Abstract
Neutrophil extracellular traps (NETs) have been described as a network of extracellular fibers composed by DNA, histones and various proteins/enzymes. Studies have demonstrated that NETs could be responsible for the trapping and elimination of a variety of infectious agents. In order to verify the presence of NETs in American tegumentary leishmaniasis (ATL) and their relationship with the presence of amastigotes we evaluated active cutaneous lesions of 35 patients before treatment by the detection of parasites, neutrophils (neutrophil elastase) and histones through immunohistochemistry and confocal immunofluorescence. Intact neutrophils could be detected in all ATL lesions. NETs were present in 27 patients (median 1.1; range from 0.1 to 23.5/mm2) with lesion duration ranging from one to seven months. NETs were in close proximity with neutrophils (r = 0.586; p = 0.0001) and amastigotes (r = 0.710; p = 0.0001). Two patterns of NET formation were detected: small homogeneously distributed networks observed in all lesions; and large structures that could be visualized at a lower magnification in lesions presenting at least 20% of neutrophils. Lesions presenting the larger NET formation showed high parasite detection. A correlation between NET size and the number of intact amastigotes was observed (p=0.02). As we detected an association between NET and amastigotes, our results suggest that neutrophil migration and NET formation could be stimulated and maintained by stimuli derived from the parasite burden/parasite antigen in the extracellular environment. The observation of areas containing only antigens not intermingled with NETs (elastase and histone) suggests that the involvement of these structures in the control of parasite burden is a dynamic process in which the formation of NETs is exhausted with the destruction of the parasites. Since NETs were also associated with granulomas, this trapping would favor the activity of macrophages in order to control the parasite burden.
Introduction
Various infectious agents are able to invade and multiply inside human cells. Protozoa of the genus Leishmania are obligate intracellular parasites of the human mononuclear phagocyte system [1], but these parasites have also been detected inside neutrophils [2], fibroblasts [3,4], endothelial cells [5,6], and dendritic cells [7].
The function of macrophages as host and effector cells during infection with Leishmania parasites has been extensively studied. However, many questions regarding the ability of this protozoan to infect other cell types remain unanswered. In this aspect, controversy still exists whether neutrophils restrain or facilitate infection with Leishmania sp [8,9]. Neutrophils are the first cells recruited to the site of infection in leishmaniasis [10,11] and their participation in the control of parasite burden has been demonstrated in experimental models. Peters et al. [9] observed rapid and persistent infiltration of neutrophils at the site of inoculation of promastigotes by sandflies in a murine model within the first hours of infection with L. major. C57BL/6 and BALB/c mice depleted of neutrophils and infected with L. major showed higher parasite burdens, greater dissemination of infection and more severe lesions than non-depleted animals [8]. However, other investigators suggested a negative role of neutrophils in murine leishmaniasis, producing more severe disease associated with a Th2 response [12], or even serving as a carrier for Leishmania entry into macrophages [13]. Afonso et al [14] observed that human necrotic, but not apoptotic, neutrophils induced in vitro leishmanicidal activity mediated by macrophages. This leishmanicidal activity was dependent on TNF-α and neutrophil elastase (NE). Ribeiro-Gomes and Sacks [15] discussed the influence of early neutrophil-Leishmania interactions on the host immune response and suggested that infection outcome critically depends on the time of neutrophil recruitment and the tissue environment in which it occurs.
A particular form of death was described for neutrophils, which occurs with the release to the extracellular milieu of a mesh formed by chromatin associated with granular and cytoplasmic proteins named netosis from Neutrophil Extracellular Traps (NETs) [16]. NETs are endowed with the properties to arrest and kill different microorganisms [16–18]. During netosis, there is disruption of the nuclear membrane and chromatin decondensation, this last feature is dependent on the activity of elastase (NE), myeloperoxidase and peptidyl arginine deiminase 4 [18–20]. The formation of NETs has been reported in studies involving different pathogens such as Mycobacterium tuberculosis [21], fungi [22–23], HIV-1 [24], Toxoplasma gondii [25], Eimeria bovis [26], Plasmodium falciparum [27], Leishmania amazonensis, L. major, L. chagasi [28] and L. donovani [29]. Therefore, the in vitro interaction of neutrophils and Leishmania promastigotes [28, 29] leads to NET formation suggesting that NETs may contribute to the containment of promastigotes at the site of inoculation, thereby facilitating their uptake by mononuclear phagocytes [29]. NETs were also detected in the active lesion of a patient with American tegumentary leishmaniasis (ATL) [28]. However, the importance of this phenomenon in human tegumentary leishmaniasis lesions is still unknown, and it remains unclear whether NETs exert effects on the control of initial parasite burden in vivo or whether this effector mechanism occurs during an already established infection when only amastigote forms are present in lesions. It has been shown that amastigote forms of L. amazonensis induce NET release upon neutrophil interaction in vitro [28]. Therefore, in the present study we investigated the presence of NETs in active established ATL lesions and their relationship with Leishmania amastigotes.
Materials and Methods
Patients
Thirty-five patients with the localized cutaneous form of ATL were studied. Etiological diagnosis was confirmed by histopathological examination and isolation in culture as previously described [30]. Parasite characterization was possible in 11 isolates and Leishmania (V.) braziliensis infection was identified for all of them. All cases were from areas of Brazil (Rio de Janeiro) where Leishmania (Viannia) braziliensis is the infecting species of greatest prevalence. Patient age, lesion duration, number and site of the lesions were described (Table 1).
Table 1. Clinical and quantitative data of cutaneous lesions from 35 patients with American tegumentary leishmaniasis.
| Patient number | Age (years) | Gender | Lesion Duration (month) | Number of lesions | Localization | MST a (mm) | Neutrophil (%) | NET/mm2 | Amastigotes/mm2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 39 | F | 2.0 | 1.0 | Lower limb | 7.0 | 1.00 | 0.00 | 0.00 |
| 2 | 20 | M | 1.0 | 1.0 | Lower Limb | ND b | 13.54 | 1.30 | 11.00 |
| 3 | 45 | M | 2.0 | 1.0 | Lower Limb | 20.0 | 11.71 | 23.50 | 0.25 |
| 4 | 18 | M | 2.0 | 1.0 | Lower limb | 12.0 | 2.90 | 0.00 | 0.09 |
| 5 | 38 | M | 4.0 | 3.0 | Upper limb | 8.0 | 9.20 | 0.00 | 0.50 |
| 6 | 46 | M | 4.0 | 1.0 | Upper limb | 14.0 | 14.70 | 0.00 | 0.60 |
| 7 | 26 | M | 2.0 | 1.0 | Upper limb | 33.0 | 7.83 | 0.50 | 0.00 |
| 8 | 22 | M | 1.0 | 1.0 | Face | ND b | 10.33 | 0.00 | 0.00 |
| 9 | 59 | F | 3.0 | 1.0 | Upper Limb | 19.0 | 10.24 | 0.00 | 0.00 |
| 10 | 64 | F | 1.0 | 1.0 | Neck | 50.0 | 14.24 | 1.40 | 5.30 |
| 11 | 37 | M | 2.0 | 1.0 | Upper Limb | 15.0 | 11.22 | 0.85 | 4.00 |
| 12 | 33 | F | 1.0 | 1.0 | Upper Limb | 9.0 | 12.94 | 1.10 | 3.00 |
| 13 | 37 | M | 2.0 | 1.0 | Lower Limb | 2.0 | 11.00 | 0.10 | 0.00 |
| 14 | 14 | F | 2.0 | 1.0 | Upper Limb | 21.0 | 22.95 | 1.52 | 0.75 |
| 15 | 18 | M | 1.0 | 1.0 | Lower Limb | 23.0 | 7.04 | 0.21 | 0.06 |
| 16 | 39 | M | 3.0 | 2.0 | Lower Limb | 34.0 | 19.05 | 0.00 | 0.00 |
| 17 | 52 | M | 1.0 | 1.0 | Upper limb | 23.0 | 6.60 | 0.25 | 0.25 |
| 18 | 55 | F | 4.0 | 1.0 | Upper Limb | 5.0 | 15.82 | 0.00 | 0.50 |
| 19 | 40 | F | 3.0 | 2.0 | Lower Limb | 17.0 | 6.79 | 0.34 | 0.17 |
| 20 | 63 | F | 3.0 | 1.0 | Trunk | 49.0 | 18.33 | 0.36 | 0.18 |
| 21 | 43 | F | 1.0 | 1.0 | Upper Limbs | ND b | 17.84 | 10.30 | 30.00 |
| 22 | 23 | M | 2.0 | 1.0 | Lower Limb | 15.0 | 7.91 | 0.40 | 0.20 |
| 23 | 63 | F | 3.0 | 1.0 | Trunk | 49.0 | 22.03 | 10.50 | 40.00 |
| 24 | 27 | F | 1.0 | 1.0 | Lower Limb | 20.0 | 9.88 | 0.42 | 0.25 |
| 25 | 16 | M | 7.0 | 1.0 | Neck | 23.0 | 22.63 | 8.70 | 16.50 |
| 26 | 19 | M | 2.0 | 2.0 | Trunk | 12.0 | 16.15 | 0.45 | 0.23 |
| 27 | 36 | M | 5.0 | 1.0 | Lower Limb | 19.0 | 11.92 | 1.10 | 1.90 |
| 28 | 46 | F | 2.0 | 1.0 | Head | 38.0 | 9.15 | 0.67 | 0.34 |
| 29 | 41 | F | 2.0 | 2.0 | Lower Limb | 18.0 | 53.58 | 2.37 | 0.43 |
| 30 | 53 | M | 2.0 | 1.0 | Lower limb | 20.0 | 16.4 | 3.00 | 0.80 |
| 31 | 31 | M | 1.0 | 1.0 | Upper limb | 24.0 | 4.30 | 0.70 | 0.30 |
| 32 | 65 | M | 1.5 | 2.0 | Upper limb | 18.0 | 29.8 | 1.80 | 0.52 |
| 33 | 25 | F | 6.0 | 1.0 | Upper limb | ND b | 1.80 | 0.14 | 0.20 |
| 34 | 49 | M | 2.0 | 6.0 | Trunk | 14.0 | 34.8 | 14.60 | 0.90 |
| 35 | 69 | F | 1.5 | 1.0 | Upper limb | 23.0 | 37.7 | 19.30 | 13.6 |
| Median | 39 | - | 2.0 | 1.0 | - | 19.0 | 11.92 | 0.5 | 0.34 |
| Min-max | 14–69 | - | 1.0–7.0 | 1.0–6.0 | - | 2.0–50.0 | 1.0–53.6 | 0–23.5 | 0–40.0 |
a MST—Montenegro skin test.
b ND—not done.
Ethics statement
The patients were included in the study after they had given formal written consent and the study was approved by the Ethics Committee of INI/FIOCRUZ (Comitê de Ética em Pesquisa do Instituto Nacional de Infectologia Evandro Chagas—CEP-INI—014/2001). When the patients were under 18 years old, informed consent was sign by parents or guardians, who also remained present throughout the clinical evaluation and diagnosis procedure.
Immunohistochemistry (IHC)
Biopsy fragments were cut into 3-μm thick sections and mounted on silanized microscope slides (DakoCytomation, Carpinteria, CA, USA). The IHC was performed as described [31,32]. For single staining, the specimens were incubated with the primary antibodies anti-neutrophil elastase (NE- DakoCytomation, Carpinteria, USA) or anti-L. braziliensis polyclonal rabbit serum (Dr. Madeira-INI/FIOCRUZ), followed by sequential steps of washes in PBS (pH 7.4) and incubation with the biotinylated secondary antibody (Zymed, San Francisco, CA, USA), the streptavidin-biotin-peroxidase complex (ABC kit, DakoCytomation), and Aminoethyl carbazole (chromogen-AEC kit, Zymed). The slides were counterstained with Mayer’s hematoxylin (Dako). Double staining was performed for the colocalization of NE and amastigotes using the Dako Envision Doublestain System according to the manufacturers instructions. The slides were examined under a light microscope (Zeiss, Germany). The percentage of neutrophils was determined by counting 500 cells as standard and the number of amastigotes and NETs per mm2 tissue was calculated using a millimeter coverslip.
Immunofluorescence and confocal microscopy
Biopsy fragments were obtained by cryosection and prepared for immunofluorescence microscopy as described [28]. Briefly, slides were rehydrated with PBS (pH 7.4) and unspecific epitopes were blocked with rabbit serum for two hours of incubation. Then, the sections were incubated with mouse anti-human NE (Calbiochem—EMD Millipore Co, Billerica, MA, USA)), anti-human DNA-histone complex (Millipore), anti-CD68 macrophages (Dako) and anti-L. braziliensis polyclonal rabbit serum, as primary antibodies. Anti-rabbit FITC-conjugated (Vector Labs), anti-mouse Alexa Fluor-546 (Calbiochem) and anti-mouse PE-conjugated were used as secondary antibodies. After staining, sections were mounted in Vectashield mounting medium (Vector Labs). Some slides were also mounted on medium containing DAPI (Fluoromount-G, eBioscience). Immunofluorescence analysis was done in a Nikon Eclipse E400 Microscopy (Nikon digital camera DXM1200F and Nikon ACT-1 software, Nikon, Japan). Confocal analysis were done in a Zeiss LSM 510 META confocal microscope (Program for Technological Development in Tools for Health—PDTIS-FIOCRUZ) using excitation at 488 nm or 546 nm and emission at 505–530 nm (band pass system) and 560 nm (long pass system). The images were processed using the program LSM Image Browser—Version 4.2.0.121 (Zeiss, Jena, Germany).
Statistical analysis
The SPSS16 for Windows program (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. The results were analyzed using the nonparametric Mann-Whitney test and Spearman’s rank correlation coefficient for comparison between groups. The data are reported as the median and range. P-values ≤0.05 were considered as significant.
Results
Clinical data (Table 1)
Lesion biopsies from 15 female and 20 male patients located on the lower limbs (n = 13), upper limbs (n = 14), face (n = 2), and trunk (n = 6), with lesion duration ranged from 1 to 7 months were studied. Clinical characteristics are shown in Table 1.
Neutrophils
Neutrophils were heterogeneously distributed in the granulomatous inflammatory infiltrate in all lesions analyzed and were also present in clusters, inside vessels or adhered to the endothelium. The percentage of neutrophils ranged from 1.0 to 53.6% (median: 11.9%) (Table 1, Fig 1A and 1B, S1–S7 Figs).
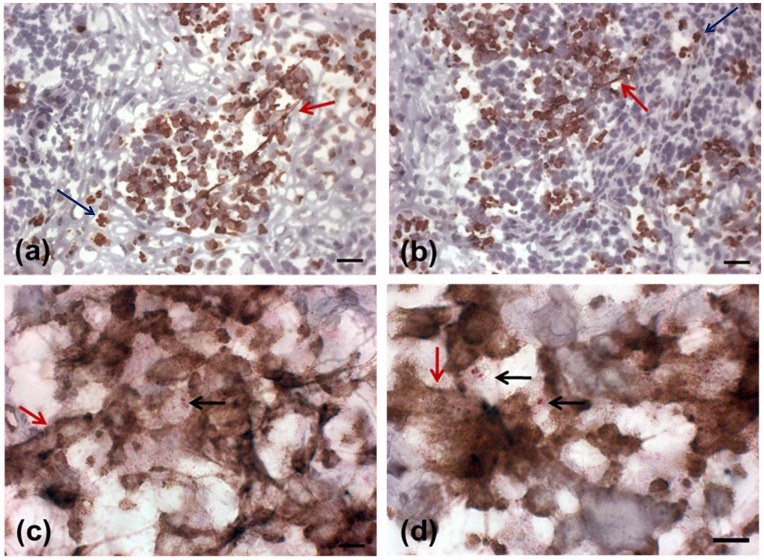
Fig 1. Detection of NETs in active lesions.
(A, B) Presence of NETs in an active lesion detected by neutrophil elastase immunostain(red arrows) in sections counterstained with Mayer`s hematoxylin (bar = 25μm). (C, D) Colocalization of neutrophils, NETs and Leishmania amastigotes. (neutrophil elastase NETs: red arrows, neutrophils: blue arrows, amastigotes: black arrows) (bar = 10μm).
Neutrophil extracellular traps (NET)
NET formation was observed in 27 (77.1%) of 35 lesions analyzed. In positive cases, the distribution of NETs/mm2 ranged from 0.1 to 23.5 (median: 1.1) and the percentage of neutrophils ranged from 1.8 to 53.6% (median: 12.9%). In patients in whom no NETs were identified, the percentage of neutrophils ranged from 1.0 to 19.1% (median: 10.3%), without significant differences between the two groups. However, a correlation was observed between the number of NETs/tissue area and the percentage of neutrophils (r = 0.586; p = 0.0001), NETs and age (r = 0.368, p = 0.03), and NET and intensity of Montenegro skin test (MST) (r = 0.372; p = 0.039).
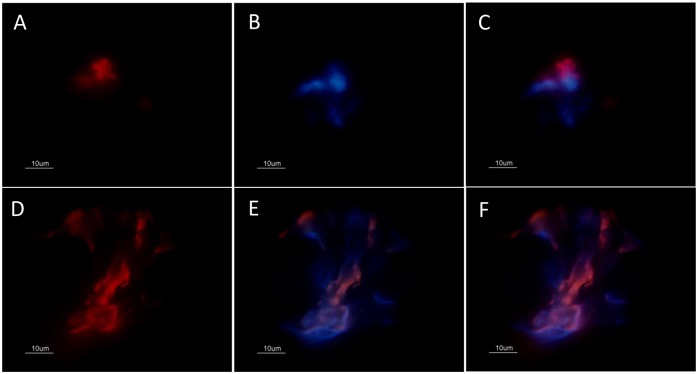
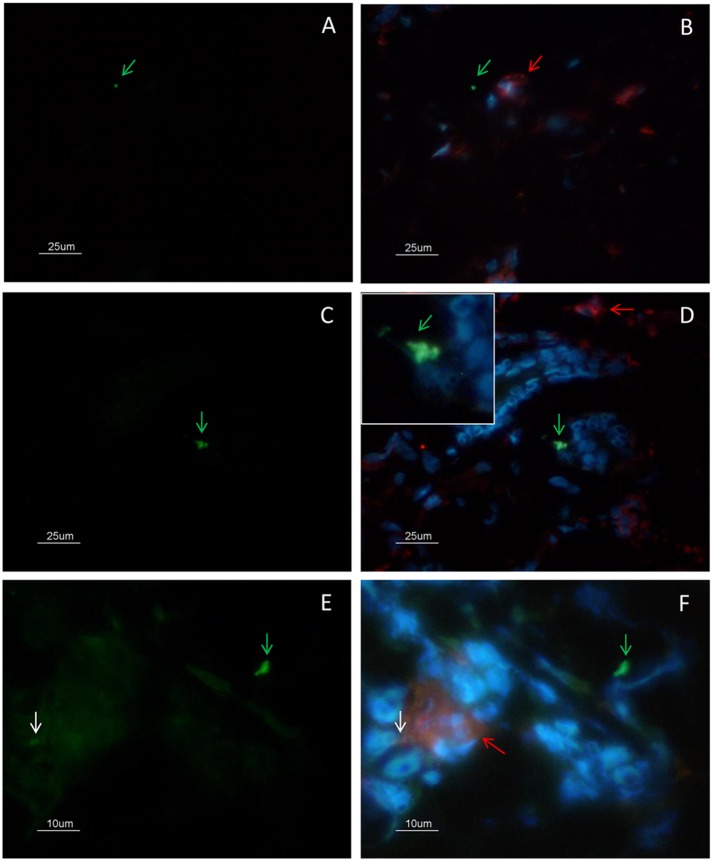
NETs were found throughout the lesion and varied in size according to the presence of neutrophils (Fig 1A–1D). Two patterns of NET formation were observed: (1) large heterogeneous webs consisting of large-diameter fibers that could be observed at low magnification (100 a 400x) identified when the percentage of neutrophils were higher than 20% (Fig 1A and 1B) and, (2) smaller homogeneous networks consisting of small-diameter fibers that could be better observed at 1000x magnification in all lesions presenting NET formation (Fig 1C and 1D). Fig 2 shows DNA and neutrophil elastase co-localization with typical features of NET formation (Fig 2D–2F), as well as neutrophil elastase positive cells presenting preserved nucleus (Fig 2A–2C). Since Leishmania amastigotes are typically found within lesion macrophages we evaluated the co-localization of macrophages and amastigotes. Amastigotes were observed outside cells (Fig 3A and 3B - green arrow) and inside CD68 negative cells (Fig 3C and 3D - zoom). We also observed amastigote antigens (white arrow) inside macrophages (Fig 3E and 3F - red arrow).
Fig 2. Colocalization analysis of neutrophil elastase and DNA by immunofluorescence.
(A) lesion stained for neutrophil elastase (red), and (B) DNA (blue). (C) merge: elastase and DNA showing a neutrophil elastase positive cell with morphologically preserved nucleus. (D) NET containing neutrophil elastase, (E) and DNA. (F) merge: elastase and DNA. All images were obtained from different regions of the same lesion with 45 days duration before diagnosis. Bar = 10μm.
Fig 3. Colocalization analysis of CD68(+) macrophages and amastigotes by immunofluorescence.
(A-B) Amastigotes (green arrow) were observed outside cells and close to macrophages (red arrow). (C-D) Amastigotes (green arrow) were observed inside CD68 negative cells (zoom). (E-F) Amastigote antigens inside macrophages (white arrow). (A-D) bar = 25μm; (E-F) bar = 10μm.
Since the presence of NETs was correlated with the number of neutrophils and since these cells might be involved in the control of parasite burden, we investigated the correlation between the presence of these structures and positivity for amastigotes and/or parasite antigens.
Amastigotes and NETs
Amastigote forms were detected by immunohistochemistry in 29 (82.9%) of 35 patients studied (Table 1). From the six remaining patients four were also negative to NET and the other two presented NET formation with small diameters (0.10 and 0.50 NETs/mm2). Among the 29 lesions positive for amastigotes, 25 (86.2%) also presented NET scaffolds., In these patients, the number of amastigotes ranged from 0.06 to 40.0 / mm2 (median: 0.5) and the number of NETs ranged from 0.14 to 23.5 /mm2 (median: 1.1). A correlation was observed between the number of amastigotes/mm2 and the number of NETs/mm2 (r = 0.710; p = 0.0001). An association between the size of NETs and number of amastigotes could be observed (p = 0.02), since the lesions presenting larger NETs (n = 7; Table 1) showed the higher parasite burden (0.43 to 40.0 amastigotes/mm2 -Median 0.90) than the lesions presenting the small NETs (0 to 30.0 amastigotes/mm2 -Median 0.25). In the lesions of four patients who presented amastigotes in the absence of NETs, 0.09, 0.6, 0.5 and 0.5 amastigotes/mm2 were observed.
When the presence of amastigotes, neutrophils and NETs were compared, we noticed that all of them were in close proximity in various areas (Fig 1C and 1D, S8–S16 Figs). A correlation was observed between the number of amastigotes/mm2 and the percentage of neutrophils (r = 0.573; p = 0.0001). Amastigotes were often intermingled with NETs stained for elastase (Fig 1C and 1D). In addition, large clusters of NETs were noted close to sites containing a large number of parasites. In order to better demonstrate the correlation between the presence of NETs and amastigotes we performed a colocalization analysis by confocal microscopy.
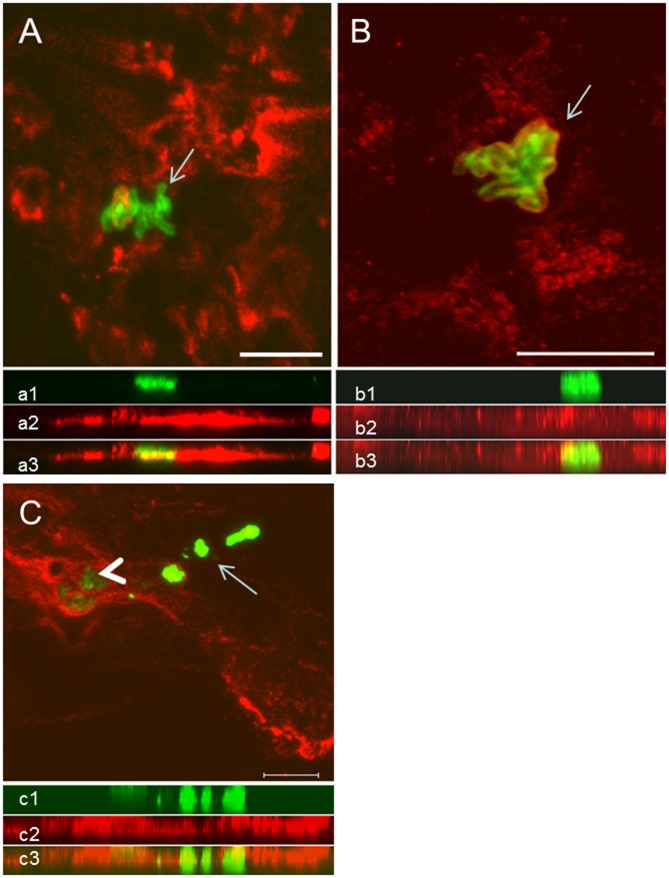
The confocal images reinforced our immunohistochemical findings since extensive areas occupied by NETs, evidenced by NE and histone staining, are in close contact with nests plenty of amastigotes (Fig 4). NETs were scattered throughout the tissue, forming webs of different shapes and diameters. Colocalization analysis of the parasites and NETs showed that most intact amastigotes were surrounded by NETs (Fig 4A–4C, S17 and S18 Figs, S1 Video). On the other hand, areas positive for Leishmania antigens in which no intact parasites could be detected were arranged in a heterogeneous fashion and were not directly associated with the presence of NETs (Fig 4C, arrow), despite the presence of a surrounding inflammatory infiltrate and small numbers of neutrophils (data not shown).
Fig 4. Colocalization analysis of NETs and amastigotes by confocal microscopy.
(A) NETs containing neutrophil elastase (red) and amastigotes (green, arrow). (B) NETs containing histone (red) and amastigotes (green, arrow). (C) Area enclosing intact amastigotes (green—arrowhead) in intimate contact with NETs labeled with anti-neutrophil elastase (red). Area containing free Leishmania sp antigens (arrow). Below each image the respective side view (Z images) of (a1, b1, c1) amastigotes (green), (a2, c2) NET-elastase, (b2) NET-histones, merged sections showing the close contact between amastigotes and NETs (a3, b3, c3). Scale bar = 10μm.
Discussion
In the present study, the characterization of NETs in the ATL lesions was done by evidencing some of its major components (DNA, NE and histones). Two patterns of NET formation were observed: 1) small homogeneously distributed webs, and 2) large structures that could be visualized at a lower magnification. Larger NETs were often identified where neutrophils were more concentrated forming clusters of cells. Brinkmann & Zychlinsky [33] described unfixed NETs as having a cloud-like appearance and occupying a space that is 10-15-fold bigger than the volume of the cells they originate from. In addition, we observed colocalization of amastigotes, neutrophils and NETs. When associated with NETs, amastigotes were intermingled with these webs, as also verified in an in vitro study of promastigote forms of L. amazonensis using electron and immunofluorescence microscopy [28]. In the present study, parasite burden was positively correlated with the number of neutrophils and with the number of NETs per tissue area. This finding suggests that in the late lesions the maintenance of neutrophil infiltration occurs in response to the presence of amastigotes in the extracellular environment. Moreover, the presence of NET and morphologically preserved neutrophils in association with amastigotes, regardless of the duration of the lesions, shows that this process might be related to a dynamic parasite clearance in the established infection. Our results suggested a dynamic process of NET formation when extracellular amastigotes are present. During cutaneous infection, amastigotes from Leishmania sp can be seen either inside or outside macrophages as we previously described [31]. The presence of neutrophils and/or NET would be important to clear extracellular amastigotes. Together with the macrophage activation and intracellular parasite destruction, the clearance of extracellular parasites by neutrophils would contribute to the parasite load control and, as a consequence, to the decrease of inflammatory process followed by lesion healing. The NET acumulation surrounding the extracellular amastigotesmay result in NET parasite trapping and, thus, impaired Leishmania dissemination, and also protozoan killing through close contact with NET toxic components. Corroborating this hypothesis, it was suggested that NETs may contribute to the containment of L. donovani promastigotes at the site of inoculation, thereby facilitating their uptake by mononuclear phagocytes [29]. Moreover, areas containing amorphous antigens of Leishmania, perhaps resulting from parasite killing were often not surrounded by NETs. The binomial NET/amastigotes could be induced by neutrophil chemotaxis which, in turn, is stimulated by extracellular amastigotes themselves and/or by IL-8 and Leishmania chemotactic factor [34,35], followed by stimulation of NET release. Finally, NET degradation would occur and macrophages could remove cell and antigen remnants. Here, we observed NETs associated to granulomatous inflammatory infiltrate in close proximity with amastigotes. Recently, Braian et al [36] analyzed human macrophages and NETs induced by Mycobacterium tuberculosis, and suggested that NETs can transfer danger signals (as that provided by Hsp72) to adjacent macrophages stimulating them to produce cytokines such as IL-6, TNF-α, IL-1β and IL-10. It has been shown that NETs activate NLRP3 inflammasome [37]. Moreover, extracellular trap formation has also been demonstrated for other types of granulocytes, such as eosinophils and mast cells [38,39], an even for macrophages [40,41]. However, as we have used specific markers for neutrophils, i.e. neutrophil elastase, colocalized with DNA, the events analyzed were due to the neutrophil extracellular traps.
Greater attention has been given over the last years to the function of neutrophils during subacute or chronic inflammatory processes. In the particular case of murine leishmaniasis, the results can vary. Lima et al [8] suggested the participation of neutrophils in the control of parasite burden since the absence of neutrophils resulted in the development of severe and disseminated disease. In contrast, Tacchini-Cottier et al [12] showed that the absence of neutrophils had a protective effect associated with a reduced Th2 response and partial resolution of lesions in susceptible BALB/c mice infected with L. major. This apparent contradiction might be due to differences in the mouse models and parasites used since Novais et al [42], studying BALB/c mice infected with L. braziliensis, observed that the elimination of neutrophils increased parasite multiplication. However, the co-inoculation of neutrophils with L. braziliensis had the opposite effect suggesting cooperation between neutrophils and macrophages associated to the production of TNF-α and superoxides. It has been well established that macrophage activation is insufficient in L. major infected BALB/c mice due to the production of IL-4 and the smaller neutrophil elastase production [43]. Furthermore, Zandbergen et al [13] showed that neutrophils may serve as vectors for the entry of Leishmania into macrophages, a model called Trojan horse. Neutrophils enter a process of apoptosis and, together with the parasite, are phagocytosed by macrophages, which became inactivated [2,13]. However, in the present study we evaluated the presence of extracellular regions of NE and histone suggesting NETosis that is independent of both apoptosis and necrosis [16,33]. In this context, using cells from resistant mice (C57Bl/6) infected with L. major, Ribeiro-Gomes et al [44] observed that the interaction between dead neutrophils and macrophages promoted the death of the parasite through the secretion of TNF-α. Fadok et al [45] demonstrated that NE was able to activate human macrophages and to induce the secretion of TNF-α. In addition, the inhibition of NE prevented the leishmanicidal activity of macrophages in a murine model, even in the presence of neutrophils, a fact suggesting the participation of NE in microbicidal activity [44]. Interestingly, during Gram-positive skin infection, Yipp et al [46] visualized live polymorphonuclear cells in vivo rapidly releasing NETs, which prevented systemic bacteria dissemination. NETosis occurred during crawling; thereby casting large areas of NETs associated with intact neutrophils that do not undergo lysis and contain phagocytosed pathogens retain the ability to multitask. In our study, active lesions of ATL also presented large areas of NETs composed NE and histones close to intact neutrophils distributed throughout the granulomatous infiltrate suggesting a probable cooperation between NETs, neutrophils and/or macrophages.
The duration of the lesions could play an important role in the relationship between neutrophil function and parasites. In this respect, during the first moment of the infection, Leishmania promastigotes may use the invasion of apoptotic neutrophils as an escape mechanism to establish infection in cells of the mononuclear phagocyte system [2,13]. Once a local inflammatory reaction is initiated, the proinflammatory environment would stimulate the degranulation and/or formation of NETs before the amastigote forms invade new cells, either neutrophils or macrophages. As a consequence, temporarily extracellular amastigotes would be destroyed, a process contributing to the reduction of local parasite burden. Our results show the presence of high numbers of neutrophils at the site of lesions and their intimate relationship with amastigotes, however it is important to mention that amastigotes could not be visualized inside neutrophils. In addition, all lesions studied already presented an important and diffusely distributed inflammatory process (data not shown and [31]).
Since their first description, the presence of NETs has been investigated in different infectious and non-infectious processes [16,47]. Within this context, three articles can be highlighted. In the first, NETs were identified in peripheral blood neutrophils of patients with malaria and associated with both severe malaria in children and mechanisms of protection in adults [27]. Knowledge about this relationship with disease severity might be important for studies investigating protection against this infection. Similarly, herein we observed positive correlations between NET formation and age, neutrophil percentage and age, as well as, NET and number of lesions. The second article, an in vitro study of the interaction between neutrophils and L. amazonensis promastigotes, demonstrated the trapping of these forms in the fiber networks [28]. In addition, the authors observed a toxic effect of histones released during the process of NET formation, reducing promastigote survival and observed extracellular regions of DNA and histone in human lesions suggesting NET activity during in vivo infection. In the third article, Bruns et al [22] showed that NETs are formed in vivo during the immune response against Aspergillus fumigatus and that their formation is a dynamic process. Although not playing a key role in the elimination of the fungus, the authors suggested that NETs exert a fungistatic effect, preventing dissemination of the fungus. In the same manner, NET could exert an additional parasiticidal effect, helping macrophages to clear parasites from the active lesions.
In conclusion, the present results suggest that 1) although neutrophils are classically identified as cells of the innate immune response, they can be detected throughout the inflammatory reaction observed in established ATL lesions; 2) the presence of NETs is directly related to the presence of intact amastigotes, irrespective of lesion duration, suggesting that their formation is maintained by the stimulus derived from the parasite burden/parasite antigen in the extracellular environment; 3) the observation of areas containing only antigens not intermingled with NETs (elastase and histone) suggests that the involvement of these structures in the control of parasite burden is a dynamic process in which the formation of these networks is exhausted with the destruction of the parasites; 4) the presence of extracellular parasite antigens could facilitate the activation of macrophages to the microbicidal stage, thus activating the control of parasite burden.
Supporting Information
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 400x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 400x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 400x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 400x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 200x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 200x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 200x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
(Figure A) Neutrophil elastase (red) and amastigotes (green); (Figure B) histone (red) and amastigotes (green); (Figure C) histone (red) and degraded amastigotes (green). Scale bar = 10um.
(PDF)
Neutrophil elastase (red) and amastigotes (green); Degraded amastigotes (arrow). Scale bar = 10um.
(PDF)
Area containing intact amastigotes (green) surrounded by NETs that contain neutrophil elastase (black arrow). Area containing free Leishmania sp antigens (white arrow). Scale bar = 10μm.
(AVI)
Acknowledgments
We thank the Program for Technological Development in Tools for Health—PDTIS-FIOCRUZ for use of their confocal facilities.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by Instituto Oswaldo Cruz-IOC (POM 2010-2011) and Instituto Nacional de Infectologia-INI (PA2010)- Fundação Oswaldo Cruz-FIOCRUZ, Fundação Nacional de Saúde/Ministério da Saúde-FUNASA/MS, Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (470886/2003-0; 471744/2012-3; 308236/2013-0); Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq-Proep (402557/2011-5); Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro-FAPERJ (E26.111.717/2012; E-26/102.456/2012, E26/102.988/2012, E26/111.230/2014, E26/203638/2014 and E-26/111.101/2014), and Coordenação de Aperfeiçomento de Pessoal de Nível Superior-CAPES (23038.005291/2011-61), Brazil. AOS is the recipient of fellowships from CNPq and FAPERJ, Brazil. The funders had no role in study design data collection and analysis, decision to publish, or preparing the manuscript.
References
- 1. Marzochi MC, Marzochi KB (1994) Tegumentary and visceral leishmaniases in Brazil: emerging anthropozoonosis and possibilities for their control. Cad Saúde Pública 10:359–75. [DOI] [PubMed] [Google Scholar]
- 2. Laskay T, van Zandbergen G, Solbach W (2008) Neutrophil granulocytes as host cells and transport vehicles for intracellular pathogens: apoptosis as infection-promoting factor. Immunobiology 213:183–91. 10.1016/j.imbio.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 3. Bogdan C, Donhauser N, Döring R, Röllinghoff M, Diefenbach A, Rittiq M. (2000) Fibroblasts as host cells in latent leishmaniosis. J Exp Med 191:2121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hespanhol RC, de Nazaré CSM, Meuser MB, de Nazareth S L, Meirelles M, Côrte-Real S. (2005) The expression of mannose receptors in skin fibroblast and their involvement in Leishmania (L.) amazonensis invasion. J Histochem Cytochem 53:35–44. [DOI] [PubMed] [Google Scholar]
- 5. Lugo-Yarbuh A, Valera M, Alarcón M, Moreno E, Premoli-Percoco G, Colasante C. (2003) [Detection of Leishmania (Viannia) braziliensis in vascular endothelium lesions of patients with localized cutaneous leishmaniasis]. Investig Clínica 44:61–76. [PubMed] [Google Scholar]
- 6. Quintella LP, Cuzzi T, Madeira M de F, Okamoto T, Schubach A de O (2009) Immunoperoxidase technique using an anti-Leishmania (L.) chagasi hyperimmune serum in the diagnosis of culture-confirmed American tegumentary leishmaniasis. Rev Inst Med Trop São Paulo 51:83–6. [DOI] [PubMed] [Google Scholar]
- 7. Von Stebut E (2007) Immunology of cutaneous leishmaniasis: the role of mast cells, phagocytes and dendritic cells for protective immunity. Eur J Dermatol Ejd 17:115–22. [DOI] [PubMed] [Google Scholar]
- 8. Lima GM, Vallochi AL, Silva UR, Bevilacqua EM, Kiffer MM, Abrahamsohn IA. (1998) The role of polymorphonuclear leukocytes in the resistance to cutaneous Leishmaniasis. Immunol Lett 64:145–51. [DOI] [PubMed] [Google Scholar]
- 9. Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhavi S et al. (2008) In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321:970–4. 10.1126/science.1159194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pompeu ML, Freitas LA, Santos ML, Khouri M, Barral-Netto M (1991) Granulocytes in the inflammatory process of BALB/c mice infected by Leishmania amazonensis. A quantitative approach. Acta Trop 48:185–93. [DOI] [PubMed] [Google Scholar]
- 11. Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. (2000) A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol 165:969–77. [DOI] [PubMed] [Google Scholar]
- 12. Tacchini-Cottier F, Zweifel C, Belkaid Y, Mukankundiye C, Vasei M, Launois P et al. (2000) An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol 165:2628–36. [DOI] [PubMed] [Google Scholar]
- 13. Van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W et al. (2004) Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol 173:6521–5. [DOI] [PubMed] [Google Scholar]
- 14. Afonso L, Borges VM, Cruz H, Ribeiro-Gomes FL, DosReis GA, Dutra AN et al. (2008) Interactions with apoptotic but not with necrotic neutrophils increase parasite burden in human macrophages infected with Leishmania amazonensis. J Leukoc Biol 84:389–96. 10.1189/jlb.0108018 [DOI] [PubMed] [Google Scholar]
- 15. Ribeiro-Gomes FL, Sacks D (2012) The influence of early neutrophil-Leishmania interactions on the host immune response to infection. Front Cell Infect Microbiol 2:59 10.3389/fcimb.2012.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abi Abdallah DS, Denkers EY (2012) Neutrophils cast extracellular traps in response to protozoan parasites. Front Immunol 3:382 10.3389/fimmu.2012.00382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS et al. (2004) Neutrophil extracellular traps kill bacteria. Science 303:1532–5. [DOI] [PubMed] [Google Scholar]
- 18. Urban CF, Reichard U, Brinkmann V, Zychlinsky A (2006) Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol 8:668–76. [DOI] [PubMed] [Google Scholar]
- 19. Neeli I, Khan SN, Radic M (2008) Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol 180:1895–902. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Li M, Stadler S, Correll S, Li P, Hayama R et al. (2009) Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 184:205–13. 10.1083/jcb.200806072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramos-Kichik V, Mondragón-Flores R, Mondragón-Castelán M, Gonzalez-Pozos S, Muñiz-Hernandez S, Rojas-Espinosa O et al. (2009) Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberc Edinb Scotl 89:29–37. [DOI] [PubMed] [Google Scholar]
- 22. Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywissen A et al. (2010) Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. Plos Pathog 6:e1000873 10.1371/journal.ppat.1000873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morgado FN, Schubach AO, Barros MBL, Conceição-Silva F (2011) The in situ inflammatory profile of lymphocutaneous and fixed forms of human sporotrichosis. Med Mycol 49:612–20. 10.3109/13693786.2011.552532 [DOI] [PubMed] [Google Scholar]
- 24. Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T et al. (2012) Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 12:109–16. 10.1016/j.chom.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 25. Abi Abdallah DS, Lin C, Ball CJ, King MR, Duhamel GE, Denkers EY. (2012) Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect Immun 80:768–77. 10.1128/IAI.05730-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Behrendt JH, Ruiz A, Zahner H, Taubert A, Hermosilla C (2010) Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria bovis. Vet Immunol Immunopathol 133:1–8. 10.1016/j.vetimm.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 27. Baker VS, Imade GE, Molta NB, Tawde P, Pam SD, Obadofin MO et al. (2008) Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in Plasmodium falciparum infected children under six years of age. Malar J 7:41 10.1186/1475-2875-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guimarães-Costa AB, Nascimento MTC, Froment GS, Soares RPP, Morgado FN, Conceição-Silva F et al. (2009) Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci USA 106:6748–53. 10.1073/pnas.0900226106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gabriel C, McMaster WR, Girard D, Descoteaux A (2010) Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J Immunol 185:4319–27. 10.4049/jimmunol.1000893 [DOI] [PubMed] [Google Scholar]
- 30. Madeira MF, Schubach A, Schubach TMP, Pacheco RS, Oliveira FS, Pereira SA et al. (2006) Mixed infection with Leishmania (Viannia) braziliensis and Leishmania (Leishmania) chagasi in a naturally infected dog from Rio de Janeiro, Brazil. Trans R Soc Trop Med Hyg 100:442–5. [DOI] [PubMed] [Google Scholar]
- 31. Morgado FN, Schubach A, Rosalino CMV, Quintella LP, Santos G, Salgueiro M et al. (2008) Is the in situ inflammatory reaction an important tool to understand the cellular immune response in American tegumentary leishmaniasis? Br J Dermatol 158:50–8. [DOI] [PubMed] [Google Scholar]
- 32. Morgado FN, Schubach A, Vasconcellos E, Azeredo-Coutinho RB, Valete-Rosalino CM, Quintella LP et al. (2010) Signs of an in situ inflammatory reaction in scars of human American tegumentary leishmaniasis. Parasite Immunol 32:285–95. 10.1111/j.1365-3024.2009.01188.x [DOI] [PubMed] [Google Scholar]
- 33. Brinkmann V, Zychlinsky A (2012) Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol 198:773–83. 10.1083/jcb.201203170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Zandbergen G, Hermann N, Laufs H, Solbach W, Laskay T (2002) Leishmania promastigotes release a granulocyte chemotactic factor and induce interleukin-8 release but inhibit gamma interferon-inducible protein 10 production by neutrophil granulocytes. Infect Immun 70:4177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Menezes MJ, Costa DJ, Clarêncio J, Miranda JC, Barral A, Barral-Netto N et al. (2008) Immunomodulation of human monocytes following exposure to Lutzomyia intermedia saliva. Bmc Immunol 9:12 10.1186/1471-2172-9-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Braian C, Hogea V, Stendahl O (2013) Mycobacterium tuberculosis- Induced Neutrophil Extracellular Traps Activate Human Macrophages. J Innate Immun 5:591–602. 10.1159/000348676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ (2013) Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol 190:1217–26. 10.4049/jimmunol.1202388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E et al. (2008) Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med 14:949–53. 10.1038/nm.1855 [DOI] [PubMed] [Google Scholar]
- 39. Von Köckritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M et al. (2008) Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 111:3070–80. 10.1182/blood-2007-07-104018 [DOI] [PubMed] [Google Scholar]
- 40. Bartneck M, Keul HA, Zwadlo-Klarwasser G, Groll J (2010) Phagocytosis independent extracellular nanoparticle clearance by human immune cells. Nano Lett 10:59–63. 10.1021/nl902830x [DOI] [PubMed] [Google Scholar]
- 41. Chow OA, von Köckritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Coqen AL et al. (2010) Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 8:445–54. 10.1016/j.chom.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Novais FO, Santiago RC, Báfica A, Khouri R, Afonso L, Borges VM et al. (2009) Neutrophils and macrophages cooperate in host resistance against Leishmania braziliensis infection. J Immunol 183:8088–98. 10.4049/jimmunol.0803720 [DOI] [PubMed] [Google Scholar]
- 43. Ribeiro-Gomes FL, Moniz-de-Souza MCA, Alexandre-Moreira MS, Dias WB, Lopes MF, Nunes MP et al. (2007) Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J Immunol 179:3988–94. [DOI] [PubMed] [Google Scholar]
- 44. Ribeiro-Gomes FL, Otero AC, Gomes NA, Moniz-De-Souza MCA, Cysne-Finkelstein L, Arnholdt AC et al. (2004) Macrophage interactions with neutrophils regulate Leishmania major infection. J Immunol 172:4454–62. [DOI] [PubMed] [Google Scholar]
- 45. Fadok VA, Bratton DL, Guthrie L, Henson PM (2001) Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol 166:6847–54. [DOI] [PubMed] [Google Scholar]
- 46. Yipp BG, Petri B, Salina D, Jenne CN, Scott BNV, Zbytnuik LD et al. (2012) Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med 18:1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guimarães-Costa AB, Nascimento MTC, Wardini AB, Pinto-da-Silva LH, Saraiva EM (2012) ETosis: A Microbicidal Mechanism beyond Cell Death. J Parasitol Res 2012:929743 10.1155/2012/929743 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 400x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 400x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 400x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 400x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 200x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 200x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary leishmaniasis. Counterstain—Meyer`s hematoxilin; 200x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
Inflammatory infiltrate in skin lesions of American tegumentary Leishmaniasis. counterstain—Meyer`s hematoxilin. 1000x magnification.
(PDF)
(Figure A) Neutrophil elastase (red) and amastigotes (green); (Figure B) histone (red) and amastigotes (green); (Figure C) histone (red) and degraded amastigotes (green). Scale bar = 10um.
(PDF)
Neutrophil elastase (red) and amastigotes (green); Degraded amastigotes (arrow). Scale bar = 10um.
(PDF)
Area containing intact amastigotes (green) surrounded by NETs that contain neutrophil elastase (black arrow). Area containing free Leishmania sp antigens (white arrow). Scale bar = 10μm.
(AVI)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.