Abstract
Purpose
Pregnancy and childbirth are normal conditions, but complications and adverse outcomes are common. Both genetic and environmental factors influence the course of pregnancy. Genetic epidemiologic research into pregnancy outcomes could be strengthened by the use of common measures, which would allow data from different studies to be combined or compared. Here, we introduce perinatal researchers to the PhenX Toolkit and the Collections related to pregnancy and childbirth.
Methods
The Pregnancy and Birth Collections were drawn from measures in the PhenX Tooklit. The lead author selected a list of measures for each Collection, which was reviewed by the remaining authors and revised on the basis of their comments. We chose the measures we thought were most relevant for perinatal research and had been linked most strongly to perinatal outcomes.
Results
The Pregnancy and Birth Health Conditions Collection includes 24 measures related to pregnancy and fertility history, maternal complications, and infant complications. The Pregnancy and Birth Outcome Risk Factors Collection includes 43 measures of chemical, medical, psychosocial, and personal factors associated with pregnancy outcomes.
Conclusions
The biological complexity of pregnancy and its sensitivity to environmental and genomic influences suggest that multidisciplinary approaches are needed to generate new insights or practical interventions. To fully exploit new research methods and resources, we encourage the biomedical research community to adopt standard measures to facilitate pooled or meta-analyses.
Keywords: Pregnancy, Birth, Risk factors, Pregnancy outcomes
Introduction
In 2008, 10%–14% of pregnant women in selected U.S. states were hospitalized at least 1 day for a pregnancy-related problem and 1%–3% were hospitalized for 7 days or longer [1,2]. Among their infants, 5–16 percent were admitted to neonatal intensive care unit [3]. Pregnancy complications can be associated with significant long term morbidity for women [4,5] and their children [6] and are a major contributor to prenatal and delivery costs [7].
Many pregnancy complications and adverse outcomes appear to result from gene-environment interactions [8–13]. To date, the findings of genetic epidemiology studies have been conflicting, inconsistent, and difficult to interpret, which is a major barrier to determining the relative contribution of specific causes of adverse pregnancy outcomes [10,11]. Researchers attending the 3rd International Workshop on Biomarkers and Preterm Birth developed research guidelines in an attempt to improve the validity and reproducibility of such studies [14]. The guidelines promoted a standardized case definition and standard minimum and optimum data sets to be used for such studies. The expected benefits of this standardization included independent validation, comparison of results between studies, and the ability to combine studies. Realizing these benefits also requires that individual variables are defined and measured comparably among studies.
Most published genetic studies of pregnancy outcomes are candidate gene studies [8–10]. Some genome-wide association studies (GWAS) of pregnancy outcomes have been published [15–17], and additional GWAS are in process [18,19]. Much research remains to be done on the genetics of pregnancy outcomes, which presents an opportunity to incorporate common measures into the bulk of perinatal genetic research. In this paper, we introduce perinatal researchers to the PhenX Toolkit (www.phenxtoolkit.org), a resource for common measures that includes measures related to pregnancy and childbirth [20,21].
PhenX
The Consensus Measures for Phenotypes and Exposures (PhenX) project was a cooperative effort of the National Human Genome Research Institute, RTI International, and the scientific community (www.phenx.org) to promote measurement and variable consistency across population-based research by providing investigators with a source of high-quality, low-burden, well-established measures [20,22]. A Steering Committee identified 21 priority research domains, defined the scope of the domains, and set guidelines for acceptable measures and protocols [23]. For each research domain, a working group of content experts was assembled, which selected (with input from the scientific community) approximately 15 measures relevant to the domain. For each measure, the working group selected one or more protocols to capture data on participant characteristics, phenotypes, clinical assessments, environmental exposures, or other traits and exposures [24]. The selected measures and protocols had to be feasible for data collection in large population studies, e.g., they had to have low implementation cost and low participant and investigator burden. Selected protocols also had to be well-established and appropriate for use by investigators who are not domain experts.
The PhenX Toolkit (www.phenxtoolkit.org) provides access to the 339 measures selected by the PhenX working groups, the associated protocols, the purpose and rationale of the measures and protocols, and data collection worksheets and data dictionaries for the protocols. The Toolkit structure is illustrated in Figure 1, and the PhenX terminology is defined in Table 1. The Toolkit’s search and browse tools help investigators find measures of interest. Some PhenX measures are relevant to multiple research domains or are essential for the interpretation of other measures. Interrelated measures are linked as essential measures, related measures, and collections (Fig. 2). Essential measures are measures that are required for correct interpretation of the measure of interest. Related measures may collect additional information on the same topic, be conceptually or biologically related to the measure of interest, or allow calculation of a derived variable. PhenX Collections are curated groups of Toolkit measures from multiple domains related by a specific topic. A measure may appear in multiple collections. This report presents two PhenX Collections related to pregnancy and childbirth.
Fig. 1.
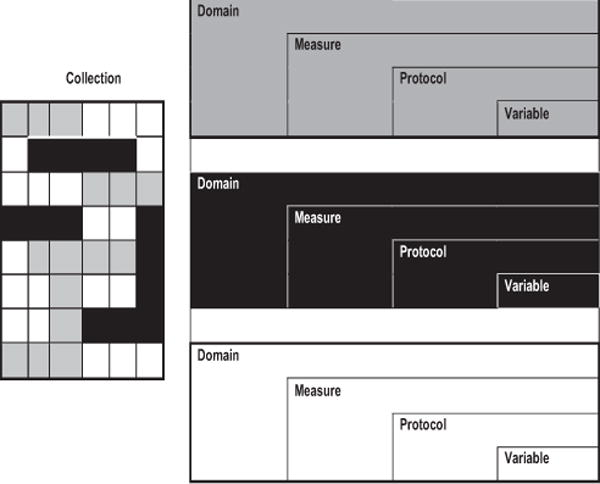
Relation of PhenX Domains, Measures, Protocols, Variables, and Collections.
Table 1.
Glossary of PhenX terms
| Term | PhenX definition |
|---|---|
| Collection | A collection of measures with a shared characteristic, target population, or topic. The measures included in a Collection may cut across research domains. |
| Domain | A field of research with a unifying theme and easily enumerated quantitative and qualitative measures (e.g., demographics, anthropometrics, organ systems, complex diseases, and lifestyle factors). |
| Measure | A standardized way of capturing data on a certain characteristic of, or relating to a study subject. |
| Protocol | A standard procedure recommended by a Working Group for investigators to collect and record a PhenX Measure. |
| Variable | A data element collected by a PhenX protocol. |
Fig. 2.
Links to related measures on the PhenX measure review screen.
The Pregnancy and Birth Collections and the Reproductive Health domain
To simplify finding pregnancy-related measures, we developed two PhenX Toolkit Collections, Pregnancy and Birth Health Conditions (shown in Table 2) and Pregnancy and Birth Outcome Risk Factors (shown in Table 3). The Pregnancy and Birth Health Conditions Collection contains measures of symptoms and outcomes associated with current or previous pregnancies, including measures of pregnancy history, normal pregnancy changes, and maternal and infant complications. The Pregnancy and Birth Outcomes Risk Factors Collection contains measures of factors and exposures associated with pregnancy complications and outcomes.
Table 2.
PhenX pregnancy and birth health conditions
| Measure | Domain |
|---|---|
| Pregnancy and fertility history | |
| Ovulation history | Cancer |
| Causes and treatments of known infertility | Reproductive Health |
| Contraceptive methods | Reproductive Health |
| Difficulty in conceiving | Reproductive Health |
| History of prepubertal development | Reproductive Health |
| Male sexual function | Reproductive Health |
| Menstrual history | Reproductive Health |
| Reproductive history | Reproductive Health |
| Sexual history | Reproductive Health |
| Maternal complications | |
| Weight loss/gain | Anthropometrics |
| Blood pressure (adult/primary) | Cardiovascular |
| Deep venous thrombosis | Cardiovascular |
| High blood pressure during pregnancy | Cardiovascular |
| Pulmonary embolism | Cardiovascular |
| Oral glucose tolerance test | Diabetes |
| Urinary creatinine assay for kidney function | Diabetes |
| Serum creatinine assay for kidney function | Diabetes |
| Urinary microalbumin assay for kidney function | Diabetes |
| Depression | Psychiatric |
| Infant complications | |
| Birth weight | Anthropometrics |
| Child head circumference | Anthropometrics |
| Personal and family history of strabismus | Ocular |
| Existence of cleft lip or palate | Oral Health |
| Male reproductive tract birth defects | Reproductive Health |
Table 3.
PhenX pregnancy and birth outcome risk factors Collection
| Measure | Domain |
|---|---|
| Chemical risks to pregnancy | |
| Alcohol: 30-day quantity and frequency | Alcohol, Tobacco, and Other Substances |
| Substances: 30-day frequency | Alcohol, Tobacco, and Other Substances |
| Tobacco: 30-day quantity and frequency | Alcohol, Tobacco, and Other Substances |
| Medications (current and past use of pain relievers, statins, and steroids) | Cancer |
| Medication inventory | Diabetes |
| Current environmental tobacco smoke exposure | Environmental Exposures |
| Caffeine intake | Nutrition and Dietary Supplements |
| Dietary supplements use | Nutrition and Dietary Supplements |
| Reproductive history | Reproductive Health |
| Urine assay for tobacco smoke exposure | Respiratory |
| Medical risks to pregnancy | |
| Cancer treatments | Cancer |
| Arrhythmia (atrial and ventricular) | Cardiovascular |
| Blood pressure (adult/primary) | Cardiovascular |
| Rheumatic fever/rheumatic heart disease | Cardiovascular |
| Personal history of type 1 and type 2 diabetes | Diabetes |
| Immunizations | Infectious Diseases and Immunity |
| Personal medical history of allergies, infectious diseases, and immunizations | Infectious Diseases and Immunity |
| Personal and family history of autoimmune and inflammatory disorders | Infectious Diseases and Immunity |
| Periodontal disease: prevalence | Oral Health |
| Causes and treatments of known infertility | Reproductive Health |
| Contraceptive methods | Reproductive Health |
| Systemic lupus erythematosus | Skin, Bone, Muscle, and Joint |
| Psychosocial risks to pregnancy | |
| Emotional Distress | Gastrointestinal |
| Chronic stress | Psychosocial |
| Perceived stress | Psychosocial |
| Discrimination | Social Environments |
| Life events | Social Environments |
| Neighborhood concentrated disadvantage | Social Environments |
| Race/ethnic residential segregation | Social Environments |
| Personal characteristics risks to pregnancy | |
| Height | Anthropometrics |
| Pregnancy weight gain | Anthropometrics |
| Weight | Anthropometrics |
| Lipid profile | Cardiovascular |
| Annual family income | Demographics |
| Current age | Demographics |
| Ethnicity | Demographics |
| Race | Demographics |
| Education | Demographics |
| History of prepubertal development | Reproductive Health |
| Reproductive history | Reproductive Health |
| Difficulty in conceiving | Reproductive Health |
| Menstrual history | Reproductive Health |
| Sexual history | Reproductive Health |
The Pregnancy and Birth Collections are most closely aligned with the Reproductive Health domain, which covered sexual development and maturation, fertility, reproduction, sexual activity and function, and sexual organ related health problems, treatment and procedures. PhenX focused on measures that could be used in a wide variety of studies. Thus, the Reproductive Health Working Group sought measures that could be used across the life span, not only during pregnancy. For example, in life course theory, the likelihood of a disease emerging at any age may be influenced by fetal exposures. However, retrospective assessment of fetal exposure is problematic. The Working Group agreed that, although not ideal, maternal recall of selected behavioral factors (e.g., smoking and alcohol) would be adequate for GWAS studies of newborns and infants; however, they could not identify valid retrospective measures of fetal exposures for studies of older children, adolescents or adults.
Locating existing protocols consistent with the PhenX selection criteria was challenging for some measures. For example, the Reproductive Health Working Group had particular difficulty identifying a suitable protocol for pregnancy history. The Working Group sought to find one comprehensive protocol, but none of the available protocols provided a comprehensive history. Rather, various protocols addressed one or two aspects of a woman’s pregnancy history, such as pregnancy planning, time to pregnancy, fertility treatment, weight gain during pregnancy, or paternity. Ultimately, the Reproductive Health Working Group recommended a modified version of the pregnancy history protocol from the Women’s Interview Study of Health [25]. Modifications included the deletion of some questions and addition of questions for smoking and alcohol during pregnancy from other sources.
Measures in the collections were selected by the lead author and revised after review by the other authors. Many factors have been associated with some aspect of the course of pregnancy. We choose the measures on the basis of their relevance for perinatal research and the strength of their link to perinatal outcomes. Additional measures within the Toolkit may be relevant to research in pregnancy and childbirth or maybe calculated from the Toolkit measures. For example, the percentile of birth weight for gestational age could be calculated from the gestational age estimated included in the Reproductive History measure and the Birth Weight measure in the Anthropometrics domain. Some measures commonly assessed in reproductive epidemiology were not in the Toolkit and therefore not available for the Pregnancy and Birth Collections.
The Pregnancy and Birth Health Conditions Collection has 24 measures and the Pregnancy and Birth Outcomes Risk Factors Collection has 43 measures. Nine measures are included in both collections, for a total of 58 measures. Although 17 (27%) of the measures in the Collections are from the Reproductive Health domain, measures of pregnancy outcomes and risk factors are found throughout the toolkit. The Pregnancy and Birth Health Conditions Collection draws measures from nine domains, and the Pregnancy and Birth Outcomes Risk Factors Collection draws measures from 18 domains. The Collections were grouped into subcollections to make the collections easier to browse.
The broad scope of PhenX, compared with the scope of the International Workshop on Biomarkers and Preterm Birth, which produced the research guidelines for genetic studies of preterm birth [14], limits the number of measures for any one topic or group. Even though the PhenX measures were not chosen specifically for pregnant women, the Pregnancy and Birth Collections provide measures for 8 of 10 the elements that Pennell et al. recommended for the minimum data set for genetic studies of preterm birth and 15 of the 33 additional measures in the optimal data set. The Collections also include measures of some congenital anomalies, another element of the optimal data set. Several measures of nutritional status are available in the Toolkit measures but were not included in the Collections. The pregnancy specific clinical elements of the Pennell datasets, such as how the preterm birth initiated, the vital status of the fetus at the onset of labor, the presence of a uterine anomaly, tocolytic therapy, and signs of maternal or neonate infection, are not included in the PhenX Toolkit.
As an example of how these Collections simplify identifying measures for a study, consider a researcher planning a study of preeclampsia. Within the Maternal Complications subcollection of the Pregnancy and Birth Health Condition Collection, the researcher would find four relevant measures: weight gain and loss from the Anthropometrics domain; blood pressure and history of high blood pressure during pregnancy from the Cardiovascular domain; and urinary creatinine and microalbumin from the Diabetes domain.
Discussion
Multifactorial disease pathways are difficult to disentangle in any complex disease. Understanding pregnancy and birth is even more complicated because maternal, paternal, and fetal genomes and environmental exposures can contribute to the course of pregnancy and its outcome. Thus, it is not surprising that genetic studies of reproductive outcomes have produced mixed results. Recent evidence suggests that predictive biomarkers may differ among subpopulations, further complicating attempts to understand the genetics of gestational age at birth [26]. Still, GWAS have brought new insights to intractable research questions, including the causal pathways of myocardial infarction [27], type 2 diabetes [28], and systematic lupus erythematosus [29] and may be able to shed similar light on reproductive outcomes.
As both Pennell et al. [14] and Hamilton et al. [20] argued, there are compelling reasons to use standard measures in genetic epidemiology studies, including the ability to combine or compare data from different studies. These reasons are particularly salient in perinatal research, where the population of interest is relatively small and the outcomes of interest may be rare. Experience with other complex disorders suggest that larger sample sizes, aided by the use of standard measures that allow pooled analyses and cross study comparison or replication, may identify and confirm more variants associated with specific pregnancy complications and outcomes [30].
Although the PhenX Toolkit is relatively new, Toolkit measures have already been incorporated into studies on a variety of topics, including one pediatric study. The Children’s Health after the Storm, a large epidemiologic study funded by the Centers for Disease Control and Prevention, is investigating short- and long-term health effects among children who resided in temporary housing units provided by the Federal Emergency Management Agency (i.e., FEMA) after Hurricanes Katrina and Rita. The study will use the following PhenX measures: height, weight, current age, gender, current educational attainment, dust collection, spirometry, peak expiratory flow rate, and urine assay for tobacco smoke.
Standard measures are not without their disadvantages [31]. They are inflexible and are often consensus-based and therefore may not work well for specialized needs or populations. The selection or development process for standard measures is often time consuming and costly, which can prevent the timely incorporation of new concepts and research. The standard measures can become irrelevant if the field moves away from the concepts they measure. Finally, standard measures may be more comprehensive than needed for a specific study, adding to the respondent burden and the cost of the study. The Data Schema and Harmonization Platform for Epidemiological Research (DataSHaPer) [32,33] project avoids these disadvantages by flexible data harmonization. Instead of recommending specific measurement protocols, they identify a core set of variables for a research area, then formally assess the suitability of harmonizing of these variables among studies. Although this approach addresses the disadvantages of rigidity, it requires a very critical assessment of the different data collection protocols to ensure they are measuring the same concepts.
The PhenX Toolkit contains a limited number of measures selected from existing measures. Working Groups could not develop new measures. No suitable existing protocol was identified for some measures. The difficulty the Reproductive Health Working Group had in identifying a comprehensive pregnancy history protocol was discussed previously. They also had difficulty locating protocols to measure testosterone replacement, selective estrogen receptor modulators, and age at receipt and number of vaccine shots for human papillomavirus. Thus, even though it is a valuable resource for standard measures, the Toolkit is not comprehensive. Some additional resources for frequently collected measures in perinatal research include questionnaires from large population-based surveys such as the Pregnancy Risk Assessment Monitoring System (www.cdc.gov/prams), and the National Survey of Family Growth (http://www.cdc.gov/nchs/nsfg.htm).
Ensuring healthy childbearing and birth outcomes continues to challenge the research, clinical, and public health communities. The biological complexity of pregnancy and its sensitivity to social, environmental, and genomic influences suggest that traditional, enisled research is unlikely to generate new insights or practical interventions [34]. New research strategies are needed that transcend traditional disciplinary boundaries and timelines. These strategies must provide optimal statistical power for systematic investigations of associations among genotypes, phenotypes, and exposures and allow for rapid and timely replication of novel findings (e.g., replication of main effects and gene-environment interaction effects).
To fully exploit new research methods and resources, we encourage the biomedical research community to adopt standard measures to facilitate pooled or meta-analyses. The PhenX Toolkit provides a catalog of common measures and measurement protocols for use in GWAS and other population-based research. Common measures will allow investigators to expand analyses beyond individual studies into areas that may expand our understanding of multiple exposure and domain interactions [22,35]. We present PhenX measures for reproduction, pregnancy, and infant health research to demonstrate how Collections of measures from multiple domains can be used to further interdisciplinary pregnancy and childbirth research. We also identify opportunities for developing and validating additional measures that may facilitate reproductive health research going forward. The development and integration of standard measures in research will help advance pregnancy research and improve the health of women and their offspring.
Acknowledgments
This work was supported by the National Human Genome Research Institute (award U01 HG004597-01).
Guidance is provided to the PhenX project by the PhenX Steering Committee: Jonathan Haines (Chair), William R. Harlan (Vice-Chair), Terri H. Beaty, Lindsay A. Farrer, Peter Kraft, Mary L. Marazita, Jose M. Ordovas, Carlos N. Pato, Erin Ramos, Margaret R. Spitz, Diane Wagener, and Michelle A. Williams. The PhenX working groups have made key contributions to this project. In particular, the authors acknowledge the expertise and significant contributions of the PhenX Reproductive Health working group: Carol J. Hogue (chair), Andrea Dunaif, Russ Hauser, Pauline Mendola, Stephanie Seminara, and Jun (Jim) Zhang.
References
- 1.Centers for Disease Control and Prevention. Indicator of whether mother went to the hospital and stayed more than 7 days for a pregnancy-related complication. Atlanta: Centers for Disease Control and Prevention; 2008. CPONDER: CDC’s PRAMS On-line Data for Epidemiologic Research. http://apps.nccd.cdc.gov/cPONDER/default.aspx?page=DisplayAllStates&state=0&year=9&category=14&variable=19. Accessed August 16, 2012. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Indicator of whether mother went to the hospital and stayed at least 1 day for a pregnancy-related complication. Atlanta: Centers for Disease Control and Prevention; 2008. CPONDER: CDC’s PRAMS On-line Data for Epidemiologic Research. http://apps.nccd.cdc.gov/cPONDER/default.aspx?page=DisplayAllStates&state=0&year=9&category=14&variable=18. Accessed August 16, 2012. [Google Scholar]
- 3.Centers for Disease Control and Prevention. After your baby was born, was he or she put in an intensive care unit? Atlanta: Centers for Disease Control and Prevention; 2008. CPONDER: CDC’s PRAMS On-line Data for Epidemiologic Research. http://apps.nccd.cdc.gov/cPONDER/default.aspx?page=DisplayAllStates&state=&year=9&category=13&variable=15. Accessed August 16, 2012. [Google Scholar]
- 4.Linne Y, Barkeling B, Rossner S. Natural course of gestational diabetes mellitus: long term follow up of women in the SPAWN study. BJOG. 2002;109(11):1227–31. doi: 10.1016/s1470-0328(02)01973-0. [DOI] [PubMed] [Google Scholar]
- 5.Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2010;23(3):199–203. doi: 10.3109/14767050903550659. [DOI] [PubMed] [Google Scholar]
- 6.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359(3):262–73. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt SK, Sneed L, Phibbs CS. Costs of newborn care in California: a population-based study. Pediatrics. 2006;117(1):154–60. doi: 10.1542/peds.2005-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anum EA, Springel EH, Shriver MD, Strauss JF., 3rd Genetic contributions to disparities in preterm birth. Pediatr Res. 2009;65(1):1–9. doi: 10.1203/PDR.0b013e31818912e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crider KS, Whitehead N, Buus RM. Genetic variation associated with preterm birth: a HuGE review. Genet Med. 2005;7(9):593–604. doi: 10.1097/01.gim.0000187223.69947.db. [DOI] [PubMed] [Google Scholar]
- 10.Williams PJ, Broughton Pipkin F. The genetics of pre-eclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):405–17. doi: 10.1016/j.bpobgyn.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orsi NM, Gopichandran N, Simpson NA. Genetics of preterm labour. Best Pract Res Clin Obstet Gynaecol. 2007;21(5):757–72. doi: 10.1016/j.bpobgyn.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Roberts CT. IFPA Award in Placentology Lecture: Complicated interactions between genes and the environment in placentation, pregnancy outcome and long term health. Placenta. 2010;31(Suppl):S47–53. doi: 10.1016/j.placenta.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Jones NM, Holzman C, Friderici KH, Jernigan K, Chung H, Wirth J, et al. Interplay of cytokine polymorphisms and bacterial vaginosis in the etiology of preterm delivery. J Reprod Immunol. 2010;87(1–2):82–9. doi: 10.1016/j.jri.2010.06.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pennell CE, Jacobsson B, Williams SM, Buus RM, Muglia LJ, Dolan SM, et al. Genetic epidemiologic studies of preterm birth: guidelines for research. Am J Obstet Gynecol. 2007;196(2):107–18. doi: 10.1016/j.ajog.2006.03.109. [DOI] [PubMed] [Google Scholar]
- 15.Haataja R, Karjalainen MK, Luukkonen A, Teramo K, Puttonen H, Ojaniemi M, et al. Mapping a new spontaneous preterm birth susceptibility gene, IGF1R, using linkage, haplotype sharing, and association analysis. PLoS Genet. 2011;7(2):e1001293. doi: 10.1371/journal.pgen.1001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horne BD, Rasmusson KD, Alharethi R, Budge D, Brunisholz KD, Metz T, et al. Genome-wide significance and replication of the chromosome 12p11.22 locus near the PTHLH gene for peripartum cardiomyopathy. Circ Cardiovasc Genet. 2011;4(4):359–66. doi: 10.1161/CIRCGENETICS.110.959205. [DOI] [PubMed] [Google Scholar]
- 17.Plunkett J, Doniger S, Orabona G, Morgan T, Haataja R, Hallman M, et al. An evolutionary genomic approach to identify genes involved in human birth timing. PLoS Genet. 2011;7(4):e1001365. doi: 10.1371/journal.pgen.1001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genome-Wide association studies of prematurity and its complications [cited August 30, 2011] 2010 Available from: http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000103.v1.p1. Accessed August 16, 2012.
- 19.ALSPAC: Genetic studies. Wellcome Trust; 2010. [August 30, 2011]. Available from: http://www.wellcome.ac.uk/Funding/Biomedical-science/Funded-projects/Major-initiatives/ALSPAC/WTDV029780.htm. Accessed August 16, 2012. [Google Scholar]
- 20.Hamilton CM, Strader LC, Pratt JG, Maiese D, Hendershot T, Kwok RK, et al. The PhenX Toolkit: Get the most from your measures. Am J Epidemiol. 2011;174(3):253–60. doi: 10.1093/aje/kwr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton CM, Strader LC, Pratt JG, Maiese D, Hendershot T, Kwok RK, et al. Hamilton et al. respond to “Consolidating Data Harmonization”. Am J Epidemiol. 2011 [Epub ahead of print] [Google Scholar]
- 22.Stover PJ, Harlan WR, Hammond JA, Hendershot T, Hamilton CM. PhenX: a toolkit for interdisciplinary genetics research. Curr Opin Lipid. 2010;21(2):136–40. doi: 10.1097/MOL.0b013e3283377395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.RTI International, National Human Genome Research Institute. PhenX Steering Committee roles and responsibilities. 2010 [September 1, 2011]. Available from: https://www.phenx.org/Default.aspx?tabid=57. Accessed August 16, 2012.
- 24.RTI International, National Human Genome Research Institute. PhenX Working Groups’ roles and responsibilities. 2010 [September 1, 2011]. Available from: https://www.phenx.org/Default.aspx?tabid=58. Accessed August 16, 2012.
- 25.Brinton LA, Daling JR, Liff JM, Schoenberg JB, Malone KE, Stanford JL, et al. Oral contraceptives and breast cancer risk among younger women. J Natl Cancer Inst. 1995;87(11):827–35. doi: 10.1093/jnci/87.11.827. [DOI] [PubMed] [Google Scholar]
- 26.Williams SM, Velez DR, Menon R. Geographic ancestry and markers of preterm birth. Expert Rev Mol Diagn. 2010;10(1):27–32. doi: 10.1586/erm.09.70. [DOI] [PubMed] [Google Scholar]
- 27.Erdmann J, Linsel-Nitschke P, Schunkert H. Genetic causes of myocardial infarction: new insights from genome-wide association studies. Deutsches Arzteblatt Int. 2010;107(40):694–9. doi: 10.3238/arztebl.2010.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frayling TM. Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet. 2007;8(9):657–62. doi: 10.1038/nrg2178. [DOI] [PubMed] [Google Scholar]
- 29.Harley IT, Kaufman KM, Langefeld CD, Harley JB, Kelly JA. Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat Rev Genet. 2009;10(5):285–90. doi: 10.1038/nrg2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juran BD, Lazaridis KN. Genomics in the post-GWAS era. Semin Liver Dis. 2011;31(2):215–22. doi: 10.1055/s-0031-1276641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feher WC, Strickland OL, Lenz ER. Standardized approaches to measurement Measurement in nursing research. 2. Philadelphia, PA: F.A. Davis Company; 1991. [Google Scholar]
- 32.Fortier I, Doiron D, Burton P, Raina P. Invited commentary: consolidating data harmonizationehow to obtain quality and applicability? Am J Epidemiol. 2011;174(3):261–4. doi: 10.1093/aje/kwr194. author reply 5–6. [DOI] [PubMed] [Google Scholar]
- 33.Fortier I, Burton PR, Robson PJ, Ferretti V, Little J, L’Heureux F, et al. Quality, quantity and harmony: the DataSHaPER approach to integrating data across bioclinical studies. Int J Epidemiol. 2010;39(5):1383–93. doi: 10.1093/ije/dyq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catalano PM, Williams MA, Wise PH, Bianchi DW, Saade GR. Scientific vision workshop on pregnancy and pregnancy outcomes. Eunice Kennedy Shriver National Institute of Child Health and Human Development; 2011. Jun, (Workshop white paper). 2011. Report No. [Google Scholar]
- 35.Hendershot T, Pan H, Haines J, Harlan WR, Junkins HA, Ramos EM, et al. Using the PhenX Toolkit to Add Standard Measures to a Study. Curr Protocols Human Genet. 2011 doi: 10.1002/0471142905.hg0121s71. Chapter 1:Unit1 21. [DOI] [PubMed] [Google Scholar]


