Abstract
Importance
Cerebral white matter hyperintensities (WMHs) are involved in the evolution of impaired mobility and executive functions. Executive functions and mobility are also associated. Thus, WMHs may impair mobility directly, by disrupting mobility-related circuits, or indirectly, by disrupting circuits responsible for executive functions. Understanding the mechanisms underlying impaired mobility in late life will increase our capacity to develop effective interventions.
Objective
To identify regional WMHs most related to slower gait and to examine whether these regional WMHs directly impact mobility, or indirectly by executive functions.
Design
Cross-sectional study. Twenty-one WMH variables (i.e., total WMH volume and WMHs in 20 tracts), gait speed, global cognition (Modified Mini-Mental State Examination; 3MS), and executive functions and processing speed (Digit-Symbol Substitution Test; DSST) were assessed. An L1–L2 regularized regression (i.e., Elastic Net model) identified the WMH variables most related to slower gait. Multivariable linear regression models quantified the association between these WMH variables and gait speed. Formal tests of mediation were also conducted.
Setting
Community-based sample.
Participants
Two hundred fifty-three adults (mean age: 83 years, 58% women, 41% black).
Main Outcome Measure
Gait speed.
Results
In older adults with an average gait speed of 0.91 m/sec, total WMH volume, WMHs located in the right anterior thalamic radiation (ATRR) and frontal corpus callosum (CCF) were most associated with slower gait. There was a >10% slower gait for each standard deviation of WMH in CCF, ATRR or total brain (standardized beta in m/sec [p value]: −0.11 [p = 0.046], −0.15 [p = 0.007] and −0.14 [p = 0.010], respectively). These associations were substantially and significantly attenuated after adjustment for DSST. This effect was stronger for WMH in CCF than for ATRR or total WMH (standardized beta in m/sec [p value]: −0.07 [p = 0.190], −0.12 [p = 0.024] and −0.10 [p = 0.049], respectively). Adjustment for 3MS did not change these associations. The mediation analyses also found that DSST significantly mediated the associations between WMHs and gait speed. Our models were adjusted for age, sex, BMI, quadriceps strength, years of education, standing height, and prevalent hypertension.
Conclusion
The impact, direct or indirect, of WMHs on gait speed depended on their location and was mediated by executive function. Thus, multi-faceted interventions targeting executive control functions as well as motor functions, such as balance and strength training, are candidates to the maintenance of mobility across the lifespan.
Keywords: White matter hyperintensities, Mobility, Cognitive function, Executive function, Gait speed, Mediation
Introduction
Impaired mobility in older adults is a significant public health concern. The prevalence of impaired mobility is 35% for community-dwelling older adults aged 70 years and older (Odenheimer et al., 1994). Reducing both the incidence and progression of impaired mobility could preserve functional independence, reduce health-care resource utilization, and sustain health-related quality of life in older adults. However, we must first gain a better understanding of the mechanisms underlying physical disability in late life to increase our capacity to develop valid screening strategies and effective interventions.
Neuroepidemiological studies highlight white matter hyperintensities (WMHs) in the evolution of impaired mobility in older adults (Annweiler and Montero-Odasso, 2012; Rosano et al., 2010; Wakefield et al., 2010; Zheng et al., 2011). White matter hyperintensities are common magnetic resonance imaging (MRI) findings among otherwise healthy older adults (Bolandzadeh et al., 2012; Breteler et al., 1994; Gunning-Dixon and Raz, 2000; Lindgren et al., 1994). These abnormalities are due to damage to the brain parenchyma (Kuo and Lipsitz, 2004), ranging from demyelination to complete axonal disruptions (Frisoni et al., 2007; Galluzzi et al., 2008). Both regional and total WMH volume are independently associated with impaired mobility, specifically, gait speed (Rosano et al., 2010; Wakefield et al., 2010).
White matter hyperintensities are also associated with impaired cognitive function, in particular, executive functions. Specifically, the prefrontal subcortical networks contain neural circuits responsible for executive functions. These circuits are located in the watershed areas and are vulnerable to lower perfusion, and are thus at high risk for WMH formation. Therefore, WMHs in prefrontal subcortical regions may further affect the tracts important for executive functions. Executive functions include the ability to concentrate, to attend selectively, and to plan and to strategize.
Of particular relevance to our current study, lower executive functions are associated with impaired mobility. It is now widely recognized that gait depends on both higher-level cognitive function (i.e., executive functions) as well as sensorimotor processes (Malouin et al., 2003; Woollacott and Shumway-Cook, 2002; Yogev-Seligmann et al., 2008). For example, Rosano et al. (2005a) demonstrated that both global cognitive function, as measured by the Modified Mini-Mental State Examination (3MS), and executive functions and information processing speed, as measured by Digit Symbol Substitution Test (DSST), are associated with impaired gait in otherwise healthy older adults.
Given the established association between WMHs, mobility, and executive functions, we hypothesize that WMHs negatively impact mobility through two central pathways: 1) directly, by disrupting mobility-related circuits (Filley, 1998; Whitman et al., 2001; Zheng et al., 2012) (i.e., direct pathway; Fig. 1); and 2) indirectly, by disrupting circuits responsible for executive functions (Guttmann et al., 2000; Starr et al., 2003) (i.e., indirect pathway; Fig. 1). It is also possible that WMH in the sensorimotor cortex is related to the executive functions performance. Therefore, we are exploring the mediating effects of cognition on both pathways.
Fig. 1.
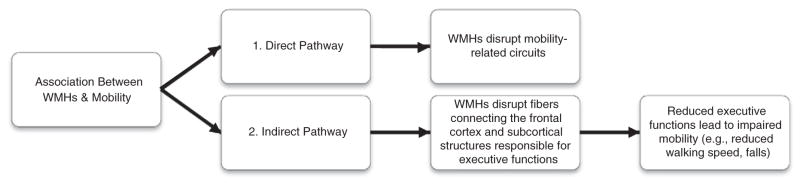
Two hypothesized pathways for the negative impact of WMHs on mobility: 1. Direct pathway—WMHs impair mobility by directly disrupting mobility-related circuits; 2. Indirect pathway—WMHs disrupt circuits responsible for cognitive function leading to impaired mobility.
If we demonstrate that the negative impact of WMHs in EF circuits on gait speed is mediated by cognitive function, then interventions targeting these networks, such as cognitive and aerobic and resistance training, should be key components in the management of older adults with impaired mobility. Both cognitive and aerobic and resistance training have been shown to be effective in promoting executive functions among older adults (Ball et al., 2002; Colcombe and Kramer, 2003; Liu-Ambrose et al., 2010; Nagamatsu et al., 2012; Verghese et al., 2010; Willis et al., 2006).
Thus, in this cross-sectional study, we examined whether WMHs directly impact mobility, or indirectly by executive functions (Fig. 1). Specifically, we seek to extend our current understanding of the relationship between WMHs and mobility by: a) identifying specific tracts in which WMH volumes are most strongly associated with gait speed, using an automated state of the art statistical method (Zou, 2005); and b) examining whether the association between WMHs and gait speed in the selected tracts is mediated by DSST or 3MS. Moreover, due to a possible effect of age, sex, body mass index (BMI), quadriceps strength, chronic pain, and hypertension on the association between WMH, cognitive function and gait speed, we are adjusting our models for these covariates. Identifying mechanisms underlying the association between WMHs and mobility will refine the focus in future research. This, in turn, will increase our capacity to identify and develop effective interventions to combat impaired mobility in older adults.
Methods
The Healthy Brain Project and participants
Our study participants were enrolled in the Healthy Brain Project (HBP). The HBP is an ancillary study on Health Aging and Body Composition (Health ABC) cohort to examine the association of structural white matter and gray matter abnormalities with age-related mobility impairment.
Among the 803 Health ABC participants alive in 2006 to 2008, 339 were eligible for inclusion in the HBP study: they walked without an assistive device, had completed the 6-meter walking test, and were eligible for MRI scanning. Three hundred nineteen Health ABC participants were ineligible for inclusion in the HBP and 145 refused to participate. Among the 339 eligible for the HBP study, 13 changed their mind after consent, 1 person died prior to scanning, and 10 were not eligible for 3 Tesla (T) scanning (i.e., 315 were included and assessed). After removal of missing data across all variables of interest, the final sample size was 253.
Independent variables: total and focal WMH volume
Brain MRIs were acquired at the MRI Research Center, University of Pittsburgh Medical Center, with a 3 T scanner. Two sequences of T1-MPRAGE and T2-FLAIR were captured. An Automated Labeling Pathway (ALP) (Wu et al., 2006) was used to quantify volumes and localization of focal WMHs. The ALP method adapts a fuzzy connected algorithm to automatically segment the WMHs. Using Johns Hopkins University White Matter Atlas that includes 20 white matter tracts (Hua et al., 2008; Wakana et al., 2007), ALP then employs a demons-based image registration technique to automate the anatomical localization of the hyperintensities. The 21 anatomical WMH variables for this study are presented in Appendix 1. The total and focal WMHs are adjusted for total brain volumes.
Dependent variable: gait speed
Gait speed is a reliable biomarker of overall health and functional status in older adults (Abellan van Kan et al., 2009). Slower gait in older adults is a significant predictor of disability and mortality (Abellan van Kan et al., 2009; Studenski et al., 2011).
The GaitMat II velocity (EQ Inc., Chalfonte, PA) measured baseline gait speed. The GaitMat II is a 4-meter long walkway with embedded pressure sensors that facilitate gait analysis. In case of missing data from GaitMat II, gait speed was obtained from walking over 3, 4 or 6 meters.
Mediators: cognitive function
We assessed global cognitive function, executive functions, and information processing speed. Global cognitive function was assessed using the 3MS (Teng and Chui, 1987). This test comprehensively evaluates cognitive domains of orientation, attention, calculation, language and short-term memory. Scores for the 3MS range from 0 to 100. Compared with the Mini-Mental State Examination, the 3MS assesses a broader range of cognitive processes.
Executive functions and information processing speed were indexed using the Digit Symbol Substitution Test (DSST) (Lezak, 1995). For this task, participants were first presented with a series of numbers (1 to 9) and their corresponding symbols. They were then asked to draw the correct symbol for any digit – placed randomly in pre-defined series – in 90 seconds. A higher number of correct answers in this time period indicated a better executive functions and processing speed.
Covariates: age, body mass index, quadriceps strength, years of education, standing height, prevalent hypertension, chronic pain
Age measured in years, sex, BMI calculated as kg/m2, years of education, and standing height in mm were added to our models. Quadriceps strength modifies the association between WMHs and gait speed (Rosano et al., 2005b, 2010). Thus, quadriceps strength was measured by the Kin-Com isometric dynamometer (Kin-Com Chattanooga, TN). We evaluated the average torque generated by the quadriceps (i.e., knee extensors) at 60 degrees per second. The mean of three trials and was used in our analysis.
Moreover, Rosano and colleagues (Buckalew et al., 2013) have shown that chronic pain might be a contributor in the association between white matter hyperintensities and disability. Therefore, chronic pain in knee, back, or leg was documented as a binary variable and added to the models as a covariate.
Prevalent hypertension for participants with average sitting systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg concurrently or before the year of MRI, was also documented as a binary variable, and was added to the models as a covariate. This variable has been shown to be associated with both WMHs and gait speed in older adults (Rosano et al., 2011).
Statistical analysis
Our statistical analyses consisted of three phases: 1) data reduction to identify WMH tracts most associated with gait speed; 2) multivariable regression models to quantify the association between the variables of interests; and 3) formal tests of mediation analysis. The multivariable regressions as well as the mediation models were adjusted for age, BMI, and quadriceps strength.
For data reduction, we used Elastic Net (Zou, 2005) to identify which of the 21 WMH variables were most associated (i.e., minimal regularized regression error using cross-validation) with gait speed. Elastic Net is an automated shrinkage and penalized statistical method. It is a preferred alternative to conventional variable selection methods, such as stepwise regression, that have been criticized for their bias, over-fitting, and exaggerated p values (Walter and Tiemeier, 2009). In Elastic Net, both L1 (i.e., the positive weighting parameter which promotes shrinkage in the regularized regression coefficients) and L2 (i.e., the weighting parameter which promotes stability on regularization) regularizations are introduced into the standard multiple linear regression model to shrink the coefficients to zero. For a given lambda (i.e., the L1 parameter) and an alpha between 0 and 1 (i.e., the L2 parameter), Elastic Net minimizes the error as presented below.
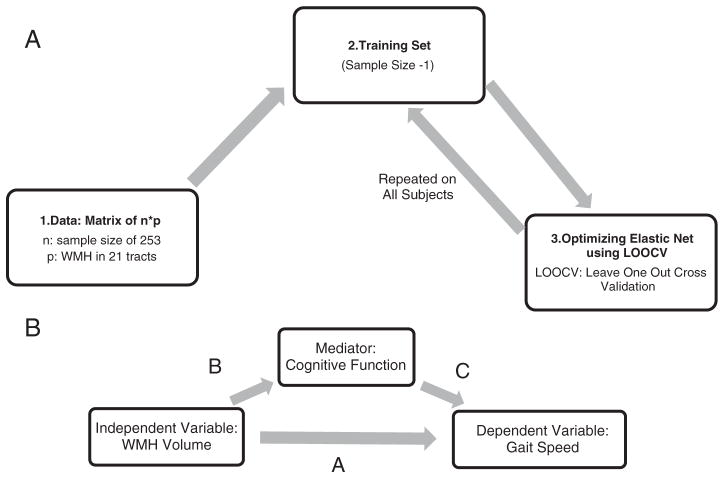
Here, Y represents gait speed for our 253 participants, and X is a 253*21 matrix of WMH volumes for 21 WMH variables. λ was set to the default value of 100, and α was set to 0.5. This analysis was performed in Matlab (R2011b, Natick, Massachusetts, The Mathworks Inc.). The process of variable selection using the Elastic Net method is illustrated in Fig. 2a and explained in greater detail in Appendix 2.
Fig. 2.
A) Variable selection using Elastic Net: Step 1 shows the n * p data where n is the sample size and p is the size of the independent variables. Step 2 employs jackknifing technique to assign one participant to the test set and the rest to the training set. This loop is required to prevent from overfitting. In Step 3, the optimized model (i.e., minimal error using cross validation) is estimated for the training set using Elastic Net. The whole process is repeated over for all the participants to avoid any bias. B) The mediation model of cognitive function for the impact of WMH volume on gait speed. The path coefficient A represents the direct effect of WMH volume on gait speed, adjusted for age, BMI, and quadriceps strength. The product of path coefficients B and C represents the indirect effect of WMH volume on gait speed through cognitive function. The total effect is the sum of the direct effect and the indirect effect (A + B * C).
For the second phase, multivariable linear regression models adjusted for age, sex, BMI, quadriceps strength, chronic pain, and prevalent hypertension were built with gait speed as the dependent variable and WMHs from specific tracts identified in phase one as independent variables. Each WMH tract was entered in a separate linear regression model, with and without adjustment for the putative mediators (e.g., DSST and MMSE).
For the third phase, mediation analyses were performed using PROCESS (Hayes, 2012), a computational macro developed for SPSS. For each WMH variable selected in phase one (i.e., data reduction) we constructed two mediation models—one for each cognitive mediating variable (i.e., 3MS and DSST). Each selected WMH variable was entered as the independent variable and gait speed as the dependent variable, while adjusting for age, BMI, and quadriceps strength. The general mediation model is illustrated in Fig. 2b.
We calculated the direct effect, indirect effect and total effect for each mediation model. The direct effect refers to the change in gait speed when WMH variable changes while the cognitive function mediators are maintained fixed (Fig. 2b: path coefficient A; the association between WMHs and gait speed adjusted for covariates and all cognitive function variables). The indirect effect refers to the change in gait speed when the independent WMH variable is maintained fixed and the cognitive mediator changes to the level it would have attained if the independent gait speed variable increased by one unit (Fig. 2b: product of path coefficients B and C). We used bootstrapping (n = 10,000) to obtain a 95% confidence interval for the indirect effect. The total effect in our linear system is the sum of direct and indirect effects of WMH volume on gait speed (i.e., the association of WMHs and gait speed adjusted for covariates; A + BC).
For each standardized model, we reported percentages of direct and indirect effect out of the total effect (i.e., direct effect * 100/total effect and indirect effect * 100/total effect), to compare the size of direct and indirect effects in each mediation model. A larger percentage for the direct or the indirect effect indicates a greater effect of the independent or the mediator variable on gait speed, respectively.
Results
Data reduction
Elastic Net selected three WMH variables that were most associated with gait speed. In addition to total WMH volume, WMHs located in right anterior thalamic radiation (ATRR) and frontal corpus callosum (CCF) were selected. Table 1 provides the descriptive statistics for all the variables of interest.
Table 1.
Descriptive statistics for covariates of age, BMI and quadriceps strength, education, height, independent WMH variables, dependent gait speed variable, and cognitive mediators.
| Variables | Number | Minimum | Maximum | Mean | Standard deviation |
|---|---|---|---|---|---|
| Age | 253 | 78 | 90 | 82.74 | 2.68 |
| % Women | 58% | ||||
| BMI | 253 | 16.88 | 39.10 | 27.25 | 4.43 |
| Quadriceps strength (newton meters) | 253 | 18 | 140 | 64.53 | 23.98 |
| Education (years) | 313 | 1 | 3 | 2.37 | 0.71 |
| Standing height (mm) | 309 | 1390.50 | 1853.50 | 1621.62 | 95.34 |
| % With chronic pain | 53% | ||||
| % With hypertension | 67% | ||||
| Total WMH volumea | 253 | 1.1 * 10−5 | 2.69 * 10−2 | 3.34 * 10−3 | 4.11 * 10−3 |
| ATRR WMH volumea | 253 | Exp −8 | 4.31 * 10−3 | 8.18 * 10−4 | 7.54 * 10−4 |
| CCF WMH volumea | 253 | Exp −8 | 6.12 * 10−3 | 5.20 * 10−4 | 7.42 * 10−4 |
| DSST | 253 | 0 | 68 | 37.10 | 13.06 |
| 3MS | 253 | 61 | 100 | 92.98 | 6.83 |
| Gait Speed (m/s) | 253 | 0. 37 | 1.50 | 0. 91 | 0. 18 |
Abbreviations: BMI: body mass index, WMH: white matter hyperintensity, ATRR: anterior thalamic radiation right, CCF: corpus callosum right, 3MS: Modified Mini-Mental State Examination, DSST: Digit Symbol Substitution Test.
WMH volumes are adjusted for total brain volume.
Multivariable regression analyses
The effects of total, ATRR and CCF WMHs on gait speed were −0.146, −0.152, and 0.114 in models adjusted for age, sex, BMI, quadriceps strength, education, and standing height (Table 2, Model 1). Since prevalent hypertension and chronic pain were also associated with slower gait (−0.240 and −0.244, p < 0.001 respectively) models were further adjusted for these variables (Table 2, Model 2). The association between WMHs and gait speed remained similar, and did not significantly modify previous results (change < 10%).
Table 2.
The results of multivariable regression analysis for the effect of three selected WMH measures on gait speed.
| WMH variables of interest: | Standardized beta [Model 1] | Standardized beta [Model 2] | Standardized beta [Model 3] | Standardized beta [Model 4] |
|---|---|---|---|---|
| Total WMHs | −0.146* [−0.257, −0.035] | −0.135* [−0.249, −0.023] | −0.1244* [−0.233, −0.015] | −0.109 [−0.218, 0.000] |
| ATRR WMHs | −0.152** [−0.262, −0.042] | −0.159*** [−0.273, −0.048] | −0.130* [−0.238, −0.021] | −0.123* [−0.230, −0.016] |
| CCF WMHs | −0.114* [−0.225, −0.002] | −0.115* [−0.227, −0.003] | −0.090 [−0.200, 0.020] | −0.073 [−0.183, 0.037] |
Model 1: Linear regression model for the effect of WMHs on gait speed, controlling for age, sex, BMI, quadriceps strength, education, and height.
Model 2: Linear regression model for the effect of WMHs on gait speed, controlling for age, sex, BMI, quadriceps strength, education, height, chronic pain, and prevalent hypertension.
Model 3: 3MS added to Linear regression Model 1 with the addition of 3MS as an independent variable.
Model 4: DSST added to Linear regression Model 1 with the addition of DSST as an independent variable.
Abbreviations: 3MS: Modified Mini-Mental State Examination, DSST: Digit-Symbol Substitution Test, WMHs: white matter hyperintensities, ATRR: anterior thalamic radiation right, CCF: corpus callosum frontal.
p < 0.05.
p < 0.01.
p < 0.005.
By contrast, the association between WMHs and gait speed substantially decreased when 3MS or DSST were added to the models (Table 2, Model 3 and 4; 15% and 25% for Total WMHs, 6% and 19% for ATR WMHs, and 21% and 35% for CCF WMHs). Results remained similar in men compared to women.
Mediation analyses
We constructed six mediation models in total; two per selected WMH variable. Overall, executive functions and information processing speed, as measured by DSST, mediated the association between WMH volume and gait speed, after adjusting for age, sex, BMI, quadriceps strength, years of education, standing height, and prevalent hypertension. Global cognitive function, as measured by 3MS, was not a significant mediator (Table 3).
Table 3.
The total, direct, and indirect effects for WMH volumes of total brain, ATRR and CCF, on gait speed are presented.
| Total effect
|
Direct effect
|
Indirect effect through 3MS
|
Indirect effect through DSST
|
||||
|---|---|---|---|---|---|---|---|
| Variables of interest: WMH volumes | Unstandardized B (95% CI) | Standardized beta (95% CI) | Unstandardized B (95% CI) | Standardized beta (% of total) | Unstandardized B (95% CI) | Unstandardized B (95% CI) | Standardized beta (% of total) |
| Model 1: Total brain | −6.90** [−11.93, −1.88] | −0.15** [−0.25, −0.04] | −4.96* [−9.87, −0.06] | −0.10* (72%) | −0.60 [−2.02,0.04] | −1.33a [−3.18, −0.35] | −0.02a (19%) |
| Model 2: ATRR | −41.31** [−68.49, −14.13] | −0.16** [−0.27, −0.05] | −32.13* [−58.57, −5.68] | −0.12* (78%) | −3.34 [−11.71, 0.23] | −5.84a [−14.93, −0.42] | −0.02a (14%) |
| Model 3:CCF | −29.42* [−57.49, −1.34] | −0.11* [−0.22, −0.005] | −17.59 [−45.04, 9.84] | −0.06 (60%) | −3.60 [−12.22, 0.00] | −8.21a [−19.79, −1.95] | −0.03a (27%) |
All three models are adjusted for age, sex, body mass index, quadriceps strength, education, height, and prevalent hypertension.
The direct effect represents the association of WMH volume on gait speed adjusted for age, sex, BMI, quadriceps strength, and prevalent hypertension, when the effect of both cognitive tests on gait speed are taken into consideration. The indirect effect represents the effect of WMH volume on gait speed through cognitive function. The total effect is the sum of the direct effect and the indirect effect.
Abbreviations: WMH: white matter hyperintensity, ATRR: anterior thalamic radiation right, CCF: corpus callosum frontal, 3MS: Modified Mini-Mental State Examination, DSST: Digit-Symbol Substitution Test.
p < 0.05.
p < 0.01.
Statistically Significant based on 95% confidence interval.
Table 3 provides the total, direct and indirect (both unstandardized and standardized beta) effects for each of the three WMH variables on gait speed, adjusted for age, sex, BMI, quadriceps strength, education, height, and prevalent hypertension. The total effect of WMH volume on gait speed was significant and negative for all three WMH variables of total brain, ATRR and CCF (standardized beta in m/sec [p value]: −0.15, −0.16, −0.11 [p < 0.05], respectively). The direct effect of WMH volume on gait speed was significant and negative for total brain and ATRR (standardized beta in m/sec [p value]: −0.10 and −0.12 [p < 0.05], respectively), but not significant for CCF (standardized beta in m/sec [p value]: −0.06 [p = 0.16]). The indirect effect of WMH volume on gait speed through executive functions and information processing speed was significant and negative for all the three WMH variables of total brain, ATRR and CCF (standardized beta in m/sec [significant based on 95% CI]: −0.02, −0.02, −0.03, respectively). Moreover, for total WMH volume, the direct effect was 72% of its total effect and the indirect effect was 19% of its total effect. For ATRR, the direct effect was 78% of its total effect and the indirect effect was 14% of its total effect. For CCF, the direct effect was not significant and the indirect effect was 27% of its total effect.
Discussion
In this cohort of community-dwelling old adults free from overt neurological conditions, total WMHs, as well as WMHs in CCF and ATRR, were most strongly associated with slower gait speed. While previous studies have consistently found an association between total WMH volume and slower gait, few studies to date have looked at focal WMHs in selected tracts. These associations were robust and independent of age, sex, body mass index, quadriceps strength, education, height, prevalent hypertension, and chronic pain.
Compared to previous studies examining the effect of focal WMHs on physical function, our current study had several new aspects; our subjects had higher average age (de Laat et al., 2011; Srikanth et al., 2010; Zheng et al., 2012), and our statistical analysis were adjusted for relevant covariates (Srikanth et al., 2010). Our study applied imaging with a high level of spatial resolution to quantify WMHs in individual white matter tracts. Other studies applied lower resolution methods and limited their analyses to overall volumes of lobar WMHs (Zheng et al., 2012) or distributed in deep versus periventricular WMHs (de Laat et al., 2011; Soumare et al., 2009; Srikanth et al., 2010). Importantly, our study provides novel insight into potential mechanistic pathways by which WMHs impact gait speed in older adults. Our results concur with previous studies that suggest WMHs in cortical regions containing projection fibers (e.g. anterior thalamic radiation) (Buckalew et al., 2013; Guttmann et al., 2000), commissural fibers (e.g. corpus callosum) (de Laat et al., 2011; Srikanth et al., 2010; Zheng et al., 2012) and association fibers (e.g. superior longitudinal fasciculus) (Zheng et al., 2012) play an important role in mobility.
Lower DSST performance, an indicator of executive dysfunction and impaired processing speed, but not global cognitive function, was a significant mediator of associations between WMH volumes and gait speed, after accounting for age, BMI, and quadriceps strength, education, and height. However, the degree by which DSST mediated the association between WMHs and gait speed depended on WMH location. Specifically, it was strongest for CCF than for ATRR or total brain WMH.
The corpus callosum is the largest white matter tract and plays a primary role in cognitive function. The CCF, or corpus callosum genu and rostrum, connects the prefrontal cortex between the two hemispheres of the brain and hence, plays a role in executive functions (Jokinen et al., 2012). Of particular relevance to DSST performance, Jokinen et al. (2007) demonstrated that overall corpus callosum atrophy was associated with impaired processing speed, and that anterior corpus callosum (genu and rostrum) atrophy was associated with impaired attention and executive functions in community-dwelling older adults with WMHs. Therefore, WMHs localized in this portion of the corpus callosum may impair mobility indirectly, because they impair executive control function.
While the association between CCF WMHs and gait was not significant after adjustment for DSST (i.e., the direct effect was not significant), the association between WMHs in the ATRR and slower gait was significant and independent of DSST (i.e., the direct effect was significant). In fact, it had a large significant direct effect (i.e., 78%) on gait speed. This finding concurs with the neuroanatomy of the ATRR. The ATRR contains fibers from superior fronto-occipital fasciculus that connects pre-motor areas with the parietal lobe (Srikanth et al., 2010). Thus, any disruption of these fibers may impair the somatosensory feedback required for gait (Srikanth et al., 2010) and thus, directly impair gait. We also found a significant indirect effect (i.e., 14%) of ATRR WMHs on gait speed through DSST performance. Duering et al. (2011) previously found that WMHs in the ATR were independently associated with executive functions and processing speed. Therefore, WMHs in ATRR may negatively impact mobility through two central pathways: 1) directly, by disrupting mobility-related circuits (Filley, 1998; Whitman et al., 2001; Zheng et al., 2012); and 2) indirectly, by impairing circuits responsible for executive functions and subsequently impairing motor control (Guttmann et al., 2000; Starr et al., 2003).
Overall, our findings highlight the importance of a multisystem assessment of slowing gait, which should include both executive functions and processing speed as well as motor pathways (Rosano et al., 2005a, 2010; Yogev-Seligmann et al., 2008) and the negative impact of WMHs on these processes. Interventions targeting these networks, for example cognitive interventions and aerobic and resistance training, may be particularly effective in promoting mobility among older adults (Ball et al., 2002; Colcombe and Kramer, 2003; Verghese et al., 2010; Willis et al., 2006). Future studies should also examine whether interventions aimed at reducing vascular risk factors (e.g., hypertension, diabetes type II, hypercholesterolemia, etc.) also improve mobility and the underlying CNS mechanisms. Although we found an association between cardiovascular factors and gait, this association did not seem to modify the relationship between WMHs and gait speed. It is possible that cardiovascular factors may impact mobility through pathways that do not include WMHs.
We highlight two key strengths of our study. First, we applied automated WMH segmentation and volume quantification method in order to localize WMHs; majority of studies only examine total WMH volume. Specifically, our WMH localization method enabled us to identify WMHs located in different white matter tracts and hence, allowed us to investigate the impact of WMH location on gait speed. Second, we applied state-of-the-art reliable statistical methods for both data reduction and mediation analysis to extend our current understanding of how WMHs impact gait speed in older adults.
We recognize the limitations of our study. The cross-sectional design limits our understanding of the temporal relationship between WMHs and slowing gait. Our study sample consisted exclusively of independent community-dwelling older adults who were without significant physical and cognitive impairments. Thus, the results of our study may not generalize beyond this population and we may have underestimated both the direct and indirect effects of WMHs on gait speed. Furthermore, we did not use diffusion tensor imaging which is more sensitive to white matter abnormalities than T2-FLAIR MRI (Zheng et al., 2012). However, we did apply a DTI-based white matter atlas to register on our MRI data and segment the WMHs in different tracts. Finally, we used a very limited neuropsychological testing battery and thus, did not have a comprehensive assessment of cognitive function. Therefore, future studies should include a broader battery to advance our understanding of which cognitive processes are most impacted by WMHs and are most relevant to mobility impairments in older adults.
Conclusion
Our current study suggests that executive functions and processing speed significantly mediate the impact of WMHs on gait speed. Current evidence suggests that both aerobic and resistance training has specific benefits for executive functions in older adults. Thus, our findings lend further support that exercise is an essential component in the maintenance of mobility across the lifespan – by improving physical function, such as balance and strength – but also cognitive function. Exercise may also have the potential to minimize the progression of WMHs and there are ongoing research exploring this possibility (Cyarto et al., 2012). Our results also suggest that mobility screening in older adults should have far greater attention to the assessment of cognitive processes of executive functions and processing speed.
Acknowledgments
This research was supported by National Institute on Aging (NIA) grant contracts N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, NIA grant R01-AG028050, and NINR grant R01-NR012459, and K23 AG28966, R01 AG029232, R01 AG037451, and P30 AG024827. This research was also supported in part by the Intramural Research Program of the National Institute of Health (NIH) NIA. N.B. was supported by Heart and Stroke Foundation of Canada for her PhD studies. T.L.A. is a Canada Research Chair in Physical Activity, Mobility and Cognitive Neuroscience. C.R. had full access to all of the data in study and responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix 1. The 21 anatomical WMH variables for this study
Total volume of WMHs
Anterior thalamic radiation, left
Anterior thalamic radiation, right
Corpus callosum, frontal
Corpus callosum, occipital
Corticospinal tract, left
Corticospinal tract, right
Cingulate, lower part left
Cingulate, lower part right
Cingulate, upper part left
Cingulate, upper part right
Inferior fronto-occipital fasciculus, left
Inferior fronto-occipital fasciculus, right
Inferior longitudinal fasciculus, left
Inferior longitudinal fasciculus, right
Entire superior longitudinal fasciculus, left
Entire superior longitudinal fasciculus, right
Superior longitudinal fasciculus, the branch to the temporal lobe, left
Superior longitudinal fasciculus, the branch to the temporal lobe, right
Uncinate fasciculus, left
Uncinate fasciculus, right
Appendix 2. Data reduction procedure
With Jackknifing resampling technique (Steps 2 to 4; Fig. 2a), the complete process was repeated for each participant in the X matrix separately (i.e., 253 times). On each run, one participant was assigned to the testing set, and the rest was assigned to the training set (Step 2; Fig. 2a). Then a Leave-One-Out Cross-Validation (LOOCV) Elastic Net was performed within the training set to minimize the mean squared error (Step 3; Fig. 2.a). The resulting optimal coefficients on training sets were tested on their independent test sets (Step 4; Fig. 2a).
Footnotes
Conflict of interest
None to disclose.
References
- Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009 Dec;13 (10):881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- Annweiler C, Montero-Odasso M. Vascular burden as a substrate for higher-level gait disorders in older adults. A review of brain mapping literature. Panminerva Med. 2012 Sep;54 (3):189–204. [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002 Nov 13;288 (18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolandzadeh N, Davis JC, Tam R, Handy TC, Liu-Ambrose T. The association between cognitive function and white matter lesion location in older adults: a systematic review. BMC Neurol. 2012;12:126. doi: 10.1186/1471-2377-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994 Jul;44 (7):1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- Buckalew N, Haut MW, Aizenstein H, et al. White matter hyperintensity burden and disability in older adults: is chronic pain a contributor? PM R. 2013 Jun;5 (6):471–480. doi: 10.1016/j.pmrj.2013.03.004. quiz 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003 Mar;14 (2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Cyarto EV, Lautenschlager NT, Desmond PM, et al. Protocol for a randomized controlled trial evaluating the effect of physical activity on delaying the progression of white matter changes on MRI in older adults with memory complaints and mild cognitive impairment: the AIBL Active trial. BMC Psychiatry. 2012;12:167. doi: 10.1186/1471-244X-12-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011 Jan;134 (Pt 1):73–83. doi: 10.1093/brain/awq343. [DOI] [PubMed] [Google Scholar]
- Duering M, Zieren N, Herve D, et al. Strategic role of frontal white matter tracts in vascular cognitive impairment: a voxel-based lesion-symptom mapping study in CADASIL. Brain. 2011 Aug;134 (Pt 8):2366–2375. doi: 10.1093/brain/awr169. [DOI] [PubMed] [Google Scholar]
- Filley CM. The behavioral neurology of cerebral white matter. Neurology. 1998 Jun;50 (6):1535–1540. doi: 10.1212/wnl.50.6.1535. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Galluzzi S, Pantoni L, Filippi M. The effect of white matter lesions on cognition in the elderly—small but detectable. Nat Clin Pract Neurol. 2007 Nov;3 (11):620–627. doi: 10.1038/ncpneuro0638. [DOI] [PubMed] [Google Scholar]
- Galluzzi S, Lanni C, Pantoni L, Filippi M, Frisoni GB. White matter lesions in the elderly: pathophysiological hypothesis on the effect on brain plasticity and reserve. J Neurol Sci. 2008 Oct 15;273 (1–2):3–9. doi: 10.1016/j.jns.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000 Apr;14 (2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Guttmann CR, Benson R, Warfield SK, et al. White matter abnormalities in mobility-impaired older persons. Neurology. 2000 Mar 28;54 (6):1277–1283. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- Hayes A. [Accessed 10/02/2013];PROCESS: a versatile computational tool for observed variable mediation, moderation, and conditional process modeling. 2012 http://www.afhayes.com/public/process2012.pdf.
- Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008 Jan 1;39 (1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen H, Ryberg C, Kalska H, et al. Corpus callosum atrophy is associated with mental slowing and executive deficits in subjects with age-related white matter hyperintensities: the LADIS Study. J Neurol Neurosurg Psychiatry. 2007 May;78 (5):491–496. doi: 10.1136/jnnp.2006.096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen H, Frederiksen KS, Garde E, et al. Callosal tissue loss parallels subtle decline in psychomotor speed. A longitudinal quantitative MRI study The LADIS Study. Neuropsychologia. 2012 Jun;50 (7):1650–1655. doi: 10.1016/j.neuropsychologia.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A: Biol Med Sci. 2004 Aug;59 (8):818–826. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. 3. Oxford University Press; New York: 1995. [Google Scholar]
- Lindgren A, Roijer A, Rudling O, et al. Cerebral lesions on magnetic resonance imaging, heart disease, and vascular risk factors in subjects without stroke. A population-based study. Stroke. 1994 May;25 (5):929–934. doi: 10.1161/01.str.25.5.929. [DOI] [PubMed] [Google Scholar]
- Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010 Jan 25;170 (2):170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp. 2003 May;19 (1):47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu LS, Handy TC, Hsu CL, Voss M, Liu-Ambrose T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med. 2012 Apr 23;172 (8):666–668. doi: 10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenheimer G, Funkenstein HH, Beckett L, et al. Comparison of neurologic changes in ‘successfully aging’ persons vs the total aging population. Arch Neurol. 1994 Jun;51 (6):573–580. doi: 10.1001/archneur.1994.00540180051013. [DOI] [PubMed] [Google Scholar]
- Rosano C, Simonsick EM, Harris TB, et al. Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology. 2005a;24 (1–2):8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005b Apr;53 (4):649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- Rosano C, Sigurdsson S, Siggeirsdottir K, et al. Magnetization transfer imaging, white matter hyperintensities, brain atrophy and slower gait in older men and women. Neurobiol Aging. 2010 Jul;31 (7):1197–1204. doi: 10.1016/j.neurobiolaging.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Longstreth WT, Jr, Boudreau R, et al. High blood pressure accelerates gait slowing in well-functioning older adults over 18-years of follow-up. J Am Geriatr Soc. 2011 Mar;59 (3):390–397. doi: 10.1111/j.1532-5415.2010.03282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumare A, Elbaz A, Zhu Y, et al. White matter lesions volume and motor performances in the elderly. Ann Neurol. 2009 Jun;65 (6):706–715. doi: 10.1002/ana.21674. [DOI] [PubMed] [Google Scholar]
- Srikanth V, Phan TG, Chen J, Beare R, Stapleton JM, Reutens DC. The location of white matter lesions and gait—a voxel-based study. Ann Neurol. 2010 Feb;67 (2):265–269. doi: 10.1002/ana.21826. [DOI] [PubMed] [Google Scholar]
- Starr JM, Leaper SA, Murray AD, et al. Brain white matter lesions detected by magnetic resonance [correction of resosnance] imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003 Jan;74 (1):94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011 Jan 5;305 (1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987 Aug;48 (8):314–318. [PubMed] [Google Scholar]
- Verghese J, Mahoney J, Ambrose AF, Wang C, Holtzer R. Effect of cognitive remediation on gait in sedentary seniors. J Gerontol A: Biol Med Sci. 2010 Dec;65 (12):1338–1343. doi: 10.1093/gerona/glq127. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007 Jul 1;36 (3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield DB, Moscufo N, Guttmann CR, et al. White matter hyperintensities predict functional decline in voiding, mobility, and cognition in older adults. J Am Geriatr Soc. 2010 Feb;58 (2):275–281. doi: 10.1111/j.1532-5415.2009.02699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Tiemeier H. Variable selection: current practice in epidemiological studies. Eur J Epidemiol. 2009;24 (12):733–736. doi: 10.1007/s10654-009-9411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman GT, Tang Y, Lin A, Baloh RW. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001 Sep 25;57 (6):990–994. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006 Dec 20;296 (23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002 Aug;16 (1):1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006 Dec 1;148 (2–3):133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008 Feb 15;23 (3):329–342. doi: 10.1002/mds.21720. quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JJ, Delbaere K, Close JC, Sachdev PS, Lord SR. Impact of white matter lesions on physical functioning and fall risk in older people: a systematic review. Stroke. 2011 Jul;42 (7):2086–2090. doi: 10.1161/STROKEAHA.110.610360. [DOI] [PubMed] [Google Scholar]
- Zheng JJ, Delbaere K, Close JC, et al. White matter hyperintensities are an independent predictor of physical decline in community-dwelling older people. Gerontology. 2012;58 (5):398–406. doi: 10.1159/000337815. [DOI] [PubMed] [Google Scholar]
- Zou H. Regilarization and variable selection via the elastic net. J R Stat Soc. 2005:301–320. [Google Scholar]