Abstract
Background
Cutaneous malignancy is associated with worse outcomes in patients with chronic lymphocytic leukemia (CLL).
Objective
To identify the incidence and recurrence rate of nonmelanoma skin cancer (NMSC) in patients with non-Hodgkin lymphoma (NHL).
Methods
NMSC incidence was calculated and Cox proportional hazard models were used to evaluate associations with risk of recurrence for patients with NHL between 1976 and 2005 who were in the Rochester Epidemiology Project research infrastructure.
Results
We identified 282 patients with CLL or small lymphocytic lymphoma and 435 with non-CLL NHL. The incidence of BCC and SCC was 1,829.3 (95% CI, 1,306.7-2,491.1) and 2,224.9 (95% CI, 1,645.9-2,941.6), respectively, in patients with CLL. The cumulative recurrence rate at 8 years after treatment with Mohs micrographic surgery was 8.3% (95% CI, 0.0%-22.7%) for BCC and 13.4% (95% CI, 0.0%-25.5%) for SCC in patients with CLL.
Limitations
This was a retrospective cohort study.
Conclusions
After Mohs micrographic surgery and standard excision of NMSC, patients with NHL had a skin cancer recurrence rate that was higher than expected. Careful treatment and monitoring of patients with NHL and NMSC are warranted.
Keywords: chronic lymphocytic leukemia, immunosuppression, malignant melanoma, Mohs surgery, non-Hodgkin lymphoma, nonmelanoma skin cancer
Introduction
Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are the most common forms of malignancy, with increased incidence in immunosuppressed patients such as those with lymphoma.1-3 The aggressiveness of nonmelanoma skin cancer (NMSC) in the clinical setting of lymphoma has been shown through increased recurrence rates and increased tumor subclinical extension.4-7
The immune system has a role in the development of subsequent neoplasia in patients with lymphoma, with skin cancer being the most common secondary malignancy.8-10 Although numerous case reports describe the potentially increased aggressiveness of skin cancer in the setting of lymphoma, only 2 case-control studies to date have demonstrated increased recurrence of BCC and SCC in patients with lymphoma.6,7
The purpose of the present study was to document the incidence and recurrence rate of NMSC in patients with non-Hodgkin lymphoma (NHL) through a population-based approach. In addition, we aimed to identify risk factors that may contribute to the potential aggressiveness of skin cancer in this unique patient population.
Methods
Institutional review board approval was obtained from Mayo Clinic and the Olmsted Medical Center as part of the Rochester Epidemiology Project (REP). REP resources include health care information for Olmsted County, Minnesota, residents from 1966 to the present. The medical records for a given patient are linked together across virtually all Olmsted County health care providers as a part of the REP records linkage system. This allows the health care information for an Olmsted County resident to be monitored continuously, regardless of changes in setting or provider. The medical records of patients identified through REP are shared among Olmsted County health care providers and can be used to identify persons with specific diseases.11,12 For all patients who resided in Olmsted County as of January 2000, it has been determined that 80% of Olmsted County residents were seen by a REP health care provider at least once within 1 year and 93% were seen within 3 years.13
We used the REP research infrastructure to identify patients with diagnosed NHL between January 1, 1976, and December 31, 2005. The disease was defined as the diagnosis of chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, or other forms of NHL. Patients who denied access to their medical records for research purposes were excluded. We reviewed the medical records and abstracted information regarding the diagnosis of secondary neoplasia, including NMSC, either before or after the NHL diagnosis was recorded. Identifying patients with NMSC was done through a diagnostic code search of all medical records in Olmsted County (part of the medical record linkage system of the REP). The presence of an NMSC was further confirmed through the presence of a pathology report or operative report, or both, in the patients’ medical records. Treatments, tumor histologic type, recurrence, and metastasis were recorded for all NMSC that developed after the NHL diagnosis. The following potential risk factors at the time of the NHL diagnosis were recorded: age, sex, previous tobacco use, and lymphoma treatments.
Statistical Analysis
Analyses were performed using the SAS software (version 9.3; SAS Institute Inc). P values less than .05 were considered statistically significant. Analyses were performed separately for patients with CLL (including small lymphocytic lymphoma) and those with non-CLL NHL. The analysis described herein for BCC outcomes was also conducted separately for SCC outcomes.
For the analysis of BCC incidence, patients were excluded if the BCC was first diagnosed before the date of the NHL diagnosis. A patient’s duration of follow-up was calculated from the date of the NHL diagnosis to the date of the first BCC diagnosis, the last follow-up, or death. Age- and sex-specific incidence density estimates were derived from the number of patients with an incident BCC diagnosis relative to the total person-years of observation; values were expressed as incidence per 100,000 person-years.14 Exact 95% confidence intervals for the incidence rates were derived by assuming that the observed number of cases followed a Poisson distribution and that the number of person-years was fixed. The cumulative incidence of BCC was estimated with Kaplan-Meier analysis. Cox proportional hazards models were used to evaluate risk factors for their association with development of BCC.
For the analysis of BCC recurrence, all primary BCC tumors of a patient were considered unless the patient had at least 1 tumor that recurred before the NHL diagnosis. The duration of follow-up for each tumor was calculated from the date of the BCC diagnosis to the date of the recurrence, last follow-up, or death. The cumulative incidence of BCC was estimated using Kaplan-Meier analysis. Risk factors were evaluated for their association with the development of recurrent BCC by fitting Cox models using a robust sandwich estimate for the covariance estimate to account for the correlation between multiple primary BCC in a patient.15
Results
We identified a cohort of 717 Olmsted County residents with the diagnosis of NHL established from January 1, 1976, through December 31, 2005. Although these data are from a number of decades ago to 2005, the diagnostic criteria for CLL has remained relatively stable, and the information obtained from the large amount of follow-up years is pertinent. It is possible, however, that the treatment regimens for lymphoma have changed significantly over this large amount of time, which could introduce a shortcoming when evaluating treatment effect on outcomes.
Patient characteristics and tumor burden are shown in Table 1. Of the 717 patients, 54 had a prior BCC and 45 had a prior SCC before their diagnosis of NHL. Among the 663 patients with no history of BCC, 73 had development of a total of 181 primary BCC tumors. Of the 672 patients with no history of SCC, 92 had development of 250 primary SCC tumors.
Table 1.
Patient Characteristics and Tumor Burden
| Characteristic | CLL (n=282) | Non-CLL NHL (n=435) | Total (N=717) |
|---|---|---|---|
| Age at first lymphoma diagnosis, mean (SD), y | 69.9 (12.7) | 61.7 (19.5) | 64.9 (17.6) |
| Male sex, No. (%) | 165 (58.5) | 237 (54.5) | 402 (56.1) |
| Tobacco use, No. (%) | 132 (46.8) | 175 (40.2) | 307 (42.8) |
| Year of first lymphoma diagnosis, No. (%) | |||
| 1976-1979 | 26 (9.2) | 21 (4.8) | 47 (6.6) |
| 1980-1984 | 25 (8.9) | 41 (9.4) | 66 (9.2) |
| 1985-1989 | 41 (14.5) | 65 (14.9) | 106 (14.8) |
| 1990-1994 | 52 (18.4) | 91 (20.9) | 143 (19.9) |
| 1995-1999 | 68 (24.1) | 98 (22.5) | 166 (23.2) |
| 2000-2005 | 70 (24.8) | 119 (27.4) | 189 (26.4) |
| Lymphoma type, No. of patients (%) | |||
| CLL | 267 (94.7) | -- | |
| SLL | 15 (5.3) | -- | |
| Diffuse large B-cell | -- | 110 (25.3) | |
| Follicular | -- | 86 (19.8) | |
| Other B-cell | -- | 41 (9.4) | |
| Othera | -- | 27 (6.2) | |
| Unspecified NHL | -- | 171 (39.3) | |
| Lymphoma treatment, No. (%) | 113 (40.1) | 361 (83.0) | 474 (66.1) |
| Prior history of NMSC, No. (%) | |||
| BCC only | 17 (6.0) | 24 (5.5) | 41 (5.7) |
| SCC only | 11 (3.9) | 21 (4.8) | 32 (4.5) |
| Both BCC and SCC | 2 (0.7) | 11 (2.5) | 13 (1.8) |
| Prior history of recurrent NMSC, No. (%) | |||
| BCC only | 2 (0.7) | 2 (0.5) | 4 (0.6) |
| SCC only | 1 (0.4) | 1 (0.2) | 2 (0.3) |
| Both BCC and SCC | 0 (0) | 1 (0.2) | 1 (0.1) |
| Duration of follow-up, mean (SD), y | 8.1 (6.3) | 7.4 (7.0) | 7.7 (6.7) |
| Subsequent primary tumors per patient | |||
| BCC | |||
| Total patients, No. with/No. at risk | 40/263 | 33/400 | 73/663 |
| No. of tumors, range | 1-15 | 1-30 | 1-30 |
| 1-9 tumors, No. of patients (%) | 38 (14.5) | 31 (7.8) | 69 (10.4) |
| ≥10 tumors, No. of patients (%) | 2 (0.8) | 2 (0.5) | 4 (0.6) |
| SCC | |||
| Total patients, No. with/No. at risk | 49/269 | 43/403 | 92/672 |
| No. of tumors, range | 1-34 | 1-5 | 1-34 |
| 1-9 tumors, No. of patients (%) | 45 (16.7) | 43 (10.7) | 88 (13.1) |
| ≥10 tumors, No. of patients (%) | 4 (1.5) | 0 (0) | 4 (0.6) |
Abbreviations: BCC, basal cell carcinoma; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin lymphoma; NMSC, nonmelanoma skin cancer; SCC, squamous cell carcinoma.
Includes Mantle cell, T-cell lymphoblastic, lymphoplasmacytic, and Burkitt lymphoma.
The incidence of BCC was 1,829.3 (95% CI, 1,306.7-2,491.1) per 100,000 person-years overall in patients with CLL and 1,086.5 (95% CI, 747.8-1,525.9) per 100,000 person-years overall in those with non-CLL NHL. The incidence of SCC was 2,224.9 (95% CI, 1,645.9-2,941.6) per 100,000 person-years overall in patients with CLL and 1,387.1 (95% CI, 1,003.7-1,868.5) per 100,000 person-years overall in those with non-CLL NHL. The cumulative incidence of BCC and SCC up to 20 years after the diagnosis of CLL or non-CLL NHL is shown in the Figure. By 20 years, the cumulative incidence of BCC was 30.6% (95% CI, 19.4%-40.1%) and 18.1% (95% CI, 11.0%-24.6%), respectively, in patients with CLL and non-CLL NHL. The cumulative incidence of SCC by 20 years was 43.2% (95% CI, 27.9%-55.3%) and 26.0% (95% CI, 16.4%-34.5%), respectively, in patients with CLL and non-CLL NHL.
Figure.
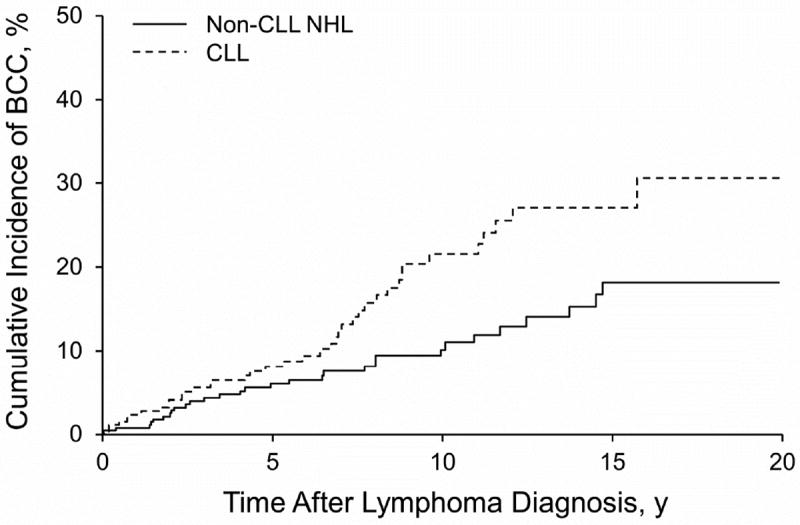
Nonmelanoma Skin Cancer per 100,000 Person-years, Stratified by Chronic Lymphocytic Leukemia (CLL) and Non-CLL Non-Hodgkin Lymphoma (NHL). Graphs show results of Kaplan-Meier Analysis for Cumulative Incidence. A, Basal cell carcinoma (BCC). B, Squamous cell carcinoma (SCC).
A total of 7 patients with BCC or SCC recurrence before the NHL diagnosis were excluded from the respective recurrence analyses. After these exclusions, the cohort consisted of 134 patients with 446 primary SCCs and 122 patients with 292 primary BCCs. All identified recurrences of BCC and SCC occurred within the first 8 years after the initial NMSC diagnosis. Twelve patients had 14 recurrences of BCC and 20 patients had 28 recurrences of SCC after the diagnosis of NHL. The overall 5- and 8-year recurrence rates for BCC in patients with CLL were 6.2% (95% CI, 1.2%-10.9%) and 8.9% (95% CI, 1.6%-15.8%), and specifically after treatment with Mohs micrographic surgery, the recurrence rates were 0% and 8.3% (95% CI, 0.0%-22.7%) (Table 2). The overall 5- and 8-year recurrence rates for SCC in patients with CLL were 9.0% (95% CI, 4.7%-13.1%) and 10.6% (95% CI, 5.3%-15.7%). After treatment with Mohs micrographic surgery, the recurrence rate was 13.4% (95% CI, 0.0%-25.5%) for both the 5-year period and the 8-year period.
Table 2.
Recurrence Rates of BCC and SCC in Patients With CLL and Non-CLL NHL, Stratified by Treatmenta
| Years After Primary NMSC | Cumulative Incidence of Recurrent BCC, % (95% CI) | Cumulative Incidence of Recurrent SCC, % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Patients With CLL
| ||||||||
| Overall (N=124; 7 events) | Mohs (n=33; 1 event) | Excision (n=44; 3 events) | C&C, ED&C, CO2 Laser (n=46; 3 events) | Overall (N=284; 19 events) | Mohs (n=47; 4 events) | Excision (n=55; 5 events) | C&C, ED&C, CO2 Laser (n=180; 10 events) | |
| 1 | 2.0 | 0.0 | 2.5 | 3.0 | 3.0 | 0.0 | 2.0 | 4.1 |
| 3 | 5.0 | 0.0 | 7.6 | 6.1 | 6.6 | 8.3 | 7.2 | 5.7 |
| 5 | 6.2 (1.2-10.9) | 0.0 (0.0-0.0) | 7.6 (0.0-15.6) | 9.3 (0.0-18.8) | 9.0 (4.7-13.1) | 13.4 (0.0-25.5) | 10.9 (0.0-20.8) | 7.0 (2.5-11.3) |
| 8b | 8.9 (1.6-15.8) (N=25) | 8.3 (0.0-22.7) (n=6) | 7.6 (0.0-15.6) (n=13) | 9.3 (0.0-18.8) (n=6) | 10.6 (5.3-15.7) (N=36) | 13.4 (0.0-25.5) (n=2) | 18.3 (0.0-33.6) (n=10) | 7.0 (2.5-11.3) (n=24) |
|
| ||||||||
| Years After Primary NMSC | Cumulative Incidence of Recurrent BCC, % (95% CI) | Cumulative Incidence of Recurrent SCC, % (95% CI) | ||||||
|
| ||||||||
|
Patients With Non-CLL NHL
| ||||||||
| Overall (N=168; 7 events) | Mohs (n=54; 1 event) | Excision (n=56; 1 event) | C&C, ED&C, CO2 Laser (n=55; 5 events) | Overall (N=162; 9 events) | Mohs (n=35; 2 events) | Excision (n=52; 4 events) | C&C, ED&C, CO2 Laser (n=72; 2 events) | |
|
| ||||||||
| 1 | 2.0 | 2.2 | 0.0 | 3.8 | 2.6 | 3.1 | 4.3 | 1.4 |
| 3 | 3.7 | 2.2 | 0.0 | 9.1 | 5.1 | 3.1 | 6.9 | 3.3 |
| 5 | 4.8 (0.9, 8.5) | 2.2 (0.0-6.4) | 2.9 (0.0-8.2) | 9.1 (0.0-17.4) | 5.1 (1.3-8.8) | 3.1 (0.0-9.0) | 6.9 (0.0-14.2) | 3.3 (0.0-7.9) |
| 8b | 6.7 (1.3, 11.7) (N=44) | 2.2 (0.0-6.4) (n=13) | 2.9 (0.0-8.2) (n=21) | 15.2 (0.0-17.4) (n=10) | 9.5 (2.3-16.1) (N=34) | 19.3 (0.0-43.9) (n=5) | 12.4 (0.0-24.2) (n=14) | 3.3 (0.0-7.9) (n=15) |
Abbreviations: BCC, basal cell carcinoma; C&C, curettage and cryotherapy; CLL, chronic lymphocytic leukemia; ED&C, electrodessication and curettage; NHL, non-Hodgkin lymphoma; SCC, squamous cell carcinoma.
The type of NMSC treatment was not available for all NMSC cases.
Patient numbers indicate those still at risk for recurrence after 8 years.
A univariate analysis of risk factors for development of NMSC after a lymphoma diagnosis was performed (Table 3). Among patients with CLL, male sex and prior lymphoma treatment were significantly associated with a higher risk of BCC development (P=.005), and a nonsignificant association was found for lymphoma treatment with SCC (P=.05). Among patients with non-CLL NHL, advancing age at lymphoma diagnosis was associated with an increased risk of BCC and SCC development, with results for SCC development similar to those among CLL patients.
Table 3.
Univariate Analysis of the Risk of Initial BCC or SCC Development After the Diagnosis of CLL or Non-CLL NHL
| Characteristic | BCC
|
SCC
|
||||
|---|---|---|---|---|---|---|
| No. of Patients | Hazard Ratio (95% CI) | P Value | No. of Patients | Hazard Ratio (95% CI) | P Value | |
| CLL | ||||||
| Sex | .005 | .10 | ||||
| Female | 107 | 1.0 | 111 | 1.0 | ||
| Male | 156 | 3.0 (1.4- 6.6) | 158 | 1.7 (0.9-3.1) | ||
| Age, y | .15 | .03 | ||||
| <50 | 24 | 1.0 | 24 | 1.0 | ||
| 50-70 | 106 | 1.3 (0.4- 3.9) | 108 | 3.6 (0.8-15.6) | ||
| >70 | 133 | 2.3 (0.8- 7.0) | 137 | 5.9 (1.4-25.4) | ||
| Tobacco use | .55 | .52 | ||||
| None | 135 | 1.0 | 136 | 1.0 | ||
| Long-term | 122 | 1.2 (0.7- 2.3) | 127 | 0.8 (0.5-1.5) | ||
| Lymphoma treatment | .01 | .05 | ||||
| None | 114 | 1.0 | 115 | 1.0 | ||
| Received treatment | 107 | 2.9 (1.2- 6.9) | 111 | 2.1 (1.0-4.5) | ||
| Non-CLL NHL | ||||||
| Sex | .46 | .14 | ||||
| Female | 186 | 1.0 | 187 | 1.0 | ||
| Male | 214 | 1.3 (0.7- 2.6) | 216 | 1.6 (0.9-3.0) | ||
| Age, y | <.001 | <.001 | ||||
| <50 | 115 | 1.0 | 116 | 1.0 | ||
| 50-70 | 139 | 4.1 (1.3- 12.5) | 143 | 4.4 (1.6-11.8) | ||
| >70 | 146 | 9.6 (3.0- 30.3) | 144 | 10.0 (3.6-27.9) | ||
| Tobacco use | .49 | .74 | ||||
| None | 233 | 1.0 | 234 | 1.0 | ||
| Long-term | 157 | 1.3 (0.6- 2.5) | 159 | 1.1 (0.6-2.0) | ||
| Lymphoma treatment | .11 | .91 | ||||
| None | 38 | 1.0 | 39 | 1.0 | ||
| Received treatment | 336 | 0.4 (0.1-1.2) | 341 | 0.9 (0.2-3.8) | ||
Abbreviations: BCC, basal cell carcinoma; CLL, chronic lymphocytic leukemia; ED&C, electrodessication and curettage; NHL, non-Hodgkin lymphoma; SCC, squamous cell carcinoma.
In a univariate analysis, the association between female sex and recurrent SCC was not significant for patients with non-CLL NHL (P=.06). In addition, sex, age, tobacco use, prior treatment of lymphoma, and NMSC treatment were not identified as significantly associated with NMSC recurrence.
Of the 134 patients with SCC, only 4 had metastatic SCC. One of these patients was a 61-year-old man with a CLL diagnosis 7 years before development of SCC on the scalp. Metastatic SCC developed 3.5 years after the initial diagnosis of SCC, and he died of metastatic SCC 10.5 months later. The second patient was a 67-year-old woman with a non-CLL NHL diagnosis 17 years before development of SCC; metastasis to the esophagus was recognized 8 days after the primary diagnosis of SCC was established. This patient died of metastatic SCC 4.5 months later. The third patient was an 80-year-old man with non-CLL NHL diagnosed 14 years after development of SCC on the neck; metastasis to the lymph nodes was recognized 3 days after the initial SCC was diagnosed. The fourth patient was a 73-year-old man with a CLL diagnosis nearly 8 years before development of SCC on the forehead; the SCC metastasized to the parotid gland and lymph nodes 2 years after the initial SCC diagnosis.
Discussion
In the past 30 years, investigators have suggested an increased incidence of solid tumors in the setting of CLL. In particular, the risk is significantly increased for malignant melanoma, soft-tissue sarcomas, and lung cancer.1,10,16-22 In 1 small study, the risk of skin cancer in the setting of CLL increased 8-fold.16 These tumors not only are more common in this clinical setting, but they also may behave more aggressively. Patients with CLL and SCC or BCC were found to have a 7- to 14-fold higher chance of recurrence after Mohs micrographic surgery in previous studies.6,7 In addition, patients with SCC and CLL are more likely to have metastatic SCC and death due to metastatic SCC.5
There are many reasons why patients with CLL have an impaired immune system; in CLL, B cells have a role in impaired host immune function.23 The expression of leukemic cells has been described previously:24
Leukemic cells have been found to express immunosuppressive factors, as well as downregulate the expression of the CD40 ligand (CD154) on activated T cells, thus interfering with the ability of T cells to interact with normal bystander B cells or other antigen-presenting cells. Furthermore, CD154 plays a role in the T-cell induction of immunoglobulin class switching, and may result in a deficient IgG level of various IgG subclasses. Many other factors also play a role in the immunosuppression seen in the setting of CLL, including low levels of complement, altered leukemic cell expression of class 2 major histocompatibility complex antigens, hypogammaglobulinemia, impaired granulocyte function, altered expression of receptor variable region genes and functional defects in bystander and other subsets of T cells. Although speculative, the ability of CLL B cells and dysfunctional T cells to suppress the antitumor response may contribute to the increased incidence of BCC and SCC noted in previous studies, and could possibly play a role in the behavior and incidence of melanoma and other more rare skin cancers in these patients as well.
Prior studies have documented the generally well-accepted recurrence rates of NMSC in the setting of Mohs micrographic surgery to be 1% for primary BCC and about 3% for primary SCC.25 The present study confirms the high incidence and comparatively worse recurrence rates of NMSC in patients with a history of lymphoma, even after Mohs micrographic surgery. Examination of risk factors for NMSC development showed that the factors most strongly correlated with NMSC in patients with NHL were age and male sex. In addition, prior lymphoma treatment was associated with development of BCC in patients with a history of CLL. Prior data are conflicting on whether chemotherapy for lymphoma affects the incidence of secondary malignancy.10,18,21,26,27
We acknowledge that the weaknesses of this study include a relatively small cohort of patients; however, we believe that the size of the cohort was sufficiently large to answer the questions at hand with statistically sound methods. Given the small sample size, though, confident comparisons of different treatment modalities would be limited. This cohort studied was also predominantly white and elderly, and thus the results of this study may not be generalizable to other, different cohorts of patients with lymphoma of various races and ages. It is nevertheless common for patients with CLL and NHL to be elderly white patients, and thus the information of this study could most likely be applied confidently to the majority of patients with these conditions. In addition, this was a retrospective cohort study; however, given the high capture rate of the REP, the biases commonly associated with retrospective studies are well accounted for in the present study. This study also has a substantial number of strengths, including that fact that it is a population-based study, which is beneficial in countering the referral bias that exists from studying a patient population seen only at a major medical referral center. In addition, given the sound nature of the REP and the access to all records of patients with lymphoma who have resided in Olmsted County during the times of the study, it is unlikely that patients were missed. Finally, given the population-based approach to this study, the incidence trends would be considered sound without the issues of reporting biases that occur in larger national databases.
Our study did not show an abnormally high metastatic rate in patients with lymphoma and SCC. We speculate that the previous study reporting this finding5 was influenced by referral bias because most patients were treated at a major academic medical center.
Earlier reports have demonstrated no influence of prior lymphoma therapy on the development of secondary cancer, including skin cancer.10 However, for our patients with CLL, a history of lymphoma treatment (of any type) was associated with increased development of BCC. Prior tobacco use did not influence skin cancer development in our patient population.
Conclusions
Patients with a history of NHL are subject to high recurrence rates of BCC and SCC, even after Mohs micrographic surgery. Careful, prompt care, appropriate management, and frequent follow-up are necessary to decrease the chance of recurrence and the possibility of subsequent metastasis.
Capsule Summary.
Patients with chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (non-CLL NHL) have increased nonmelanoma skin cancer (NMSC) incidence.
CLL and non-CLL NHL patients with NMSC treated surgically had higher recurrence rates, even after Mohs.
Prompt and aggressive margin control is necessary to decrease recurrence risk and subsequent skin cancer metastasis.
Acknowledgments
Dr Brewer has been a recipient of the Dermatology Foundation Career Development Award for the study of lymphoma-associated skin cancer.
Funding/Support: This study was supported in part by Dermatology Foundation (J.D.B. was recipient of the DF Clinical Development Award).
Abbreviations
- BCC
basal cell carcinoma
- CLL
chronic lymphocytic leukemia
- NHL
non-Hodgkin lymphoma
- NMSC
nonmelanoma skin cancer
- REP
Rochester Epidemiology Project
- SCC
squamous cell carcinoma
Footnotes
Financial Disclosure: None reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adami J, Frisch M, Yuen J, Glimelius B, Melbye M. Evidence of an association between non-Hodgkin’s lymphoma and skin cancer. BMJ. 1995;310(6993):1491–1495. doi: 10.1136/bmj.310.6993.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Athas WF, Hunt WC, Key CR. Changes in nonmelanoma skin cancer incidence between 1977-1978 and 1998-1999 in Northcentral New Mexico. Cancer Epidemiol Biomarkers Prev. 2003;12(10):1105–1108. [PubMed] [Google Scholar]
- 3.Berg JW. The incidence of multiple primary cancers. I. Development of further cancers in patients with lymphomas, leukemias, and myeloma. J Natl Cancer Inst. 1967;38(5):741–752. [PubMed] [Google Scholar]
- 4.Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29(2):129–134. doi: 10.1046/j.1524-4725.2003.29034.x. [DOI] [PubMed] [Google Scholar]
- 5.Mehrany K, Weenig RH, Lee KK, Pittelkow MR, Otley CC. Increased metastasis and mortality from cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. J Am Acad Dermatol. 2005;53(6):1067–1071. doi: 10.1016/j.jaad.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 6.Mehrany K, Weenig RH, Pittelkow MR, Roenigk RK, Otley CC. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140(8):985–988. doi: 10.1001/archderm.140.8.985. [DOI] [PubMed] [Google Scholar]
- 7.Mehrany K, Weenig RH, Pittelkow MR, Roenigk RK, Otley CC. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31(1):38–42. doi: 10.1111/j.1524-4725.2005.31006. [DOI] [PubMed] [Google Scholar]
- 8.Levine PH, Hoover R. The emerging epidemic of non-Hodgkin’s lymphoma: current knowledge regarding etiological factors. Cancer Epidemiol Biomarkers Prev. 1992;1(6):515–517. [PubMed] [Google Scholar]
- 9.Manusow D, Weinerman BH. Subsequent neoplasia in chronic lymphocytic leukemia. JAMA. 1975;232(3):267–269. [PubMed] [Google Scholar]
- 10.Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27(6):904–910. doi: 10.1200/JCO.2008.17.5398. Epub 2008 Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. doi: 10.1093/ije/dys195. Epub 2012 Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergstralh EJ, Offord KP, Kosanke JL, Augstine GA. PERSONYRS: a SAS procedure for person year analyses. Rochester (MN): Department of Health Sciences Research; 1986. Technical report series no. 31. [Google Scholar]
- 15.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–1078. [Google Scholar]
- 16.Greene MH, Hoover RN, Fraumeni JF., Jr Subsequent cancer in patients with chronic lymphocytic leukemia: a possible immunologic mechanism. J Natl Cancer Inst. 1978;61(2):337–340. [PubMed] [Google Scholar]
- 17.Levi F, Randimbison L, Te VC, La Vecchia C. Non-Hodgkin’s lymphomas, chronic lymphocytic leukaemias and skin cancers. Br J Cancer. 1996;74(11):1847–1850. doi: 10.1038/bjc.1996.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Travis LB, Curtis RE, Hankey BF, Fraumeni JF., Jr Second cancers in patients with chronic lymphocytic leukemia. J Natl Cancer Inst. 1992;84(18):1422–1427. doi: 10.1093/jnci/84.18.1422. [DOI] [PubMed] [Google Scholar]
- 19.Wiernik PH. Second neoplasms in patients with chronic lymphocytic leukemia. Curr Treat Options Oncol. 2004;5(3):215–223. doi: 10.1007/s11864-004-0013-7. [DOI] [PubMed] [Google Scholar]
- 20.Hall P, Rosendahl I, Mattsson A, Einhorn S. Non-Hodgkin’s lymphoma and skin malignancies: shared etiology? Int J Cancer. 1995;62(5):519–522. doi: 10.1002/ijc.2910620505. [DOI] [PubMed] [Google Scholar]
- 21.Travis LB, Curtis RE, Boice JD, Jr, Hankey BF, Fraumeni JF., Jr Second cancers following non-Hodgkin’s lymphoma. Cancer. 1991;67(7):2002–2009. doi: 10.1002/1097-0142(19910401)67:7<2002::aid-cncr2820670729>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Travis LB, Curtis RE, Glimelius B, et al. Second cancers among long-term survivors of non-Hodgkin’s lymphoma. J Natl Cancer Inst. 1993;85(23):1932–1937. doi: 10.1093/jnci/85.23.1932. [DOI] [PubMed] [Google Scholar]
- 23.Aslakson CJ, Lee G, Boomer JS, Gilman-Sachs A, Kucuk O, Beaman KD. Expression of regeneration and tolerance factor on B cell chronic lymphocytic leukemias: a possible mechanism for escaping immune surveillance. Am J Hematol. 1999;61(1):46–52. doi: 10.1002/(sici)1096-8652(199905)61:1<46::aid-ajh9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 24.Brewer JD. Skin cancer in patients with non-Hodgkin’s lymphoma. [February 26, 2014];Expert Rev Dermatol. 2010 5(5):525–533. http://informahealthcare.com/doi/abs/10.1586/edm.10.48. [Google Scholar]
- 25.Rowe DE, Carroll RJ, Day CL., Jr Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15(4):424–431. doi: 10.1111/j.1524-4725.1989.tb03249.x. [DOI] [PubMed] [Google Scholar]
- 26.Molica S. Second neoplasms in chronic lymphocytic leukemia: incidence and pathogenesis with emphasis on the role of different therapies. Leuk Lymphoma. 2005;46(1):49–54. doi: 10.1080/10428190400007524. [DOI] [PubMed] [Google Scholar]
- 27.Morton LM, Curtis RE, Linet MS, et al. Second malignancy risks after non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol. 2010;28(33):4935–4944. doi: 10.1200/JCO.2010.29.1112. Epub 2010 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]


