Abstract
Background
Mild cognitive impairment (MCI) is a well-recognized risk factor for dementia and represents a vital opportunity for intervening. Exercise is a promising strategy for combating cognitive decline, by improving both brain structure and function. Specifically, aerobic training (AT) improved spatial memory and hippocampal volume in healthy community-dwelling older adults. In older women with probable MCI, we previously demonstrated that both resistance training (RT) and AT improved memory. In this secondary analysis, we investigated: 1) the effect of both RT and AT on hippocampal volume; and 2) the association between change in hippocampal volume and change in memory.
Methods
Eighty-six females aged 70 to 80 years with probable MCI were randomly assigned to a six-month, twice-weekly program of: 1) AT, 2) RT, or 3) Balance and Tone Training (BAT; i.e., control). At baseline and trial completion, participants performed a 3T magnetic resonance imaging scan to determine hippocampal volume. Verbal memory and learning was assessed by Rey’s Auditory Verbal Learning Test.
Results
Compared with the BAT group, AT significantly improved left, right, and total hippocampal volumes (p≤0.03). After accounting for baseline cognitive function and experimental group, increased left hippocampal volume was independently associated with reduced verbal memory and learning performance as indexed by loss after interference (r=0.42, p=0.03).
Conclusion
Aerobic training significantly increased hippocampal volume in older women with probable MCI. More research is needed to ascertain the relevance of exercise-induced changes in hippocampal volume on memory performance in older adults with MCI.
Keywords: Mild cognitive impairment, aerobic exercise, memory, hippocampus, brain volume
INTRODUCTION
Worldwide, one new case of dementia is detected every four seconds.1 Currently, 35.6 million people have dementia and by 2050, this number will increase to 115.4 million.1 Thus, the societal value of developing effective intervention strategies cannot be overstated.2
Mild cognitive impairment (MCI) is a well-recognized risk factor for dementia and may represent the prodromal stage of Alzheimer’s disease (AD).3,4 Older adults with MCI develop AD at a rate of 10–30% annually,5,6 while those without MCI develop dementia at a rate of 1–2% annually.5 MCI is characterized by cognitive decline that is greater than expected for an individual’s age and education level, but does not significantly interfere with everyday function.7 Thus, we propose that MCI represents a critical window of opportunity for intervening and altering the trajectory of both cognitive decline and loss of functional independence in older adults.
Exercise is a promising strategy for combating cognitive decline. There are two distinct forms of exercise training: 1) aerobic training, which aims at improving cardiovascular fitness (e.g., running), and 2) resistance training, which aims to increase muscle mass, strength, and power (e.g., lifting weights). Both types of exercise training enhance cognitive performance and functional plasticity in healthy community-dwelling older adults.8–11
Emerging evidence also suggests that exercise has cognitive benefits for older adults with MCI. Specifically, a six-month aerobic training program significantly improved conflict resolution, processing speed, and verbal fluency in older women with amnesic MCI. We previously demonstrated that six months of resistance training improved executive functions while both resistance training and aerobic training improved memory in older women with probable MCI.12,13 Furthermore, we showed that resistance training positively impacted the regional patterns of functional brain plasticity during associative memory task performance in this high-risk population.12
However, no intervention study has examined the impact of exercise on brain structure (e.g., volume) in older adults with MCI. With respect to dementia risk, the hippocampus is a brain structure of intense interest. The hippocampus is sensitive to aging effects 14 and significant hippocampal atrophy is a hallmark of AD.15,16 Thus, understanding the effect of exercise on the hippocampus will increase our appreciation of the role exercise may play in dementia prevention.
To date, aerobic training has been shown to significantly increase hippocampal volume among healthy community-dwelling older adults.17 Specifically, Erickson and colleagues 17 demonstrated that a 12-month, 3-days/week walking program significantly increased both left and right hippocampal volumes (2.12% and 1.97%, respectively). This was equivalent to reversing age-related loss in hippocampal volume by up to two years.17 To our knowledge, no study has examined whether exercise, including either resistance training or aerobic training, have similar benefits on the hippocampus among older adults with MCI.
Thus, using neuroimaging data from a proof-of-concept single-blinded randomized controlled trial (NCT00958867) of exercise,12,18 we conducted a secondary analysis to assess: 1) the effects of both resistance training and aerobic training on hippocampal volume among older women with probable MCI; and 2) the association between change in hippocampal volume and change in memory performance. We hypothesized that both types of exercise training would increase hippocampal volume after six months of training.
METHODS
Study Design
We conducted a 26-week, single-blinded, randomized trial of exercise (NCT00958867; EXercise for Cognition and Everyday Living (EXCEL)) at the University of British Columbia with assessments at baseline, mid-point, and trial completion. Assessors were trained by the research team and blinded to group allocation of the participants. Magnetic resonance imaging (MRI) data were acquired at baseline and trial completion in a subset of eligible individuals.
Participants
Our study only included women due to potential sex differences in cognitive response to exercise.19 From April 2009 to August 2009, we recruited participants using advertisements in local media and a memory clinic. Individuals were screened by a standardized telephone interview and by a 30-minute in-person assessment. Participants with both single- and multi-domain MCI (lower performance in multiple cognitive domains) were included in this study.
Women who lived in Vancouver, Canada, were eligible for the study if they: 1) were 70 to 80 years old; 2) were living independently in their own home; 3) scored ≥ 24/30 on the Mini-Mental State Examination (MMSE);20 4) scored < 26/30 on the Montreal Cognitive Assessment (MoCA);21 5) answered “yes” to the question “Do you have any difficulty with your memory?”;22 6) Scored ≥ 6/8 on the Lawton and Brody Instrumental Activities of Daily Living;23 7) had a visual acuity of at least 20/40, with or without corrective lenses; and 8) obtained their physician’s clearance to start a supervised exercise program.
We excluded those who: 1) had a current medical condition for which exercise is contraindicated; 2) had participated regularly in resistance training or aerobic training in the last six months; 3) had a neurodegenerative disease and/or stroke; 4) had a diagnosed psychiatric condition (e.g., depression); 5) had a diagnosis of dementia of any type; 6) did not speak and understand English fluently; or 7) were on oestrogen replacement therapy.
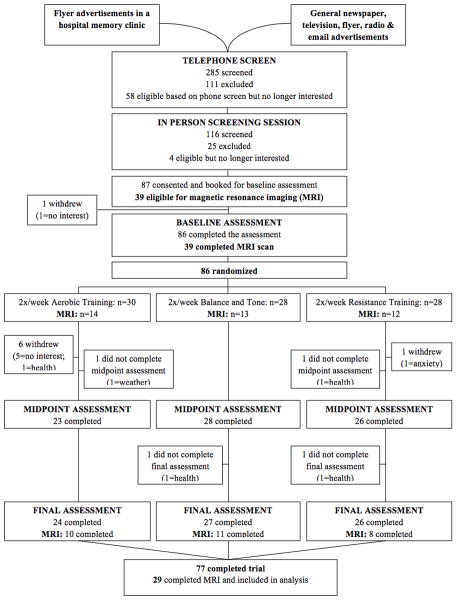
Figure 1, the CONSORT (Consolidated Standards of Reporting Trial) flowchart shows the number and distribution of participants. Ethical approval was obtained from the Vancouver Coastal Health Research Institute and the University of British Columbia’s Clinical Research Ethics Board. All participants provided written informed consent.
Figure 1.
CONSORT flowchart for study participants.
Descriptive Variables
Current level of physical activity was determined by the Physical Activities Scale for the Elderly (PASE) self-report questionnaire.24 The 15-item Geriatric Depression Scale 25 was used to screen for depression. The Functional Comorbidity Index was calculated to estimate the degree of comorbidity associated with physical functioning.26 We used the Lawton and Brody 23 Instrumental Activities of Daily Living Scale to subjectively assess ability to perform daily activities.
MRI Acquisition and Segmentation of Hippocampus
The primary outcome measure in this secondary analysis was hippocampal volume. MRI eligibility was based on a standard screening form. MRI acquisition was conducted at the UBC MRI Research Centre using a Philips Achieva 3.0T MRI scanner with an eight-channel sensitivity encoding head coil (SENSE factor=2.4) and parallel imaging. At baseline and trial completion, structural T1-weighted MRI images (TR=8 ms, TE=3.7 ms, bandwidth=2.26 kHz, voxel size=1 mm3) were obtained.
To calculate hippocampal volume, the T1-weighted structural images were analyzed using FIRST 27, a model-based segmentation and registration tool developed as a part of FMRIB Software Library (FSL; version 5.0, September 2012). The models used in FIRST are constructed from 317 manually-segmented images. The manual labels are constructed from 317 manually-segmented images. The manual labels are parameterized as surface meshes and modeled as a point distribution model. FIRST searches through linear combinations of shape models of variation for the most probable shape instance given the observed intensities in a T1-weighted image.
Using this method, an affine registration to MNI space was performed with 1mm resolution and 12 degrees of freedom. Then, the left and right hippocampi were segmented using lower boundary limits of 52.5 and 16.5, and upper boundary limits of 53.5 and 17.5, respectively. The individual quality of the segmentations was checked manually. No boundary adjustments were necessary. To calculate the total hippocampal volume, left and right hippocampal volumes were added together.
Verbal Memory and Learning
The Rey Auditory Verbal Learning Test (RAVLT) 28 assessed verbal memory and learning. Participants were read a list of 15 common words five times. Immediately after each time, they were required to recall as many words as possible. After the fifth trial, an interference list was presented, after which participants had to spontaneously recall the original words. Finally, participants were required to spontaneously recall the original words after a 20 minute delay. Scores were calculated as the total number of words recalled: 1) across the five trials (i.e., total acquisition); 2) after the interference list (i.e., recall after interference); 3) on the fifth trial minus after the interference (i.e., loss after interference); 4) after the delay (i.e., long delay free recall); and 5) in the final list that contained 60 words (i.e., recognition).
Randomization
The randomization sequence was generated by www.randomization.com and was concealed until group allocation. This sequence was held independently and remotely by the Research Coordinator. Participants were enrolled and randomized by the Research Coordinator to the twice-weekly exercise groups: resistance training (RT), aerobic training (AT), or balance and tone (BAT).
Sample Size
The required sample size was calculated based on predictions of six-month changes in the primary outcome measure, the Stroop Test. We predicted 12% improvement for both RT and AT and 10% deterioration in the BAT group (i.e., control group) based on our previous work in seniors aged 70+ years with a significant history of falls 29 and healthy community-dwelling senior women aged 65–75 years.8 Assuming a 15% attrition rate and an alpha level of ≤0.05, 29 participants per group ensured a power of 0.80.
Exercise Intervention
Exercise classes began one month after baseline assessments. RT and BAT classes were held at a fully-equipped gym in a research centre and were led by certified fitness instructors who received additional training from the study investigators. The classes were 60 minutes in duration (10-minute warm-up, 40 minutes of core content, and 10-minute cool down). Attendance was recorded daily, which was used to calculate compliance (i.e., percentage of total classes attended).
Resistance Training Program
Both a Keiser ® Pressurized Air system and free-weights were used.8 The Keiser-based exercises consisted of biceps curls, triceps extension, seated row, latissimus dorsi pull downs, leg press, hamstring curls, and calf raises. The intensity of the training stimulus was at a work range of 6–8 repetitions (two sets). The training stimulus was subsequently increased using the 7RM method – when two sets of 6–8 repetitions were completed with proper form and without discomfort. Other key strength exercises included mini-squats, mini-lunges, and lunge walks. Participants subjectively monitored the intensity of their workouts by the Borg’s Rating of Perceived Exertion (RPE).30 The Borg’s RPE scale subjectively indicates the participant’s perceived intensity during exercise. The scale ranges from six (i.e., very, very light perceived exertion) to 20 (i.e., very, very hard perceived exertion).30 Participants were asked to aim for 13–15 on the RPE scale during the RT sessions.
Aerobic Training Program
An outdoor walking program was used. The intensity of the training stimulus was at approximately 40% of one’s age-specific target heart rate (i.e., heart rate reserve; HRR) and progressed over the first 12 weeks to the range of 70% to 80% of HRR. Exercise intensity was monitored through heart rate monitors. Participants also monitored the intensity of their workouts by Borg’s RPE 30 and the “talk” test.31,32 Participants were asked to aim for 13–15 on the RPE scale during the AT sessions. Furthermore, when participants were able to speak comfortably during the AT session, they were asked to increase their walking speed (as able).
Balance and Tone Program
The program consisted of stretching exercises, range of motion exercises, balance exercises functional and relaxation techniques.8 Other than bodyweight, no additional loading (e.g., hand weights, etc.) was applied. This group served to control for confounding variables such as physical training received by traveling to the training centres, social interaction, and changes in lifestyle secondary to study participation.
Adverse Effects
Participants were questioned about the presence of any adverse effects, such as musculoskeletal pain or discomfort, at each exercise session. Instructors monitored participants for symptoms of angina and shortness of breath during the exercise classes.
Statistical Analysis
All analyses were “full analysis set” 33 (defined as the analysis set which is as complete and as close as possible to the intention-to-treat ideal of including all randomised participants).
Between-group differences in hippocampal (left, right, and total) volume were compared by multiple linear regression analysis. In the models, baseline hippocampal volume and baseline MoCA score were included as covariates. Two planned simple contrasts were performed. These contrasts were employed to assess differences between: 1) the AT group and the BAT group; and 2) the RT group and the BAT group. If a contrast was significant, single-sample t-tests were used to assess whether mean change in hippocampal volume (left, right, and total) differed from zero. Change was calculated as trial completion volume minus baseline volume.
Pearson correlations were calculated to examine the relationship between change in hippocampal volume and change in RAVLT performance. Change in hippocampal volume and RAVLT performance was calculated as: trial completion value minus baseline value. Baseline MoCA score and experimental group were used as covariates in these correlation analyses. The overall alpha was set at p<0.05.
RESULTS
Participants
Thirty-nine out of the eighty-six participants who consented and were randomized in the EXCEL study underwent MRI scanning at baseline. At trial completion, 10 of the 39 MRI participants dropped out, and 29 participants completed a second MRI scan. Baseline characteristics of the 39 MRI participants are reported in Table 1. There were no significant between-group differences at baseline.
Table 1.
Participant Characteristics at Baseline (N=39).
| Variable | AT (n=14) | RT (n=12) | ABAT (n=13) |
|---|---|---|---|
|
| |||
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 76.07 (3.43) | 73.75 (3.72) | 75.46 (3.93) |
|
| |||
| Weight (kg) | 61.74 (6.84) | 63.28 (7.51) | 64.83 (13.82) |
|
| |||
| Height (cm) | 158.76 (5.81) | 161.55 (8.09) | 157.46 (8.06) |
|
| |||
| Education (%) | |||
| • Less than grade 9 | 0 (0) | 0 (0) | 0 (0) |
| • Grade 9–12 without certificate or diploma | 2 (14.30) | 0 (0) | 3 (23.10) |
| • High school certificate or diploma | 4 (28.60) | 4 (33.30) | 2 (15.40) |
| • Trades or professional certificate or diploma | 1 (7.10) | 1 (8.30) | 3 (23.10) |
| • University certificate or diploma | 3 (21.40) | 1 (8.30) | 4 (30.80) |
| • University Degree | 4 (28.60) | 6 (50.00) | 1 (7.70) |
|
| |||
| Physical Activity Scale for the Elderly | 132.60 (59.42) | 145.36 (77.94) | 157.33 (93.99) |
|
| |||
|
Geriatric Depression Scale (max score of 15) |
0.71 (1.33) | 1.17 (1.95) | 0.46 (.88) |
|
| |||
|
Functional Comorbidities Index (max score of 18) |
2.57 (1.34) | 2.00 (1.21) | 2.46 (1.66) |
|
| |||
|
Instrumental Activities of Daily Living (max score of 8) |
8.00 (0.00) | 7.92 (0.29) | 7.85 (0.55) |
|
| |||
|
Montreal Cognitive Assessment (MoCA) (max score of 30) |
21.86 (3.11) | 21.36 (3.61)*** | 23.00 (2.71) |
|
| |||
|
Mini-Mental State Examination (MMSE) (max score of 30) |
27.54 (1.51)* | 26.67 (2.64) | 27.17 (1.85)** |
n=13,
n=12,
n=11
We previously reported that the participants of the AT group significantly improved general balance and mobility performance, as measured by the Short Physical Performance Battery, 34 and cardiovascular capacity, as measured by the Six-Minute Walk Test, 35 compared with the BAT group (p≤0.04).12
Exercise Compliance
Compliance (i.e., percentage of classes attended) was 60% for the AT group, 54% for the RT group, and 59% for the BAT group. Compliance was not significantly different between the groups.12,13
Hippocampal Volume
Baseline, trial completion, and change in hippocampal volumes are reported in Table 2. At baseline, there is no significant between-group difference for exercise groups (i.e., AT and RT). The AT group had significantly increased total hippocampal volume compared with the BAT group (p=0.01) at trial completion. Accordingly, both left and right hippocampal volumes improved significantly over the course of the intervention in the AT group compared with the BAT group (both p=0.03). Single-sample t-tests indicated that mean change in hippocampal volume (left, right, and total) within the AT group were not significantly different from zero (p≥0.09), indicating hippocampal volume maintenance rather than increase over the six-month period.
Table 2.
Hippocampal volumes segmented from T1-weighted MRI scans using FSL FIRST (N=29).
| Variable | AT (n=10) | RT (n=8) | BAT (n=11) |
|---|---|---|---|
|
| |||
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Hippocampus Volume Baseline (mm3) | |||
| • Left hippocampus | 3158.20 (500.41) | 3016.25 (528.93) | 3003.64 (476.32) |
| • Right hippocampus | 3337.10 (656.34) | 3189.50 (481.25) | 3053.64 (638.16) |
| • Total hippocampus | 6495.30 (1072.08) | 6205.75 (954.27) | 6057.27 (1089.61) |
|
| |||
| Hippocampus Volume 6 months (mm3) | |||
| • Left hippocampus | 3336.50 (454.99)* | 3027.88 (468.76) | 3027.73 (563.30) |
| • Right hippocampus | 3419.70 (539.57)* | 3043.00 (564.82) | 2923.64 (626.61) |
| • Total hippocampus | 6756.20 (952.25)* | 6070.88 (974.35) | 5951.36 (1117.94) |
|
| |||
| Hippocampal Volume Change (mm3) | |||
| • Left hippocampus | 178.30 (297.56) | 11.63 (183.55) | 24.10 (209.70) |
| • Right hippocampus | 82.60 (437.39) | −146.50 (317.70) | −130.00 (186.21) |
| • Total hippocampus | 260.90 (607.96) | −134.88 (411.09) | −105.91 (220.56) |
significantly different from BAT (p<0.05), 95% CI for difference = [136.437, 918.126]
Compared with the BAT group, the RT group did not demonstrate a significant increase in total hippocampal volume at trial completion (p=0.76). Accordingly, neither left nor right hippocampal volumes improved significantly in the RT group compared with the BAT group (p=0.83 and p=0.70, respectively).
Change in Hippocampal Volume and Change in RAVLT Performance
Baseline, trial completion, and change in RAVLT performance are reported in Table 3. After accounting for baseline MoCA score and experimental group, change in left hippocampal volume was significantly and positively associated with change in loss after interference over the six-month intervention period (see Figure 2 and Table 4; r(25)=0.42, p=0.03). Thus, increased left hippocampal volume was associated with greater loss in number of words recalled post-interference (i.e., poorer performance). Change in left hippocampal volume explained 16% of the total variance in change in loss after interference; total variance explained was 27%. No other significant associations between change in hippocampal volume and change in RAVLT performance were found (Table 4).
Table 3.
Scores for verbal memory and learning (Rey Auditory Visual Learning Test) (N=29).
| Variables* | AT (n=10) | RT (n=8) | BAT (n=11) |
|---|---|---|---|
|
| |||
| Mean (SD) | Mean (SD) | Mean (SD) | |
| RAVLT Baseline | |||
| • Total acquisition | 7.56 (1.63) | 8.38 (2.00) | 7.33 (1.37) |
| • Recall after interference | 7.10 (3.25) | 7.88 (2.64) | 6.64 (2.46) |
| • Loss after interference** | 3.20 (1.62) | 3.63 (2.45) | 2.82 (2.32) |
| • Long delay free recall | 7.10 (3.38) | 8.00 (2.00) | 6.73 (2.83) |
| • Recognition | 12.70 (2.58) | 13.25 (2.32) | 13.45 (1.29) |
|
| |||
| RAVLT 6 months | |||
| • Total acquisition | 8.16 (2.68) | 9.68 (1.55) | 8.04 (1.72) |
| • Recall after interference | 8.10 (3.35) | 9.25 (2.60) | 7.64 (3.29) |
| • Loss after interference** | 2.30 (1.16) | 2.63 (1.92) | 2.82 (1.99) |
| • Long delay free recall | 7.20 (4.34) | 9.75 (3.24) | 7.90 (3.31)*** |
| • Recognition | 13.30 (1.06) | 13.14 (1.46) | 13.73 (1.19)*** |
|
| |||
| RAVLT Change score | |||
| • Total acquisition | 0.60 (2.28) | 1.30 (1.79) | 0.71 (1.29) |
| • Recall after interference | 1.00 (2.40) | 1.38 (2.97) | 1.00 (2.10) |
| • Loss after interference** | −0.90 (1.60) | −1.00 (3.07) | 0.00 (2.53) |
| • Long delay free recall | 0.10 (2.96) | 1.75 (2.76) | 0.80 (2.57)*** |
| • Recognition | 0.60 (2.12) | −0.43 (2.70)**** | 0.27 (1.19) |
Higher score indicates better performance (except for loss after interference)
Loss after interference calculated as trial 5 score minus trial 6 score
n=11
n=7
Figure 2.
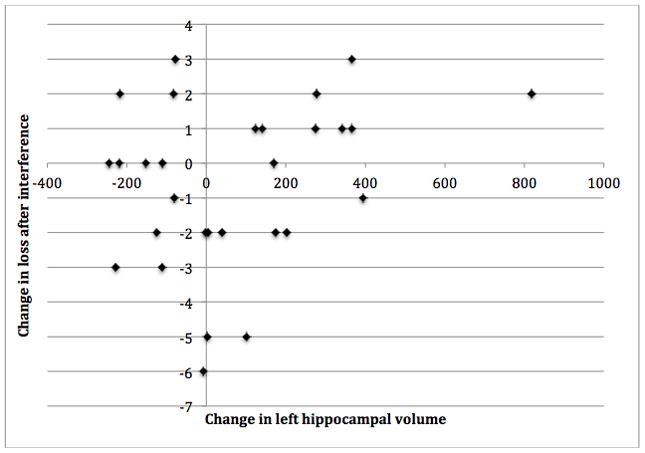
Positive partial correlation between change in left hippocampal volume and change in loss after interference (Rey Auditory Visual Learning Test) (N=29).
Table 4.
Correlation between change in Rey Auditory Visual Learning Test scores with change in hippocampal volume (N=29).*
| Δ RAVLT | Δ Total HV | ΔLeft HV | Δ Right HV |
|---|---|---|---|
| r (p-value) | r (p-value) | r (p-value) | |
| Δ Total Acquisition | −0.04 (0.86) | 0.09 (0.65) | −0.11 (0.58) |
| Δ Recall after Interference | −0.34 (0.08) | −0.37 (0.06) | −0.19 (0.34) |
| Δ Loss after Interference | 0.15 (0.44) | 0.42 (0.03)*** | −0.10 (0.63) |
| Δ Long Delay Free Recall** | −0.27 (0.19) | −0.28 (0.17) | −0.16 (0.43) |
| Δ Recognition** | −0.24 (0.23) | −0.19 (0.35) | −0.19 (0.35) |
Abbreviation: HV = Hippocampal volume
Controlled for baseline MoCA and experimental group
N=28
p<0.05
Δ calculated as trial completion value minus baseline value
Adverse Events
Results of the Chi Square test indicated no significant between-group differences (p=0.54) in the proportion of participants reporting adverse events. Specifically, one episode of shortness of breath that was resolved with rest was recorded for the RT group, two non-injuries falls were recorded for the AT group, and two non-injuries falls and one episode of shortness of breath that resolved with rest were reported in the BAT group.
DISCUSSION
In 70 to 80 year-old community-dwelling women with probable MCI, six months of twice-weekly aerobic training significantly increased the total hippocampus volume, relative to twice-weekly balance and toning exercises. We also found that increased left hippocampal volume was significantly associated with reduced verbal memory performance over the six-month intervention period. To our knowledge, our study is the first to examine the effect of exercise, both aerobic training and resistance training, on hippocampal volume in older women with probable MCI.
Our primary finding of the positive impact of aerobic training on hippocampal volume concurs and extends previous observations.17 Aerobic training may increase hippocampal volume by increasing levels of brain derived neurotrophic factor which stimulate neurogenesis and increase the complexity of the dendritic network. 17,36 Erickson and colleagues 17 demonstrated that 12 months of thrice-weekly aerobic training significantly increased both left and right hippocampal volume among healthy older adults. Notably, we observed a 5.6% volume increase in the left hippocampus, a 2.5% increase in the right hippocampus, and a 4% increase in total hippocampus. These volume changes exceed those observed by Erickson and colleagues, who reported a 2.12% increase in the left hippocampus and a 1.97% increase in the right hippocampus.17 The greater benefit observed in this study may relate to participant characteristics. First, our study included older adults with probable MCI while Erickson and colleagues 17 included cognitively-healthy older adults. Second, we included women only while both men and women were included in the study by Erickson and colleagues.17 Notably, a previous meta-analysis suggested that the cognitive benefits of aerobic training may be greater for women than men.19 Importantly, this sex-difference in cognitive response to aerobic training has been demonstrated among older adults with MCI.37 It is possible this sex-difference extends to exercise-induced changes in brain structure.
Our finding that increased left hippocampal volume was significantly associated with greater loss after interference over the six-month intervention period was unexpected. In the same study sample, we previously demonstrated that aerobic training significantly reduced loss after interference as measured by the RAVLT.18 It has been hypothesized that the left hippocampus is more involved in episodic or autobiographical memory while the right hippocampus is implicated in spatial memory.38 Our current and previously reported results correspond with these hypothesized hemispheric differences. Thus, we had expected to observe that increased left hippocampal volume to be significantly associated with better retention of the 15-word list. However, Eyler and colleagues 39 recently concluded that a simple model of greater brain volumes result in better cognitive performance may not be accurate. Other factors such as white matter degeneration, may significantly moderate the association between brain volume and cognitive performance. Of particular relevance to our study, among individuals with MCI or AD, the connectivity between the hippocampus and other brain areas are greatly disrupted by white matter abnormalities 40. Thus, increasing hippocampus volume alone in these populations may not result in improved memory performance. Regardless, we are very cautious in our interpretation of this result and emphasize that this facet of the study, requires further investigation.
In contrast to aerobic training, resistance training did not have a significant effect on hippocampal volume. However, in a rat study, both aerobic training and resistance training made positive impacts on the hippocampus and hippocampus-dependent memory performance, but through divergent pathways.36 As our study is the first to explore the effect of resistance training on hippocampal volume and hippocampal-dependent memory in older adults with MCI, future studies are needed to further elucidate these relationships.
The conclusions of our proof-of-concept randomized controlled trial are tempered by our exclusion of men and those older or younger than 70–80 years old. Future studies with larger sample sizes and greater in-depth characterization of MCI subtypes (i.e., single- versus multi-domain MCI) are needed to corroborate our current findings and to extend our understanding of the role of exercise training in promoting cognitive and neural brain plasticity in older adults with MCI.
Conclusions and future recommendations
The clinical entity of MCI represents a vital opportunity for intervening and altering the trajectory of both cognitive decline and loss of functional independence. Our proof-of-concept study suggests that aerobic training may be effective in maintaining hippocampal volume in this high-risk population. Given the growing evidence that exercise is beneficial for both cognitive and brain health, physical activity should be a standard recommendation for all older adults regardless of cognitive status.
What are the new findings?
With respect to dementia risk, the hippocampus is a brain structure of intense interest. We demonstrated that six months of aerobic training significantly increased hippocampal volume in older women with probable mild cognitive impairment (MCI).
Increased hippocampal volume was associated with reduced verbal learning and memory performance.
Exercise appears to be an effective intervention strategy to combat cognitive decline in those with MCI.
How might it impact on clinical practice in the near future?
Cognitive decline is a major health care and societal issue; MCI represents a critical period to intervene against dementia.
Exercise is a low-risk and cost-effective intervention strategy.
Our research suggests that twice-weekly aerobic exercise can positively impact hippocampal volume – a structure essential for memory and yet, sensitive to both aging and neurodegeneration. Therefore, we recommend that exercise be implemented at a population-level to combat cognitive decline
Acknowledgments
Funding for this study was provided to T.L. Ambrose by The Pacific Alzheimer’s Research Foundation. Teresa Liu-Ambrose is a Canada Research Chair in Physical Activity, Mobility, and Cognitive Neuroscience. Niousha Bolandzadeh is a HSFC Doctoral trainee. Lindsay Nagamatsu is a MSFHR Senior Graduate trainee and a Natural Sciences and Engineering Research Council of Canada Doctoral trainee. Chun Liang Hsu is an Alzheimer’s Society Research Program Doctoral trainee. Jennifer Davis is a MSFHR and a CIHR Post-Doctoral Fellow. Funding for Lisanne ten Brinke was provided by University Maastricht and the Dutch Alzheimer Association (Alzheimer Nederland).
Footnotes
Contributorship Statements
TLA: Study concept and design, acquisition of data, analysis and interpretation of data, preparation of manuscript, and critical review of manuscript. CLH, LN, JD: Acquisition of data, interpretation of data, preparation of manuscript, and critical review of the manuscript. LTB and NB: Analysis and interpretation of data, preparation of manuscript, and critical review of the manuscript.
Competing Interests
There was no question of competing interests.
References
- 1.World Health Organization, Alzheimer’s Disease International. Dementia: A Public Health Authority. 2012. [Google Scholar]
- 2.Brookmeyer R, Johnson E, Ziegler-Graham K, et al. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s and Dementia. 2007;3:186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 3.Feldman HH, Jacova C. Mild cognitive impairment. Am J Geriatr Psychiatry. 2005;13:645–55. doi: 10.1176/appi.ajgp.13.8.645. [DOI] [PubMed] [Google Scholar]
- 4.Burns A, Zaudig M. Mild cognitive impairment in older people. Lancet. 2002;360:1963–5. doi: 10.1016/S0140-6736(02)11920-9. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 6.Busse A, Bischkopf J, Riedel-Heller SG, et al. Mild cognitive impairment: prevalence and incidence according to different diagnostic criteria. Results of the Leipzig Longitudinal Study of the Aged (LEILA75+) Br J Psychiatry. 2003;182:449–54. [PubMed] [Google Scholar]
- 7.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Archives of neurology. 2001;58:1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 8.Liu-Ambrose T, Nagamatsu LS, Graf P, et al. Resistance training and executive functions: a 12-month randomized controlled trial. Archives of internal medicine. 2010;170:170–8. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu-Ambrose T, Nagamatsu LS, Voss MW, et al. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiology of aging. 2012;33:1690–98. doi: 10.1016/j.neurobiolaging.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–21. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassilhas RC, Viana VA, Grassmann V, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39:1401–07. doi: 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- 12.Nagamatsu LS, Handy TC, Hsu CL, et al. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Archives of internal medicine. 2012;172:666–8. doi: 10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagamatsu LS, Chan A, Davis JC, et al. Physical activity improves verbal and spatial memory in older adults with probable mild cognitive impairment: a 6-month randomized controlled trial. Journal of aging research. 2013;2013:861893. doi: 10.1155/2013/861893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golomb J, de Leon MJ, Kluger A, et al. Hippocampal atrophy in normal aging. An association with recent memory impairment. Archives of neurology. 1993;50:967–73. doi: 10.1001/archneur.1993.00540090066012. [DOI] [PubMed] [Google Scholar]
- 15.Mungas D, Harvey D, Reed BR, et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–71. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR, Jr, Wiste HJ, Vemuri P, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133:3336–48. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagamatsu LS, Chan A, Davis JC, et al. Physical activity improves verbal and spatial memory in older adults with probable mild cognitive impairment: a 6-month randomized controlled trial. Journal of aging research. 2013;2013:861893. doi: 10.1155/2013/861893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 22.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–37. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 23.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 24.Washburn RA, McAuley E, Katula J, et al. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–51. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 25.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–11. [PubMed] [Google Scholar]
- 26.Groll DL, To T, Bombardier C, et al. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Patenaude B, Smith SM, Kennedy DN, et al. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–22. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lezak MD. Neuropsychological Assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- 29.Liu-Ambrose T, Donaldson MG, Ahamed Y, et al. Otago home-based strength and balance retraining improves executive functioning in older fallers: a randomized controlled trial. J Am Geriatr Soc. 2008;56:1821–30. doi: 10.1111/j.1532-5415.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 30.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–81. [PubMed] [Google Scholar]
- 31.Persinger R, Foster C, Gibson M, et al. Consistency of the talk test for exercise prescription. Med Sci Sports Exerc. 2004;36:1632–6. [PubMed] [Google Scholar]
- 32.Foster C, Porcari JP, Anderson J, et al. The talk test as a marker of exercise training intensity. J Cardiopulm Rehabil Prev. 2008;28:24–30. doi: 10.1097/01.HCR.0000311504.41775.78. [DOI] [PubMed] [Google Scholar]
- 33.ICH Expert Working Group. ICH Harmonised Tripartite Guideline: Statistical Principals in Clinical Trials. Statistics in Medicine. 1999;18:1905–42. [PubMed] [Google Scholar]
- 34.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. 1995;332:556–62. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123:387–98. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 36.Cassilhas RC, Lee KS, Fernandes J, et al. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–17. doi: 10.1016/j.neuroscience.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 37.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–9. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–41. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 39.Eyler LT, Sherzai A, Kaup AR, et al. A review of functional brain imaging correlates of successful cognitive aging. Biological psychiatry. 2011;70:115–22. doi: 10.1016/j.biopsych.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowley J, Fonov V, Wu O, et al. White matter abnormalities and structural hippocampal disconnections in amnestic mild cognitive impairment and Alzheimer’s disease. PLoS One. 2013;8:e74776. doi: 10.1371/journal.pone.0074776. [DOI] [PMC free article] [PubMed] [Google Scholar]