Abstract
Background
Inflammation is linked to cognitive decline in midlife, but the neural basis for this link is unclear. One possibility is that inflammation associates with adverse changes in brain morphology, which accelerates cognitive aging and later dementia risk. Clear evidence is lacking, however, regarding whether inflammation relates to cognition in midlife via changes in brain morphology. Accordingly, the current study examines whether associations of inflammation with cognitive function are mediated by variation in cortical gray matter volume among midlife adults.
Methods
Plasma levels of interleukin (IL)-6 and C-reactive protein (CRP), relatively stable markers of peripheral systemic inflammation, were assessed in 408 community volunteers aged 30–54 years. All participants underwent structural neuroimaging to assess global and regional brain morphology and completed neuropsychological tests sensitive to early changes in cognitive function. Measurements of brain morphology (regional tissue volumes and cortical thickness and surface area) were derived using Freesurfer.
Results
Higher peripheral inflammation was associated with poorer spatial reasoning, short term memory, verbal proficiency, learning and memory, and executive function, as well as lower cortical gray and white matter volumes, hippocampal volume and cortical surface area. Mediation models with age, sex and intracranial volume as covariates showed cortical gray matter volume to partially mediate the association of inflammation with cognitive performance. Exploratory analyses of body mass suggested that adiposity may be a source of the inflammation linking brain morphology to cognition.
Conclusions
Inflammation and adiposity might relate to cognitive decline via influences on brain morphology.
Keywords: Inflammation, interleukin-6, C-reactive protein, gray matter volume, cognitive decline, brain atrophy, cognitive aging
INTRODUCTION
Aging engenders declines in multiple cognitive domains, including episodic and working memory, attention, and executive functioning (Salthouse, 2004). Typically, these cognitive declines begin in the late 20s and progress across adulthood (Salthouse, 2004). Trajectories of cognitive aging are heterogeneous, however, with some individuals showing minimal declines and others more precipitous deterioration (Raz et al., 2005). Understanding factors that account for individual differences in cognitive aging is critical because age-related impairments in cognitive function confer risk for dementia (Barberger-Gateau et al., 1999; De Lepeleire et al., 2004), injuries (Sattin, 1992), hospitalization, and death (Bennett, 1997).
Preclinical changes in brain morphology precede and may contribute to cognitive declines among healthy aging individuals, as well as predict risk for dementia (Burgmans et al., 2009; den Heijer et al., 2006; Driscoll et al., 2009; Kramer et al., 2007; Persson et al., 2012; Raz et al., 2005; Walhovd et al., 2011). These changes include reductions in total brain and gray matter volume and in white matter integrity that accompany advancing age (Burgmans et al., 2009; DeCarli et al., 2005; Fjell et al., 2013; Kennedy and Raz, 2009; Raji et al., 2012; Raz et al., 2005; Ryan et al., 2011). On average, reductions in gray matter volume begin around age 35 and accelerate thereafter (Fjell et al., 2013; Hedman et al., 2012), associating with concomitant and future declines in cognitive function (Burgmans et al., 2009; Fjell and Walhovd, 2010; Kramer et al., 2007). Individuals differ in rates of age-related brain atrophy, with accelerated gray matter loss associated with earlier progression to dementia (Whitwell et al., 2008). Thus, gray matter atrophy represents a midlife preclinical predictor of cognitive aging. At present, however, the biological bases for early deterioration of global and regional gray matter volumes remain unclear. On a cellular level, explanations for brain atrophy in aging are manifold and include apoptosis, axonal degeneration, reduced dendritic spine density, dendritic simplification, cell shrinkage, and reduced vascularity (von Bohlen und Halbach, 2010). However, factors that affect these cellular changes and consequent changes in gross morphology remain to be determined. In this regard, recent evidence suggests that inflammation may play a critical role.
Peripheral inflammatory mediators (e.g., interleukin (IL)-6) can cross the blood-brain barrier to modulate central inflammatory processes that result in neurodegeneration and impair cognitive function (Arai et al., 2001; Ek et al., 2001; Heyser et al., 1997; Monje et al., 2003; Poluektova et al., 2005; Pugh et al., 1998; Richwine et al., 2008; Trapero and Cauli, 2014; Yirmiya and Goshen, 2011). Within the brain, proinflammatory cytokines and their receptors are expressed by many cell subtypes throughout the cerebral cortex (Hampel et al., 2005). Chronic increases in peripheral inflammation, such as those that accompany aging, “prime” microglia to switch to an inflammatory phenotype, increasing central inflammatory responses to peripheral inflammation (Chen et al., 2008; Perry et al., 2007; Ye and Johnson, 1999) and possibly playing a pathogenic role in age-related neurocognitive decline. Human studies support this possibility, with increases in circulating mediators of inflammation, whether the result of exogenous administration or acute/chronic inflammatory conditions, relating inversely to disturbances of, attention, memory, executive and global cognitive function (Bucks et al., 2008; Capuron et al., 1999; Kozora et al., 2001; Krabbe et al., 2005; Reichenberg et al., 2001; Smith et al., 1988). Poorer cognitive function also associates with peripheral inflammation among the wellfunctioning elderly (Marioni et al., 2009; Rafnsson et al., 2007; Schram et al., 2007; Weaver et al., 2002; Wright et al., 2006; Yaffe et al., 2003), with higher levels predicting future cognitive decline in some (Marioni et al., 2009; Rafnsson et al., 2007; Schram et al., 2007; Tilvis et al., 2004; Weaver et al., 2002; Yaffe et al., 2003), but not all studies (Alley et al., 2008; Dik et al., 2005; Teunissen et al., 2003). We have extended this evidence previously by showing an inverse association of circulating IL-6 with performance on memory and executive function tasks among cognitively normal adults of mean age 43 years (Marsland et al., 2006), raising the possibility that systemic inflammation represents an early marker of cognitive risk.
Findings from studies of healthy older adults show inverse associations of markers of systemic inflammation with aspects of brain morphology that decline with age, including total brain volume (Jefferson et al., 2007), total gray matter volume (Satizabal et al., 2012), temporal lobe volume (Bettcher et al., 2012; Taki et al., 2013), hippocampal volume (Satizabal et al., 2012) and white matter integrity (Wersching et al., 2010). Our earlier work extended these findings to a midlife community sample, showing associations of higher IL-6 and CRP with lower total gray matter volume and white matter integrity throughout the brain, and with lower regional gray matter volume of the hippocampus and PFC (Gianaros et al., 2013; Marsland et al., 2008; Verstynen et al., 2013). However, it is still unknown whether these global or regional aspects of brain structure provide a plausible pathway linking inflammation to preclinical neurocognitive decline among midlife adults.
Accordingly, the goals of this study were (1) to confirm inverse associations of markers of systemic inflammation (IL-6 and CRP) with a broad range of cognitive functions and with global and regional measures of brain structure among midlife adults and (2) to extend our prior work by examining whether global or regional measures of gray matter volume statistically mediate associations between inflammation and cognitive function. In regard to brain structure, our early work was informed by animal models and focused on relationships between inflammation and the structure and function of the hippocampus (Marsland et al., 2008). More recently, however, we have found inflammation associated with global aspects of brain morphology as well (Gianaros et al., 2013; Verstynen et al., 2013). In the current study we explored two possibilities. One is that inflammatory associations are localized to specific brain regions; the other is that they are global. Finally, in light of evidence that adipocytes are a primary source of circulating IL-6 (Mohamed-Ali et al., 1997) and that body fat covaries positively with IL-6 (Bermudez et al., 2002; Khaodhiar et al., 2004) and inversely with gray matter volume (Marsland et al., 2008; Taki, 2008), cognitive function (Marsland et al., 2006), and accelerated cognitive aging (Ho et al., 2011), we explored whether inflammation accounts for associations of BMI with brain atrophy and associated cognitive performance.
METHODS AND MATERIALS
Participants
Participants were 408 adults 30–54 years of age drawn from the Adult Health and Behavior Project – Phase 2 (AHAB-II) project (see Table 1). AHAB-II is an epidemiological registry of biobehavioral correlates of cardiovascular disease risk among midlife adults. Participants were recruited between 2008 and 2011 by mass-mail solicitation from Western Pennsylvania (principally Allegheny County). To be eligible, participants had to be in good general health and working at least 25 hours/week outside of the home (a substudy involving this cohort focused on occupational stress and CHD risk). Participants were excluded if they (a) had a history of cardiovascular disease, schizophrenia or bipolar disorder, chronic hepatitis, renal failure, major neurological disorder, chronic lung disease, or hypertension (BP ≥ 160/100 mm Hg); (b) reported drinking ≥ 35 portions of alcohol per week; (c) took fish-oil supplements (because of the requirements for another sub-study); (d) were prescribed insulin or glucocorticoid, anti-arrhythmic, antihypertensive, lipid-lowering, psychotropic, or prescription weight-loss medications; (e) were pregnant or lactating; (f) had less than 8th grade reading skills; or (g) were shift workers. Remaining exclusion criteria were claustrophobia, presence of medical devices, implants, or other metal objects in or on the body that could not be removed, tattooed eyeliners, or a body habitus prohibiting MR scanning.
Table 1.
Characteristics of the Sample (n = 408)
| Characteristic | Mean (SD) or % |
|---|---|
| Sex (%) | 47% male |
| Age (years) | 42.8 (7.3) |
| Race (%) | 83% white, 15% black |
| Education (years) | 17.0 (2.9) |
| BMI (kg/m2: mean +/− SD) | 26.7 (5.0) |
| Current smokers (%) | 15% |
| Alcohol Use (drinks/week) | 3.27 (4.7) |
| Systolic blood pressure | 115 (11) |
| IL-6 (pg/ml) | 1.10 (.97) |
| CRP (ng/ml) | 1.51 (1.86) |
BMI – body mass index; IL-6: interleukin-6; CRP: C-reactive protein
Of the 490 participants in AHAB-II, 448 had reliable measures of CRP and IL-6, excluding 8 individuals who did not have blood drawn, 36 taking medications known to impact immune function (e.g., cold medications/antihistamines) and 1 individual with rheumatoid arthritis. In addition, we excluded 9 individuals with CRP levels greater than 10 ng/ml, which is outside the normal range and suggests acute illness at the time of blood draw (Biasucci, 2004; Pearson et al., 2003). Of the remaining 439 participants, brain imaging data were available for 408. An initial examination of subjects included in the analyses (n = 408) versus those excluded (n = 82) revealed no systematic differences in age, sex, race, SBP, education, smoking status, current alcohol use, or cognitive performance. The investigation was approved by the Institutional Review Board of the University of Pittsburgh; all participants were paid for their participation.
Protocol and Measures
Measures for this study were circulating markers of inflammation, neuropsychological assessments of cognitive function, brain structural imaging data, body mass index (BMI), demographic characteristics, systolic blood pressure (SBP), and smoking status. Plasma levels of IL-6 and CRP were assessed from blood samples drawn between 7:30AM and 12:35PM (M = 9:16AM+/−0:54 min). Prior to the blood draw, participants were asked to fast for 8 hours, avoid vigorous exercise for 12 hours and alcohol for 24 hours, and refrain from using tobacco products that morning. The blood draw was rescheduled if the participant reported symptoms of acute infection or use of antibiotics or antivirals in the previous 2 weeks. Plasma samples were frozen at −80°C until analysis in batches. IL-6 levels were determined in duplicate by high sensitivity quantitative sandwich enzyme immunoassay kit (R & D Systems, Minneapolis, MN) run according to manufacturer’s directions. CRP was measured at the University of Vermont’s Laboratory of Clinical Biochemistry Research with the BNII nephelometer from Dade Behring utilizing a particle enhanced immunonephelometric assay. Average inter- and intra- assay coefficient of variation was <10%. Natural-log transformation was applied to IL-6 and CRP to correct non-normal distributions. Because plasma IL-6 and CRP were correlated (r = .48), a composite measure of systemic inflammation was calculated for each participant by averaging Z-transformed values. Primary analyses employed the composite marker of inflammation; however, correlations of each circulating marker with cognitive and structural brain measures are also presented. There was no significant association of time of blood draw with levels of either inflammatory marker or the composite measure.
Assessment of Cognitive Function
Neuropsychological tests were selected on the basis of their ability to assess domains of cognitive functioning known to decline with age, including (1) spatial reasoning (Block Design and Matrix Reasoning subtests from the Wechsler Abbreviated Scale of Intelligence (WASI) (Coorporation, 1999; Wechsler, 1997a)), (2) working memory (Digit Span subtest from the Wechsler Adult Intelligence Scale – III (Wechsler, 1997a)) and the Spatial Span subtest from the Wechsler Memory Scale-III (Wechsler, 1997b)), (3) visuomotor processing speed (Part A of the Trail Making Test (Reitan, 1985), and the Stroop Color-Word Test (Golden, 1978)), (4) verbal proficiency (Vocabulary and Similarities subtests from the WASI), (5) verbal learning and memory (Four Word Short-Term Memory Test (Kobayashi et al., 2010) and the Rey Verbal Learning Test (Rey, 1958)), and (6) executive function (the Trail Making Test (Reitan, 1985) and the Stroop Color-Word Test (Golden, 1978)). See Supplementary Material for details regarding neuropsychological testing. To derive a single measure for each cognitive function, principal components analyses with varimax rotation were conducted on the subtests within each domain. In each case, the subtests loaded on a single factor (See Table 2 for factor loadings, Eigenvalues ranged from 1.64–1.97 and percentage of variance explained from 58–83%). For analysis, we created a factor scale for each domain, calculated as the unit-weighted averages of standardized subtest scores.
Table 2.
Partial correlations between inflammation and performance on 6 domains of cognitive function covarying age and sex. False discovery rate (FDR) is computed for multiple comparison correction.
| Subtests | Factor Analysis | Inflammation | IL-6 | CRP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor Loading |
Variance explained |
r | p | FDR | n | r | p | FDR | n | r | p | FDR | n | ||
| Spatial Reasoning |
Block Design Matrix | .91 | |||||||||||||
| reasoning | .91 | 82% | −0.19 | <.001 | 0.006 | 404 | −0.18 | <.001 | 0.006 | 402 | −0.14 | 0.004 | 0.01 | 404 | |
| Processing Speed |
Stroop -Part 1 | .87 | |||||||||||||
| Stroop - Part 2 | .88 | ||||||||||||||
| Trail Making - Part A | −.63 | 64% | −0.05 | 0.33 | 0.32 | 365 | −0.02 | 0.66 | 0.66 | 363 | −0.07 | 0.19 | 0.2 | 365 | |
| Short Term Memory |
Spatial Span - forward | .77 | |||||||||||||
| Spatial span - back | .70 | ||||||||||||||
| Digit span- forward | .75 | ||||||||||||||
| Digit Span-back | .82 | 58% | −0.14 | 0.008 | 0.012 | 375 | −0.14 | 0.01 | 0.01 | 373 | −0.09 | 0.08 | 0.08 | 375 | |
| Verbal Proficiency |
Vocabulary | .91 | |||||||||||||
| Similarities | .91 | 83% | −0.22 | <.001 | 0.006 | 404 | −0.20 | <.001 | 0.006 | 402 | −0.16 | 0.001 | 0.004 | 404 | |
| Verbal learning and memory |
Four Word Memory | .82 | |||||||||||||
| Rey - Trial A7 | .80 | ||||||||||||||
| Rey - Trial B1 | .80 | 66% | −0.15 | 0.004 | 0.008 | 372 | −0.11 | 0.03 | 0.04 | 370 | −0.14 | 0.007 | 0.009 | 372 | |
| Executive Function |
Stroop Color-Word | .89 | |||||||||||||
| Stroop Interference | .89 | ||||||||||||||
| Trail Making- Part B- A | −.53 | 62% | −0.11 | 0.04 | 0.05 | 364 | −0.06 | 0.23 | 0.28 | 362 | −0.12 | 0.025 | 0.025 | 364 | |
Magnetic Resonance Imaging
High resolution T1-weighted structural MRI data were collected using magnetization-prepared rapid gradient echo sequence (MPRAGE) on a 3T Trio TIM scanner (Siemens, Erlangen, Germany) equipped with a 12-channel head coil. MRI data were preprocessed and analyzed with methods detailed in Supplementary Material. The FreeSurfer 5.3.0 software package (http://surfer.nmr.mgh.harvard.edu) was used to measure cortical surface area, cortical thickness, and volumetric data (Fischl and Dale, 2000). Total volumes of cortical lobes were computed as the sum of cortical regions compromising each lobe for the left and right hemispheres (See Supplementary Material).
Control Variables
A number of variables were considered that might contribute to associations between inflammation, brain structure and neurocognitive function. These included age, sex, race (coded as Caucasian [1] and Other [2]), SBP, years of education, smoking status (coded as current smoker [1] versus ex/non-smoker[0]) and current alcohol use (number of alcoholic drinks in the past 7 days). Ex-smoking was defined as not current smoker, but a lifetime history of smoking > 100 cigarettes. Finally, height and weight were recorded to calculate BMI (weight (kg)/(height (m))
Data Analysis
All analyses were performed using SPSS for Windows (version 21). Partial correlation analyses controlling for sex, age, and, in the case of brain structure, intracranial volume (ICV) tested associations of inflammation with domains of cognitive function and with global (total and cortical gray and white matter volumes) and regional (gray matter volumes of the 4 cortical lobes and of subcortical structures) measures of brain structure. In addition, the use of Freesurfer permitted us to conduct exploratory analyses parsing the contributions of cortical surface area and thickness. To control for multiple testing, all correlations were subjected to correction for false discovery rate.
Path analyses tested whether observed relationships of inflammation with cognitive function were statistically mediated by variation in gray matter volume. Here, we first examined associations of structural measures with cognitive functions in analyses that controlled for age, sex, and ICV. Next, mediation analyses were conducted using a product-of-coefficient regression approach with nonparametric bootstrapping (5000 iterations) to attain 95% confidence intervals (CIs) for mediation effects (Preacher and Hayes, 2008). This bootstrapped mediation analysis approach was performed with the PROCESS v2.13 macro designed for SPSS (Preacher and Hayes, 2008). See Supplementary Material for details of path models. In these analyses, the total gray matter volumes of all four lobes were treated as mediators in the same model (i.e., they were entered simultaneously for multiple mediator modeling). This method enabled the examination of whether the total effect and/or the volumes of the four cortical lobes uniquely accounted for (mediated) the associations of inflammation with cognitive function. Finally, exploratory analyses tested whether race, SBP, education, smoking status, and BMI contributed to an indirect pathway linking inflammation to cognitive function via gray matter volume. We also conducted exploratory analyses examining associations of BMI with cognitive function and gray matter volume to test the possible role of inflammation as a statistical mediator.
RESULTS
Inflammation and Cognitive Performance
In analyses that controlled for age and sex, inflammation associated inversely with performance on tests of spatial reasoning, short-term memory, verbal proficiency, verbal learning and memory, and executive function (Table 2; Figure 1). In all cases except executive function, significant associations were retained following control for multiple testing.
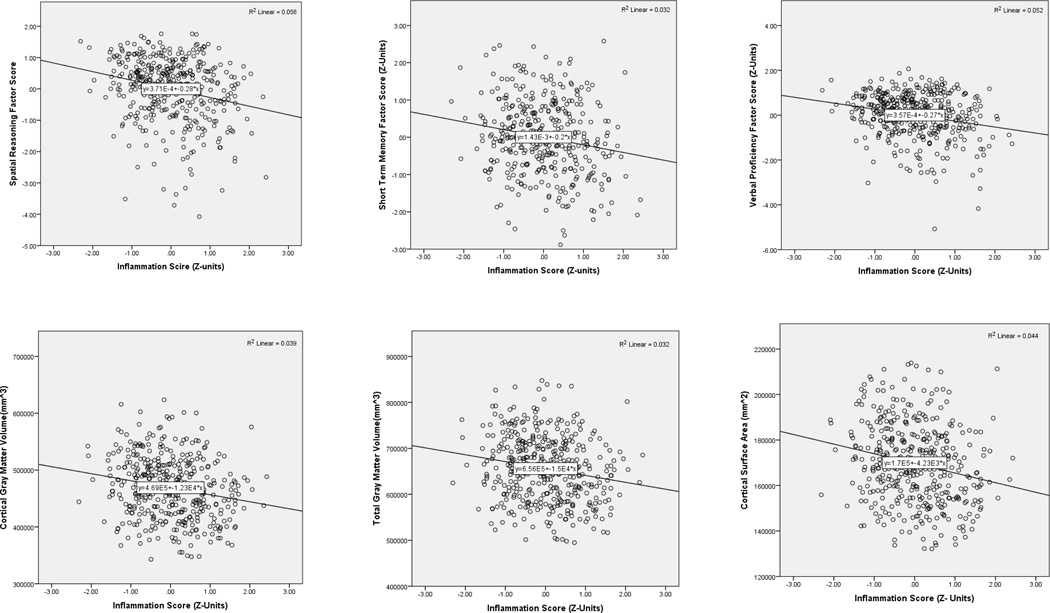
Figure 1.
Scatterplots showing bivariate associations of the Inflammation Score (mean circulating IL-6 and CRP in Z-units) with factor scores on tests of spatial reasoning, short term memory and verbal proficiency (N’s = 375–404)
Inflammation and Brain Morphology
As expected, inflammation associated inversely with total gray and cortical gray and white matter volumes and total volumes of all four cortical lobes in analyses that controlled for age, sex, and ICV (see Table 3). There was no evidence for regional specificity at this level of examination (cortical lobes). However, exploratory analyses that controlled for age, sex, and ICV revealed some evidence of specificity among subcortical structures, with inflammation relating inversely to volume of the hippocampus, pallidum and thalamus, but not other regions, such as the amygdala (Supplemental Table 1). Inflammation also associated with total cortical surface area (r = −.19, pFDR-corrected<.01) and surface areas of the frontal, occipital, parietal, and temporal lobes (r’s = −.13 to −.19, p’sFDR-corrected < 01). There were no significant associations of inflammatory markers with measures of cortical thickness.
Table 3.
Partial correlations between total volumes of cerebral cortex and inflammation measures covarying age, sex, and intracranial volume. False discovery rate (FDR) is computed for multiple comparison correction.
| Inflammatory Scores N=403 |
IL-6 N=401 |
CRP N=403 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Volume | r | p | FDR | r | p | FDR | r | p | FDR |
| Total gray matter | −.15 | .002 | .005 | −.10 | .04 | .05 | −.16 | .001 | .007 |
| Cortical gray matter | −.17 | .001 | .007 | −.12 | .02 | .06 | −.18 | <.001 | .007 |
| Cortical white matter | −.18 | <.001 | .007 | −.12 | .02 | .05 | −.19 | <.001 | .007 |
| Frontal Lobe | −.15 | .003 | .004 | −.08 | .10 | .12 | −.17 | <.001 | .007 |
| Occipital lobe | −.10 | .04 | .04 | −.07 | .16 | .16 | −.11 | .02 | .02 |
| Parietal lobe | −.16 | .002 | .005 | −.13 | .008 | .06 | −.14 | .005 | .006 |
| Temporal lobe | −.15 | .002 | .005 | −.11 | .03 | .05 | −.16 | .001 | .007 |
Does Brain Structure Mediate the Association of Inflammation with Cognitive function?
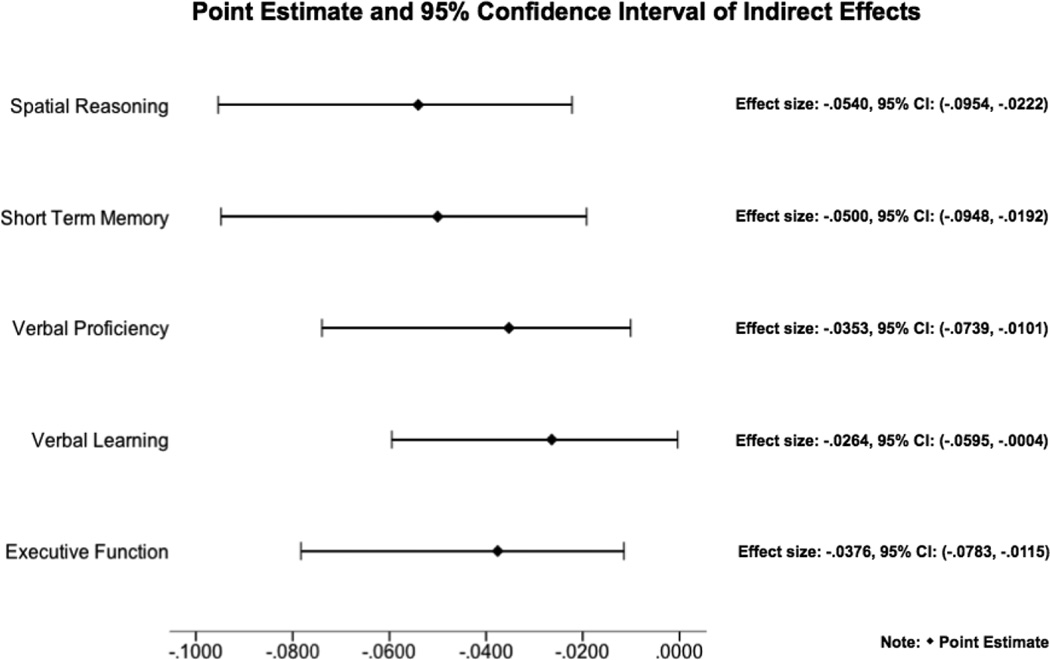
Results supported testing global and regional gray matter volumes as mediators linking inflammation to poorer spatial reasoning, short-term memory, verbal proficiency, verbal learning, and executive function. In analyses that controlled for age, sex, and ICV, total and cortical gray matter volumes associated positively with all of the inflammation-related cognitive functions (Table 4). Mediation models showed a significant total indirect effect, with the combined effects of the 4 cortical lobes partially mediating the association of inflammation with all of the examined cognitive functions (See Figure 2 for ab indirect effect sizes and confidence intervals; all p’s < .05). On analysis of spatial reasoning, verbal proficiency and verbal learning/memory only, mediation analyses also revealed a significant association of inflammation with cognitive function (direct c’ paths) that was independent of the volumes of the four cortical lobes (indirect ab paths) (spatial reasoning direct effect = −.14, p = .007; verbal proficiency direct effect = −.20, p <.001, and verbal learning direct effect = −.14, p = .01). These findings suggest that inflammation relates to cognitive function in part – but not entirely – via its association with global cortical structure. An examination of the separate contributions of the 4 cortical lobes in the mediation analyses showed gray matter volume of the temporal lobe to be a significant independent mediator of associations between inflammation and spatial reasoning (ab indirect effect = −.03; SE = −.02; 95% CI = −.07, −.004; p <.05) and short term memory (ab indirect effect = −.04; SE = −.02; 95% CI = −.08, −.01; p <.05) in models that controlled for volumes of the other three cortical lobes, as well as age, sex and ICV. No other cortical lobes were significant independent mediators (Supplemental Table 2). Exploratory analyses were conducted to examine cortical white matter volume as a mediator linking inflammation to cognitive function. Preliminary analyses controlling for age, sex and ICV showed a significant association of cortical white matter with spatial reasoning and STM (Table 4). However, mediation models showed no significant indirect pathway, suggesting that white matter volume does not contribute to the association of inflammation with the assessed cognitive functions.
Table 4.
Partial correlations between brain volumes and cognitive domains covarying age, sex, and intracranial volume. False discovery rate (FDR) is computed for multiple comparison correction.
| Total Volume | Spatial Reasoning N = 403 |
STM N = 374 |
Verbal Proficiency N = 403 |
Verbal learn/memory N = 371 |
Executive Function N = 363 |
|---|---|---|---|---|---|
| Total gray matter | .259** | .238** | .189** | .147* | .179** |
| Cortical gray matter | .274** | .241** | .192** | .156* | .169** |
| Cortical white matter | .118* | .114* | .046 | −.027 | .105 |
| Frontal Lobe | .183** | .170** | .111* | .096 | .069 |
| Occipital lobe | .215** | .180** | .154** | .153* | .138* |
| Parietal lobe | .251** | .211** | .191** | .131* | .174** |
| Temporal lobe | .268** | .252** | .207** | .159* | .199** |
pFDR-corrected < .005,
pFDR-corrected < .05
Figure 2.
Point estimates and 95% confidence intervals of indirect effects from mediation analyses.
Ancillary Analyses exploring covariates
Results of correlational analyses examining covariates are presented in Table 5. After controlling for age and sex, greater inflammation associated with non-white race, fewer years of education, current smoking, and SBP. In general, cognitive performance associated positively with white race, more years of education, not smoking, and lower SBP. When examining brain morphology, partial correlations controlling for age, sex and ICV revealed no significant associations of education with global or regional gray or white matter volumes. In contrast, non-white race, higher SBP, and, to a lesser extent, current smoking, were associated with lower gray matter volumes. There were no significant associations of number of alcoholic drinks in the last 7 days with inflammation, cognitive performance or brain morphology.
Table 5.
Partial correlations of health covariates with inflammation, cognitive functions, and cortical gray matter volume after controlling for age and sex (and intracranial volume on analysis of gray matter volume). False discovery rate (FDR) is computed for multiple comparison correction (N’s = 364–404)
| Total Volume | Inflammation | Cortical gray matter volume |
Spatial Reasoning |
Short term memory |
Verbal Proficiency |
Verbal learn/memory |
Executive Function |
|---|---|---|---|---|---|---|---|
| Race 1 | −.150** | .239** | .450** | .368** | .420** | .278** | .269** |
| Education (years) | −.179** | .076 | .321** | .208** | .522** | .306** | .149* |
| Smoking status2 | .195** | −.099 | −.197** | −.026 | −.197** | −.155* | .008 |
| Alcohol (drinks/week) | −.032 | −.017 | .016 | .060 | .047 | .045 | .054 |
| Body mass index (kg/m2) | .443** | −.175** | −.209** | −.108 | −.220** | −.056 | −.140* |
| Blood pressure (mmHg) | .155** | −.133* | −.147** | −.071 | −.175** | .008 | −.139* |
pFDR-corrected < .005,
pFDR-corrected < .05
European American = 1, Other race = 2
1 = current smoker, 0 = former/ never smoker
Based on associations of covariates with cortical gray matter volume, mediation analyses were rerun including age, sex, ICV, race, smoking status and SBP as covariates. The pattern of results did not change on analysis of spatial reasoning or short term memory, with total gray matter volume (and volume of the temporal lobe) partially mediating the association of inflammation with cognitive performance (spatial reasoning indirect effect = −.02, SE = .01; 95% CI = −.05 and −.002; p <.05; Short term memory indirect effect = −.02, SE = .01, 95% CI = −.05 and −.0003, p < .05). On analysis of verbal proficiency and executive function, findings no longer supported total cortical gray matter volume as a mediator of the association of inflammation with cognitive function; however, volume of the temporal lobe continued as a significant indirect path after controlling for the 6 covariates and the volume of the other 3 cortical lobes. Finally, for verbal proficiency and verbal learning, the mediation analyses also revealed significant direct path effects (c’ paths), showing associations of inflammation with cognitive function after controlling for age, sex, ICV, race, smoking status, SBP and the volumes of the four cortical lobes corresponding to the indirect paths.
The Role of Body Mass
As expected, BMI related positively to inflammation and inversely to cortical gray matter volume and performance on all cognitive functions except verbal leaning (See Table 5). Hence, we reran the mediation analyses including age, sex, ICV and BMI as covariates. Results no longer supported cortical gray matter volume as a significant mediator of associations between inflammation and any of the cognitive functions. These findings suggest that the relationship of inflammation with cognitive function via cortical gray matter is largely related to variance in BMI.
To further examine covariance of BMI and inflammation, we conducted mediation analyses examining whether associations of BMI with cortical gray matter volume and cognitive function are mediated by inflammation. Results showed a significant indirect effect, with inflammation partially mediating the association of BMI with cortical gray matter volume after controlling for age, sex, and ICV (ab indirect effect size = −309.9, SE = 146.3; 95% CI −599 and − 31; p < .05). Furthermore, inflammation also mediated associations of BMI with spatial reasoning, verbal proficiency, and verbal learning (spatial reasoning indirect effect = −.01, SE = .005; 95% CI = −.02 and −.001; p <.05; verbal proficiency indirect effect = −.01, SE = .005; 95% CI = −.02 and −.003, p < .05; verbal learning indirect effect = −.01, SE = .005; 95% CI - −.03 and − .004). These results suggest that BMI relates to cognitive function in part via its positive association with inflammation. Exploratory analyses were conducted to examine whether associations of BMI with cortical white matter volume (r = −.12, p = .01) were also mediated by inflammation. Results showed a significant indirect effect with inflammation statistically mediating the association of BMI with cortical white matter volume after controlling for age, sex and ICV (ab indirect effect size = −549.5, SE = 202.0; 95% CI −951.2 and −170.4; p < .05).
DISCUSSION
The present study examined relationships of peripheral inflammation to (1) performance on cognitive tests assessing spatial reasoning, short term memory, verbal proficiency, verbal learning and memory, visuomotor speed, and executive function and (2) global and regional measures of brain morphology among a community sample of 408 adults aged 30–54 years. After controlling for age and sex, we replicated and extended our earlier findings (Marsland et al., 2006) showing inverse associations of inflammation with performance across all of the cognitive domains except visuomotor processing speed. These associations are consistent with findings from studies of older adults, showing IL-6 and CRP to associate inversely with cognitive performance (Marioni et al., 2009; Rafnsson et al., 2007; Schram et al., 2007; Tilvis et al., 2004). Based on the present findings and our earlier study (Marsland et al., 2006), systemic inflammation appears to account for 2–5% of the variance in cognitive function, net other explanatory factors (e.g., age and sex). Our findings show these relationships apparent even in midlife, a period when trajectories of cognitive aging that precede clinical impairment are also first noted (Hedman et al., 2012; Salthouse, 2004). This raises the possibility that inflammation contributes to the preclinical cognitive decline that accompanies normal midlife aging.
The current findings also show an inverse association of peripheral inflammation with aspects of brain morphology that atrophy with age and presage cognitive decline, including total gray, and cortical gray and white matter volumes and volumes of all four cortical lobes. Results do not provide strong evidence for regional specificity at the level of the cerebral cortex; however, an exploratory examination of the volume of subcortical structures revealed inverse associations of inflammation with volumes of the hippocampus, pallidum, and thalamus, but not with other regions, such as the amygdala. These findings also replicate our earlier work, showing inverse associations of inflammatory markers with both global measures of gray matter volume and white matter integrity, and with regional gray matter volume of the hippocampus among healthy midlife adults (Gianaros et al., 2013; Marsland et al., 2008; Verstynen et al., 2013). Recent evidence shows a similar relationship of IL-6 and CRP with total gray matter and hippocampal volumes among older adults (Bettcher et al., 2012; Satizabal et al., 2012; Taki et al., 2013). Preclinical declines in brain size are widely thought to begin in the third decade of life, contributing to the cognitive declines that accompany healthy aging and risk for dementia (Burgmans et al., 2009; den Heijer et al., 2006; Driscoll et al., 2009; Raz et al., 2005; Walhovd et al., 2011). Thus, the current findings raise the possibility that inflammation relates to midlife cognitive function in part via its inverse association with brain structure.
Our primary findings support associations of inflammation with global aspects of cortical morphology; however, exploratory analyses also raise the possibility of hippocampal and subcortical regional specificity. As noted above, we observed inverse associations of inflammation with volumes of the hippocampus, pallidum, and thalamus, but not with other regions. Prior work, including our own (Marsland et al., 2008), has focused on the hippocampus because of its marked vulnerability to ‘shrinkage’ in typical aging, its key role in memory function, and its systematic morphological associations with future memory decline in healthy aging and with cognitive impairment and dementia risk in later life (Driscoll et al., 2009). It is possible that the hippocampus is particularly susceptible to the effects of inflammation. Indeed, evidence shows that although proinflammatory cytokines and their receptors are expressed by astroglia, microglia, neurons and endothelial cells throughout the brain (Sawada et al., 1993), they are present at higher concentrations in the hippocampus and cortex (Gadient and Otten, 1994; Hampel et al., 2005; Schobitz et al., 1994; Vitkovic et al., 2000).These distributional patterns may foster both local (regional) and global (distributed) inflammatory effects. It is also possible that the Freesurfer automated image analysis does a better job of segmenting some subcortical structures than others, resulting in the observed inconsistent associations. The use of surface-based morphometry permitted a novel examination of whether cortical thickness and/or surface area contributed to the association of inflammation with cortical volume. Growing evidence shows that cortical thickness and surface are genetically and developmentally independent characteristics with distinct cellular mechanisms that combine to determine cortical volume(Panizzon et al., 2009). Neurons in the cerebral cortex are organized into ontogenetic columns that run perpendicular to the surface of the brain (Mountcastle, 1997). Cortical surface area is thought to reflect the number of these columns, whereas cortical thickness is influenced by the number of cells within the columns (Rakic, 1988). The current results suggest that the lower cortical volume that associates with midlife inflammation reflects less cortical surface area rather than a lesser cortical thickness. Reductions in cortical surface area have been shown to accompany normal brain aging (Dickerson et al., 2009; Hogstrom et al., 2013; Lemaitre et al., 2012), rather than pathological brain changes, which associate with reductions in cortical thickness rather than surface area (Dickerson et al., 2009). The cellular bases for age-related reductions in surface area remain unclear; however, it is proposed that they could result from loss of dendritic size and complexity and/or demyelination (Hogstrom et al., 2013; Lemaitre et al., 2012). The possibility that inflammation contributes to these or other histological changes that accompany early stages of normal brain aging warrants further investigation.
We employed mediation analyses to examine the possibility that inflammation relates to cognitive function in part via its association with brain structure. Results provide initial support for this possibility, with total cortical gray matter volume partially mediating the association of inflammation with spatial reasoning, short-term memory, verbal proficiency, verbal learning, and executive function in analyses that controlled for age, sex, and ICV. Findings also provide some—albeit circumscribed—support for regional specificity, with gray matter volume of the temporal lobe partially mediating associations of inflammation with spatial reasoning and shortterm memory. On examination, spatial reasoning and short-term memory associations were independent of age, sex, ICV, race, education, smoking status, and SBP. For verbal proficiency and executive function, mediation by total cortical gray matter volume lost significance with race, education, smoking status and SBP in the model; however, gray matter volume of the temporal lobe remained a significant independent mediator. Overall, the current findings suggest that low grade systemic inflammation contributes to preclinical cognitive decline via global or regional aspects of brain structure, with inflammation-related variation in brain morphology associating with midlife cognitive function. These findings may also help to explain the accelerated cognitive aging that accompanies pathologies that manifest with chronic systemic inflammation (e.g. (Kozora et al., 2001).
Three pathways have been identified by which peripheral proinflammatory cytokines can access the central nervous system (CNS): (1) cytokines can cross the blood brain barrier by active transport mechanisms in the choroid plexus and the circumventricular organs (Banks and Kastin, 1991; Trapero and Cauli, 2014); (2) cytokines can bind to their cognate receptors on endothelial cells within the brain microvasculature, stimulating a central response (Ek et al., 2001; Yirmiya and Goshen, 2011); and (3) cytokines can indirectly induce changes within the CNS via activation of vagal-nerve sensory afferents (McAfoose and Baune, 2009; Yirmiya and Goshen, 2011). Within the CNS, proinflammatory cytokines and their receptors are expressed by astroglia, microglia, neurons and endothelial cells throughout the brain, with the highest concentrations in the hippocampus and cerebral cortex (Hampel et al., 2005; Schobitz et al., 1994). When stimulated by proinflammatory cytokines, microglia adopt an inflammatory phenotype characterized by the production of high levels of TNF-α and IL-6 (Sparkman et al., 2006; Van Dam et al., 1995). Animal studies show this neuroinflammation to interfere with neurogenesis (Monje et al., 2003), synaptic plasticity (Poluektova et al., 2005; Tancredi et al., 2000), neurotransmission (Trapero and Cauli, 2014), and dendritic branching (Richwine et al., 2008), resulting in neurodegeneration (Campbell et al., 1997) and dendritic atrophy (Richwine et al., 2008) that is thought to contribute to brain atrophy and associated cognitive disturbances (Trapero and Cauli, 2014). One limitation of the current study is that we are unable to identify which of these mechanisms might be accounting for observed effects.
Adults recruited for the current study were relatively healthy and free from symptoms of infection at the time of testing, yet wide variation in markers of systemic inflammation were noted (range for IL-6 = .06 – 9.83 pg/ml and for CRP = .15 – 9.9 ng/ml). Consistent with existing literature (Kiecolt-Glaser et al., 2003; Krabbe et al., 2004), inflammatory markers related positively to age (IL-6: r = .19, p < .001; CRP: r = .12, p = .02). Variation within this subclinical range is known to predict risk for a range of age-associated diseases, including cardiovascular disease, type 2 diabetes, frailty and general functional decline (e.g.,(Ferrucci et al., 1999; Luc et al., 2003; Pradhan et al., 2001; Ridker et al., 2000a; Ridker et al., 2000b). The current findings raise the possibility that midlife inflammation may also be a marker of accelerated cognitive aging.
Many cells other than immune cells contribute to circulating levels of inflammatory mediators. Of particular note, adipose tissue is a potent source of circulating IL-6 (Mohamed-Ali et al., 1997). The current findings suggest that body fat is a source of the inflammation that associates with cortical gray and white matter volume and cognitive function. Consistent with our prior findings and those of others (Elias et al., 2003; Gunstad et al., 2006; Gunstad et al., 2007; Ho et al., 2011; Khaodhiar et al., 2004; Marsland et al., 2008; Marsland et al., 2006; Taki, 2008; Willette and Kapogiannis, 2014), here, BMI associated with greater inflammation, poorer cognitive function, and lower cortical gray and white matter volume. Furthermore, results of mediation analyses suggest that inverse associations of body mass with cognitive function may be secondary to inflammation-related decreases in cortical volume, raising the possibility that inflammation contributes to the poorer cognitive performance and accelerated cognitive decline that accompanies obesity (Ho et al., 2011; Willette and Kapogiannis, 2014). These associations may help to explain the accelerated cognitive aging that accompanies pathologies that manifest with chronic systemic inflammation.
These novel findings should be interpreted in context of recognized study limitations. First, the cross-sectional design precludes causal inferences and, in particular, whether peripheral inflammation predicts temporal decline in brain size and/or cognitive performance. It is conceivable that the greater systemic inflammation relating to brain atrophy to cognitive function derives from the CNS and is a marker of subclinical neuroinflammation (Licastro et al., 2000). It is also possible that inflammation is a marker of individual differences in cognitive ability and brain structure across the lifespan. Alternatively, observed associations could be independently related to a third factor, such as genetic vulnerability or related vascular or metabolic processes. Second, although evidence suggests that levels of systemic inflammation are relatively stable over extended periods (Alley et al., 2007; Knudsen et al., 2008; Rao et al., 1994), we employed only one assessment of systemic inflammation typical of epidemiological studies. Third, future work would benefit from the use of cognitive tests that engage discrete brain regions to shed further light on associations of inflammation with regional brain morphology and function. Although the current cognitive battery was relatively comprehensive, many of the employed tests engage multiple brain networks and we did not have a focused test of psychomotor reaction time and associated cortico-striatal circuit dysfunction, which have been demonstrated to relate to aging and inflammation (Brydon et al., 2008; Raz et al., 2003). Finally, in the ideal, future longitudinal investigations would commence in early adulthood and employ serial assessments of inflammation and brain morphology to examine predictors of future cognitive decline.
In sum, the current findings in a relatively large sample make a novel contribution to our understanding of inflammatory neurobiology of cognitive function and are consistent with the possibility that inflammation accelerates midlife cognitive decline in part via its influence on brain morphology.
Supplementary Material
Highlights.
Markers of inflammation associate inversely with cognitive functions among midlife adults
Markers of inflammation associate inversely with global cortical structure among midlife adults
Cortical gray matter volume partially mediates inflammation with cognitive function.
Adiposity may be one source of the inflammation linking brain morphology to cognition.
Inflammation might relate to cognitive decline via influences on brain morphology.
Acknowledgments
This work was supported by National institutes of Health grants PO1 HL040962 (SBM) and R01 HL089850 (PJG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alley DE, Crimmins E, Bandeen-Roche K, Guralnik J, Ferrucci L. Three-year change in inflammatory markers in elderly people and mortality: the Invecchiare in Chianti study. Journal of the American Geriatrics Society. 2007;55:1801–1807. doi: 10.1111/j.1532-5415.2007.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley DE, Crimmins EM, Karlamangla A, Hu P, Seeman TE. Inflammation and rate of cognitive change in high-functioning older adults. The journals of gerontology. Series A, Biological sciences and medical sciences. 2008;63:50–55. doi: 10.1093/gerona/63.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Matsuki N, Ikegaya Y, Nishiyama N. Deterioration of spatial learning performances in lipopolysaccharide-treated mice. Japanese journal of pharmacology. 2001;87:195–201. doi: 10.1254/jjp.87.195. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Blood to brain transport of interleukin links the immune and central nervous systems. Life sciences. 1991;48:PL117–PL121. doi: 10.1016/0024-3205(91)90385-o. [DOI] [PubMed] [Google Scholar]
- Barberger-Gateau P, Fabrigoule C, Helmer C, Rouch I, Dartigues JF. Functional impairment in instrumental activities of daily living: an early clinical sign of dementia? Journal of the American Geriatrics Society. 1999;47:456–462. doi: 10.1111/j.1532-5415.1999.tb07239.x. [DOI] [PubMed] [Google Scholar]
- Bennett KM. A longitudinal study of wellbeing in widowed women. International journal of geriatric psychiatry. 1997;12:61–66. doi: 10.1002/(sici)1099-1166(199701)12:1<61::aid-gps465>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arteriosclerosis, thrombosis, and vascular biology. 2002;22:1668–1673. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]
- Bettcher BM, Wilheim R, Rigby T, Green R, Miller JW, Racine CA, Yaffe K, Miller BL, Kramer JH. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain, behavior, and immunity. 2012;26:103–108. doi: 10.1016/j.bbi.2011.07.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasucci LM. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: clinical use of inflammatory markers in patients with cardiovascular diseases: a background paper. Circulation. 2004;110:e560–e567. doi: 10.1161/01.CIR.0000148983.88334.80. [DOI] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biological psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucks RS, Gidron Y, Harris P, Teeling J, Wesnes KA, Perry VH. Selective effects of upper respiratory tract infection on cognition, mood and emotion processing: a prospective study. Brain, behavior, and immunity. 2008;22:399–407. doi: 10.1016/j.bbi.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Burgmans S, van Boxtel MP, Smeets F, Vuurman EF, Gronenschild EH, Verhey FR, Uylings HB, Jolles J. Prefrontal cortex atrophy predicts dementia over a six-year period. Neurobiology of aging. 2009;30:1413–1419. doi: 10.1016/j.neurobiolaging.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Campbell IL, Stalder AK, Chiang CS, Bellinger R, Heyser CJ, Steffensen S, Masliah E, Powell HC, Gold LH, Henriksen SJ, Siggins GR. Transgenic models to assess the pathogenic actions of cytokines in the central nervous system. Molecular psychiatry. 1997;2:125–129. doi: 10.1038/sj.mp.4000225. [DOI] [PubMed] [Google Scholar]
- Capuron L, Lamarque D, Dantzer R, Goodall G. Attentional and mnemonic deficits associated with infectious disease in humans. Psychological medicine. 1999;29:291–297. doi: 10.1017/s0033291798007740. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain, behavior, and immunity. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorporation TP. Wechsler Abbreviated Scale if Intelligence (WASI) manual. San Antonio, TX: 1999. [Google Scholar]
- De Lepeleire J, Aertgeerts B, Umbach I, Pattyn P, Tamsin F, Nestor L, Krekelbergh F. The diagnostic value of IADL evaluation in the detection of dementia in general practice. Aging & mental health. 2004;8:52–57. doi: 10.1080/13607860310001613338. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D'Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiology of aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Geerlings MI, Hoebeek FE, Hofman A, Koudstaal PJ, Breteler MM. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Archives of general psychiatry. 2006;63:57–62. doi: 10.1001/archpsyc.63.1.57. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, Buckner RL. Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiology of aging. 2009;30:432–440. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64:1371–1377. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek M, Engblom D, Saha S, Blomqvist A, Jakobsson PJ, Ericsson-Dahlstrand A. Inflammatory response: pathway across the blood-brain barrier. Nature. 2001;410:430–431. doi: 10.1038/35068632. [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. Journal of the American Geriatrics Society. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Reviews in the neurosciences. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Holland D, Dale AM, Walhovd KB. Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiology of aging. 2013;34:2239–2247. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadient RA, Otten U. Expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat brain during postnatal development. Brain research. 1994;637:10–14. doi: 10.1016/0006-8993(94)91211-4. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD. Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cereb Cortex. 2013;23:2058–2071. doi: 10.1093/cercor/bhs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JC. Stroop color and word test. Chicago, IL: 1978. [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Gordon E. Obesity is associated with memory deficits in young and middle-aged adults. Eating and weight disorders : EWD. 2006;11:e15–e19. doi: 10.1007/BF03327747. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Hampel H, Haslinger A, Scheloske M, Padberg F, Fischer P, Unger J, Teipel SJ, Neumann M, Rosenberg C, Oshida R, Hulette C, Pongratz D, Ewers M, Kretzschmar HA, Moller HJ. Pattern of interleukin-6 receptor complex immunoreactivity between cortical regions of rapid autopsy normal and Alzheimer's disease brain. European archives of psychiatry and clinical neuroscience. 2005;255:269–278. doi: 10.1007/s00406-004-0558-2. [DOI] [PubMed] [Google Scholar]
- Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Human brain mapping. 2012;33:1987–2002. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, Dinov ID, Stein JL, Rosano C, Toga AW, Thompson PM. The effects of physical activity, education, and body mass index on the aging brain. Human brain mapping. 2011;32:1371–1382. doi: 10.1002/hbm.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogstrom LJ, Westlye LT, Walhovd KB, Fjell AM. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cereb Cortex. 2013;23:2521–2530. doi: 10.1093/cercor/bhs231. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Massaro JM, Wolf PA, Seshadri S, Au R, Vasan RS, Larson MG, Meigs JB, Keaney JF, Jr, Lipinska I, Kathiresan S, Benjamin EJ, DeCarli C. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology. 2007;68:1032–1038. doi: 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN. Journal of parenteral and enteral nutrition. 2004;28:410–415. doi: 10.1177/0148607104028006410. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen LS, Christensen IJ, Lottenburger T, Svendsen MN, Nielsen HJ, Nielsen L, Horslev-Petersen K, Jensen JE, Kollerup G, Johansen JS. Pre-analytical and biological variability in circulating interleukin 6 in healthy subjects and patients with rheumatoid arthritis. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2008;13:59–78. doi: 10.1080/13547500701615017. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Nakano K, Tago H, Niwa S. Development of a simplified Short-Term Memory recall Test (STMT) and its clinical evaluation. Aging clinical and experimental research. 2010;22:157–163. doi: 10.1007/BF03324790. [DOI] [PubMed] [Google Scholar]
- Kozora E, Laudenslager M, Lemieux A, West SG. Inflammatory and hormonal measures predict neuropsychological functioning in systemic lupus erythematosus and rheumatoid arthritis patients. Journal of the International Neuropsychological Society : JINS. 2001;7:745–754. doi: 10.1017/s1355617701766106. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Experimental gerontology. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Reichenberg A, Yirmiya R, Smed A, Pedersen BK, Bruunsgaard H. Low-dose endotoxemia and human neuropsychological functions. Brain, behavior, and immunity. 2005;19:453–460. doi: 10.1016/j.bbi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weiner MW, Chui HC. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiology of aging. 2012;33:617, e611–e619. doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, Casadei V, Grimaldi LM. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer's disease: peripheral inflammation or signals from the brain? Journal of neuroimmunology. 2000;103:97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, Arveiler D, Fruchart JC, Ducimetiere P. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:1255–1261. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- Marioni RE, Stewart MC, Murray GD, Deary IJ, Fowkes FG, Lowe GD, Rumley A, Price JF. Peripheral levels of fibrinogen, C-reactive protein, and plasma viscosity predict future cognitive decline in individuals without dementia. Psychosomatic medicine. 2009;71:901–906. doi: 10.1097/PSY.0b013e3181b1e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biological psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Petersen KL, Sathanoori R, Muldoon MF, Neumann SA, Ryan C, Flory JD, Manuck SB. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosomatic medicine. 2006;68:895–903. doi: 10.1097/01.psy.0000238451.22174.92. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neuroscience and biobehavioral reviews. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. The Journal of clinical endocrinology and metabolism. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain : a journal of neurology. 1997;120(Pt 4):701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nature reviews. Immunology. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Persson J, Pudas S, Lind J, Kauppi K, Nilsson LG, Nyberg L. Longitudinal structure-function correlates in elderly reveal MTL dysfunction with cognitive decline. Cereb Cortex. 2012;22:2297–2304. doi: 10.1093/cercor/bhr306. [DOI] [PubMed] [Google Scholar]
- Poluektova L, Meyer V, Walters L, Paez X, Gendelman HE. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 2005;52:344–353. doi: 10.1002/glia.20253. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA : the journal of the American Medical Association. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior research methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain, behavior, and immunity. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Rumley A, Lowe GD, Fowkes FG. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery Study. Journal of the American Geriatrics Society. 2007;55:700–707. doi: 10.1111/j.1532-5415.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- Raji CA, Lopez OL, Kuller LH, Carmichael OT, Longstreth WT, Jr, Gach HM, Boardman J, Bernick CB, Thompson PM, Becker JT. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiology of aging. 2012;33:834, e837–e816. doi: 10.1016/j.neurobiolaging.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rao KM, Pieper CS, Currie MS, Cohen HJ. Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. American journal of clinical pathology. 1994;102:802–805. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, Acker JD. Differential aging of the human striatum: longitudinal evidence. AJNR. American journal of neuroradiology. 2003;24:1849–1856. [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Archives of general psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. THe Halstead-Reitan Neuropsychological Test Battery. Tuscon, AZ: 1985. [Google Scholar]
- Rey A. L'Examin Clinique en Psychologie. Paris, France: 1958. [Google Scholar]
- Richwine AF, Parkin AO, Buchanan JB, Chen J, Markham JA, Juraska JM, Johnson RW. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology. 2008;33:1369–1377. doi: 10.1016/j.psyneuen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. The New England journal of medicine. 2000a;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000b;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Ryan L, Walther K, Bendlin BB, Lue LF, Walker DG, Glisky EL. Age-related differences in white matter integrity and cognitive function are related to APOE status. NeuroImage. 2011;54:1565–1577. doi: 10.1016/j.neuroimage.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. What and when of cognitive aging. Current Directions in Psychological Science. 2004;13:140–144. [Google Scholar]
- Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology. 2012;78:720–727. doi: 10.1212/WNL.0b013e318248e50f. [DOI] [PubMed] [Google Scholar]
- Sattin RW. Falls among older persons: a public health perspective. Annual review of public health. 1992;13:489–508. doi: 10.1146/annurev.pu.13.050192.002421. [DOI] [PubMed] [Google Scholar]
- Sawada M, Itoh Y, Suzumura A, Marunouchi T. Expression of cytokine receptors in cultured neuronal and glial cells. Neuroscience letters. 1993;160:131–134. doi: 10.1016/0304-3940(93)90396-3. [DOI] [PubMed] [Google Scholar]
- Schobitz B, De Kloet ER, Holsboer F. Gene expression and function of interleukin 1, interleukin 6 and tumor necrosis factor in the brain. Progress in neurobiology. 1994;44:397–432. doi: 10.1016/0301-0082(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Schram MT, Euser SM, de Craen AJ, Witteman JC, Frolich M, Hofman A, Jolles J, Breteler MM, Westendorp RG. Systemic markers of inflammation and cognitive decline in old age. Journal of the American Geriatrics Society. 2007;55:708–716. doi: 10.1111/j.1532-5415.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- Smith A, Tyrrell D, Coyle K, Higgins P. Effects of interferon alpha on performance in man: a preliminary report. Psychopharmacology. 1988;96:414–416. doi: 10.1007/BF00216072. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, Uchida S, Kawashima R, Fukuda H. Relationship between body mass index and gray matter colume in 1,428 health individuals. Obesity. 2008;16:119–124. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Wu K, Kakizaki M, Tsuji I, Kawashima R, Fukuda H. Correlation between high-sensitivity C-reactive protein and brain gray matter volume in healthy elderly subjects. Human brain mapping. 2013;34:2418–2424. doi: 10.1002/hbm.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancredi V, D'Antuono M, Cafe C, Giovedi S, Bue MC, D'Arcangelo G, Onofri F, Benfenati F. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. Journal of neurochemistry. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- Teunissen CE, van Boxtel MP, Bosma H, Bosmans E, Delanghe J, De Bruijn C, Wauters A, Maes M, Jolles J, Steinbusch HW, de Vente J. Inflammation markers in relation to cognition in a healthy aging population. Journal of neuroimmunology. 2003;134:142–150. doi: 10.1016/s0165-5728(02)00398-3. [DOI] [PubMed] [Google Scholar]
- Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. The journals of gerontology. Series A, Biological sciences and medical sciences. 2004;59:268–274. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- Trapero I, Cauli O. Interleukin 6 and cognitive dysfunction. Metabolic brain disease. 2014 doi: 10.1007/s11011-014-9551-2. [DOI] [PubMed] [Google Scholar]
- Van Dam AM, Bauer J, Tilders FJ, Berkenbosch F. Endotoxin-induced appearance of immunoreactive interleukin-1 beta in ramified microglia in rat brain: a light and electron microscopic study. Neuroscience. 1995;65:815–826. doi: 10.1016/0306-4522(94)00549-k. [DOI] [PubMed] [Google Scholar]
- Verstynen TD, Weinstein A, Erickson KI, Sheu LK, Marsland AL, Gianaros PJ. Competing physiological pathways link individual differences in weight and abdominal adiposity to white matter microstructure. NeuroImage. 2013;79:129–137. doi: 10.1016/j.neuroimage.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Molecular psychiatry. 2000;5:604–615. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O. Involvement of BDNF in age-dependent alterations in the hippocampus. Frontiers in aging neuroscience. 2010;2 doi: 10.3389/fnagi.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiology of aging. 2011;32:916–932. doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Third Edition: Administration and scoring manual. San Antonio, TX: 1997a. [Google Scholar]
- Wechsler D. THe WMS-III administration and scoring manual. San Antonio, TX: 1997b. [Google Scholar]
- Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M, Conty M, Minnerup J, Ringelstein EB, Berger K, Deppe M, Knecht S. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74:1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Shiung MM, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70:512–520. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette AA, Kapogiannis D. Does the brain shrink as the waist expands? Ageing research reviews. 2014 doi: 10.1016/j.arr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CB, Sacco RL, Rundek T, Delman J, Rabbani L, Elkind M. Interleukin-6 is associated with cognitive function: the Northern Manhattan Study. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2006;15:34–38. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. Journal of neuroimmunology. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, behavior, and immunity. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.