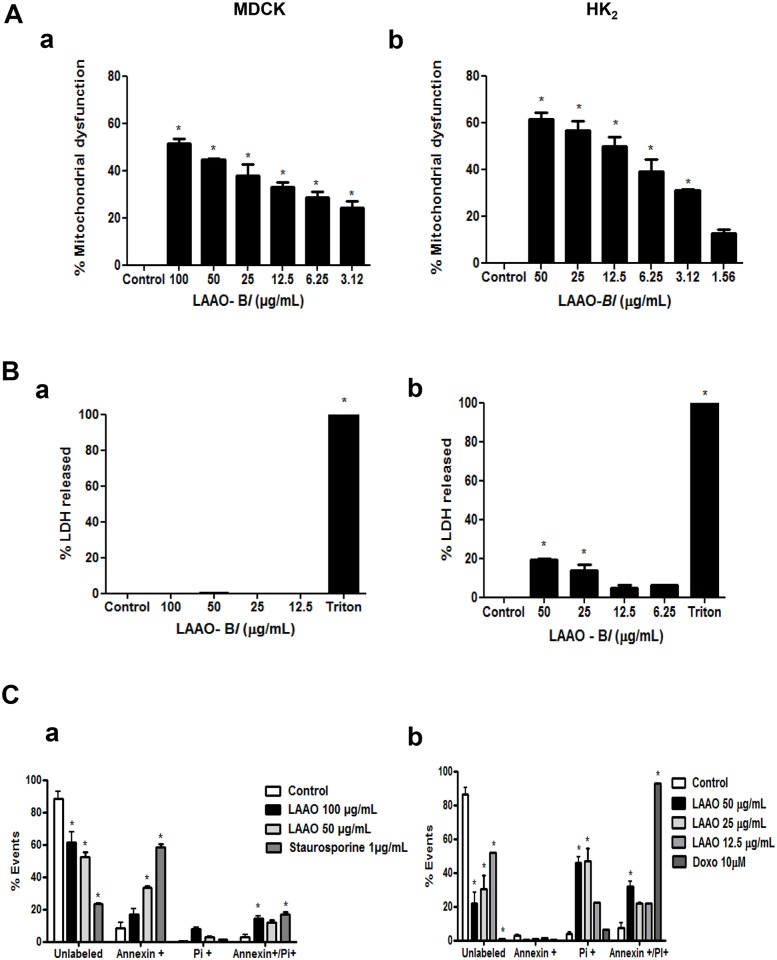
Previous studies reported that Bothrops leucurus venom was able to induce nephrotoxicity in the isolated perfused kidney from rats and was cytotoxic against renal tubular epithelial cells [2]. However, the cytotoxicity of LAAO-Bl, a major component of B. leucurus venom is poorly understood and studied. In order to clarify the mechanisms of nephrotoxicity and to compare whether LAAO-Bl has the same effects observed previously with total venom [2], cell death assays were conducted using two renal tubular cell lines such as MDCK and HK-2. In these cells treated with LAAO-Bl, 1.56–100 μg/mL for 12 h, there was a decrease in their viability in a concentration-dependent manner (Fig 1A, a-b). We next evaluated if necrosis was implicated in the cellular viability decrease observed by analyzing lactate dehydrogenase (LDH) release. Interestingly, in MDCK cells LDH release was not observed after 12 h of LAAO-Bl exposure (Fig 1B, a) while LAAO-Bl induced an apparent membrane rupture in HK-2 cells at the highest concentrations studied (50 and 25 μg/mL) when compared with untreated cells (Fig 1B, b). Aiming to identify the cell death modalities that were involved in our results we analyzed different markers of apoptosis and necrosis. The exposure of phosphatidylserine (PS) residues on the outer leaflet of the plasma membrane is usually considered a hallmark of apoptosis. Thus, cell staining with FITC-labeled Annexin V, which binds to PS, is considered a marker of early apoptotic events. Propidium iodide (PI) is a DNA intercalating agent that can be incorporated into cells only after major cell membrane damages (PI+). Hence, the combination of AnnV and PI cell staining can be used to distinguish apoptotic and necrotic cell death. Thus, annexin V/PI staining was applied to detect apoptotic/necrotic cells after LAAO-Bl treatment. In MDCK cells, LAAO-Bl significantly increased the percentage of early apoptotic (Annexin-V+, PI-), necrotic (Annexin-V-, PI+) and secondary necrotic cells (Annexin-V+, PI+) when compared with control untreated cells (Fig 1C, a). In HK-2 cells, in accordance with data obtained in the LDH-release assay, the Annexin-V-PI loading cell analysis demonstrated an increase in necrotic (PI+ cells) and secondary necrotic cells (Annexin-V+, PI+) in a concentration-dependent manner (Fig 1C, b). Treatment with staurosporine (1 μg/mL) and doxorubicin (10 μM) for 12h were used as positive controls.
Fig 1. LAAO-Bl induces necrosis and apoptosis in renal epithelial cell lines.
(A) MDCK (a) and HK-2 (b) cells were treated with LAAO-Bl (1.56–50/100 μg/mL) for 12 h. After treatment, cell viability was determined by MTT. (B) Percentage release of the lactate dehydrogenase enzyme of MDCK (a) and HK-2 (b) cells treated with the indicated concentrations of LAAO-Bl. Triton (7%) was used as positive control. (C) Annexin V+/PI-, Annexin V+/PI+ and AnnexinV-/PI+-populations were analyzed by flow cytometry in MDCK (a) and HK-2 (b) cells for 12 h after treatment with LAAO-Bl. Staurosporine (1 μg/mL) and doxorubicin (10 μM) for 12h were used as positive controls. All data are representative of three independent experiments in triplicate and are expressed as mean ± S.E.M. *Significantly different from control group (p<0.05, one way ANOVA, Dunnett post-test).
LAAO-Bl exposure induces mitochondrial hyperpolarization in MDCK cells
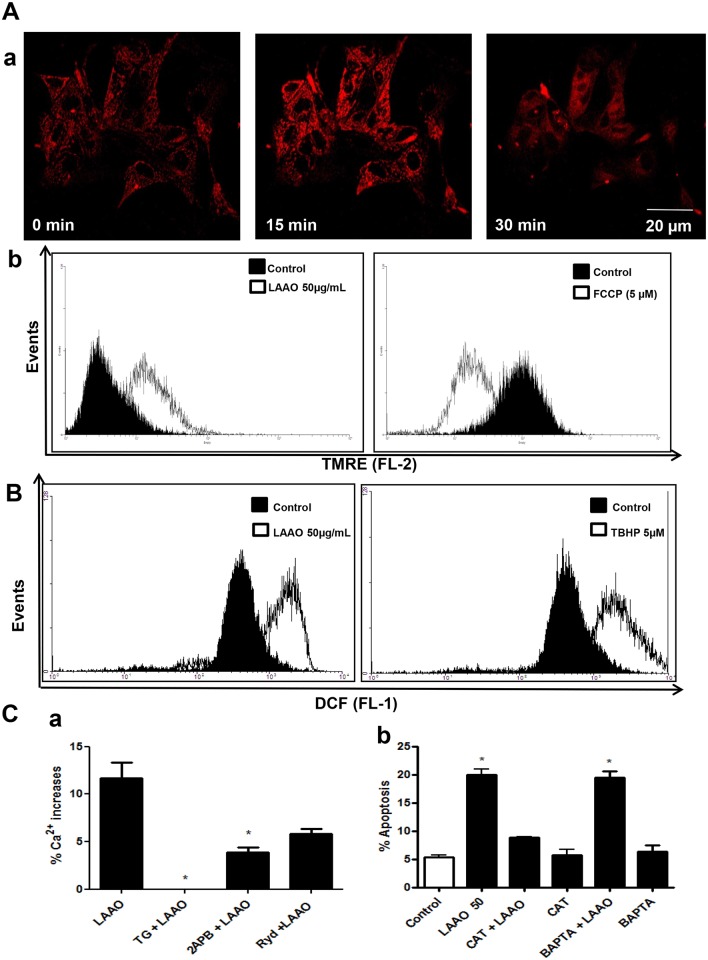
To verify the LAAO-Bl effects on mitochondrial transmembrane potential (ΔΨm) cells were stained with the mitochondrial specific probe, tetramethylrhodamine ethyl ester (TMRE) and analyzed in a real-time confocal microcopy experiment and by flow cytometry. The images of the TMRE fluorescence changes observed in MDCK cells show a clear increase in fluorescence due to mitochondria hyperpolarization upon exposure of these cells to LAAO-Bl (Fig 2A, a). The increase of TMRE fluorescence was also observed in flow cytometry experiments (Fig 2A, b). The FCCP, nonspecific protonophore and uncoupler of oxidative phosphorylation, was used as a control. As expected, upon addition of FCCP (5 μM), TMRE fluorescence decreased immediately indicating mitochondrial depolarization.
Fig 2. LAAO-Bl exposure induces mitochondrial hyperpolarization, intracellular Ca2+ mobilization and ROS generation in MDCK cells.
(A) Changes on ΔΨm after LAAO-Bl (50 μg/mL) stimulation were measured by TMRE fluorescence (25 nM, 15 min at 37°C) and detected by confocal microscopy or flow cytometry. (a) Confocal images from cells TMRE loaded were collected at baseline and seconds after exposure with LAAO-Bl showing the increase of ΔΨm in MDCK cells in relation to basal levels. (b) Representative histograms obtained from MDCK cells TMRE loaded and exposed with LAAO-Bl (50 μg/mL). The uncoupler FCCP (5 μM) was used as positive control (b). (B) Intracellular LAAO-Bl (50 μg/mL) induced ROS generation in MDCK cells. After treatment for 12 h, cells were incubated with DCFH-DA (5 μM, 30 min at 37°C) then examined by flow cytometer. ROS generation was expressed as a ratio of relative fluorescent intensity compared to the control group. t-BHP (5 μM) was used as positive control. (C) (a) Cytosolic Ca2+ increase (%) induced by LAAO-Bl (50 μg/mL) was monitored in a real-time fluorescence microscope. Relative increase in maximum cytosolic Ca2+ response (%) evoked by LAAO-Bl (50 μg/mL) or after pre-incubation with TG (2 μM, 20 min) (TG + LAAO-Bl) or 2-APB (100 μM, 10 min) (2-APB + LAAO-Bl) or with Ryanodine (Ryd., 20 μM, 20 min) (Ryd. + LAAO-Bl). (b) The summary data quantifies the flow cytometry analysis of cells loaded with propidium iodide (PI, 50 μg/mL) under treatment with LAAO-Bl (50 μg/mL) for 12 hrs, in the presence or absence of catalase (100 μg/mL) or BAPTA-AM (20 μM). The data represent the mean ± S.E.M of at least three independent experiments. *Significantly different from control group (p<0.05, one way ANOVA, Dunnett post-test).
Cytosolic calcium and ROS generation involvement on the cytotoxicity and death induced by LAAO-Bl to renal epithelial cell
To determine the possible involvement of ROS production in the mechanism of action of LAAO-Bl. The ROS levels upon LAAO-Bl exposure were analyzed. For this purpose cells were stained with the 7′-Dichlorofluorescin diacetate (DCFH-DA) a cell-permeable non-fluorescent probe that turns to highly fluorescent 2′,7′-dichlorofluorescein upon oxidation. Fluorescence changes were analyzed using flow cytometry. LAAO-Bl was able to induce an increase of intracellular ROS levels as showed by the significant shift of the peak (shift in relative fluorescence intensity) when compared with untreated control group (Fig 2B). The oxidant agent tertButyl hydroperoxide-(t-BHP) was included as positive control. The participation of ROS on LAAO-Bl-mediated apoptosis was also evaluated in cells pre-treated with catalase (100 μg/mL, 30 min), a H2O2 scavenger. There was a significantly reduction in the percentage of cell death induced by LAAO-Bl under this condition (Fig 2C, b), suggesting the importance of ROS production in apoptosis LAAO-Bl-induced.
We next evaluated the intracellular Ca2+ levels in response to LAAO-Bl. In cells loaded with the intracellular calcium indicator Fura-2-acetoxymethyl ester (Fura-2AM), LAAO-Bl evoked a cytosolic Ca2+ increase. As the endoplamastic reticulum (ER) is the mainly source for cytosolic calcium release, the same study was done in cells treated before with thapsigargin (TG, 2 μM). Notably, the cytosolic Ca2+ mobilization by LAAO-Bl was decreased in this situation due to the inhibition of ER Ca2+ pump, SERCA. To define the mechanism which LAAO-Bl mediates Ca2+ release from ER, elucidating the possible involvement of channels in these responses, MDCK cells were pre-treated with different ER Ca2+ channel inhibitors, such as ryanodine (Ryd, 20 μM, 20 min) and 2-APB (100 μM, 10 min), that inhibit the Ca2+ release from RyR and IP3R, respectively. Our results showed that Ryd and 2-APB were able to decrease the Ca2+ rises LAAO-Bl-induced, suggesting a prominent regulation of these channels in LAAO-Bl-mediated Ca2+ increases (Fig 2C, a). To further identify the role of Ca2+ on apoptosis LAAO-Bl-mediated, we pre-treated the cells with BAPTA-AM (20 μM, 30 min), a Ca2+ chelator. Our data showed that apoptosis induced by LAAO-Bl in MDCK cells was not dependent on cytosolic Ca2+ release (Fig 2C, b).
LAAO-Bl activates Bax, caspases 3 and 7 in epithelial kidney cells
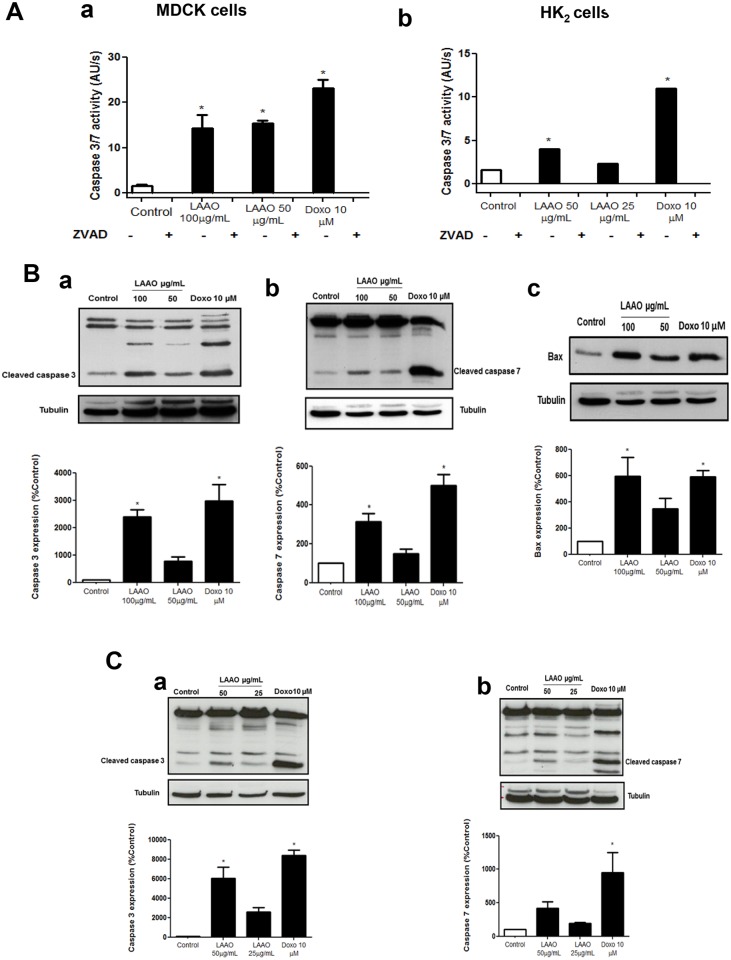
Our findings showed that LAAO-Bl induced a significant increase in the activities of caspase-3 or 7 in both cell lines (MDCK or HK-2), which were inhibited by pre-treatment with zVAD (10 μM, 30 min), respectively (Fig 3A a-b). However, this inhibition was unable to protect cells (Data not shown). Furthermore, LAAO-Bl induced the upregulation of cleaved caspase-3, caspase- 7 and the pro-apoptotic protein Bax after 12 h of exposure in a concentration-dependent manner (Fig 3B, a-c) in MDCK or in HK-2 cells (Fig 3C, a-b). Doxorubicin (Dox) 10 μM was used as a positive control.
Fig 3. LAAO-Bl activates Bax, caspases 3 and 7 in epithelial kidney cells.
(A) Caspase-3/7 activity was determined in the presence of the fluorogenic Ac-DEVD-afc substrate after treatment with LAAO-Bl (12 h) in the presence or absence of zVAD (10 μM) in MDCK (a) and HK-2 cells (b). (B-C) Western blot analyses were performed to detect the effects of LAAO-Bl on changes in Caspase-3 (a), -7 (b) and Bax (c) expressions from total protein of MDCK (B) and Caspase-3 (a), -7 (b) HK-2 (C) cell extracts. Percentage of Caspase-3 (a), -7 (b) and Bax (c) expression in MDCK (B) and caspase-3 (a) and caspase-7 (b) HK2 (C) treated with LAAO-Bl for 12 h. Data normalized in relation to Control group. Protein bands were analyzed and normalized to the densitometric values of α-tubulin. Doxorubicin was used as positive control (10 μM). Data are representative results of three independent experiments.
LAAO-Bl induced alterations in rat isolated kidney
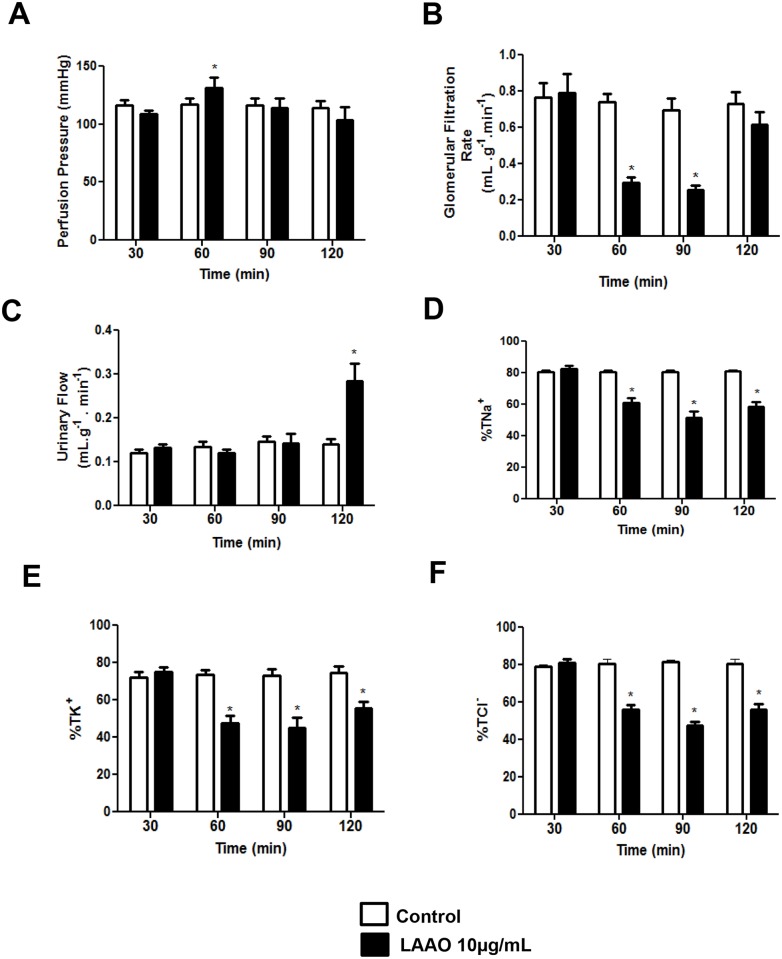
Consistently with previous data, we used this experimental model in order to clarify the involvement of the LAAO-Bl in the renal effects of Bothrops leucurus venom. LAAO-Bl (10 μg/mL) increased perfusion pressure at 60 min and returned to normal at 90 min of perfusion (Fig 4A). There were no significant differences in the renal vascular resistance (RVR) after the infusion of LAAO-Bl when compared with control group. The glomerular filtration rate (GFR) decreased at 60 and 90 min of infusion and normalized at 120 min (Fig 4B). Urinary flow (UF) increased in the period of 120 min (Fig 4C). LAAO-Bl reduced significantly sodium, potassium and chloride tubular transport in the periods of 60, 90 and 120 min (Fig 4D–4F).
Fig 4. LAAO-Bl induced alterations in rat isolated kidney.
Effects of LAAO isolated of Bothrops leucurus venom on perfusion pressure (A), glomerular filtration rate (B), urinary flow (C), sodium, potassiun and chloride (D, E, F) tubular transport percentage. LAAO-Bl was added to the system 30 min after the beginning of each perfusion. The data represent the mean ± S.E.M of at least six independent experiments. *Significantly different from control group (p<0.05, one way ANOVA, Bonferroni post-test).
Discussion
Acute renal failure is a common complication of Bothrops viper envenomation. High venom concentration at the renal tissue, direct action of venom components on renal tubules and renal epithelial cells, hemoglobinuria, vascular occlusion and oxidative stress are among factors incriminated in the pathogenesis of renal failure [12].
In order to understand the molecular mechanisms involved in the LAAO-Bl nephrotoxicity, we evaluated its cytotoxic to two renal tubule cell lines, HK-2, isolated from human tubule proximal [13] and MDCK, isolated from canine tubule distal [14]. Using Annexin/PI assay, it was observed that LAAO-Bl induced death in both cell lines, probably by different mechanisms. This result is in accordance with the literature showing possible involvement of apoptosis and necrosis in LAAO different cell types response [5, 15, 16, 17, 18, 19].
The tubular epithelial cells are the main targets of acute injury kidney for venoms [20]. Apoptosis, usually responsible for loss of kidney function, could be induced by many factors, including mitochondrial dysfunction, oxidative stress, Ca2+ imbalance and energy deprivation [13]. Some studies showed that mitochondrial dysfunction play an important role in renal cell damage by snake venoms [21, 2]. Here, we showed that LAAO-Bl (50 μg/mL) induced mitochondrial hyperpolarization in MDCK cells accompanied by ROS generation. In addition, some investigations suggested that ΔΨm increases after induction of apoptosis, initiated by, e.g., ROS, Ca2+, Bax and ceramides [22, 23].
Since the apoptosis induced by LAAO is mainly attributed with a H2O2 generation [24, 25, 26, 27, 28, 29]. Here, intracellular H2O2 and ROS increase was monitored with DCFH-DA after stimulation with LAAO-Bl (50 μg/mL) in MDCK cells. Furthermore, H2O2 removal by catalase decreased, approximately, 50% of the apoptotic effects, suggesting that H2O2 plays a main role to induce apoptosis LAAO-Bl-mediated. Interestingly, the cytosolic Ca2+ rises mediated by LAAO-Bl (50 μg/mL) were from endoplasmic reticulum (ER) via IP3 receptors, since ROS and oxidative stress disrupt the ER function, inducing Ca2+ rises [30]. Although Ca2+ participates of cell damage, BAPTA-AM was not able to reduce apoptosis LAAO-Bl-regulated.
Two major apoptotic signaling pathways, including the intrinsic and extrinsic signals, converge onto caspases to initiate cell death [31]. In this study, we demonstrated that LAAO-Bl triggered the activation of caspase 3 and 7 in MDCK and HK-2 cells. Furthermore, we showed that LAAO treatment up-regulated the expression of pro-apoptotic protein Bax. Other reports showed that LAAO induced apoptosis through caspase-dependent [5, 24, 32, 33].
To verify the effect of LAAO-Bl in the kidney without interference of systemic factors, we used perfusion in the rat kidney. LAAO- Bl (10 μg/mL) showed an increase in perfusion pressure and urine flow, with no effect in renal vascular resistance. It was observed a decrease in glomerular filtration rate. The tubular transport of sodium, potassium and chloride was reduced significantly. The loss of tubular epithelial cells function induced for LAAO- Bl can explain some of the renal effects observed in this study, such as increased of the urine flow and reducing the transport of electrolytes. Using the same model, we showed that B. leucurus venom also reduced the GFR, but decreased the PP and UF. This venom also reduced the sodium and potassium tubular transport [2].
Among several enzymes in Bothrops venom, L-amino acid oxidase, phospholipase A2, metalloprotease and sphingomyelinase are believed to contribute to renal toxicity [34]. In addition, perfusion of isolated rat kidneys with LAAO [35], C type lectins [36, 37], phospholipase A2 (PLA2) myotoxins [38, 39] and thrombin-like enzyme [40] from Bothrops venoms caused renal function disturbances.
In conclusion, we showed that LAAO-Bl induced intense alterations in renal physiology, assessed in the isolated rat kidney. These direct nephrotoxicity effects are in agreement with loss of tubular epithelial cell function, as it has been demonstrated that LAAO-Bl induced apoptosis in tubular epithelial cells by oxidative stress.
Our results have important implications for understanding the mechanisms of nephrotoxicity triggered by this family of enzymes. Since these molecular events are responsible for acute kidney injury caused by ophidism, it contributes to develop drugs and more effective therapeutic strategies.
Acknowledgments
Isabel C. O. Morais gratefully acknowledges CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior Brazilian Ministry of Education); for providing a 1-year scholarship to perform this study at Centro de Investigación Príncipe Felipe, Valencia, Spain. The authors wish to thank Dr Tatiana Guevara and Dr Hanako Hirata for their excellent scientific and technical assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants provided by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior By ICOM Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (08/11515-3 by SSS; 10/51647-6 by CB), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) by AMCM HSAM, Fundação de Amparo à Pesquisa do Estado do Ceará (FUNCAP) by AMCM HSAM, Centro de Investigación Príncipe Felipe, Valencia, Spain by MOC EPP. Isabel C. O. Morais gratefully acknowledges CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior Brazilian Ministry of Education) for providing a 1-year scholarship to perform this study at Centro de Investigación Príncipe Felipe, Valencia, Spain. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sousa LF, Nicolau CA, Peixoto PS, Bernardoni JL, Oliveira SS, Portes-Junior JA, et al. Comparison of phylogeny, venom composition and neutralization by antivenom in diverse species of bothrops complex. PLoS Negl Trop. 2013; 9:e2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Morais IC, Torres AF, Pereira GJ, Pereira TP, Pessoa Bezerra de Menezes RR, Mello CP, et al. Bothrops leucurus venom induces nephrotoxicity in the isolated perfused kidney and cultured renal tubular epithelia. Toxicon. 2013; 61: 38–46. 10.1016/j.toxicon.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 3. de Moura AA, Kayano AM, Oliveira GA, Setúbal SS, Ribeiro JG, Barros NB, et al. Purification and biochemical characterization of three myotoxins from Bothrops mattogrossensis snake venom with toxicity against Leishmania and tumor cells. Biomed Res Int. 2014; 2014: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campos LB, Pucca MB, Roncolato EC, Bertolini TB, Netto JC, Barbosa JE. In vitro comparison of enzymatic effects among Brazilian Bothrops spp. Venoms. Toxicon. 2013; 76:1–10. [DOI] [PubMed] [Google Scholar]
- 5. Lee ML, Fung SY, Chung I, Pailoor J, Cheah SH, Tan NH. King cobra (Ophiophagus hannah) venom L-amino acid oxidase induces apoptosis in PC-3 cells and suppresses PC-3 solid tumor growth in a tumor xenograft mouse model. Int J Med Sci. 2014; 6:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tan NH, Fung SY. Snake venom L-amino acid oxidase In: Mackessy S. (Ed.), Handbook of Venoms and Toxins of Reptiles; 2009. pp. 219–232. [Google Scholar]
- 7. Guo C, Liu S, Yao Y, Zhang Q, Sun M-Z. Past decade study of snake venom L-amino acid oxidase. Toxicon. 2012; 60:302–311. 10.1016/j.toxicon.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 8. Naumann GB, Silva LF, Silva L, Faria G, Richardson M, Evangelista K, et al. Cytotoxicity and inhibition of platelet aggregation caused by an l-amino acid oxidase from Bothrops leucurus venom. Biochim Biophys Acta. 2011; 7:683–94. [DOI] [PubMed] [Google Scholar]
- 9. Torres AFC, Dantas RT, Menezes RRPPB, Toyama MH, Filho ED, Oliveira MF, et al. Antimicrobial activity of an L-amino acid oxidase isolated from Bothrops leucurus snake venom. J. Venom. Anim. Toxins incl. 2010; 16: 614–622. [Google Scholar]
- 10. Smaili SS, Russell JT. Permeability transition pore regulates both mitochondrial membrane potential and agonist-evoked Caþ2 signals in oligodendrocyte progenitors. Cell Calcium. 1999; 26: 121–130. [DOI] [PubMed] [Google Scholar]
- 11. Bowman RH. Gluconeogenesis in the isolated perfused rat kidney. J.Biol. Chem. 1970; 245: 1604–1612. [PubMed] [Google Scholar]
- 12. Martines MS, Mendes MM, Shimizu MH, Melo Rodrigues V, de Castro I, Filho SR, et al. Effects of Schizolobium parahyba extract on experimental Bothrops venom-induced acute kidney injury. PLoS One. 2014; 9: e86828 10.1371/journal.pone.0086828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin M, Li L, Zhang Y, Zheng L, Xu M, Rong R, et al. Baicalin ameliorates H2O2 induced cytotoxicity in HK-2 cells through the inhibition of ER stress and the activation of Nrf2 signaling. Int J Mol Sci. 2014; 7:12507–12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collares-Buzato CB, Le Sueur LP, Cruz-Hofling MA. Impairment of the Cell-to-Matrix Adhesion and Cytotoxicity Induced by Bothrops moojeni Snake Venom in Cultured Renal Tubular Epithelia. Toxicol. Appl. Pharmacol. 2002; 181: 124–132. [DOI] [PubMed] [Google Scholar]
- 15. Marcussi S, Stábeli RG, Santos-Filho NA, Menaldo DL, Silva Pereira LL, Zuliani JP, et al. Genotoxic effect of Bothrops snake venoms and isolated toxins on human lymphocyte DNA. Toxicon. 2013; 65:9–14. 10.1016/j.toxicon.2012.12.020 [DOI] [PubMed] [Google Scholar]
- 16. de Melo Alves Paiva R, de Freitas Figueiredo R, Antonucci GA, Paiva HH, de Lourdes Pires Bianchi M, Rodrigues KC, et al. Cell cycle arrest evidence, parasiticidal and bactericidal properties induced by L-amino acid oxidase from Bothrops atrox snake venom. Biochimie. 2011; 5: 941–947. [DOI] [PubMed] [Google Scholar]
- 17. Ciscotto P, Machado de Avila RA, Coelho EA, Oliveira J, Diniz CG, Farias LM, et al. Antigenic, microbicidal and antiparasitic properties of an l-amino acid oxidase isolated from Bothrops jararaca snake venom. Toxicon. 2009; 53: 330–341. 10.1016/j.toxicon.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 18. Torii S, Yamane K, Mashima T, Haga N, Yamamoto K, Fox JW, et al. Molecular cloning and functional analysis of apoxin I, a snake venom-derived apoptosis-inducing factor with L-amino acid oxidase activity. Biochemistry. 2000; 39: 3197–3205. [DOI] [PubMed] [Google Scholar]
- 19. Sun MZ, Guo C, Tian Y, Chen D, Greenaway FT, Liu S. Biochemical, functional and structural characterization of Akbu-LAAO: a novel snake venom L-amino acid oxidase from Agkistrodon blomhoffii ussurensis. Biochimie. 2010; 92: 343–349. 10.1016/j.biochi.2010.01.013 [DOI] [PubMed] [Google Scholar]
- 20. Castro I, Burdmann EA, Seguro AC, Yu L. Bothrops venom induces direct renal tubular injury: role for lipid peroxidation and prevention by antivenom. Toxicon. 2004; 43: 833–839. [DOI] [PubMed] [Google Scholar]
- 21. Mello CP, Morais IC, Menezes RR, Pereira GJ, Torres AF, Lima DB, et al. Bothropoides insularis venom cytotoxicity in renal tubular epithelia cells. Toxicon. 2014; 88:107–114. 10.1016/j.toxicon.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 22. Joo HK, Lee YR, Park MS, Choi S, Park K, Lee SK, et al. Mitochondrial APE1/Ref-1 suppressed protein kinase C-induced mitochondrial dysfunction in mouse endothelial cells. Mitochondrion. 2014; 17: 42–49. 10.1016/j.mito.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 23. Liu D, Yang J, Li Y, Zhang M, Wang L. Cd-induced apoptosis through the mitochondrial pathway in the hepatopancreas of the freshwater crab Sinopotamon henanense. PLoS One. 2013; 22;8(7):e68770 10.1371/journal.pone.0068770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alves RM, Antonucci GA, Paiva HH, Cintra AC, Franco JJ, Mendonça-Franqueiro EP, et al. Evidence of caspase-mediated apoptosis induced by L-amino acid oxidase isolated from Bothrops atrox snake venom. Comp Biochem Physiol A Mol Integr Physiol. 2008; 4: 542–550. [DOI] [PubMed] [Google Scholar]
- 25. Samel M, Vija H, Ronnholm G, Siigur J, Kalkkinen N, Siigur E. Isolation and characterization of an apoptotic and platelet aggregation inhibiting L-amino acid oxidase from Vipera berus berus (common viper) venom. Biochim Biophys Acta. 2006; 4:707–714. [DOI] [PubMed] [Google Scholar]
- 26. Sun LK, Yoshii Y, Hyodo A, Tsurushima H, Saito A, Harakuni T, et al. Apoptotic effect in the glioma cells induced by specific protein extracted from Okinawa Habu (Trimeresurus flavoviridis) venom in relation to oxidative stress, Toxicol. In Vitro. 2003; 2: 169–177. [DOI] [PubMed] [Google Scholar]
- 27. Zhang L, Wei LJ. ACTX-8, a cytotoxic L-amino acid oxidase isolated from Agkistrodon acutus snake venom, induces apoptosis in Hela cervical cancer cells. Life Sci. 2007; 80:1189–1197. [DOI] [PubMed] [Google Scholar]
- 28. Stabeli RG, Sant’Ana CD, Ribeiro PH, Costa TR, Ticli FK, Pires MG, et al. Cytotoxic L-amino acid oxidase from Bothrops moojeni: biochemical and functional characterization. Int. J. Biol. Macromol. 2007; 41: 132–140. [DOI] [PubMed] [Google Scholar]
- 29. Toyama MH, Toyama DO, Passero LFD, Laurenti MD, Corbett CE, Tomokane TY, et al. Isolation of a new L-amino acid oxidase from Crotalus durissus cascavella venom. Toxicon. 2006; 47: 47–57. [DOI] [PubMed] [Google Scholar]
- 30. Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease.Antioxid Redox Signal. 2014; 3: 396–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mondragón L, Orzáez M, Sanclimens G, Moure A, Armiñán A, Sepúlveda P, et al. Modulation of cellular apoptosis with apoptotic protease-activating factor 1 (Apaf-1) inhibitors. J Med Chem. 2008; 3: 521–529. [DOI] [PubMed] [Google Scholar]
- 32. Deolindo P, Teixeira-Ferreira AS, DaMatta RA, Alves EW. Lamino acid oxidase activity present in fractions of Bothrops jararaca venom is responsible for the induction of programmed cell death in Trypanosoma cruzi. Toxicon. 2010; 56: 944–955. 10.1016/j.toxicon.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 33. Zhang L, Wu WT. Isolation and characterization of ACTX-6: a cytotoxic L-amino acid oxidase from Agkistrodon acutus snake venom. Nat. Prod. Res. 2008; 22: 554–563. 10.1080/14786410701592679 [DOI] [PubMed] [Google Scholar]
- 34. Sitprija V, Sitprija S. Renal effects and injury induced by animal toxins. Toxicon. 2012; 5:943–953. [DOI] [PubMed] [Google Scholar]
- 35. Braga MD, Martins AM, Amora DN, de Menezes DB, Toyama MH, Toyama DO, et al. Purification and biological effects of L-amino acid oxidase isolated from Bothrops insularis venom. Toxicon. 2008; 2:199–207. [DOI] [PubMed] [Google Scholar]
- 36. Havt A, Toyama MH, do Nascimento NR, Toyama DO, Nobre AC, Martins AM, et al. A new C-type animal lectin isolated from Bothrops pirajai is responsible for the snake venom major effects in the isolated kidney. Int J Biochem Cell Biol. 2005; 1:130–141. [DOI] [PubMed] [Google Scholar]
- 37. Braga MD, Martins AM, Amora DN, de Menezes DB, Toyama MH, Toyama DO, et al. Purification and biological effects of C-type lectin isolated from Bothrops insularis venom. Toxicon. 2006; 8:859–867. [DOI] [PubMed] [Google Scholar]
- 38. Barbosa PS, Martins AM, Havt A, Toyama DO, Evangelista JS, Ferreira DP, et al. Renal toxicity of Bothrops moojeni snake venom and its main myotoxins. Toxicon. 2005; 4: 376–386. [DOI] [PubMed] [Google Scholar]
- 39. Barbosa PS, Martins AM, Alves RS, Amora DN, Martins RD, Toyama MH, et al. The role of indomethacin and tezosentan on renal effects induced by Bothrops moojeni Lys49 myotoxin I. Toxicon. 2006; 8:831–837. [DOI] [PubMed] [Google Scholar]
- 40. Braga MD, Martins AM, de Menezes DB, Barbosa PS, Evangelista JS, Toyama MH, et al. Purification and biological activity of the thrombin-like substance isolated from Bothrops insularis venom. Toxicon. 2007; 3:329–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.