Abstract
Previously we showed that exposure of threespine stickleback (Gasterosteus aculeatus) to the endocrine disruptor perchlorate results in pronounced structural changes in thyroid and gonad, while surprisingly, whole-body thyroid hormone concentrations remain unaffected. To test for hormone titer variations on a finer scale, we evaluated the interactive effects of time (diel and reproductive season) and perchlorate exposure on whole-body contents of triiodothyronine (T3), thyroxine (T4), and 11-ketotestosterone (11-KT) in captive stickleback. Adult stickleback were exposed to 100 ppm perchlorate or control water and sampled at four-hour intervals across the 24-hour day and at one time-point (1100 h) weekly across the reproductive season (May-July). Neither whole-body T3 nor T4 concentration significantly differed across the day in control or perchlorate treated stickleback. Across the reproductive season, whole-body T3 concentration remained stable while T4 significantly increased. However, neither hormone concentration was significantly affected by perchlorate, verifying our previous studies. The concentration of whole-body 11-KT, a major fish androgen, displayed significant diel variation and also steadily declined across the reproductive season in untreated males; perchlorate exposure did not influence the concentration of 11-KT in either diel or reproductive season schedules. Diel and reproductive season variations in 11-KT content in male stickleback are likely related to reproductive physiology and behavior. The observed increase in T4 content across the reproductive season may be reflective of increased energy investment in reproduction near the end of the life cycle.
Keywords: diel, Gasterosteus aculeatus, 11-ketotestosterone, reproductive season, thyroid hormone
1. Introduction
Perchlorate, a water soluble anion, is a known inhibitor of thyroid hormone (TH) synthesis (De Groef et al., 2006; Goleman et al., 2002; Leung et al., 2010; Wu et al., 2012). Perchlorate appears in a variety of sources relevant to human health, including contaminated drinking water, milk, and leafy vegetables (Dasgupta et al., 2006; Urbansky, 2002). Environmental perchlorate largely comes from releases associated with its production, storage and use as an oxidizer for rocket fuel, artillery and a number of consumer products. The concentration of perchlorate in contaminated ground and surface water is generally in the parts per billion (ppb) range but can be in the parts per thousand range in some highly contaminated areas (Sanchez et al., 2007; Theodorakis et al., 2006). At the biochemical level, perchlorate competes with iodide at the sodium iodide symporter (NIS, also known as SLC5A5) in epithelial cells (thyrocytes) of thyroid follicles and thus disrupts normal TH synthesis, resulting in hypothyroidism in a variety of species (Lawrence et al., 2000).
Recent studies show that some fish species reorganize the architecture of their thyroid tissue in response to chronic perchlorate exposure (Furin et al., in review; Petersen et al., 2015; Schmidt et al., 2012). Specifically, perchlorate reduces individual thyroid follicle size and increases the overall number of follicles, potentially serving as a mechanism for increasing the available surface area for NIS transporters in thyrocytes. Surprisingly, threespine stickleback (Gasterosteus aculeatus) that were chronically exposed to 100 ppm perchlorate from fertilization maintained normal whole-body levels of total TH (T3 and T4) (Petersen et al., 2015) despite perchlorate's known mechanism of competition with iodide at the NIS (Leung et al., 2010; Wolff, 1998). Zebrafish (Danio rerio) treated with perchlorate display similar changes in thyroid tissue morphology (Mukhi et al., 2007; Schmidt et al., 2012), but in contrast to stickleback, show significant reductions in whole-body T4 concentrations (Mukhi and Patiño, 2007; Schmidt et al., 2012). Given the disparity in TH responses to perchlorate between fish species, it is possible that underlying molecular mechanisms of the response to this contaminant differ among species. An additional explanation for the failure to detect effects of perchlorate on TH levels in stickleback (Petersen et al., 2015) could be related to the experimental time-course (i.e., effects could have been masked by diel fluctuations in hormone contents).
The effects of perchlorate are not restricted to thyroid structure and function in fishes. For example, perchlorate disrupts sexual development in some species (Bernhardt and von Hippel, 2008; Bernhardt et al., 2006; Furin et al., in review; Mukhi et al., 2007; Petersen et al., 2015). Interestingly, perchlorate-induced alteration in gonadogenesis is not consistent across fish taxa; perchlorate induces feminization in zebrafish (Mukhi et al., 2007), while in threespine stickleback it induces masculinization (Bernhardt et al., 2006). Perchlorate-induced reproductive effects in threespine stickleback also include a pronounced increase in 11-ketotestosterone (11-KT, the major fish androgen; (Borg, 1994)) in early developing fish and hyperplasia of spermatocytes (Petersen et al., 2015). Cross-talk between the hypothalamic-pituitary-thyroid (HPT) and hypothalamic-pituitary-gonadal (HPG) axes may, in part, explain the widespread phenotypic effects of perchlorate (Duarte-Guterman et al.; Flood and Langlois, 2014).
Photoperiod strongly affects the regulation of multiple endocrine axes in stickleback, including the HPT and HPG axes (Kitano et al., 2010; O'Brien et al., 2012). Seasonal change in photoperiod is a potent environmental cue for regulating a suite of physiological responses, including patterns of hormone synthesis and secretion. Recent work in fishes and other species has demonstrated that TH production and secretion is responsive to changes in photoperiod and can vary on a seasonal basis (Comeau et al., 2000; Dardente et al., 2014; Nakane and Yoshimura, 2014; Yoshimura, 2013). Likewise, increased day-length initiates reproduction in many fishes and is associated with seasonal increases in the synthesis of 11-KT, a primary driver of secondary sexual characteristics, sexual behavior, and spermatogenesis (Borg, 1994). Alaskan populations of threespine stickleback, our study species, experience extreme seasonally-dependent changes in photoperiod. The early summer season in south-central Alaska is characterized by a long photophase (19 h), a correspondingly truncated scotophase (5 h), and is coincident with the peak of reproduction in this species (Bell and Foster, 1994). We targeted threespine stickleback as our platform of investigation because it is an established aquatic model for ecotoxicology (including perchlorate exposure) that is broadly distributed across the northern hemisphere and has abundant molecular and genetic resources available for studying in-depth mechanisms of toxicity.
The interaction of temporal (diel or reproductive season) variation in hormone concentration and perchlorate exposure is not well understood in threespine stickleback. The objectives of this study were to characterize the variation in whole-body TH and 11-KT contents within a day and across the reproductive season in captive adult stickleback exposed to natural changes in lighting, and to determine if exposure to perchlorate modifies patterns of hormone variation. Due to perchlorate's known effects on the HPT and HPG endocrine axes in stickleback, we hypothesized that perchlorate is an effective modulator of whole-body TH and 11-KT contents.
2. Methods
2.1. Fish Collections & Housing Conditions
Wild anadromous threespine stickleback (hereafter, stickleback) were collected from Westchester Lagoon, Alaska (N 61.207815°, W 149.924987° and N 61.204378°, W 149.912140°) in May 2012. Fish were caught using 0.64 cm unbaited minnow traps and adults (~2 years old) were separated by sex based on nuptial coloration and housed separately in outdoor 1514 L static system pools at the University of Alaska Anchorage. Overhead tarps protected pools from rain, but still allowed natural daylight to enter the water column. Due to the protracted periods of civil twilight of this latitude in summer, complete darkness was never observed for the duration of the experiment. Water quality measurements (temperature (10 ±3°C), pH (6.5 ±0.5), ammonia (~0)) were collected using a YSI multiprobe (Yellow Springs, OH) and API water testing kits (Mars Fishcare, Chalfont, PA). Daily fluctuations in water temperature within the outdoor experimental pools were relatively negligible and are representative of summer temperatures recorded at the field collection sites. Water chemistry was tested once per week for the duration of the experiment and was adjusted with water changes if deviations beyond the aforementioned ranges were observed. All animal protocols were approved by the UAA Institutional Animal Care and Use Committee (IRB reference # 159870-1). Field work was conducted under an Alaska Department of Fish & Game scientific collection permit (SF-2010-029).
2.2 Experimental Design and Exposures
Adult stickleback were chronically exposed to either 100 ppm sodium perchlorate (NaClO4, Acros Organics, 99% purity, Pittsburgh, PA) dissolved in dechlorinated and biologically conditioned tap water or to dechlorinated and biologically conditioned tap water alone (control). The exposure level of perchlorate (100 ppm) selected for this study is environmentally-relevant to some contaminated areas of the US. The fish were subsequently sampled over two experimental regimens: diel (0300, 0700, 1100, 1500, 1900, 2300 hours on four independent days, Fig. 1) and across an entire reproductive season (22 May - 3 July 2012, one sample each of seven weeks at the 1100 h time-point, Fig. 1). Perchlorate concentration was monitored weekly with an Acorn Ion 6 meter (Oakton Instruments, Vernon Hills, IL) with a perchlorate ISE electrode (Cole-Parmer, Vernon Hills, IL). Perchlorate concentrations measured in control tanks were below the minimum detection limit (0.7 ppm) of the electrode. Males and females were maintained in separate pools for the duration of the exposures to minimize the potential for confounding effects related to reproductive behavior. Six replicate pools were used per treatment/sex combination. Fish density was kept constant in the pools for the entire experiment by replacing sampled fish with spine-clipped stickleback of the same sex collected from Rabbit Slough, Alaska (N 61.5595°, W 149.2583°). During each collection period, whole fish were immediately snap-frozen in liquid nitrogen and stored at −80°C until analyses were conducted. The use of whole-body homogenates rather than plasma allowed the analysis of multiple hormones per fish despite their small size.
Figure 1.
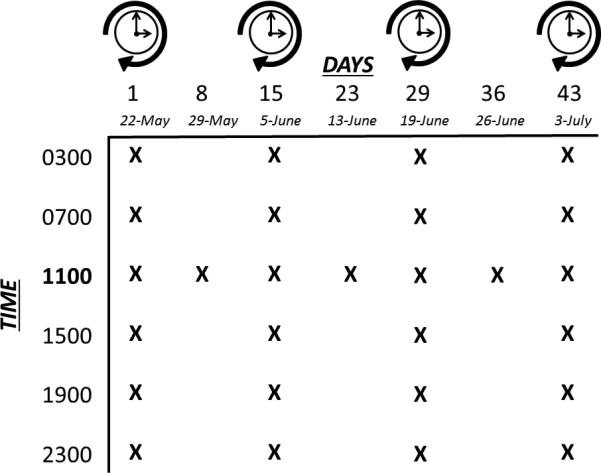
Experimental sampling schedule with diel and reproductive season components. Clock icons indicate days with continuous sampling at four hour intervals.
2.3 TH Assays
Whole stickleback were homogenized and THs were extracted according to methods in Petersen et al. (2015). Briefly, 0.5 g aliquots of whole-body homogenate were extracted with barbital and then re-extracted for further purification. Commercially available enzyme-linked immunosorbant assay (ELISA) kits (Total T3 and T4, MP Biomedicals, Santa Ana, CA) that were previously validated in our laboratory (Petersen et al., 2015) were used to assess the concentrations of T3 and T4 in whole-body homogenates following resuspension in enzyme immunoassay buffer (0.1M PBS, 0.15M NaCl, 0.1% BSA, pH 7.4). Manufacturer-provided standards and an inter-assay pooled homogenate were assayed in parallel with samples on every 96-well plate. Samples were assayed in triplicate for T3 and in duplicate for T4, to control for the higher T3 assay variation. Absorbance was measured on a plate reader at 450 nm (SpectraMax 340PC, Molecular Devices, Sunnyvale, CA) and hormone concentrations were determined by extrapolation from a standard curve. T3 standards ranged from 0-6 ng/ml while T4 standards ranged from 0-100 ng/ml. The intra- and inter-assay variability for T3 was 9.5% and 33.0%, respectively. The intra- and inter-assay variability for T4 was 5.4% and 14.2%, respectively. Individual samples were adjusted using a correction factor determined from readings of the pooled stickleback homogenate to account for inter-assay variation.
2.4 11-KT Assay
11-KT was extracted from a subset of male whole-body homogenates and assayed using an ELISA kit (11-KT, Cayman Chemicals, Lexington, KY) as described by Petersen et al. (2015). Males were exclusively targeted for this assay because expression of this steroid is sexually dimorphic, with the highest expression observed in reproductively active males (Mayer et al., 1990). Briefly, 0.15 g aliquots of whole-body homogenate were extracted with diethyl ether and the extract was diluted at least 20X using the manufacturer-provided buffer. Subsequently, samples, standards, and an inter-assay pooled homogenate were assayed in duplicate on a 96-well plate by reading absorbance on a microplate reader at 450 nm (SpectraMax 340PC, Molecular Devices, Sunnyvale, CA). 11-KT standards ranged from 0-50 pg/ml. Diel samples were all collected from Day 29 males, while reproductive season samples were all collected at the 1100 h time-point. The intra- and inter-assay variability for 11-KT was 5.4% and 9.8%, respectively. Measured values were corrected for inter-assay variation using values obtained from a pooled stickleback homogenate that was assayed on each plate.
2.5 Statistical Analyses
All data were tested for normality and homogeneity of variance using Levene's test and the Shapiro-Wilk test, respectively. Results for transformed and non-transformed data were consistent and non-transformed analyses are presented here. Analysis of variance (ANOVA) was performed separately for diel and reproductive season datasets with time and/or day and 100 ppm perchlorate vs. control as fixed factors, tank as a random factor, and [T3], [T4], or [11-KT] as the response variable in the models. Sex was included as a fixed factor in the linear models for THs but was excluded from the 11-KT model because only males were analyzed. Tukey's HSD post-hoc multiple comparisons of means was used to identify significant differences between treatment groups. Statistical analyses were performed in IBM SPSS (v. 22); all differences were considered significant at P<0.05.
3. Results
3.1 Whole-body TH contents did not vary across the 24-hour day; however, T4 content significantly increased across the reproductive season
Diel
Neither perchlorate nor sex significantly impacted the regulation of either T3 or T4 content (Figs. 2-3). The diel model included the fixed effect of sampling date, which was significant for T3 (F3, 1634 = 2.997, P=0.030). This significant effect was due to a significantly lower concentration of T3 on Day 1 than Day 29 (P=0.021, Fig. 2). Sampling date was also a significant factor for T4 (F3, 1634 = 35.443, P<0.001, Fig. 3). The interaction of day × time was significant for T4 concentration (F15, 1634 = 1.900, P=0.019) but not for T3.
Figure 2.
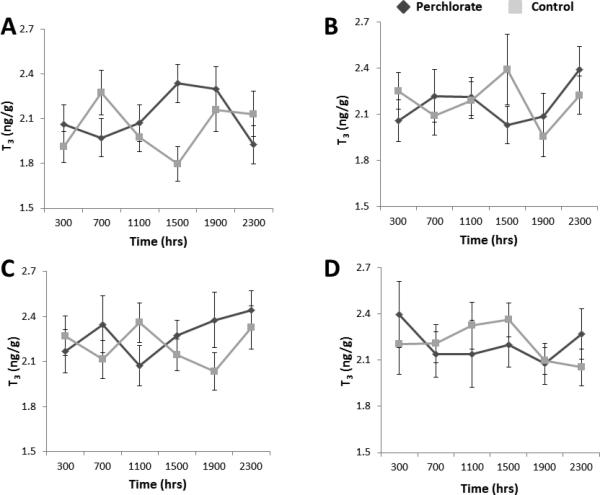
(A-D). Means ± SE for whole-body T3 concentration in stickleback following chronic exposure to 100 ppm perchlorate or control conditions on Day 1 (A), Day 15 (B), Day 29 (C), and Day 43 (D).
Figure 3.
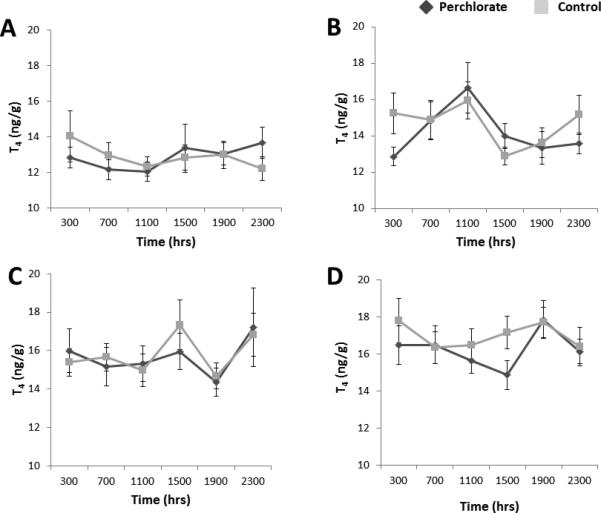
(A-D). Means ± SE for whole-body T4 concentration in stickleback following chronic exposure to 100 ppm perchlorate or control conditions on Day 1 (A), Day 15 (B), Day 29 (C), and Day 43 (D).
Reproductive Season
The reproductive season model included only the 1100 h time-point. As with the diel results, neither perchlorate nor sex significantly impacted the regulation of whole-body T3 or T4 contents across the reproductive season. There was a significant effect of day on T4 concentration (F6, 476 = 7.035, P<0.001). Specifically, T4 content significantly increased across the reproductive season, peaking at Day 43 (Fig. 4B). T3 content did not significantly change across the reproductive season (Fig. 4A).
Figure 4.
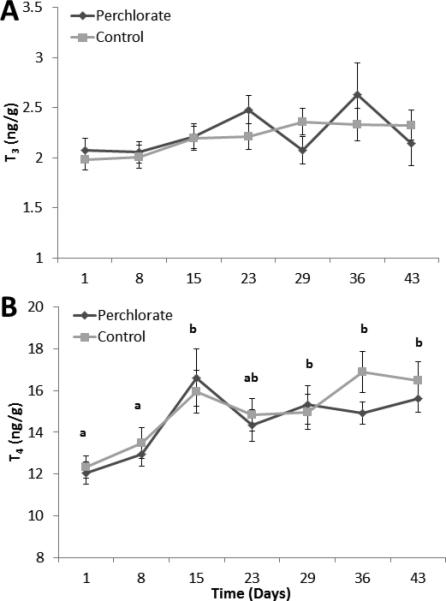
(A-B). Means ± SE for whole-body T3 (A) and T4 (B) in stickleback that were chronically exposed to 100 ppm perchlorate or control conditions and sampled at 1100 h across the reproductive season (May-July). Different letters indicate significant differences between groups evaluated by post-hoc Tukey HSD test (p<0.05) following ANOVA.
3.2 Whole-body 11-KT content exhibited significant diel variation and decreased across the reproductive season
Diel
Perchlorate-treated males did not significantly differ across the 24-hour day in concentration of whole-body homogenate 11-KT from control males. Time of day was a significant factor for 11-KT concentration (F5, 201 = 2.568, P= 0.028). 11-KT values peaked at 1100 h, when they were significantly higher than both 0700 h (P = 0.047) and 1900 h (P = 0.024, Fig. 5A).
Figure 5.
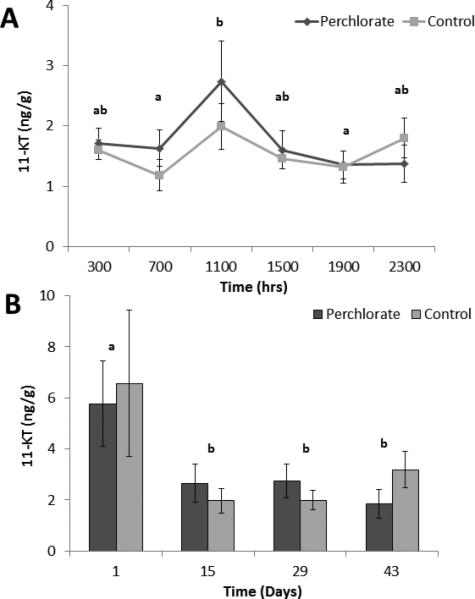
(A-B). Means ± SE for whole-body 11-KT in male stickleback that were chronically exposed to 100 ppm perchlorate or control conditions at 4 hour intervals throughout Day 29 of the exposure (A) and across the reproductive season (B). Different letters indicate significant differences between groups evaluated by post-hoc Tukey HSD test (p<0.05) following ANOVA.
Reproductive Season
Perchlorate-treated males did not significantly differ across the reproductive season in concentrations of whole-body homogenate 11-KT from control males. There was a significant effect of day on T4 concentrations (F3, 59 = 5.874, P=0.001). 11-KT concentration was significantly higher early in the reproductive season compared to later in the season (Fig. 5B).
4. Discussion
4.1 Whole-body TH content does not vary on a diel schedule
Within vertebrates, such as teleosts, THs are important modulators of a variety of essential physiological processes including growth and development (Power et al., 2001). Diel regulation of circulating TH is observed in temperate and high-latitude fish species including red drum (Sciaenops ocellatus) and salmonids (Gomez et al., 1997; Leiner et al., 2000; Leiner and MacKenzie, 2001, 2003; Spieler, 1979). Generally, while plasma T4 concentrations fluctuate widely across diel schedules, corresponding concentrations of T3 remain stable. In red drum, T4 concentrations exhibit high amplitude oscillations that increase 10-fold from baseline values every 12 hours (Leiner et al., 2000) and under constant lighting conditions this rhythm free-runs and thus is likely under endogenous circadian clock control (Leiner and MacKenzie, 2001). Rainbow trout (Oncorhynchus mykiss) robustly increase T4 plasma concentrations every four hours while T3 levels remain unchanged (Gomez et al., 1997). We found no evidence of significant diel variation in stickleback whole-body TH content. Instead, stickleback TH (T3 and T4) content remained relatively stable within a given day. However, the observed variation in TH between individual fish was high in this study and may have contributed to our inability to detect significant daily variation. Similar to rainbow trout (Gomez et al., 1997), sex-specific differences in the regulation of daily TH contents were not found in this study.
The majority of investigations of temporal changes in TH regulation in fishes have measured circulating THs in the plasma (Comeau et al., 2000; Leiner et al., 2000; Leiner and MacKenzie, 2001). The small size of stickleback and temporal schedule of our study precluded our sampling for plasma. Plasma T4 has been shown to transiently increase (up to 2-fold) in fish during blood collections due to handling stress (Brown et al., 1978) and may obscure diel TH variations. However, it is possible that the whole-body homogenates used in the current study lacked the sensitivity to detect subtle differences in TH regulation, especially for those fractions of TH (e.g., free) that are responsible for physiologic action and are typically at low concentrations. This limitation may be amplified for hormones that are primarily stored and later secreted from a gland, such as T4. Stored T4 within the thyroid follicles likely compromised our ability to detect fine diel variations in other forms of TH (circulating, receptor-bound, intra- and extra-cellular, excreted) that could be indicative of synthesis and degradation processes (Eales and Brown, 1993). In contrast, T3 is almost exclusively found in the bloodstream and peripheral tissues since most of the conversion of T4 to T3 in fish occurs extrathyroidally via outer-ring deiodination (Brown et al., 2004). For this reason, we expect that whole-body T3 content is likely more representative of diel patterns of T3 variation. This is supported by our finding that T3 whole-body content is stable across diel schedules, a finding that is consistent with many fish studies that found stable circulating T3 levels across the day (Eales, 1985; Gomez et al., 1997; Leiner et al., 2000). This fine-tuned regulation of T3 may be a result of a shorter half-life (e.g., higher metabolism) and the ability of deiodinase enzymes (mainly type II) to efficiently utilize available T4 for conversion to T3. Clearly, a detailed investigation of the relationships among various pools of TH across the day and associated deiodinase activity in target tissues of fish would be an informative avenue of future research.
4.2 11-KT content exhibits diel variation in male stickleback
Daily sex steroid secretion patterns in vertebrates facilitate changes in physiology and behavior. Male mammals and birds demonstrate strong diel variation in plasma testosterone levels (El Omari et al., 1989; Laucht et al., 2011). The primary androgen in fish, 11-KT, is known to influence reproductive behavior (Borg, 1994; Kindler et al., 1989; Matsumoto et al., 2012) and is highly induced in male stickleback during the reproductive season (Mayer et al., 1990). Induction of 11-KT stimulates spermatogenesis (Borg, 1994) and therefore is thought to be critical for the maturation of male gonads. In the current study, we found that whole-body 11-KT content varied significantly on a diel schedule. Specifically, 11-KT concentrations peaked (2 ng/g) at the 1100 h time-point and tapered off within a few hours. Similarly, carp (Cyprinus carpio) exhibit the highest concentrations of 11-KT during the light phase between 1000 and 1200 h (Bieniarz et al., 1986) while Japanese char (Salvelinus leucomaenis) display peak serum 11-KT concentration late in the light phase, just prior to darkness (Yamada et al., 2002). In contrast, Lorenzi et al. (2008) found that 11-KT concentrations in environmental water samples of male or female bluebanded gobies (Lythrypnus dalli) indicated no diel pattern of regulation.
Daily variation in secretion of sex steroids may influence how other hormones in the body affect various biochemical pathways. For example, sex steroids can act as modulators of the general stress response (hypothalamus–pituitary–adrenal/interrenal axis, (Aloisi and Bonifazi, 2006)) through estrogenic regulation of corticotropin-releasing hormone gene expression (Vamvakopoulos and Chrousos, 1993). It has also been shown that rainbow trout implanted with 11-KT display lower cortisol levels in response to stressful environments (Pottinger et al., 1996; Young et al., 1996). We did not evaluate the cross-talk between the various stickleback endocrine axes in this study, but it is intriguing to consider how cross-talk might influence the effects of contaminants, especially in viewing contaminants as stressors.
4.3 T4 and 11-KT contents are inversely correlated across the reproductive season
In the current study, we found that 11-KT content in males peaked at the beginning of the reproductive season (late May) while T4 content peaked at the end of the reproductive season (early July). This inverse relationship is logical in the context of stickleback reproductive behavior and physiology. 11-KT is an androgen that stimulates stickleback male reproductive phenotypes, including nest building and kidney hypertrophy (Jakobsson et al., 1999; Páll et al., 2002a). Nests are used as a place for courtship, spawning and parental care, and therefore need to be built in the early reproductive season (Páll et al., 2002b). Páll et al. (2005) observed a significant decline in plasma 11-KT concentrations of male stickleback in the late parental phase of the reproductive cycle and attributed this decrease to energetic savings related to reduced glue protein (spiggin) production. Similarly, 11-KT plasma concentrations peak in male plainfin midshipmen (Porichthys notatus) early in the reproductive season and become nearly undetectable later in the post-nesting period (Sisneros et al., 2004). This decrease in androgen levels in type I male midshipmen is thought to mediate the transition in reproductive behaviors (from courtship to parental care) over the course of the breeding season. Male blenniid fish (Rhabdoblennius nitidus) caring for eggs also exhibit lower 11-KT levels than male fish without eggs (Matsumoto et al., 2012).
Alternate explanations for the early peak of 11-KT and abrupt decline may be related to the experimental design of this study, captivity stress, or natural timing of reproductive regression. Because males and females were separated for the duration of the experiment, it is possible that 11-KT concentrations were higher when males were in the presence of both sexes in their natural habitat prior to collection. Captivity may have acted as a stressor and thereby stimulated the secretion of glucocorticoids and subsequent down-modulation of the reproductive axis. Indeed, results from investigations of other free-living vertebrates (e.g., arctic ground squirrel (Urocytellus parryii)) clearly indicate the social influence of both males and females on androgen secretion (Buck and Barnes, 2003) and in some fishes, 11-KT concentrations can also increase when males are isolated from females due to the dynamics of social hierarchy (Galhardo and Oliveira, 2014; Oliveira et al., 2002). In the current study, the housing of males with other males with no access to nest-building material resulted in little aggression among individuals and may have contributed to the observed decline in whole-body 11-KT content. The variation in 11-KT concentration among individual male fish was high in this study, which may be due to differences in reproductive activity. Following reproduction, photoperiod may still be increasing, yet animals of some species may become photorefractory and exhibit suppression of the HPG axis and subsequent regression of the gonads (Yoshimura, 2013).
Seasonal regulation of THs in fishes can be variable due to the diversity of life histories, life cycle events, and the distribution of environments experienced by individuals in different taxa. TH concentrations and reproductive status have been positively correlated in fish (Cyr and Eales, 1996) and other animals (Nakane and Yoshimura, 2014; Yoshimura, 2013). TH is generally elevated during the reproductive season in many animals. In this study, we found that adult stickleback sharply increased (1.5-fold increase) whole-body T4 content across the reproductive season (peak in July). This intra-seasonal change in whole-body T4 content is comparable to that observed in other anadromous fish (Specker et al., 1992) and may be related to reproductive output. Anadromous stickleback return to fresh water to spawn before the end of their life cycle, after about two years of residence in the ocean (Bell and Foster, 1994). Because TH plays a role in many reproductive processes, and energetic resources are differentially allocated to reproduction in stickleback during this time, elevated T4 content could reflect reproductive output. In contrast, a study in field-collected cod (Gadus morhua) found the lowest serum TH levels in the summer season, with photoperiod and metabolism identified as the primary drivers for T4 and T3 concentrations, respectively (Comeau et al., 2000). Similar to our study, seasonal changes in salmon T4 plasma concentrations were more pronounced than those of T3 (Larsen et al., 2011).
THs (primarily T4) are critical modulators of smoltification in salmon (Barron, 1986), a physiological transformation that takes place in preparation for adult life in the ocean. This includes the remodeling of gills with mitochondria-rich cells for salt secretion (Hwang and Lee, 2007). It is unknown whether THs play a role in osmotic preparation for anadromous adult teleost fish that are returning to freshwater habitat to spawn. Interestingly, anadromous stickleback administered exogenous T4 rapidly display a preference for fresh water (Baggerman, 1957). In this study, the elevated T4 content observed in stickleback that were at the end of their life cycle was probably driven by reproduction rather than osmoregulation. However, seasonal changes in plasma TH levels of smolting salmon can be asynchronous with tissue TH concentrations (Specker et al., 1992), which highlights the ambiguity associated with the interpretation of whole-body TH data.
4.4 Perchlorate exposure does not modify variation in TH and 11-KT contents
Because perchlorate competitively inhibits iodide import at the NIS in thyrocytes of mammals (Wolff, 1998), we predicted that TH content in stickleback whole-body homogenates would decrease following exposure to perchlorate. Instead, we found that whole-body TH contents (T3 and T4) remained stable in response to acute (days) and chronic (> 1 month) perchlorate exposure. Nevertheless, our findings are consistent with our recent studies in stickleback that have assessed TH concentrations following chronic exposure to perchlorate (Furin et al., in review; Petersen et al., 2015). Even following 43 days of perchlorate exposure, stickleback maintained normal levels of TH that did not significantly differ from controls, suggesting a compensatory mechanism for the inhibitory effects of perchlorate on thyroid function. Alternatively, perchlorate's observed effects in stickleback are driven primarily through a mechanism independent of the NIS, such as other SLC5 transporters.
Petersen et al. (2015) observed that chronic exposure to perchlorate from fertilization causes stickleback to redefine their thyroid tissue architecture, possibly to more efficiently import iodide to the thyroid follicles. This compensatory mechanism may also be occurring in adult stickleback used in the current study, but we did not test this hypothesis. Interestingly, Furin et al. (in review) found that a critical window for thyroid tissue plasticity in response to perchlorate exposure exists early in developing stickleback (at least until day 42 post fertilization). It is also possible that differences in TH concentrations were not observed in whole-body homogenates because sufficient TH may be sequestered as thyroglobulin within follicles of the thyroid that then release T4 into circulation as needed. We think that the sequestration/release explanation is unlikely given the duration of the study (43 days). However, a significant decrease in zebrafish whole-body T4 content following chronic exposure to ~10 ppm perchlorate was observed after 16-weeks, but not at 12-weeks (Mukhi et al., 2005; Mukhi and Patiño, 2007). Although the TH concentrations we measured are comparable to previous studies in fishes using whole-body homogenates (Crane et al., 2004; Mukhi and Patiño, 2007; Petersen et al., 2015; Tagawa and Hirano, 1989), our data suggest that whole-body contents of TH are not biologically informative as a sole endpoint for understanding the effects of perchlorate on TH regulation.
11-KT increases in stickleback males as they enter the reproductive season (Mayer et al., 1990; Sebire et al., 2007) and functions to stimulate male typical reproductive behaviors and physiologies including nest-building and kidney hypertrophy associated with spiggin production (Jakobsson et al., 1999; Páll et al., 2002a, b). Our previous work indicates that chronic perchlorate exposure increases whole-body contents of 11-KT in stickleback embryos but not adults (Petersen et al., 2015), which is consistent with the lack of an effect of perchlorate on 11-KT concentrations in wild-caught adult male fish in the current study. Sex steroids can have both organizational and activational effects (Adkins-Regan, 2012) and thus can influence physiology, behavior and developmental processes differently across life stages (Arnold, 2009). The disparity in the 11-KT response between early versus late life stages suggests that the effect of perchlorate on 11-KT is organizational and mediated by developmental pathways. Thus, in developing fish, perchlorate exposure may result in permanent phenotypic alterations whereas in adults with established gonads, perchlorate exposure may not alter 11-KT expression.
4.5 Conclusions
We characterized the patterns of whole-body hormone content (THs and 11-KT) across diel and reproductive season (May-July) timescales in combination with exposure to the endocrine disrupting compound, perchlorate, in stickleback. We found that perchlorate exposure does not perturb patterns of whole-body TH and 11-KT content over diel and reproductive season schedules in adult stickleback. Consistent with many other studies of perchlorate and other contaminants, we found that effects of exposure on adults pale in comparison to exposure during early development. However, it is important to emphasize that whole-body hormone content is a limited sole biological endpoint for studies focused on elucidating perchlorate's effects on hormone regulation. Approaches that include additional endpoints (e.g., tissue histology, gene expression) as supplements to whole-body hormone content data are necessary to achieve a more accurate physiological representation of how perchlorate impacts endocrine function.
In this study, we found that 11-KT content exhibited significant diel variation while TH contents remained stable. The diel regulation of thyroid stimulating hormone (TSH), an important modulator of TH synthesis and secretion, may be more robust than that of TH (MacKenzie et al., 2009) and is a target for future studies. TSH cycling generally precedes and likely drives the secretion patterns of TH (Jones et al., 2013; Jordan, 1980). In red drum, TSH mRNA expression cycles are antiphase to T4 cycling, lending support for this hypothesis (Jones et al., 2013). Across the reproductive season, we found that T4 and 11-KT showed significant changes in whole-body content, but in opposite directions, with 11-KT peaking early and T4 peaking late. Overall, this study demonstrates the need to build upon our knowledge of hormone homeostasis in fishes, how emerging contaminants modulate regulation of hormones, and what the downstream physiological consequences are for the organism.
Highlights.
Perchlorate does not alter whole-body TH and 11-KT contents in adult stickleback.
Significant diel variation was found in 11-KT content of male stickleback.
Thyroxine and 11-KT contents are inversely regulated across the reproductive season.
Reproductive physiology and behavior may be drivers for TH and androgen regulation.
Acknowledgments
Kristin Cheney, Tory Adams, and Kerry Tope assisted with animal maintenance, exposures, and sample collection. Kristin Cheney, Kate Backstrum, Nicholas Herrick, Tyler Samse, and Dayanna Lau assisted with extractions and ELISAs. Christoff Furin and Ann Petersen provided insightful discussions and access to unpublished data. Richard Bernhardt assisted with experimental design and setup. Two anonymous reviewers provided suggestions that greatly improved this manuscript. Funding was provided by NIH grant number 1RO1ES017039.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins-Regan E. Hormonal organization and activation: Evolutionary implications and questions. General and Comparative Endocrinology. 2012;176:279–285. doi: 10.1016/j.ygcen.2011.12.040. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Bonifazi M. Sex hormones, central nervous system and pain. Hormones and Behavior. 2006;50:1–7. doi: 10.1016/j.yhbeh.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Arnold AP. The organizational–activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Hormones and Behavior. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggerman B. An experimental study on the timing of breeding and migration in the three-spined stickleback, Gasterosteus aculeatus L. Archives Neerlandaises de Zoologie. 1957;12:105–318. [Google Scholar]
- Barron MG. Endocrine control of smoltification in anadromous salmonids. Journal of Endocrinology. 1986;108:313–319. doi: 10.1677/joe.0.1080313. [DOI] [PubMed] [Google Scholar]
- Bell MA, Foster SA. The evolutionary biology of threespine stickleback. Oxford University Press; New York: 1994. [Google Scholar]
- Bernhardt RR, von Hippel FA. Chronic perchlorate exposure impairs stickleback reproductive behaviour and swimming performance. Behaviour. 2008;145:527–559. doi: 10.1163/156853908792451511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt RR, von Hippel FA, Cresko WA. Perchlorate induces hermaphroditism in threespine sticklebacks. Environmental Toxicology and Chemistry. 2006;25:2087–2096. doi: 10.1897/05-454r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieniarz K, Sokolowska M, Fostier A, Epler P. Daily changes in blood serum levels of 17 beta-estradiol and 11-ketotestosterone in the mature carp Cyprinus carpio L. Chronobiologia. 1986;13:23–27. [PubMed] [Google Scholar]
- Borg B. Androgens in teleost fishes. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology. 1994;109:219–245. [PubMed] [Google Scholar]
- Brown S, Fedoruk K, Eales JG. Physical injury due to injection or blood removal causes transitory elevations of plasma thyroxine in rainbow trout, Salmo gairdneri. Canadian Journal of Zoology. 1978;56:1998–2003. [Google Scholar]
- Brown SB, Adams BA, Cyr DG, Eales JG. Contaminant effects on the teleost fish thyroid. Environmental Toxicology and Chemistry. 2004;23:1680–1701. doi: 10.1897/03-242. [DOI] [PubMed] [Google Scholar]
- Buck CL, Barnes BM. Androgen in free-living arctic ground squirrels: seasonal changes and influence of staged male-male aggressive encounters. Hormones and Behavior. 2003;43:318–326. doi: 10.1016/s0018-506x(02)00050-8. [DOI] [PubMed] [Google Scholar]
- Comeau LA, Campana SE, Hanson JM, Chouinard GA. Seasonal changes of thyroid hormones in field-collected Atlantic cod in relation to condition indices, water temperature and photoperiod. Journal of Fish Biology. 2000;57:571–588. [Google Scholar]
- Crane HM, Pickford DB, Hutchinson TH, Brown JA. Developmental changes of thyroid hormones in the fathead minnow, Pimephales promelas. General and Comparative Endocrinology. 2004;139:55–60. doi: 10.1016/j.ygcen.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Cyr D, Eales JG. Interrelationships between thyroidal and reproductive endocrine systems in fish. Reviews in Fish Biology and Fisheries. 1996;6:165–200. [Google Scholar]
- Dardente H, Hazlerigg DG, Ebling FJ. Thyroid hormone and seasonal rhythmicity. Frontiers in Endocrinology (Lausanne) 2014;5:19. doi: 10.3389/fendo.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta PK, Dyke JV, Kirk AB, Jackson WA. Perchlorate in the United States. Analysis of relative source contributions to the food chain. Environmental Science & Technology. 2006;40:6608–6614. doi: 10.1021/es061321z. [DOI] [PubMed] [Google Scholar]
- De Groef B, Decallonne BR, Van der Geyten S, Darras VM, Bouillon R. Perchlorate versus other environmental sodium/iodide symporter inhibitors: potential thyroid-related health effects. European Journal of Endocrinology. 2006;155:17–25. doi: 10.1530/eje.1.02190. [DOI] [PubMed] [Google Scholar]
- Duarte-Guterman P, Navarro-Martín L, Trudeau VL. Mechanisms of crosstalk between endocrine systems: Regulation of sex steroid hormone synthesis and action by thyroid hormones. General and Comparative Endocrinology. doi: 10.1016/j.ygcen.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Eales JG. The peripheral metabolism of thyroid hormones and regulation of thyroidal status in poikilotherms. Canadian Journal of Zoology. 1985;63:1217–1231. [Google Scholar]
- Eales JG, Brown SB. Measurement and regulation of thyroidal status in teleost fish. Reviews in Fish Biology and Fisheries. 1993;3:299–347. [Google Scholar]
- El Omari B, Lacroix A, Saboureau M. Daily and seasonal variations in plasma LH and testosterone concentrations in the adult male hedgehog (Erinaceus europaeus). Journal of Reproduction and Fertility. 1989;86:145–155. doi: 10.1530/jrf.0.0860145. [DOI] [PubMed] [Google Scholar]
- Flood DE, Langlois VS. Crosstalk between the thyroid hormone and androgen axes during reproductive development in Silurana tropicalis. General and Comparative Endocrinology. 2014 doi: 10.1016/j.ygcen.2014.03.037. [DOI] [PubMed] [Google Scholar]
- Furin CG, von Hippel FA, Cresko WA, Buck CL, Postlethwait J, O'Hara TM. Developmentally critical windows for histomorphology biomarkers of fish thyroid tissue and gonad maturity with exposure to sodium perchlorate General and Comparative Endocrinology. in review. [Google Scholar]
- Galhardo L, Oliveira RF. The effects of social isolation on steroid hormone levels are modulated by previous social status and context in a cichlid fish. Hormones and Behavior. 2014;65:1–5. doi: 10.1016/j.yhbeh.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Goleman WL, Carr JA, Anderson TA. Environmentally relevant concentrations of ammonium perchlorate inhibit thyroid function and alter sex ratios in developing Xenopus laevis. Environmental Toxicology and Chemistry. 2002;21:590–597. [PubMed] [Google Scholar]
- Gomez JM, Boujard T, Boeuf G, Solari A, Le Bail PY. Individual diurnal plasma profiles of thyroid hormones in rainbow trout (Oncorhynchus mykiss) in relation to cortisol, growth hormone, and growth rate. General and Comparative Endocrinology. 1997;107:74–83. doi: 10.1006/gcen.1997.6897. [DOI] [PubMed] [Google Scholar]
- Hwang P-P, Lee T-H. New insights into fish ion regulation and mitochondrion-rich cells. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2007;148:479–497. doi: 10.1016/j.cbpa.2007.06.416. [DOI] [PubMed] [Google Scholar]
- Jakobsson S, Borg B, Haux C, Hyllner SJ. An 11-ketotestosterone induced kidney-secreted protein: the nest building glue from male three-spined stickleback, Gasterosteus aculeatus. Fish Physiology and Biochemistry. 1999;20:79–85. [Google Scholar]
- Jones RA, Cohn WB, Miller TC, Jaques JT, MacKenzie DS. Cyclic mRNA expression of thyrotropin subunits and deiodinases in red drum, Sciaenops ocellatus. General and Comparative Endocrinology. 2013;194:248–256. doi: 10.1016/j.ygcen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Jordan D, Rousset B, Perrin F, Fournier M, Orgiazzi J. Evidence for circadian variations in serum thyrotropin, 3,5,3-triiodothyronine, and thyroxine in the rat. Endocrinology. 1980;107:1245–1248. doi: 10.1210/endo-107-4-1245. [DOI] [PubMed] [Google Scholar]
- Kindler PM, Philipp DP, Gross MR, Bahr JM. Serum 11-ketotestosterone and testosterone concentrations associated with reproduction in male bluegill (Lepomis macrochirus: Centrarchidae). General and Comparative Endocrinology. 1989;75:446–453. doi: 10.1016/0016-6480(89)90180-9. [DOI] [PubMed] [Google Scholar]
- Kitano J, Lema SC, Luckenbach JA, Mori S, Kawagishi Y, Kusakabe M, Swanson P, Peichel CL. Adaptive divergence in the thyroid hormone signaling pathway in the stickleback radiation. Current Biology. 2010;20:2124–2130. doi: 10.1016/j.cub.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen DA, Swanson P, Dickhoff WW. The pituitary-thyroid axis during the parr-smolt transformation of Coho salmon, Oncorhynchus kisutch: quantification of TSH beta mRNA, TSH, and thyroid hormones. General and Comparative Endocrinology. 2011;171:367–372. doi: 10.1016/j.ygcen.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Laucht S, Dale J, Mutzel A, Kempenaers B. Individual variation in plasma testosterone levels and its relation to badge size in House Sparrows Passer domesticus: It's a night-and-day difference. General and Comparative Endocrinology. 2011;170:501–508. doi: 10.1016/j.ygcen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Lawrence JE, Lamm SH, Pino S, Richman K, Braverman LE. The effect of short-term low- dose perchlorate on various aspects of thyroid function. Thyroid : official journal of the American Thyroid Association. 2000;10:659–663. doi: 10.1089/10507250050137734. [DOI] [PubMed] [Google Scholar]
- Leiner KA, Han GS, MacKenzie DS. The effects of photoperiod and feeding on the diurnal rhythm of circulating thyroid hormones in the red drum, Sciaenops ocellatus. General and Comparative Endocrinology. 2000;120:88–98. doi: 10.1006/gcen.2000.7539. [DOI] [PubMed] [Google Scholar]
- Leiner KA, MacKenzie DS. The effects of photoperiod on growth rate and circulating thyroid hormone levels in the red drum, Sciaenops ocellatus: evidence for a free-running circadian rhythm of T4 secretion. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2001;130:141–149. doi: 10.1016/s1095-6433(01)00373-7. [DOI] [PubMed] [Google Scholar]
- Leiner KA, Mackenzie DS. Central regulation of thyroidal status in a teleost fish: Nutrient stimulation of T4 secretion and negative feedback of T3. Journal of Experimental Zoology Part A: Comparative Experimental Biology. 2003;298A:32–43. doi: 10.1002/jez.a.10255. [DOI] [PubMed] [Google Scholar]
- Leung AM, Pearce EN, Braverman LE. Perchlorate, iodine and the thyroid. Best Practice and Research in Clinical Endocrinology & Metabolism. 2010;24:133–141. doi: 10.1016/j.beem.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi V, Earley RL, Rodgers EW, Pepper DR, Grober MS. Diurnal patterns and sex differences in cortisol, 11-ketotestosterone, testosterone, and 17-estradiol in the bluebanded goby (Lythrypnus dalli). General and Comparative Endocrinology. 2008;155:438–446. doi: 10.1016/j.ygcen.2007.07.010. [DOI] [PubMed] [Google Scholar]
- MacKenzie DS, Jones RA, Miller TC. Thyrotropin in teleost fish. General and Comparative Endocrinology. 2009;161:83–89. doi: 10.1016/j.ygcen.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Yabuno A, Kiros S, Soyano K, Takegaki T. Changes in male courtship intensity and androgen levels during brood cycling in the blenniid fish Rhabdoblennius nitidus. Journal of Ethology. 2012;30:387–394. [Google Scholar]
- Mayer I, Borg B, Schulz R. Seasonal changes in and effect of castration/androgen replacement on the plasma levels of five androgens in the male three-spined stickleback, Gasterosteus aculeatus L. General and Comparative Endocrinology. 1990;79:23–30. doi: 10.1016/0016-6480(90)90084-y. [DOI] [PubMed] [Google Scholar]
- Mukhi S, Carr JA, Anderson TA, Patiño R. Novel biomarkers of perchlorate exposure in zebrafish. Environmental Toxicology and Chemistry. 2005;24:1107–1115. doi: 10.1897/04-270r.1. [DOI] [PubMed] [Google Scholar]
- Mukhi S, Patiño R. Effects of prolonged exposure to perchlorate on thyroid and reproductive function in zebrafish. Toxicological Sciences. 2007;96:246–254. doi: 10.1093/toxsci/kfm001. [DOI] [PubMed] [Google Scholar]
- Mukhi S, Torres L, Patiño R. Effects of larval–juvenile treatment with perchlorate and co- treatment with thyroxine on zebrafish sex ratios. General and Comparative Endocrinology. 2007;150:486–494. doi: 10.1016/j.ygcen.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Nakane Y, Yoshimura T. Universality and diversity in the signal transduction pathway that regulates seasonal reproduction in vertebrates. Frontiers in Neuroscience. 2014;8 doi: 10.3389/fnins.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CS, Bourdo R, Bradshaw WE, Holzapfel CM, Cresko WA. Conservation of the photoperiodic neuroendocrine axis among vertebrates: evidence from the teleost fish, Gasterosteus aculeatus. General and Comparative Endocrinology. 2012;178:19–27. doi: 10.1016/j.ygcen.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AVM. Social modulation of androgen levels in male teleost fish. Comparative Biochemistry and Physiology Part B: Biochemistry & Molecular Biology. 2002;132:203–215. doi: 10.1016/s1096-4959(01)00523-1. [DOI] [PubMed] [Google Scholar]
- Páll MK, Hellqvist A, Schmitz M, Olsson PE, Mayer I, Borg B. Changes in reproductive physiology and behaviour over the nesting cycle in male three-spined sticklebacks. Journal of Fish Biology. 2005;66:1400–1410. [Google Scholar]
- Páll MK, Mayer I, Borg B. Androgen and behavior in the male three-spined stickleback, Gasterosteus aculeatus i. Changes in 11-ketotestosterone levels during the nesting cycle. Hormones and Behavior. 2002a;41:377–383. doi: 10.1006/hbeh.2002.1777. [DOI] [PubMed] [Google Scholar]
- Páll MK, Mayer I, Borg B. Androgen and behavior in the male three-spined stickleback, Gasterosteus aculeatus: ii. Castration and 11-ketoandrostenedione effects on courtship and parental care during the nesting cycle. Hormones and Behavior. 2002b;42:337–344. doi: 10.1006/hbeh.2002.1820. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Dillon D, Bernhardt RA, Torunsky R, Postlethwait JH, von Hippel FA, Buck CL, Cresko WA. Perchlorate disrupts reproductive development in threespine stickleback without changing whole-body levels of thyroid hormone. General and Comparative Endocrinology. 2015;210:130–144. doi: 10.1016/j.ygcen.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottinger TG, Carrick TR, Hughes SE, Balm PHM. Testosterone, 11-ketotestosterone, and estradiol-17 modify baseline and stress-induced interrenal and corticotropic activity in trout. General and Comparative Endocrinology. 1996;104:284–295. doi: 10.1006/gcen.1996.0173. [DOI] [PubMed] [Google Scholar]
- Power DM, Llewellyn L, Faustino M, Nowell MA, Björnsson BT, Einarsdottir IE, Canario AVM, Sweeney GE. Thyroid hormones in growth and development of fish. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2001;130:447–459. doi: 10.1016/s1532-0456(01)00271-x. [DOI] [PubMed] [Google Scholar]
- Sanchez CA, Blount BC, Valentin-Blasini L, Krieger RI. Perchlorate, thiocyanate, and nitrate in edible cole crops (Brassica sp.) produced in the lower Colorado River region. Bulletin of Environmental Contamination and Toxicology. 2007;79:655–659. doi: 10.1007/s00128-007-9292-6. [DOI] [PubMed] [Google Scholar]
- Schmidt F, Schnurr S, Wolf R, Braunbeck T. Effects of the anti-thyroidal compound potassium-perchlorate on the thyroid system of the zebrafish. Aquatic Toxicology. 2012;109:47–58. doi: 10.1016/j.aquatox.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Sebire M, Katsiadaki I, Scott AP. Non-invasive measurement of 11-ketotestosterone, cortisol and androstenedione in male three-spined stickleback (Gasterosteus aculeatus). General and Comparative Endocrinology. 2007;152:30–38. doi: 10.1016/j.ygcen.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Knapp R, Bass AH. Seasonal variation of steroid hormone levels in an intertidal-nesting fish, the vocal plainfin midshipman. General and Comparative Endocrinology. 2004;136:101–116. doi: 10.1016/j.ygcen.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Specker JL, Brown CL, Bern HA. Asynchrony of changes in tissue and plasma thyroid hormones during the parr-smolt transformation of coho salmon. General and Comparative Endocrinology. 1992;88:397–405. doi: 10.1016/0016-6480(92)90234-b. [DOI] [PubMed] [Google Scholar]
- Spieler RE. Diel rhythms of circulating prolactin, cortisol, thyroxine, and triiodothyronine levels in fishes: A review. Reviews in Canadian Biology. 1979:301–315. [Google Scholar]
- Tagawa M, Hirano T. Changes in tissue and blood concentrations of thyroid hormones in developing chum salmon. General and Comparative Endocrinology. 1989;76:437–443. doi: 10.1016/0016-6480(89)90140-8. [DOI] [PubMed] [Google Scholar]
- Theodorakis C, Rinchard J, Anderson T, Liu F, Park J-W, Costa F, McDaniel L, Kendall R, Waters A. Perchlorate in fish from a contaminated site in east-central Texas. Environmental Pollution. 2006;139:59–69. doi: 10.1016/j.envpol.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Urbansky E. Perchlorate as an environmental contaminant. Environmental Science and Pollution Research. 2002;9:187–192. doi: 10.1007/BF02987487. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. The Journal of Clinical Investigation. 1993;92:1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. Perchlorate and the thyroid gland. Pharmacological Reviews. 1998;50:89–106. [PubMed] [Google Scholar]
- Wu F, Zhou X, Zhang R, Pan M, Peng KL. The effects of ammonium perchlorate on thyroid homeostasis and thyroid-specific gene expression in rat. Environmental toxicology. 2012;27:445–452. doi: 10.1002/tox.20655. [DOI] [PubMed] [Google Scholar]
- Yamada H, Satoh R, Ogoh M, Takaji K, Fujimoto Y, Hakuba T, Chiba H, Kambegawa A, Iwata M. Circadian changes in serum concentrations of steroids in Japanese char Salvelinus leucomaenis at the stage of final maturation. Zoological Sciences. 2002;19:891–898. doi: 10.2108/zsj.19.891. [DOI] [PubMed] [Google Scholar]
- Yoshimura T. Thyroid hormone and seasonal regulation of reproduction. Frontiers in Neuroendocrinology. 2013;34:157–166. doi: 10.1016/j.yfrne.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Young G, Thorarensen H, Davie PS. 11-ketotestosterone suppresses interrenal activity in rainbow trout (Oncorhynchus mykiss). General and Comparative Endocrinology. 1996;103:301–307. doi: 10.1006/gcen.1996.0125. [DOI] [PubMed] [Google Scholar]


